Introduction
Osteosarcoma is the most common primary malignant
bone tumor (1). It is derived
from primitive mesenchymal cells and commonly develops in the
distal femur and proximal tibia (2). Osteosarcoma predominantly affects
young people, in particular, adolescents aged below 20 years
(3,4). The prognosis of osteosarcoma
patients is poor with an overall 5-year survival rate below 30% in
cases showing relapse and metastasis (5).
At present, neoadjuvant chemotherapy is the
predominant treatment for osteosarcoma. Neoadjuvant chemotherapy
combines surgery with pre- or post-operative multi-agent
chemotherapy. Most patients require intensive chemotherapy with a
combination of drugs such as methotrexate, cisplatin, doxorubicin,
ifosfamide and etoposide (6).
This leads to chemotherapy toxicity in normal tissues and cellular
chemo-resistance, which prevent the long-term application of this
treatment (7,8). Introduction of several targeted
therapeutic agents has markedly improved the results of preclinical
and clinical trials on osteosarcoma (9). However, the high cost of these
therapeutic agents limit their widespread application. Therefore,
safe and effective therapeutic approaches are urgently needed for
treating osteosarcoma.
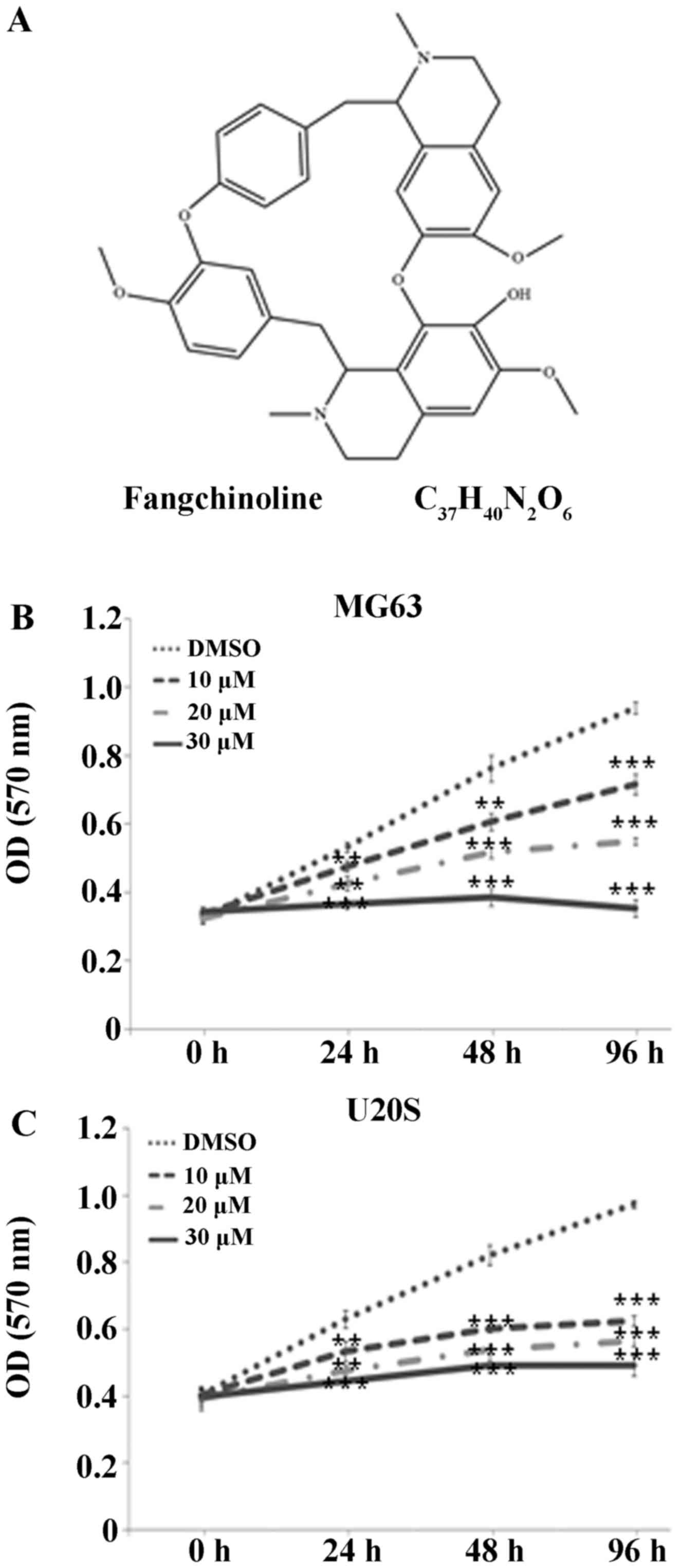
Fangchinoline is a bisbenzylisoquinoline alkaloid
with a complex structure (Fig.
1A) and is extracted from an alkaloid tetrandrine, found in a
traditional Chinese medicinal plant belonging to the Menispermaceae
family. Increasing evidence suggests that fangchinoline posesses
various pharmacological activities (10,11). Current research has focused on the
antitumor effects of fangchinoline and has shown that it exerts
antitumor effects on lung cancer (12), breast cancer (13,14), hepatocellular carcinoma (15), chronic myelogenous leukemia
(16), glioblastoma (17), gastric cancer (18), and prostate cancer cells (19). However, the antitumor effects of
fangchinoline on osteosarcoma cells are unclear.
Therefore, the present study was designed to
evaluate the effects of fangchinoline on osteosarcoma cell lines
MG63 and U20S. The study evaluated the effects of fangchinoline on
the proliferation, apoptosis, migration and invasion of
osteosarcoma cells in vitro and on their tumorigenesis in
vivo and determined the possible underlying mechanism of
action.
Materials and methods
Cell culture
Human osteosarcoma cell lines MG63 and U20S were
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco BRL, Gaithersburg, MD, USA)
supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin-streptomycin in an incubator with a humidified
atmosphere of 5% CO2. Cell medium was replaced with
fresh medium containing dimethyl sulfoxide (DMSO) or 10, 20 or 30
µM fangchinoline for the following experiments. Changes in
the morphology of the cells were determined using an inverted
phase-contrast microscope.
Cell proliferation assay
Cell viability was evaluated by performing
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide (MTT)
assay, as described previously (20). Briefly, MG63 or U20S cells were
seeded in 96-well plates(cell density, 1×104 cells/well)
containing 0.1 ml medium supplemented with the indicated
concentration of fangchino-line and were incubated for 24, 48 and
96 h. At each time point, 0.01 ml MTT solution (5 mg/ml in PBS) was
added to each well, and the cells were incubated for 4 h at 37°C.
The medium was replaced with DMSO for 10 min to solubilize
crystals, and optical densities (ODs) were measured at 570 nm.
Hoechst 33258 staining
MG63 cells were treated with DMSO or 10, 20 or 30
µM fangchinoline for 24 h. Next, the cells were fixed with
4% polyoxymethylene, washed twice with PBS, and then incubated with
10 µg/ml Hoechst 33258 solution in the dark for 5 min at
room temperature. Finally, the cells were washed three times with
PBS and were observed under a fluorescence microscope.
Flow cytometry
Cell apoptosis was assessed using Annexin
V-fluorescein isothiocyanate or Annexin V-propidiumiodide (Annexin
V-FITC/Annexin V-PI) apoptosis detection kit (Thermo Fisher
Scientific, Waltham, MA, USA). MG63cells were harvested, stained
with Annexin V-FITC or Annexin V-PI, and analyzed using a FACScan
flow cytometer (Becton-Dickinson, Franklin Lakes, NJ, USA). Cells
showing Annexin V(+)/PI(−) staining were considered as early
apoptotic cells and those showing Annexin V(+)/PI(+) staining were
considered as late apoptotic cells.
Scratch wound healing assay
MG63 cells were cultured in 6-well plates until
confluency and were incubated in serum-free DMEM overnight before
wounding. Scratches were made using a pipette tip, and the cells
were treated with a medium containing DMSO or 10, 20 or 30
µM fangchinoline at 37°C for 24 and 48 h. Images of the
wounded area were obtained and quantified using an optical
microscope.
Transwell assay
Transwell assay was performed to evaluate cell
invasion. The upper part of each Transwell chamber was coated with
1×105 cells cultured in 0.1 ml serum-free DMEM
supplemented with DMSO or 10, 20 or 30 µM fangchinoline, and
the lower part of the chamber was filled with 0.6 ml DMEM
containing 10% FBS. After incubation for 24 h at 37°C, the cells in
the upper part of chamber were removed. Cells invading the lower
part of the chamber were fixed, stained, and counted using a
high-power microscope.
Western blot analysis
Total cellular proteins were extracted from
1×106 cultured cells using 100 µl RIPA lysis
buffer. Next, 60 µg of the protein samples were resolved by
performing SDS-PAGE and were transferred onto nitrocellulose
membranes through electroblotting. The membranes were blocked using
5% non-fat dry milk for 1 h and were probed by incubating overnight
with the primary antibodies at 4°C. Next, the membranes were washed
and incubated with HRP-conjugated secondary antibodies
(Sigma-Aldrich, St. Louis, MO, USA) for 1 h. Immunoreactivity was
detected using Western Lighting Ultra (ECL; Pierce Technology,
Rockford, IL, USA). The following primary antibodies were used: i)
rabbit polyclonal antibody against caspase-9 (ab32068) and
monoclonal antibody against caspase-3 (ab32351) (Abcam, Cambridge,
UK) at a dilution of 1:5,000; ii) rabbit polyclonal antibody
against GAPDH (sc-25778) and monoclonal antibodies against
phosphoinositide 3-kinase (PI3K; sc-7175), Akt (sc-135829), and
Aktp-Thr308 (sc-135650) (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) at a dilution of 1:1,000; and iii) rabbit
monoclonal antibodies against cyclin D1 (60186-1-1g), MMP-2
(10373-2-AP), and MMP-9 (10375-2-AP) (ProteinTech Group, Inc.,
Rosemont, IL, USA) at a dilution of 1:1,000.
Animal model of osteosarcoma
Six-week-old femaleBalb/c-nu-nu mice were purchased
from Guangxi Medical University (Guangxi, China). All animal
experimental procedures were approved by the Ethics Committee
(2016/025) of the Shaoxing People's Hospital (Zhejiang, China) and
were performed as described previously (12). The mice were randomly divided into
fangchinoline treatment and control groups, with six mice in each
group. All the mice were maintained under sterile conditions at
20–25°C room temperature, 50–60% relative humidity, and a 12 h
light/dark cycle and were fed a sterilized diet. After habituation
for 7 days, the mice were anesthetized by intraperitoneally
injecting 12 mg/kg chloral hydrate. Next, 0.2 ml of the U20S cell
suspension (cell density, 1×107 cells/ml) was
subcutaneously injected into the left armpit of each mouse. Tumors
became palpable 1 week after xenografting. Next, 0.1 ml
fangchinoline solution (0.5 mg/ml) or 0.1 ml DMSO solution was
locally injected into the tumors of each mouse three times per
week. The mice were sacrificed 4 weeks after xenografting, and
their tumors were harvested and weighed. The length and width of
the tumors were measured to calculate tumor volume (tumor volume =
length × width2/2).
Histological staining
The kidneys and livers of the mice were harvested,
fixed, and embedded in paraffin. Histological sections were
prepared for hematoxylin and eosin (H&E) staining, and images
were obtained using a light microscope.
Statistical analysis
Data for all groups are presented as mean ± SD.
Differences between the groups were evaluated using one-way
analysis of variance (ANOVA) with LSD test, and P<0.05 was
considered statistically significant. Statistical analysis was
performed using SPSS software version 19.0.
Results
Fangchinoline decreases the viability of
osteosarcoma cells
Fangchinoline markedly suppressed the proliferation
of human osteosarcoma cell lines MG63 and U20S in a dose-dependent
manner. The MTT assay showed that the ODs of the MG63 cells treated
with 10, 20 and 30 µM fangchinoline significantly decreased
to 0.72±0.03 (P<0.001), 0.55±0.01 (P<0.001), and 0.35±0.03
(P<0.001), respectively, after 96 h compared with the OD of the
control cells (0.94±0.02; Fig.
1B). Similar trends were observed at the 24 and 48 h time
points. The experiment was repeated using U20S cells, which
responded similarly to the inhibitory effects of fangchinoline
(Fig. 1C).
Fangchinoline increases the apoptosis of
osteosarcoma cells
Fangchinoline treatment significantly accelerated
the apoptosis of MG63 and U20S cells in a dose-dependent manner.
Fangchinoline-untreated MG63 and U20S cells were rhombus-like and
angular, as determined by performing inverted phase-contrast
microscopy, and were attached to the culture plates along with few
dead cells that were round and suspended (Fig. 2A). Treatment with 10, 20 and 30
µM fangchinoline markedly increased the number of apoptotic
cells to 177±19.1 (P<0.001), 322±21.5 (P<0.001), and 418±8.9
(P<0.001), respectively, compared with that in the control cells
(8.7±5.7; Fig. 2B). Similar
trends were observed for U20S cells (Fig. 2C and D). Hoechst 33258 staining
showed that fangchinoline treatment of MG63 cells induced the
formation of apoptotic nuclei and condensed their chromatin, which
appeared bright blue (Fig. 2E).
Results of the flow cytometry showed that the percentages of late
apoptotic cells increased to 10.35±0.55 (P=0.001), 12.77±0.69
(P<0.001) and 30.56±0.80% (P<0.001) in the 10, 20 and 30
µM fangchinoline-treated MG63 cells, respectively, compared
with 7.23%±0.15% in the control cells (Fig. 2F). A similar trend was observed
for the percentages of early apoptotic MG63 cells.
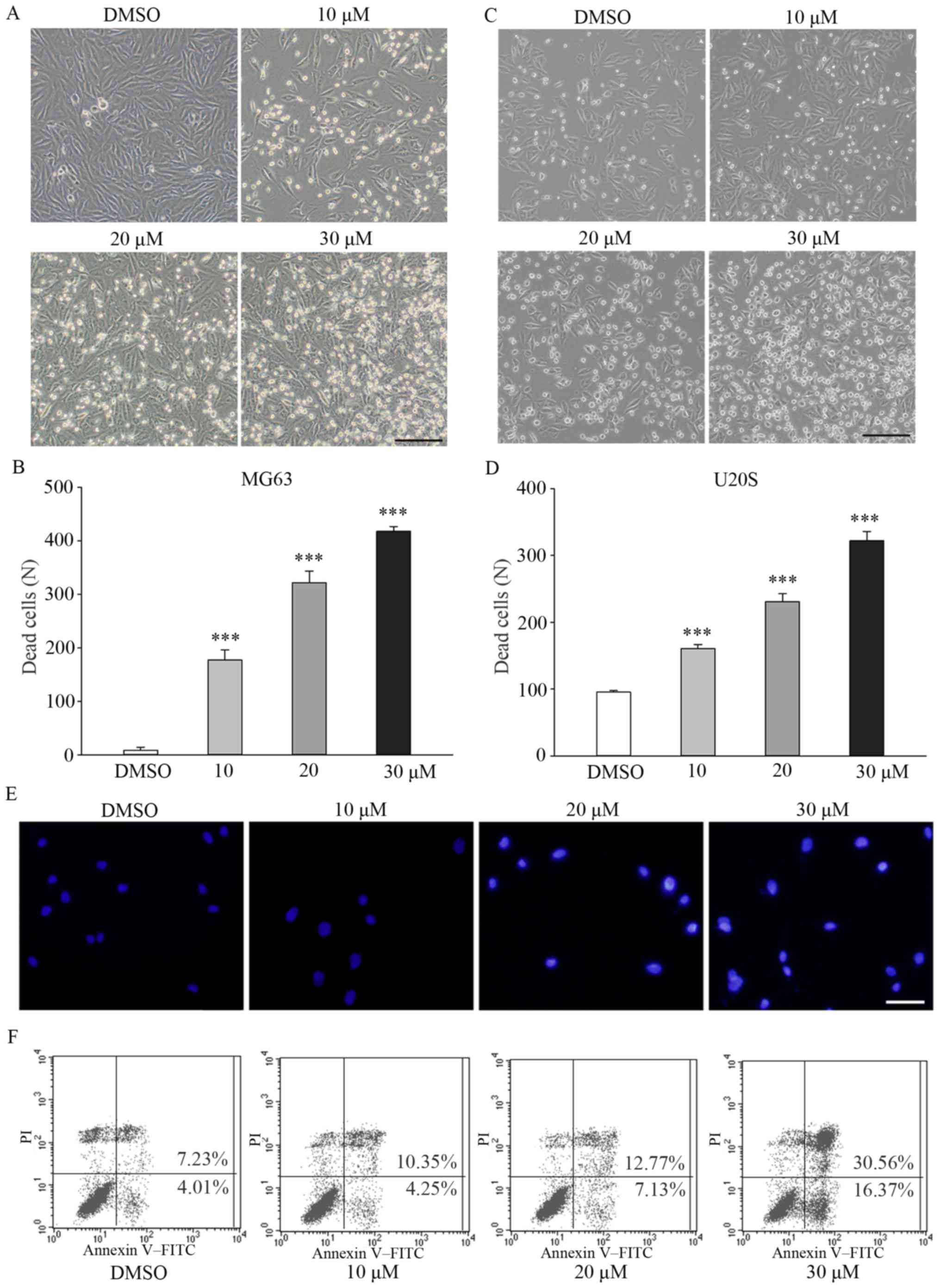 | Figure 2Fangchinoline increases the apoptosis
of osteosarcoma cells. (A) Changes in the morphology of MG63 cells
treated with DMSO or 10, 20 or 30 µM fangchinoline for 48 h
were determined using an inverted phase-contrast microscope; scale
bar, 100 µm. (B) Dead MG63 cells were counted, and results
are presented as mean ± SD. (C) Changes in the morphology of U20S
cell treated with DMSO or 10, 20 or 30 µM fangchinoline for
48 h were determined using an inverted phase-contrast microscope;
scale bar, 100 µm. (D) Dead U20S cells were counted, and
results are presented as mean ± SD; ***P<0.001. (E)
MG63 cells were preincubated with DMSO or 10, 20 or 30 µM
fangchinoline for 48 h. Next, the cells were stained with Hoechst
33258 solution and were observed under a fluorescence microscope;
scale bar, 50 µm. (F) Flow cytometric analysis of MG63 cells
treated with 0, 10, 25 or 30 µM fangchinoline for 24 h.
Annexin V(+)/PI(−) cells were considered as early apoptotic
cells. |
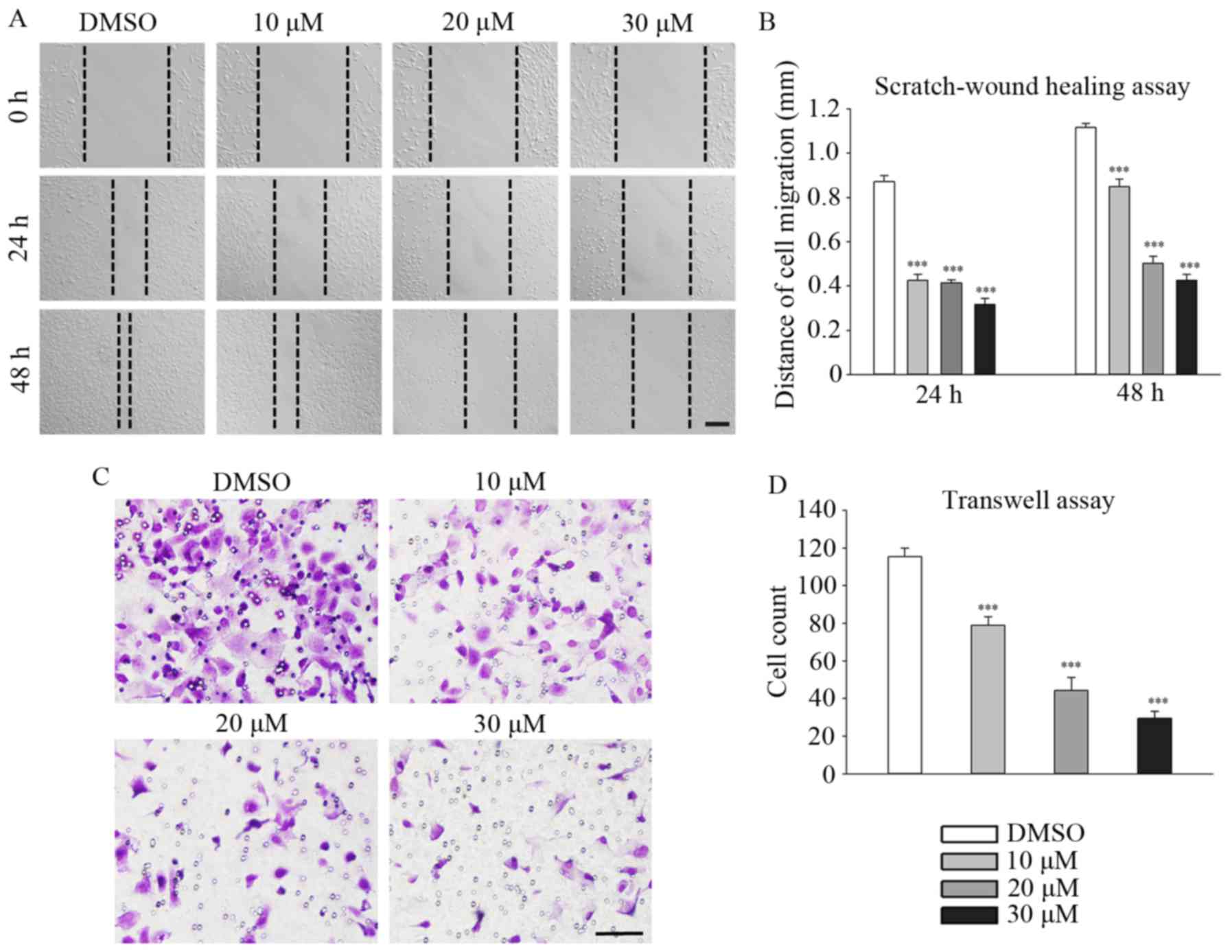
Fangchinoline suppresses the migration
and invasion of MG63 cells
Fangchinoline significantly inhibited the migration
and invasion of MG63 cells in a dose-dependent manner. Distances
traveled by 10, 20 and 30 µM fangchinoline-treated MG63
cells decreased to 0.86±0.03 (P<0.001), 0.50±0.03 (P<0.001),
and 0.43±0.03 mm (P<0.001), respectively, compared with the
distance travelled by the control cells (1.18±0.02 mm) at the 48 h
time point (Fig. 3A and B).
Similar trends were observed at the 24 h time point. The Transwell
assay showed that the number of 10, 20 and 30 µM
fangchinoline-treated MG63 cells traversing through the
polycarbonate membrane decreased to 79±4.6 (P<0.001), 44±7.0
(P<0.001) and 30±3.5 (P<0.001), respectively, compared with
the number of control cells (115±4.5; Fig. 3C and D).
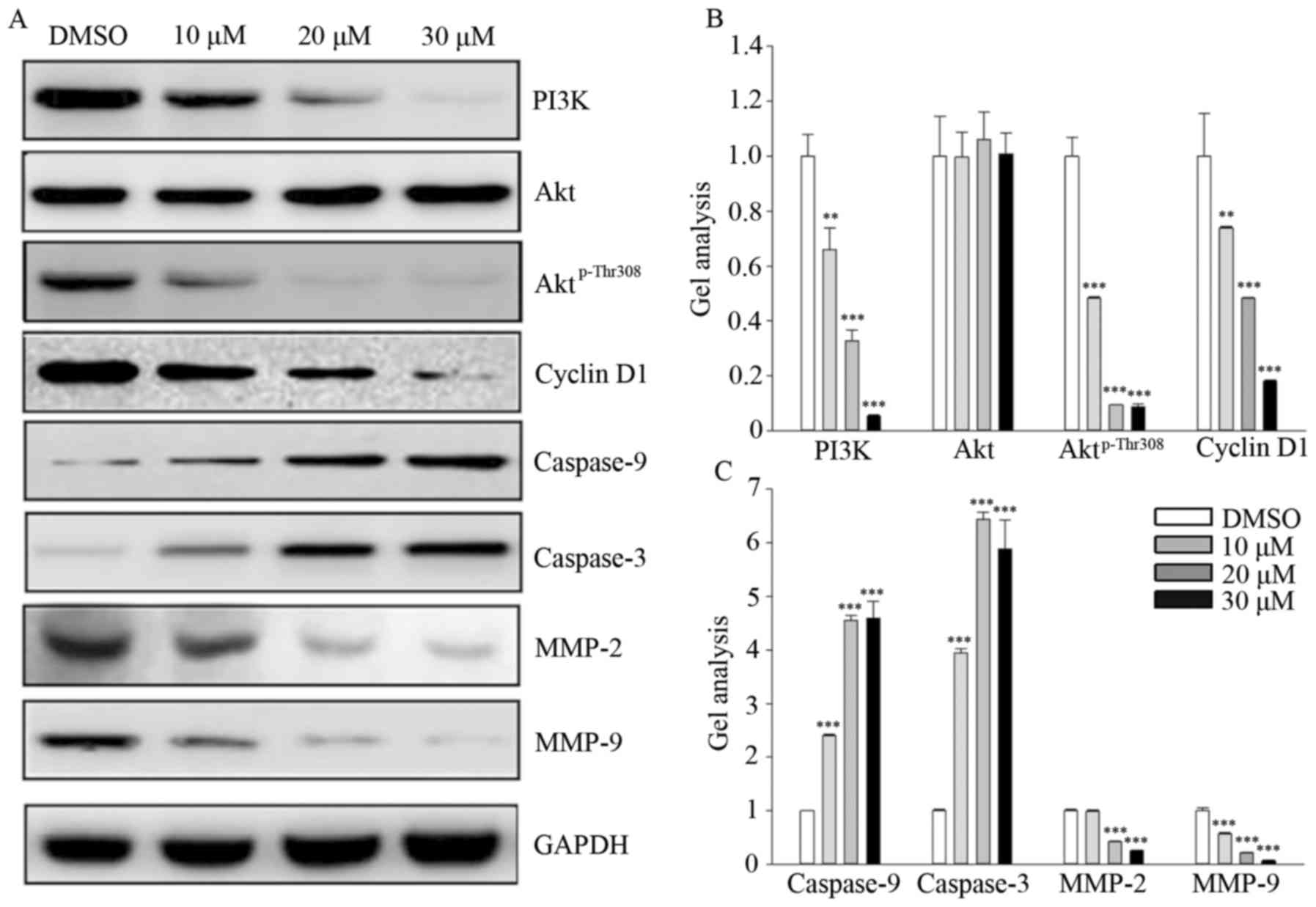
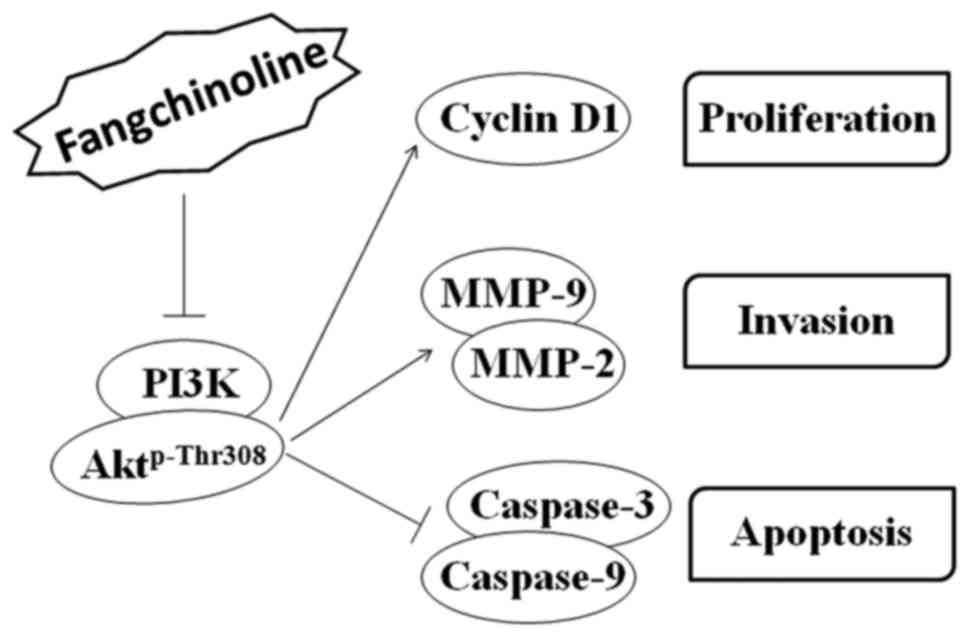
Fangchinoline inhibits the PI3K/Akt
signaling pathway
Fangchinoline treatment markedly decreased PI3K and
Aktp-Thr308 expression in a dose-dependent manner but
did not alter the total Akt level. Moreover, fangchinoline
treatment markedly decreased the level of cell proliferation
regulator cyclin D1 but obviously increased the expression of
caspase-9 and caspase-3. In contrast, fangchinoline treatment
significantly decreased MMP-2 and MMP-9 expression in the MG63
cells (Fig. 4A–C).
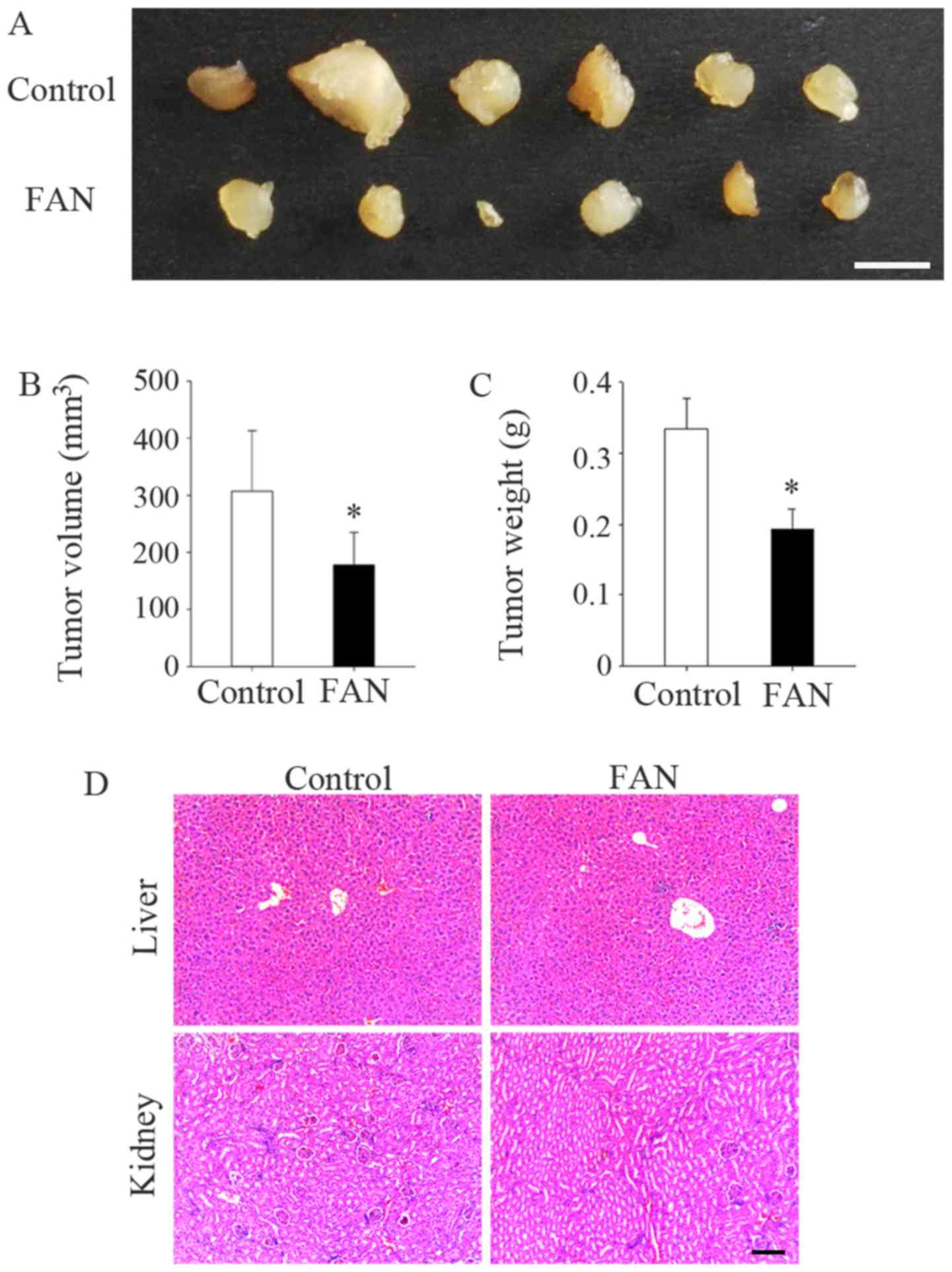
Fangchinoline inhibits the tumorigenesis
of MG63 cells in vivo
The average size (178.23±23.28 mm3,
P=0.043) and weight (0.19±0.03 g, P=0.034) of tumors in the
fangchinoline-treated mice were significantly decreased compared
with these parameters in the control mice (307.31±43.29
mm3 and 0.33±0.04 g, respectively; Fig. 5A–C). In addition, none of the mice
died during the experimental period. Moreover, no histological
impact was observed on the kidneys and livers of mice in both the
fangchinoline and control groups (Fig. 5D).
Discussion
Previous studies have reported that fangchinoline
exerts antitumor effects on several tumor cell lines by inhibiting
proliferation and by inducing apoptosis. However, its effects on
osteosarcoma cells have not been elucidated to date. In the present
study, we observed that fangchinoline suppressed the proliferation,
migration and invasion of osteosarcoma cells lines MG63 and U20S in
a dose-dependent manner. We speculate that these effects are
associated with the inhibition of PI3K and its downstream signaling
pathways. Moreover, we observed that fangchinoline inhibited the
growth of subcutaneous osteosarcoma tumors in vivo. These
results suggest that fangchinoline exerts antitumor effects against
osteosarcoma cell lines.
Chemotherapeutic drugs such as paclitaxel exert an
obvious killing effect on osteosarcoma cells (21). However, drug resistance and toxic
side-effects after long-term application remain the main obstacles
for their clinical use. Fangchinoline is a well-known traditional
Chinese medicine that is chemically extracted from the alkaloid
tetrandrine. It is an effective agent for tumor treatment and
prevention, with no significant toxicity or side-effects (18). Research on the functions of
fangchinoline will provide a potential new treatment for
osteosarcoma, with important clinical significance.
The PI3K/Akt signaling pathway mediates various
cellular functions, including cell proliferation, apoptosis and
invasion. The PI3K/Akt pathway plays a central role in regulating
the functions of osteosarcoma cells (22) and is an important target for
treating different tumors (23–25). Fangchinoline was found to
effectively suppress the proliferation of SGC7901, MKN45, and human
gastric cancer cell lines that highly express PI3K but to exert a
negligible effect on human embryonic kidney 293 cells and normal
cells that express low levels of PI3K (18), suggesting that fangchinoline is a
novel inhibitor of PI3K and exerts antitumor effects by suppressing
PI3K. Consistently, we found that fangchinoline treatment
significantly decreased the expression of PI3K and
Aktp-Thr308 in a dose-independent manner. These findings
may explain why traditional Chinese medicines show negligible
toxicity against normal cells. Tumor cells grow rapidly because of
their activated cell cycle progression. Cyclin D1, a downstream
target of the PI3K/Akt signaling pathway, is a key regulator of the
G1-S checkpoint (26,27). Previous studies have shown that
fangchinoline inhibits cell cycle progression in breast cancer
cells by inhibiting the Akt/GSK-3β/cyclin D1 signaling pathway
(13). Moreover, levels of cyclin
D3 and cyclin E and cyclin-dependent kinase 2, 4 and 6 are
decreased and levels of their endogenous inhibitors
p21WAF1 and p27Kip1 are increased in
fangchinoline-treated breast cancer cells (14). In the present study, cyclin D1
expression markedly decreased after fangchinoline treatment. These
results suggest that fangchinoline prevents cell cycle progression
in osteosarcoma cells by inhibiting the PI3K/Akt/cyclin D1
signaling pathway, thereby inhibiting the proliferation of these
cells.
Enhancement of tumor cell apoptosis is another
effective strategy for treating tumors. Fangchinoline was found to
promote the early apoptosis of human glioblastoma cells by
inhibiting Akt-mediated Bax and caspase-9 expression (17), suggesting that fangchinoline
serves as a caspase agonist and can be used as an alternative
treatment for tumors. Interestingly, another study showed that
fangchinoline did not induce apoptotic cell death but induced
autophagic cell death in hepatoma cell lines HepG2 and PLC/PRF-5
through the p53/sestrin2/AMPK signaling pathway (15). These results suggest that
fangchinoline induces cell death through different mechanisms and
exerts specific effects on different tumor cells. In the present
study, we observed that fangchino-line significantly accelerated
the apoptosis of MG63 cells and markedly decreased the expression
of caspase-8 and caspase-3. However, further studies are needed to
elucidate whether fangchinoline induces autophagic cell death in
osteosarcoma cells. Therefore, it is reasonable to speculate that
fangchinoline enhances the apoptosis of osteosarcoma cells.
Besides accelerated proliferation, osteosarcoma
cells show early metastasis. During metastasis, MMPs play a major
role in degrading the extracellular matrix, thus allowing tumor
cells to migrate and accelerate metastatic progression (28). A previous study showed that
fangchino-line effectively inhibited the proliferation and invasion
of gastric cancer cell lines BGC823 and SGC7901 by inhibiting
PI3K-induced MMP-9 and MMP-2 expression (18). Similarly, we observed that
fangchinoline significantly suppressed the migration and invasion
of MG63 cells by downregulating MMP-9 and MMP-2 expression. This
finding suggests that fangchinoline can be used as an
antimetastatic agent. Moreover, fangchinoline suppressed the
migratory and invasive potential of lung cancer cell line A549 by
inhibiting the FAK/MMP-9/MMP-2 pathway (12). However, it is unclear whether
fangchinoline targets PI3K in A549 cells. These findings suggest
that fangchinoline inhibits MMPs by inhibiting different upstream
signaling pathways. As fangchinoline downregulated the expression
of MMPs and PI3K/Akt in the present study, we speculate that
fangchinoline inhibits the migration and invasion of osteosarcoma
cells by inhibiting the PI3K/Akt/MMP-9/MMP-2 signaling pathway.
An animal model of osteosarcoma was used to evaluate
the antitumor effect of fangchinoline in vivo. Fangchinoline
was previously found to inhibit the growth of lung tumors formed by
A549 cells in a nude mouse model (12). Similarly, fangchinoline
significantly suppressed the growth of subcutaneous osteosarcoma
tumors in the present study. Moreover, fangchinoline treatment did
not exert any toxic side-effects, as evidenced by the consistent
histology of the kidneys and livers of mice in both the
fangchinoline-treatment and control groups.
However, the present study has several limitations.
First, a positive control group was required, such as
cisplatin-treated osteosarcoma cells. Second, fangchinoline was
injected locally into the tumors of the mouse models of
osteosarcoma in the present study; however, it is unclear whether
intravenous or oral administration of fangchinoline will exert the
same effect. Third, the results of the H&E staining did not
precisely show the effect of fangchinoline on the physiological
functions of the livers and kidneys of treated mice. Moreover,
further clinical trials are needed to assess whether systemic
treatment with moderate doses of fangchinoline affects bone
metabolism.
In conclusion, the present study showed that
fangchinoline suppressed the proliferation, migration, and invasion
and enhanced the apoptosis of osteosarcoma cells. In addition,
fangchinoline inhibited the growth of osteosarcoma tumors in
vivo by possibly inhibiting PI3K and Akt and their downstream
signaling pathways (Fig. 6).
Thus, fangchinoline may serve as an auxiliary therapeutic agent for
treating osteosarcoma.
Acknowledgments
The present study was supported by the Natural
Science Foundation of Zhejiang Province (nos. LQ16H160013 and
LY15H060005) and the National Natural Science Foundation of China
(no. 81572126).
References
|
1
|
Picci P: Osteosarcoma (osteogenic
sarcoma). Orphanet J Rare Dis. 2:62007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jones KB: Osteosarcomagenesis: Modeling
cancer initiation in the mouse. Sarcoma. 2011:6941362011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stiller CA, Craft AW and Corazziari I:
Survival of children with bone sarcoma in Europe since 1978:
Results from the EUROCARE study. Eur J Cancer. 37:760–766. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu PK, Chen WM, Chen CF, Lee OK, Haung CK
and Chen TH: Primary osteogenic sarcoma with pulmonary metastasis:
Clinical results and prognostic factors in 91 patients. Jpn J Clin
Oncol. 39:514–522. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Group TEES: ESMO/European Sarcoma Network
Working Group: Bone sarcomas: ESMO Clinical Practice Guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 25(Suppl 3):
iii113–iii123. 2014. View Article : Google Scholar
|
|
7
|
Yang R, Sowers R, Mazza B, Healey JH,
Huvos A, Grier H, Bernstein M, Beardsley GP, Krailo MD, Devidas M,
et al: Sequence alterations in the reduced folate carrier are
observed in osteosarcoma tumor samples. Clin Cancer Res. 9:837–844.
2003.PubMed/NCBI
|
|
8
|
Guo W, Healey JH, Meyers PA, Ladanyi M,
Huvos AG, Bertino JR and Gorlick R: Mechanisms of methotrexate
resistance in osteosarcoma. Clin Cancer Res. 5:621–627.
1999.PubMed/NCBI
|
|
9
|
Shaikh AB, Li F, Li M, He B, He X, Chen G,
Guo B, Li D, Jiang F, Dang L, et al: Present advances and future
perspectives of molecular targeted therapy for osteosarcoma. Int J
Mol Sci. 17:5062016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma W, Nomura M, Takahashi-Nishioka T and
Kobayashi S: Combined effects of fangchinoline from Stephania
tetrandra Radix and formononetin and calycosin from Astragalus
membranaceus Radix on hyperglycemia and hypoinsulinemia in
streptozotocin-diabetic mice. Biol Pharm Bull. 30:2079–2083. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gülçin I, Elias R, Gepdiremen A, Chea A
and Topal F: Antioxidant activity of bisbenzylisoquinoline
alkaloids from Stephania rotunda: Cepharanthine and fangchinoline.
J Enzyme Inhib Med Chem. 25:44–53. 2010. View Article : Google Scholar
|
|
12
|
Guo B, Su J, Zhang T, Wang K and Li X:
Fangchinoline as a kinase inhibitor targets FAK and suppresses
FAK-mediated signaling pathway in A549. J Drug Target. 23:266–274.
2015. View Article : Google Scholar
|
|
13
|
Wang CD, Yuan CF, Bu YQ, Wu XM, Wan JY,
Zhang L, Hu N, Liu XJ, Zu Y, Liu GL, et al: Fangchinoline inhibits
cell proliferation via Akt/GSK-3beta/cyclin D1 signaling and
induces apoptosis in MDA-MB-231 breast cancer cells. Asian Pac J
Cancer Prev. 15:769–773. 2014. View Article : Google Scholar
|
|
14
|
Xing Z, Zhang Y, Zhang X, Yang Y, Ma Y and
Pang D: Fangchinoline induces G1 arrest in breast cancer cells
through cell-cycle regulation. Phytother Res. 27:1790–1794. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang N, Pan W, Zhu M, Zhang M, Hao X,
Liang G and Feng Y: Fangchinoline induces autophagic cell death via
p53/sestrin2/AMPK signalling in human hepatocellular carcinoma
cells. Br J Pharmacol. 164:731–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Chen J, Wang L, Huang Y, Leng Y
and Wang G: Fangchinoline induces G0/G1 arrest by modulating the
expression of CDKN1A and CCND2 in K562 human chronic myelogenous
leukemia cells. Exp Ther Med. 5:1105–1112. 2013.PubMed/NCBI
|
|
17
|
Guo B, Xie P, Su J, Zhang T, Li X and
Liang G: Fangchinoline suppresses the growth and invasion of human
glioblastoma cells by inhibiting the kinase activity of Akt and
Akt-mediated signaling cascades. Tumour Biol. 37:2709–2719. 2016.
View Article : Google Scholar
|
|
18
|
Tian F, Ding D and Li D: Fangchinoline
targets PI3K and suppresses PI3K/AKT signaling pathway in SGC7901
cells. Int J Oncol. 46:2355–2363. 2015.PubMed/NCBI
|
|
19
|
Li D, Lu Y, Sun P, Feng LX, Liu M, Hu LH,
Wu WY, Jiang BH, Yang M, Qu XB, et al: Inhibition on proteasome β1
subunit might contribute to the anti-cancer effects of
fangchinoline in human prostate cancer cells. PLoS One.
10:e01416812015. View Article : Google Scholar
|
|
20
|
Li X, Huang T, Jiang G, Gong W, Qian H and
Zou C: Synergistic apoptotic effect of crocin and cisplatin on
osteosarcoma cells via caspase induced apoptosis. Toxicol Lett.
221:197–204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsai HC, Huang CY, Su HL and Tang CH: CTGF
increases drug resistance to paclitaxel by upregulating survivin
expression in human osteosarcoma cells. Biochim Biophys Acta.
1843:846–854. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/Akt signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li YJ, Dong BK, Fan M and Jiang WX: BTG2
inhibits the proliferation and metastasis of osteosarcoma cells by
suppressing the PI3K/AKT pathway. Int J Clin Exp Pathol.
8:12410–12418. 2015.
|
|
24
|
Du S and Yang L: ClC-3 chloride channel
modulates the proliferation and migration of osteosarcoma cells via
AKT/GSK3β signaling pathway. Int J Clin Exp Pathol. 8:1622–1630.
2015.
|
|
25
|
Ma X, Sun W, Shen J, Hua Y, Yin F, Sun M
and Cai Z: Gelsolin promotes cell growth and invasion through the
upregulation of p-AKT and p-38 pathway in osteosarcoma. Tumour
Biol. 37:7165–7174. 2016. View Article : Google Scholar
|
|
26
|
Nakagami H, Kawamura K, Sugisaka K, Sekine
M and Shinmyo A: Phosphorylation of retinoblastoma-related protein
by the cyclin D/cyclin-dependent kinase complex is activated at the
G1/S-phase transition in tobacco. Plant Cell. 14:1847–1857. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Keenan SM, Lents NH and Baldassare JJ:
Expression of cyclin E renders cyclin D-CDK4 dispensable for
inactivation of the retinoblastoma tumor suppressor protein,
activation of E2F, and G1-S phase progression. J Biol Chem.
279:5387–5396. 2004. View Article : Google Scholar
|
|
28
|
Libra M, Scalisi A, Vella N, Clementi S,
Sorio R, Stivala F, Spandidos DA and Mazzarino C: Uterine cervical
carcinoma: Role of matrix metalloproteinases (Review). Int J Oncol.
34:897–903. 2009. View Article : Google Scholar : PubMed/NCBI
|