Introduction
Fluid shear stress (FSS) is the blood flow-induced
force per unit area of the blood vessel walls, which results in the
vascular remodeling of blood vessels (1–4).
The type of FSS may be laminar and disturbed, and the endothelial
cell response varies with the type of FSS acting on the vessel wall
(5,6). Thus, vascular endothelial cells
undergo structural alterations to adapt to the blood flow-induced
FSS, thereby maintaining the homeostasis of the organ system
(7).
The uterus is a special organ that undergoes
periodic changes in blood vessels during the menstruation cycle and
pregnancy (8,9). Pregnancy is characterized by active
neovascularization and vascular remodeling, with a progressive
increase in the maternal blood volume (8,10).
There is a simultaneous increase in the blood flow to the uterus,
and the endometrial vessels experience vascular remodeling to
accommodate the increased blood flow (8,11).
Through vascular remodeling, the placenta that delivers maternal
blood to the fetus is formed stably (12–14). The failure in vascular remodeling
at this stage may be associated with pre-eclampsia and fetal growth
retardation. Thus, vascular remodeling at the early stage of
pregnancy is a necessary process for the development of the fetus
(15). Recent studies have shown
that endothelial cells may undergo changes in their morphologies
and cytoskeletal structures to adapt to FSS (16–18). Furthermore, vascular remodeling by
FSS is attributed to the expression of the vascular endothelial
growth factor receptor-3 (VEGFR-3) (18,19).
VEGFR-3 is a receptor of VEGF-C/D and a close
homolog of VEGFR-2 (20,21). It is predominantly expressed on
lymphatic cells and vessels (21). Studies have indicated that the
VEGF-C-mediated activation of VEGFR-3 results in the initiation of
lymph angiogenesis through proliferation and migration of lymphatic
cells (22,23). It has been reported that VEGFR-3
is expressed in the endometrium of the uterus (24) and that VEGFR-3 expression was
spatiotemporally similar to that of VEGFR-2 in the uterus during
pregnancy (25).
A recent study demonstrated that angiogenesis
associated with VEGFR-3 failed to occur in the absence of VEGFR-2
(26). In addition, it has been
reported that VEGFR-3-mediated FSS regulates the remodeling of the
arteries (18), suggestive of a
plausible role of VEGFR-2 and VEGFR-3 in the process of vascular
remodeling through the same or different stimulation. However, the
mechanisms underlying vascular remodeling mediated via VEGFR-3
expression in the uterus during pregnancy are not yet fully
understood.
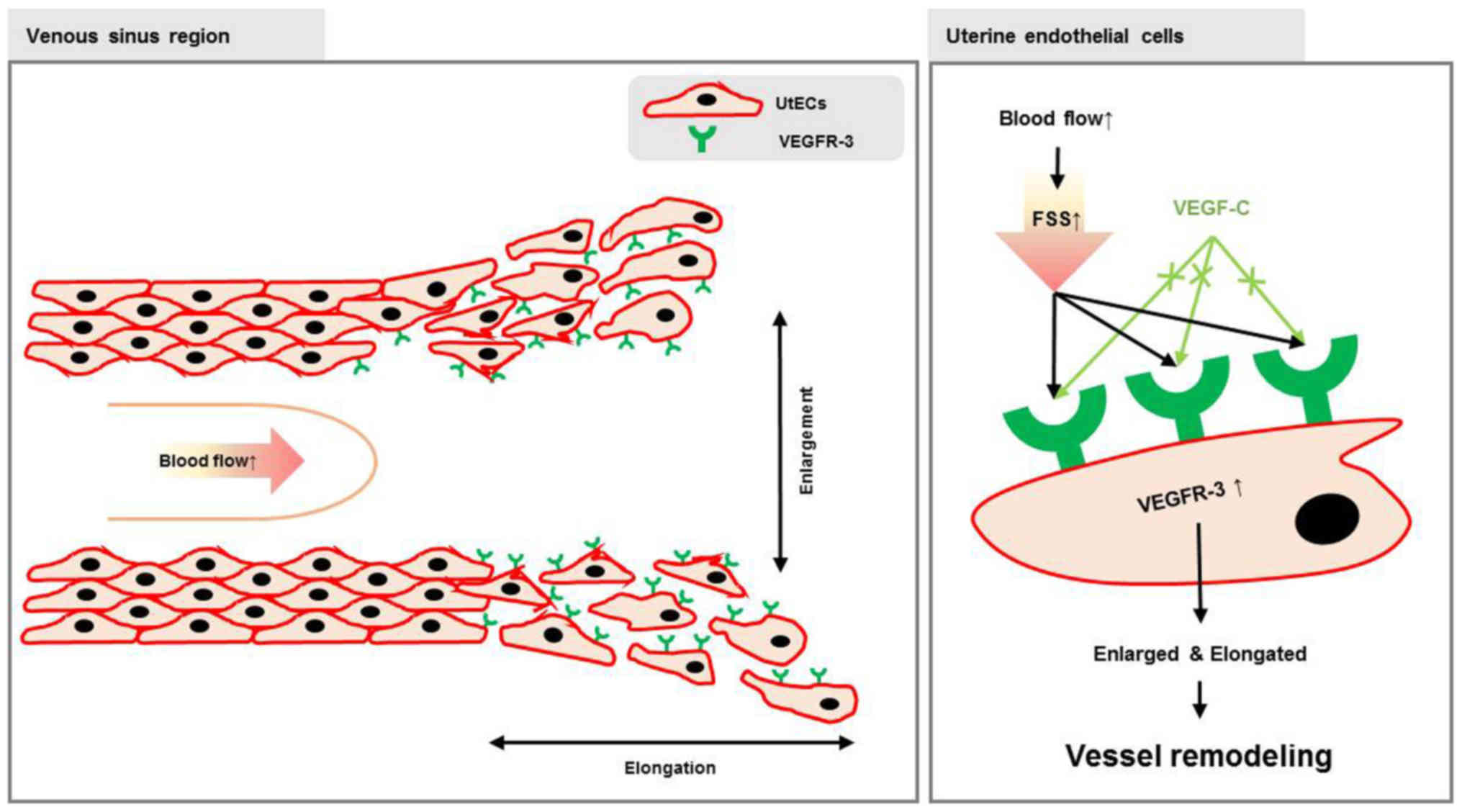
According to previous studies (18,26), we expected that VEGFR-3 expressed
during pregnancy may be associated with FSS. In this study, we
investigated the association between FSS and VEGFR-3, and the role
of VEGFR-3 in vascular remodeling in the uterus during pregnancy.
We demonstrate that VEGFR-3 is expressed in vascular endothelial
cells of the endometrium in the uterus during pregrancy. We used
lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1)
staining to determine the expression of VEGFR-3 in lymphatic cells
of the uterus. VEGFR-3 expression in the endometrium was distinct
from the region expressing LYVE-1, while its expression in the
myometrium coincided with the region expressing LYVE-1.
Furthermore, we investigated the response of endothelial cells to
FSS using an in vitro FSS model (19) and found that VEGFR-3 expression in
human uterine microvascular endothelial cells (HUtMECs) is
dependent on FSS.
Materials and methods
Mice
All animal experiments were performed following
approval from the Animal Care Committees of Chonbuk National
University, Iksan, Korea. Specific pathogen-free C57BL/6 mice were
purchased from the Samtako Bio Korea (Osan, Korea). All mice were
transferred and bred in pathogen-free animal facilities and fed a
standard diet (PMI Laboratory Diet, Richmond, IN, USA) and provided
with water ad libitum. Female mice (weighing 20 ± 1.25 g;
aged 7 weeks; n=40) and male mice (weighing 23 ± 0.83 g; aged 8
weeks; n=10) were used in this study; female mice were mated with
male mice. Copulation was indicated by the presence of vaginal
plugs the following morning, and the plug day was designated as 0.5
days post coitum (dpc). The animals were divided into 4 groups as
follows: the estrus non-pregnancy (ENP) group, and the 4.5, 6.5 and
8.5 dpc groups.
Histological analysis
The mice were sacrificed by the cervical dislocation
method on the indicated days. Segments of the uterus containing
implanted embryos were fixed in 4% paraformaldehyde (PFA) for 4 h,
followed by overnight dehydration in 20% sucrose solution.
Dehydrated samples were embedded with tissue-freezing medium
(Leica, Wetzlar, Germany) and the frozen blocks were cut into
20-µm-thick sections. The samples were blocked with 5%
donkey (or goat) serum in 0.03% Triton X-100 in phosphate-buffered
saline (PBST) and incubated for 4 h at room temperature with the
following primary antibodies: anti-CD31 (hamster, clone 2H8; cat.
no. MAB1398Z; Millipore, Temecula, CA, USA), anti-VEGFR-3 (goat
polyclonal; cat. no. AF743; R&D Systems, Minneapolis, MN, USA),
anti-VE-cadherin (mouse monoclonal; cat. no. sc-9989; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), anti-LYVE-1 (rabbit,
cat. no. 11-034; AngioBio, Del Mar, CA, USA), and anti-VEGF-C (goat
polyclonal; cat. no. sc-1881; Santa Cruz Biotechnology, Inc.).
Following incubation, the samples were washed 3–5 times and
incubated for 2 h at room temperature with the following secondary
antibodies: Cy3-conjugated anti-hamster IgG (127-165-160; Jackson
ImmunoResearch Laboratories, West Grove, PA, USA), fluorescein
isothiocyanate (FITC)-conjugated anti-mouse IgG (A90-216F; Bethyl
Laboratories, Montgomery, TX, USA), FITC-conjugated anti-goat IgG
(ab6881; Abcam, Cambridge, MA, USA) and Cy3-conjugated anti-rabbit
IgG (711-615-152; Jackson ImmunoResearch Laboratories). Nuclei were
stained with 4′,6-diamidino-2-phenylindole (DAPI). Samples were
mounted in fluorescent mounting medium (DAKO) and immunofluorescent
images acquired using a Zeiss LSM510 confocal fluorescence
microscope (Carl Zeiss, Oberkochen, Germany). The samples were
overnight fixed in 4% PFA for hematoxylin and eosin (H&E)
staining. The tissue was processed using standard procedures and
embedded in paraffin. Tissue blocks were cut into 3-µm-thick
sections and subjected to H&E staining.
Morphometric analysis
Densities and different sizes of blood vessels in
the uterus of pregnant mice were analyzed using photographic
analysis with ImageJ software (http://rsb.info.nih.gov/ij) and LSM Image Browser
(Carl Zeiss). The CD31-positive blood vessels were measured in the
venous sinus region (VSR) and presented as percentage per measured
area. Different sized blood vessels were measured in the
mesometrial region (MR) of the uterus at 6.5 and 8.5 dpc.
Cell culture
HUtMECs purchased from Lonza Group, Ltd. (Basel,
Switzerland) (CC-2564) were grown in endothelial cell growth medium
(EGM-2 MV BulletKit, CC-3202) and used at passage 3–4 in all the
experiments.
FSS model
The FSS model for in vitro experiments was
used as previously described (19). The shear stress across each
monolayer was approximated as the maximal wall shear stress as
follows: where α is the radius of orbital
rotation (1.25 cm), ϱ and η are the density (1.0 g/ml) and
viscosity (7.5×10−3 dynes/sec/cm) of the medium,
respectively, and f is the frequency of rotation
(rotations/sec). Using this equation, a shear stress of 10
dynes/cm2 was achieved at a rotating frequency of 91 rpm
(rotation/min), which was within the range of physiological shear
stress (0–20 dynes/cm2). HUtMECs at the same passage
stage and not subjected to shear stress were incubated in the same
incubator and served as the static controls. The alignment of the
HUtMECs was detected using a Nikon Eclipse TS 100 microscope with a
digital camera system under a 10X objective.
RNA extraction and reverse
transcription-quantitative (real-time) polymerase chain reaction
(RT-qPCR)
Total RNA was extracted from the uterus using
TRIzol® reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer's instructions. The extracted RNA (2
µg) was reverse transcribed into cDNA using SuperScript II
Reverse Transcriptase (Invitrogen). Quantitative PCR was performed
using Bio-Rad™ CFX96 Real-Time PCR detection system (Bio-Rad,
Hercules, CA, USA) with the following primers: VEGFR-3 forward,
5′-CCTGAAGAAGATCGCTGTTC-3′ and reverse, 5′-GAGAGCTGGTTCCTGGAGAT-3′;
and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; internal
control) forward, 5′-TGCCTCCTGCACCACCAACT-3′ and reverse,
5′-CGCCTGCTTCACCACCTTC-3′.
Western blot analysis
The uterus tissue and cells were homogenized in cold
radioimmunoprecipitation assay (RIPA) buffer supplemented with
protease inhibitor cocktail on the indicated days. Each protein was
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred onto nitrocellulose
membranes. After blocking with 5% skim milk, the membranes were
incubated overnight with goat polyclonal anti-VEGFR-3 (dilution
1:1,000; cat. no. sc-321; Santa Cruz Biotechnology, Inc.) and mouse
monoclonal anti-β-actin antibody (dilution 1:1,000; cat. no. A5441;
Sigma-Aldrich, St. Louis, MO, USA) at 4°C. Following incubation,
and then probed with horseradish peroxidase (HRP)-conjugated
secondary antibody [goat anti-mouse (dilution 1:5,000; cat. no.
ADI-SAB-100-J) and goat anti-rabbit (dilution 1:5,000; cat. no.
ADI-SAB-300); both from Enzo Life Sciences, Inc., Farmingdale, NY,
USA] for 2 h at room temperature and the signal developed with the
enhanced chemiluminescence HRP substrate (Millipore) was detected
using the Fusion FX7 acquisition system (Vilbert Lourmat,
Eberhardzell, Germany).
Immunocytochemistry
The HUtMECs were cultured on glass slides, fixed
with cold acetone, and blocked with 5% donkey serum in
Tris-buffered saline, 0.1% Tween-20 (TBST). The cells were
incubated with goat polyclonal anti-VEGFR-3 (goat polyclonal; cat.
no. AF743; R&D Systems) and mouse monoclonal anti-VE-cadherin
(mouse monoclonal; cat. no. sc-9989; Santa Cruz Biotechnology,
Inc.) at 4°C, followed by treatment with FITC-conjugated anti-goat
IgG (cat. no. ab6881; Abcam) and Cy3-conjugated anti-mouse IgG
(cat. no. 715-165-150; Jackson ImmunoResearch Laboratories). Nuclei
were stained with DAPI (cat. no. BML-AP402; Enzo Life Sciences,
Inc.). Samples were mounted in a fluorescent mounting medium and
immunofluorescent images were acquired using a Zeiss LSM510
confocal fluorescence microscope.
Statistical analysis
Values are presented as the means ± standard
deviation (SD). Statistical significance between groups was
determined using an unpaired Student's t-test or one-way analysis
of variance (ANOVA) followed by the Student-Newman-Keuls test.
Statistical significance was set at p<0.05 or p<0.01.
Results
The endometrium undergoes gradual
vascular remodeling during early pregnancy
We obtained a fertilized uterine sample to observe
morphological changes in the uterus during early pregnancy
(Fig. 1). According to the embryo
morphology, we divided the uterus into the MR and anti-mesometrial
region (AMR); MR was further subdivided into the uterus lumen (UL)
region and VSR. Immunofluorescence staining using CD31 antibody, an
endothelial cell marker, was performed to observe changes in blood
vessels in the uterus of pregnant mice. As shown in Fig. 1A, the changes in blood vessels
within the uterus of pregnant mice were observed. A higher
magnification revealed significant changes in the vascular diameter
and size of the VSR. The blood vessel density (BVD) in the VSR
gradually increased by 14.7 and 27.3% at 6.5 and 8.5 dpc,
respectively, as compared with that at 4.5 dpc (Fig. 1B). Within the MR, the number of
blood vessels with a diameter >300 µm increased, while
those with a diameter <300 µm decreased at 8.5 dpc
compared to 6.5 dpc (Fig. 1C). In
addition, significant morphological changes were observed in the
uterus during early pregnancy (Fig.
1D). There was a gradual increase in the proportion of embryos
in the uterus, and the vascular lumen of the MR became enlarged and
elonged. In particular, changes in the VSR within the uterus were
easily noticeable (Fig. 1E).
These results demonstrate that blood vessels in the endometrium of
uterus undergo neovascularization and vascular remodeling during
pregnancy.
 | Figure 1Changes (vascular remodeling)
occurring in the endometrium during pregnancy. (A) Images showing
CD31+ BVs in the uterus at ENP, 4.5, 6.5 and 8.5 days
post coitum (dpc). Scale bars, 500 µm. Each numbered
magnification image (square-dotted line) shows endometrial
CD31+ BVs in the VSR. Scale bars, 100 µm. (B)
Comparisons of CD31+ BV densities (BVD, %) in the VSR at
4.5, 6.5 and 8.5 dpc. Each group, n=5–6. *p<0.05 vs.
4.5 dpc; **p<0.01 vs. 4.5 dpc by unpaired t-test. (C)
Comparisons of numbers of different sized BVs in the MR at 6.5 and
8.5 dpc. Each group, n=5–6. *p<0.05 vs. 6.5 dpc;
**p<0.01 vs. 6.5 dpc by unpaired t-test. (D)
Cross-sectioned uterus from 6.5 to 8.5 dpc stained with hematoxylin
and eosin. Scale bars, 500 µm. (E) Magnified images showing
enlarged and elongated vascular lumen (arrowheads) in the uterus at
6.5 to 8.5 dpc. Scale bars, 100 µm. MR, mesometrial region;
AMR, anti-mesometrial region; UL, uterus lumen; VSR, venous sinus
region; Em, embryo; BVs, blood vessels. |
Expression of VEGFR-3 in the endometrium
gradually increases during pregnancy
It has been reported that vascular remodeling within
the uterus is controlled with progesterone-mediated VEGF-A/VEGFR-2
signaling (25). In addition, it
has been shown that angiogenesis associated with VEGF-3 fails to
occur in the absence of VEGFR-2 (26). Therefore, we hypothesized that
VEGFR-3 plays a role in the vascular remodeling of the uterus
during pregnancy. Our results revealed an increase in the
expression of VEGFR-3 in the uterus during the post-implantation
periods (Fig. 2C). In comparison
to the level observed 4.5 dpc, VEGFR-3 expression exhibited an
increase of approximately 60% at 8.5 dpc (Fig. 2D). Furthermore, VEGFR-3 was
predominantly expressed in the myometrium and MR of the endometrium
(Fig. 2A). In particular, VEGFR-3
exhibited a strong expression in the VSR and myometrium. VEGFR-3
expression in the endometrium coincided with a CD31-positive region
(Fig. 2B). These results suggest
that VEGFR-3 expression in the endometrium may be involved in
vascular remodeling during post-implantation periods.
VEGFR-3 expression in the endometrium is
not associated with lymphatic vasculature and is independent of
VEGF-C expression
VEGFR-3 is known to be strongly expressed in
lymphatic endothelial cells. Therefore, we evaluated the expression
of VEGFR-3 in lymphatic cells by immunofluorescence staining of
VEGFR-3 using LYVE-1 (a lymphatic endothelial cell marker)
(Fig. 3A). We confirmed VEGFR-3
expression in the region of the myometrium which stained positive
for LYVE-1. On the contrary, VEGFR-3 expression in the endometrium
failed to coincide with the region exhibiting LYVE-1 expression
(Fig. 3C). Furthermore, VEGF-C
and LYVE-1 were co-stained to evaluate the expression of VEGF-C, a
ligand for VEGFR-3, in the lymphatic endothelial cells of the
uterus (Fig. 3B and D). The
staining results confirmed that VEGF-C exhibited no detectable
expression in the endometrium. In addition, the protein expression
of VEGF-C was hardly detected (Fig.
3E). We confirmed the same results as those of a previous study
(25). Taken together, these
results demonstrate that VEGFR-3 is expressed in vascular
endothelial cells undergoing vascular remodeling during early
pregnancy and is independent of the expression of its ligand,
VEGF-C.
FSS induces VEGFR-3 expression in
HUtMECs
To determine whether VEGFR-3 expression in vascular
endothelial cells of the endometrium actually responds to FSS,
HUtMECs were used to observe the effects of FSS on vascular
endothelial cells. The FSS experiments were performed using the
method described in a previous study (19). Our results revealed that VEGFR-3
expression in the vascular endothelial cells was regulated by FSS.
Changes in the morphology of the HUtMECs were observed at varying
FSS strengths (Fig. 4A); an
increase in FSS resulted in the alignment of cells in the direction
of the flow. Cells subjected to direct FSS exhibited a strong
expression of VEGFR-3 around the nucleus and exhibited
morphological changes (Fig. 4F).
Furthermore, FSS increased the mRNA expression of VEGFR-3, as well
as its protein expression (Fig. 4B
and D). Based on the static controls, in the cells exposed to
FSS at 10 and 20 dyne/cm2, mRNA expression increased by
approximately 85.4 and 100.5%, respectively, and the protein
expression increased by approximately 12.9 and 85.8%, respectively
(Fig. 4C and E). In addition,
VEGF-C was not found to be expressed in the HUtMECs (Fig. 4G). These results confirm that the
vascular remodeling induced by FSS in vascular endothelial cells is
dependent on VEGFR-3 expression, but not on that of its ligand,
VEGF-C.
Discussion
Vascular remodeling is a process that occurs to
maintain the homeostasis of blood vessels by adapting to changes in
blood flow and is an essential process for the survival of vascular
endothelial cells on blood vessels (7). Thus, neovascularization and vascular
remodeling are frequently observed in developing tissues or organs
(8,9). The uterus is a special organ that
experiences periodic vascular remodeling due to menstruation
(8,10,11). The uterus exhibits marked
neovascularization and vascular remodeling during early pregnancy,
which promotes the stable development of the embryo (13,14). The failure of this process may be
associated with pre-eclampsia, miscarriage and fetal growth
restriction (25,27). Therefore, remodeling of the blood
vessels occurring in the early stages of pregnancy is a very
important process for embryonic development. During pregnancy, the
maternal body experiences increased levels of hormones, such as
estrogen and progesterone and increased body temperature and blood
flow (8,25). Due to changes in blood flow, the
blood vessel wall is subject to stimulations, such as increased
blood pressure and FSS, through which the blood vessel is remodeled
to adapt to the changed blood flow (3,4,17).
A previous study demonstrated that progesterone
governs vascular remodeling via VEGF-A/VEGFR-2 signaling during
early pregnancy (25). The
expression of VEGFR-3 in the uterus is spatiotemporally similar to
that of VEGF-2. However, the association between VEGFR-3 and
vascular remodeling during early pregnancy is not yet fully
understood. Recently, FSS has been shown to activate VEGFR-3 and
regulate vascular remodeling in a VEGFR-3-dependent manner
(18). In addition, it has been
demonstrated angiogenesis associated with VEGFR-3 failed to occur
in the absence of VEGFR-2 (26).
All these data suggest that VEGFR-3 may play an important role in
vascular remodeling within the uterus during pregnancy and that FSS
may regulate its expression. Thus, we hyopthesized that
VEGFR-3-related signaling may be involved in vascular
remodeling.
In this study, we examined the association between
FSS and VEGFR-3 expression in uterus of pregnant mice (Fig. 5). Prior to demonstrating our
prediction, we observed vascular remodeling within the uterus and
identified areas where vascular remodeling was actively occurring
(Fig. 1). As expected, VSR
characterized by active vascular remodeling exhibited a high
expression level of VEGFR-3 (Fig.
2). We confirmed VEGFR-3 expression in the uterus of pregnant
mice and observed an increase in its expression during
post-implantation periods (Fig.
2). Both blood and lymph vessels in the uterus were positive
for VEGFR-3 expression. VEGFR-3 expression in the myometrium
coincided with the region expressing LYVE-1; however, in the
endometrium, VEGFR expression was confirmed in the CD31-positive
region (Fig. 3A and C). VEGF-C
and LYVE-1 were co-stained to evaluate the expression of VEGF-C in
lymphatic endothelial cells of the uterus (Fig. 3B and D). As a result, it was
confirmed that VEGF-C was barely observed in the uterus. Our
results revealed that VEGFR-3 expression in vascular endothelial
cells of the endometrium was independent of VEGF-C expression. We
confirmed the effect of FSS on the expression of VEGFR-3 in
vascular endothelial cells using a previously described shear
stress model (19). Changes in
FSS increased the expression of VEGFR-3 and induced morphological
changes (cell elongation) in HUtMECs.
Taken together, both progesterone-mediated
VEGF-A/VEGFR-2 signaling and FSS-induced VEGFR-3 expression are
thought to be involved in the regulation of vascular remodeling in
the uterus during pregnancy. However, the correlation between
VEGFR-3 and vascular remodeling was not clarified clearly. Further
studies are required to focus on the relationship between VEGFR-3
and vascular remodeling. Nevertheless, our results indicated that
VEGFR-3 expression is observed in vascular endothelial cells of the
uterus and that blood flow-induced FSS contribute to the process of
vascular remodeling through the regulation of VEGFR-3 expression.
Thus, changes in blood flow can affect the intrauterine environment
and regulation of VEGFR-3 expression to induce vascular remodeling
may prevent pre-eclampsia, miscarriage and fetal growth
restriction.
Abbreviations:
|
FSS
|
fluid shear stress
|
|
HUtMECs
|
human uterine microvascular
endothelial cells
|
|
MR
|
mesometrial region
|
|
AMR
|
anti-mesometrial region
|
|
UL
|
uterus lumen
|
|
VSR
|
venous sinus region
|
|
Em
|
embryo
|
|
BVD
|
blood vessel density
|
Acknowledgements
This study was supported by the National Research
Foundation of Korea funded by the Ministry of Science, ICT and
Future Planning (2015R1A1A1A05001546, to J.-W.S.) and by the Korean
Institute of Planning and Evaluation for Technology in Food,
Agriculture, Forestry and Fisheries (IPET) through Agriculture,
Food and Rural Affairs Research Center Support Program, funded by
Ministry of Agriculture, Food and Rural Affairs (MAFRA;
716002-7).
References
|
1
|
Baeyens N, Bandyopadhyay C, Coon BG, Yun S
and Schwartz MA: Endothelial fluid shear stress sensing in vascular
health and disease. J Clin Invest. 126:821–828. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galie PA, Nguyen DH, Choi CK, Cohen DM,
Janmey PA and Chen CS: Fluid shear stress threshold regulates
angiogenic sprouting. Proc Natl Acad Sci USA. 111:7968–7973. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ghaffari S, Leask RL and Jones EA: Flow
dynamics control the location of sprouting and direct elongation
during developmental angiogenesis. Development. 142:4151–4157.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Davies PF: Hemodynamic shear stress and
the endothelium in cardiovascular pathophysiology. Nat Clin Pract
Cardiovasc Med. 6:16–26. 2009. View Article : Google Scholar
|
|
5
|
Chiu JJ and Chien S: Effects of disturbed
flow on vascular endothelium: Pathophysiological basis and clinical
perspectives. Physiol Rev. 91:327–387. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heo KS, Fujiwara K and Abe J: Shear stress
and atherosclerosis. Mol Cells. 37:435–440. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang J and Friedman MH: Adaptive response
of vascular endothelial cells to an acute increase in shear stress
frequency. Am J Physiol Heart Circ Physiol. 305:H894–H902. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Osol G and Moore LG: Maternal uterine
vascular remodeling during pregnancy. Microcirculation. 21:38–47.
2014. View Article : Google Scholar
|
|
9
|
Mandala M and Osol G: Physiological
remodelling of the maternal uterine circulation during pregnancy.
Basic Clin Pharmacol Toxicol. 110:12–18. 2012. View Article : Google Scholar
|
|
10
|
Nakamura H, Hosono T, Minato K, Hamasaki
T, Kumasawa K and Kimura T: Importance of optimal local uterine
blood flow for implantation. J Obstet Gynaecol Res. 40:1668–1673.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang L, Qiao J, Li R, Zhen X and Liu Z:
Role of endometrial blood flow assessment with color Doppler energy
in predicting pregnancy outcome of IVF-ET cycles. Reprod Biol
Endocrinol. 8:1222010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rai A and Cross JC: Development of the
hemochorial maternal vascular spaces in the placenta through
endothelial and vasculogenic mimicry. Dev Biol. 387:131–141. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sipos PI, Rens W, Schlecht H, Fan X,
Wareing M, Hayward C, Hubel CA, Bourque S, Baker PN, Davidge ST, et
al: Uterine vasculature remodeling in human pregnancy involves
functional macrochimerism by endothelial colony forming cells of
fetal origin. Stem Cells. 31:1363–1370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soares MJ, Chakraborty D, Kubota K, Renaud
SJ and Rumi MA: Adaptive mechanisms controlling uterine spiral
artery remodeling during the establishment of pregnancy. Int J Dev
Biol. 58:247–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roberts JM and Escudero C: The placenta in
preeclampsia. Pregnancy Hypertens. 2:72–83. 2012.PubMed/NCBI
|
|
16
|
Conway DE, Breckenridge MT, Hinde E,
Gratton E, Chen CS and Schwartz MA: Fluid shear stress on
endothelial cells modulates mechanical tension across VE-cadherin
and PECAM-1. Curr Biol. 23:1024–1030. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Steward R Jr, Tambe D, Hardin CC, Krishnan
R and Fredberg JJ: Fluid shear, intercellular stress, and
endothelial cell alignment. Am J Physiol Cell Physiol.
308:C657–C664. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baeyens N, Nicoli S, Coon BG, Ross TD, Van
den Dries K, Han J, Lauridsen HM, Mejean CO, Eichmann A, Thomas JL,
et al: Vascular remodeling is governed by a VEGFR3-dependent fluid
shear stress set point. eLife. 4:e046452015. View Article : Google Scholar :
|
|
19
|
dela Paz NG, Walshe TE, Leach LL,
Saint-Geniez M and D'Amore PA: Role of shear-stress-induced VEGF
expression in endothelial cell survival. J Cell Sci. 125:831–843.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shibuya M: Vascular endothelial growth
factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A
crucial target for anti- and pro-angiogenic therapies. Genes
Cancer. 2:1097–1105. 2011. View Article : Google Scholar
|
|
21
|
Davydova N, Harris NC, Roufail S,
Paquet-Fifield S, Ishaq M, Streltsov VA, Williams SP, Karnezis T,
Stacker SA and Achen MG: Differential receptor binding and
regulatory mechanisms for the lymphangiogenic growth factors VEGF-C
and VEGF-D. J Biol Chem. 291:27265–27278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rutkowski JM, Ihm JE, Lee ST, Kilarski WW,
Greenwood VI, Pasquier MC, Quazzola A, Trono D, Hubbell JA and
Swartz MA: VEGFR-3 neutralization inhibits ovarian
lymphangiogenesis, follicle maturation, and murine pregnancy. Am J
Pathol. 183:1596–1607. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Coso S, Bovay E and Petrova TV: Pressing
the right buttons: Signaling in lymphangiogenesis. Blood.
123:2614–2624. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Taylor A, Showeil R, Trivedi P,
Horimoto Y, Bagwan I, Ewington L, Lam EW and El-Bahrawy MA:
Expression profiling and significance of VEGF-A, VEGFR2, VEGFR3 and
related proteins in endometrial carcinoma. Cytokine. 68:94–100.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim M, Park HJ, Seol JW, Jang JY, Cho YS,
Kim KR, Choi Y, Lydon JP, Demayo FJ, Shibuya M, et al: VEGF-A
regulated by progesterone governs uterine angiogenesis and vascular
remodelling during pregnancy. EMBO Mol Med. 5:1415–1430. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zarkada G, Heinolainen K, Makinen T,
Kubota Y and Alitalo K: VEGFR3 does not sustain retinal
angiogenesis without VEGFR2. Proc Natl Acad Sci USA. 112:761–766.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saito S and Nakashima A: A review of the
mechanism for poor placentation in early-onset preeclampsia: The
role of autophagy in trophoblast invasion and vascular remodeling.
J Reprod Immunol. 101–102:80–88. 2014. View Article : Google Scholar
|