Introduction
Atopic dermatitis (AD) is a chronic allergic
inflammatory skin disease, characterized by pruritic, skin
thickening, erythema, and eczematous skin lesions (1). The interaction of multiple factors
such as environmental factors, skin barrier function, and the
immune system is attributed to the pathogenesis of AD (2). Among the environmental factors,
Dermatophagoides farinae (D. farinae) is a type of
house dust mite and is a common environmental allergen associated
with human AD. D. farinae extract (DFE) is known to
contribute to the pathogenesis of AD by inducing both acute and
chronic AD lesions (3–5). The major mechanistic studies on AD
point to the imbalance of Th1 and Th2 responses in favor of Th2
responses (4,6). Other studies have reported that the
acute and chronic phases of AD are predominantly a Th2 and Th1
response, respectively (1,7,8).
It is also known that immunoglobulin E (IgE) production is
associated with Th2 cellular response, whereas IgG2a is associated
with Th1 response (1). The
importance of Th1 and Th2 cytokines in skin inflammation has been
demonstrated (4,9). The major cytokines released from Th1
and Th2 cells are interferon-γ (IFN-γ) and interleukin-4 (IL-4)
(10). IL-4 and IFN-γ play
critical roles in isotype switching to IgE and IgG2a, respectively
(1).
Keratinocyte activation is a feature of the
pathogenesis of the acute and chronic stages of AD (11). The keratinocytes of AD patients
exhibit a propensity for an exaggerated cytokine/chemokine
expression, a phenomenon that may be relevant in promoting and
maintaining inflammation (12).
Therefore, the suppression of keratinocyte activation is a target
for the treatment of AD (13).
The treatment of AD mainly consists of topical
steroid creams and oral steroids as immunosuppressants (14). However, chronic usage of steroids
can cause thinning of the skin, leading to cracking and bleeding
(15). Hence, drugs with no side
effects for the treatment of AD are still being extensively
explored. Recently, many natural products have been reported to
exhibit anti-inflammatory properties and have the potential to
treat skin inflammatory disorders, especially AD (16,17).
Diospyros kaki (Ebenaceae) is a well-known
conventional medicinal herb (18). D. kaki calyx is also
generally used as a traditional medicine in Asia to relieve asthma,
chronic bronchitis, and cough symptoms (19,20). D. kaki calyx contains
various biologically active compounds, such as stearic acid,
palmitic acid, succinic acid, syringic acid, vanillic acid, gallic
acid, kaempferol, trifolin, β-hydroxyursolic acid, friedelin,
oleanolic acid, quercetin, β-sitosterol and ursolic acid (21). Among the various compounds, gallic
acid, quer-cetin, β-sitosterol and oleanolic acid are already known
to possess anti-AD potential (4,22–24). In spite of various studies
regarding the biological effects of D. kaki, the anti-AD
effect of D. kaki calyx has not yet been reported. The aim
of the present study was to assure the beneficial effects of
aqueous extract of D. kaki calyx (AEDKC) on AD and to define
the underlying mechanisms of these effects.
Materials and methods
Animals
Six-week-old female BALB/c mice were purchased from
SLC Inc. (Hamamatsu, Japan). The mice were housed with 5 mice/cage
in a laminar air flow room maintained at a temperature of 22±2°C, a
relative humidity of 55±5% and a 12 h light:dark cycle throughout
the study. The care and treatment of the mice were in accordance
with the guidelines established by the Public Health Service Policy
on the Humane Care and Use of Laboratory Animals and were approved
by the Institutional Animal Care and Use Committee of Kyungpook
National University.
Preparation of AEDKC, reagents and cell
culture
D. kaki calyx used in this study was
purchased from the Oriental drug store Bohwa Dang (Jeonju, Korea)
and identified by Dr D.K. Kim at the College of Pharmacy, Woosuk
University. A voucher specimen (no. WSP-15-098) was deposited at
the Herbarium of the College of Pharmacy, Woosuk University. The
sample was extracted with purified water at 70°C for 5 h (2 times)
in a water bath. Then the extract was filtered, lyophilized, and
then kept at 4°C. The yield of dried extract from starting crude
materials was ~10.1%.
For the animal experiments, the dried residue was
dissolved in phosphate-buffered saline (PBS). DFE (Greer
Laboratories, Lenoir, NC, USA) and 2,4-dinitrochlorobenzene (DNCB)
were used as antigen and hapten for the induction of AD-like skin
lesions, respectively. All other reagents were purchased from Sigma
(St. Louis, MO, USA) unless otherwise stated. DFE was dissolved in
PBS containing 0.5% Tween-20. DNCB was dissolved in an
acetone/olive oil (1:3) solution. Recombinant human tumor necrosis
factor-α (TNF-α) and IFN-γ were purchased from R&D Systems
(Minneapolis, MN, USA).
A human keratinocyte cell line, HaCaT, was
maintained in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) and antibiotics (100
U/m penicillin G, 100 µg/ml streptomycin) at 37°C under 5%
CO2. Passages 3–6 were used throughout the study.
Induction of AD-like lesions in the ears
of mice
AD-like lesions were induced by DFE and DNCB
according to previous studies (4,5).
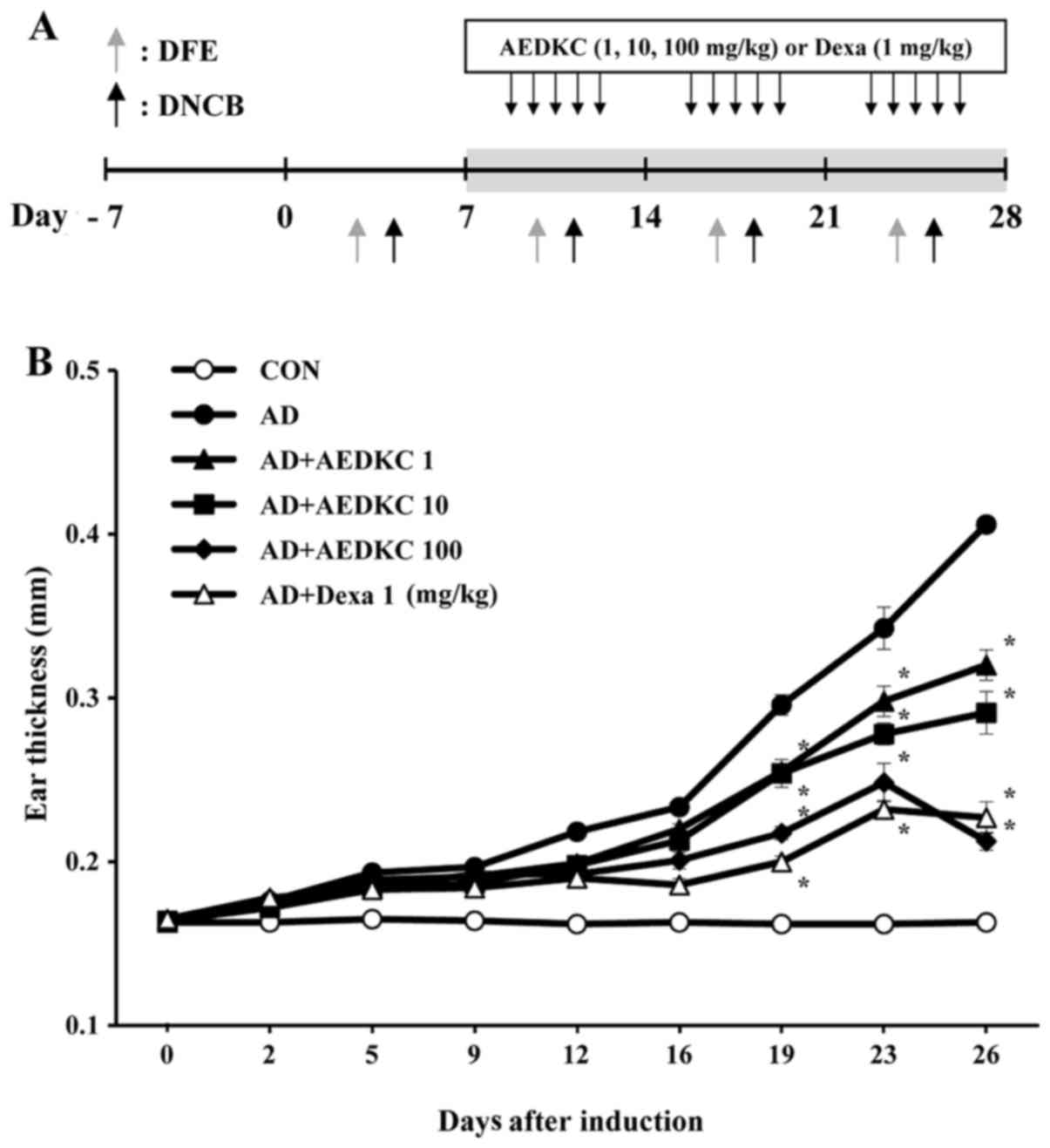
The schematic experimental procedure is described in Fig. 1A. Female BALB/c mice were divided
into 6 groups (n=5): vehicle, DFE/DNCB plus vehicle, DFE/DNCB plus
AEDKC (1, 10 and 100 mg/kg), or dexamethasone (Dexa, 1 mg/kg). Mice
were anesthetized with ketamine and the surfaces of both ear lobes
were very gently stripped 4 times with surgical tape (Nichiban,
Tokyo, Japan). Then, 20 µl of DNCB (1%) was painted on each
ear and then with 20 µl of DFE (10 mg/ml) 4 days later.
DFE/DNCB exposure was repeated once a week rotationally for 4
weeks. Two weeks after the first induction, tail bleeding was
performed to evaluate the serum IgE level. After the confirmation
of AD, as indicated by the IgE level, AEDKC was orally administered
until the end of the 4-week induction (5 times/week). Ear thickness
was measured the following day at the same time after DFE or DNCB
application using a dial thickness gauge (Mitutoyo, Co., Tokyo,
Japan).
On day 28, blood samples were collected by orbital
puncture. After the blood had clotted at room temperature, it was
centrifuged at 400 x g for 15 min at 4°C, and the serum was
isolated. The serum was stored at −80°C for additional analysis.
The ear of each mice was removed and subjected to histopathological
analysis. Serum IgE and IgG2a levels were measured using an
enzyme-linked immunosorbent assay (ELISA) kit (BD Biosciences,
Oxford, UK) according to the manufacturer's instructions. For the
detection of DFE-specific IgE, 96-well plates (Nunc, Wiesbaden,
Germany) were coated with 10 mg DFE in PBS. The DFE-specific IgE
level was indicated by the OD value.
Histological observation
The ears were fixed with 10% formaldehyde and
embedded in paraffin. Sections (5 µm) were stained with
hematoxylin and eosin (H&E) and toluidine blue (TB). To measure
Infiltrated lymphocytes, thickening of the epidermis, and fibrosis
in the dermis, skin sections were stained with H&E and the
stained fields were observed by microscopy. To measure mast cell
infiltration, skin sections were stained with TB, and the number of
mast cells in the 5 sites chosen at random was counted. Eosinophils
were counted in a blinded manner in 10 high-power fields at a
magnification of ×400. Epidermal and dermal thickness of the
H&E-stained sections was analyzed under a magnification of
×200. Thickness was measured in 5 randomly chosen fields from each
sample.
Histamine assay
Histamine content was measured following the
o-phthaldialdehyde spectrofluorometric procedure of a previous
study (25). The blood from mice
was centrifuged at 400 x g for 15 min, and the serum was diluted
with PBS and withdrawn to measure the histamine content.
Fluorescence intensity was measured using 355-nm excitation and
450-nm filters and the fluorescence spectrometer LS-50B
(Perkin-Elmer, Norwalk, CT, USA).
qPCR
To detect the expression of cytokines, qPCR was
performed using the Thermal Cycler Dice TP850 (Takarabio Inc.,
Shiga, Japan) according to the manufacturer's protocol. HaCaT cells
(1×105 cells/24-well plate) were pretreated with AEDKC
for 1 h, and then stimulated with TNF-α (10 ng/ml) and IFN-γ (10
ng/ml) for 6 h. Total cellular RNA was isolated from cells as
described in a previous study (4). Briefly, 1 µl of cDNA (100
ng), 1 µl of a sense and antisense primer solution (0.4
µM), 12.5 µl of SYBR Premix Ex Taq (Takarabio Inc),
and 9.5 µl of nuclease-free water were mixed together to
obtain a final 25 µl reaction mixture in each reaction tube.
The conditions for PCR were similar to those of a previous study
(4). The normalization and
quantification of mRNA expression were performed using TP850
software supplied by the manufacturer.
Western blot analysis
Samples for western blot analysis were prepared in
accordance with a previous study (26). Briefly, HaCaT cells
(2×106 cells/6-well plate) were pretreated with AEDKC
for 1 h and then stimulated with TNF-α (10 ng/ml) and IFN-γ (10
ng/ml) for 30 min to activate signal transducer and activator of
transcription 1 (STAT1) and nuclear factor-κB (NF-κB). Cells were
rinsed once with ice-cold PBS, and total cell lysates were
collected in 100 µl of lysis buffer. The lysates were spun
in a micro-centrifuge for 20 min at 4°C, and the supernatant was
collected. Proteins were subjected to 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then
transferred to nitrocellulose membranes. The membranes were stained
with reversible Ponceau S to ensure equal loading of samples onto
the gels. Nuclear and cytosolic p65 NF-κB and IκBα were assayed
using anti-NF-κB (p65; sc-109) and anti-IκBα (sc-371) antibodies
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
respectively. The phosphorylation of STAT1 was detected using
anti-STAT1 (#9172) and anti-phospho-STAT1 (#9167) antibodies (Cell
Signaling Technology, Beverly, MA, USA). Anti-β-actin (sc-8432;
mouse monoclonal; 1:1,000) was from Santa Cruz Biotechnology, Inc.
Immunodetection was conducted using Supersignal West Pico
Chemiluminescent Substrate (Thermo Fisher Scientific, Waltham, MA,
USA).
Statistical analysis
Statistical analyses were performed using Prism 5
(GraphPad Software, San Diego, CA, USA). Treatment effects were
analyzed using one-way analysis of variance followed by Dunnett's
test. A p-value <0.05 indicates a statistically significant
difference.
Results
Effects of AEDKC on histopathological
observations
To investigate the efficacy of AEDKC on AD-like skin
lesions, a DFE/DNCB-induced AD-like model was used. During the
induction period, the ear swelling of mice was measured after 24 h
of DFE or DNCB induction (Fig.
1B). The tendency of ear swelling of mice in each group was
similar until after 2 weeks. After 19 days of AD induction, oral
administration of AEDKC considerably reduced ear thickness.
DFE/DNCB treatment during the induction period (4 weeks) evoked
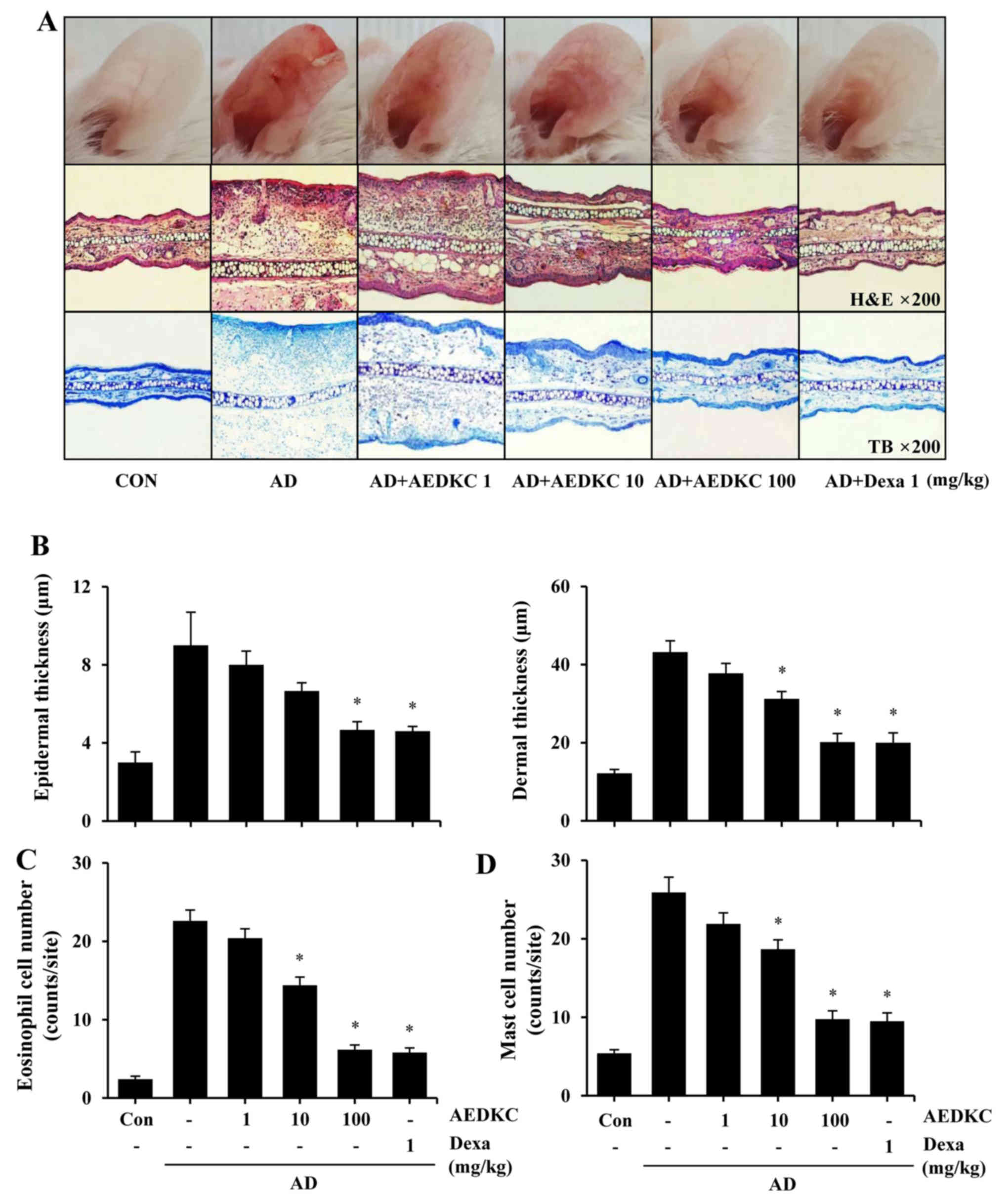
severe AD-like skin lesions (Fig.
2A). Mouse ears became red and swollen after DFE/DNCB exposure.
However, oral treatment of AEDKC relieved these symptoms compare to
the AD mice. Dexamethasone (Dexa) was used as a positive control
drug. Oral administration of AEDKC five consecutive days a week
during 3 weeks did not alter the body weight of mice (data not
shown), indicating that AEDKC exhibited no toxic effects.
Histological analysis showed that AEDKC treatment
significantly suppressed erythema and infiltration of acute
inflammatory cells compared with the AD mice (Fig. 2B–D). Epidermis thickening is
regarded as an important factor that contributes to ear swelling
(22). Compared to the AD mice,
AEDKC considerably decreased DFE/DNCB-induced epidermal and dermal
thickness (Fig. 2B) and
infiltration of eosinophils (Fig.
2C). Mast cell-derived inflammatory mediators and histamine
contribute to itching and inflammation in AD (7). Thus, mast cell infiltration into the
AD site and serum histamine level were measured; AEDKC attenuated
both mast cell infiltration (Fig.
2D) and serum histamine (Fig.
3A).
Effects of AEDKC on serum
immunoglobulin
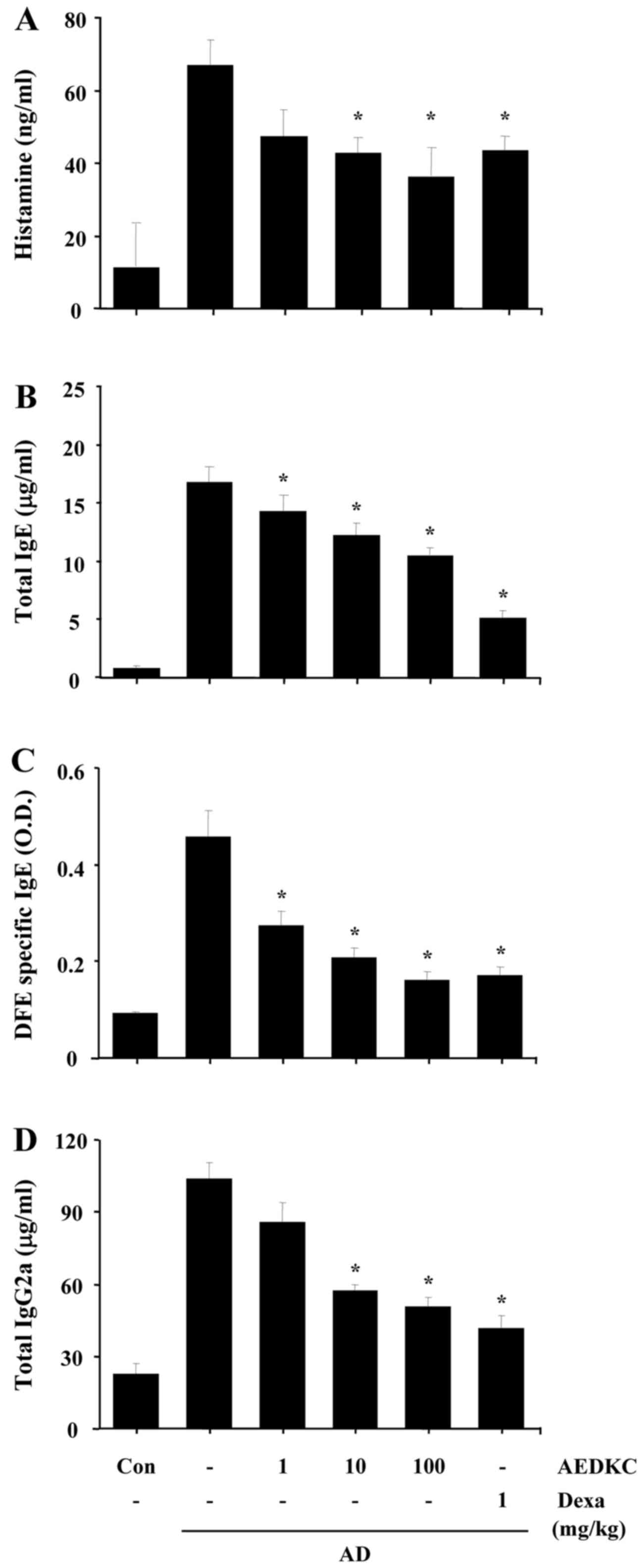
We previously reported a substantial increase in
serum immunoglobulin and cytokines in DFE/DNCB-induced AD mice
(4). To distinguish the role of
AEDKC on the Th1 and Th2 response, we examined individually the
serum levels of IgG2a and IgE (total and DFE-specific). Compared
with the AD mice, the levels of total IgE, DFE-specific IgE, and
IgG2a were markedly decreased in the serum of mice treated with
AEDKC (Fig. 3B–D).
Effects of AEDKC on keratinocyte
activation
After establishing the inhibitory effect of AEDKC on
AD mice, the keratinocyte model was used to ascertain the molecular
mechanism and biological function of AEDKC. Keratinocytes have been
broadly used to imitate the AD environment in vitro
(27). They exhibit a similar
immune response during the development of skin disorders, such as
AD-like skin lesions (28).
First, we evaluated the cytotoxicity of AEDKC by exposing HaCaT
cells to various concentrations of AEDKC for 24 h. In the MTT
assay, AEDKC did not exert cytotoxicity at concentrations up to
1,000 µg/ml in the keratinocytes (data not shown). To
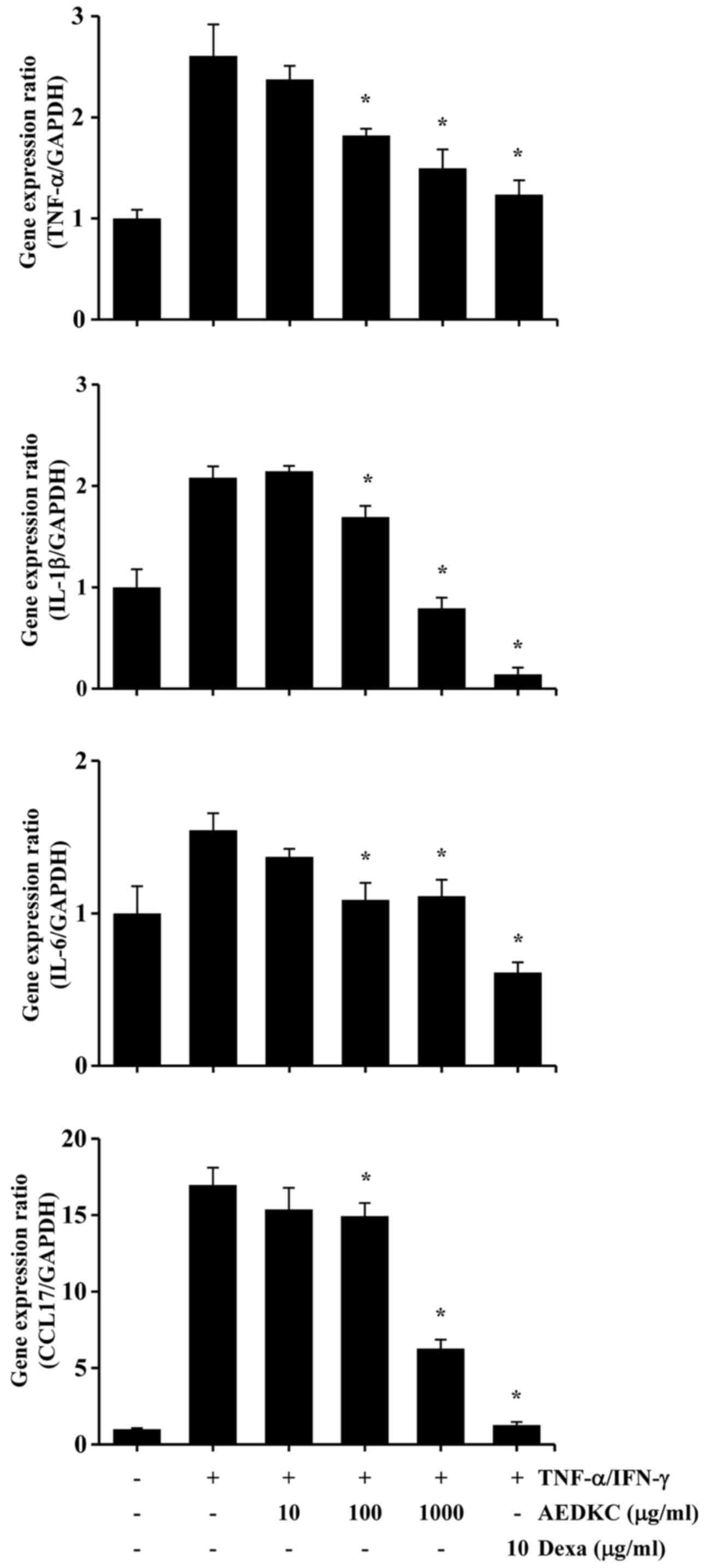
examine the effect of AEDKC on pro-inflammatory cytokines and a
chemokine, HaCaT cells were pretreated with AEDKC for 1 h, followed
by the stimulation with TNF-α/IFN-γ for 6 h. The results of qPCR
indicated that AEDKC inhibited TNF-α/IFN-γ-induced gene expression
of TNF-α, IL-1β, IL-6 and CCL17 in the HaCaT cells (Fig. 4).
Thereafter, the regulatory effect of AEDKC on the
expression of pro-inflammatory cytokines and a chemokine was
examined. To establish the mechanism responsible for the inhibitory
effect of AEDKC, we investigated the effect of AEDKC on
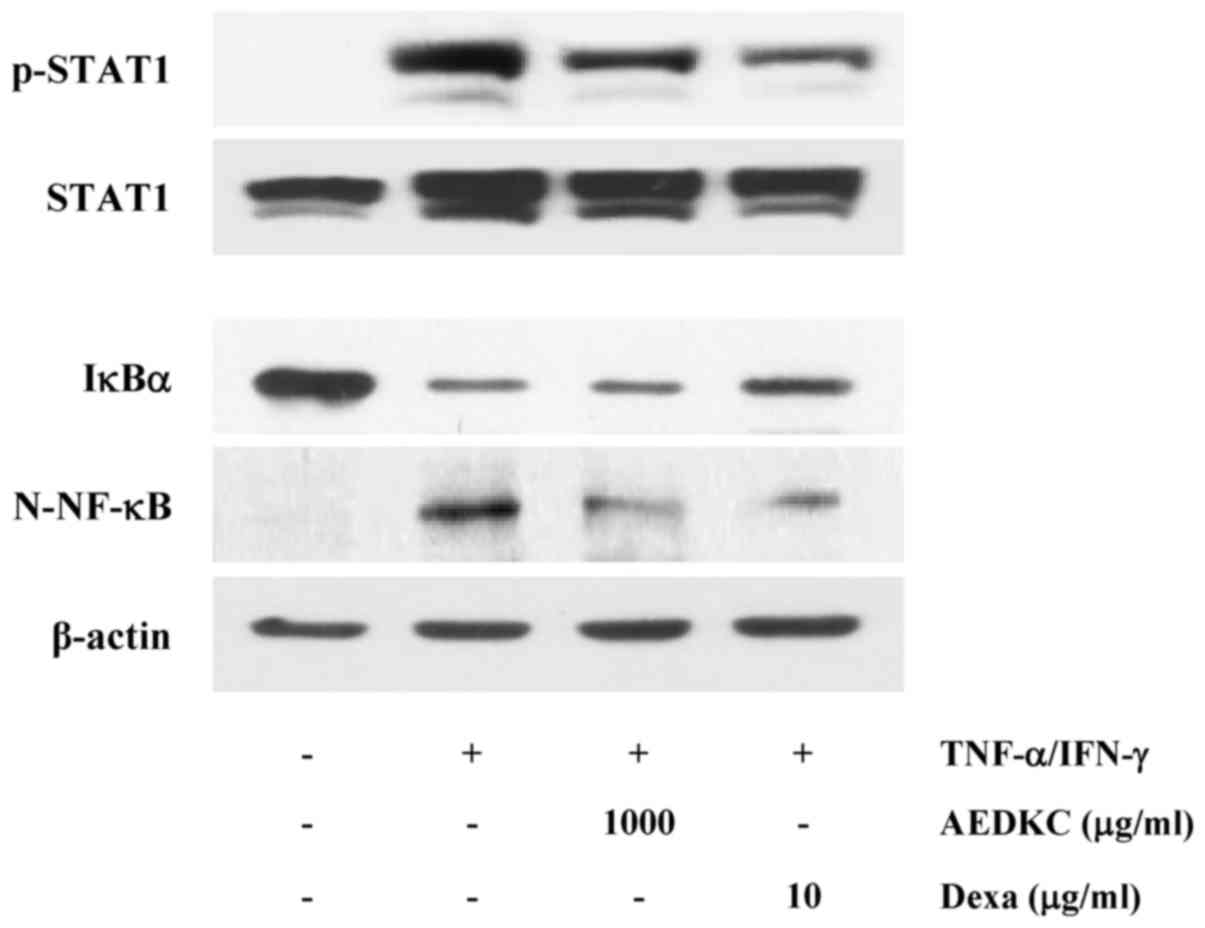
TNF-α/IFN-γ-induced activation of STAT1 and NF-κB. Previous studies
have reported that the STAT and NF-κB signaling pathways contribute
to the production of pro-inflammatory cytokines (TNF-α, IL-1β and
IL-6) and chemokine (CCL17) in TNF-α/IFN-γ-induced HaCaT cells
(29,30). As shown in Fig. 5, activation of STAT1 and NF-κB was
reduced by AEDKC (1,000 µg/ml).
Discussion
Various parts of Diospyros kaki have been
widely used as a herbal medicine including the treatment of
allergic inflammation (17,31). D. kaki folium ameliorates
transepidermal water loss in AD and allergic skin symptoms
(32). D. kaki calyx has
been generally used to relieve asthma, cough symptoms and chronic
bronchitis (19,20). Various ingredients of D.
kaki calyx have been reported (39). Among them, gallic acid, oleanolic
acid, quercetin, and β-sitosterol are known to act as biological
active compounds (4,22–24). Based on the known various
pharmacological activity of D. kaki, the role of AEDKC on
AD-like skin lesions was evaluated using in vivo and in
vitro models.
To examine the effect of AEDKC on AD-like skin
lesions, we adopted a DFE/DNCB-induced AD mouse model. This AD
model is often used by many researchers due to the reproducibility
and AD-like characteristics involved in both Th1 and Th2 responses
as in human AD patients exposed to DFE, a common allergen (1,4,9).
We previously reported that this mouse model exhibits phenotypes
reminiscent of both acute and chronic AD lesions, including
spongiosis, epidermal hyperplasia, fibrosis and infiltration of
inflammatory cells (eosinophils and mast cells) (4). In the present study, we confirmed
that mice epicutaneously sensitized with DFE/DNCB exhibited ear
redness and swelling, hyperplasia and dysregulated differentiation
of the epidermis, and infiltration of dermal inflammatory cells.
Oral administration of AEDKC relieved the typical and histological
changes such as intense ear thickness, dysregulated differentiation
of the epidermis, dermal and epidermal thickness, epidermal
hyperplasia, and infiltration of inflammatory cells. Mast cells are
key effector cells in patients with IgE receptor (FcεRI)-bearing
immediate allergic disorders (33). Activation of mast cells leads to
the release of mediators such as cytokines and histamine. Histamine
mainly induces pruritus and edema; thus, it is likely to be a
crucial mediator in AD patients (34). In addition, serum histamine levels
have been reported to be significantly higher in AD patients than
that in normal human skin (35).
The present results indicate that oral administration of AEDKC
reduced serum histamine levels and the pathogenesis of skin lesions
in AD.
In AD condition, keratinocytes release a
characteristic form of cytokines/chemokines after pro-inflammatory
cytokine exposure (36). TNF-α
and IFN-γ can synergistically induce important cytokines for AD
symptoms in keratinocytes, and this experimental model has been
widely used to mimic the AD environment in vitro (4,27).
Several studies have reported that CCL17 is overexpressed in the
serum of AD patients, and that the severity of AD is strongly
correlated with the chemokine levels (37,38). Dexamethasone is an effective
immunosuppressive medication widely used in the treatment of AD
(39). Thus, it was used as a
positive control. The present results indicated that AEDKC
treatment suppressed TNF-α/IFN-γ-induced TNF-α, IL-1β, IL-6 and
CCL17. Compared to the effects of dexamethasone, AEDKC at a high
dose showed a similar immune suppressive effect in
keratinocytes.
STAT1 and NF-κB in the cytoplasm translocate into
the nucleus, where they participate in the expression of
pro-inflammatory genes (30).
CCL17 promoters contain STAT1 and NF-κB binding sequences, and
these transcription factors mediate the transcription of genes
(30). In this study, we
demonstrated that AEDKC inhibited the signaling pathways involved
in the activation of STAT1 and NF-κB. AEDKC suppressed STAT1
phosphorylation. Furthermore, AEDKC inhibited the degradation of
IκBα and nuclear translocation of NF-κB. These results indicate
that AEDKC exerts inhibitory effect on CCL17 via the downregulation
of both STAT1 and NF-κB. This study provides evidence that AEDKC
has suppressive effects on TNF-α/IFN-γ-induced expression of
pro-inflammatory cytokines and chemokine by the blocking of STAT1
and NF-κB. CCL17 is adjusted by the STAT1 and NF-κB pathways in
keratinocytes; thus, the present results indicate that AEDKC may
reduce AD-like skin lesions by suppressing CCL17.
In this study, we demonstrated that AEDKC suppressed
the development of AD-like skin lesions in both in vivo and
in vitro models. AEDKC inhibited the cytokines and chemokine
involved in AD via blocking NF-κB and STAT1 signaling pathways in
keratinocytes. Taken together, AEDKC is a potential effective
treatment for AD and could be used as a pharmacological agent or
food supplement.
Acknowledgments
This study was supported by the National Research
Foundation of Korea (nos. 2014R1A5A2009242, 2012M3A9B6055416 and
2016R1A2B4008513), KRIBB Research Initiative Program (KGM4251723),
and High Value-added Food Technology Development Program, Ministry
of Agriculture, Food and Rural Affairs.
References
|
1
|
Bieber T: Atopic dermatitis. Ann Dermatol.
22:125–137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boguniewicz M and Leung DY: Atopic
dermatitis: A disease of altered skin barrier and immune
dysregulation. Immunol Rev. 242:233–246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dai X, Sayama K, Tohyama M, Shirakata Y,
Hanakawa Y, Tokumaru S, Yang L, Hirakawa S and Hashimoto K: Mite
allergen is a danger signal for the skin via activation of
inflammasome in keratinocytes. J Allergy Clin Immunol.
127:806–14.e1. 42011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi JK, Oh HM, Lee S, Park JW, Khang D,
Lee SW, Lee WS, Rho MC and Kim SH: Oleanolic acid acetate inhibits
atopic dermatitis and allergic contact dermatitis in a murine
model. Toxicol Appl Pharmacol. 269:72–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kwon HK, Lee CG, So JS, Chae CS, Hwang JS,
Sahoo A, Nam JH, Rhee JH, Hwang KC and Im SH: Generation of
regulatory dendritic cells and CD4+Foxp3+ T
cells by probiotics administration suppresses immune disorders.
Proc Natl Acad Sci USA. 107:2159–2164. 2010. View Article : Google Scholar
|
|
6
|
Kim JY, Jeong MS, Park MK, Lee MK and Seo
SJ: Time-dependent progression from the acute to chronic phases in
atopic dermatitis induced by epicutaneous allergen stimulation in
NC/Nga mice. Exp Dermatol. 23:53–57. 2014. View Article : Google Scholar
|
|
7
|
Oyoshi MK, He R, Kumar L, Yoon J and Geha
RS: Cellular and molecular mechanisms in atopic dermatitis. Adv
Immunol. 102:135–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsushima H, Hayashi S and Shimada S:
Skin scratching switches immune responses from Th2 to Th1 type in
epicutaneously immunized mice. J Dermatol Sci. 32:223–230. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Novak N: New insights into the mechanism
and management of allergic diseases: Atopic dermatitis. Allergy.
64:265–275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoon SY, Kang HB, Ko YE, Shin SH, Kim YJ,
Sohn KY, Han YH, Chong S and Kim JW:
1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol (EC-18) Modulates Th2
Immunity through Attenuation of IL-4 Expression. Immune Netw.
15:100–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kaiko GE and Foster PS: New insights into
the generation of Th2 immunity and potential therapeutic targets
for the treatment of asthma. Curr Opin Allergy Clin Immunol.
11:39–45. 2011. View Article : Google Scholar
|
|
12
|
Girolomoni G and Pastore S: Epithelial
cells and atopic diseases. Curr Allergy Asthma Rep. 1:481–482.
2001. View Article : Google Scholar
|
|
13
|
Trautmann A, Akdis M, Schmid-Grendelmeier
P, Disch R, Bröcker EB, Blaser K and Akdis CA: Targeting
keratinocyte apoptosis in the treatment of atopic dermatitis and
allergic contact dermatitis. J Allergy Clin Immunol. 108:839–846.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wollenberg A and Ehmann LM: Long term
treatment concepts and proactive therapy for atopic eczema. Ann
Dermatol. 24:253–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hsu CJ and Wang LF: Emerging treatment of
atopic dermatitis. Clin Rev Allergy Immunol. 33:199–203. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi JH, Jin SW, Park BH, Kim HG, Khanal
T, Han HJ, Hwang YP, Choi JM, Chung YC, Hwang SK, et al: Cultivated
ginseng inhibits 2,4-dinitrochlorobenzene-induced atopic
dermatitis-like skin lesions in NC/Nga mice and TNF-α/IFN-γ-induced
TARC activation in HaCaT cells. Food Chem Toxicol. 56:195–203.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim HH, Kim DS, Kim SW, Lim SH, Kim DK,
Shin TY and Kim SH: Inhibitory effects of Diospyros kaki in a model
of allergic inflammation: Role of cAMP, calcium and nuclear
factor-κB. Int J Mol Med. 32:945–951. 2013.PubMed/NCBI
|
|
18
|
Jung HG, Kim HH, Paul S, Jang JY, Cho YH,
Kim HJ, Yu JM, Lee ES, An BJ, Kang SC, et al:
Quercetin-3-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranoside
suppresses melanin synthesis by augmenting p38 MAPK and CREB
signaling pathways and subsequent cAMP downregulation in murine
melanoma cells. Saudi J Biol Sci. 22:706–713. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bei W, Zang L, Guo J, Peng W, Xu A, Good
DA, Hu Y, Wu W, Hu D, Zhu X, et al: Neuroprotective effects of a
standardized flavonoid extract from Diospyros kaki leaves. J
Ethnopharmacol. 126:134–142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sa YS, Kim SJ and Choi HS: The
anticoagulant fraction from the leaves of Diospyros kaki L. has an
antithrombotic activity. Arch Pharm Res. 28:667–674. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Singh S and Joshi h: Diospyros kaki
(Ebenaceae): A Review. Asian J Res Pharm Sci. 1:55–58. 2011.
|
|
22
|
Tsang MS, Jiao D, Chan BC, Hon KL, Leung
PC, Lau CB, Wong EC, Cheng L, Chan CK, Lam CW, et al:
Anti-inflammatory activities of pentaherbs formula, berberine,
gallic acid and chlorogenic acid in atopic dermatitis-like skin
inflammation. Molecules. 21:5192016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karuppagounder V, Arumugam S,
Thandavarayan RA, Sreedhar R, Giridharan VV and Watanabe K:
Molecular targets of quercetin with anti-inflammatory properties in
atopic dermatitis. Drug Discov Today. 21:632–639. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han NR, Kim HM and Jeong HJ: The
β-sitosterol attenuates atopic dermatitis-like skin lesions through
downregulation of TSLP. Exp Biol Med (Maywood). 239:454–464. 2014.
View Article : Google Scholar
|
|
25
|
Bae Y, Lee S and Kim SH: Chrysin
suppresses mast cell-mediated allergic inflammation: Involvement of
calcium, caspase-1 and nuclear factor-κB. Toxicol Appl Pharmacol.
254:56–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee S, Yun HS and Kim SH: The comparative
effects of mesoporous silica nanoparticles and colloidal silica on
inflammation and apoptosis. Biomaterials. 32:9434–9443. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leung DYM, Boguniewicz M, Howell MD,
Nomura I and Hamid QA: New insights into atopic dermatitis. J Clin
Invest. 113:651–657. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guttman-Yassky E, Nograles KE and Krueger
JG: Contrasting pathogenesis of atopic dermatitis and psoriasis -
part I: Clinical and pathologic concepts. J Allergy Clin Immunol.
127:1110–1118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim SY, Sohn EJ, Kim DW, Jeong HJ, Kim MJ,
Kang HW, Shin MJ, Ahn EH, Kwon SW, Kim YN, et al: Transduced
PEP-1-FK506BP ameliorates atopic dermatitis in NC/Nga mice. J
Invest Dermatol. 131:1477–1485. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kwon DJ, Bae YS, Ju SM, Goh AR, Youn GS,
Choi SY and Park J: Casuarinin suppresses TARC/CCL17 and MDC/CCL22
production via blockade of NF-κB and STAT1 activation in HaCaT
cells. Biochem Biophys Res Commun. 417:1254–1259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie C, Xie Z, Xu X and Yang D: Persimmon
(Diospyros kaki L.) leaves: A review on traditional uses,
phytochemistry and pharmacological properties. J Ethnopharmacol.
163:229–240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Matsumoto M, Kotani M, Fujita A, Higa S,
Kishimoto T, Suemura M and Tanaka T: Oral administration of
persimmon leaf extract ameliorates skin symptoms and transepidermal
water loss in atopic dermatitis model mice, NC/Nga. Br J Dermatol.
146:221–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oka T, Rios EJ, Tsai M, Kalesnikoff J and
Galli SJ: Rapid desensitization induces internalization of
antigen-specific IgE on mouse mast cells. J Allergy Clin Immunol.
132:922–32. e1–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kawakami T, Ando T, Kimura M, Wilson BS
and Kawakami Y: Mast cells in atopic dermatitis. Curr Opin Immunol.
21:666–678. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Greaves MW: Antihistamines in dermatology.
Skin Pharmacol Physiol. 18:220–229. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Akdis CA, Akdis M, Bieber T,
Bindslev-Jensen C, Boguniewicz M, Eigenmann P, Hamid Q, Kapp A,
Leung DY, Lipozencic J, et al: Diagnosis and treatment of atopic
dermatitis in children and adults: European Academy of Allergology
and Clinical Immunology/American Academy of Allergy, Asthma and
Immunology/PRACTALL Consensus Report. J Allergy Clin Immunol.
118:152–169. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shimada Y, Takehara K and Sato S: Both Th2
and Th1 chemokines (TARC/CCL17, MDC/CCL22, and Mig/CXCL9) are
elevated in sera from patients with atopic dermatitis. J Dermatol
Sci. 34:201–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jahnz-Rozyk K, Targowski T, Paluchowska E,
Owczarek W and Kucharczyk A: Serum thymus and activation-regulated
chemokine, macrophage-derived chemokine and eotaxin as markers of
severity of atopic dermatitis. Allergy. 60:685–688. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schmitt J, von Kobyletzki L, Svensson A
and Apfelbacher C: Efficacy and tolerability of proactive treatment
with topical corticosteroids and calcineurin inhibitors for atopic
eczema: Systematic review and meta-analysis of randomized
controlled trials. Br J Dermatol. 164:415–428. 2011. View Article : Google Scholar
|