Introduction
Nonsyndromic complete or incomplete cleft of the lip
and palate is one of the most frequent congenital defects occurring
in humans. The incidence of this malformation is influenced by
ethnic origin, genetics and by environmental factors (1). This malformation bears the burden of
serious medical, aesthetic and psychosocial consequences. Notably,
a genetic link between this disorder and risk of cancer in the
affected person has been identified (2). Clinical studies have demonstrated
that an early surgical repair of the cleft lip results in the most
favourable outcome. The cleft should be reconstructed as soon as
possible, preferably during the first postnatal week. This strategy
appears to be successful in terms of improved healing, as well as
minimized scar formation (3–5).
However, even this very early timing of cheiloplasty is unable to
fully prevent future growth irregularities typical of this
malformation (6,7). The excellent results of early
cheiloplasty are not surprising, as the prenatal period (the first
two trimesters) is associated with the fully regenerative type of
healing. Fetal healing in the last trimester of pregnancy, as well
as early postnatal healing, is also notably rapid and associated
with minimal scar formation (8,9).
This marked difference between prenatal/neonatal and adult types of
wound healing was widely evaluated, and explained by reduced
readiness of fetuses and neonates to development of inflammation.
Interleukin-10 (IL-10) inhibiting the immune response is considered
the principal cytokine regulating immune response of this stage
(10–13).
However, the underlying mechanism of the
hypertrophic form of scarring remains unclear with the majority of
research focusing on the pro-fibrotic growth factor, transforming
growth factor-β1 (TGF-β1). TGF-β signaling is complex and context
dependent. The TGF-β family of growth factors is involved in
numerous processes in wound healing: Inflammation, angiogenesis
stimulation, fibroblast proliferation, collagen synthesis, and
deposition and remodelling of the new extracellular matrix. It has
been suggested that three known isoforms of TGF-β (−1, −2 and −3)
exert different temporal effects on wound healing and scarring, and
any disruption in their expression pattern may result in
hypertrophic scar formation (14). Fibroblasts from hypertrophic scars
indeed demonstrated increased TGF-β1 expression levels; however,
they were also demonstrated to exhibit prolonged expression of the
TGF-β receptors compared with normal skin, thus enhancing
downstream signaling and target gene activation (15). Interestingly, animals lacking SMAD
family member (Smad)3, a principal component of the signaling
cascade, demonstrated improved wound-healing (achieved by the
increased rate of re-epithelization and reduced infiltration of
monocytes) (16).
Scarless wound healing is also associated with a
reduction of the production of growth factors, which influence
fibroblasts and keratinocytes (17), and with changes in extracellular
matrix remodelling (18). Fetal
keratinocytes differ from their adult counterparts structurally and
functionally (19). Suspensions
of keratinocytes prepared from neonatal epidermis contain a pool of
very small keratinocytes (diameter, 4 µm) that express
vimentin (20). This size
determinant is a classical marker of clone-forming ability in human
keratinocytes (21). These cells
appear to be morphologically similar to very small stem cells
(22). The ability of small
keratinocytes isolated from the neonates to re-epithelise the
experimental defect in the confluent culture of epithelial cells
improves in vitro when compared with keratinocytes prepared
from adult donors (23).
The neonatal fibroblasts are highly active and
influence diverse cells in their environment. Notably, adult
keratinocytes acquire properties of neonatal keratinocytes,
including the occurrence of very small ones under their influence
(23). This result is supported
by the observation that the neonatal fibroblasts, as well as their
products, exert a distinct anti-fibrotic effect (19,24). This broad activity is to be
expected, as fibroblasts are drivers of the embryonic development
of epidermal appendages. Under conditions of normal development,
fibroblasts determine what the newly formed appendage structure
will be, such as hair, tooth, scale or feather (25). Under pathological conditions,
cancer-associated fibroblasts are drivers of the aggressiveness of
squamous epithelium-originated cancer. The crosstalk between
epithelial cells and fibroblasts over the course of wound healing
and in cancer are quite similar (26,27).
In the present study, the differences between
neonatal cleft lip fibroblasts (NCF) and older child cleft lip
fibroblasts (OCCF) were investigated, with emphasis on the cleft
lip repair strategy and prognosis. Our previous results (20,23) demonstrated a high expression level
of nestin, a marker of immature cells, and paradoxically also the
presence of smooth muscle actin (SMA), a marker of terminally
differentiated cells, in neonatal fibroblasts. Thus, a detailed
analysis of the expression levels of nestin and SMA was performed
in neonates. In addition, the neonatal dermal fibroblasts were
further investigated with a focus on possible overlap with a
molecular signature typical for neural crest-originated hair
follicle stem cells (20). This
aspect seems to be developmentally relevant, with respect to the
origin of (ecto-) mesenchymal cells from the neural crest in the
head region. These data include analysis of expression profiles of
neonatal, child, and adult fibroblasts as well as the fibroblasts
harvested from scars.
Materials and methods
Isolation of cells and cell culture
Dermal fibroblasts were isolated from residual skin
during cleft lip reconstructive surgery as follows: Neonates (age,
1–16 days), 24 samples; older children (age, 3 months-6 years), 8
samples; control samples of adults (age, 23–77 years), 8 samples (4
from the breast and 4 from the face); scars from the trunk of adult
patients (age, 38 and 78 years), 2 samples. The skin samples were
acquired at the Ear, Nose and Throat (ENT) Department of the
University Hospital in Motol and from the Department of Plastic
Surgery, University Hospital Královské Vinohrady (Prague, Czech
Republic). The samples of the scars were obtained from the
Department of Dermatovenerology, General University Hospital
(Prague, Czech Republic). All samples were collected under Local
Ethics Committee approval in accordance with the ethical standards
of the Institutional and National Research Committee, and according
to the 1964 Helsinki declaration and its later amendments or
comparable ethical standards. The informed consent was obtained
from all individual participants, or their legal representatives in
the case of minors, included in the present study. Certain samples
were cryoprotected using Tissue-Tek (Sakura Finetek Europe B.V.,
Zoeterwoude, The Netherlands) and frozen in liquid nitrogen. The 10
µm sections were prepared using CryoCut (Reichert-Jung,
Viena, Austria) and were used for immunohistochemistry. The
processing of skin samples for cell cultivation and the isolation
of fibroblast populations were described in detail by Krejčí et
al (20). Residual skin
samples were cut to very small sections (up to 1 mm3)
which were shortly digested in 0.25% trypsin (Sigma-Aldrich,
Prague, Czech Republic) for 20 min at 37°C and then transferred
into CellBIND 6-well plates (Corning, Schiphold Rijk, The
Netherlands). Skin explants were cultured in minimal volume of
Dulbecco's modified Eagle's medium (DMEM) supplemented with
antibiotics (penicillin 10 U/ml and streptomycin 100 µg/ml)
10% of fetal bovine serum (FBS) (all from Biochrom, Berlin,
Germany) and 100 µg/ml gentamycin (Sigma-Aldrich) at 37°C
and 5% CO2 in humidified incubator. Fibroblasts
emigrated from tissue samples were harvested by 1:1 v/v mixtures of
0.25% trypsin and 0.02% ethylenediaminetetraacetic acid (EDTA;
Biochrom, Berlin, Germany). Isolated fibroblasts were characterized
by their negativity for leukocyte marker, cluster of
differentiation (CD)45, melanocyte markers (melanoma antigen A and
HMB-45), keratins, and positivity for vimentin. The absence of
bacterial contamination was continuously controlled by
visualization under a microscope. The absence of mycoplasma was
verified by staining with 4′,6-diamidino-2-phenylindole (DAPI;
Merck KGaA, Darmstadt, Germany). Routinely, all fibroblast
populations were cultured in DMEM supplemented with antibiotics
(penicillin 10 U/ml and streptomycin 100 µg/ml) and 10% FBS
at 37°C and 5% CO2 in a humidified incubator.
Immunocytochemistry
Fibroblasts were inoculated at a density of 1,500
cells/cm2 on the coverslips, placed in 8-well dishes
(Nunc; Thermo Fisher Scientific, Inc., Waltham, Ma, USA) and
cultured in DMEM for 4 days (for nestin, octamerbinding
transcription factor 4 and Nanog) or to full confluence (generally
7 day for SMA). The culture medium was refreshed every two
days.
Fibroblasts that were adherent to coverslips were
fixed by 5% (w/v) paraformaldehyde in phosphate-buffered saline
(PBS; pH 7.2), washed three times with PBS and permeabilized with
0.05% Triton X-100 (Sigma-Aldrich; Merck KGaA). The non-specific
binding of immunoglobulins via Fc receptors was blocked by 3.3%
non-immune porcine serum (Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA). The employed antibodies are presented in Table I (antibody dilutions were
according to the supplier's instructions). The specificity of the
immunocytochemical reaction was evaluated by use of isotype
controls or irrelevant antibodies rather than specific antibodies.
The cell nuclei were counterstained with DAPI. The specimens were
mounted in Vectashield (Vector Laboratories, Ltd., Peterborough,
UK) and analyzed using an Eclipse 90i fluorescence microscope
(Nikon Corp., Tokyo, Japan) equipped with a ProgRes MF Cool camera
(Jenoptik Optical Systems GmbH, Jena, Germany) and NIS-Elements
AR4.40.00 computer-assisted image analysis system (Laboratory
Imaging, Prague, Czech Republic).
 | Table IAntibodies used for
immunohistochemical, immunocytochemical and western blot
analysis. |
Table I
Antibodies used for
immunohistochemical, immunocytochemical and western blot
analysis.
| Primary
antibody | Supplier
(location) | Secondary
antibody/fluorochrome | Supplier
(location) |
|---|
Nestin/M
immunohistochemistry
MAB5326; 1:200 | Merck KGaA
(Darmstadt, Germany) | Goat
anti-mouse/TRITC 115-025-044; 1:30 | Jackson
ImmunoResearch Laboratories, Inc. (West Grove, PA, USA) |
Nestin/P
HPA007007; 1:200
Oct-4/P
P0056; 1:200 | Sigma-Aldrich
(Merck KGaA; Darmstadt, Germany) | Swine
anti-rabbit/FITC F0205; 1:30 | Dako (Agilent
Technologies, Inc., Santa Clara, CA, USA) |
CD45/M
C7556; 1:50
Vimentin/M
M0725; 1:50 | Dako (Agilent
Technologies, Inc., Santa Clara, CA, USA) | Goat
anti-mouse/TRITC T5393; 1:30 | Sigma-Aldrich
(Merck KGaA; Darmstadt, Germany) |
Smooth muscle
actin/M
M0851; 1:50 | | | |
Nanog/M
AF1997; 1:15 | R&D Systems,
Inc. (Minneapolis, MN, USA) | Donkey
anti-goat/TRITC Sc-2094; 1:200 | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) |
Melan A/M
MA5-14168; 1:100
HMB-45 (anti-melanosome)/M 081050 ready to use antibody | Invitrogen (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) | Goat
anti-mouse/TRITC 115-025-044; 1:30 | Sigma-Aldrich
(Merck KGaA; Darmstadt, Germany) |
Wide spectrum
cytokeratin/P
Ab9377; 1:100 | Abcam (Cambridge,
UK) | Swine
anti-rabbit/FITC F0205; 1:30 | Dako (Agilent
Technologies, Inc., Santa Clara, CA, USA) |
α-Tubulin
(loading control)
T9026; 1:500 | Sigma-Aldrich
(Merck KGaA; Darmstadt, Germany) | Goat
anti-mouse/horseradish peroxidase Sc-516102; 1:2,000 | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) |
Western blot analysis
The cells of each fibroblast population were seeded
at a density of 1,000 cells/cm2 into 10-cm diameter
Petri dishes (Corning, Inc., Corning, NY, USA) and cultured for 7
days (95–100% confluence). The culture medium was refreshed every
two days. The cell lysates were harvested according to the standard
protocol in NP-40 cell lysis buffer (Thermo Fisher Scientific,
Inc.). The supernatant was collected into a fresh microtube
containing Protease Inhibitor Cocktail (Sigma-Aldrich; Merck KGaA).
The total protein concentration was detected using the Bradford
method for protein quantitation (28). Samples were resolved by
1-dimensional sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) gels according to standard techniques.
Equal quantities of total protein (10 µg) were subjected to
12% SDS-PAGE (electrophoresis for 60 min, 100 V, in cold room at
4°C) and transferred onto polyvinylidene difluoride membranes. The
membranes were subsequently blocked with 5% goat serum
(Sigma-Aldrich) for 1 h and probed with specific SMA primary
antibody (dilution, 1:1,000; overnight at 4°C), followed by the
appropriate horseradish peroxidase-conjugated secondary antibody
(dilution, 1:5,000; 60 min at room temperature). Proteins were
detected using the KPL TrueBlue blotting detection reagents
(BioVendor Laboratory Medicine, Inc., Brno, Czech Republic).
Detection of IL-6, IL-8 and TGF-β1 by
enzyme-linked immunosorbent assay (ELISA)
The cells of each fibroblast population were seeded
at a density of 30,000 cells/cm2 into two wells (two
technical replicates) of a 6-well plate (TPP Techno Plastic
Products AG, Trasadingen, Switzerland). The next day, the culture
medium was removed and 2.5 ml fresh DMEM was placed in each well.
After 24 h, the conditioned medium was collected (each replicate
separately), filtered through a 0.2-µm microstrainer to
remove the floating cells, divided into 500-µl aliquots and
stored at −20°C. Using Human IL-8, IL-6 and TGF-β1 kits (cat. nos.
El1008-1, El1006-1 and ET3102-1; BioVendor Laboratory Medicine
Inc.), the concentration of the proteins was detected according to
the producer's instructions. The final intensity of yellow
coloration was measured at a wavelength of 450 nm using a Universal
Microplate Reader (Bio-Tek Instruments, Inc., Winooski, VT,
USA).
Cell proliferation assay and TGF-β
treatment/inhibition
Fibroblasts from different donors [neonates with
cleft lip with (n=2) or without (n=2) family history; children,
n=2; adult facial (n=2)/breast (n=2) fibroblasts] were seeded at a
density of 750 cells/well in 96-well plates. To achieve full
adherence, cells were incubated at 37°C overnight in DMEM with 10%
FBS. The next day, the culture medium was changed to media
containing following active substances. TGF-β signaling blockade
was performed selectively using small drug inhibitor SB-431542
(Sigma-Aldrich) at a concentration of 10 µmol/l, which
completely abrogates phosphorylation of SMAD2 in fibroblasts
(29). The control cells were
maintained in DMEM plus 10% FBS. Recombinant human TGF-β1, TGF-β2
and TGF-β3 (all Sigma-Aldrich) were dissolved in 4 mM HCl with 0.1%
of bovine serum albumin at a concentration 10 ng/µl and, for
the experiments, they were used at a final concentration of 10
ng/ml DMEM. Cell proliferation was continuously monitored using an
IncuCyte ZOOM Kinetic live cell imaging system (Essen BioScience,
Ann Arbor, MI, USA) and measured as the phase object confluence
percentage. All experiments were performed in triplicate and
monitored for one week. Graphs were plotted and aligned (at 15%
confluence) in the R statistical environment. The growth of cells
was compared after 96 h, where the growth curves were in the log
phase.
Microarray analysis
The cells of each fibroblast population were seeded
at a density of 1,000 cells/cm2 into two 6-cm diameter
Petri dishes (Corning, Inc.) and cultured for 7 days (95–100%
confluence). The culture medium was changed every two days and 24 h
before harvest. The cells were washed twice with Dulbecco's PBS
(Biochrom GmbH, Berlin, Germany), and 350 µl buffer RLT
(Qiagen GmbH, Hilden, Germany) and 2-mercaptoethanol
(Sigma-Aldrich; Merck KGaA) was added. Using automatic pipette, the
cell lysates (two technical replicates of each population) were
collected into small Eppendorf tubes, immediately frozen and stored
at −80°C.
Total RNA was isolated using an RNeasy micro kit
(cat. no. 74004; Qiagen, Inc., Valencia, CA, USA) according to the
procedure for animal cells. The quantity of RNA was measured using
a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies; Thermo
Fisher Scientific, Inc.). The quality of the RNA was analyzed using
an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc.). The RNA
samples with an RNA integrity number >9 were used for further
analysis.
Illumina HumanHT-12 v4 chips (Illumina, Inc., San
Diego, CA, USA) were used for the microarray analysis according to
the standard protocol. Total RNA (150 ng) was amplified using an
Illumina TotalPrep RNA amplification kit (cat. no. AMIL1791;
Ambion; Thermo Fisher Scientific, Inc.), and 0.75 µg of the
amplified RNA was hybridized on the chips according to the
manufacturer's protocol. The analysis was performed in three
biological replicates for the adult scar fibroblast group, nine
biological (plus three technical) replicates for the adult breast
fibroblast group, five biological replicates for the adult facial
fibroblast group, seven biological (plus six technical) replicates
for the OCCF group, and 24 biological (plus 18 technical)
replicates for the NCF group.
The raw data was preprocessed using GenomeStudio
software (version 1.9.0.24624; Illumina, Inc.) and further analyzed
using the oligo (30) and limma
(31) packages of the
bioconductor (32) within the R
environment (33). Briefly, the
transcription profiles were background corrected using a
normal-exponential model, quantile normalized and variance
stabilized using base 2 logarithmic transformation. A moderated
t-test was used to detect differentially expressed transcripts
(after fitting a linear model I~group * gender within limma). A
Storey's q-value of <0.1 (34)
and a minimum of a 2-fold change in expression intensity were
required to consider genes as differentially transcribed. The
Minimum Information About a Microarray Experiment compliant data
was deposited to the ArrayExpress database (accession no.
E-MTAB-5385).
Gene set enrichment analysis (GSEA) was performed
using Fisher's exact test on gene sets defined by Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathways (35) and the terms of the Gene Ontology
(GO) (36). The gene sets were
compared with the set of differentially expressed genes in the
respective comparisons. Only the KEGG pathways (resp. GO terms)
with GSEA p<0.005, a minimal overlap of six genes, and odds
ratio >3 (resp. five) were considered to be statistically
significant.
Statistical analysis
Statistical analysis was performed using
Paleontological Statistics (version 3.14; University of Oslo, Oslo,
Norway) and the nonparametric Kruskal-Wallis test (by ranks) was
performed for pairwise comparisons. p<0.05 was considered to
indicate a statistically significant difference.
Results
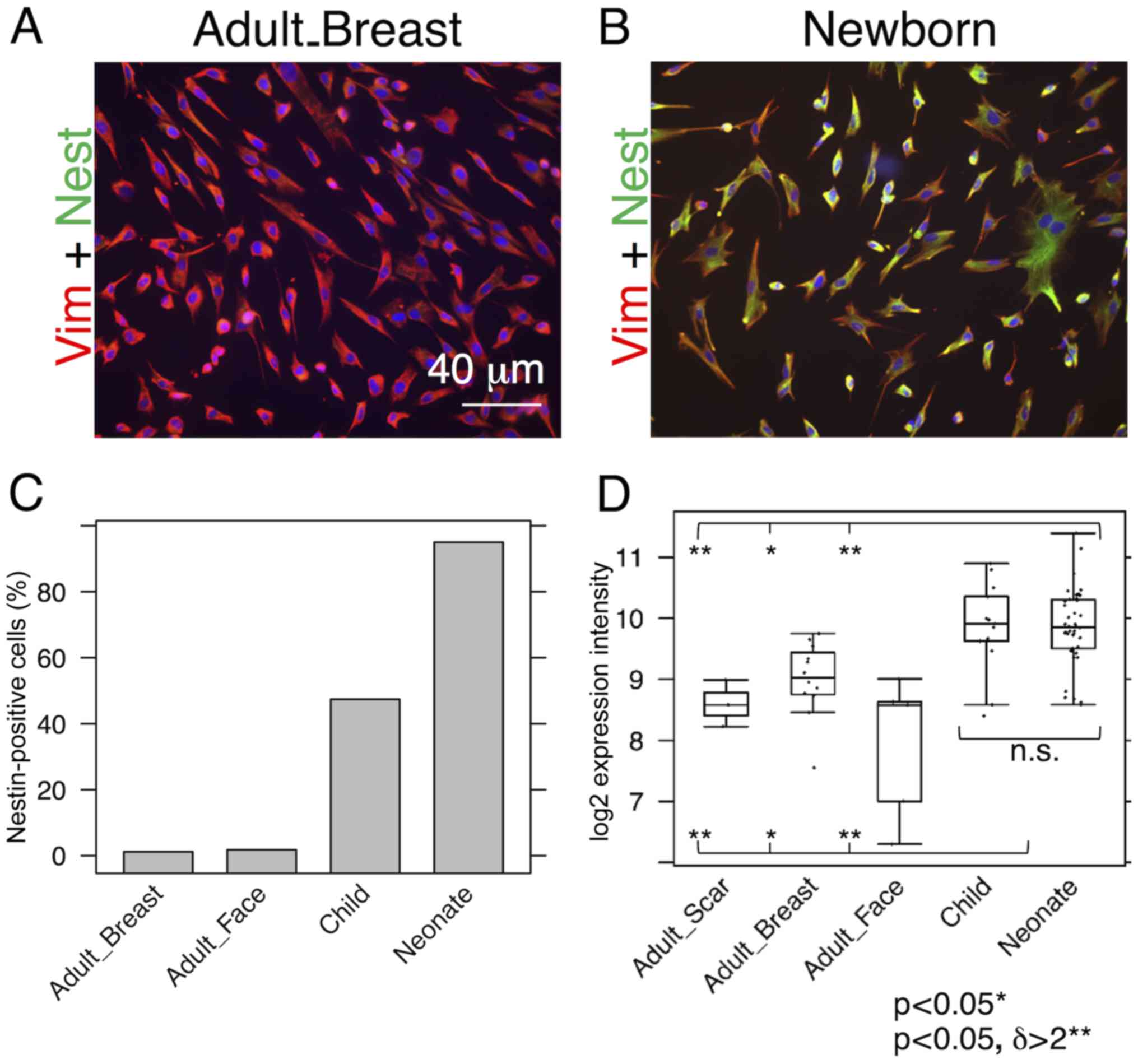
Nestin and SMA are expressed by neonatal
fibroblasts
While almost all NCF isolates exhibited distinct
signals for nestin, the signal in the OCCF group was strongly
reduced and the number of nestin-positive cells in the samples from
normal adult face or breast tissue was negligible (Fig. 1A–C). Similar profiles were
observed in the transcriptomics data (Fig. 1D) and confirmed in the sections of
the human dermis (Fig. 2). The
high incidence of fibroblasts expressing nestin in NCF populations
corresponded well with the presence of cells with nuclei that were
positive for pluripotency markers, Oct4 and Nanog (Fig. 3A–C). This was observed in cultures
with or without SMA exhibiting myofibroblasts. No obvious
correlation of nestin, Nanog homeobox and POU
class 5 homeobox 1 was detected in transcriptomic data
(Fig. 3D and E). In comparison to
the fibroblasts isolated from normal adult breast and face tissue
samples, the NCF and OCCF groups often exhibited signals for SMA,
as detected by immunocytochemistry (Fig. 4A and B). However, the expression
level of this protein was even stronger, as western blot analyses
of NCF lysates exhibited SMA even in the populations that were
morphologically negative for the presence of SMA in its typical
fibrillary pattern (also termed stress fibres) as detected by
immunofluorescence (Fig. 4C).
Overall, 50% of NCF and OCCF samples exhibited signals for
fibrillar SMA (Fig. 4D). The
transcriptomic activity of actin α2, smooth muscle, aorta, the gene
coding for SMA, was also elevated in the NCF and OCCF groups when
compared with adult cells, predominantly from the face (Fig. 4E). In addition, the NCF and OCCF
groups expressed a greater quantity of actin γ2, smooth muscle,
enteric when compared with adult breast or scar fibroblast samples
(Fig. 4F).
 | Figure 4Expression levels of SMA in (A) adult
breast and (B) neonatal fibroblasts as quantified by
immunohistochemistry and (C) WB analysis of representative neonatal
and adult fibroblasts. (D) Proportion of SMA-positive cells
detected by immunocytochemistry. (E) ACTA2 and (F)
ACTG2 gene activities are also demonstrated. Potential
statistical significance between tested types of fibroblasts was
marked by use of line segments connecting tested cell types;
**p<0.05 indicates a statistically significant
difference and a minimum of a 2-fold (δ>2) change. SMA, smooth
muscle actin; WB, western blotting; ACTA2, actin α2, smooth muscle,
aorta; ACTG2, actin γ2, smooth muscle, enteric; αTUB, α tubulin;
mRNA, messenger RNA. |
Expression levels of IL-6 and IL-8 differ
between fibroblast groups
As it is known that the transition of fibroblasts to
SMA-positive myofibroblasts is strongly stimulated by TGF-β1
(37), this cytokine was measured
by ELISA in the media conditioned by all of the investigated
fibroblasts. No significant differences between the NCF and OCCF
groups, as well as the adult fibroblasts were identified in the
expression level of TGF-β1 (data not shown). IL-6, IL-8 and C-X-C
motif chemokine ligand-1 (CXCL-1) are produced by fibroblasts
during the course of wound healing, as well as by cancer-associated
fibroblasts and they influence the low differentiation status of
normal human keratinocytes (38).
Production of IL-6 by NCF was significantly higher than by adult
fibroblasts (Fig. 5A). No
difference in production of IL-8 among the investigated cell types
was observed (Fig. 5C). In the
case of CXCL-1, none of the evaluated fibroblast types produced
detectable quantities of this chemokine to the medium (data not
shown). At the transcript level, NCF and, in particular, OCCF
transcribed the IL-6 gene more than the adult breast
fibroblasts [3-fold upregulated; (p<0.05) (Fig. 5B)]. By contrast, the IL-8
gene was expressed to a lesser extent in the NCF and OCCF groups
when compared with in adult breast fibroblasts [threefold
downregulated; (p<0.05, resp. p=0.06) (Fig. 5D)]. No change in CXCL1 expression
was detected.
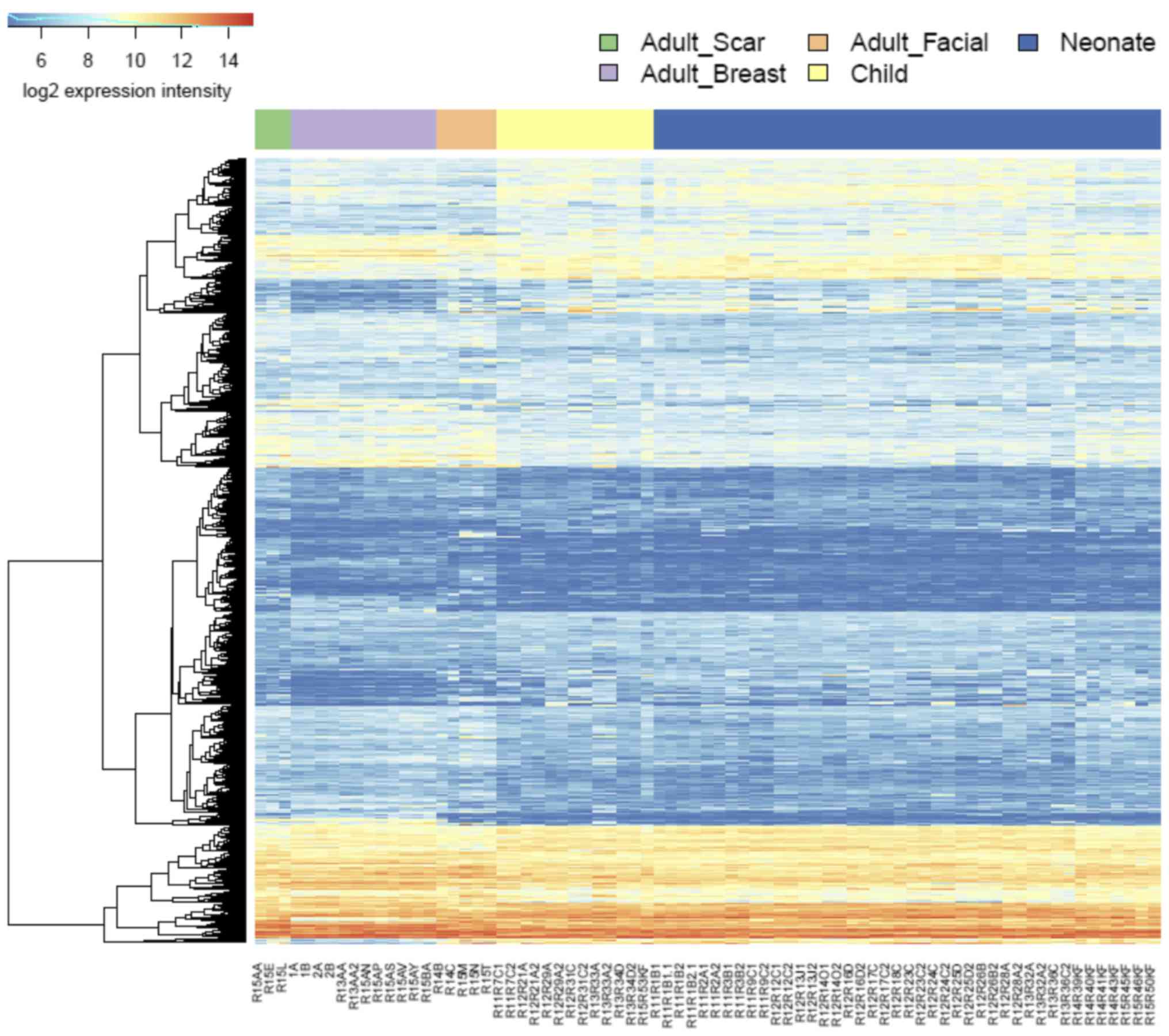
Transcription profiling reveals the
involvement of TGF-β signaling
NCF group differed from adult cells isolated from
the face in transcription activity of hundreds of genes. Similar
differences were identified between the OCCF group and fibroblasts
prepared from adult face skin, and in comparison of NCF and OCCF
groups, respectively, to the fibroblasts isolated from the chest
(Fig. 6; supplementary data,
https://www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-5385/).
While the changes between the NCF and OCCF groups, and adult breast
cells were dominated by the changes in signaling pathways, the
differences between the NCF and OCCF groups, and adult facial
fibroblasts were dominated by the changes in metabolic pathways
(https://www.ebi.ac.uk/array-express/experiments/E-MTAB-5385/).
This fact reflects the common ontological origin of the facial
cells (NCF, OCCF and adult facial fibroblasts), which differs from
the adult breast fibroblast. It is widely accepted that facial
mesenchyme is originated from the neural crest (termed
ectomesenchyme) and so it differs, concerning its origin, from
fibroblasts in other parts of the body (25). The transcriptomic analysis indeed
demonstrated a difference between fibroblasts prepared from the
face and breast in 364 genes (https://www.ebi.ac.uk/array-express/experiments/E-MTAB-5385/).
The differentially expressed genes included members of the homeobox
gene family (Fig. 7) (39). Their expression intensity was
independent of the disease/cleft status.
Among the most dysregulated KEGG pathways, which
distinguished the adult samples and neonate/child samples were
advanced glycation endproducts-receptor for advanced glycation
endproducts (AGE-RAGE) signaling (hsa04933), TGF-β signaling
(hsa04350) and TNF signaling pathway (hsa04668). Closer analysis of
the AGE-RAGE signaling pathway revealed that the changes in this
pathway accumulate in its TGF-β module (TGF-β2, TGF-β3 and TGF-βR2)
(Fig. 8) and
inflammation-associated modules (IL-8, matrix metalloproteinase-2
and vascular endothelial growth factor A). Thus, there was
significant enrichment of differentially expressed genes involved
in TGF-β signaling. These findings were supported by the GSEA
analysis on the GO terms. In the comparison of adult facial
fibroblasts to nCF, pronounced changes were observed in the term,
GO:0071604, TGF-β production, with large changes in
transcription of the genes CD2 associated protein (CD2AP),
CD34 molecule (CD34), hypoxia inducible factor 1α subunit
(HIF1A), CD24 molecule (CD24),
prostaglandin-endoperoxide synthase 2 (PTGS2) and MET
proto-oncogene, receptor tyrosine kinase (MET).
Additionally, GO term analysis revealed shared changes in the
expression of genes involved in skeletal development, mesenchyme
proliferation and microtubule polymerization. Adult breast
fibroblasts differed from the neonatal and child facial fibroblasts
in the expression of genes connected to chemotaxis and organ
development, including heart morphogenesis and neural crest
development, (https://www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-5385/).
The latter of the above-mentioned changes were also observed in the
comparison of adult breast and facial fibroblasts. This was
expected for the neural crest genes; however, the observation of
changes in organs (for example, heart development) were not
anticipated. The largest contribution to detection of heart
development by GSEA had the heart and neural crest derivatives
expressed 2 (Hand2) gene, a transcription factor involved in the
development of various organs. Notably, the Hand2 gene was
associated with cleft palate in mice (40). No significant dysregulation of the
GO terms associated with TNF signaling was observed, which may have
been caused by a lack of statistical strength. TGF-β signaling,
however, reoccurred in the GSEA analyses on the GO terms and KEGG
pathways; therefore, this pathway became the study focus (Fig. 9).
 | Figure 9Kyoto Encyclopedia of Genes and
Genomes and TGF-β signaling pathway with changes between adult
facial fibroblasts and neonate fibroblasts denoted by color code.
TGF-β family member binds to the type II receptor and recruits type
I, whereby type II receptor phosphorylates and activates type I.
The type I receptor, in turn, phosphorylates receptor-activated
Smads (R-Smads: Smad1, Smad2, Smad3, Smad5 and Smad8). Once
phosphorylated, R-Smads associate with the co-mediator smad, smad4
and the heteromeric complex then translocates into the nucleus. In
the nucleus, smad complexes activate specific genes via cooperative
interactions with other DNA-binding and coactivator (or
co-repressor) proteins. TGF-β, transforming growth factor-β; Smad,
SMAD family member. |
Virtually no differences between the
transcription of genes in NCF and OCCF were observed
The NCFs significantly differed from fibroblasts
prepared from scar located on the body in 994 genes and a similar
difference (807 genes) was observed during the comparison of OCCFs
and adult scar fibroblasts (Fig.
6). The GSEA analysis results were similar to the comparison of
the adult fibroblasts to NCF and OCCF, with a notable exception of
the p53 signaling pathway and apoptosis (sestrin 1, cyclin
dependent kinase inhibitor 2A and
phorbol-12-myristate-13-acetate-induced protein 1). Finally,
only subtle differences between NCF and OCCF were observed: only
two genes were significantly different between these fibroblasts
and only one of them was protein-coding (structural maintenance
of chromosomes 6).
Differences in sensibility to TGF-β
inhibition
TGF-β signaling was determined as the most likely
difference between adult and child fibroblasts. Furthermore, a high
incidence of myofibroblasts was observed in cultures of NCFs and
OCCFs, which supported the decision to focus the study on the TGF
family. No differences in the expression level of TGF-β1 were
observed; however, the activity of TGF-β2, TGF-β3, and receptor
TGF-βR2 in the NCF and OCCF groups appears to be functionally
relevant (Fig. 8A–C). In NCFs and
OCCFs, the activity of the SMAD3 gene, a product of which is
downstream of the TGF-β signaling cascade, supported this finding
(Fig. 8E). Certain differences
between adult fibroblasts obtained from breast and facial samples
were observed, namely in the case of TGF-β3 and TGF-α (Fig. 8B and D). In addition, changes in
the transcriptional activity of the genes that likely regulate
TGF-β signaling (CD2AP, CD34, HIF1A,
CD24, PTGS2 and MET) were observed. The role
of TGF-β3 and inhibitor of TGF-β signaling in the growth of NCFs
and OCCFs, and adult fibroblasts was also analyzed. The results
demonstrated that TGF-β3 exerted no influence on the cell culture
growth; however, the adult cells and OCCF were sensitive to TGF-β
receptor inhibition. NCF were insensitive in this case (Fig. 10).
Discussion
The observation that NCF and OCCF strongly express
SMA, and are therefore regarded as myofibroblasts, represents the
main finding of the present study. Myofibroblasts frequently
originate as a result of the transition of local fibroblasts in
various locations. This transition appears to be triggered by an
excess of TGF-β (37,41,42). Myofibroblasts also represent the
common element of tumour stroma, and positively influence tumour
growth and spread. Furthermore, similar cells are present in
granulation tissue of the wound and influence the process of
healing, including wound contraction (27). The observations at mRNA level from
the present study demonstrate dysregulation of TGF-β signaling with
upregulation of transcripts for TGF-β2 and TGF-β3, and
downregulation of receptor, TGF-βR2. Etiopathogenesis of the
orofacial cleft is associated with aberrant TGF-β signaling
(43–45). In addition, TGF-β appears to
influence the phenotype of fibroblasts isolated from cleft palate
(46). Low production of IL-6 and
IL-8 is associated with scarless wound healing and a particularly
low secretion of IL-6 and IL-8 is a specific feature of normal
neonatal fibroblasts (47,48).
This data indicates that NCFs and OCCFs are influenced by the cleft
status.
Expression profiles of fibroblasts isolated from the
breast and face are distinct, this difference is maintained despite
the orofacial cleft status and age of the donor. The facial
fibroblasts originate from neural crest derived ectomesenchyme and,
in comparison with fibroblasts of other origin and different
nature, they are quite devoid of classical HOX gene
expression (49–51). The present analysis demonstrated
low activity of a subgroup of homeobox genes in facial fibroblasts
when compared with fibroblasts from the breast tissue.
Based upon the analysis performed in the present
study, the boundary between improved and almost scarless healing as
referred to by aesthetic surgeons in cleft lip restoration
(52) was not defined. However,
the therapeutic window may be larger or site specific, as
demonstrated by regeneration of the digit tip (53). The expression profiles of NCFs and
OCCFs are practically identical and it must be noted that they
differ from normal fibroblasts due to the high incidence of
SMA-positive fibroblasts/myofibroblasts, typical of wound healing
and stromal cancer-associated fibroblasts (27). Similar profiles of cleft palate
and cancer have also been observed by others (54). Cancer-associated fibroblasts
produce IL-6 and IL-8, which influence differentiation patterns of
human keratinocytes to elements with low differentiation status
(38). NCF/OCCF also produce
these factors, and secrete TGF-β3 and exhibit a reduced quantity of
TGF-βR2, which is associated with anti-fibrotic and
anti-myofibroblast formation activity (55–59).
In conclusion, fibroblasts isolated from the cleft
lip, as the most frequent congenital malformation of the face,
reflect the disease status. Expression levels of nestin and changes
in TGF-β signaling, which influence SMA expression levels in
fibroblasts, participate in improved wound healing following
neonatal cheiloplasty. Furthermore, the neural crest
ectomesenchyme-derived fibroblasts significantly differ from
fibroblasts isolated from the chest.
Acknowledgments
The authors would like to thank Marie Jindráková and
Šárka Kocourková, MSc, for their technical assistance. The present
study was supported by the Grant Agency of the Czech Republic
(grant. no. P304-13-20293S), Charles University (project of
Specific University research and PROGRES Q28/F1 and UNCE 204013),
Ministry of Education, Youth and Sports of CR within the National
Sustainability Program II (project BIOCEV-FAR; registration no.
LQ1604), and project BIOCEV (grant no. CZ.1.05/1.1.00/02.0109). The
present study is a result of the following project implementation:
The equipment for metabolomic and cell analyses (grant no.
CZ.1.05/2.1.00/19.0400) supported by the Research and Development
for Innovations operational Program, co-financed by the European
regional development fund and the state budget of the Czech
Republic. Access to computing and storage facilities, owned by
parties and projects contributing to the National Grid
Infrastructure MetaCentrum, were provided under the program
Projects of Large Research, Development and Innovations
Infrastructures (CESNET LM2015042) was greatly appreciated.
References
|
1
|
Panamonta V, Pradubwong S, Panamonta M and
Chowchuen B: Global Birth Prevalence of Orofacial Clefts: A
Systematic Review. J Med Assoc Thai. 98(Suppl 7): S11–S21.
2015.
|
|
2
|
Dunkhase E, Ludwig KU, Knapp M, Skibola
CF, Figueiredo JC, Hosking FJ, Ellinghaus E, Landi MT, Ma H,
Nakagawa H, et al: Nonsyndromic cleft lip with or without cleft
palate and cancer: Evaluation of a possible common genetic
background through the analysis of GWAS data. Genom Data. 10:22–29.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Galinier P, Salazard B, Deberail A,
Vitkovitch F, Caovan C, Chausseray G, Acar P, Sami K, Guitard J and
Smail N: Neonatal repair of cleft lip: A decision-making protocol.
J Pediatr Surg. 43:662–667. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harris PA, Oliver NK, Slater P, Murdoch L
and Moss AL: Safety of neonatal cleft lip repair. J Plast Surg Hand
Surg. 44:231–236. 2010. View Article : Google Scholar
|
|
5
|
Borský J, Velemínská J, Jurovčík M, Kozák
J, Hechtová D, Tvrdek M, Černý M, Kabelka Z, Fajstavr J, Janota J,
et al: Successful early neonatal repair of cleft lip within first 8
days of life. Int J Pediatr Otorhinolaryngol. 76:1616–1626. 2012.
View Article : Google Scholar
|
|
6
|
Dadáková M, Cagáňová V, Dupej J,
Hoffmannová E, Borský J and Velemínská J: Three-dimensional
evaluation of facial morphology in pre-school cleft patients
following neonatal cheiloplasty. J Craniomaxillofac Surg.
44:1109–1116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hoffmannova E, Bejdová Š, Borský J, Dupej
J, Cagáňová V and Velemínská J: Palatal growth in complete
unilateral cleft lip and palate patients following neonatal
cheiloplasty: Classic and geometric morphometric assessment. Int J
Pediatr Otorhinolaryngol. 90:71–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coolen NA, Schouten KCWM, Boekema BKHL,
Middelkoop E and Ulrich MMW: Wound healing in a fetal, adult, and
scar tissue model: A comparative study. Wound Repair Regen.
18:291–301. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yates CC, Hebda P and Wells A: Skin wound
healing and scarring: Fetal wounds and regenerative restitution.
Birth Defects Res C Embryo Today. 96:325–333. 2012. View Article : Google Scholar
|
|
10
|
Kieran I, Knock A, Bush J, So K, Metcalfe
A, Hobson R, Mason T, O'Kane S and Ferguson M: Interleukin-10
reduces scar formation in both animal and human cutaneous wounds:
Results of two preclinical and phase II randomized control studies.
Wound Repair Regen. 21:428–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gourevitch D, Kossenkov AV, Zhang Y, Clark
L, Chang C, Showe LC and Heber-Katz E: Inflammation and its
correlates in regenerative wound healing: An alternate perspective.
Adv Wound Care (New Rochelle). 3:592–603. 2014. View Article : Google Scholar
|
|
12
|
King A, Balaji S, Le LD, Crombleholme TM
and Keswani SG: Regenerative wound healing: The role of
interleukin-10. Adv Wound Care (New Rochelle). 3:315–323. 2014.
View Article : Google Scholar
|
|
13
|
Walraven M, Talhout W, Beelen RHJ, van
Egmond M and Ulrich MM: Healthy human second-trimester fetal skin
is deficient in leukocytes and associated homing chemokines. Wound
Repair Regen. 24:533–541. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu L, Saulis AS, Liu WR, Roy NK, Chao JD,
Ledbetter S and Mustoe TA: The temporal effects of anti-TGF-beta1,
2, and 3 monoclonal antibody on wound healing and hypertrophic scar
formation. J Am Coll Surg. 201:391–397. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmid P, Itin P, Cherry G, Bi C and Cox
DA: Enhanced expression of transforming growth factor-beta type I
and type II receptors in wound granulation tissue and hypertrophic
scar. Am J Pathol. 152:485–493. 1998.PubMed/NCBI
|
|
16
|
Ashcroft GS, Yang X, Glick AB, Weinstein
M, Letterio JL, Mizel DE, Anzano M, Greenwell-Wild T, Wahl SM, Deng
C, et al: Mice lacking Smad3 show accelerated wound healing and an
impaired local inflammatory response. Nat Cell Biol. 1:260–266.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dang CM, Beanes SR, Soo C, Ting K, Behaim
P, Hendrick MH and Lorenz HP: Decreased expression of growth factor
isoforms and receptors during scarless repair. Plast Reconstr Surg.
111:1969–1979. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dang CM, Beanes SR, Lee H, Zhang X, Soo C
and Ting K: Scarless fetal wounds are associated with an increased
matrix metalloproteinase-to-tissue-derived inhibitor of
metalloproteinase ratio. Plast Reconstr Surg. 111:2273–2285. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tan KK, Salgado G, Connolly JE, Chan JK
and Lane EB: Characterization of fetal keratinocytes, showing
enhanced stem cell-like properties: A potential source of cells for
skin reconstruction. Stem Cell Reports. 3:324–338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Krejčí E, Kodet O, Szabo P, Borský J,
Smetana K Jr, Grim M and Dvořánková B: In vitro differences of
neonatal and later postnatal keratinocytes and dermal fibroblasts.
Physiol Res. 64:561–569. 2015.
|
|
21
|
Barrandon Y and Green H: Cell size as a
determinant of the clone-forming ability of human keratinocytes.
Proc Natl Acad Sci USA. 82:5390–5394. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shin DM, Suszynska M, Mierzejewska K,
Ratajczak J and Ratajczak MZ: Very small embryonic-like stem-cell
optimization of isolation protocols: An update of molecular
signatures and a review of current in vivo applications. Exp Mol
Med. 45:e562013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mateu R, Živicová V, Krejčí ED, Grim M,
Strnad H, Vlček Č, Kolář M, Lacina L, Gál P, Borský J, et al:
Functional differences between neonatal and adult fibroblasts and
keratinocytes: Donor age affects epithelial-mesenchymal crosstalk
in vitro. Int J Mol Med. 38:1063–1074. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pratsinis H, Armatas A, Dimozi A, Lefaki
M, Vassiliu P and Kletsas D: Paracrine anti-fibrotic effects of
neonatal cells and living cell constructs on young and senescent
human dermal fibroblasts. Wound Repair Regen. 21:842–851. 2013.
View Article : Google Scholar
|
|
25
|
Gilbert SF: The central nervous system and
the epidermis. Developmental Biology. 6th edition. Sunderland MA:
Sinauer Associates; Sunderland: pp. 379–410. 2000
|
|
26
|
Ng YZ, Pourreyron C, Salas-Alanis JC,
Dayal JH, Cepeda-Valdes R, Yan W, Wright S, Chen M, Fine JD and
Hogg FJ: Fibroblast-derived dermal matrix drives development of
aggressive cutaneous squamous cell carcinoma in patients with
recessive dystrophic epidermolysis bullosa. Cancer Res.
72:3522–3534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lacina L, Plzak J, Kodet O, Szabo P,
Chovanec M, Dvorankova B and Smetana K Jr: Cancer microenvironment:
What can we learn from the stem cell niche. Int J Mol Sci.
16:24094–24110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kruger NJ: Basic protein and peptide
protocols. Methods in Molecular biology. Walker JM: 32. Humana
Press Inc; Totowa, NJ: pp. 9–15. 1994
|
|
29
|
Inman GJ, Nicolás FJ, Callahan JF, Harling
JD, Gaster LM, Reith AD, Laping NJ and Hill CS: SB-431542 is a
potent and specific inhibitor of transforming growth factor-beta
superfamily type I activin receptor-like kinase (ALK) receptors
ALK4, ALK5, and ALK7. Mol Pharmacol. 62:65–74. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carvalho BS and Irizarry RA: A framework
for oligonucleotide microarray preprocessing. Bioinformatics.
26:2363–2367. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: Limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huber W, Carey VJ, Gentleman R, Anders S,
Carlson M, Carvalho BS, Bravo HC, Davis S, Gatto L, Girke T, et al:
Orchestrating high-throughput genomic analysis with Bioconductor.
Nat Methods. 12:115–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
RC Team: R: A Language and environment for
statistical computing. R Foundation for Statistical Computing;
Vienna, Austria: 2016, https://www.R-project.org/.
|
|
34
|
Storey JD and Tibshirani R: Statistical
significance for genomewide studies. Proc Natl Acad Sci USA.
100:9440–9445. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kanehisa M, Furumichi M, Tanabe M, Sato Y
and Morishima K: KEGG: New perspectives on genomes, pathways,
diseases and drugs. Nucleic Acids Res. 45:D353–D361. 2017.
View Article : Google Scholar :
|
|
36
|
Gene Ontology Consortium: Gene Ontology
Consortium: Going forward. Nucleic Acids Res. 43:D1049–D1056. 2015.
View Article : Google Scholar :
|
|
37
|
Dvořánková B, Szabo P, Lacina L, Gal P,
Uhrova J, Zima T, Kaltner H, André S, Gabius HJ, Syková E, et al:
Human galectins induce conversion of dermal fibroblasts into
myofibroblasts and production of extracellular matrix: Potential
application in tissue engineering and wound repair. Cells Tissues
Organs. 194:469–480. 2011. View Article : Google Scholar
|
|
38
|
Kolář M, Szabo P, Dvořánková B, Lacina L,
Gabius HJ, Strnad H, Sáchová J, Vlček C, Plzák J, Chovanec M, et
al: Upregulation of IL-6, IL-8 and CXCL-1 production in dermal
fibroblasts by normal/malignant epithelial cells in vitro:
Immunohistochemical and transcriptomic analyses. Biol Cell.
104:738–751. 2012. View Article : Google Scholar
|
|
39
|
Holland PWH, Booth HAF and Bruford EA:
Classification and nomenclature of all human homeobox genes. BMC
Biol. 5:472007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xiong W, He F, Morikawa Y, Yu X, Zhang Z,
Lan Y, Jiang R, Cserjesi P and Chen Y: Hand2 is required in the
epithelium for palatogenesis in mice. Dev Biol. 330:131–141. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hinz B, Phan SH, Thannickal VJ, Galli A,
Bochaton-Piallat ML and Gabbiani G: The myofibroblast: One
function, multiple origins. Am J Pathol. 170:1807–1816. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brenmoehl J, Miller SN, Hofmann C, Vogl D,
Falk W, Schölmerich J and Rogler G: Transforming growth factor-β1
induces intestinal myofibroblast differentiation and modulates
their migration. World J Gastroenterol. 15:1431–1442. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ito Y, Yeo JY, Chytil A, Han J, Bringas P
Jr, Nakajima A, Shuler CF, Moses HL and Chai Y: Conditional
inactivation of Tgfbr2 in cranial neural crest causes cleft palate
and calvaria defects. Development. 130:5269–5280. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ichikawa E, Watanabe A, Nakano Y, Akita S,
Hirano A, Kinoshita A, Kondo S, Kishino T, Uchiyama T, Niikawa N,
et al: PAX9 and TGFB3 are linked to susceptibility to nonsyndromic
cleft lip with or without cleft palate in the Japanese:
Population-based and family-based candidate gene analyses. J Hum
Genet. 51:38–46. 2006. View Article : Google Scholar
|
|
45
|
Iwata J, Parada C and Chai Y: The
mechanism of TGF-β signaling during palate development. Oral Dis.
17:733–744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Baroni T, Bellucci C, Lilli C, Pezzetti F,
Carinci F, Becchetti E, Carinci P, Stabellini G, Calvitti M, Lumare
E, et al: Retinoic acid, GABA-ergic, and TGF-β signaling systems
are involved in human cleft palate fibroblast phenotype. Mol Med.
12:237–245. 2006. View Article : Google Scholar
|
|
47
|
Satish L and Kathju S: Cellular and
molecular characteristics of scarless versus fibrotic wound
healing. Dermatol Res Pract. 2010:7902342010.
|
|
48
|
Bermudez DM, Canning DA and Liechty KW:
Age and proinflammatory cytokine production: Wound-healing
implications for scar-formation and the timing of genital surgery
in boys. J Pediatr Urol. 7:324–331. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Whiting J: Craniofacial abnormalities
induced by the ectopic expression of homeobox genes. Mutat Res.
396:97–112. 1997. View Article : Google Scholar
|
|
50
|
Creuzet S, Couly G and Le Douarin NM:
Patterning the neural crest derivatives during development of the
vertebrate head: Insights from avian studies. J Anat. 207:447–459.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mallo M and Alonso CR: The regulation of
Hox gene expression during animal development. Development.
140:3951–3963. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
McHeik JN, Sfalli P, Bondonny JM and
Levard G: Early repair for infants with cleft lip and nose. Int J
Pediatr Otorhinolaryngol. 70:1785–1790. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shieh SJ and Cheng TC: Regeneration and
repair of human digits and limbs: Fact and fiction. Regeneration
(Oxf). 2:149–168. 2015. View Article : Google Scholar
|
|
54
|
Wang H, Qiu T, Shi J, Liang J, Wang Y,
Quan L, Zhang Y, Zhang Q and Tao K: Gene expression profiling
analysis contributes to understanding the association between
non-syndromic cleft lip and palate, and cancer. Mol Med Rep.
13:2110–2116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Eslami A, Gallant-Behm CL, Hart DA, Wiebe
C, Honardoust D, Gardner H, Häkkinen L and Larjava HS: Expression
of integrin alphavbeta6 and TGF-beta in scarless vs scar-forming
wound healing. J Histochem Cytochem. 57:543–557. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhu X, Li L, Zou L, Zhu X, Xian G, Li H,
Tan Y and Xie L: A novel aptamer targeting TGF-β receptor II
inhibits transdifferentiation of human tenon's fibroblasts into
myofibroblast. Invest Ophthalmol Vis Sci. 53:6897–6903. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chang Z, Kishimoto Y, Hasan A and Welham
NV: TGF-β3 modulates the inflammatory environment and reduces scar
formation following vocal fold mucosal injury in rats. Dis Model
Mech. 7:83–91. 2014. View Article : Google Scholar
|
|
58
|
Sriram S, Gibson DJ, Robinson P, Pi L,
Tuli S, Lewin AS and Schultz G: Assessment of anti-scarring
therapies in ex vivo organ cultured rabbit corneas. Exp Eye Res.
125:173–182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liang C, Li X, Zhang L, Cui D, Quan X and
Yang W: The anti-fibrotic effects of microRNA-153 by targeting
TGFBR-2 in pulmonary fibrosis. Exp Mol Pathol. 99:279–285. 2015.
View Article : Google Scholar : PubMed/NCBI
|