Introduction
Inflammatory bowel disease (IBD) is a group of
complex intestinal disorders that includes ulcerative colitis (UC)
and Crohn's disease (CD), both of which are characterized by
chronic inflammation and damage, and the destruction of tissue
architecture. Overall morbidity is relatively high in developed
countries, and an increasing prevalence in the developing world has
been noted, particularly for CD (1). In contrast to UC, CD is
characterized by patchy, transmural inflammation, which may lead to
perforation or stricture of the digestive tract. CD is defined by
age at onset, and the location and pattern of disease, which are
combined in the Vienna classification (2). Genetic and environmental factors all
play important roles in CD, although a definitive pathogenesis
remains unknown.
Intestinal fibrosis, defined as an excessive
synthesis of connective tissue in the intestinal wall, is a common
complication of CD that is associated with long-term enervating
consequences that impair the quality of life of patients. Although
surgical treatment is not curative for CD, it is still required for
approximately 70% of CD patients if medical treatments are
ineffective or to ameliorate complications, such as obstruction
caused by intestinal fibrosis. One of the key points for reducing
the rate of re-operation is to alleviate fibrosis at the site of
anastomosis.
The primary therapy for CD is pharmaceutical-based,
which is evolving rapidly with many new biological agents under
investigation. Possessing both immunomodulatory and
anti-inflammatory effects, triptolide (TPL), an active component of
Tripterygium wilfordii Hook F., has been used as a
therapeutic agent for a number of autoimmune diseases, including
CD, for several years. A previous study showed that extracts of
Tripterygium wilfordii Hook F. exhibited therapeutic
activity in mild or moderately active CD (3). Many mechanisms of TPL have been
reported in previous studies, including the inhibition of the
expression of XPB (a subunit of TFIIH) (4), the Toll-like receptor (TLR)/nuclear
factor (NF)-κB signaling pathway (5) and the tumor necrosis factor
(TNF)-α/TNFR2 signaling pathway (6). Recently, a novel model involving
interleukin (IL)-10−/− mice undergoing ICR has provided
a novel method with which to investigate fibrosis following
anastomosis in CD (7).
TPL was used as a therapeutic substance in this new
model of IL-10−/− mice undergoing ICR in the present
study. The aim of this study was to investigate the therapeutic
effects of TPL in ameliorating fibrosis following anastomosis.
Materials and methods
Animals and surgical procedure
Male C3H/HeJBir IL-10−/− and wild-type
(WT) mice (8 weeks old) were purchased from Jackson Laboratory (Ben
Harbor, ME, USA) and raised under specific pathogen-free (SPF)
conditions at the Medical School of Southeast University, Nanjing,
China. A total of 18 IL-10−/− mice were evenly and
randomly divided into 3 groups as follows: the control group (no
intervention), the ST-ICR group (ICR and intraperitoneally injected
saline) and the TT-ICR group (ICR and intraperitoneally injected
TPL). A total of 6 WT mice of matched ages were assigned to the WT
group (no intervention). Mice were fasted 1 day before the
beginning of the experiment. Surgeries were performed under sterile
conditions with the assistance of a surgical microscope (×7
magnification). An intestinal segment comprising of 5 cm of small
bowel proximal to the ileocecal junction and 2 cm distal to the
proximal colon was resected after ligating the mesentery. The
intestinal continuity was restored with a single-layered,
interrupted, end-to-end anastomosis with a 9-0 monofilament suture.
Animals surviving less than 4 days after surgery were excluded. All
protocols for this animal research were approved by the Ethics
Committee of Southeast University.
Administration protocol of drugs and
assessment of disease activity
TPL (chemical structure shown in Fig. 1; Solarbio, Beijing, China) with a
purity ≥99% was dissolved in dimethyl sulfoxide (DMSO; Solarbio) at
a concentration of 0.0035 mg/ml. A 0.07 mg/kg body weight dose of
TPL was selected as the appropriate dose to inject mice
intraperitoneally in the TT-ICR group every other day for 8
consecutive weeks, as previously described (6). Mice in the ST-ICR group were
administered equal amounts of saline.
A disease activity index (DAI) was employed to
evaluate the status of the mice after undergoing ICR for 8 weeks.
Three parameters were taken into account as previously described:
weight loss, stool consistency and bleeding (8). Each clinical parameter was assigned
a value ranging from 0–4 (with 4 being the most severe). The final
value of the DAI was calculated as (the sum of the scores for
weight loss, stool consistency and bleeding)/3.
Tissue collection
A total of 8 weeks after the beginning of the
experiment, the mice were weighed and sacrificed by decapitation. A
1 cm segment of the small intestine (SI) spanning the ileocecal
junction in the WT and control groups and a 1 cm segment of the SI
proximal to the anastomosis in the ICR groups was collected for
histological and immunohistochemical analyses, reverse
transcription-quantitative PCR (RT-qPCR), ELISA and western blot
analysis.
Histological analysis
The samples were fixed in 10% neutral-buffered
formalin for 24 h and then embedded in paraffin and sectioned at a
thickness of 5 µm. Xylene was used to remove the paraffin;
the slides were then cleared with alcohol and subjected to H&E
(hematoxylin and eosin) and Masson's trichrome staining (both from
Servicebio, Wuhan, China). The evaluation of histological
inflammation scores (0–4, with 4 being the most severe
inflammation) based on H&E staining was performed in a blinded
manner according to previously described criteria (9,10).
This well-validated grading system is based on the severity and
extent of leukocyte infiltration, epithelial hyperplasia,
architectural distortion and depletion of goblet cells.
Masson's trichrome staining was used to visualize
collagen accumulation. Fibrosis scoring was conducted based on the
extent of collagen deposition on a scale from 0–3, with 0
representing no collagen deposition. The details of the grading
criteria were designed as follows: 0, no significant collagen
deposition; 1, increased collagen deposition in submucosa and
mucosa; 2, increased collagen deposition in submucosa, mucosa and
muscular layer; 3, increased collagen deposition throughout all
layers, including serosa. All scoring was performed by a single
qualified pathologist who was blinded to the intervention.
Western blot analysis
Protein was extracted from the SI tissue using lysis
buffer (150 mM NaCl; 0.5% sodium deoxycholate; 50 mM Tris, pH 8.0,
containing 1.0% NP-40; 1.5 mM EDTA; 10% glycerol) supplemented with
a protease inhibitor cocktail (Bioss, Beijing, China) on ice.
Supernatants were collected and centrifuged at 15,000 × g for 20
min at 4°C. The protein concentration was measured using a BCA
protein assay kit (Bioss). A total of 20 µg of total protein
was resolved by 10% sodium dodecylsulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to polyvinylidene
difluoride (PVDF) membranes by electroblotting at 4°C. After
blocking with 5% skim milk for 2 h at room temperature, the
membranes were incubated with primary antibodies overnight at 4°C.
Following washing with TBST 3 times, the membranes were then
incubated with HRP-conjugated secondary antibody (Cat. no.
bs-0369M-HRP, Bioss) at a dilution rate of 1:10,000 for 1 h at room
temperature. After washing with TBST 3 times, the membranes were
then incubated with ECL (Amresco, LLC, Solon, OH, USA) and analyzed
with a FluorChem FC system (Alpha Innotech, San Leandro, CA, USA).
The levels of protein expression were normalized to those of
β-actin. The primary antibodies used were as follows: rabbit
anti-mouse procollagen-I (Cat. no. ab64409; Abcam, Cambridge, UK),
procollagen-III (GTX39505; Genetex, Irvine, CA, USA) and heat shock
protein 70 (HSP70; Cat. no. ab79852; Abcam) monoclonal antibodies.
All primary antibodies were used at a dilution of 1:200.
Immunohistochemistry
Slides were prepared as described above in the
'Histological analysis' section. Following incubation with 3%
H2O2 and 10% methanol in 0.01 M PBS for15
min, the sections were blocked with 10% normal goat serumin 0.01 M
PBS for 1 h and incubated at 4°C overnight with a monoclonal
antibody to mouse anti-CD4 (Cat. no. ab183685, Abcam)
diluted 1:100 with 5% normal goat serum in Tris-Triton buffer. The
sections were then incubated at room temperature for 1 h with goat
anti-mouse (Cat. no. 115-505-003; Jackson Laboratory, Ben Harbor,
ME, USA) 1:500 with Tris-Triton buffer. Finally, sections were
visualized using the avidin-biotin DAB kit from Vector
Laboratories, Inc. (Burlingame, CA, USA). Slice images
(magnification, ×100) were captured with a digital camera (Leica,
Mannheim, Germany), which was linked to a microscope and managed
with OPTIMAS™ software version 6.1 (Optima Corp., Doral, FL, USA).
The analyses of the number of cells per area (12,234
µm2) were performed in 3 random areas.
RNA isolation and RT-qPCR
Total intestinal tissue RNA was isolated using the
TRIzol® Plus RNA Purification kit (Invitrogen, Carlsbad,
CA, USA) according to the manufacturer's instructions. 18S RNA was
used as an internal control. qPCR was performed using the
SYBR® Premix Ex Taq™ II PCR kit (Takara Bio, Inc., Otsu,
Japan).
A total of 4 mg of DNase-treated (Ambion, Austin,
TX, USA) RNA was reverse transcribed into cDNA using oligo (dT)
primers and reverse transcriptase (Promega Corp., Madison, WI, USA)
under standard conditions. β-actin served as a control for HSP70.
RT-PCR was performed using the StepOne™ and StepOnePlus™
Real-Time PCR System (Applied Biosystems Life Technologies, Foster
City, CA, USA). Cycle thresholds for each test mRNA were recorded
and normalized to a control. The denaturation process occurred at
95°C for 2 min, and then PCR amplification was performed with 40
cycles at 95°C for 15 sec and annealing at 60°C for 1 min. The
relative expression of microRNA/miRNA-16-1 (miR-16-1 or HSP70 was
measured using the ΔΔCq method. The abundance of miR-16-1 or
HSP70mRNA was presented as the fold change from the mean expression
levels in WT mice. The sequences of the primers are listed in
Table I.
 | Table IPrimer sequences for PCR. |
Table I
Primer sequences for PCR.
| Gene | Forward primer | Reverse primer |
|---|
| miR-16-1 |
5′-CCGCTCGAGTGCAGGCCATATTGTGCTGCC-3′ |
5′-TCCCCGCGGATTGTCTTCTAAGCTCTGTTC-3′ |
| 18S RNA |
5′-GTAACCCGTTGAACCCCATT-3′ |
5′-CCATCCAATCGGTAGTAGCG-3′ |
| HSP70 |
5′-GGCTGATCGGCCGCAAGTT-3′ |
5′-AACTGCACCCACTTCCCAGTC-3′ |
| β-actin |
5′-ACCACAGCTGAGAGGGAAATCG-3′ |
5′-AGAGGTCTTTACGGATGTCAACG-3′ |
Cytokine-specific ELISA
Protein was extracted from the intestinal tissue by
homogenization (0.5mgtissue/ml) in homogenization buffer with a
protease inhibitor (Bioss). Homogenized tissue samples were
collected following centrifugation at 20,000 × g at 4°C for 30 min,
and the supernatants were then stored at −80°C. The concentrations
of TGF-β1, TNF-α and IL-6 were evaluated using a kit from Abcam
according to the manufacturer's instructions. Values were expressed
as pg/mg protein.
Statistical analysis
Statistical significance among groups were
calculated with one-way ANOVA testing: parametric data were
assessed with a Bonferroni post hoc test and non-parametric data
were assessed with the Kruskal-Wallis test with the Dunn's post hoc
test (SPSS 21.0; IBM SPSS, Armonk, NY, USA). The data are expressed
as the means ± standard deviation (SD). A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
TPL alleviates clinical symptoms and
intestinal macroscopic changes in mice subjected to
anastomosis
The IL-10−/− mouse represents a
well-established animal model of CD owing to its persistent and
dominant pro-inflammatory systemic immune profile. The model using
IL-10−/− mice undergoing ICR was adopted in our study to
investigate post-operative anastomotic inflammation and fibrosis in
CD.
The mice in the ST-ICR group exhibited progressive
weight loss, severe diarrhea, bowel wall edema and thickening,
whereas no obvious signs of such symptoms were observed in the WT
and control groups. In the mice in the TT-ICR group, slight
diarrhea and weight loss were observed shortly after surgery, while
bowel function recovered promptly following treatment with TPL, and
no serious complications were observed in the mice who survived
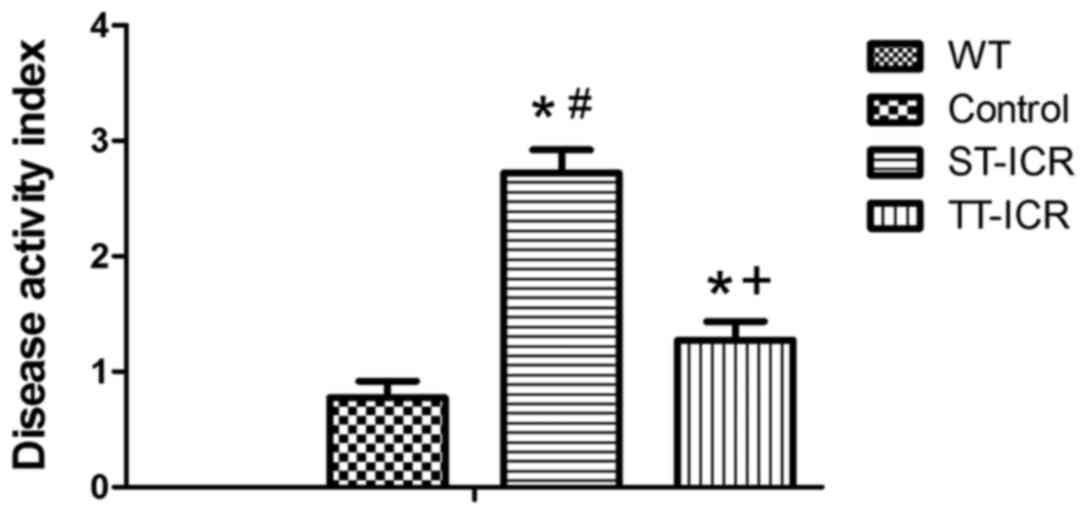
>2 weeks after surgery. The DAI score of each group at the 8th
week after surgery is shown in Fig.
2. No significant differences were observed between the WT and
control groups (P>0.05). The DAI score in the ST-ICR group was
significantly higher than that in the control group (P<0.05);
however, a reduction was observed following treatment with TPL
(P<0.05).
The bowels of the mice in the WT and control groups
showed no macroscopic signs of inflammation, whereas the intestinal
tract and, in particular, the anastomosis site of the mice in the
ST-ICR group removed 8 weeks after surgery revealed evident
hyperemia and inflammation (Fig.
3). Specifically, significant anastomotic lumen stenosis was
observed in the mice in the ST-ICR group, while ICR-induced
intestinal inflammation and anastomotic stenosis were effectively
attenuated by TPL, although intestinal walls immediately adjacent
to the anastomosis site still exhibited mild thickening in the mice
in the TT-ICR group.
TPL attenuates post-surgical inflammation
and anastomotic fibrosis in IL-10−/− mice that underwent
ICR
H&E staining and inflammation scores were
employed to determine the severity of anastomotic inflammation. No
obvious inflammation or destruction of the tissue architecture was
observed in the WT or control groups. A total of 8 weeks after
undergoing ICR, significant anastomotic microvilli swelling,
epithelial hyperplasia, and architectural distortion accompanied by
leukocytic infiltration were observed in the ST-ICR group compared
to the control group, and all of these pathological abnormalities
were significantly attenuated by treatment with TPL (Fig. 4A). The inflammation score, based
on H&E staining of the anastomosis site, was further
investigated in IL-10−/− mice (Fig. 4B). Compared with the control
group, significantly higher inflammation scores of the anastomosis
site were observed in the ST-ICR group (P<0.05), and a reduction
was observed after following treatment with TPL (P<0.05).
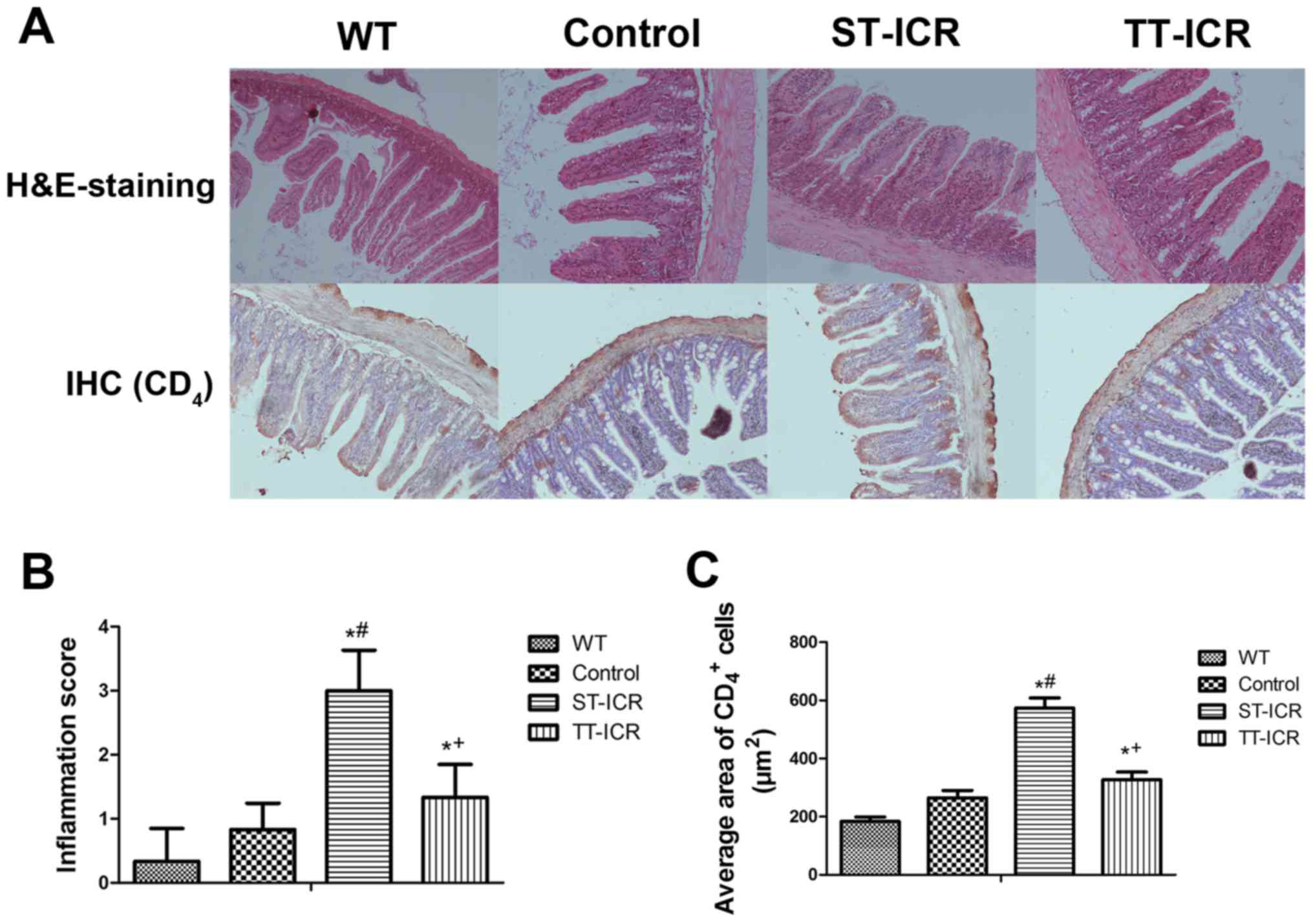 | Figure 4H&E staining and IHC of
CD4 were employed to evaluate the severity of
inflammation at the site of anastomosis in IL-10−/− mice
that underwent ICR. (A) Histopathological changes at the site of
anastomosis in each group. H&E staining was performed to
visualize the degree of inflammation at the site of anastomosis
(magnification, ×100); IHC was used to demonstrate the infiltration
of CD4+ cells (magnification, ×100). (B)
Anastomotic inflammation score of each group; (C) result of IHC for
number of CD4+ cells evaluated by image
analysis (total area per field = 12,234 µm2). The
data are presented as the average ± SD of 6 independent
experiments. *P<0.05, significantly different from
the WT group; #P<0.05, significantly different from
the control group; +P<0.05, significantly different
from the ST-ICR group. IHC, immunohistochemistry; ICR, ileocecal
resection; WT, wild-type; ST-ICR, saline-treated-ICR group; TT-ICR,
triptolide-treated ICR group. |
In patients with CD, over-activated Th1/Th17 type T
cells are the main resources that induce local inflammatory
reactions by producing pro-inflammatory cytokines; therefore, as a
specific marker of Th cells, CD4 was examined by
immunohistochemistry (Fig. 4A).
The area of CD4+ cell infiltration in the
anastomosis site of the ST-ICR group was significantly larger than
that in the WT and control groups (P<0.05; Fig. 4C), and CD4+
cells were found infiltrating throughout the entire intestinal
wall, particularly in the submucosa and mucosa. By contrast,
following treatment with TPL, the CD4+ area
was diminished and showed no significant difference compared with
the control group (P>0.05).
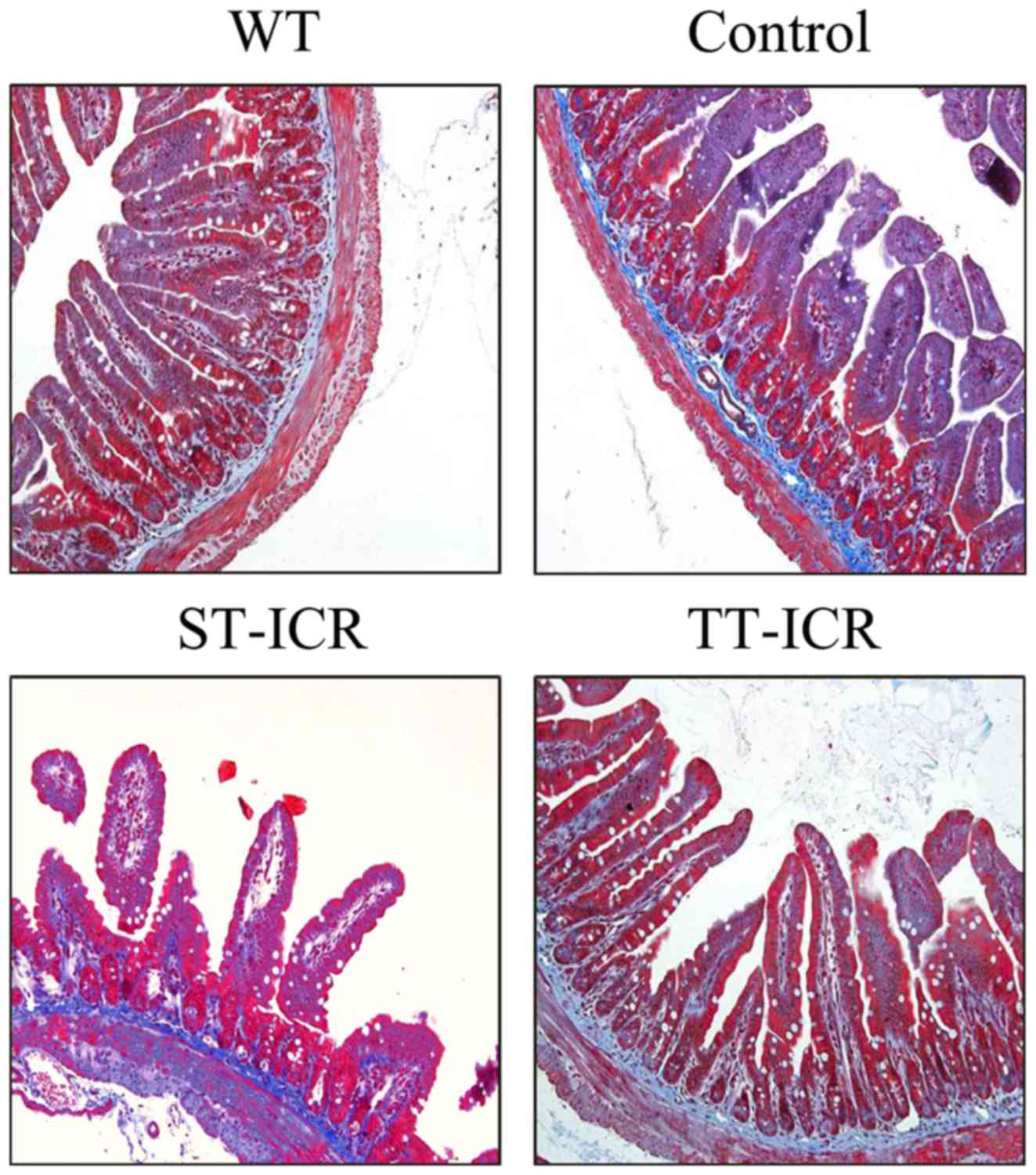
Collagen, which is the main component of the
extracellular matrix (ECM), is mainly synthesized by fibrotic cells
and indicates the severity of fibrosis (11). In this study, to determine whether
intestinal fibrosis was the cause of luminal stenosis, the
accumulation of collagen was visualized in the anastomosis site
with Masson's staining 8 weeks after performing ICR (Fig. 5). No obvious deposition of
collagen was observed in the WT and control groups, whereas
significant collagen deposition throughout all layers of the
intestinal wall was observed in the ST-ICR group compared to the WT
and control groups. Once collagen synthesis becomes uncontrollable,
thickening and stiffness of the bowel wall and luminal stenosis may
be present; however, TPL treatment effectively attenuated the
excessive accumulation of collagen. Furthermore, compared with the
control group, the fibrosis scores in the ST-ICR group were
significantly increased (P<0.05), whereas a significant
reduction was observed in the TT-ICR group (P<0.05; Fig. 6A).
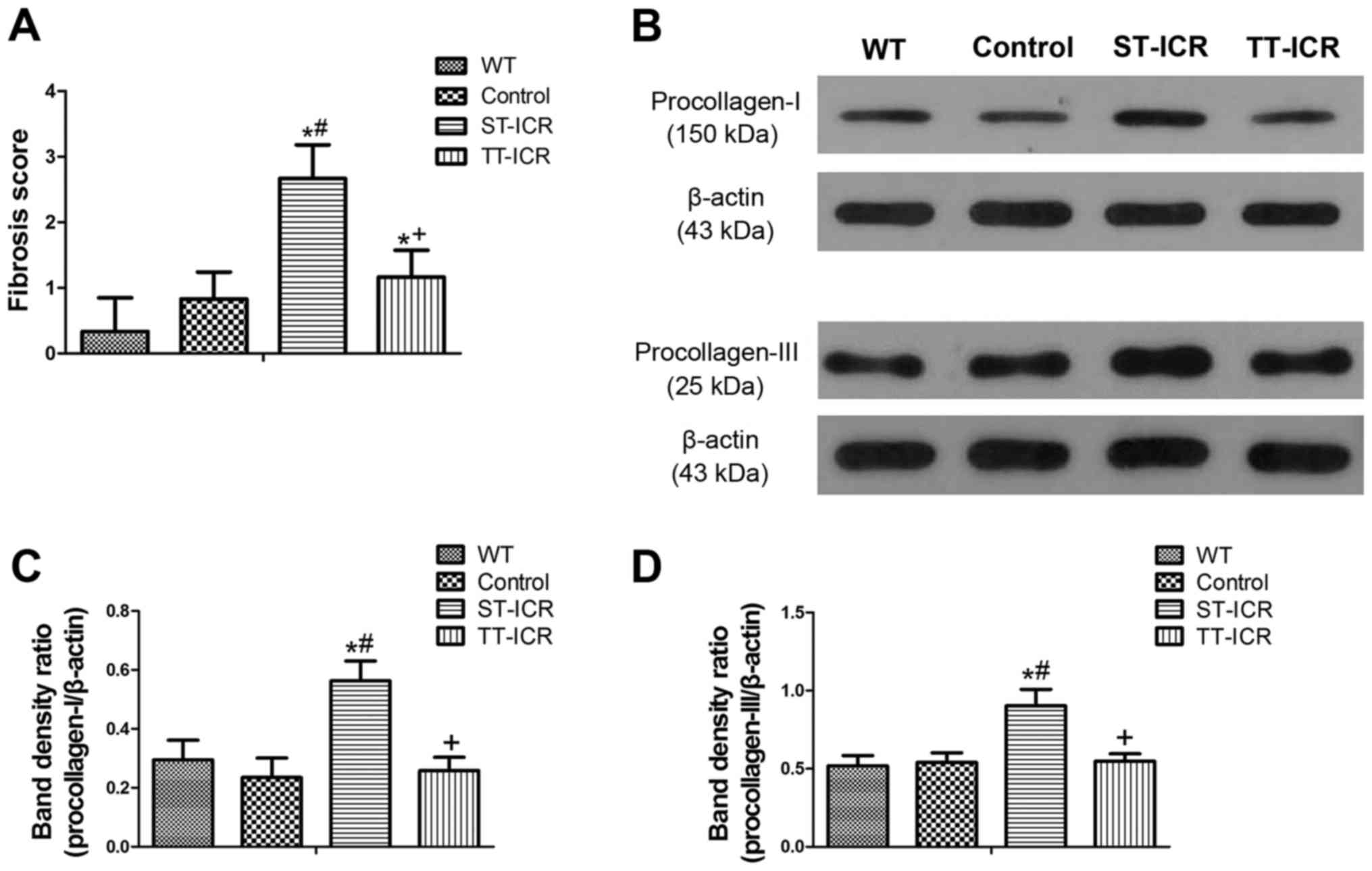
Collagen molecules are synthesized from larger
precursor proteins, known as procollagens. Thus, the synthesis rate
of fibrillar collagens was further determined by measuring
procollagen I and III by western blot analysis. As shown in
Fig. 6B–D, no noteworthy
differences were detected between the WT and control groups
(P>0.05). Compared with the WT and control groups, the
upregulated levels of procollagen I and III in the ST-ICR group
were significantly decreased by TPL treatment (P<0.05). These
results indicate that the balance between the synthesis and
catabolism of ECM was altered in the IL-10−/− mice that
underwent ICR.
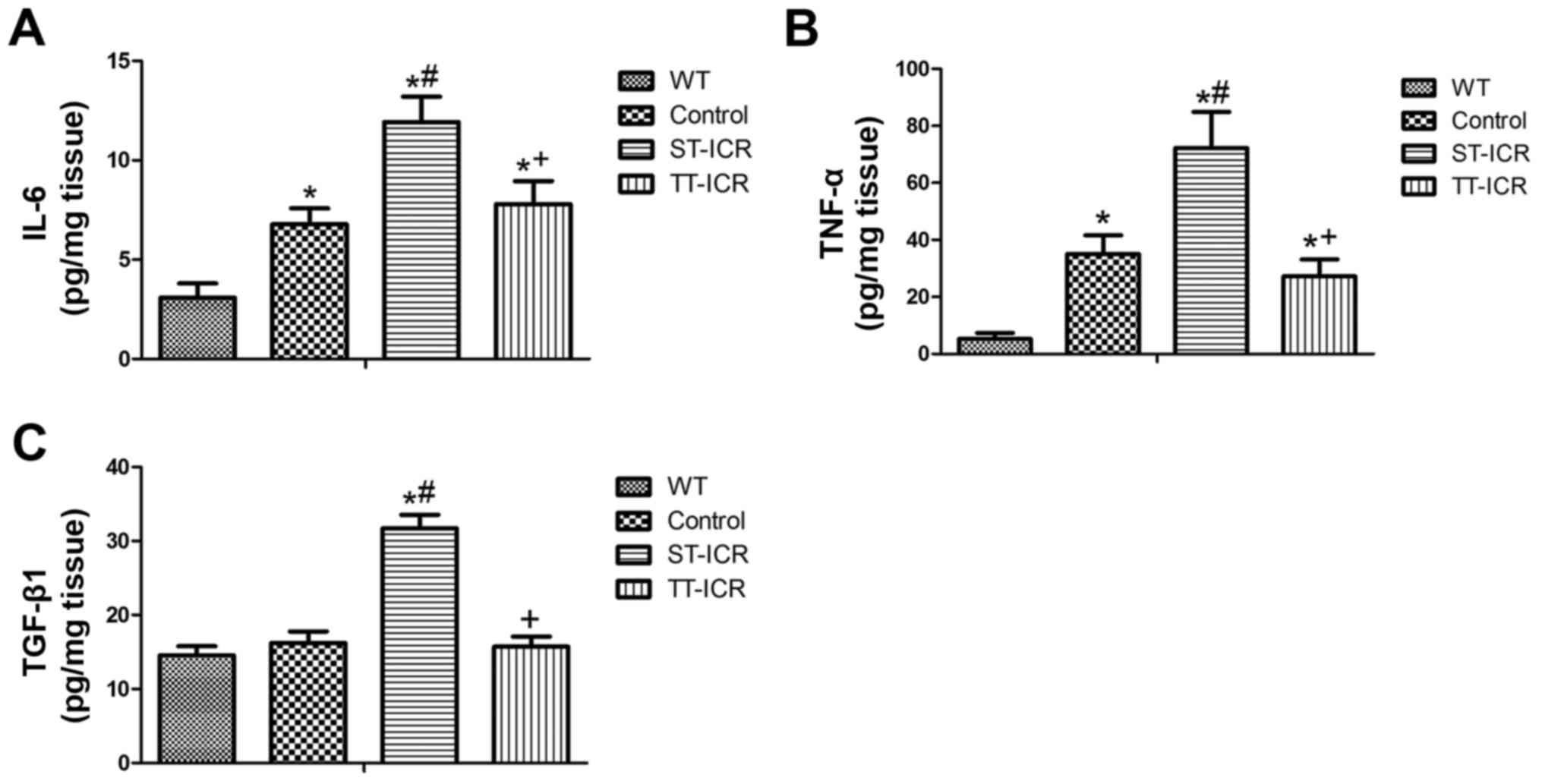
TPL inhibits the secretion of
inflammatory and fibrotic cytokines
IL-6, TNF-α and TGF-β1 are efficient risk indicators
and are closely related to the disease activity of CD. TGF-β1, the
most potent activator of fibroblasts, is crucial for the
progression of fibrosis (12–14). In this study, to further verify
the degree and pathogenesis of inflammation and anastomotic
fibrosis in the IL-10−/− mice that underwent ICR,
cytokine-specific ELISAs were utilized to investigate the
expression of IL-6, TNF-α and TGF-β1 (Fig. 7). With the increasing severity of
inflammation and fibrosis, the anastomotic concentrations of
TGF-β1, IL-6 and TNF-α all increased. Following treatment with TPL,
these levels were all significantly decreased (P<0.05). Of note,
although no significant difference in inflammation and fibrosis was
detected between the WT and control groups in macroscopic or
histopathologic views, the levels of IL-6 and TNF-α in the control
group were significantly higher than those in the WT group
(P<0.05).
TPL attenuates ileocolonic anastomotic
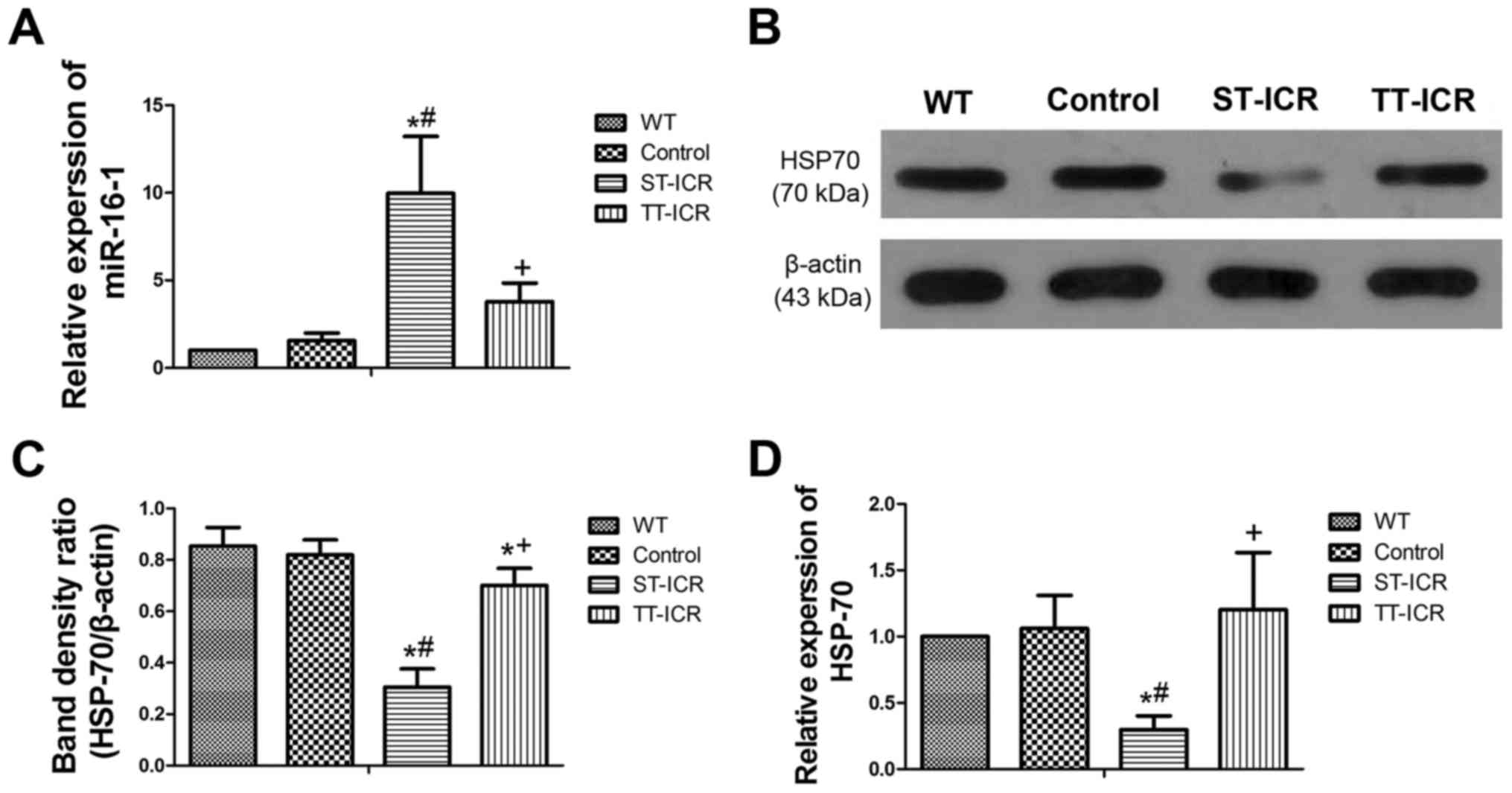
fibrosis by regulatingthemi R-16-1/HSP70 pathway
As miR-16-1 is closely associated with the activity
of CD (15,16) and can be regulated by TPL
(17); the level of miR-16-1 was
further investigated by RT-qPCR (Fig.
8A). The level of miR-16-1 in the ST-ICR group was
significantly elevated compared with the WT group (P<0.05).
Compared with the control group, a higher level of miR-16-1 at the
anastomosis site was observed in the ST-ICR group (P<0.05),
although this increase was reversed by TPL (P<0.05). Therefore,
we demonstrated that the expression of miR-16-1 can be effectively
inhibited by TPL, and the DAI score can be reduced through the
inhibition of miR-16-1.
As a main member of the HSP family, HSP70 can
inhibit inflammation and fibrosis through mechanisms, such as the
suppression of the expression of cytokines, regulating
myofibroblast and T cell differentiation, and the inhibition of
epithelial cell apoptosis (18,19). More importantly, miR-16-1 targets
the 3′ untranslated region (3′UTR) of HSP70 and reduces HSP70
expression (20). Therefore, in
this study, RT-qPCR and western blot analysis were used to
investigate the level of HSP70 and verify the association between
HSP70 and miR-16-1 (Fig. 8B–D).
The level of HSP70 decreased along in the mice with anastomotic
fibrosis severity at both the mRNA and protein level. Compared with
the control group, the level of HSP70 in the ST-ICR group was
significantly downregulated (P<0.05). However, a sharp rise in
HSP70 expression was observed following treatment with TPL
(P<0.05), which partly results from the inhibition of
miR-16-1.
Discussion
CD is a chronic inflammatory gastrointestinal
disorder that can affect any portion of the gastrointestinal tract,
particularly the terminal ileum. Chronic and persistent
inflammation induces intestinal fibrosis, and severe fibrosis can
result in intestinal stenosis, which can lead to obstructions that
require surgery or repeat surgery (21). The most frequent surgical strategy
for patients with CD is ICR and ileum-colon anastomosis; however,
patients undergoing ICR and re-anastomosis tend to have a higher
relapse rate compared with patients with stricturoplasties or
isolated small bowel resections. Recurrence frequently occurs
around the anastomotic sites and the proximal intestine (22). The concrete mechanisms underlying
post-surgical anastomotic fibrosis and stricture remain unknown.
Therefore, further studies using animal models are warranted. Among
numerous animal models, the IL-10−/− mouse model has
features of Th1-/Th17-type inflammation similar to CD and has been
extensively applied to study the pathogenesis of CD (23). As a novel model to study
post-surgical pathological changes of anastomosis, the model of
IL-10−/− mice undergoing ICR was constructed (7). In our study, no significant
difference in either inflammation or fibrosis was detected between
the WT and control groups; however, of note, the IL-6 and TNF-α
levels in the control group were significantly higher than those in
the WT group. We hypothesize that this may result from the innate
immune features of IL-10−/− mice and environmental
factors. IL-10 can inhibit the production of cytokines, including
IFN-γ, IL-1, IL-6 and TNF-α, from pro-inflammatory cells;
therefore, this suppressive effect may be weakened by a lack of
IL-10. However, intestinal inflammation and fibrosis in the
IL-10−/− mice is intestinal-content dependent
(particularly bacteria or bacterial products) (7); thus, the IL-10−/− mice
used in our study, which were raised under SPF conditions, suffered
only from local colitis and slight collagen accumulation.
Therefore, we hypothesize that IL-10−/− mice, which
underwent ICR and were raised under SPF conditions, are appropriate
models to investigate the pathogenesis of surgery-associated
complications, particularly post-surgical anastomotic inflammation
and fibrosis. In the present study, the upregulated inflammation
and fibrosis score, the larger area of CD4+
cell infiltration, as well as the overexpression of procollagen I
and III in mice in the ST-ICR group, proved that the
IL-10−/− mice that underwent ICR, which have many
features in common with CD, can develop sustained and significant
post-operative inflammation and anastomotic fibrosis. The
pathogenesis can be attributed to mechanisms including impaired
blood flow around the anastomosis site, an absence of the ileocecal
valve and subsequent exposure of the anastomosis to luminal
contents (7), or changes in the
systemic immune response (22),
as well as others. We showed that IL-10−/− mice that
underwent ICR, models that precisely mimic post-surgical intestinal
lesions of CD, provide an appropriate experimental platform with
which to study the pathogenesis of inflammation and anastomotic
fibrosis in patients with CD undergoing ICR.
The fibrotic process comprises a series of abnormal
biological behaviors, including inflammatory cell infiltration,
cytokine release, fibroblast differentiation and proliferation, and
an imbalance in ECM synthesis and degradation. Previous studies
have demonstrated the anti-inflammatory activity of TPL; however,
an anti-fibrosis effect of this agent was still uncovered, and the
therapeutic effects of TPL, attenuating fibrosis following
intestinal anastomosis in this new model of CD have not been
previously investigated, at least to the best of our knowledge. We
demonstrated that following treatment with TPL, the inflammation
and fibrosis levels at anastomosis sites of the mice in the TT-ICR
groups were significantly attenuated compared with those in the
ST-ICR group; additionally, TPL effectively inhibited the
infiltration of CD4+ T cells and the
synthesis of collagen at the anastomosis site. The elevated
expression levels of TNF-α, IL-6 and TGF-β1 were also reduced by
TPL. As an effective risk-indicator of disease activity in CD, IL-6
can regulate T cell differentiation and activation, promote
fibroblast proliferation and increase fibroblast collagen and
tissue inhibitor of metalloproteinases-1 (TIMP-1) synthesis. TNF-α
is also closely related to the process of fibrosis. A previous
study demonstrated that TNF-α found in chronic inflammatory
conditions inhibits collagen phagocytosis and induces tissue
fibrosis (12). Additionally, by
reducing chemokine production by fibroblasts, TPL can also limit
inflammatory cell infiltration (24). Matrix metalloproteinases (MMPs)
are involved in the catabolism of ECM, and the activity of MMPs is
restricted by TIMPs; imbalances between TIMPs and MMPs may result
in disorders of ECM metabolism (25). TLRs, which are a family of pattern
recognition receptors, represent receptors of the innate immune
system. They activate downstream inflammatory responses and
initiate the acquired immune response. The dysregulation of TLRs
may induce chronic inflammation and tissue impairment in IBD. TPL
can inhibit MMP expression and augment TIMP expression. Moreover,
the level of TLR-2 and TLR-4 can also be reduced by TPL in the
intestines of patients with CD (5). In mice with collagen-induced
arthritis, TPL was found to inhibitIL-1β, IL-6, TNF-α, MMP-13 and
MMP-3 expression and augment TIMP-1 and TIMP-2 expression, thereby
suppressing the inflammatory reaction and preventing the
development of the fibrotic process (26). In IL-1β-treated human
intervertebral disc cells, TPL significantly suppressed the
expression of numerous genes, including IL-6, IL-8, MMP-1, MMP-2,
MMP-3, MMP-13, TLR-2 and TLR-4 (27). TGF-β, which plays important roles
in wound healing, the regulation of the immune system, metabolism
of connective tissue, fibrosis and cancer progression, can drive
fibrosis through multiple mechanisms, including the activation of
the TGF-β/Smad signaling pathway and epithelial to mesenchymal
transition (EMT) (13). TPL can
inhibit ECM protein synthesis by fibroblasts by suppressing Smad2
activation (28). A previous
study found that TPL treatment markedly inhibited the expression of
TGF-β1 and attenuated cardiac fibrosis (29). TPL has also been reported to
inhibit the TGF-β-induced phosphorylation of Smad2 and Smad3, but
to increase the level of Smad7 (30). Additionally, the
NF-κB/TNF-α/VCAM-1, TLR4-induced NF-κB/IL-1β and
TGF-β1/α-SMA/Vimentin signaling pathways are all important targets
of TPL (31). In particular, we
have noticed that there are many miRNAs regulated by TPL, such as
miR-344b-3p, miR-30b-3p (32) and
miR-155 (33); as miR-16-1 is
also an important target of TPL (17) and relates to the activity of CD,
we hypothesized that the anti-fibrotic effects of TPL may be
mediated partly through the inhibition of the expression
ofmiR-16-1.
miRNAs are small non-coding RNA molecules that
comprise approximately 22 nucleotides and function as inhibitors of
gene expression by primarily binding to complementary sites on the
3′UTR of target mRNAs. miR-16-1, which was first reported to be
associated with chronic lymphocytic lymphomain 2002(34), can regulate numerous cellular
biological behaviors, including cell proliferation,
differentiation, cell cycle regulation and apoptosis (35). Specifically, miR-16-1 has an
increased expression both in the mucosa of the terminal ileum and
in the peripheral blood of patients with active CD compared with
healthy individuals (15,16). The association between miR-16-1
and anastomotic fibrosis in patients with CD has not been
previously investigated, at least to the best of our knowledge. A
previous study showed that TPL can inhibit the expression of
miR-16-1 in a time- and dose-dependent manner (17), which is in accordance with the
findings of the present study. In our study, the expression of
miR-16-1 was significantly inhibited by TPL, and the level of
miR-16-1positively correlated with the severity of inflammation and
fibrosis in anastomosis. These results suggested that TPL may
attenuate intestinal fibrosis partly through the downregulation of
miR-16-1. Previous studies have tried to elucidate the association
between miR-16-1 and inflammation and fibrosis. miR-16-1-expressing
macrophages were found to promote the activation of purified
CD4+ T cells (36). A recent study demonstrated that
HCV infection results in the overexpression of miR-16-1 and then
inhibits the expression of Smad7 in the progression of liver
fibrosis (37). Additionally,
miR-16-1 is thought to be related to EMT, a significant contributor
to the development of fibrosis (38). In addition, miR-16-1 functions
through multiple mechanisms in a tissue- and cell-specific manner;
for example, miR-16(-1) regulates T cell activation, proliferation
and apoptosis by targeting Bcl2, CCND1, Erbb3, mTOR, Rictor and
Runx1 (39–41). Recently, a study proved that
miR-16-1 targets the 3′UTR of HSP70 and thus reduces HSP70
expression, and HSP70 was found to be crucial in the alleviation of
intestinal damage (42,43). Therefore, we further attempted to
explore changes in HSP70 expression in this new model.
HSPs, which can be found in all eukaryotes and
prokaryotes, are a family of highly conserved proteins that play
important roles in cellular proliferation and differentiation and
oncogenesis (44,45). As the primary member of the HSP
family, HSP70 has been shown to be involved in the pathogenesis of
numerous chronic autoimmune diseases, including CD (18,46). In the present study, the level of
HSP70 decreased in mice with inflammation and fibrosis following
ICR; however a sharp rise in HSP70 expression was observed
following treatment with TPL, which partly results from the
inhibition of miR-16-1. Besides, there is also a tight connection
between HSP70 and anastomotic inflammation and fibrosis.
Furthermore, the levels of IL-6, TGF-β1 and TNF-α were all
significantly elevated at the anastomosis site in the mice in the
ST-ICR group and negatively correlated with the level of HSP70, and
reversions were observed following treatment with TPL. As the
TGF-β/Smad pathway and EMT are all considered to be closely related
to the level of TGF-β, the over expression of HSP70 has been found
to inhibit EMT by exerting domain-specific effects on Smad3
activation and nuclear translocation as well as increasing Smad7
expression in a dose-dependent manner (47). Additionally, HSP70 inhibits TGF-β
signal transduction by interacting with Smad2; the over expression
of HSP70 can inhibit the phosphorylation and nuclear translocation
of Smad2and blocks TGF-β-induced EMT (48). The overexpression of HSP70 has
also been found to prevent the synthesis of cytokines, including
IL-6 and TNF-α, and protects against TNF-α- and IL-6-induced
intestinal damage (42,43). Furthermore, HSP70 is involved in
numerous signaling pathways, including nuclear factor-κB, Src, Akt
and Raf (49). Through mechanisms
such as regulating myofibroblast and T cell differentiation and
inhibiting epithelial cell apoptosis, HSP70 is believed to
effectively inhibit tissue inflammation and fibrosis (18,19,49,50). We demonstrated that through the
inhibition of the expression of miR-16-1, TPL significantly
upregulated the HSP70 levels, and thus prevented the development of
the fibrotic process in IL-10−/− mice undergoing
ICR.
In conclusion, to the very best of our knowledge,
the present study is the first to use the novel model of
IL-10-deficient mice undergoing ICR to investigate the treatment
utility of TPL on anastomotic fibrosis. The present study
demonstrates that TPL is an effective substance against CD and
exerts a protective role against post-surgical anastomotic
fibrosis. The miR-16-1/HSP70 signaling pathway, which is an
important target of TPL, is a valuable therapeutic approach for CD
that deserves further investigation.
Acknowledgments
This research was supported by funding from the
National Natural Science Foundation of China (grant no. 81500421).
We would sincerely like to thank Professor Qiu-Rong Li (Jinling
Hospital), Dr Bao-Cai Wang (Technical University, Munich) and
Professor Feng Gao (Medical School of Southeast University), as
well as their team for their technical assistance.
References
|
1
|
Bernstein CN, Fried M, Krabshuis JH, Cohen
H, Eliakim R, Fedail S, Gearry R, Goh KL, Hamid S, Khan AG, et al:
World Gastroenterology Organization practice guidelines for the
diagnosis and management of IBD in 2010. Inflamm Bowel Dis.
16:112–124. 2010. View Article : Google Scholar
|
|
2
|
Carter MJ and Lobo AJ: Guidelines for the
management of inflammatory bowel disease in adults. Gut. 53(Suppl
5): V1–V16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ren J, Tao Q, Wang X, Wang Z and Li J:
Efficacy of T2 in active Crohn's disease: A prospective study
report. Dig Dis Sci. 52:1790–1797. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Titov DV, Gilman B, He QL, Bhat S, Low WK,
Dang Y, Smeaton M, Demain AL, Miller PS, Kugel JF, et al: XPB, a
subunit of TFIIH, is a target of the natural product triptolide.
Nat Chem Biol. 7:182–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu C, Shan T, Feng A, Li Y, Zhu W, Xie Y,
Li N and Li J: Triptolide ameliorates Crohn's colitis is associated
with inhibition of TLRs/NF-κB signaling pathway. Fitoterapia.
82:709–715. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wei X, Gong J, Zhu J, Wang P, Li N, Zhu W
and Li J: The suppressive effect of triptolide on chronic colitis
and TNF-alpha/TNFR2 signal pathway in interleukin-10 deficient
mice. Clin Immunol. 129:211–218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rigby RJ, Hunt MR, Scull BP, Simmons JG,
Speck KE, Helmrath MA and Lund PK: A new animal model of
postsurgical bowel inflammation and fibrosis: The effect of
commensal microflora. Gut. 58:1104–1112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murthy SN, Cooper HS, Shim H, Shah RS,
Ibrahim SA and Sedergran DJ: Treatment of dextran sulfate
sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis
Sci. 38:1722–1734. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berg DJ, Davidson N, Kühn R, Müller W,
Menon S, Holland G, Thompson-Snipes L, Leach MW and Rennick D:
Enterocolitis and colon cancer in interleukin-10-deficient mice are
associated with aberrant cytokine production and CD4(+)
TH1-likeresponses. J Clin Invest. 98:1010–1020. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rath HC, Herfarth HH, Ikeda JS, Grenther
WB, Hamm TE Jr, Balish E, Taurog JD, Hammer RE, Wilson KH and
Sartor RB: Normal luminal bacteria, especially Bacteroides species,
mediate chronic colitis, gastritis, and arthritis in HLA-B27/human
beta2 microglobulin transgenic rats. J Clin Invest. 98:945–953.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eckes B, Zigrino P, Kessler D, Holtkötter
O, Shephard P, Mauch C and Krieg T: Fibroblast-matrix interactions
in wound healing and fibrosis. Matrix Biol. 19:325–332. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chou DH, Lee W and McCulloch CA: TNF-alpha
inactivation of collagen receptors: Implications for fibroblast
function and fibrosis. J Immunol. 156:4354–4362. 1996.PubMed/NCBI
|
|
13
|
Morikawa M, Derynck R and Miyazono K:
TGF-β and the TGF-β family: Context-dependent roles in cell and
tissue physiology. Cold Spring Harb Perspect Biol. 8:82016.
View Article : Google Scholar
|
|
14
|
Lochhead P, Khalili H, Ananthakrishnan AN,
Richter JM and Chan AT: Association between circulating levels of
c-reactive protein and interleukin-6 and risk of inflammatory bowel
disease. Clin Gastroenterol Hepatol. 14:818–824. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iborra M, Bernuzzi F, Correale C, Vetrano
S, Fiorino G, Beltrán B, Marabita F, Locati M, Spinelli A, Nos P,
et al: Identification of serum and tissue micro-RNA expression
profiles in different stages of inflammatory bowel disease. Clin
Exp Immunol. 173:250–258. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paraskevi A, Theodoropoulos G,
Papaconstantinou I, Mantzaris G, Nikiteas N and Gazouli M:
Circulating MicroRNA in inflammatory bowel disease. J Crohn's
Colitis. 6:900–904. 2012. View Article : Google Scholar
|
|
17
|
Meng HT, Zhu L, Ni WM, You LS, Jin J and
Qian WB: Triptolide inhibits the proliferation of cells from
lymphocytic leukemic celllines in association with downregulation
of NF-κB activity and miR-16-1*. Acta Pharmacol Sin.
32:503–511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bellaye PS, Burgy O, Causse S, Garrido C
and Bonniaud P: Heat shock proteins in fibrosis and wound healing:
Good or evil? Pharmacol Ther. 143:119–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hauet-Broere F, Wieten L, Guichelaar T,
Berlo S, van der Zee R and Van Eden W: Heat shock proteins induce T
cell regulation of chronic inflammation. Ann Rheum Dis. 65(Suppl
3): iii65–iii68. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Z and Cheng Y: miR-16-1 promotes the
aberrant α-synuclein accumulation in parkinson disease via
targeting heat shock protein 70. Scientific World Journal.
2014:9383482014.
|
|
21
|
Li C and Kuemmerle JF: Mechanisms that
mediate the development of fibrosis in patients with Crohn's
disease. Inflamm Bowel Dis. 20:1250–1258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Borowiec AM, Sydora BC, Doyle J, Guan LL,
Churchill TA, Madsen K and Fedorak RN: Small bowel fibrosis and
systemic inflammatory response after ileocolonic anastomosis in
IL-10null mice. J Surg Res. 178:147–154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kühn R, Löhler J, Rennick D, Rajewsky K
and Müller W: Interleukin-10-deficient mice develop chronic
enterocolitis. Cell. 75:263–274. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Li J, Liu Y, Wang P and Jia H:
Inhibition of zymosan-induced cytokine and chemokine expression in
human corneal fibroblasts by triptolide. Int J Ophthalmol. 9:9–14.
2016.PubMed/NCBI
|
|
25
|
Burger D, Rezzonico R, Li JM, Modoux C,
Pierce RA, Welgus HG and Dayer JM: Imbalance between interstitial
collagenase and tissue inhibitor of metalloproteinases 1 in
synoviocytes and fibroblasts upon direct contact with stimulated T
lymphocytes: Involvement of membrane-associated cytokines.
Arthritis Rheum. 41:1748–1759. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin N, Liu C, Xiao C, Jia H, Imada K, Wu H
and Ito A: Triptolide, a diterpenoid triepoxide, suppresses
inflammation and cartilage destruction in collagen-induced
arthritis mice. Biochem Pharmacol. 73:136–146. 2007. View Article : Google Scholar
|
|
27
|
Klawitter M, Quero L, Klasen J, Liebscher
T, Nerlich A, Boos N and Wuertz K: Triptolide exhibits
anti-inflammatory, anti-catabolic as well as anabolic effects and
suppresses TLR expression and MAPK activity in IL-1β treated human
intervertebral disc cells. Eur Spine J. 21(Suppl 6): S850–S859.
2012. View Article : Google Scholar
|
|
28
|
Zhu B, Wang YJ, Zhu CF, Lin Y, Zhu XL, Wei
S, Lu Y and Cheng XX: Triptolide inhibits extracellular matrix
protein synthesis by suppressing the Smad2 but not the MAPK pathway
in TGF-beta1-stimulated NRK-49F cells. Nephrol Dial Transplant.
25:3180–3191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Z, Qu X, Ni Y, Zhang K, Dong Z, Yan
X, Qin J, Sun H, Ding Y, Zhao P, et al: Triptolide protects rat
heart against pressure overload-induced cardiac fibrosis. Int J
Cardiol. 168:2498–2505. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen M, Lv Z, Huang L, Zhang W, Lin X, Shi
J, Zhang W, Liang R and Jiang S: Triptolide inhibits TGF-β1-induced
cell proliferation in rat airway smooth muscle cells by suppressing
Smad signaling. Exp Cell Res. 331:362–368. 2015. View Article : Google Scholar
|
|
31
|
Guo X, Xue M, Li CJ, Yang W, Wang S, Ma Z,
Zhang X, Wang X, Zhao R, Chang B and Chen LM: Protective effects of
triptolide on TLR4 mediated autoimmune and inflammatory response
induced myocardial fibrosis in diabetic cardiomyopathy. J
Ethnopharmacol. 193:333–344. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang CB, Wei MG, Tu Y, Zhu H, Li CQ, Jing
WM and Sun W: Triptolide attenuates podocyte injury by regulating
expression of miRNA-344b-3p and miRNA-30b-3p in rats with
adriamycin-induced nephropathy. Evidence-based complementary and
alternative medicine. eCAM. 2015:pp. 1078142015, https://doi.org/10.1155/2015/107814.
|
|
33
|
Wu R, Li Y, Guo Z, Gong J, Zhu W, Li N and
Li J: Triptolide ameliorates ileocolonic anastomosis inflammation
in IL-10 deficient mice by mechanism involving suppression of
miR-155/SHIP-1 signaling pathway. Mol Immunol. 56:340–346. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Calin GA, Dumitru CD, Shimizu M, Bichi R,
Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, et al:
Frequent deletions and down-regulation of micro-RNA genes miR15 and
miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci
USA. 99:15524–15529. 2002. View Article : Google Scholar
|
|
35
|
Li F, Xu Y, Deng S, Li Z, Zou D, Yi S, Sui
W, Hao M and Qiu L: MicroRNA-15a/16-1 cluster located at chromosome
13q14 is downregulated but displays different expression pattern
and prognostic significance in multiple myeloma. Oncotarget.
6:38270–38282. 2015.PubMed/NCBI
|
|
36
|
Jia X, Li X, Shen Y, Miao J, Liu H, Li G
and Wang Z: MiR-16 regulates mouse peritoneal macrophage
polarization and affects T-cell activation. J Cell Mol Med.
20:1898–1907. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhu B, Wei XX, Wang TB, Zhou YC, Liu AM
and Zhang GW: Increased miR-16 expression induced by hepatitis C
virus infection promotes liver fibrosis through downregulation of
hepatocyte growth factor and Smad7. Arch Virol. 160:2043–2050.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi L, Jackstadt R, Siemens H, Li H,
Kirchner T and Hermeking H: p53-induced miR-15a/16-1 and AP4 form a
double-negative feedback loop to regulate epithelial-mesenchymal
transition and metastasis in colorectal cancer. Cancer Res.
74:532–542. 2014. View Article : Google Scholar
|
|
39
|
Liu L, Walker EA, Kissane S, Khan I,
Murray PI, Rauz S and Wallace GR: Gene expression and miR profiles
of human corneal fibroblasts in response to dexamethasone. Invest
Ophthalmol Vis Sci. 52:7282–7288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Marcais A, Blevins R, Graumann J, Feytout
A, Dharmalingam G, Carroll T, Amado IF, Bruno L, Lee K, Walzer T,
et al: microRNA-mediated regulation of mTOR complex components
facilitates discrimination between activation and anergy in CD4T
cells. J Exp Med. 211:2281–2295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rouse M, Rao R, Nagarkatti M and
Nagarkatti PS: 3,3′-diindolylmethane ameliorates experimental
autoimmune encephalomyelitis by promoting cell cycle arrest and
apoptosis in activated T cells through microRNA signaling pathways.
J Pharmacol Exp Ther. 350:341–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Van Molle W, Wielockx B, Mahieu T, Takada
M, Taniguchi T, Sekikawa K and Libert C: HSP70 protects against
TNF-induced lethal inflammatory shock. Immunity. 16:685–695. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Meng K, Liu Q, Dou Y and Huang Q: Prior
peritoneal lavage with hot 0.9% saline induces HSP70 expression and
protects against cerulein-induced acute pancreatitis in rats. Mol
Biol Rep. 40:1443–1449. 2013. View Article : Google Scholar
|
|
44
|
Sherman MY and Gabai VL: Hsp70 in cancer:
Back to the future. Oncogene. 34:4153–4161. 2015. View Article : Google Scholar :
|
|
45
|
Murphy ME: The HSP70 family and cancer.
Carcinogenesis. 34:1181–1188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Abou El Azm AR, Yousef M, Kobtan A, Awad
A, Elkassas G and Elfert A: Colonic mucosal expression of
heat-shock proteins may have a potential prognostic value in
ulcerative colitis. Arab journal of gastroenterology: the official
publication of the Pan-Arab Association of Gastroenterology. 16:pp.
20–24. 2015, https://doi.org/10.1016/j.ajg.2015.02.005.
View Article : Google Scholar
|
|
47
|
Zhou Y, Mao H, Li S, Cao S, Li Z, Zhuang
S, Fan J, Dong X, Borkan SC, Wang Y, et al: HSP72 inhibits Smad3
activation and nuclear translocation in renal
epithelial-to-mesenchymal transition. J Am Soc Nephrol. 21:598–609.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li Y, Kang X and Wang Q: HSP70 decreases
receptor-dependent phosphorylation of Smad2 and blocks
TGF-beta-induced epithelial-mesenchymal transition. Journal of
genetics and genomics = Yi chuan xue bao. 38:111–116. 2011.
View Article : Google Scholar
|
|
49
|
Tao Y, Hart J, Lichtenstein L, Joseph LJ,
Ciancio MJ, Hu S, Chang EB and Bissonnette M: Inducible heat shock
protein 70 prevents multifocal flat dysplastic lesions and invasive
tumors in an inflammatory model of colon cancer. Carcinogenesis.
30:175–182. 2009. View Article : Google Scholar :
|
|
50
|
Mao H, Li Z, Zhou Y, Li Z, Zhuang S, An X,
Zhang B, Chen W, Nie J, Wang Z, et al: HSP72 attenuates renal
tubular cell apoptosis and interstitial fibrosis in obstructive
nephropathy. Am J Physiol Renal Physiol. 295:F202–F214. 2008.
View Article : Google Scholar : PubMed/NCBI
|