Introduction
Astrocytoma is one of the most malignant tumors
worldwide and its incidence is increasing. Despite progress being
made in diagnosis and treatment over the past decades, its
prognosis remains poor (1). The
balance between tumor suppressors and oncogenes is very important
during carcinogenesis and the progression of this malignant
disease. Finding novel oncogenes which play an important role in
its development would be helpful for the development of novel
therapeutics (2,3).
The MAPK/ERK signaling pathway is a highly conserved
intracellular pathway that plays vital roles in the transmission of
signals to the cell nucleus; these signals then transcriptionally
regulate genes that are involved in various cellular processes,
including cell invasion and metastasis (4–6).
The invasion and metastasis of tumor cells, and the related
degradation of the extracellular matrix (ECM) are closely
associated with the development of malignant tumors. ECM
degradation by extracellular proteinases is a key step and matrix
metalloproteinase (MMPs) are primarily responsible for ECM
degradation. In particular, MMP9 has been shown to play critical
roles in the invasion and metastasis of many tumors (7–9)
apart from astrocytoma.
The phosphatase and transactivator eyes absent (Eya)
family encodes a group of transcription cofactors for eye
development, which play important roles during organ development
through multiple signaling pathways, including the epidermal growth
factor receptor (EGFR), transforming growth factor (TGF), Hedgehog
and Notch pathways (10–12). Recent studies have indicated their
roles in human cancer proliferation and metastasis (13,14). Eya2 has been shown to be
upregulated in ovarian cancer and to correlate with poor overall
patient survival (15). It has
also been demonstrated that Eya2 depletion inhibits the viability,
growth and migration of HPV-16 transformed cervical keratinocytes
(16). These studies suggested
that Eya2 is a potential cancer-related protein during cancer
development and progression. To date, its expression and biological
roles in human astrocytoma remains unexplored, however. In this
study, we examined the clinical significance of Eya2 in 90 cases of
astrocytoma using immunohistochemistry. We also investigated its
biological characteristics in human astrocytoma cell lines.
Materials and methods
Patients and specimens
The study protocol was approved by the Institutional
Review Board of the First Affiliated Hospital of China Medical
University. A total of 90 cases of archived primary tumor specimens
embedded in paraffin were obtained from the Department of
Pathology. For 15 of the 90 patients, paraffin-embedded adjacent
non-cancerous normal brain tissues were also obtained. These
specimens were from 90 patients diagnosed with astrocytoma who
underwent resection at the First Affiliated Hospital of China
Medical University between 2012 and 2014. Informed consent was
obtained from all these patients. Histological diagnosis was
performed and the astrocytoma tissues were classified as grades
I-IV according to the WHO classification guidelines (2007).
Immunohistochemistry
Surgically excised astrocytoma specimens were fixed
in 10% neutral buffered formalin, embedded in paraffin, and
4-µm-thick sections were prepared. Astrocytoma tissue
sections were used for immunostaining with the Maixin Elivision
plus kit (Fuzhou Maixin Biotechnology Co., Ltd., Fuzhou, China).
The sections were deparaffinized and antigen retrieval was
performed in citrate buffer for 2 min using an autoclave.
Endogenous peroxidase was then blocked using hydrogen peroxide.
Eya2 rabbit polyclonal antibody (1:300 dilution; 11314-1-AP;
Proteintech Inc., Chicago, IL, USA) was used to incubate the tissue
sections at 4°C overnight. Incubation with polymer secondary
antibody was performed after washing with phosphate-buffered saline
(PBS). The DAB plus kit (Fuzhou Maixin Biotechnology Co., Ltd.) was
used to develop the staining. Counterstaining was carried out using
hematoxylin, and the sections were dehydrated in alcohol before
mounting.
Strong nuclear staining was regarded as positive.
Samples with <15% strong nuclear staining were regarded as
having a negative/low expression. Tumor samples with ≥15% strong
nuclear staining were considered to have a Eya2 high
expression.
Cell culture and transfection
The U251, U87, A172 cell lines were obtained from
the American Type Culture Collection (ATCC; Manassas, VA, USA). The
cells were cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan, UT,
USA). The astrocytoma cells were cultured in sterilized bottles.
the Eya2 plasmid was obtained from OrigGene Technologies, Inc.
(Rockville, MD, USA) and transfection was performed using
Lipofectamine 3000 reagent. siRNA against Eya2 (Eya2 siRNA), Six1
siRNA and control siRNA were all purchased from Dharmacon (GE
Healthcare Dharmacon Inc., Lafayette, CO, USA). DharmaFECT reagent
(GE Healthcare Dharmacon Inc.) was used to transfect the siRNA. ERK
inhibitor (PD98059) was purchased from Sigma-Aldrich (St. Louis,
MO, USA). The cells were treated with PD98059 at the concentration
of 20 µM for 6 h.
Reverse transcription-quantitative PCR
(RT-qPCR)
RNA was isolated from the cells and tissues using
TRIzol reagent (Life Technology, St. Louis, MO, USA) according to
the manufacturer's instructions. cDNA was obtained using the
PrimerScript RT Master Mix kit (Takara, Dalian, China). 20
µl of reverse-transcription reaction solution consisted of 4
µl 5X RT Master Mix and 800 ng RNA. The reaction procedure
was 85°C, 2 min and 37°C, 30 min. Quantitative PCR was performed
using SYBR-Green PCR Master mix (Takara Bio, Inc., Shiga, Japan) in
a total volume of 20 µl on 7900 Real-Time PCR system
(Applied Biosystems, Foster City, CA, USA) as follows: 95°C for 30
sec, 40 cycles of 95°C for 5 sec, 60°C for 30 sec. β-actin was used
as the reference gene. The relative levels of gene expression were
calculated using the 2−ΔΔCq method. The sequences of the
primers are listed as follows: Eya2 forward,
5′-CACTCCCTGAAGGCACTAAACCTCATC-3′ and reverse,
5′-CTGCATTATCCTCTCGAAGCAGCTCTC-3′; cyclin D1 forward,
5′-TGGAGGTCTGCGAGGAACA-3′ and reverse,
5′-TTCATCTTAGAGGCCACGAACAT-3′; cyclin E forward,
5′-AGCCAGCCTTGGGACAATAAT-3′ and reverse,
5′-GAGCCTCTGGATGGTGCAAT-3′; MMP9 forward,
5′-CCTCTGGAGGTTCGACGTGA-3′ and reverse,
5′-TAGGCTTTCTCTCGGTACTGGAA-3′; actin forward,
5′-ATAGCACAGCCTGGATAGCAACGTAC-3′ and reverse,
5′-CACCTTCTACAATGAGCTGCGTGTG-3′.
Matrigel invasion assay
Cell invasion assay was performed using a Transwell
chamber. The inserts were coated with 18 µl Matrigel from BD
Biosciences (San Jose, CA, USA) with a dilution rate of 1:4. At 48
h after siRNA transfection, the cells were trypsinized and 100
µl of serum-free DMEM were used to dilute cells, which was
added to the upper chamber followed by incubation for a further 20
h. DMEM with 15% FBS was added to the lower chamber. The cells that
passed through the filter were fixed, stained with hematoxylin and
counted under a microscope (BX53; Olympus, Tokyo, Japan).
Colony formation assay and cell counting
kit-8 (CCK-8) cell viability assay
For colony formation assay, following transfection,
the cells were plated into 6-cm dishes and cultured for 2 weeks.
The cells were then stained with Giemsa (Sigma-Aldrich). to observe
the colonies. The colonies with >50 cells were counted using a
microscope (CX23; Olympus).
For CCK-8 assay, the cells were seeded in 96-well
plates and 20 µl CCK-8 solution were added to each well.
CCK-8 solution was incubated with the cells for 4 h, and the medium
was then removed, and the remaining formazan was dissolved using
DMSO and measured at 450 nm using a microplate reader (5119100,
Multiskan™ FC; Thermo Fisher Scientific, Inc., Carlsbad, CA,
USA).
Western blot analysis
Total proteins were extracted using Pierce cell
lysis buffer (Pierce, Thermo Fisher Scientific, Inc., Rockford, IL,
USA). The proteins were quantified by the Bradford method. A total
of 50 µg of protein was transferred onto PVDF membranes
following separation by SDS-PAGE. The membranes were incubated at
4°C overnight with primary antibodies against Eya2 (1:1,000;
11314-1-AP; Proteintech), Six1 (1:1,000; SAB2104425;
Sigma-Aldrich), cyclin D1 (2978), cyclin E (20808), p-ERK (9101),
ERK (9102), MMP9 (3852) and GAPDH (2118) (1:1,000; all from Cell
Signaling Technology, Inc., Boston, MA, USA). Following incubation
with HRP-coupled anti-mouse (sc-2789)/rabbit (sc-2357) IgG
(1:1,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at
37°C for 2 h, the bound proteins were visualized using an ECL kit
(Pierce) and detected using a DNR Bio-Imaging System (DNR
Bio-Imaging Systems, Ltd., Jerusalem, Israel). The densitometry of
western bands was measured using ImageJ software.
Immunoprecipitation
A sufficient amount of antibody was added to 500
µg protein and gently rotated at 4°C overnight. The
immunocomplex was captured by adding 25 µl protein A/G
agarose beads (Cell Signaling Technology, Inc.) and rotated at 4°C
for 2 to 3 h. The mixture was centrifuged at 1,500 × g for 5 min
and the precipitate was washed 3 times with cold PBS, resuspended
in sample buffer and boiled for 5 min to dissociate the
immunocomplex from the beads. The supernatant was then collected by
centrifugation (1,500 × g for 5 min at 4°C) and subjected to
western blot analysis.
Statistical analysis
SPSS version 16.0 software (SPSS Inc., Chicago, IL,
USA) for Windows was used for all statistical analyses. The
Chi-square test was used to investigate the clinical data. A t-test
was used to compare other data. A value of p<0.05 was considered
to indicate a statistically significant difference. The experiments
were repeated 3 times.
Results
Eya2 is upregulated in human astrocytoma
tissues and correlates with tumor grade
We examined Eya2 protein expression in 90 cases of
astrocytoma tissues and 15 adjacent non-cancerous tissues using
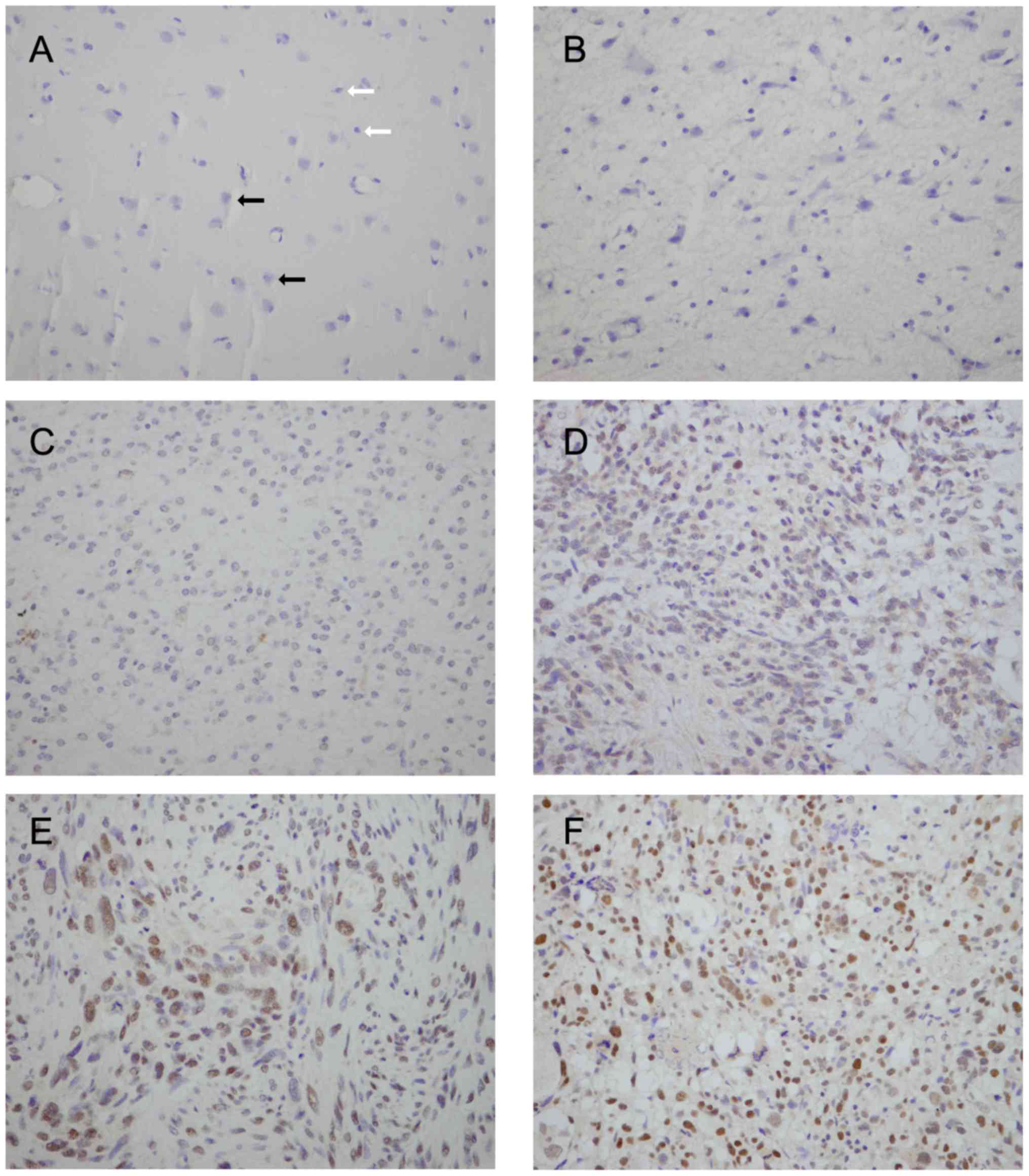
immunohistochemistry. We found a negative/low expression of Eya2 in
glial cells in normal tissue (Fig.
1A). Positive nuclear Eya2 staining was observed in 36.7%
(33/90) of the astrocytoma tissues (Fig. 1B–F). We also analyzed the
correlation between Eya2 expression and clinical characteristics. A
high Eya2 expression was found to positively correlate with an
advanced tumor grade. As shown in Table I, the positive rates of Eya2
protein in grade I (pilocytic astrocytoma), grade II (diffuse
astrocytoma), grade III (anaplastic astrocytoma) and grade IV
(glioblastoma) astrocytoma were 0% (0/8), 26.7% (8/30), 45.5%
(10/22) and 50% (15/30), respectively (p=0.03). No correlation was
found between Eya2 and age (p=0.62) and gender (p=0.82).
 | Table IThe association between eyes absent 2
(Eya 2) and the clinical characteristics of astrocytoma. |
Table I
The association between eyes absent 2
(Eya 2) and the clinical characteristics of astrocytoma.
| Parameters | Patients | Eya2 expression
| P-value |
|---|
| Low | High |
|---|
| Age (years) | | | | 0.62 |
| <50 | 68 | 42 | 26 (38.2%) | |
| ≥50 | 22 | 15 | 7 (31.8%) | |
| Gender | | | | 0.82 |
| Male | 58 | 36 | 22 (37.9%) | |
| Female | 32 | 21 | 11 (34.3%) | |
| Grades | | | | 0.03 |
| I | 8 | 8 | 0 (0%) | |
| II | 30 | 22 | 8 (26.7%) | |
| III | 22 | 12 | 10 (45.5%) | |
| IV | 30 | 15 | 15 (50%) | |
Eya2 regulates the proliferation
andinvasion of astrocytoma cell lines
In order to explore the biological function of Eya2
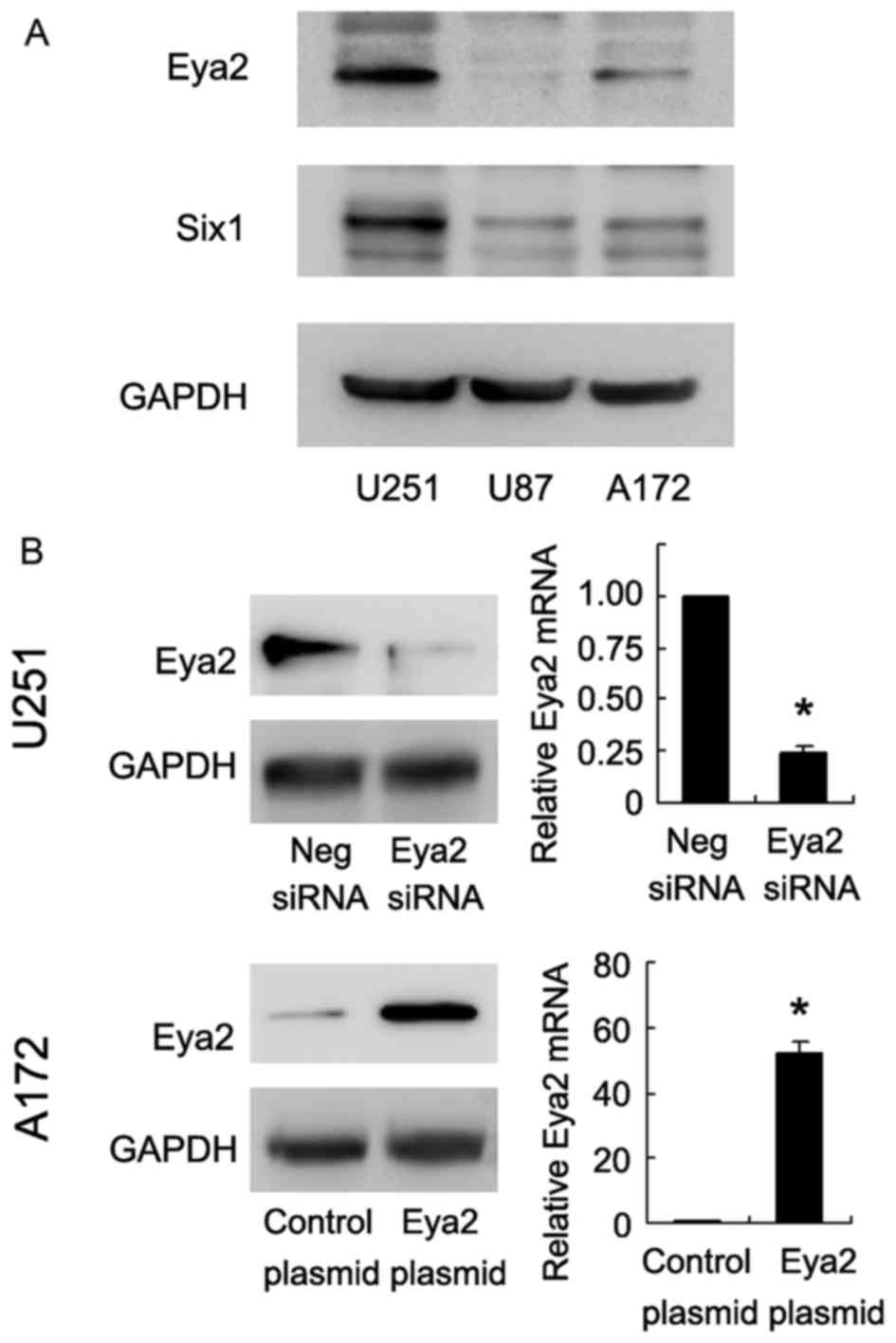
in astrocytoma cells, we analyzed its expression level in 3
astrocytoma cell lines (U251, U87 and A172) (Fig. 2A). Transfection with the Eya2
expression plasmid was performed in the A172 cell line, which was
fouond to have a low endogenous expression of Eya2. siRNA knockdown
was carried out in the U251 cell line, which was found to have a
high expression of Eya2 (Fig.
1A). As shown in Fig. 2B, the
protein and mRNA levels of Eya2 were significantly increased
following transfection with the expression plasmid, and were
decreased following transfection with siRNA.
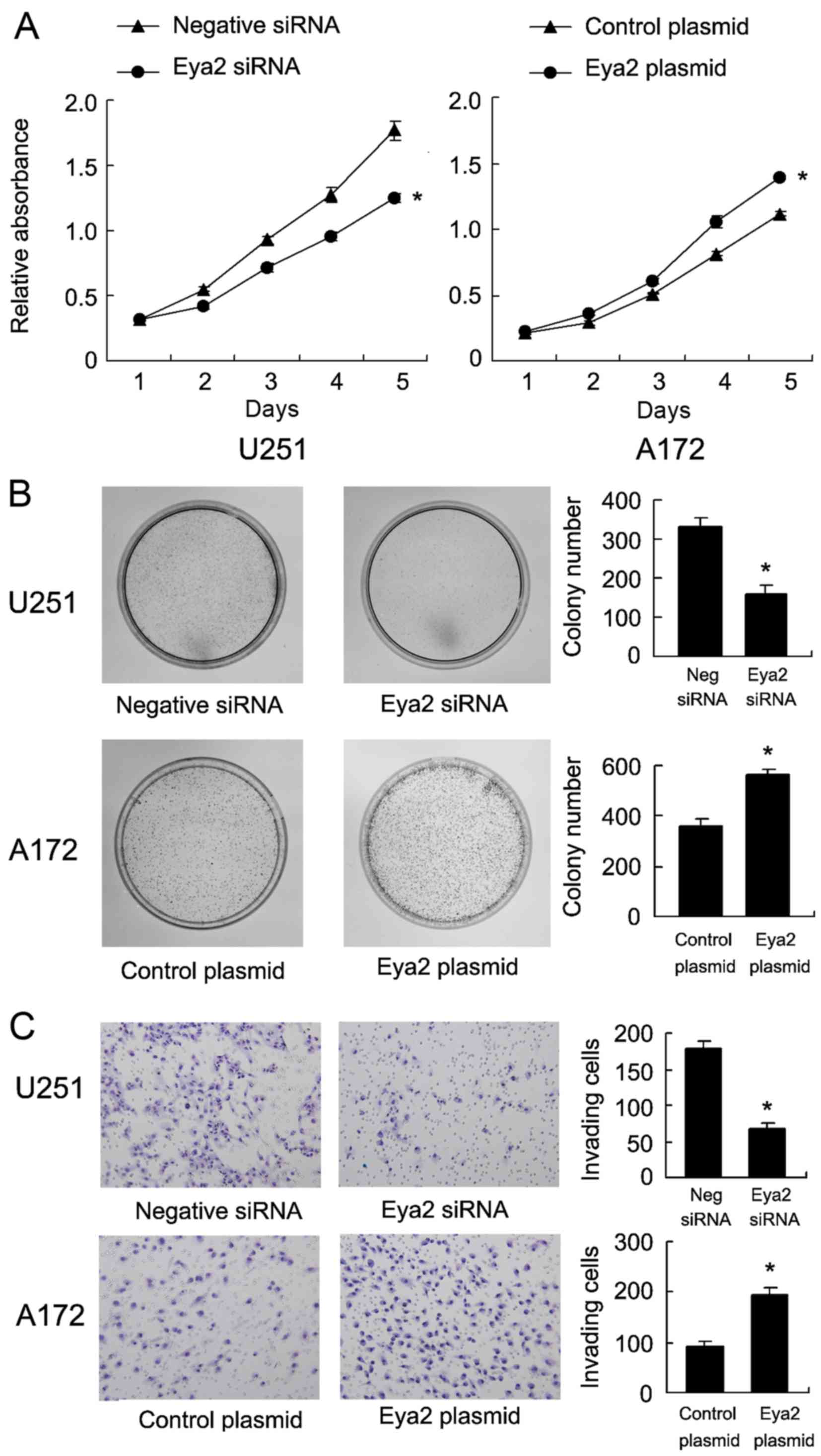
The role of Eya2 in cell growth was examined by
CCK-8 cell viability assay (Fig.
3A). The results revealed that transfection with the Eya2
expression plasmid increased the cell growth rate and the silencing
of Eya2 inhibited cell proliferation. Colony formation assay
revealed that transfection with the Eya2 expression plasmid
significantly increased the colony number in the A172 cells. On the
contrary, the knockdown of Eya2 in the U251 cells significantly
decreased the colony number (Fig.
3B). Tran swell invasion assay was also performed to examine
the role of Eya2 in the invasion of both the A172 and U251 cell
lines. The results revealed that the knockdown of Eya2 decreased
the number of invading U251 cells, and that Eya2 overexpression in
the A172 cells facilitated cell invasion (Fig. 3C).
Eya2 regulates cell cycle progression and
related protein expression
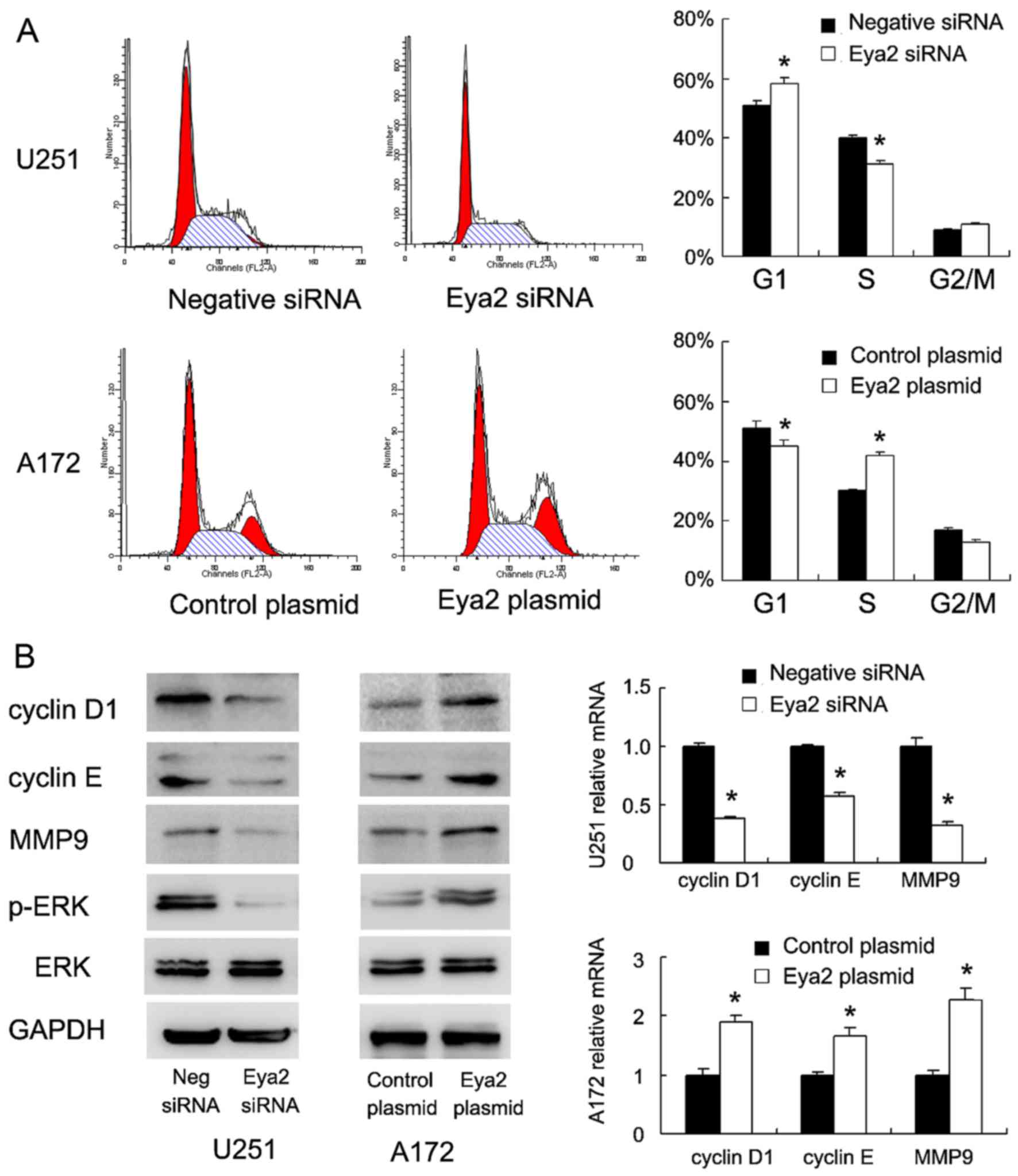
The above-mentioned results indicated that Eya2
promoted astrocytoma cell proliferation and invasion. Thus, we
further examined the effect of Eya2 on cell cycle progression. As
shown in Fig. 4A, Eya2
overexpression in the A172 cells facilitated the G1/S transition,
while the knockdown of Eya2 in the U251 cells decreased the
percentage of cells in the S phase. We further examined the levels
of cell cycle-related proteins and found that Eya2 overexpression
positively regulated cyclin D1 and cyclin E mRNA and protein
expression (Fig. 4B). However,
the knockdown of Eya2 in the U251 cell line downregulated the mRNA
and protein expression of cyclin D1 and cyclin E. In addition, Eya2
overexpression upregulated the mRNA and protein levels of MMP9, a
protein that is closely associated with cancer invasiveness
(Fig. 4B).
Eya2 regulates cell invasion through ERK
signaling
To explore the potential mechanisms of the Eya2
regulation of cell invasion, we examined the changes in several
signaling pathways previously reported to be involved in cancer
invasion (17–21). We found that Eya2 overexpression
upregulated ERK phosphorylation, while Eya2 knockdown downregulated
ERK phosphorylation (Fig. 4B),
suggesting that the ERK pathway may be responsible for the
invasion-promoting effects of Eya2. To confirm the role of ERK as
the mediator of invasiveness, the ERK inhibitor, PD98059 (20
µM, 6 h), was used to treat the A172 cells transfected with
the Eya2 expression plasmid. As shown in Fig. 5A, treatment with the ERK inhibitor
blocked the promoting effect of Eya2 on cell invasion. In addition,
western blot analysis and PCR revealed that treatment with the ERK
inhibitor abolished the effect of Eya2 on MMP9 upregulation in the
A712 cell line at both the mRNA and protein level (Fig. 5B).
Eya2 interacts with Six1 protein in U251
cells
Eya2 has been previously regarded as a Six1 partner,
which has been reported to be an important cancer-related protein
(22,29). To confirm this,
immunoprecipitation was performed. We immunoprecipitated Eya2 from
U251 cell lysate and analyzed it for Six1 binding. As shown in
Fig. 5C, Eya2
co-immunoprecipitated with Six1 in the U251 cells. We also examined
the protein expression of Six1 in 3 astrocytoma cell lines. We
found that Six1 expression was high in the U251 cells and low in
the U87 and A172 cells, which closely correlated with the Eya2
levels (Fig. 2A). To validate the
involvement of Six1 in the biological role of Eya2, we adopted Six1
siRNA to deplete its endogenous expression in the A172 cells
(Fig. 5D). Western blot analysis
and real-time PCR analysis demonstrated that the depletion of Six1
down-regulated MMP9 expression in the A172 cells. In addition, Eya2
overexpression failed to upregulated MMP9 mRNA and protein in the
Six1-depleted cells, which indicated Six1 that is required for the
biological effects of Eya2.
Discussion
Eya2 overexpression has been reported in several
human malignancies (15,16,23). In this study, we found that Eya2
protein was overexpressed in 36.7% of human astrocytoma specimens,
and its expression was higher in grade III and IV astrocytomas than
in grade I and II astrocytomas. Using siRNA and plasmid
transfection, we demonstrated that the knockdown of Eya2
significantly inhibited the U251 cell growth rate and colony
formation ability. Matrigel invasion assay revealed that Eya2
overexpression promoted the invasive ability, while Eya2 knockdown
inhibited the invasive ability. Since Eya2 overexpression
accelerated and its depletion inhibited astrocytoma cell growth and
colony formation, we examined the changes in cell cycle
progression. In accordance with the above data, Eya2 facilitated
cell cycle transition, particularly during the G1/S checkpoint. We
also found that Eya2 overexpression upregulated the level of cyclin
D1 and cyclin E, which are key regulators of cell cycle
progression, particularly during the G1/S checkpoint. Previous
studies have demonstrated that cyclin D1/E are important cancer
markers during astrocytoma progression (24,25). Thus, Eya2 promotes astrocytoma
cell proliferation possibly through the regulation of cell
cycle-related proteins. It was has been reported that Eya2 is
required to mediate the pro-metastatic functions of Six1 via the
induction of transforming growth factor (TGF)-β signaling (14). Eya2 also serves as an oncogene in
cervical carcinogenesis by promoting viability, migratory capacity
and anchorage-independent growth (16). Eya2 also serves as an oncogene in
breast cancer cells (23). Our
biological results are in accordance with those of the
above-mentioned previous studies indicating Eya2 is an oncogene
which can regulate both the proliferation and invasion of
astrocytoma cells.
The molecular mechanisms of action of Eya2 related
to the promotion of cell invasion were also investigated by
examining related proteins and signaling pathways. We found that
Eya2 overexpression positively regulated ERK signaling, and
regulated MMP9 mRNA and protein expression. ERK activation has been
demonstrated to facilitate astrocytoma invasion and metastasis via
the upregulation of MMP family proteins (26–28). Importantly, treatment with the ERK
inhibitor, PD98059, abolished the promoting effects of Eya2 on both
cell invasion and MMP9 protein expression. These results indicated
that Eya2 promoted astrocytoma invasion possibly through ERK
signaling.
Eya2 has been reported as the binding partner of
Six1, which serves as an oncoprotein in multiple types of cancer,
including astrocytoma (29–32). Six1 and Eya2 have been reported to
be critical for metastasis in a breast cancer model (14,29). Six1 has also been reported to
promote cancer proliferation and invasion through the regulation of
cell cycle protein and ERK signaling (30,33,34). Thus, we hypothesized that the
effects of Eya2 on ERK signaling and MMP9 are dependent on its
association with Six1. To validate this hypothesis, we first
confirmed that Eya2 can interact with Six1 protein using
immunoprecipitation. Second, we found that Six1 protein levels
closely correlated with Eya2 expression in the cell lines. Third,
the depletion of Six1 by siRNA blocked the promoting effect of Eya2
on MMP9 expression. Taken together, these results suggest that Eya2
exerts its oncogenic effects by interacting with Six1 protein and
activating ERK/MMP9 signaling.
In conclusion, the present study demonstrated that
Eya2 was overexpressed in human astrocytoma and correlated with an
advanced tumor grade. Eya2 interacts with Six1 and promotes
astrocytoma cell proliferation and invasion through the
upregulation of cyclin proteins and ERK signaling.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krupkova O Jr, Loja T, Redova M, Neradil
J, Zitterbart K, Sterba J and Veselska R: Analysis of nuclear
nestin localization in cell lines derived from neurogenic tumors.
Tumour Biol. 32:631–639. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zadran S, Amighi A, Otiniano E, Wong K and
Zadran H: ENTPD5-mediated modulation of ATP results in altered
metabolism and decreased survival in gliomablastoma multiforme.
Tumour Biol. 33:2411–2421. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chi F, Wu R, Jin X, Jiang M and Zhu X:
HER2 induces cell proliferation and invasion of non-small-cell lung
cancer by upregulating COX-2 expression via MEK/ERK signaling
pathway. Onco Targets Ther. 9:2709–2716. 2016.PubMed/NCBI
|
|
5
|
Jiang Q, Pan Y, Cheng Y and Li H, Liu D
and Li H: Lunasin suppresses the migration and invasion of breast
cancer cells by inhibiting matrix metalloproteinase-2/-9 via the
FAK/Akt/ERK and NF-κB signaling pathways. Oncol Rep. 36:253–262.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ko HS, Park BJ, Choi SK, Kang HK, Kim A,
Kim HS, Park IY and Shin JC: STAT3 and ERK signaling pathways are
implicated in the invasion activity by oncostatin M through
induction of matrix metalloproteinases 2 and 9. Yonsei Med J.
57:761–768. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dong DD, Zhou H and Li G: ADAM15 targets
MMP9 activity to promote lung cancer cell invasion. Oncol Rep.
34:2451–2460. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kalhori V and Törnquist K: MMP2 and MMP9
participate in S1P-induced invasion of follicular ML-1 thyroid
cancer cells. Mol Cell Endocrinol. 404:113–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jacob A, Jing J, Lee J, Schedin P, Gilbert
SM, Peden AA, Junutula JR and Prekeris R: Rab40b regulates
trafficking of MMP2 and MMP9 during invadopodia formation and
invasion of breast cancer cells. J Cell Sci. 126:4647–4658. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Silver SJ, Davies EL, Doyon L and Rebay I:
Functional dissection of eyes absent reveals new modes of
regulation within the retinal determination gene network. Mol Cell
Biol. 23:5989–5999. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumar JP and Moses K: EGF receptor and
Notch signaling act upstream of Eyeless/Pax6 to control eye
specification. Cell. 104:687–697. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kenyon KL, Ranade SS, Curtiss J, Mlodzik M
and Pignoni F: Coordinating proliferation and tissue specification
to promote regional identity in the Drosophila head. Dev Cell.
5:403–414. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krishnan N, Jeong DG, Jung SK, Ryu SE,
Xiao A, Allis CD, Kim SJ and Tonks NK: Dephosphorylation of the
C-terminal tyrosyl residue of the DNA damage-related histone H2A.X
is mediated by the protein phosphatase eyes absent. J Biol Chem.
284:16066–16070. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Farabaugh SM, Micalizzi DS, Jedlicka P,
Zhao R and Ford HL: Eya2 is required to mediate the pro-metastatic
functions of Six1 via the induction of TGF-β signaling,
epithelial-mesenchymal transition, and cancer stem cell properties.
Oncogene. 31:552–562. 2012.
|
|
15
|
Zhang L, Yang N, Huang J, Buckanovich RJ,
Liang S, Barchetti A, Vezzani C, O'Brien-Jenkins A, Wang J, Ward
MR, et al: Transcriptional coactivator Drosophila eyes absent
homologue 2 is up-regulated in epithelial ovarian cancer and
promotes tumor growth. Cancer Res. 65:925–932. 2005.PubMed/NCBI
|
|
16
|
Bierkens M, Krijgsman O, Wilting SM, Bosch
L, Jaspers A, Meijer GA, Meijer CJ, Snijders PJ, Ylstra B and
Steenbergen RD: Focal aberrations indicate EYA2 and hsa-miR-375 as
oncogene and tumor suppressor in cervical carcinogenesis. Genes
Chromosomes Cancer. 52:56–68. 2013. View Article : Google Scholar
|
|
17
|
Bai G, Chu J, Eli M, Bao Y and Wen H:
PAQR3 overexpression suppresses the aggressive phenotype of
esophageal squamous cell carcinoma cells via inhibition of ERK
signaling. Biomed Pharmacother. 94:813–819. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J, Chen S and Ni B: Upregulation of
HINT2 inhibits non-small cell lung cancer cell invasion via
COX-2/PGE2-mediated activation of β-catenin signaling. Oncol Res.
Aug 11–2017.Epub ahead of print. View Article : Google Scholar
|
|
19
|
Song J, Guan Z, Li M, Sha S, Song C, Gao Z
and Zhao Y: MicroRNA-154 inhibits the growth and invasion of
gastric cancer cells by targeting DIXDC1/WNT signaling. Oncol Res.
Aug 11–2017.Epub ahead of print.
|
|
20
|
Liao Y, Yuan S, Chen X, Zhu P, Li J, Qin L
and Liao W: Up-regulation of BRCA1-associated RING domain 1
promotes hepatocellular carcinoma progression by targeting Akt
signaling. Sci Rep. 7:76492017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin H, Xie Q, Guo X, Xu J, Wang A, Li J,
Zhu J, Wu XR, Huang H and Huang C: p63α protein upregulates heat
shock protein 70 expression via E2F1 transcription factor 1,
promoting Wasf3/Wave3/MMP9 signaling and bladder cancer invasion. J
Biol Chem. Aug 9–2017.Epub ahead of print. View Article : Google Scholar
|
|
22
|
Patrick AN, Cabrera JH, Smith AL, Chen XS,
Ford HL and Zhao R: Structure-function analyses of the human
SIX1-EYA2 complex reveal insights into metastasis and BOR syndrome.
Nat Struct Mol Biol. 20:447–453. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu J, Xu X, Kang L, Zhou L, Wang S, Lu J,
Cheng L, Fan Z, Yuan B, Tian P, et al: miR-30a suppresses breast
cancer cell proliferation and migration by targeting Eya2. Biochem
Biophys Res Commun. 445:314–319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abdullah JM, Ahmad F, Ahmad KA, Ghazali
MM, Jaafar H, Ideris A, Ali AM, Omar AR, Yusoff K, Lila MA, et al:
Molecular genetic analysis of BAX and cyclin D1 genes in patients
with malignant glioma. Neurol Res. 29:239–242. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arato-Ohshima T1 and Sawa H:
Over-expression of cyclin D1 induces glioma invasion by increasing
matrix metalloproteinase activity and cell motility. Int J Cancer.
83:387–392. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Velpula KK, Rehman AA, Chelluboina B,
Dasari VR, Gondi CS, Rao JS and Veeravalli KK: Glioma stem cell
invasion through regulation of the interconnected ERK, integrin α6
and N-cadherin signaling pathway. Cell Signal. 24:2076–2084. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Das G, Shiras A, Shanmuganandam K and
Shastry P: Rictor regulates MMP-9 activity and invasion through
Raf-1-MEK-ERK signaling pathway in glioma cells. Mol Carcinog.
50:412–423. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lakka SS, Jasti SL, Gondi C, Boyd D,
Chandrasekar N, Dinh DH, Olivero WC, Gujrati M and Rao JS:
Downregulation of MMP-9 in ERK-mutated stable transfectants
inhibits glioma invasion in vitro. Oncogene. 21:5601–5608. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Blevins MA, Towers CG, Patrick AN, Zhao R
and Ford HL: The SIX1-EYA transcriptional complex as a therapeutic
target in cancer. Expert Opin Ther Targets. 19:213–225. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Z, Tian T, Lv F, Chang Y, Wang X, Zhang
L, Li X, Li L, Ma W, Wu J, et al: Six1 promotes proliferation of
pancreatic cancer cells via upregulation of cyclin D1 expression.
PLoS One. 8:e592032013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ono H, Imoto I, Kozaki K, Tsuda H, Matsui
T, Kurasawa Y, Muramatsu T, Sugihara K and Inazawa J: SIX1 promotes
epithelial-mesenchymal transition in colorectal cancer through ZEB1
activation. Oncogene. 31:4923–4934. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tian T, Li A, Lu H, Luo R, Zhang M and Li
Z: Six1 promotes glioblastoma cell proliferation and invasion by
upregulation of connective tissue growth factor. Am J Cancer Res.
5:1823–1830. 2015.PubMed/NCBI
|
|
33
|
Iwanaga R, Wang CA, Micalizzi DS, Harrell
JC, Jedlicka P, Sartorius CA, Kabos P, Farabaugh SM, Bradford AP
and Ford HL: Expression of Six1 in luminal breast cancers predicts
poor prognosis and promotes increases in tumor initiating cells by
activation of extracellular signal-regulated kinase and
transforming growth factor-beta signaling pathways. Breast Cancer
Res. 14:R1002012. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Coletta RD, Christensen K, Reichenberger
KJ, Lamb J, Micomonaco D, Huang L, Wolf DM, Müller-Tidow C, Golub
TR, Kawakami K, et al: The Six1 homeoprotein stimulates
tumorigenesis by reactivation of cyclin A1. Proc Natl Acad Sci USA.
101:6478–6483. 2004. View Article : Google Scholar : PubMed/NCBI
|