The estimated prevalence of IBS ranges between 5 and
20% of the population worldwide (1,2,4,6,8–10,17–22), with marked geographical variations
(9). Using Rome II criteria, the
prevalence is 11.5% in Europe (9), 12.1% in Canada (23), 4.7% in the USA (24), 6.9% in Australia (25), 34% in Egypt (26), 4% in India (27,28) and 4.6–5.6% in China (29). The prevalence of IBS in
Scandinavian countries is as follows: 10.5% in Denmark (30), 14.5% in Sweden (31), 8% in the south of Norway (32) and 25% in the north of Norway
(33).
IBS is mainly diagnosed clinically based on the
presenting symptoms due to the lack of biochemical,
histopathological or radiological diagnostic tests (3,4,34,35). The symptoms of IBS comprise
abdominal pain or discomfort, abdominal bloating or distension and
alterations in the stool (1,4,34,36). Based on the predominant bowel
movements, patients with IBS are classified into
diarrhea-predominant (IBS-D), constipation-predominant (IBS-C) and
mixed diarrhea and constipation (37,38). Rome IV criteria are the currently
used symptom-based diagnostic criteria (39).
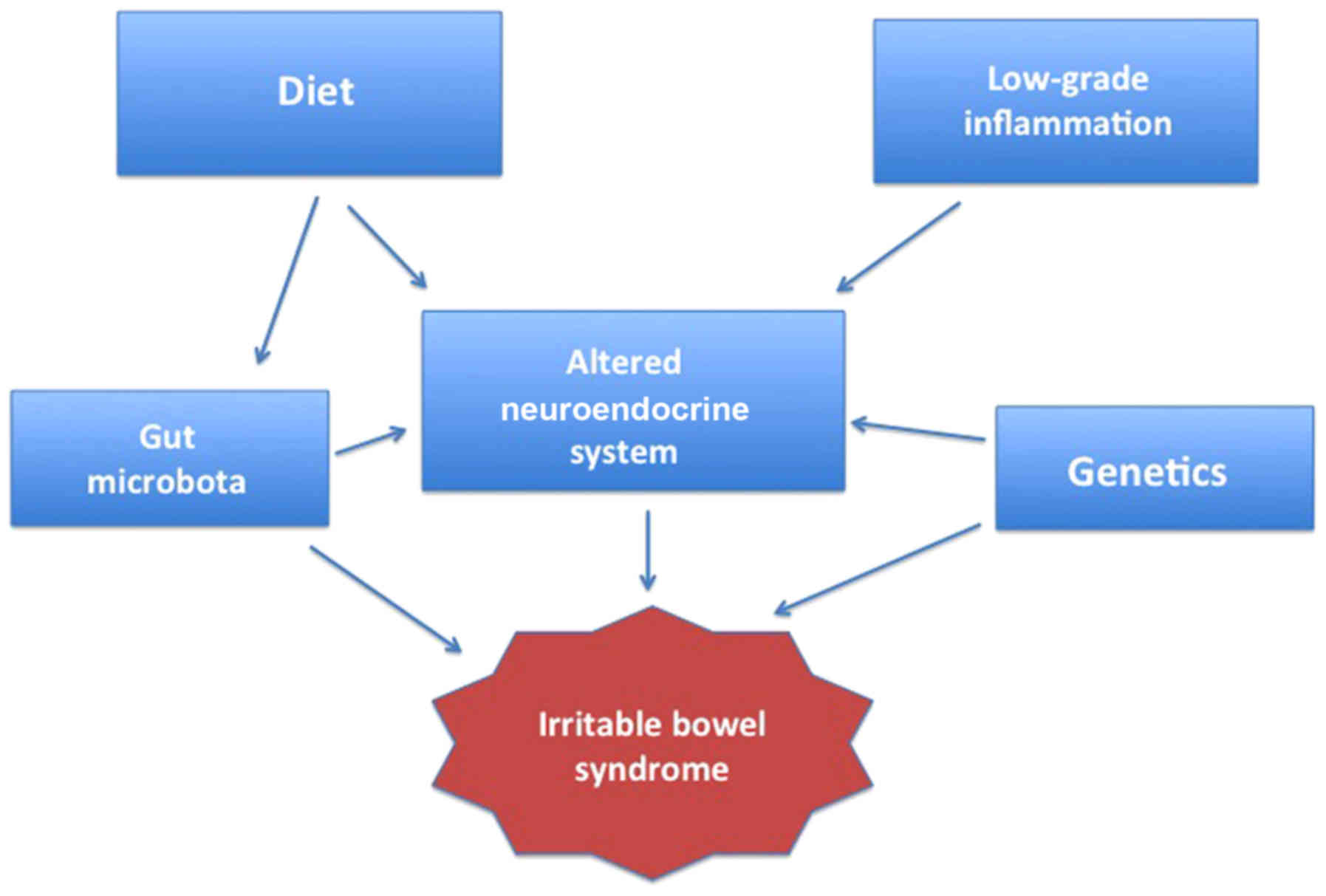
Several factors seem to be involved in the
pathogenesis of IBS, including hereditariness, diet, mucosal
low-grade inflammation, GI microbiota and abnormal endocrine cells
in the GI tract (Fig. 1)
(4,35,54–78). This review discusses the effects
of applying individual dietary guidance on symptoms, quality of
life and GI endocrine cells in patients with IBS.
More than two-thirds of patients with IBS associate
the development of their symptoms with the consumption of certain
foodstuffs (71–73), such as milk and other dairy
products, wheat products, caffeine, certain meats, cabbage, onion,
peas, beans, tomatoes, hot spices and fried foods (13,52,71,79), as well as raw vegetables, raw
broccoli, paprika, leeks, garlic and mushrooms (52). These foodstuffs are rich in the
poorly absorbed rapidly fermentable oligosaccharides,
disaccharides, monosaccharides and polyols (FODMAPs) (13,52,80–82). The time delay between the
consumption of food until the appearance of IBS symptoms varies,
with 28% of patients experiencing symptoms within 15 min of eating,
and 93% reporting that the symptoms become more severe between 15
min and 3 h (71).
Carbohydrates constitute the largest source of
energy for humans, ranging from 40 to 80% of the total energy
requirements (81). There are two
types of dietary carbohydrates, namely long-chain carbohydrates
(starch, resistant starch and non-starch polysaccharides) and
short-chain (sugars, polyols and oligosaccharides) (80). Long-chain carbohydrates provide
benefits of fecal bulking, accelerating colonic transit and causing
slight acidification of the luminal milieu (80). On the other hand, short-chain
carbohydrates, also referred to as prebiotics, stimulate the growth
of beneficial bacteria in the colon (Bifidobacteria and
Lactobacillus) and include fructans (fructo-oligosaccharides
and inulin) and galacto-oligosaccharides (80,83). Prebiotics help to reduce the risk
of GI infections (84), improve
laxation (85) and calcium
absorption (86), preserve the GI
mucosal barrier (87) and
stimulate the immune system of the GI tract that may reduce the
risk of colon cancer (demonstrated in animals) (88).
The microbiota in the colon ferments undigested
long-chain carbohydrates and produces gases (carbon dioxi de,
hydrogen and/or methane) and short-chain fatty acids (butyrate,
which is an important source of energy for colonocytes and the
colonic microbiota) (80,89–91). The poorly absorbed short-chain
carbohydrates increase the volume of fluid in the bowel through
osmosis (80,91,92), which results in a natural laxative
effect in healthy individuals and diarrhea in patients with IBS
(80). The gut microbiota in
patients with IBS comprises fewer Lactobacillus and
Bifidobacterium spp. and more Clostridium spp. that
ferment FODMAPs and fiber and produce gas, leading to luminal
distension with symptoms of abdominal pain and bloating (93–95).
The importance of both the presence of specific
types of FODMAPs and the total content of FODMAPs in the diet
should be noted (75,80,81). Some foods contain several types of
FODMAPs; for example, white onion contains excess fructose,
raffinose, nystose and kestose, which are particularly problematic
for patients with IBS (81). The
main clinical problems associated with the total content of a major
FODMAP are likely to be due to fructans in vegetables and, to a
lesser extent, to free fructose and sorbitol in fruits (81).
It has been reported that foods containing a higher
proportion of dietary fiber, particularly water-soluble fiber, can
help to prevent diseases, such as coronary heart disease, diabetes,
irritable bowel disease and colon cancer and obesity (96, and refs
therein).
The lipolysis of triglycerides comprising >12
carbon atoms is initiated in the stomach with the formation of
emulsions of finely dispersed lipids that bind to gastric lipase.
This process of fat digestion is completed in the duodenum by
pancreatic lipase and releases fatty acids and monoglycerides. The
lipid components (fatty acids and monoglycerides) then form
water-soluble micelles with conjugated bile acids and are absorbed
across the enterocyte membrane. The triglycerides are then
reassembled and incorporated into chylomicrons and transported via
the lymphatic system. On the other hand, medium-chain triglycerides
(comprising 8–12 carbon atoms) are absorbed directly into the
bloodstream without the need for luminal lipolysis and micelle
formation. Under physiological conditions, no dietary fat enters
the colon since fat is digested and completely absorbed in the
small bowel.
Patients with IBS have abnormal lipid-dependent
motor dysfunction that affects the small intestine, but not the
colon (109). These patients
also exhibit increased small intestine sensitivity to lipid
exposure, which induces symptoms of bloating (89,109), fullness and nausea at lower
nutrient loads, and enhances GI sensitivity to mechanical
distension (89). In addition,
intraluminal fat in the small intestine of patients with IBS
impairs gas transit and results in the development of gas retention
(bloating) and abdominal distension, particularly in the jejunum
(109). Lipids also exacerbate
rectal hypersensitivity (89,110) and increase the perception of
rectal distension in patients with IBS (89,111), causing pain in patients with
IBS-C patients, but urgency in patients with IBS-D (89).
Some patients with IBS reportedly relate the
development of their symptoms to the consumption of fat-rich meals.
However, no consistent differences in dietary fat consumption have
been observed between patients with IBS and healthy controls
(52,89).
Almost 20% of the dietary protein enters the distal
colon and undergoes putrefaction by colonic bacteria to produce
ammonia, amines, phenols and sulfides (112,113). Ammonia is essential for
bacterial metabolism and protein synthesis. Branched-chain fatty
acids (isovalerate and isobutyrate) (113,114) and short-chain fatty acids
(butyrate) (113,115) are produced in the distal part of
the colon in the absence of carbohydrate fermentation (which occurs
at the proximal colon) (113).
Another product of protein putrefaction is sulfur-containing gas
(hydrogen sulfide), which has a foul odor (112). The products of protein
putrefaction are potentially harmful, phenols are carcinogenic in
other systems and hydrogen sulfide is toxic to the epithelium.
However, only the malodorous flatus is of concern to patients with
IBS, and no definitive effects of malabsorbed protein on intestine
motility or visceral hypersensitivity have been identified
(112).
Gluten is a mixture of two proteins (gliadin and
glutenin) that is found in wheat, barley and rye (112). Consuming gluten activates the
immune system so as to change the function of the mucosal barrier
that increases intestinal permeability, a condition known as celiac
disease that presents with symptoms mimicking IBS. A gluten-free
diet usually reduces bowel frequency in patients with IBS-D who are
positive for human leukocyte antigen (HLA)-DQ2/8 due to the
reduction in intestinal permeability (116). In addition, a gluten-free diet
improves the symptoms of IBS due to the associated reduced intake
of FODMAPs in wheat rather than of gluten in foods with gluten as a
common component (117).
Natural chemicals, such as amines, glutamates and
salicylates occur in foods. Salicylates are found in plants whereas
amines and glutamates are products of protein breakdown in animal
meat (118). Food additives,
such as glutamates are used as flavor enhancers and benzoates,
sulfites, and nitrates are used as preservatives (118). These bioactive chemicals
interact with the GI luminal chemoreceptors and influence the
function of the enteric nervous system of the GI tract (112). A diet that is low in these
chemicals may be beneficial to patients with IBS, whereas there is
no evidence of the benefits of reducing caffeine or ethanol
consumption (112).
The GI endocrine cells are scattered among the
epithelial cells lining the GI lumen (61,119–121). They comprise almost 1% of all
epithelial cells in the GI tract and are considered to be the
largest endocrine organ in the body (121–123). All epithelial cell types in the
GI tract (including GI endocrine cells) originate from pluripotent
stem cells with an endodermal origin (124–133). Gastrointestinal stem cells
differentiate into endocrine cells over a period of 2–6 days
(134,135). The GI endocrine cells project
specialized microvilli into the GI lumen to sense the luminal
contents (mainly nutrients) and release specific hormones into the
lamina propria (61,119,120,136–145).
There are at least 15 different types of GI
endocrine cells in the GI tract releasing different types of
hormones (2,127). The types of released hormones
depend on the types of sensed nutrients: protein and fat trigger
the release of serotonin; somatostatin, ghrelin, polypeptide YY
(PYY) and carbohydrates suppress ghrelin release; and carbohydrates
and fat trigger the release of oxyntomodulin (enteroglucagon)
(2,93,146). Particular GI endocrine cells are
located either in specific parts of the GI tract or throughout the
GI tract (93,127,146,147). Cells producing gastrin and
ghrelin are found in the stomach, those producing secretin,
cholecystokinin and gastric inhibitory peptide are found in the
duodenum, those producing oxyntomodulin (enteroglucagon) and PYY
are located in the lower small and large intestines, and those
producing serotonin and somatostatin are found throughout the GI
tract (93,127,146,147). The functions of the different
hormones are summarized in Table
I (2).
Several functions of the GI tract, such as motility,
secretion, absorption, microcirculation, local immune defense, cell
proliferation and food intake, are regulated by the interactions of
GI endocrine cells with themselves and with the enteric nervous
system, independent of the central nervous system but also
communicating and integrated with it (2,119,146,148–151). The GI endocrine cells release
their hormones that exert their effects via endocrine signaling
(through the bloodstream to distant targets), paracrine or
autocrine signaling (locally), synaptic signaling or
neuroendocrinally (being released from synapses into the
bloodstream) (2,93,146,148).
There is increasing evidence of an altered
neuroendocrine system, namely the GI endocrine cells, being a cause
of IBS, since the densities of different types of GI endocrine
cells in the different parts of the GI tract are abnormal in
patients with IBS (4,35,61–70). Such alterations are responsible
for abnormal functions of the GI tract, such as visceral
hypersensitivity, dysmotility and abnormal secretion, all of which
are characteristics of IBS (35).
Several studies have shown that a low-FODMAP diet
can improve the symptoms experienced by patients with IBS (78,90,91,152–157) and that these patients tend to
comply with consuming such a diet; one study found that >75% of
patients were compliant (75).
However, consuming such a diet over a long period of time is
associated with several complications, such as a lack of nutrients
(52), and changes in the fecal
microbiota (158,159). Many patients with IBS make a
conscious choice to avoid certain foodstuffs, some of which belong
to the FODMAPs group. However, they also tend to 'unknowingly'
consume other foodstuffs that are rich in FODMAPs and avoid food
sources that are important to their health (52). A Norwegian study on food
intolerance and IBS found that 62% of the included subjects limited
or excluded some foodstuffs from their daily meals, while 12% made
drastic changes in their diet that could result in nutritional
deficiencies in the long term (72). Patients with IBS tend to have low
intakes of calcium, potassium, magnesium, vitamin A, vitamin
B12 and vitamin B2 (52,160–162). Avoiding such adverse effects of
the long-term consumption of a low-FODMAP diet therefore requires
the administration of dietary guidance (75).
Educating patients can facilitate changes in their
behavior for the purpose of disease management and prevention
(163). Patients with IBS are
particularly interested in learning about dietary modifications,
coping strategies and the causes of the disease (163). Dietary guidance can be
administered individually, whereby patients are provided with
information via one-to-one consultations; this approach is useful
due to the tolerance to different FODMAPs, varying widely between
individual patients (164). When
being provided with individual dietary guidance, patients are
scheduled to attend several relatively short sessions, administered
by a physician, nurse or nutritionist, to receive information
concerning IBS and the appropriate foodstuffs they should consume
to reduce their symptoms. The main emphasis should be on consuming
foodstuffs that are low in FODMAPs and water-insoluble fiber, and
changing the proportions of protein (increase), fat (decrease) and
carbohydrates (decrease). Providing several sessions of
consultation gives the patients reassurance and confidence while
they are receiving dietary guidance. Using a daily diary to
register the daily consumption of food and fluids and the
accompanying IBS symptoms (if any) helps in identifying which
foodstuffs worsen the symptoms experienced by the patients
(52,165,166).
Individual dietary guidance helps patients with IBS
to choose dietary items that are low in FODMAPs and water-insoluble
fiber (166). The effects of
this approach on symptoms and the quality of life of patients with
IBS have been assessed using several questionnaires, such as the
Birmingham IBS Symptom Questionnaire, the Irritable Bowel Syndrome
- Quality of Life (IBS-QOL) questionnaire, and the Short-Form
Nepean and Dyspepsia Index (SF-NDI) quality-of-life questionnaire
(52,166). The Birmingham IBS symptom
questionnaire is disease-specific and measures the symptoms
experienced by IBS patients, namely pain, diarrhea and constipation
(167). IBS symptoms, as
assessed by the Birmingham IBS symptom questionnaire, improve
significantly after receiving dietary guidance, particularly the
symptoms of pain (52,166) and diarrhea (166), but not constipation (52,166).
The IBS-QOL questionnaire is IBS-specific and
assesses physical and psychosocial functioning as a result of IBS
(168,169). The SF-NDI questionnaire is a
disease-specific questionnaire that assesses the health-related
quality of life and was constructed and validated primarily in
patients with dyspepsia (170).
However, a validated version of the questionnaire translated into
Norwegian was demonstrated to perform well in patients with IBS
(171). The total scores, as
assessed by the IBS-QOL and SF-NDI questionnaires, showed
significant improvements in the quality of life of patients with
IBS after they received dietary guidance (52,166).
Individual dietary guidance helps to reduce IBS
symptoms, improving the quality of life of patients with IBS
(52,165,166), and ensures that they have an
adequate intake of necessary vitamins and minerals, thus avoiding
multiple nutrition deficiencies (52,166).
At the cellular level, changing the diet through
individual dietary guidance changes the densities of different GI
endocrine cells in different parts of the GI tract (172–175), as shown in Figs. 2Figure 3Figure 4–5. Chromogranin A (CgA) is considered to
be a general marker of endocrine cells (176–178). As previously demonstrated, the
densities of CgA-immunoreactive cells are abnormal in the stomach,
duodenum, ileum and colon of patients with IBS, and dietary
guidance tends to change these densities toward the values measured
in healthy control subjects (173,179,180). These changes are considered to
be brought about by changes in different endocrine cells in the
respective parts of the GI tract after providing dietary guidance.
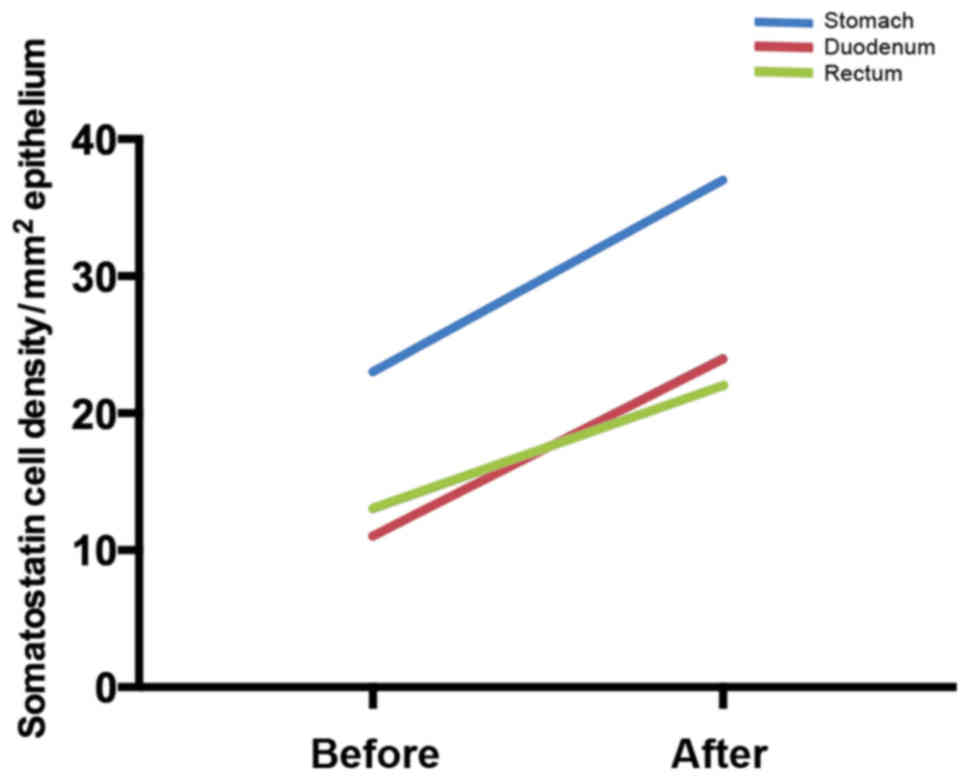
For example, in the stomach, the densities of several endocrine
cells (gastrin, ghrelin, serotonin and somatostatin) changed toward
the values measured in healthy control subjects after providing
dietary guidance, but only somatostatin cell density showed a
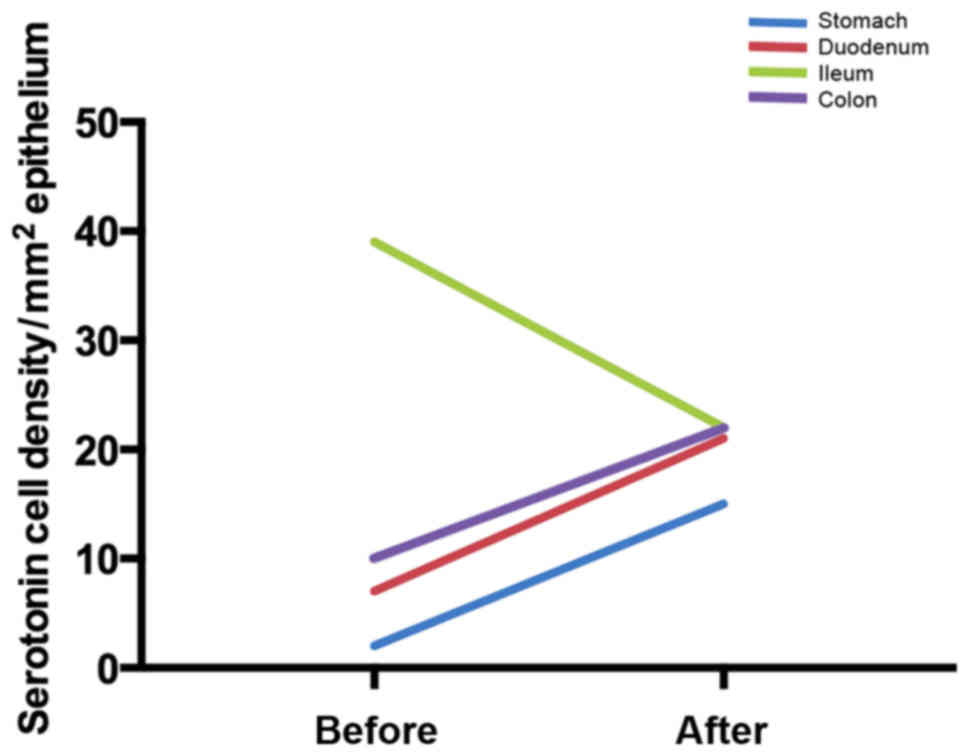
significant change in the gastric corpus (181). The densities of serotonin cells
in the duodenum and ileum changed significantly (172,175), and the density of somatostatin
cells in the duodenum changed significantly after providing dietary
guidance toward that measured in healthy control subjects (175). After providing dietary guidance,
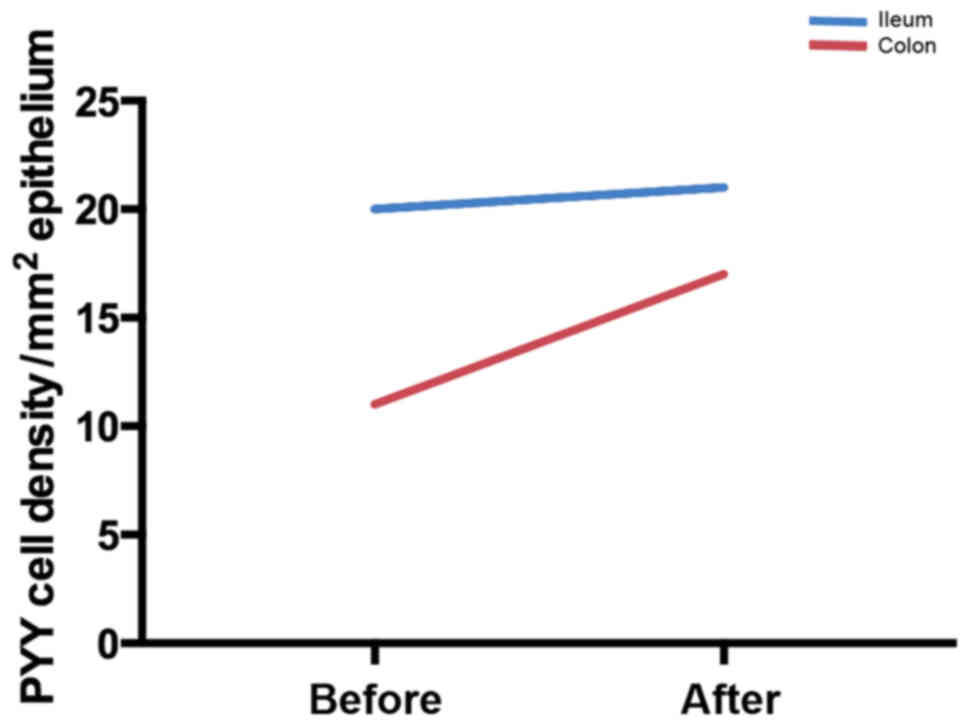
the densities of serotonin and PYY cells tend to normalize in
different segments of the colon, with the density of somatostatin
cells increasing in the rectum (174).
The observation that dietary guidance can alter the
densities of several GI endocrine cells in patients with IBS,
particularly serotonin, PYY and somatostatin cells, to approach
those in healthy control subjects suggests that these changes
underlie the reduction in pain and the improvement in diarrhea as
quantified using the Birmingham IBS symptom questionnaire, and
consequently the improvement in the quality of life of these
patients.
Patients with IBS typically report that food
aggravates their IBS symptoms. The interactions between specific
types of foodstuffs and GI endocrine cells result in changes in
these cell densities. Providing individual dietary guidance about a
low FODMAP intake, high soluble-fiber intake, and changing the
proportions of protein, fat and carbohydrates helps to reduce the
symptoms experienced by IBS patients and to improve their quality
of life. This improvement is accompanied by changes in the
densities of the GI endocrine cells where some densities are
restored back to the normal values. These observations emphasize on
the role of GI endocrine cells in the pathophysiology of IBS, and
support the provision of dietary guidance as a first-line treatment
for managing IBS (76).
The studies conducted by the authors and cited in
this review were supported by grants from Helse-Vest (grant no.
911976) and Helse-Fonna (grant no. 40415).
|
1
|
Thompson WG: A world view of IBS.
Irritable Bowel Syndrome: Diagnosis and Treatment. Camilleri M and
Spiller RC: WB Saunders; Edinburgh: pp. 17–26. 2002
|
|
2
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Irritable Bowel Syndrome. Nova Science Publishers; New
York, NY: 2012
|
|
3
|
Camilleri M, Heading RC and Thompson WG:
Clinical perspectives, mechanisms, diagnosis and management of
irritable bowel syndrome. Aliment Pharmacol Ther. 16:1407–1430.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-Salhy M: Irritable bowel syndrome:
diagnosis and pathogenesis. World J Gastroenterol. 18:5151–5163.
2012.PubMed/NCBI
|
|
5
|
Kay L, Jørgensen T and Jensen KH: The
epidemiology of irritable bowel syndrome in a random population:
prevalence, incidence, natural history and risk factors. J Intern
Med. 236:23–30. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Drossman DA, Li Z, Andruzzi E, Temple RD,
Talley NJ, Thompson WG, Whitehead WE, Janssens J, Funch-Jensen P,
Corazziari E, et al: U.S. householder survey of functional
gastro-intestinal disorders. Prevalence, sociodemography, and
health impact. Dig Dis Sci. 38:1569–1580. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kennedy TM, Jones RH, Hungin AP,
O'flanagan H and Kelly P: Irritable bowel syndrome,
gastro-oesophageal reflux, and bronchial hyper-responsiveness in
the general population. Gut. 43:770–774. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Everhart JE and Renault PF: Irritable
bowel syndrome in office-based practice in the United States.
Gastroenterology. 100:998–1005. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hungin AP, Whorwell PJ, Tack J and Mearin
F: The prevalence, patterns and impact of irritable bowel syndrome:
an international survey of 40,000 subjects. Aliment Pharmacol Ther.
17:643–650. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
O'Keefe EA, Talley NJ, Zinsmeister AR and
Jacobsen SJ: Bowel disorders impair functional status and quality
of life in the elderly: a population-based study. J Gerontol A Biol
Sci Med Sci. 50:M184–M189. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gralnek IM, Hays RD, Kilbourne AM, Chang L
and Mayer EA: Racial differences in the impact of irritable bowel
syndrome on health-related quality of life. J Clin Gastroenterol.
38:782–789. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thompson WG, Heaton KW, Smyth GT and Smyth
C: Irritable bowel syndrome in general practice: prevalence,
characteristics, and referral. Gut. 46:78–82. 2000. View Article : Google Scholar
|
|
13
|
El-Salhy M, Ostgaard H, Gundersen D,
Hatlebakk JG and Hausken T: The role of diet in the pathogenesis
and management of irritable bowel syndrome (Review). Int J Mol Med.
29:723–731. 2012.PubMed/NCBI
|
|
14
|
Mitchell CM and Drossman DA: Survey of the
AGA membership relating to patients with functional
gastrointestinal disorders. Gastroenterology. 92:1282–1284. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schuster MM: Defining and diagnosing
irritable bowel syndrome. Am J Manag Care. 7(Suppl 8): S246–S251.
2001.PubMed/NCBI
|
|
16
|
El-Salhy M, Hatlebakk JG, Gilja OH and
Hausken T: Irritable bowel syndrome: recent developments in
diagnosis, pathophysiology, and treatment. Expert Rev Gastroenterol
Hepatol. 8:435–443. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Talley NJ, Gabriel SE, Harmsen WS,
Zinsmeister AR and Evans RW: Medical costs in community subjects
with irritable bowel syndrome. Gastroenterology. 109:1736–1741.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thompson WG and Heaton KW: Functional
bowel disorders in apparently healthy people. Gastroenterology.
79:283–288. 1980.PubMed/NCBI
|
|
19
|
Bordie AK: Functional disorders of the
colon. J Indian Med Assoc. 58:451–456. 1972.PubMed/NCBI
|
|
20
|
Jones R and Lydeard S: Irritable bowel
syndrome in the general population. BMJ. 304:87–90. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harvey RF, Salih SY and Read AE: Organic
and functional disorders in 2000 gastroenterology outpatients.
Lancet. 1:632–634. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wilson S, Roberts L, Roalfe A, Bridge P
and Singh S: Prevalence of irritable bowel syndrome: a community
survey. Br J Gen Pract. 54:495–502. 2004.PubMed/NCBI
|
|
23
|
Thompson WG, Irvine EJ, Pare P, Ferrazzi S
and Rance L: Functional gastrointestinal disorders in Canada: first
population-based survey using Rome II criteria with suggestions for
improving the questionnaire. Dig Dis Sci. 47:225–235. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saito YA, Locke GR, Talley NJ, Zinsmeister
AR, Fett SL and Melton LJ III: A comparison of the Rome and Manning
criteria for case identification in epidemiological investigations
of irritable bowel syndrome. Am J Gastroenterol. 95:2816–2824.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boyce PM, Koloski NA and Talley NJ:
Irritable bowel syndrome according to varying diagnostic criteria:
are the new Rome II criteria unnecessarily restrictive for research
and practice? Am J Gastroenterol. 95:3176–3183. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abdulmajeed A, Rabab MA, Sliem HA and
Hebatallah NE: Pattern of irritable bowel syndrome and its impact
on quality of life in primary health care center attendees, Suez
governorate, Egypt. Pan Afr Med J. 9:52011.PubMed/NCBI
|
|
27
|
Ghoshal UC, Abraham P, Bhatt C, Choudhuri
G, Bhatia SJ, Shenoy KT, Banka NH, Bose K, Bohidar NP, Chakravartty
K, et al: Epidemiological and clinical profile of irritable bowel
syndrome in India: report of the Indian Society of Gastroenterology
Task Force. Indian J Gastroenterol. 27:22–28. 2008.PubMed/NCBI
|
|
28
|
Makharia GK, Verma AK, Amarchand R,
Goswami A, Singh P, Agnihotri A, Suhail F and Krishnan A:
Prevalence of irritable bowel syndrome: a community based study
from northern India. J Neurogastroenterol Motil. 17:82–87. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu J and Hou X: A review of the irritable
bowel syndrome investigation on epidemiology, pathogenesis and
pathophysiology in China. J Gastroenterol Hepatol. 26(Suppl 3):
88–93. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rasmussen S, Jensen TH, Henriksen SL,
Haastrup PF, Larsen PV, Søndergaard J and Jarbøl DE: Overlap of
symptoms of gastroesophageal reflux disease, dyspepsia and
irritable bowel syndrome in the general population. Scand J
Gastroenterol. 50:162–169. 2015. View Article : Google Scholar
|
|
31
|
Kjellström L, Molinder H, Agréus L, Nyhlin
H, Talley NJ and Andreasson A: A randomly selected population
sample undergoing colonoscopy: prevalence of the irritable bowel
syndrome and the impact of selection factors. Eur J Gastroenterol
Hepatol. 26:268–275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vandvik PO, Lydersen S and Farup PG:
Prevalence, comorbidity and impact of irritable bowel syndrome in
Norway. Scand J Gastroenterol. 41:650–656. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Breckan RK, Asfeldt AM, Straume B,
Florholmen J and Paulssen EJ: Prevalence, comorbidity, and risk
factors for functional bowel symptoms: a population-based survey in
Northern Norway. Scand J Gastroenterol. 47:1274–1282. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Longstreth GF, Thompson WG, Chey WD,
Houghton LA, Mearin F and Spiller RC: Functional bowel disorders.
Gastroenterology. 130:1480–1491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
El-Salhy M, Gundersen D, Gilja OH,
Hatlebakk JG and Hausken T: Is irritable bowel syndrome an organic
disorder? World J Gastroenterol. 20:384–400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hahn BA, Yan S and Strassels S: Impact of
irritable bowel syndrome on quality of life and resource use in the
United States and United Kingdom. Digestion. 60:77–81. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Spiller R, Aziz Q, Creed F, Emmanuel A,
Houghton L, Hungin P, Jones R, Kumar D, Rubin G, Trudgill N, et al:
Clinical Services Committee of The British Society of
Gastroenterology: Guidelines on the irritable bowel syndrome:
mechanisms and practical management. Gut. 56:1770–1798. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tillisch K, Labus JS, Naliboff BD, Bolus
R, Shetzline M, Mayer EA and Chang L: Characterization of the
alternating bowel habit subtype in patients with irritable bowel
syndrome. Am J Gastroenterol. 100:896–904. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Drossman DA: Functional gastrointestinal
disorders: history, pathophysiology, clinical features and Rome IV.
Gastroenterology. Feb 19–2016.Epub ahead of print. View Article : Google Scholar
|
|
40
|
Harvey RF, Mauad EC and Brown AM:
Prognosis in the irritable bowel syndrome: a 5-year prospective
study. Lancet. 1:963–965. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
García Rodríguez LA, Ruigómez A, Wallander
MA, Johansson S and Olbe L: Detection of colorectal tumor and
inflammatory bowel disease during follow-up of patients with
initial diagnosis of irritable bowel syndrome. Scand J
Gastroenterol. 35:306–311. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nørgaard M, Farkas DK, Pedersen L,
Erichsen R, de la Cour ZD, Gregersen H and Sørensen HT: Irritable
bowel syndrome and risk of colorectal cancer: a Danish nationwide
cohort study. Br J Cancer. 104:1202–1206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Whitehead WE, Burnett CK, Cook EW III and
Taub E: Impact of irritable bowel syndrome on quality of life. Dig
Dis Sci. 41:2248–2253. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schmulson MRG and Kershenobich D: Los
pacientes con trastornos funcionales digestivos (TFD) tienen major
compromiso de la calidad de vida (CV) evaluadas por el SF-36
comparados con pacientes con hepatitis C y pancreatitis cronica.
Rev Gastroenterol Mex. 65(Suppl): 50–51. 2000.In Spanish.
|
|
45
|
Martin R, Barron JJ and Zacker C:
Irritable bowel syndrome: toward a cost-effective management
approach. Am J Manag Care. 7(Suppl 8): S268–S275. 2001.PubMed/NCBI
|
|
46
|
Spiegel BM: The burden of IBS: looking at
metrics. Curr Gastroenterol Rep. 11:265–269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
American Gastroenterological Association:
The burden of gastrointestinal diseases. Bethesda, MD: 2001
|
|
48
|
Sandler RS, Everhart JE, Donowitz M, Adams
E, Cronin K, Goodman C, Gemmen E, Shah S, Avdic A and Rubin R: The
burden of selected digestive diseases in the United States.
Gastroenterology. 122:1500–1511. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li FX, Patten SB, Hilsden RJ and
Sutherland LR: Irritable bowel syndrome and health-related quality
of life: a population-based study in Calgary, Alberta. Can J
Gastroenterol. 17:259–263. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Thompson WG, Longstreth GF, Drossman DA,
Heaton KW, Irvine EJ and Müller-Lissner SA: Functional bowel
disorders and functional abdominal pain. Gut. 45(Suppl 2):
II43–II47. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Spanier JA, Howden CW and Jones MP: A
systematic review of alternative therapies in the irritable bowel
syndrome. Arch Intern Med. 163:265–274. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ostgaard H, Hausken T, Gundersen D and
El-Salhy M: Diet and effects of diet management on quality of life
and symptoms in patients with irritable bowel syndrome. Mol Med
Rep. 5:1382–1390. 2012.PubMed/NCBI
|
|
53
|
Canavan C, West J and Card T: Review
article: the economic impact of the irritable bowel syndrome.
Aliment Pharmacol Ther. 40:1023–1034. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Saito YA: The role of genetics in IBS.
Gastroenterol Clin North Am. 40:45–67. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lee YJ and Park KS: Irritable bowel
syndrome: emerging paradigm in pathophysiology. World J
Gastroenterol. 20:2456–2469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Grover M, Camilleri M, Smith K, Linden DR
and Farrugia G: On the fiftieth anniversary. Postinfectious
irritable bowel syndrome: mechanisms related to pathogens.
Neurogastroenterol Motil. 26:156–167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ishihara S, Tada Y, Fukuba N, Oka A,
Kusunoki R, Mishima Y, Oshima N, Moriyama I, Yuki T, Kawashima K,
et al: Pathogenesis of irritable bowel syndrome - review regarding
associated infection and immune activation. Digestion. 87:204–211.
2013. View Article : Google Scholar
|
|
58
|
Lin HC: Small intestinal bacterial
overgrowth: a framework for understanding irritable bowel syndrome.
JAMA. 292:852–858. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hong SN and Rhee PL: Unraveling the ties
between irritable bowel syndrome and intestinal microbiota. World J
Gastroenterol. 20:2470–2481. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Balsari A, Ceccarelli A, Dubini F, Fesce E
and Poli G: The fecal microbial population in the irritable bowel
syndrome. Microbiologica. 5:185–194. 1982.PubMed/NCBI
|
|
61
|
El-Salhy M: Ghrelin in gastrointestinal
diseases and disorders: a possible role in the pathophysiology and
clinical implications (Review). Int J Mol Med. 24:727–732. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
El-Salhy M, Gilja OH, Gundersen D,
Hatlebakk JG and Hausken T: Endocrine cells in the ileum of
patients with irritable bowel syndrome. World J Gastroenterol.
20:2383–2391. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
El-Salhy M, Gundersen D, Hatlebakk JG,
Gilja OH and Hausken T: Abnormal rectal endocrine cells in patients
with irritable bowel syndrome. Regul Pept. 188:60–65. 2014.
View Article : Google Scholar
|
|
64
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Low-grade inflammation in the rectum of patients with
sporadic irritable bowel syndrome. Mol Med Rep. 7:1081–1085. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
El-Salhy M, Gundersen D, Ostgaard H,
Lomholt-Beck B, Hatlebakk JG and Hausken T: Low densities of
serotonin and peptide YY cells in the colon of patients with
irritable bowel syndrome. Dig Dis Sci. 57:873–878. 2012. View Article : Google Scholar :
|
|
66
|
El-Salhy M, Lomholt-Beck B and Hausken T:
Chromogranin A as a possible tool in the diagnosis of irritable
bowel syndrome. Scand J Gastroenterol. 45:1435–1439. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
El-Salhy M, Mazzawi T, Gundersen D and
Hausken T: Chromogranin A cell density in the rectum of patients
with irritable bowel syndrome. Mol Med Rep. 6:1223–1225. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
El-Salhy M and Rauma J: Low density of
ghrelin cells in the oxyntic mucosa correlated to slow gastric
emptying in patients with type 1 diabetes. Mol Med Rep. 2:893–896.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
El-Salhy M, Vaali K, Dizdar V and Hausken
T: Abnormal small-intestinal endocrine cells in patients with
irritable bowel syndrome. Dig Dis Sci. 55:3508–3513. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
El-Salhy M, Wendelbo IH and Gundersen D:
Reduced chromogranin A cell density in the ileum of patients with
irritable bowel syndrome. Mol Med Rep. 7:1241–1244. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Simrén M, Månsson A, Langkilde AM,
Svedlund J, Abrahamsson H, Bengtsson U and Björnsson ES:
Food-related gastrointestinal symptoms in the irritable bowel
syndrome. Digestion. 63:108–115. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Monsbakken KW, Vandvik PO and Farup PG:
Perceived food intolerance in subjects with irritable bowel
syndrome - etiology, prevalence and consequences. Eur J Clin Nutr.
60:667–672. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Dainese R, Galliani EA, De Lazzari F, Di
Leo V and Naccarato R: Discrepancies between reported food
intolerance and sensitization test findings in irritable bowel
syndrome patients. Am J Gastroenterol. 94:1892–1897. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Heizer WD, Southern S and McGovern S: The
role of diet in symptoms of irritable bowel syndrome in adults: a
narrative review. J Am Diet Assoc. 109:1204–1214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Gibson PR and Shepherd SJ: Evidence-based
dietary management of functional gastrointestinal symptoms: the
FODMAP approach. J Gastroenterol Hepatol. 25:252–258. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Gibson PR: Food intolerance in functional
bowel disorders. J Gastroenterol Hepatol. 26(Suppl 3): 128–131.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Barrett JS and Gibson PR: Fermentable
oligosaccharides, disaccharides, monosaccharides and polyols
(FODMAPs) and nonallergic food intolerance: FODMAPs or food
chemicals? Therap Adv Gastroenterol. 5:261–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
de Roest RH, Dobbs BR, Chapman BA, Batman
B, O'Brien LA, Leeper JA, Hebblethwaite CR and Gearry RB: The low
FODMAP diet improves gastrointestinal symptoms in patients with
irritable bowel syndrome: a prospective study. Int J Clin Pract.
67:895–903. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Nanda R, James R, Smith H, Dudley CR and
Jewell DP: Food intolerance and the irritable bowel syndrome. Gut.
30:1099–1104. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Biesiekierski JR, Rosella O, Rose R, Liels
K, Barrett JS, Shepherd SJ, Gibson PR and Muir JG: Quantification
of fructans, galacto-oligosacharides and other short-chain
carbohydrates in processed grains and cereals. J Hum Nutr Diet.
24:154–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Muir JG, Rose R, Rosella O, Liels K,
Barrett JS, Shepherd SJ and Gibson PR: Measurement of short-chain
carbohydrates in common Australian vegetables and fruits by
high-performance liquid chromatography (HPLC). J Agric Food Chem.
57:554–565. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Muir JG, Shepherd SJ, Rosella O, Rose R,
Barrett JS and Gibson PR: Fructan and free fructose content of
common Australian vegetables and fruit. J Agric Food Chem.
55:6619–6627. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Gibson GR, Probert HM, Loo JV, Rastall RA
and Roberfroid MB: Dietary modulation of the human colonic
microbiota: updating the concept of prebiotics. Nutr Res Rev.
17:259–275. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Gibson GR, McCartney AL and Rastall RA:
Prebiotics and resistance to gastrointestinal infections. Br J
Nutr. 93(Suppl 1): 31–34. 2005. View Article : Google Scholar
|
|
85
|
Szajewska H, Ruszczyński M and Radzikowski
A: Probiotics in the prevention of antibiotic-associated diarrhea
in children: a meta-analysis of randomized controlled trials. J
Pediatr. 149:367–372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Cashman K: Prebiotics and calcium
bioavailability. Curr Issues Intest Microbiol. 4:21–32.
2003.PubMed/NCBI
|
|
87
|
Kleessen B and Blaut M: Modulation of gut
mucosal biofilms. Br J Nutr. 93(Suppl 1): 35–40. 2005. View Article : Google Scholar
|
|
88
|
Rafter J, Bennett M, Caderni G, Clune Y,
Hughes R, Karlsson PC, Klinder A, O'Riordan M, O'Sullivan GC,
Pool-Zobel B, et al: Dietary synbiotics reduce cancer risk factors
in polypectomized and colon cancer patients. Am J Clin Nutr.
85:488–96. 2007.PubMed/NCBI
|
|
89
|
Feinle-Bisset C and Azpiroz F: Dietary
lipids and functional gastrointestinal disorders. Am J
Gastroenterol. 108:737–747. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ong DK, Mitchell SB, Barrett JS, Shepherd
SJ, Irving PM, Biesiekierski JR, Smith S, Gibson PR and Muir JG:
Manipulation of dietary short chain carbohydrates alters the
pattern of gas production and genesis of symptoms in irritable
bowel syndrome. J Gastroenterol Hepatol. 25:1366–1373. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Shepherd SJ and Gibson PR: Fructose
malabsorption and symptoms of irritable bowel syndrome: guidelines
for effective dietary management. J Am Diet Assoc. 106:1631–1639.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Barrett JS, Gearry RB, Muir JG, Irving PM,
Rose R, Rosella O, Haines ML, Shepherd SJ and Gibson PR: Dietary
poorly absorbed, short-chain carbohydrates increase delivery of
water and fermentable substrates to the proximal colon. Aliment
Pharmacol Ther. 31:874–882. 2010.PubMed/NCBI
|
|
93
|
El-Salhy M, Gilja OH, Gundersen D,
Hatlebakk JG and Hausken T: Interaction between ingested nutrients
and gut endocrine cells in patients with irritable bowel syndrome
(Review). Int J Mol Med. 34:363–371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kassinen A, Krogius-Kurikka L, Mäkivuokko
H, Rinttilä T, Paulin L, Corander J, Malinen E, Apajalahti J and
Palva A: The fecal microbiota of irritable bowel syndrome patients
differs significantly from that of healthy subjects.
Gastroenterology. 133:24–33. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Si JM, Yu YC, Fan YJ and Chen SJ:
Intestinal microecology and quality of life in irritable bowel
syndrome patients. World J Gastroenterol. 10:1802–1805. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Talukder S: Effect of dietary fiber on
properties and acceptance of meat products: a review. Crit Rev Food
Sci Nutr. 55:1005–1011. 2015. View Article : Google Scholar
|
|
97
|
Bijkerk CJ, Muris JW, Knottnerus JA, Hoes
AW and de Wit NJ: Systematic review: the role of different types of
fibre in the treatment of irritable bowel syndrome. Aliment
Pharmacol Ther. 19:245–251. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Quartero AO, Meineche-Schmidt V, Muris J,
Rubin G and de Wit N: Bulking agents, antispasmodic and
antidepressant medication for the treatment of irritable bowel
syndrome. Cochrane Database Syst Rev. 18:CD0034602005.
|
|
99
|
Akehurst R and Kaltenthaler E: Treatment
of irritable bowel syndrome: a review of randomised controlled
trials. Gut. 48:272–282. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ford AC, Moayyedi P, Lacy BE, Lembo AJ,
Saito YA, Schiller LR, Soffer EE, Spiegel BM and Quigley EM: Task
Force on the Management of Functional Bowel Disorders American
College of Gastroenterology monograph on the management of
irritable bowel syndrome and chronic idiopathic constipation. Am J
Gastroenterol. 109(Suppl 1): S2–S26. 2014. View Article : Google Scholar
|
|
101
|
Parr NJ, Grime JS, Baxter JN, Critchley M
and Mackie CR: Small bowel resistances and the gastroduodenal
brake. Gut. 28:950–954. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
White CM, Poxon V and Alexander-Williams
J: Effects of nutrient liquids on human gastroduodenal motor
activity. Gut. 24:1109–1116. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Vidon N, Chaussade S, Merite F, Huchet B,
Franchisseur C and Bernier JJ: Inhibitory effect of high caloric
load of carbohydrates or lipids on human pancreatic secretions: a
jejunal brake. Am J Clin Nutr. 50:231–236. 1989.PubMed/NCBI
|
|
104
|
Vidon N, Pfeiffer A, Franchisseur C, Bovet
M, Rongier M and Bernier JJ: Effect of different caloric loads in
human jejunum on meal-stimulated and nonstimulated biliopancreatic
secretion. Am J Clin Nutr. 47:400–405. 1988.PubMed/NCBI
|
|
105
|
Cunningham KM, Daly J, Horowitz M and Read
NW: Gastrointestinal adaptation to diets of differing fat
composition in human volunteers. Gut. 32:483–486. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Boyd KA, O'Donovan DG, Doran S, Wishart J,
Chapman IM, Horowitz M and Feinle C: High-fat diet effects on gut
motility, hormone, and appetite responses to duodenal lipid in
healthy men. Am J Physiol Gastrointest Liver Physiol.
284:G188–G196. 2003. View Article : Google Scholar
|
|
107
|
Lin HC, Zhao XT and Wang L: Jejunal brake:
inhibition of intestinal transit by fat in the proximal small
intestine. Dig Dis Sci. 41:326–329. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Spiller RC, Trotman IF, Higgins BE, Ghatei
MA, Grimble GK, Lee YC, Bloom SR, Misiewicz JJ and Silk DB: The
ileal brake - inhibition of jejunal motility after ileal fat
perfusion in man. Gut. 25:365–374. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Salvioli B, Serra J, Azpiroz F and
Malagelada JR: Impaired small bowel gas propulsion in patients with
bloating during intestinal lipid infusion. Am J Gastroenterol.
101:1853–1857. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Simrén M, Abrahamsson H and Björnsson ES:
Lipid-induced colonic hypersensitivity in the irritable bowel
syndrome: the role of bowel habit, sex, and psychologic factors.
Clin Gastroenterol Hepatol. 5:201–208. 2007. View Article : Google Scholar
|
|
111
|
Caldarella MP, Milano A, Laterza F, Sacco
F, Balatsinou C, Lapenna D, Pierdomenico SD, Cuccurullo F and Neri
M: Visceral sensitivity and symptoms in patients with constipation-
or diarrhea-predominant irritable bowel syndrome (IBS): effect of a
low-fat intraduodenal infusion. Am J Gastroenterol. 100:383–389.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Gibson PR, Barrett JS and Muir JG:
Functional bowel symptoms and diet. Intern Med J. 43:1067–1074.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Windey K, De Preter V and Verbeke K:
Relevance of protein fermentation to gut health. Mol Nutr Food Res.
56:184–196. 2012. View Article : Google Scholar
|
|
114
|
Smith EA and Macfarlane GT: Dissimilatory
amino acid metabolism in human colonic bacteria. Anaerobe.
3:327–337. 1997. View Article : Google Scholar
|
|
115
|
Blachier F, Mariotti F, Huneau JF and Tomé
D: Effects of amino acid-derived luminal metabolites on the colonic
epithelium and physiopathological consequences. Amino Acids.
33:547–562. 2007. View Article : Google Scholar
|
|
116
|
Vazquez-Roque MI, Camilleri M, Smyrk T,
Murray JA, Marietta E, O'Neill J, Carlson P, Lamsam J, Janzow D,
Eckert D, et al: A controlled trial of gluten-free diet in patients
with irritable bowel syndrome-diarrhea: effects on bowel frequency
and intestinal function. Gastroenterology. 144:903–911. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Talley NJ: Dietary modification as a
treatment for irritable bowel syndrome. Gastroenterol Hepatol.
8:552–554. 2012.
|
|
118
|
Gibson PR and Shepherd SJ: Food choice as
a key management strategy for functional gastrointestinal symptoms.
Am J Gastroenterol. 107:657–667. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Sandström O and El-Salhy M: Ageing and
endocrine cells of human duodenum. Mech Ageing Dev. 108:39–48.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Sternini C, Anselmi L and Rozengurt E:
Enteroendocrine cells: a site of 'taste' in gastrointestinal
chemosensing. Curr Opin Endocrinol Diabetes Obes. 15:73–78. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Moran GW, Leslie FC, Levison SE,
Worthington J and McLaughlin JT: Enteroendocrine cells: neglected
players in gastrointestinal disorders? Therap Adv Gastroenterol.
1:51–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Moran-Ramos S, Tovar AR and Torres N:
Diet: friend or foe of enteroendocrine cells - how it interacts
with enteroendocrine cells. Adv Nutr. 3:8–20. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Buffa R, Capella C, Fontana P, Usellini L
and Solcia E: Types of endocrine cells in the human colon and
rectum. Cell Tissue Res. 192:227–240. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Barker N and Clevers H: Tracking down the
stem cells of the intestine: strategies to identify adult stem
cells. Gastroenterology. 133:1755–1760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Sandstrom O and El-Salhy M: Age-related
changes in neuroendocrine system of the gut. A possible role in the
pathogenesis of gastrointestinal disorders in the elderly.
Minireview based on a doctoral thesis. Ups J Med Sci. 106:81–97.
2001.
|
|
126
|
Barker N, van de Wetering M and Clevers H:
The intestinal stem cell. Genes Dev. 22:1856–1864. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
May CL and Kaestner KH: Gut endocrine cell
development. Mol Cell Endocrinol. 323:70–75. 2010. View Article : Google Scholar :
|
|
128
|
Korinek V, Barker N, Moerer P, van
Donselaar E, Huls G, Peters PJ and Clevers H: Depletion of
epithelial stem-cell compartments in the small intestine of mice
lacking Tcf-4. Nat Genet. 19:379–383. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Cheng H and Leblond CP: Origin,
differentiation and renewal of the four main epithelial cell types
in the mouse small intestine. V. Unitarian theory of the origin of
the four epithelial cell types. Am J Anat. 141:537–561. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Fontaine J, Le Lièvre C and Le Douarin NM:
What is the developmental fate of the neural crest cells which
migrate into the pancreas in the avian embryo? Gen Comp Endocrinol.
33:394–404. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Le Douarin NM and Teillet MA: The
migration of neural crest cells to the wall of the digestive tract
in avian embryo. J Embryol Exp Morphol. 30:31–48. 1973.PubMed/NCBI
|
|
132
|
Rawdon BB and Andrew A: Origin and
differentiation of gut endocrine cells. Histol Histopathol.
8:567–580. 1993.PubMed/NCBI
|
|
133
|
Hoffman J, Kuhnert F, Davis CR and Kuo CJ:
Wnts as essential growth factors for the adult small intestine and
colon. Cell Cycle. 3:554–557. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Höcker M and Wiedenmann B: Molecular
mechanisms of enteroendocrine differentiation. Ann N Y Acad Sci.
859:160–174. 1998. View Article : Google Scholar
|
|
135
|
Inokuchi H, Fujimoto S and Kawai K:
Cellular kinetics of gastrointestinal mucosa, with special
reference to gut endocrine cells. Arch Histol Jpn. 46:137–157.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Tolhurst G, Reimann F and Gribble FM:
Intestinal sensing of nutrients. Handb Exp Pharmacol. 209:309–335.
2012. View Article : Google Scholar
|
|
137
|
Lee J, Cummings BP, Martin E, Sharp JW,
Graham JL, Stanhope KL, Havel PJ and Raybould HE: Glucose sensing
by gut endocrine cells and activation of the vagal afferent pathway
is impaired in a rodent model of type 2 diabetes mellitus. Am J
Physiol Regul Integr Comp Physiol. 302:R657–R666. 2012. View Article : Google Scholar
|
|
138
|
Parker HE, Reimann F and Gribble FM:
Molecular mechanisms underlying nutrient-stimulated incretin
secretion. Expert Rev Mol Med. 12:e12010. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Raybould HE: Nutrient sensing in the
gastrointestinal tract: possible role for nutrient transporters. J
Physiol Biochem. 64:349–356. 2008. View Article : Google Scholar
|
|
140
|
San Gabriel A, Nakamura E, Uneyama H and
Torii K: Taste, visceral information and exocrine reflexes with
glutamate through umami receptors. J Med Invest. 56(Suppl):
209–217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Rudholm T, Wallin B, Theodorsson E,
Näslund E and Hellström PM: Release of regulatory gut peptides
somatostatin, neurotensin and vasoactive intestinal peptide by acid
and hyperosmolal solutions in the intestine in conscious rats.
Regul Pept. 152:8–12. 2009. View Article : Google Scholar
|
|
142
|
Sternini C: Taste receptors in the
gastrointestinal tract. IV. Functional implications of bitter taste
receptors in gastrointestinal chemosensing. Am J Physiol
Gastrointest Liver Physiol. 292:G457–G461. 2007. View Article : Google Scholar
|
|
143
|
Buchan AM: Nutrient tasting and signaling
mechanisms in the gut III. Endocrine cell recognition of luminal
nutrients. Am J Physiol. 277:G1103–G1107. 1999.PubMed/NCBI
|
|
144
|
Montero-Hadjadje M, Elias S, Chevalier L,
Benard M, Tanguy Y, Turquier V, Galas L, Yon L, Malagon MM,
Driouich A, et al: Chromogranin A promotes peptide hormone sorting
to mobile granules in constitutively and regulated secreting cells:
role of conserved N- and C-terminal peptides. J Biol Chem.
284:12420–12431. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Shooshtarizadeh P, Zhang D, Chich JF,
Gasnier C, Schneider F, Haïkel Y, Aunis D and Metz-Boutigue MH: The
antimicrobial peptides derived from chromogranin/secretogranin
family, new actors of innate immunity. Regul Pept. 165:102–110.
2010. View Article : Google Scholar
|
|
146
|
El-Salhy M, Seim I, Chopin L, Gundersen D,
Hatlebakk JG and Hausken T: Irritable bowel syndrome: the role of
gut neuroendocrine peptides. Front Biosci. 4:2783–2800. 2012.
|
|
147
|
Gunawardene AR, Corfe BM and Staton CA:
Classification and functions of enteroendocrine cells of the lower
gastrointestinal tract. Int J Exp Pathol. 92:219–231. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
El-Salhy M: The possible role of the gut
neuroendocrine system in diabetes gastroenteropathy. Histol
Histopathol. 17:1153–1161. 2002.PubMed/NCBI
|
|
149
|
Debas HT and Mulvihill SJ: Neuroendocrine
design of the gut. Am J Surg. 161:243–249. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Goyal RK and Hirano I: The enteric nervous
system. N Engl J Med. 334:1106–1115. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
McConalogue K and Furness JB:
Gastrointestinal neurotransmitters. Baillieres Clin Endocrinol
Metab. 8:51–76. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Gearry RB, Irving PM, Barrett JS, Nathan
DM, Shepherd SJ and Gibson PR: Reduction of dietary poorly absorbed
short-chain carbohydrates (FODMAPs) improves abdominal symptoms in
patients with inflammatory bowel disease-a pilot study. J Crohn's
Colitis. 3:8–14. 2009. View Article : Google Scholar
|
|
153
|
Halmos EP, Power VA, Shepherd SJ, Gibson
PR and Muir JG: A diet low in FODMAPs reduces symptoms of irritable
bowel syndrome. Gastroenterology. 146:67–75. 2014. View Article : Google Scholar
|
|
154
|
Shepherd SJ1, Parker FC, Muir JG and
Gibson PR: Dietary triggers of abdominal symptoms in patients with
irritable bowel syndrome: randomized placebo-controlled evidence.
Clin Gastroenterol Hepatol. 6:765–771. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Staudacher HM, Lomer MC, Anderson JL,
Barrett JS, Muir JG, Irving PM and Whelan K: Fermentable
carbohydrate restriction reduces luminal bifidobacteria and
gastrointestinal symptoms in patients with irritable bowel
syndrome. J Nutr. 142:1510–1518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Staudacher HM, Whelan K, Irving PM and
Lomer MC: Comparison of symptom response following advice for a
diet low in fermentable carbohydrates (FODMAPs) versus standard
dietary advice in patients with irritable bowel syndrome. J Hum
Nutr Diet. 24:487–495. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Wilder-Smith CH, Materna A, Wermelinger C
and Schuler J: Fructose and lactose intolerance and malabsorption
testing: the relationship with symptoms in functional
gastrointestinal disorders. Aliment Pharmacol Ther. 37:1074–1083.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Halmos EP, Christophersen CT, Bird AR,
Shepherd SJ, Gibson PR and Muir JG: Diets that differ in their
FODMAP content alter the colonic luminal microenvironment. Gut.
64:93–100. 2015. View Article : Google Scholar
|
|
159
|
Hustoft TN, Hausken T, Ystad SO, Valeur J,
Brokstad K, Hatlebakk JG and Lied GA: Effects of varying dietary
content of fermentable short-chain carbohydrates on symptoms, fecal
microenvironment, and cytokine profiles in patients with irritable
bowel syndrome. Neurogastroenterol Motil. 29:2017. View Article : Google Scholar
|
|
160
|
Böhn L, Störsrud S and Simrén M: Nutrient
intake in patients with irritable bowel syndrome compared with the
general population. Neurogastroenterol Motil. 25:23–30. 2013.
View Article : Google Scholar
|
|
161
|
Ligaarden SC, Lydersen S and Farup PG:
Diet in subjects with irritable bowel syndrome: a cross-sectional
study in the general population. BMC Gastroenterol. 12:612012.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Williams EA, Nai X and Corfe BM: Dietary
intakes in people with irritable bowel syndrome. BMC Gastroenterol.
11:92011. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Halpert A, Dalton CB, Palsson O, Morris C,
Hu Y, Bangdiwala S, Hankins J, Norton N and Drossman D: National
Survey on Patient Educational Needs in IBS and Development and
Validation of the Patient Educational Needs Questionnaire (PEQ):
What patients know about irritable bowel syndrome (IBS) and what
they would like to know. National Survey on Patient Educational
Needs in IBS and development and validation of the Patient
Educational Needs Questionnaire (PEQ). Am J Gastroenterol.
102:1972–1982. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
El-Salhy M and Gundersen D: Diet in
irritable bowel syndrome. Nutr J. 14:362015. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
El-Salhy M, Lillebø E, Reinemo A, Salmelid
L and Hausken T: Effects of a health program comprising
reassurance, diet management, probiotics administration and regular
exercise on symptoms and quality of life in patients with irritable
bowel syndrome. Gastroenterol Insights. 2:1–6. 2010. View Article : Google Scholar
|
|
166
|
Mazzawi T, Hausken T, Gundersen D and
El-Salhy M: Effects of dietary guidance on the symptoms, quality of
life and habitual dietary intake of patients with irritable bowel
syndrome. Mol Med Rep. 8:845–852. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Roalfe AK, Roberts LM and Wilson S:
Evaluation of the Birmingham IBS symptom questionnaire. BMC
Gastroenterol. 8:302008. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Patrick DL, Drossman DA, Frederick IO,
DiCesare J and Puder KL: Quality of life in persons with irritable
bowel syndrome: development and validation of a new measure. Dig
Dis Sci. 43:400–411. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Drossman DA, Patrick DL, Whitehead WE,
Toner BB, Diamant NE, Hu Y, Jia H and Bangdiwala SI: Further
validation of the IBS-QOL: a disease-specific quality-of-life
questionnaire. Am J Gastroenterol. 95:999–1007. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Talley NJ, Verlinden M and Jones M:
Quality of life in functional dyspepsia: responsiveness of the
Nepean Dyspepsia Index and development of a new 10-item short form.
Aliment Pharmacol Ther. 15:207–216. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Arslan G, Lind R, Olafsson S, Florvaag E
and Berstad A: Quality of life in patients with subjective food
hypersensitivity: applicability of the 10-item short form of the
Nepean Dyspepsia Index. Dig Dis Sci. 49:680–687. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Mazzawi T and El-Salhy M: Dietary guidance
and ileal enteroendocrine cells in patients with irritable bowel
syndrome. Exp Ther Med. 12:1398–1404. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Mazzawi T, Gundersen D, Hausken T and
El-Salhy M: Increased gastric chromogranin A cell density following
changes to diets of patients with irritable bowel syndrome. Mol Med
Rep. 10:2322–2326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Mazzawi T, Hausken T, Gundersen D and
El-Salhy M: Dietary guidance normalizes large intestinal endocrine
cell densities in patients with irritable bowel syndrome. Eur J
Clin Nutr. 70:175–181. 2016. View Article : Google Scholar :
|
|
175
|
Mazzawi T and El-Salhy M: Changes in
duodenal enteroendocrine cells in patients with irritable bowel
syndrome following dietary guidance. Exp Biol Med. 2017. View Article : Google Scholar
|
|
176
|
Deftos LJ: Chromogranin A: its role in
endocrine function and as an endocrine and neuroendocrine tumor
marker. Endocr Rev. 12:181–187. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Taupenot L, Harper KL and O'Connor DT: The
chromogranin-secretogranin family. N Engl J Med. 348:1134–1149.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Wiedenmann B and Huttner WB: Synaptophysin
and chromogranins/secretogranins - widespread constituents of
distinct types of neuroendocrine vesicles and new tools in tumor
diagnosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 58:95–121.
1989. View Article : Google Scholar
|
|
179
|
Mazzawi T and El-Salhy M: Changes in small
intestinal chromogranin A-immunoreactive cell densities in patients
with irritable bowel syndrome after receiving dietary guidance. Int
J Mol Med. 37:1247–1253. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Mazzawi T, Gundersen D, Hausken T and
El-Salhy M: Increased chromogranin a cell density in the large
intestine of patients with irritable bowel syndrome after receiving
dietary guidance. Gastroenterol Res Pract. 2015:8238972015.
View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Mazzawi T, Hausken T, Gundersen D and
El-Salhy M: Effect of dietary management on the gastric endocrine
cells in patients with irritable bowel syndrome. Eur J Clin Nutr.
69:519–524. 2015. View Article : Google Scholar :
|