Introduction
Metadherin (MTDH), also known as astrocyte elevated
gene-1 (AEG-1) or LYRIC, is a protein encoded by the MTDH
gene. MTDH is frequently overexpressed in cancers of the breast,
prostate, ovary and endometrium, and is associated with poor
clinical outcomes (1,2). MTDH influences several oncogenic
signaling pathways and transcription factors, such as the Ras, Myc,
PI3K/AKT, nuclear factor-κB (NF-κB), mitogen-activated protein
kinase (MAPK) and Wnt pathways (3,4),
suggesting that MTDH plays an important role in disease development
and progression.
MTDH is highly basic and contains a transmembrane
domain and multiple nuclear localization signals, with reported
localizations in the cell membrane, cytoplasm, nucleus, nucleolus
and endoplasmic reticulum. Not surprisingly, distinct interaction
partners have been reported for the various locales, allowing MTDH
to contribute to a diverse range of intracellular events (4). For example, MTDH has been shown to
interact with several proteins to drive tumorigenesis, including
NF-κB, promyelocytic leukaemia zinc finger (PLZF), BRCA2- and
CDKN1A (p21Cip1/Waf-1/mda-6)-interacting protein α (BCCIPα) and
staphylococcal nuclease and tudor domain containing 1 (SND1)
(5–8). MTDH also interacts with the steroid
hormone receptors RXRs (RXRα, β, γ) to negatively regulate RXR
function and RXR-mediated transcriptional regulation (9). Recently, some reports have suggested
that MTDH overexpression inversely correlates with the estrogen
receptor (ER) status, and MTDH may induce resistance to tamoxifen
in ERα-positive breast cancer cells (10–12).
In this study, we examined the potential association
between MTDH and ER. Estrogens control multiple cellular functions
via estrogen receptors (ER-α and ER-β), modular proteins that
belong to the steroid/nuclear receptor family of intracellular
homeostatic regulators. Ligand binding in the cytosol results in
conformation changes that promote receptor translocation into the
nucleus, thereby promoting gene expression or repression through
interactions with chromatin and transcriptional co-regulatory
complexes. In this study, we found that the expression of a number
of genes correlated with MTDH expression in ER positive cancers
from the Cancer Genome Atlas (TCGA) dataset, and we also found a
transient interaction of MTDH with ERα following estrogen
treatment.
Materials and methods
The Cancer Genome Atlas (TCGA) data
analysis
Gene expression data from the RNA sequencing
(RNA-seq) experiments were downloaded from the TCGA (https://portal.gdc.cancer.gov/) data portal for
breast and endometrial cancer. For RNA-seq only level 3 data was
available. Data at level 3 are already preprocessed, normalized and
log2-transformed (for more details see https://cancergenome.nih.gov/abouttcga/about-data/datalevelstypes).
In addition, metadata and clinical data were extracted and
downloaded to identify clinical parameters, such as endometrial
histological types (serous vs. endometrioid), and molecular
characteristics, such as ER-positive vs. ER-negative breast cancer.
Table I shows the patients
included in the analysis.
 | Table INumber of genes correlated with MTDH
expression (p<10−6). |
Table I
Number of genes correlated with MTDH
expression (p<10−6).
| Endometrial cancer
(18,023 genes)
| Breast cancer (17,660
genes)
|
|---|
ER-Pos
(endometrioid)
(N=142) | ER-Neg
(serous)
(N=38) | ER-Pos
(N=705) | ER-Neg
(N=207) |
|---|
| Significant
r2 with MTDH | 4479 | 88 | 4,451 | 332 |
| % Correlation | 24.9% | 0.5% | 25.2% | 1.9% |
To abstract unique gene expression data we used the
Biometric Research Branch (BRB) ArrayTools (version 2.13.2 for X64
systems) software suite. BRB-ArrayTools was developed by Dr Richard
Simon and colleagues, and is an integrated package for
visualization and statistical analysis utilizing Excel (Microsoft,
Redmond, WA, USA) as the front end, with tools developed in the R
statistical system. MTDH expression was correlated with the
expression of all other genes in breast and endometrial RNA-seq
experiments using rank-based Spearman correlation to allow for
non-linear relationships between several gene expression values. To
control the false discovery rate (FDR) we used the q-value for
statistical significance (qFDR) and Bonferroni correction for
multiple comparisons. A significant correlation was considered a
correlation with a p-value <10−6 after controlling
for multiple comparisons.
Cell culture and generation of
MTDH-deficient cells
The human endometrial cancer cell line, ECC1
(American Type Culture Collection; ATCC, Manassas, VA, USA), was
maintained in RPMI-1640 medium supplemented with 10% fetal bovine
serum (FBS) and 1% penicillin/streptomycin, MCF-7 cells (ATCC) were
maintained in Dulbecco's modified Eagle's medium (DMEM) medium
supplemented with 10% FBS and 1% penicillin/streptomycin (all from
Gibco-BRL, Carlsbad, CA, USA); all cells were incubated at 37°C in
a humidified atmosphere of 5% CO2. Genomic deletions of
MTDH were created in the ECC1 cells using 3 pairs of chimeric
single guide RNAs (sgRNAs) as previously described (13). Briefly, sgRNA oligos were
synthesized and cloned into the lentiCRISPR transfer plasmid for
virus production. Viral vectors were produced in 293T cells (ATCC),
followed by infection of ECC1 cells as described. MTDH-deficient
ECC1 cells were selected with 5 µg/ml puromycin after 48 h
of transduction. MTDH-deficient ECC1 cells were grown at low
density for 2 weeks to select clones. MTDH expression was
determined by western blot analysis to identify clones with no or
low MTDH protein expression.
Microarray assay and data analysis
ECC1 and MTDH-deficient ECC1 cells were incubated in
RPMI-1640 supplemented with 5% charcoal stripped FBS for 24 h then
treated with 10 nM estrogen (Sigma-Aldrich, St. Louis, MO, USA) or
treated with ethanol as a control for 24 h. Total RNA was isolated
using the Qiagen RNeasy mini kit (Qiagen, Valencia, CA, USA). RNA
sample preparation for hybridization and the subsequent
hybridization to the Illumina beadchips were performed at the
University of Iowa IIHG Genomics Division using the manufacturer's
recommended protocols. Briefly, 100 ng total RNA were converted to
amplified biotin-aRNA using the Epicentre TargetAmp-Nano Labeling
kit for Illumina Expression BeadChip (Illumina, Inc., San Diego,
CA, USA). The amplified Biotin-aRNA product was purified through a
Qiagen RNeasy MinElute Cleanup column (Qiagen) according to
modifications from Epicentre (Madison, WI, USA). Subsequently, 750
ng of this product were mixed with Illumina hybridization buffer,
placed onto Illumina-Human H12 v4 BeadChips (part no. BD-103-0204),
and incubated at 58°C for 17 h, with rocking, in an Illumina
hybridization oven. Following hybridization, the arrays were
washed, blocked and then stained with streptavidin-Cy3 (Amersham/GE
Healthcare, Piscataway, NJ, USA) according to the Illumina
Whole-Genome Gene Expression Direct Hybridization Assay protocol.
Beadchips were scanned with the Illumina iScan system (ID #N0534)
and data were collected using the GenomeStudio software v2011.1.
Microarray data have been deposited at GEO (www.ncbi.nlm.nih.gov/geo) under the accession no.
GSE78182. The microarray data were analyzed for data summarization,
normalization and quality control by using BRB ArrayTools (version
2.13.2 for X64 systems) software suite. To select the
differentially expressed genes, we used threshold values of ≥2 and
≤−2-fold-change and a Benjamini-Hochberg corrected p-value of
0.001. The data were log2-transformed and median centred by genes
using the Adjust Data function of Cluster 3.0 software, and then
further analyzed with hierarchical clustering with average linkage.
The micro-array experiment was performed using biological
triplicates.
Immunoprecipitation (IP) and western blot
analysis
The ECC1 cells were incubated in RPMI-1640 and the
MCF-7 cells were incubated in DMEM supplemented with 5% charcoal
stripped FBS for 24 h then treated with 10 nM estrogen for 0, 1, 2
and 4 h. For IP, the cells were lysed in 400 µl of NP-40
buffer and 5% of the lysates were saved as input. BCA protein assay
was used to determine the protein concentrations of each lysate. A
total of 1 mg of the protein lysates were incubated with 1
µg primary antibodies followed by incubation with protein
A/G agarose beads (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), which were then washed 4 times with NP-40 buffer.
Immuno-complexes were boiled in 2X Laemmli sample buffer for 5 min
before they were subjected to sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE), transferred onto nitrocellulose
membranes and immunoblotted with MTDH antibody (cat. no. 40-6500;
Invitrogen) or ERα antibody (cat. no. sc-543; Santa Cruz
Biotechnology, Inc.). The procedures for cytoplasmic and nuclear
extraction were carried out according the manufacture'rs
instructions (Active Motif, Carlsbad, CA, USA), and the fractions
were then immunoprecipitated with ERα antibody, followed by
immunoblotting with ERα or MTDH antibodies.
Immunostaining
Breast cancer and endometrial cancer specimens
obtained from the Tissue Procurement Core were subjected to
immunohistochemical staining for either ER or MTDH as previously
described (13).
RT-qPCR
Four significant genes from microarray data were
randomly selected and validated by RT-qPCR analysis in the ECC1
cells. Total RNA was isolated using the Qiagen RNeasy mini kit
(Qiagen), and reverse transcription with SuperScript®
III First-Strand Synthesis system (Invitrogen). SYBR-Green-based
real-time PCR (SYBR-Green I; ABI, Warrington, UK) was used to
quantitatively detect the expression of Phosphatidic Acid
Phosphatase Type 2B (PPAP2B), Plexin Domain Containing 2 (PLXDC2),
Transmembrane And Tetratricopeptide Repeat Containing 1 (TMTC1),
Protease, Serine 23 (PRSS23) and 18S RNA. The fold variation in the
RNA samples was calculated using the 2−ΔΔct method
following normalization with 18S. The primer sequences were as
follows: PPAP2B forward, 5′-TGA GAG CAT CAA GTA CCC ACT-3′ and
reverse, 5′-ACG TAG GGG TTC TGA ATC GTC-3′; PLXDC2 forward, 5′-TTC
TCA AGG CGG TAG ACA CGA-3′ and reverse, 5′-CGA TCT GAG TGT TAT TGT
CCT GC-3′; TMTC1 forward, 5′-ACG GTG TCT CCC TTC TTC TTG-3′ and
reverse, 5′-ATT GCT CGA CTT GTC TTG CTT-3′; PRSS23 forward, 5′-CAG
TGT CAT AAG GGA ACT CCA C-3′ and reverse, 5′-CCT GAG TCT CGG TGT
TGG G-3′; 18S forward, 5′-AAC TTT CGA TGG TAG TCG CCG-3′ and
reverse, 5′-CCT TGG ATG TGG TAG CCG TTT-3′.
Online tools
Gene ontology (GO) analyses were based on the online
tool PANTHER (http://pantherdb.org/).
Results
The expression of a number of genes
correlates with MTDH expression in ER-positive endometrial cancer
and breast cancer
Using the Cancer Genome Atlas (TCGA) dataset, with a
cut-off set at p-value <10−6 between endometrial
cancer and serous endometrial cancer, or between ER-positive and
ER-negative breast cancer, we found that the expression of 24.9% of
the genes correlated with MTDH expression in endometrial cancer,
and the expression of 25.2% of the genes correlated with MTDH
expression in ER-positive breast cancer (Table I). By contrast, only approximately
0.5 or 1.9% of the genes correlated with MTDH expression in serous
endometrial cancer and ER-negative breast cancer, respectively.
These results indicate that MTDH may be associated with ER or
estrogen-regulated gene expression.
Microarray analysis of estrogen-treated
ECC1 cells and MTDH-deficient ECC1 cells
To further examine the association between MTDH and
ER, genomic deletions of MTDH were created in ECC1 cells
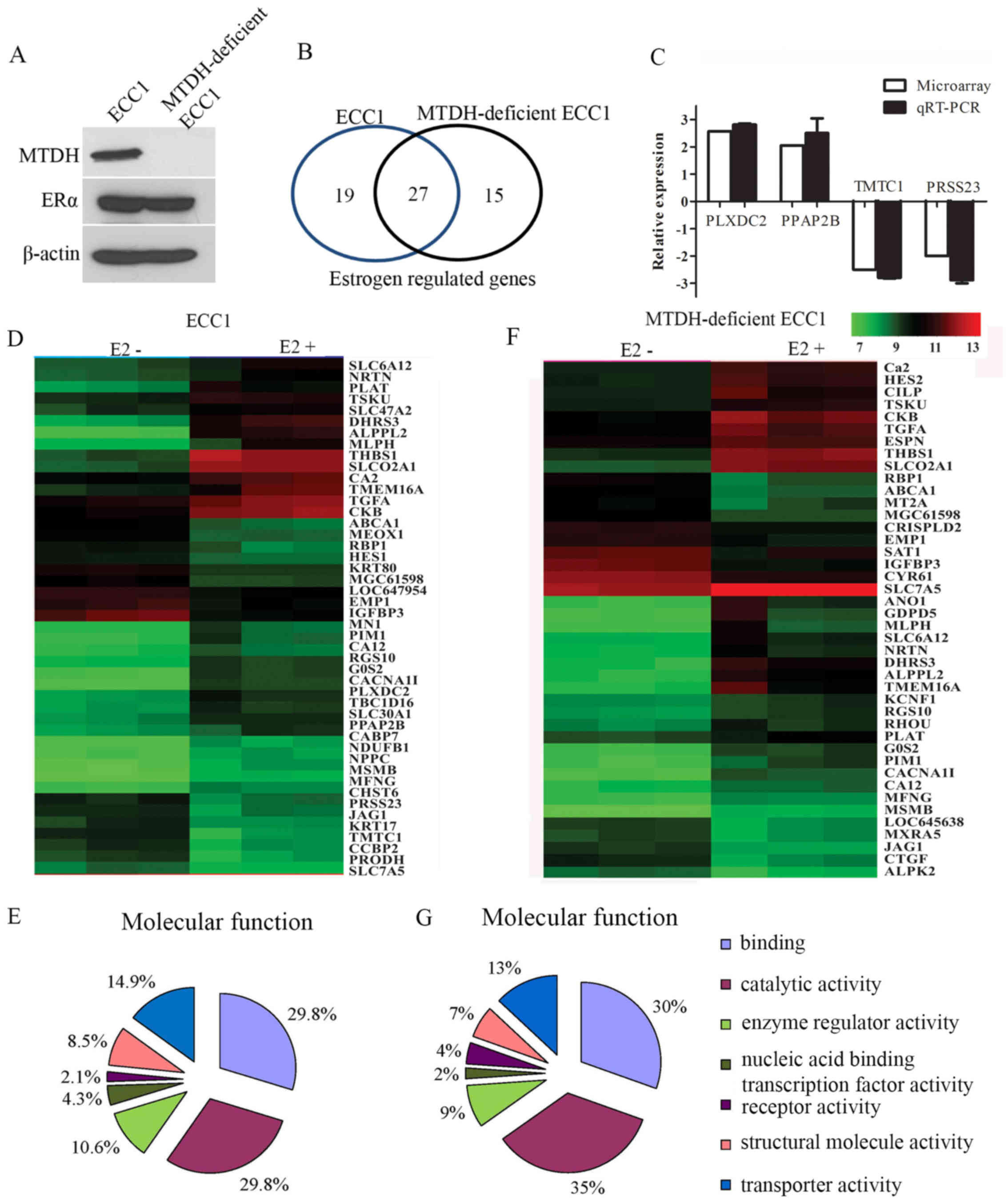
(MTDH-deficient ECC1 cells). The expression levels of MTDH and ER
were detected by western blot analysis in these cells (Fig. 1A), and the differences in gene
expression between estrogen-treated parental ECC1 cells and
MTDH-deficient ECC1 cells were then investigated using microarray
assay. By using threshold values of p<0.001 and fold-change ≥2
or ≤−2, the ECC1 cells had 46 and the MTDH-deficient ECC1 cells had
42 significantly altered genes induced by estrogen treatment
(Fig. 1B). Of note, 27 genes were
induced by estrogen in both parental the ECC1 and MTDH-deficient
ECC1 cells (Table II). These
genes are estrogen induced and not related with MTDH status, and
thus these genes may be associated with the function of estrogen
receptors independent of MTDH. There were 19 estrogen-regulated
genes that were dependent on MTDH expression (Table III). Molecular network analysis
did not find that these differentially expressed genes had direct
interactions with MTDH (data not shown). We also found 15 genes
significantly regulated by estrogen treatment in the MTDH-deficient
ECC1 cells (Table IV).
 | Table IIMTDH-independent gene expression
regulated by estrogen in ECC1 cells (MTDHWT) and MTDH-deficient
ECC1 (MTDH KO) cells (p<0.001 and fold-change ≥2 or ≤−2). |
Table II
MTDH-independent gene expression
regulated by estrogen in ECC1 cells (MTDHWT) and MTDH-deficient
ECC1 (MTDH KO) cells (p<0.001 and fold-change ≥2 or ≤−2).
| UniqueID | Name | Map Location | Fold-change
(treat/no-treat)
|
|---|
| MTDH WT | MTDH KO |
|---|
| ALPPL2 | Alkaline phosphatase,
placental-like 2 (ALPPL2) | 2q37.1c | 12.06 | 7.50 |
| THBS1 | Thrombospondin 1
(THBS1) | 15q14d | 9.50 | 6.45 |
| SLCO2A1 | Solute carrier
organic anion transporter family, member 2A1 (SLCO2A1) | 3q22.1e | 9.45 | 8.33 |
| DHRS3 |
Dehydrogenase/reductase (SDR family)
member 3 (DHRS3) | 1p36.22a-p36.21d | 7.92 | 9.38 |
| MLPH | Melanophilin (MLPH),
transcript variant 2 | 2q37.3b | 5.19 | 4.60 |
| CACNA1I | Calcium channel,
voltage-dependent, T type, alpha 1I subunit (CACNA1I), transcript
variant 1 | 22q13.1d | 4.18 | 3.81 |
| G0S2 | G0/G1 switch 2
(G0S2) | 1q32.2b | 4.04 | 3.81 |
| PLAT | Plasminogen
activator, tissue (PLAT), transcript variant 1 | 8p11.21a | 3.66 | 2.02 |
| TMEM16A | Transmembrane protein
16A (TMEM16A) | 11q13.3b-q13.3c | 3.60 | 8.52 |
| CKB | Creatine kinase,
brain (CKB) | 14q32.33a | 3.41 | 3.68 |
| PIM1 | Pim-1 oncogene
(PIM1) | 6p21.2c | 3.01 | 3.59 |
| CA2 | Carbonic anhydrase II
(CA2) | 8q21.2b | 2.82 | 3.29 |
| RGS10 | Regulator of
G-protein signaling 10 (RGS10), transcript variant 1 | 10q26.11d | 2.80 | 2.16 |
| TSKU | Tsukushin (TSKU),
mRNA. | 11q13.5c | 2.64 | 2.42 |
| TGFA | Transforming growth
factor alpha (TGFA), transcript variant 2 | 2p13.3c | 2.52 | 2.86 |
| SLC6A12 | Solute carrier family
6 (neurotransmitter transporter, betaine/GABA), member 12
(SLC6A12) | 12p13.33d | 2.41 | 4.11 |
| CA12 | Carbonic anhydrase
XII (CA12), transcript variant 1 | 15q22.2b | 2.30 | 2.28 |
| MFNG | MFNG O-fucosylpeptide
3-beta-N-acetylglucosaminyltransferase (MFNG) | 22q13.1a | 2.15 | 2.10 |
| SLC7A5 | Solute carrier family
7 (cationic amino acid transporter, y+ system), member 5
(SLC7A5) | 16q24.2b | 2.07 | 2.22 |
| MSMB | Microseminoprotein
beta (MSMB), transcript variant PSP57 | 10q11.23b | 2.03 | 2.08 |
| NRTN | Neurturin
(NRTN) | 19p13.3b | 2.03 | 3.89 |
| JAG1 | Jagged 1 (Alagille
syndrome) (JAG1) | 20p12.2a | −2.18 | −2.41 |
| RBP1 | Retinol binding
protein 1, cellular (RBP1) | 3q23a | −2.19 | −2.98 |
| MGC61598 | PREDICTED: Homo
sapiens similar to ankyrin-repeat protein Nrarp (MGC61598) | 9q34.3f | −2.22 | −2.16 |
| EMP1 | Epithelial membrane
protein 1 (EMP1) | 12p13.1b | −2.23 | −2.29 |
| ABCA1 | ATP-binding
cassette, sub-family A (ABC1), member 1 (ABCA1) | 9q31.1d | −2.83 | −2.81 |
| IGFBP3 | Insulin-like growth
factor binding protein 3 (IGFBP3), transcript variant 2 | 7p13b | −3.32 | −3.82 |
 | Table IIIList of differently expressed
MTDH-dependent genes regulated by estrogen in ECC1 cells
(p<0.001 and fold-change ≥2 or ≤−2). |
Table III
List of differently expressed
MTDH-dependent genes regulated by estrogen in ECC1 cells
(p<0.001 and fold-change ≥2 or ≤−2).
| UniqueID | Fold-change
(treat/no-treat) | Name | MapLocation |
|---|
| PLXDC2 | 2.57 | Plexin domain
containing 2 (PLXDC2) |
10p12.32a-p12.31c |
| MN1 | 2.54 | Meningioma
(disrupted in balanced translocation) 1 (MN1) | 22q12.1b |
| NPPC | 2.36 | Natriuretic peptide
precursor C (NPPC) | 2q37.1b |
| TBC1D16 | 2.27 | TBC1 domain family,
member 16 (TBC1D16), mRNA. | 17q25.3d |
| SLC30A1 | 2.21 | Solute carrier
family 30 (zinc transporter), member 1 (SLC30A1) | 1q32.3a |
| SLC47A2 | 2.17 | Solute carrier
family 47, member 2 (SLC47A2), transcript variant 1 | 17p11.2d |
| CABP7 | 2.06 | Calcium binding
protein 7 (CABP7) | 22q12.2a |
| PPAP2B | 2.05 | Phosphatidic acid
phosphatase type 2B (PPAP2B), transcript variant 2 | 1p32.2c |
| NDUFB1 | 2.03 | NADH dehydrogenase
(ubiquinone) 1β subcomplex, 1, 7 kDa (NDUFB1) | 14q32.12b |
| LOC647954 | −2.00 | PREDICTED: Homo
sapiens misc_RNA (LOC647954) | 5q23.2b |
| PRODH | −2.00 | Proline
dehydrogenase (oxidase) 1 (PRODH) | 22q11.21b |
| CHST6 | −2.00 | Carbohydrate
(N-acetylglucosamine 6-O) sulfotransferase 6 (CHST6) | 16q23.1a |
| HES1 | −2.01 | Hairy and enhancer
of split 1, (Drosophila) (HES1) | 3q29c |
| PRSS23 | −2.01 | Protease, serine,
23 (PRSS23) | 11q14.2a |
| MEOX1 | −2.34 | Mesenchyme homeobox
1 (MEOX1), transcript variant 3 | 17q21.31b |
| CCBP2 | −2.44 | Chemokine binding
protein 2 (CCBP2) | 3p22.1a |
| TMTC1 | −2.49 | Transmembrane and
tetratricopeptide repeat containing 1 (TMTC1) | 12p11.22a |
| KRT80 | −2.58 | Keratin 80 (KRT80),
transcript variant 1 | 12q13.13d |
| KRT17 | −3.02 | Keratin 17
(KRT17) | 17q21.2b |
 | Table IVMTDH negatively regulated gene
expression regulated by estrogen in MTDH-deficient ECC1 cells
(p<0.001 and fold-change ≥2 or ≤–2). |
Table IV
MTDH negatively regulated gene
expression regulated by estrogen in MTDH-deficient ECC1 cells
(p<0.001 and fold-change ≥2 or ≤–2).
| UniqueID | Fold-change
(treat/no-treat) | Name | MapLocation |
|---|
| ANO1 | 6.46 | Anoctamin 1,
calcium activated chloride channel (ANO1), transcript variant
1 |
11q13.3b-q13.3c |
| GDPD5 | 5.85 |
Glycerophosphodiester phosphodiesterase
domain containing 5 (GDPD5) |
11q13.4c-q13.5a |
| HES2 | 3.24 | Hairy and enhancer
of split 2 (Drosophila) (HES2) | 1p36.31a |
| CILP | 2.90 | Cartilage
intermediate layer protein, nucleotide pyrophosphohydrolase
(CILP) | 15q22.31b |
| RHOU | 2.07 | Ras homolog gene
family, member U (RHOU) | 1q42.13c |
| KCNF1 | 2.02 | Potassium
voltage-gated channel, subfamily F, member 1 (KCNF1) | 2p25.1c |
| ESPN | 2.01 | Espin (ESPN) | 1p36.31a |
| MXRA5 | −2.00 | Matrix-remodelling
associated 5 (MXRA5) | Xp22.33b |
| CRISPLD2 | −2.07 | Cysteine-rich
secretory protein LCCL domain containing 2 (CRISPLD2) | 16q24.1a |
| LOC645638 | −2.08 | PREDICTED: Homo
sapiens misc_RNA (LOC645638), miscRNA | 17q23.1a |
| MT2A | −2.21 | Metallothionein 2A
(MT2A) | 16q13b |
| ALPK2 | −2.22 | Alpha-kinase 2
(ALPK2) |
18q21.31b-q21.32a |
| SAT1 | −2.44 | Spermidine/spermine
N1-acetyltransferase 1 (SAT1) | Xp22.11a |
| CTGF | −2.45 | Connective tissue
growth factor (CTGF) | 6q23.2b |
| CYR61 | −2.50 | Cysteine-rich,
angiogenic inducer, 61 (CYR61) | 1p22.3e |
We randomly selected 2 upregulated and 2
downregulated genes and validated the expression changes in the
ECC1 cells by RT-qPCR. The fold-change of these genes was similar
with the microarray data (Fig.
1C). The differential gene expression induced by estrogen in
the ECC1 and MTDH-deficient ECC1 cells was represented graphically
in a heatmap (Fig. 1D and F).
Inputting 46 differentially expressed genes induced
by estrogen in the ECC1 cells into the online tool PANTHER
(http://pantherdb.org/) identified 47 terms
belonging to molecular functions (Fig. 1E) and 90 terms belonging to
biological processes (data not shown). Inputting 42 differentially
expressed genes induced by estrogen in the MTDH-deficient ECC1
cells into the online tool PANTHER identified 46 terms belonging to
molecular functions (Fig. 1G) and
97 terms belonging to biological processes (data not shown). The
most representative molecular functions were binding and catalytic.
On the other hand, the most significant biological processes
involving the ER interacting proteins identified are represented by
metabolic processes and cellular process (data not shown).
ER and MTDH interact in the nucleus
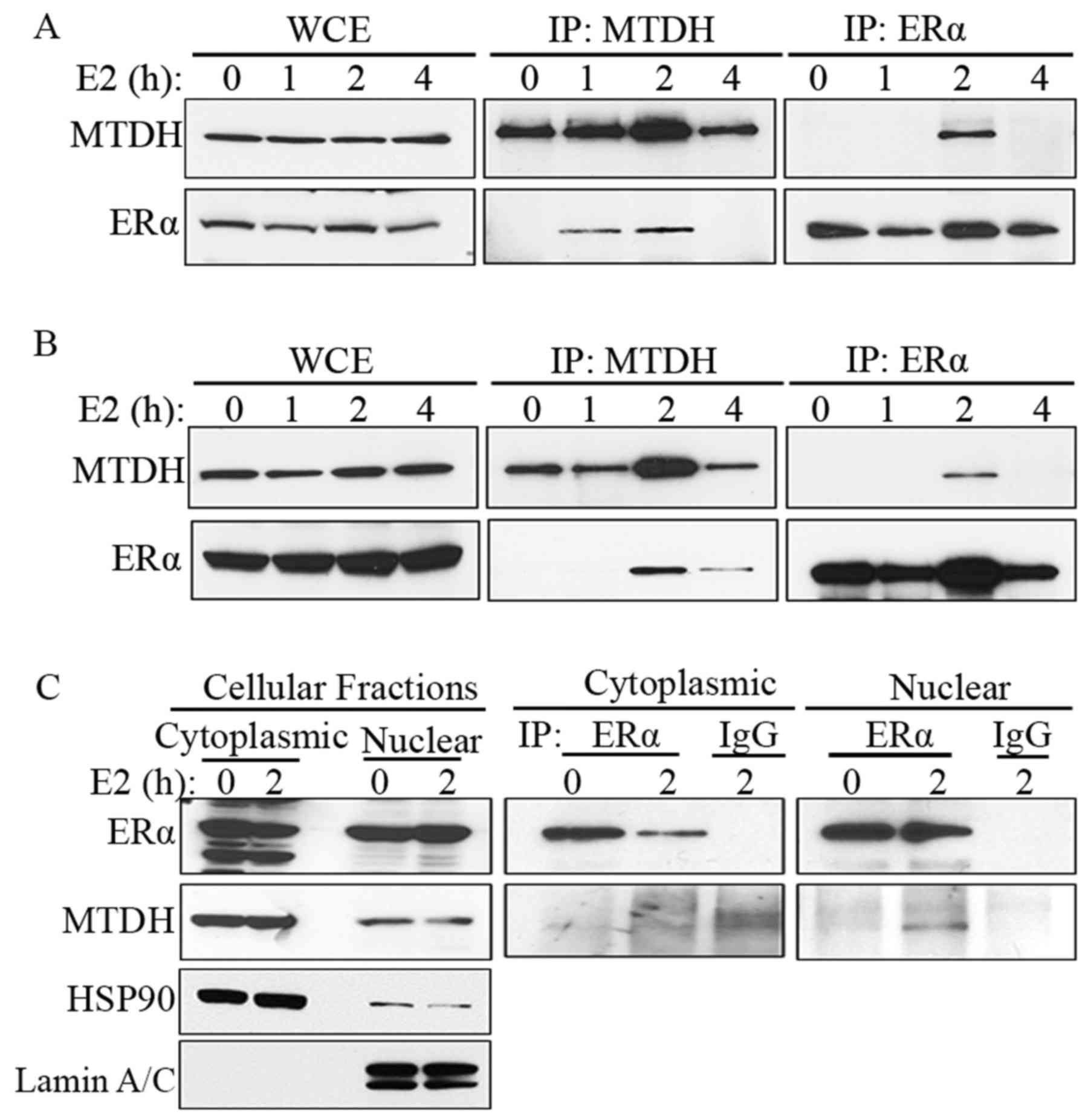
In order to define the involvement of MTDH in ER or
estrogen-regulated gene expression, the ECC1 endometrial cancer
cells were treated with 10 nM estrogen for 0, 1, 2 and 4 h, and the
interaction of MTDH and ERα was queried using IP assays. The IP
results revealed that MTDH and ERα can 'pull down' each other
following estrogen treatment for 2 h (Fig. 2A). We acquired similar results in
the MCF-7 cells (Fig. 2B).
We then wished to determine where MTDH and ERα
interact in the cell. Cytoplasmic and nuclear fractions of ECC1
cells treated with 10 nM estrogen were immunoprecipitated with
anti-ERα antibody, followed by immunoblotting with ERα or MTDH
antibody. The results indicated that the interaction of MTDH and
ERα occurs in the nucleus (Fig.
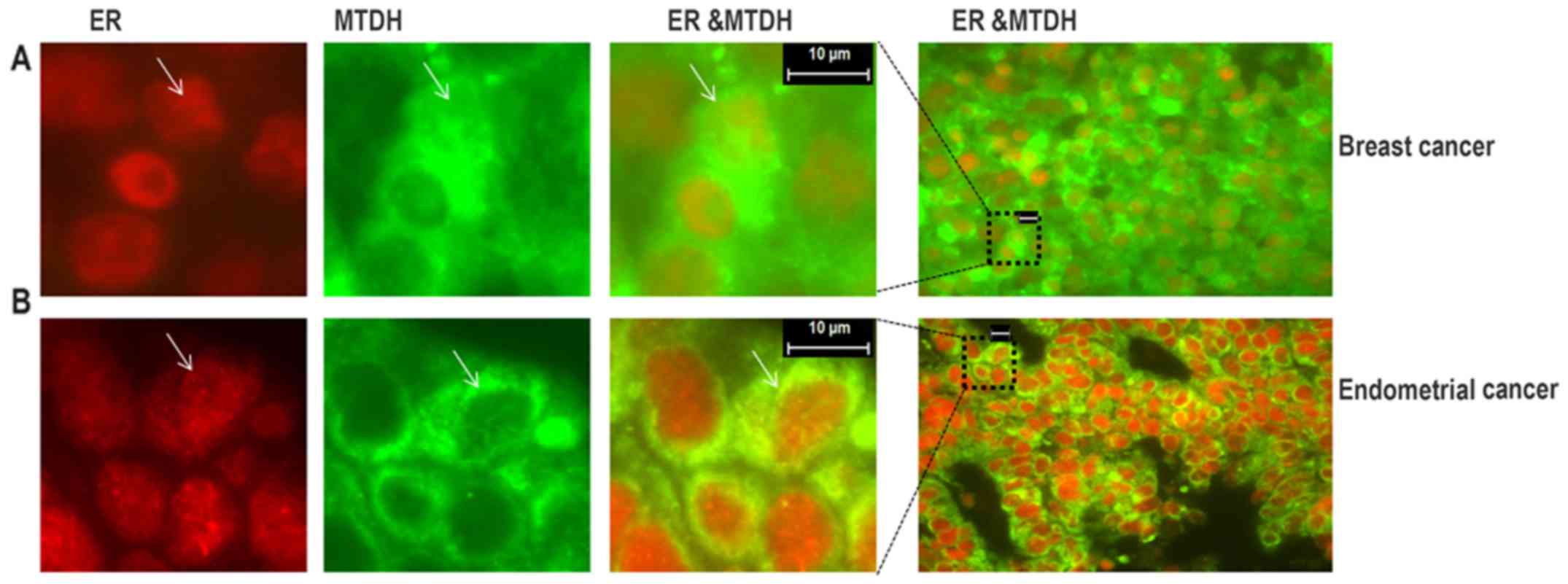
2C). Furthermore, we detected the co-localization of MTDH and
ER by immunostaining in a subset of cells in ER-positive breast
cancer and endometrial cancer tissues (Fig. 3).
Discussion
Cancer progression is a multifactorial process that
includes alterations in proliferation, cell death, apoptosis,
genetic instability, and changes in the expression and activity of
tumor suppressor genes, oncogenes, growth factors and cell adhesion
molecules (14–16). The overexpression of MTDH is
frequently observed in most human cancers, and several studies have
suggested that MTDH may play a pivotal role in the pathogenesis,
invasion, metastasis, angiogenesis, progression and regulation of
apoptosis. Estrogen receptors are overexpressed in approximately
70% of breast and endometrium tumors. Similar to MTDH, estrogen
signaling through ER promotes cell proliferation and survival and
inhibits apoptosis, thereby promoting tumorigenesis. In this study,
we found that the expression of many genes correlated with the
expression of MTDH in ER-positive cancers using TCGA data, and we
defined an interaction between MTDH and ER by co-IP. Our data
suggest that the interaction is dependent on estrogen and is a
short-term process.
ERs are involved in pathological processes,
including breast cancer, endometrial cancer, osteoporosis,
hypertension and coronary artery diseases (17–19). One of the main risk factors for
endometrial cancer is high levels of estrogen (20). In this study, we used the human
endometrial cancer cell line, ECC1, and MTDH-deficient ECC1 cells,
treated with or without estrogen, and assessed the gene expression
profiles by microarray assay. Our results demonstrated that most
estrogen-induced genes were common in the ECC1 cells and
MTDH-deficient ECC1 cells, indicating that approximately 60% of the
genes induced by estrogen were unrelated with the MTDH status, and
that MTDH may only participate in parts of ER function.
Previous studies have indicated that MTDH interacts
with and activates the transcription factor, NF-κB, to regulate
gene expression (3,8,21).
In this study, we found that MTDH transiently interacted with ER in
the nucleus of cancer cells, the interaction is dependent on
estrogen and it is a short-term process. Based on this time course,
the biological function of this transient interaction may relate to
the transcriptional activity of ERα. Indeed, the long-standing
dogma in the hormone receptor field is that ligand binding and
receptor-mediated transcriptional activation are highly transient
events. Microarray data indicated that MTDH had some effect on
estrogen-regulated genes, and they jointly control the expression
of a subset of ER-regulated genes. MTDH thus may play an important
role early in ER-mediated transcriptional regulation. Further
studies on understanding the interaction of MTDH with ER and the
related genes may provide new insight into the function of MTDH and
ER in tumors.
Acknowledgments
This study was supported by National Institutes of
Health (NIH) Grants R01CA184101 (to X.M. and K.K.L.) and R01CA99908
(to K.K.L.) and by the Department of Obstetrics and Gynecology
Research Development Fund (to K.K.L.). Microarray data presented
herein were obtained at the Genomics Division of the Iowa Institute
of Human Genetics which is supported, in part, by the University of
Iowa Carver College of Medicine and the Holden Comprehensive Cancer
Center and its National Cancer Institute Award P30CA086862. It
should be noted that K.W.T. is a co-owner of Immortagen, Inc.
References
|
1
|
Wang Z, Wei YB, Gao YL, Yan B, Yang JR and
Guo Q: Metadherin in prostate, bladder, and kidney cancer: A
systematic review. Mol Clin Oncol. 2:1139–1144. 2014.PubMed/NCBI
|
|
2
|
Song H, Li C, Lu R, Zhang Y and Geng J:
Expression of astrocyte elevated gene-1: A novel marker of the
pathogenesis, progression, and poor prognosis for endometrial
cancer. Int J Gynecol Cancer. 20:1188–1196. 2010. View Article : Google Scholar
|
|
3
|
Emdad L, Sarkar D, Su ZZ, Randolph A,
Boukerche H, Valerie K and Fisher PB: Activation of the nuclear
factor kappaB pathway by astrocyte elevated gene-1: Implications
for tumor progression and metastasis. Cancer Res. 66:1509–1516.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoo BK, Emdad L, Lee SG, Su ZZ,
Santhekadur P, Chen D, Gredler R, Fisher PB and Sarkar D: Astrocyte
elevated gene-1 (AEG-1): A multifunctional regulator of normal and
abnormal physiology. Pharmacol Ther. 130:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wan L, Lu X, Yuan S, Wei Y, Guo F, Shen M,
Yuan M, Chakrabarti R, Hua Y, Smith HA, et al: MTDH-SND1
interaction is crucial for expansion and activity of
tumor-initiating cells in diverse oncogene- and carcinogen-induced
mammary tumors. Cancer Cell. 26:92–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ash SC, Yang DQ and Britt DE: LYRIC/AEG-1
overexpression modulates BCCIPalpha protein levels in prostate
tumor cells. Biochem Biophys Res Commun. 371:333–338. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thirkettle HJ, Mills IG, Whitaker HC and
Neal DE: Nuclear LYRIC/AEG-1 interacts with PLZF and relieves
PLZF-mediated repression. Oncogene. 28:3663–3670. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sarkar D, Park ES, Emdad L, Lee SG, Su ZZ
and Fisher PB: Molecular basis of nuclear factor-kappaB activation
by astrocyte elevated gene-1. Cancer Res. 68:1478–1484. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Srivastava J, Robertson CL, Rajasekaran D,
Gredler R, Siddiq A, Emdad L, Mukhopadhyay ND, Ghosh S, Hylemon PB,
Gil G, et al: AEG-1 regulates retinoid X receptor and inhibits
retinoid signaling. Cancer Res. 74:4364–4377. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Su P, Zhang Q and Yang Q:
Immunohistochemical analysis of Metadherin in proliferative and
cancerous breast tissue. Diagn Pathol. 5:382010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tokunaga E, Nakashima Y, Yamashita N,
Hisamatsu Y, Okada S, Akiyoshi S, Aishima S, Kitao H, Morita M and
Maehara Y: Overexpression of metadherin/MTDH is associated with an
aggressive phenotype and a poor prognosis in invasive breast
cancer. Breast Cancer. 21:341–349. 2014. View Article : Google Scholar
|
|
12
|
Xu C, Kong X, Wang H, Zhang N, Kong X,
Ding X, Li X and Yang Q: MTDH mediates estrogen-independent growth
and tamoxifen resistance by downregulating PTEN in MCF-7 breast
cancer cells. Cell Physiol Biochem. 33:1557–1567. 2014. View Article : Google Scholar
|
|
13
|
Kavlashvili T, Jia Y, Dai D, Meng X, Thiel
KW, Leslie KK and Yang S: Inverse relationship between progesterone
receptor and Myc in endometrial cancer. PLoS One. 11:e01489122016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roberts JM and Gammill H: Pre-eclampsia
and cardiovascular disease in later life. Lancet. 366:961–962.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mendelsohn ME and Karas RH: The protective
effects of estrogen on the cardiovascular system. N Engl J Med.
340:1801–1811. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gennari L, Merlotti D, De Paola V, Calabrò
A, Becherini L, Martini G and Nuti R: Estrogen receptor gene
polymorphisms and the genetics of osteoporosis: A HuGE review. Am J
Epidemiol. 161:307–320. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saso S, Chatterjee J, Georgiou E, Ditri
AM, Smith JR and Ghaem-Maghami S: Endometrial cancer. BMJ.
343:d39542011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khuda II II, Koide N, Noman AS, Dagvadorj
J, Tumurkhuu G, Naiki Y, Komatsu T, Yoshida T and Yokochi T:
Astrocyte elevated gene-1 (AEG-1) is induced by lipopolysaccharide
as toll-like receptor 4 (TLR4) ligand and regulates TLR4
signalling. Immunology. 128(Suppl 1): e700–e706. 2009. View Article : Google Scholar : PubMed/NCBI
|