Introduction
The central nervous system, through the sympathetic
and parasympathetic pathways, regulates the function of the enteric
nervous system (ENS); the latter mainly governs the signaling from
the central nervous system to the ENS following gastrointestinal
injury. The ENS can function independently of the central nervous
system via the myenteric and submucosal plexuses. A recent
demonstrated that atrial fibrillation ablation leads to the
functional impairment of gastric emptying, which may be mediated by
damage to the vagal nerve (1).
Vagal nerve stimulation increases enteric glial activation, which
protects the intestines from burn-induced injury (2). The stomach is dominated by the vagal
nerve, and its sensory information is transmitted via the afferent
vagal nerve to the nucleus tractus solitarius, which modulates
sensory information from multiple locations throughout the central
nervous system (3).
Glial cell line-derived neurotrophic factor (GDNF)
is a growth factor that promotes neuronal survival and
differentiation, and markedly enhances nerve regeneration following
severe nerve damage (4). GDNF
also plays a protective role in hepatic steatosis in mice by
decreasing liver fat content (5).
GDNF regulates the phosphatidylinositol 3-kinase/protein kinase B
(PI3K/Akt) and extracellular signal-regulated kinase 1/2
(ERK1/2/mitogen-activated protein kinase (MAPK) pathways. These
signals modulate cellular survival via the PI3K/Akt pathway and
neuronal differentiation via the ERK1/2 MAPK pathway. Evidence
indicates that GDNF and the PI3K/Akt signaling pathway are involved
in enteric neuropathy (6,7), and electroacupuncture at acupoint
ST-36 leads to enteric neuronal regeneration through GDNF and the
PI3K/Akt signaling pathway in the diabetic rat colon (8). Nevertheless, limited information is
available on the release of GDNF and its downstream pathway in the
stomachs of vagotomized rats.
In recent years, increasing attention has been paid
to gastric electrical stimulation (GES), a promising alternative
treatment for functional gastrointestinal disorders. GES, when
applied with optimal parameters, has an analgesic effect on
visceral pain through the opioid system and the inhibition of
spinal afferent neuronal activity (9). In a previous study, GES using trains
of short pulses was shown to attenuate apomorphine-induced emetic
responses via the activation of the amygdale (10). In addition, GES has been shown to
alleviate symptoms (such as nausea, loss of appetite and early
satiety) in 75% of patients with refractory gastroparesis (11). Of note, GES, when applied with the
appropriate parameters, can inhibit gastric motility in healthy
dogs, representing a potential treatment for obesity (12).
However, the effect of synchronized dual-pulse GES
(SGES) on the communication between enteric glial cells (EGCs) and
enteric neurons and the possible involvement of GDNF with enteric
neurons through the PI3K/Akt pathway in a vagotomized rat stomach
are unknown. Thus, the aim of this study was to evaluate the
effects of SGES on the apoptosis of enteric neurons, and to further
investigate whether GDNF and the PI3K/Akt pathway are involved in
the communication between EGCs and enteric neurons.
Materials and methods
Animals
Male Sprague-Dawley rats weighing 250–350 g were
obtained from the Experimental Animal Center of Hubei province,
China and were used in the present study. The animals were housed
under normal laboratory conditions at 22°C under a 12-h dark/light
cycle and were given food and water ad libitum. The rats
received humane care, and the experiment strictly abided by the
ethical guidelines and approval of the Animal Care and Use
Committee of Tongji Medical College, Huazhong University of Science
and Technology.
SGES consisted of a long pulse (300 msec, 4 mA) and
5 short pulses (0.33 msec, 4 mA, 100 Hz). SGES was applied to the
vagotomized rats while in a fasting state. The rats subjected to
early subdiaphragmatic vagotomy (ESDV group) were divided into 2
groups: one group was treated with short-term SGES (ESDV + SSGES
group) for 30 min/day over a period of 2 weeks, and the other group
was a sham SGES (ESDV group) group containing mice that were
treated for 30 min/day over a period of 2 weeks. Similarly, the
rats subjected to terminal subdiaphragmatic vagotomy (TSDV) were
divided into 2 groups: the long-term SGES (TSDV + LSGES) group was
treated for 30 min/day over a period of 4 weeks and the sham SGES
(TSDV) group was a time-matched control. The control group was
treated for laparotomy alone without vagotomy or SGES. The animals
underwent subdiaphragmatic vagotomy. After 24 h of fasting, the
subdiaphragmatic esophagus was exposed, and ventral and dorsal
truncal vagotomy was carried out. Approximately 1 cm of the
bilateral vagus innervating the stomach was cut off to prevent
nerves regeneration. A pair of stimulating electrodes (United
States Surgical, a division of Tyco Healthcare Group LP) was
implanted in the middle of the greater curvature of the stomach.
After allowing 7 days for recovery, the experimental rats were
subjected to electrical stimulation. The rats were sacrificed at 2
and 6 weeks, and specimens of the antrum were carefully collected.
Each specimen was cut into several parts. One part was kept at
−80°C for use in western blot analysis and RT-qPCR, and a second
part was placed in 4% paraformaldehyde for immunofluorescence
staining and terminal deoxynucleotidyltransferase-mediated dUTP
nick-end labelling (TUNEL) assay.
RNA extraction, cDNA synthesis and
RT-qPCR
The total RNA of the antrum was isolated using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and First-Strand
cDNA was synthesized using PrimeScript™ RT Master Mix (Perfect
Real-Time) (Takara, Otsu, Japan) according to the instructions of
the manufacturer. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
served as an internal control. The primer sequences used were as
follows: connexin 43 (Cx43) forward, 5′-AGGAGTTCCACCAACTTTGGC-3′
and reverse, 5′-TGGAGTAGGCTTGGACCTTGTC-3′; GDNF forward,
5′-AATGTCACTGACTTGGGTTTGG-3′ and reverse,
5′-CGTTTAGCGGAATGCTTTCTTA-3′; PGP9.5 forward,
5′-TGGAGATTAACCCCGAGATGC-3′ and reverse,
5′-GAGTTCCCGATGGTCTGCTTC-3′; pan-Akt forward,
5′-ATCGTGTGGCAAGATGTGTATGA-3′ and reverse,
5′-CAAAATACCTGGTGTCGGTCTCA-3′; GAPDH forward,
5′-GTATGACTCTACCCACGGCAAGT-3′ and reverse,
5′-TTCCCGTTGATGACCAGCTT-3′. RT-PCR was performed using the
SYBR-Green PCR master mix. All reactions were performed in
duplicate in a 10-μl volume containing 1 μl cDNA, 5
μl SYBR-Green reaction mix (Qiagen, Hilden, Germany), 0.5
μl sense primer, 0.5 μl antisense primer (both from
Invitrogen), and 3 μl ddH2O. The reaction
conditions used were 95°C for 10 min, followed by 40 cycles of 95°C
for 15 sec and 60°C for 1 min. All reactions were performed using
an ABI-StepOne Real-Time system (Applied Biosystems, Carlsbad, CA,
USA). Relative changes in gene expression were confirmed using the
2−ΔΔCq method.
Western blot analysis
Fresh-frozen antrum samples were homogenized by
mechanical crushing in radioimmunoprecipitation assay (RIPA) buffer
containing protease inhibitor. Following incubation on ice for 30
min, the mixture was centrifuged at 12,000 rpm for 10 min at 4°C,
and the supernatants used as the total protein fraction. Protein
concentrations were determined using a bicinchoninic acid (BCA)
protein assay kit.
The supernatants were diluted with loading buffer
and the proteins were denatured at 95°C for 10 min. Lysates
equivalent to 60 μg of protein lysates were then resolved
using 8–12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE), and the separated proteins were
transferred onto polyvinylidene difluoride (PVDF) membranes.
Non-specific binding areas of the membranes were blocked with 5%
non-fat dry milk or 5% bovine serum albumin (BSA) in Tris-buffered
saline containing 0.1% Tween-20 (TBST) for 1 h. Subsequently, the
membranes were incubated overnight at 4°C with antibodies against
GDNF (1:300; cat. no. ab18956; Abcam, Cambridge, UK), PGP9.5
(1:10,000; cat. no. NB110-58869; NovusBio, Littleton, CO, USA),
p-Akt (1:1,000; cat. no. 4060), pan-Akt (1:1,000; cat. no. 13038)
(both from Cell Signaling Technology, Danvers, MA, USA), Cx43
(1:1,000; cat. no. A2163; ABclonal Technology, Hubei, China) and
GAPDH (1:5,000; cat. no. A01020; Abbkine, Inc., Redlands, CA, USA).
After washing 3 times in TBST, the membranes were incubated with
HRP-linked secondary antibody [HRP-labeled goat anti-rabbit IgG
(1:5,000; cat. no. ANT020; AntGene Biotech Co., Ltd., Wuhan,
China), HRP-labeled goat anti-mouse IgG (1:5,000; cat. no. ANT019;
AntGene Biotech Co., Ltd.) and HRP-labeled goat anti-rabbit IgG
(1:5,000; cat. no. GGHL-5P; Immunology Consultants Laboratory,
Inc., Portland, OR, USA)] for 60 min at room temperature. After 3
further washes, the protein bands were detected using an enhanced
chemiluminescence agent (ECL reagents). Densitometry analysis was
performed using ImageJ software (Media Cybernetics, Inc.,
Rockville, MD, USA).
Immunofluorescence staining
The antrum tissue was labeled to determine the
expression of GDNF, GFAP and PGP9.5. The harvested specimens were
immersed in 4% paraformaldehyde for 24 h, embedded in paraffin in a
vacuum, and sliced into 5-μm-thick sections. The sections
were then dewaxed and hydrated in xylene and ethanol solutions.
After boiling for 2 min for antigen retrieval, the tissues were
incubated with 5% BSA for 30 min at room temperature, followed by
incubation with primary antibodies to GDNF (1:50), GFAP (1:300)
(both from Abcam), and PGP9.5 (1:300; NovusBio) overnight at 4°C.
The sections were rinsed in phosphate-buffered saline (PBS) 3 times
the following day and then incubated with secondary antibodies
[Alexa Fluor 488-Donkey Anti-Rabbit IgG (1:100; cat. no. ANT024),
Alexa Fluor 594-Donkey Anti-Goat IgG (1:100; cat. no. ANT031) and
Alexa Fluor 488-Donkey Anti-Mouse IgG (1:200; cat. no. ANT029) (all
from AntGene Biotech Co., Ltd.)], in the dark for 90 min at room
temperature. Subsequently, the sections were treated with
4′,6-diamidino-2-phenylindole (DAPI; 1:1500) for 10 min washed in
PBS 3 times, and then sealed with a fluorescence quenching agent.
The immunolabeled tissues were observed using a confocal laser
scanning microscope (Nikon, Tokyo, Japan).
Apoptosis was detected with TUNEL labeling according
to the manufacturer's instructions (Roche, Mannheim, Germany).
Briefly, the sections were dewaxed, rehydrated and incubated with
TUNEL reaction buffer (labeling solution: enzyme solution, 9:1)
under dark and humid conditions. The sections were then washed to
remove unbound fluorescein-dUTP and incubated with DAPI to
counterstain the nuclei. Finally, the specimens were observed using
a confocal laser scanning microscope (Nikon); 5 random fields were
captured in each section.
Statistical analysis
All data are expressed as the means ± SEM, and
one-way ANOVA was performed to assess the difference between the
control group and the vagotomized rat subgroups, followed by LSD or
Dunnett's T3 analysis. A p-value <0.05 was considered to
indicate a statistically significant difference. SPSS version 17
software was used for all statistical analyses.
Results
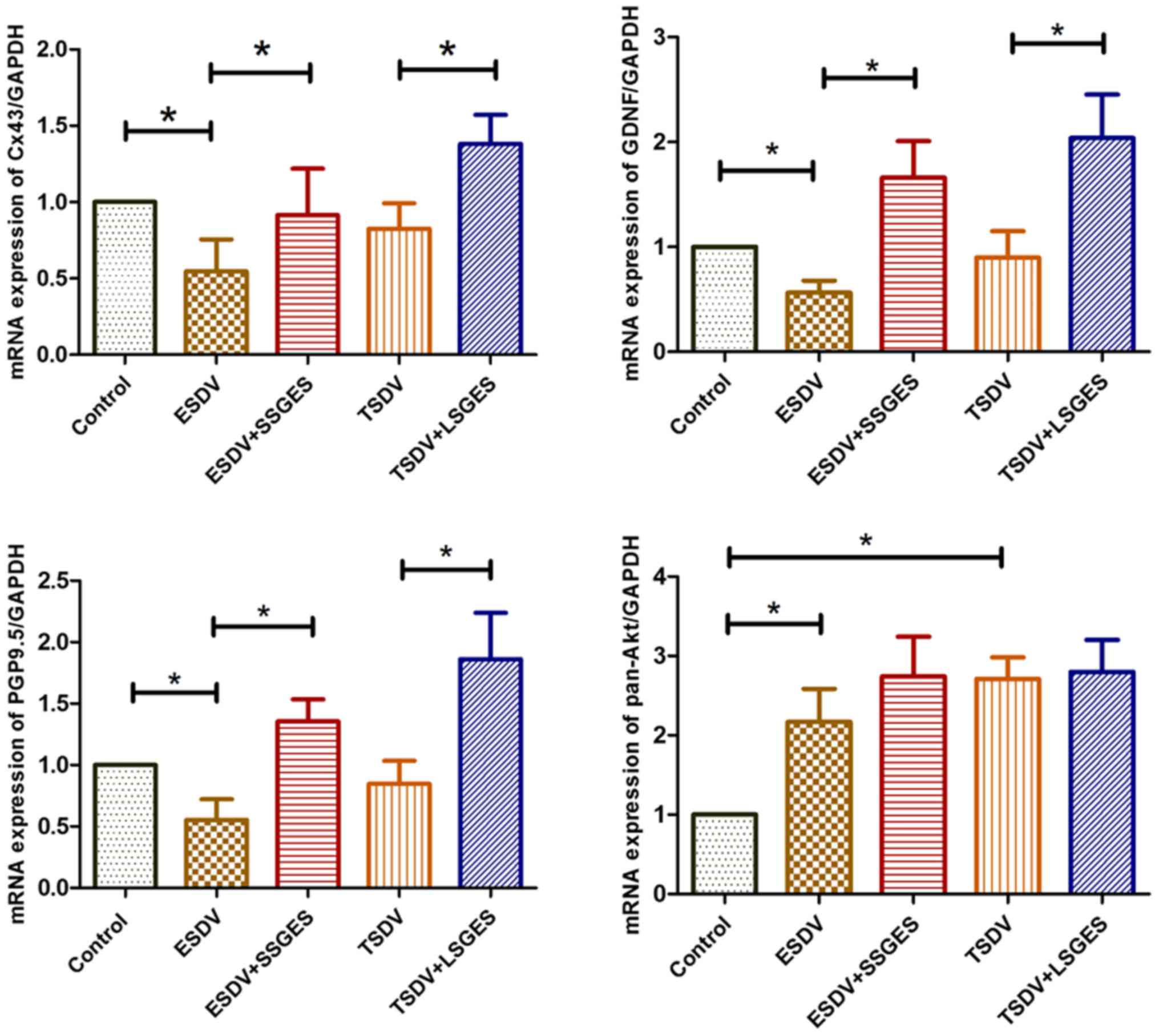
mRNA levels of Cx43, GDNF, pan-Akt and
PGP9.5
As shown in Fig.
1, the mRNA levels of Cx43, GDNF, pan-Akt, and PGP9.5 in the
antrum tissue were assessed. The result sof RT-qPCR revealed that
the levels of Cx43, GDNF and PGP9.5 were markedly decreased in the
early vagotomized group (ESDV group) compared with the control
group (all p<0.05). Conversely, both short-term SGES (SSGES) and
long-term SGES (LSGES) significantly enhanced the expression levels
of Cx43, GDNF and PGP9.5 compared with the matched vagotomized
groups (all p<0.05). The expression of pan-Akt in the
vagotomized groups was markedly upregulated compared with the
control group (both p<0.05), whereas there was no difference
between the vagotomized groups and the SGES groups (both
p=1.00).
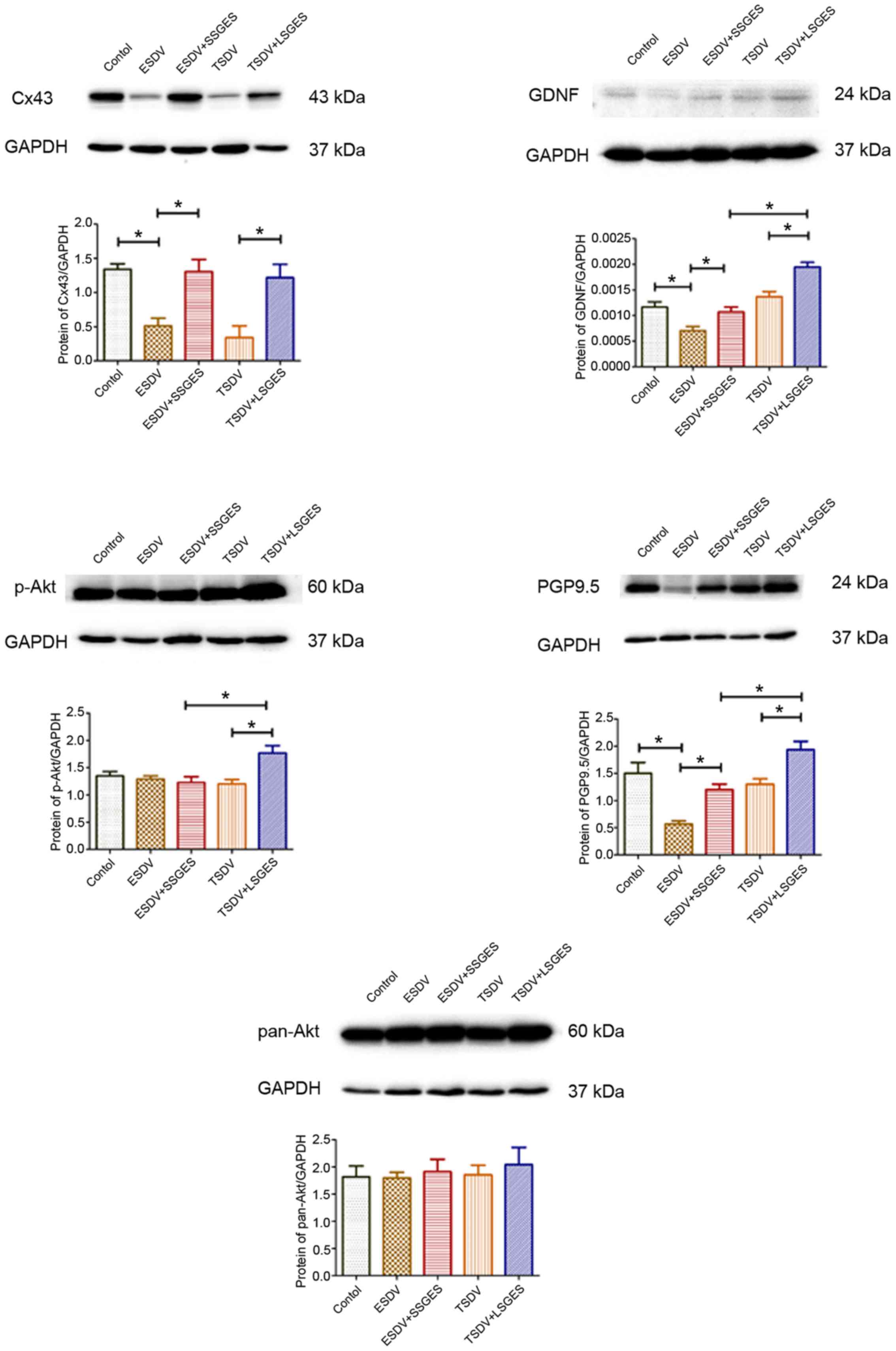
Protein levels detected by western blot
analysis
The protein levels of Cx43, GDNF, p-Akt, pan-Akt and
PGP9.5 are depicted in Fig. 2.
The expression levels of Cx43, GDNF and PGP9.5 in the early
vagotomized group were markedly lower than those in the control
group (0.51325±0.06376 vs. 1.33943±0.04699, p=0.004;
0.00070±0.00005 vs. 0.00117±0.00020, p=0.022; 0.56762±0.03344 vs.
1.50193±0.11532, p= 0.043). Short-term SGES obviously upregulated
the levels of these proteins compared with the corresponding
age-matched vagotomized groups (1.30507±0.10339 vs.
0.51325±0.06376, p=0.028; 0.00107±0.00005 vs. 0.00070±0.00005,
p=0.045; 1.20183±0.05780 vs. 0.56762±0.03344, p= 0.01). Long-term
SGES significantly increased the expression of p-Akt compared with
the terminal vagotomized group (1.76987±0.07826 vs.
1.20364±0.04708, p=0.034). However, no significant difference was
detected in pan-Akt expression was observed between the vagotomized
groups and the SGES groups (all p>0.05).
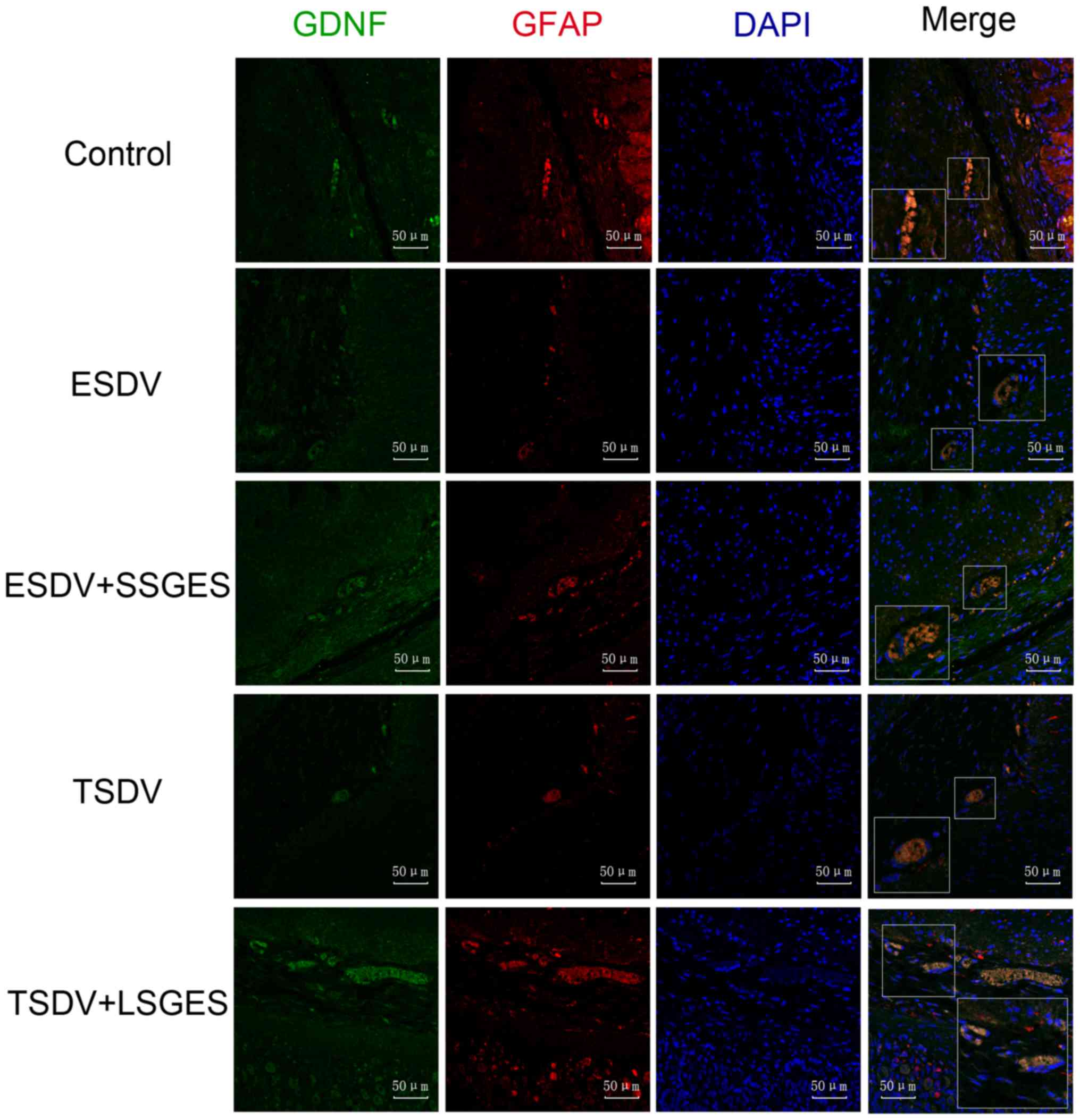
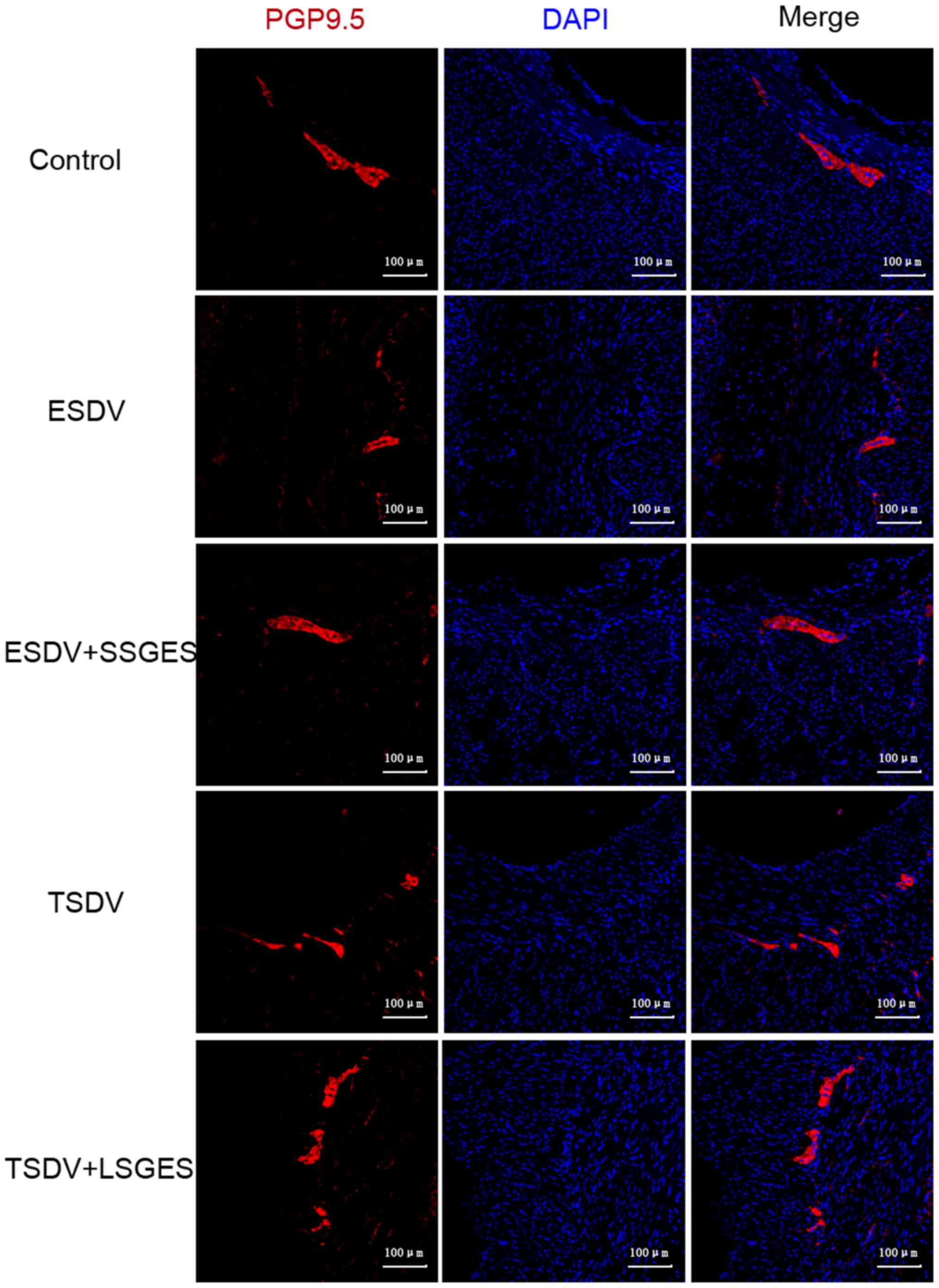
Immunofluorescence labeling of GDNF, GFAP
and PGP9.5
The immunofluorescence staining of GDNF, GFAP and
PGP9.5 is shown in Figs. 3 and
4. As shown in Fig. 3, GDNF and GFAP co-expression in
the vagotomized groups was markedly lower than that in the control
group in the myenteric plexus (all p<0.05). Conversely, long and
short-term SGES significantly increased the expression levels of
GDNF and GFAP compared with the levels in the vagotomized groups
(all p<0.05). Consistently, the change in PGP9.5 expression was
similar to that observed for GDNF and GFAP (Fig. 4).
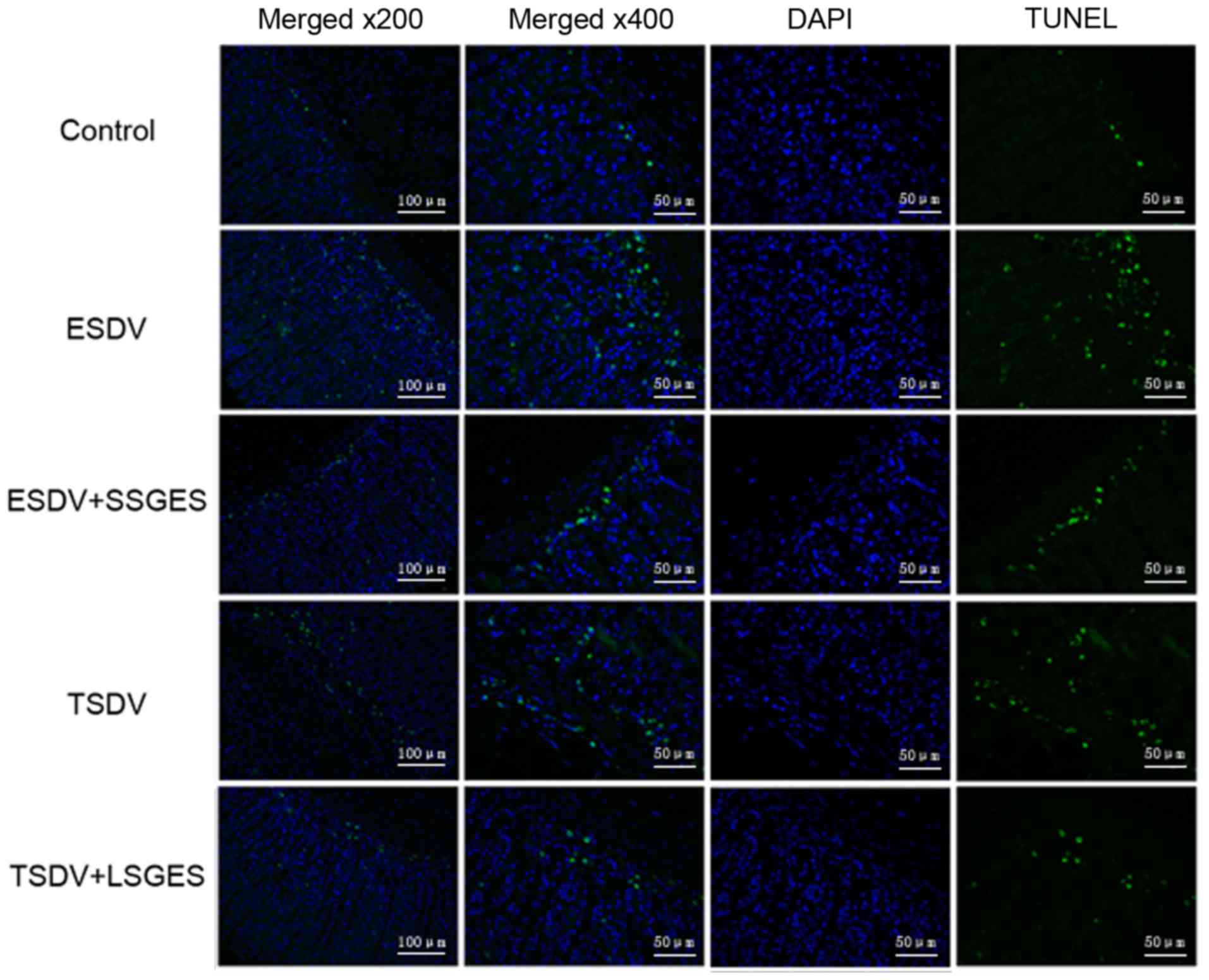
Assessment of cell apoptosis by TUNEL
assay
As shown in Fig.
5, few TUNEL-positive cells were observed in the control group.
However, more positive cells were found in the early and terminal
vagotomized groups compared with the control group (p<0.05). In
addition, short- and long-term SGES markedly decreased the number
of TUNEL-positive cells compared with those in the matched
vagotomized groups (p<0.05).
Discussion
GES has been applied to alleviate gastrointestinal
symptoms and promote gastrointestinal motility; however, the
mechanisms involved in the effects of SGES remain elusive. In the
present study, we found that SGES enhanced the release of GDNF
following the upregulation of its downstream pathway, PI3K/Akt, and
the inhibition of enteric neuronal loss in the antrum tissue.
The severity of enteric neuronal loss parallels
gastrointestinal motility defects in zebrafish (13). Gastric and small intestinal
motility damage is related to a decrease in the number of neuronal
nitric oxide synthase (nNOS)-immunoreactive myenteric neurons
following chronic alcohol consumption (14). In rats with streptozotocin
(STZ)-induced diabetes, the change in aquaporin 1 immunoreactive
neurons may lead to gastrointestinal dysfunction (15). Moreover, the survival and
neurogenesis of transplanted neural stem cells in the adult gut are
key issues in the use of stem cell therapy for treating
gastrointestinal motility disorders (16). In this study, we found that the
expression of PGP9.5, an enteric neuronal marker, was significantly
decreased, and more TUNEL-positive cells were found in the
vagotomized rats. These results likely indicate injury to or the
loss of enteric neurons following vagotomy and may partly explain
the role of gastrointestinal dysmotility in enteric
neuropathies.
GDNF is located in glia or Schwann cells in the
human gut (17). GDNF and
neurturin play prominent roles in the survival and proliferation of
enteric neurons and glial progenitors in vitro (18); the ENS completely fails to mature
in mice lacking GDNF (19).
Abundant GDNF synthesis has been found in enterocytes, promoting
wound healing and barrier maturation (20). Disorders of gastrointestinal
motility have been associated with signaling components of GDNF in
enteric neuropathies (21). In
addition, GDNF contributes to synaptophysin and synaptobrevin
expression and formatted neuronal varicosities, which induces
functional neuronal networks (22). A previous study indicated that
GDNF and brain-derived neurotrophic factor expression levels are
upregulated and that cellular apoptosis is attenuated by
acupuncture in the hippocampi of rats experiencing hypoxia-ischemia
(23). Furthermore, GDNF has a
neurotrophic effect on diabetes-induced neuronal apoptosis in the
hippocampus (24) and enhances
the migration of colon cancer cells (25) via the PI3K/Akt pathway.
In the present study, we demonstrated that GDNF
expression was markedly decreased in the stomachs of rats subjected
to early vagotomy, and SGES upregulated GDNF and p-Akt expression.
Additionally, no change was observed in the pan-Akt levels in
either the vagotomized groups or the SGES groups. These data
indicate that GDNF and p-Akt probably play a role in the damage to
enteric neurons, and that SGES likely protected the loss of enteric
neurons via GDNF and the PI3K/Akt pathway.
In recent years, EGCs have been increasingly
regarded as crucial partners of enteric neurons in the regulation
of gastrointestinal function and structure. Enteric glia-neuron
communication is beneficial to neuronal maintenance, survival and
function. As a result, evidence indicates that enteric glial cells
release neurotransmitters, such as reduced glutathione and
15d-PGJ2, which exert neuroprotective effects (26,27). Enteric glial ablation or
disruption is involved in the dysfunction of enteric neurons that
contributes to intestinal motility disorders (28,29). Indeed, the present study
demonstrated that GFAP expression, specifically its expression in
EGCs in the antrum tissue, was decreased and followed almost the
same trend as PGP9.5 in the vagotomized groups. We speculate that
the injured EGCs contributed to neuronal damage as a dense network
of EGCs was packed around the enteric neurons.
GES is used in the treatment of gastrointestinal
dysfunction including nausea, vomiting, early satiety, abdominal
bloating and loss of appetite. GES has been shown to alleviate
symptoms, such as nausea, loss of appetite and early satiety, with
optimal responses observed in 75% of patients with refractory
gastroparesis (11). The effect
of GES may be involved in central, neuronal and hormonal mechanisms
that GES activates in gastric distension-sensitive neurons and in
the improved expression of cholecystokinin in the hippocampus
(30). GES applied with the
appropriate parameters has become a promising treatment for
gastrointestinal functional disorders. In the present study, we
demonstrated that SGES with a long pulse (300 msec, 4 mA) followed
by 5 short pulses (0.3 msec, 100 Hz, 4 mA) markedly upregulated
GDNF expression together with p-Akt and PGP9.5, and reduced the
number of TUNEL-positive cells. Our previous studies demonstrated
that SGES activated EGCs and improved gastric emptying (31,32). This result indicates that SGES
restores injured ENS and improves gastric motility.
Intriguingly, a previous study demonstrated that
hepatic electrical stimulation decreased fasting and fed blood
glucose in normal and diabetic rats, a result that may be mediated
by the release of glucagon-like peptide-1 (GLP-1) (33). Moreover, another previous study
demonstrated that SGES using optically appropriate parameters
improved vagotomy-induced impairment in gastric accommodation
resulting from vagotomy (34). It
has also been shown that SGES improves gastric motility, an effect
that may be related to the activation of enteric glial cells
(31).
Cx43 belongs to the connexin family of gap junction
proteins and is widespread in the body. Cx43, which is limited to
enteric glia in the myenteric plexus of the mouse colon, directly
mediates Ca2+ responses and indirectly contributes to
whole-gut transit (35). In
addition, Cx43 serves as a major gap junction protein that
deteriorates in inflamed white matter in multiple sclerosis
(36). Our data indicated that
Cx43 expression was markedly reduced in the vagotomized groups and
that short- or long-term SGES increased Cx43 expression. Our
previous study found that SGES improved gastric emptying in
vagotomized rats through the activation of enteric glial cells
(32). More research is required
to determine whether SGES enhances the cross-talk between enteric
glial cells via the Cx43 protein and further promotes the release
of GDNF.
GES is used in the treatment of certain diseases.
Numerous studies have shown that the use of GES with appropriate
parameters is an effective method for the treatment of refractory
gastroparesis resulting from gastric neuropathy (37–39). Of note, GES contributes to
significant weight loss as the result of changes in eating behavior
(40) and the activation neurons
in the hypothalamus (41).
Neurodegenerative diseases of the central nervous system and injury
to the central nervous system lead to delayed gastric emptying.
Gastric emptying is delayed in Parkinson's disease, probably as the
result of neurodegeneration in the dorsal motor nucleus of the
vagus nerve (42,43). Delayed gastric emptying occurrs in
patients with traumatic brain injury, and treatment with prokinetic
drug is needed to relieve enteral feeding tolerance (44). Spinal cord injury can lead to
damage to gastric motility and emptying (45). Collectively, these findings
indicate that GES has therapeutic potential for treating
gastrointestinal dysfunction due to ENS or in central nervous
system.
In conclusion, in this study, we demonstrated that
GDNF, p-Akt and PGP9.5 expression was decreased and more
TUNEL-positive cells were observed in the vagotomized rats. These
results imply that GDNF and the PI3K/Akt signaling pathway play
vital roles in neuropathy of the gastrointestinal tract. Moreover,
SGES upregulated the expression of GDNF, p-Akt and PGP9.5 in the
vagotomized rats and inhibited the apoptosis of enteric neurons.
This finding suggests that SGES may be a promising treatment for
enteric neuropathies and functional gastrointestinal disorders.
Acknowledgments
This study was supported by a grant from the
National Natural Science Foundation of China (project no:
81170342).
References
|
1
|
Lakkireddy D, Reddy YM, Atkins D,
Rajasingh J, Kanmanthareddy A, Olyaee M, Dusing R, Pimentel R,
Bommana S and Dawn B: Effect of atrial fibrillation ablation on
gastric motility: the atrial fibrillation gut study. Circ
Arrhythmia Electrophysiol. 8:531–536. 2015. View Article : Google Scholar
|
|
2
|
Costantini TW, Bansal V, Krzyzaniak M,
Putnam JG, Peterson CY, Loomis WH, Wolf P, Baird A, Eliceiri BP and
Coimbra R: Vagal nerve stimulation protects against burn-induced
intestinal injury through activation of enteric glia cells. Am J
Physiol Gastrointest Liver Physiol. 299:G1308–G1318. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Holmes GM: Upper gastrointestinal
dysmotility after spinal cord injury: Is diminished vagal sensory
processing one culprit? Front Physiol. 3:2772012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tajdaran K, Gordon T, Wood MD, Shoichet MS
and Borschel GH: A glial cell line-derived neurotrophic factor
delivery system enhances nerve regeneration across acellular nerve
allografts. Acta Biomater. 29:62–70. 2016. View Article : Google Scholar
|
|
5
|
Taba Taba Vakili S, Kailar R, Rahman K,
Nezami BG, Mwangi SM, Anania FA and Srinivasan S: Glial cell
line-derived neurotrophic factor-induced mice liver defatting: A
novel strategy to enable transplantation of steatotic livers. Liver
Transpl. 22:459–467. 2016. View
Article : Google Scholar
|
|
6
|
Du F, Wang L, Qian W and Liu S: Loss of
enteric neurons accompanied by decreased expression of GDNF and
PI3K/Akt pathway in diabetic rats. Neurogastroenterol Motil.
21:1229-e1142009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anitha M, Gondha C, Sutliff R, Parsadanian
A, Mwangi S, Sitaraman SV and Srinivasan S: GDNF rescues
hyperglycemia-induced diabetic enteric neuropathy through
activation of the PI3K/Akt pathway. J Clin Invest. 116:344–356.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du F and Liu S: Electroacupuncture with
high frequency at acupoint ST-36 induces regeneration of lost
enteric neurons in diabetic rats via GDNF and PI3K/AKT signal
pathway. Am J Physiol Regul Integr Comp Physiol. 309:R109–R118.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun Y, Tan Y, Song G and Chen JD: Effects
and mechanisms of gastric electrical stimulation on visceral pain
in a rodent model of gastric hyperalgesia secondary to chemically
induced mucosal ulceration. Neurogastroenterol Motil. 26:176–186.
2014. View Article : Google Scholar
|
|
10
|
Yu X, Tu L, Lei P, Song J, Xu H and Hou X:
Antiemesis effect and brain fMRI response of gastric electrical
stimulation with different parameters in dogs. Neurogastroenterol
Motil. 26:1049–1056. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heckert J, Sankineni A, Hughes WB,
Harbison S and Parkman H: Gastric electric stimulation for
refractory gastroparesis: A prospective analysis of 151 patients at
a single center. Dig Dis Sci. 61:168–175. 2016. View Article : Google Scholar
|
|
12
|
Song GQ, Zhu H, Lei Y, Yuan C, Starkebaum
W, Yin J and Chen JD: Gastric electrical stimulation optimized to
inhibit gastric motility reduces food intake in dogs. Obes Surg.
25:1047–1055. 2015. View Article : Google Scholar
|
|
13
|
Kuhlman J and Eisen JS: Genetic screen for
mutations affecting development and function of the enteric nervous
system. Dev Dyn. 236:118–127. 2007. View Article : Google Scholar
|
|
14
|
Bagyánszki M, Krecsmarik M, De Winter BY,
De Man JG, Fekete E, Pelckmans PA, Adriaensen D, Kroese AB, Van
Nassauw L and Timmermans JP: Chronic alcohol consumption affects
gastrointestinal motility and reduces the proportion of neuronal
NOS-immunoreactive myenteric neurons in the murine jejunum. Anat
Rec (Hoboken). 293:1536–1542. 2010. View
Article : Google Scholar
|
|
15
|
Ishihara E, Nagahama M, Naruse S, Semba R,
Miura T, Usami M and Narita M: Neuropathological alteration of
aquaporin 1 immunoreactive enteric neurons in the
streptozotocin-induced diabetic rats. Auton Neurosci. 138:31–40.
2008. View Article : Google Scholar
|
|
16
|
Liu W, Yue W and Wu R: Overexpression of
Bcl-2 promotes survival and differentiation of neuroepithelial stem
cells after transplantation into rat aganglionic colon. Stem Cell
Res Ther. 4:72013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bar KJ, Facer PA, Williams NS, Tam PK and
Anand PR: Glial-derived neurotrophic factor in human adult and
fetal intestine and in Hirschsprung's disease. Gastroenterology.
4:1381–1385. 1997. View Article : Google Scholar
|
|
18
|
Heuckeroth RO, Lampe PA Jr, Johnson EM Jr
and Milbrandt J: Neurturin and GDNF promote proliferation and
survival of enteric neuron and glial progenitors in vitro. Dev
Biol. 200:116–129. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sánchez MP, Silos-Santiago I, Frisén J, He
B, Lira SA and Barbacid M: Renal agenesis and the absence of
enteric neurons in mice lacking GDNF. Nature. 5686:70–73. 1996.
View Article : Google Scholar
|
|
20
|
Meir M, Flemming S, Burkard N, Bergauer L,
Metzger M, Germer CT and Schlegel N: Glial cell line-derived
neurotrophic factor promotes barrier maturation and wound healing
in intestinal epithelial cells in vitro. Am J Physiol Gastrointest
Liver Physiol. 309:G613–G624. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barrenschee M, Böttner M, Hellwig I, Harde
J, Egberts JH, Becker T and Wedel T: Site-specific gene expression
and localization of growth factor ligand receptors RET, GFRα1 and
GFRα2 in human adult colon. Cell Tissue Res. 354:371–380. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Böttner M, Harde J, Barrenschee M, Hellwig
I, Vogel I, Ebsen M and Wedel T: GDNF induces synaptic vesicle
markers in enteric neurons. Neurosci Res. 77:128–136. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Lan R, Wang J, Li XY, Zhu DN, Ma
YZ, Wu JT and Liu ZH: Acupuncture reduced apoptosis and upregulated
BDNF and GDNF expression in hippocampus following hypoxia-ischemia
in neonatal rats. J Ethnopharmacol. 172:124–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu G, Wang X, Wu S, Li X and Li Q:
Neuroprotective effects of puerarin on
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson's
disease model in mice. Phytother Res. 28:179–186. 2014. View Article : Google Scholar
|
|
25
|
Huang SM, Chen TS, Chiu CM, Chang LK, Liao
KF, Tan HM, Yeh WL, Chang GR, Wang MY and Lu DY: GDNF increases
cell motility in human colon cancer through VEGF-VEGFR1
interaction. Endocr Relat Cancer. 21:73–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abdo H, Derkinderen P, Gomes P, Chevalier
J, Aubert P, Masson D, Galmiche JP, Vanden Berghe P, Neunlist M and
Lardeux B: Enteric glial cells protect neurons from oxidative
stress in part via reduced glutathione. FASEB J. 24:1082–1094.
2010. View Article : Google Scholar
|
|
27
|
Abdo H, Mahé MM, Derkinderen P,
Bach-Ngohou K, Neunlist M and Lardeux B: Bach-NgohouK, NeunlistM
and LardeuxB: The omega-6 fatty acid derivative
15-deoxy-Δ12,14-prostaglandin J2 is involved in neuroprotection by
enteric glial cells against oxidative stress. J Physiol.
590:2739–2750. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nasser Y, Fernandez E, Keenan CM, Ho W,
Oland LD, Tibbles LA, Schemann M, MacNaughton WK, Rühl A and
Sharkey KA: Role of enteric glia in intestinal physiology: Effects
of the gliotoxinfluorocitrate on motor and secretory function. Am J
Physiol Gastrointest Liver Physiol. 291:G912–G927. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aubé AC, Cabarrocas J, Bauer J, Philippe
D, Aubert P, Doulay F, Liblau R, Galmiche JP and Neunlist M:
Changes in enteric neurone phenotype and intestinal functions in a
transgenic mouse model of enteric glia disruption. Gut. 55:630–637.
2006. View Article : Google Scholar
|
|
30
|
Xu L, Sun X, Lu J, Tang M and Chen JD:
Effects of gastric electric stimulation on gastric distention
responsive neurons and expressions of CCK in rodent hippocampus.
Obesity. 16:951–957. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang W, Wang N, Shi X and Chen J:
Synchronized dual pulse gastric electrical stimulation induces
activation of enteric glial cells in rats with diabetic
gastroparesis. Gastroenterol Res Pract. 2014:9640712014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang N, Song S and Chen J: Syncronized
dual pulse gastric electrical stimulation improves gastric emptying
and activates enteric glial cells via upregulation of GFAP and
S100B with different courses of subdiaphragmatic vagotomy in rats.
Mol Med Rep. 15:3826–3832. 2017.PubMed/NCBI
|
|
33
|
Chen J, Pasricha PJ, Yin J, Lin L and Chen
JD: Hepatic electrical stimulation reduces blood glucose in both
type-I and type-II diabetic rats. Neurogastroenterol Motil.
22:1109-e2862010. View Article : Google Scholar
|
|
34
|
Chen J, Koothan T and Chen JD:
Synchronized gastric electrical stimulation improves
vagotomy-induced impairment in gastric accommodation via the
nitrergic pathway in dogs. Am J Physiol Gastrointest Liver Physiol.
296:G310–G318. 2009. View Article : Google Scholar :
|
|
35
|
McClain JL, Grubišić V, Fried D,
Gomez-Suarez RA, Leinninger GM, Sévigny J, Parpura V and Gulbransen
BD: Ca2+ responses in enteric glia are mediated by
connexin-43 hemichannels and modulate colonic transit in mice.
Gastroenterology. 2:497–507. 2014. View Article : Google Scholar
|
|
36
|
Brand-Schieber E, Werner P, Iacobas DA,
Iacobas S, Beelitz M, Lowery SL, Spray DC and Scemes E: Connexin43,
the major gap junction protein of astrocytes, is down-regulated in
inflamed white matter in an animal model of multiple sclerosis. J
Neurosci Res. 80:798–808. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Abell T, McCallum R, Hocking M, Koch K,
Abrahamsson H, Leblanc I, Lindberg G, Konturek J, Nowak T, Quigley
EM, et al: Gastric electrical stimulation for medically refractory
gastroparesis. Gastroenterology. 125:421–428. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brody F, Vaziri K, Saddler A, Ali A,
Drenon E, Hanna B, Akin E, Gonzalez F and Soffer E: Gastric
electrical stimulation for gastroparesis. J Am Coll Surg.
207:533–538. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chu H, Lin Z, Zhong L, McCallum RW and Hou
X: Treatment of high-frequency gastric electrical stimulation for
gastroparesis. J Gastroenterol Hepatol. 27:1017–1026. 2012.
View Article : Google Scholar
|
|
40
|
Horbach T, Thalheimer A, Seyfried F,
Eschenbacher F, Schuhmann P and Meyer G: abiliti®
closed-loop gastric electrical stimulation system for treatment of
obesity: clinical results with a 27-month follow-up. Obes Surg.
25:1779–1787. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang J, Tang M and Chen JD: Gastric
electrical stimulation for obesity: The need for a new device using
wider pulses. Obesity (Silver Spring). 17:474–480. 2009. View Article : Google Scholar
|
|
42
|
Goetze O, Nikodem AB, Wiezcorek J, Banasch
M, Przuntek H, Mueller T, Schmidt WE and Woitalla D: Predictors of
gastric emptying in Parkinson's disease. Neurogastroenterol Motil.
18:369–375. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Marrinan S, Emmanuel AV and Burn DJ:
Delayed gastric emptying in Parkinson's disease. Mov Disord.
29:23–32. 2014. View Article : Google Scholar
|
|
44
|
Dickerson RN, Mitchell JN, Morgan LM,
Maish GO III, Croce MA, Minard G and Brown RO: Disparate response
to metoclopramide therapy for gastric feeding intolerance in trauma
patients with and without traumatic brain injury. JPEN J Parenter
Enteral Nutr. 33:646–655. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Qualls-Creekmore E, Tong M and Holmes GM:
Time-course of recovery of gastric emptying and motility in rats
with experimental spinal cord injury. Neurogastroenterol Motil.
22:62–69. e27–e28. 2010.
|