Introduction
Mesenchymal stem cells (MSCs) derived from various
sources are valuable in regenerative medicine, including bone
repair, because they can differentiate into multiple cell lineages,
including osteoblasts, chondrocytes and adipocytes (1,2).
MSCs derived from different tissues have similar characteristics,
but differ in their molecular profiles and differentiation
potential (3).
Recently, MSCs have been applied to bone tissue
engineering with a regenerative medicine approach (4). Osteogenic differentiation of MSCs is
intricately regulated by multiple transcription factors and various
cytokines and hormones (5–7).
Previously, we found that distal-less homeobox 5 (DLX5), a
homeodomain transcription factor encoded by a mammalian homolog of
one of the Drosophila distal-less (DLL/DLX) genes that
regulates the development of multiple cell types, is only expressed
in MSCs with osteogenic potential (3). The discovery led us to examine
whether DLX5 is critically involved in the differentiation
of MSCs into osteoblasts.
Homeobox-containing genes play a key role as
regulators of skeletal development (8). DLX genes that encode
homeobox-containing transcription factors function in several
developmental processes, including osteoblast development (9,10).
DLX5, which is involved in developing bone, cartilage, and
teeth, is a member of the distal-less homeobox domain family
(11–14). Overexpression of DLX5 is
known to stimulate bone differentiation, and DLX5-null mice
exhibit abnormal osteogenesis (15–18). Although numerous studies strongly
suggest that DLX5 is involved in osteogenesis, its
functional role in this process is still obscure.
Here, we investigated the regulatory role of
DLX5 in osteogenic differentiation of bone marrow- and cord
blood-derived MSCs by examining the effects of DLX5
inhibition and the expression levels of osteogenesis-associated
genes, including bone morphogenetic protein 2 (BMP2) and
runt-related transcription factor 2 (RUNX2). BMP2 and
RUNX2 play essential roles in bone development and
maintenance by collaborating with other signaling molecules;
however, they are insufficient to induce osteogenic differentiation
(19,20).
The aim of this study was to examine the key
regulators of osteogenesis in MSCs. DLX5 is regulated by
BMP2, an inducer of osteogenesis (21,22). To investigate the effects of DLX5
on osteogenic differentiation of MSCs, we examined osteogenic
factors (DLX5 and RUNX2), chondrogenic factors
[BMP7 and sex determining region Y-box 9 (SOX9)], and
adipogenic factors [peroxisome proliferator-activated receptor γ
(PPARG) and CCAAT-enhancer binding protein α
(C/EBPA)]. We demonstrated that the induction of DLX5
led to osteoblast differentiation with the expression of several
osteoblast markers, whereas the knockdown of DLX5 expression
inhibited the osteogenesis of MSCs. Our data indicate that
DLX5 is the master transcription factor stimulating the
osteogenic factor RUNX2 through BMP2 signaling during
osteogenesis.
Furthermore, we aimed to ascertain whether
activation of DLX5 and/or BMP2 signaling by certain
chemicals could induce osteogenic differentiation in MSCs.
Tanshinone IIA is a major active phytochemical derived from
phenanthren-equinone, which can be isolated from the roots of
Salvia miltiorrhiza. It was found to enhance
BMP2-stimulated differentiation of C2C12 cells into
osteoblasts via p38 activation (23). For the first time, we evaluated
the effect of tanshinone IIA on the differentiation of MSCs into
osteoblasts. This study demonstrated that tanshinone IIA affects
osteogenesis from MSCs by augmenting DLX5.
These findings may be important for regenerative
medicine, facilitating an increase in MSCs with osteogenic
potential. Further, tanshinone IIA, as a small-molecule activator
of DLX5 and BMP signaling, could be one of the key
molecules in DLX5-induced osteogenesis of MSCs.
Materials and methods
Cells
Bone marrow and umbilical cord blood were collected
from healthy donors after obtaining written informed consent. This
study was approved by the Institutional Review Boards of Severance
Hospital of Yonsei University Health System, Seoul, Korea. As
previously described, mononuclear cells were isolated by
Ficoll-Hypaque density gradient centrifugation (Pharmacia Biotech,
Uppsala, Sweden) and the MSCs were cultured using the plastic
adherence method (24). The cells
were cultured at 37°C with 5% CO2, and the medium
[DMEM-low glucose supplemented with 10% fetal bovine serum (FBS)
and 1% penicillin/streptomycin (P/S) (all from Invitrogen,
Carlsbad, CA, USA)] was changed every 3 or 4 days. Tanshinone IIA
from Sigma-Aldrich (St. Louis, MO, USA) was used for this study.
During cultivation, cells were photographed under an inverted phase
microscope (Olympus IX-71; Olympus, Tokyo, Japan) to compare
morphologies.
Differentiation
To cause MSCs to differentiate into osteoblasts,
chondrocytes, and adipocytes, bone marrow- and cord blood-derived
MSCs were cultured in osteogenic induction medium, chondrogenic
induction medium, and adipogenic induction medium for 3 weeks
(Cambrex, Lonza, MD, USA). Osteoinductive medium-treated cells were
used as the control. The medium was changed every 3 or 4 days, and
the cells intended for chondrogenic differentiation were treated
with 10 ng/ml transforming growth factor (TGF)-β3 (Cambrex)
whenever the medium was replaced. For analysis, the induced cells
were stained by Von Kossa to confirm osteogenesis, safranin O to
confirm chondrogenesis, and Oil Red O to confirm adipogenesis.
Images of the stained cells were captured using a phase microscope
(Olympus IX-71; Olympus).
RT-PCR
Total RNA was extracted using TRIzol reagent, and
standard reverse transcription (RT) was carried out using
transcriptase II (both from Invitrogen). RT-PCR was performed using
PCR primers (Bioneer, Daejeon, Korea) and annealing temperatures
listed in Table I. Glyceraldehyde
3-phosphate dehydrogenase (GAPDH) was used as an internal
control. The signal intensity of the product was normalized to the
respective GAPDH signal intensity. Osteoinductive
medium-treated cells were used as control.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene name | Primer sequences
(5′-3′) | Annealing
temperature (°C) | Product size
(bp) |
|---|
| BMP2 | Forward:
CGAGGTCCTGAGCGAGTTCGAG | | |
| Reverse:
TGGCAGTAAAAGGCGTGATACC | 60 | 838 |
| RUNX2 | Forward:
GACCAGTCTTACCCCTCCTACC | | |
| Reverse:
CTGCCTGGCTCTTCTTACTGAG | 58 | 190 |
| DLX5 | Forward:
ACCATCCGTCTCAGGAATCG | | |
| Reverse:
ACCTTCTCTGTAATGCGGCC | 60 | 384 |
| GAPDH | Forward:
GTGGTCTCCTCTGACTTCAACA | | |
| Reverse:
CTCTTCCTCTTGTGCTCTTGCT | 62 | 210 |
| BMP7 | Forward:
CCAACGTCATCCTGAAGAAATAC | | |
| Reverse:
GCTTGTAGGATCTTGTTCATTGG | 60 | 271 |
| SOX9 | Forward:
GCCGGGCAAGGCTGACCTGAAG | | |
| Reverse:
TTCTGGTGGTCGGTGTAGTCGT | 62 | 605 |
| PPARG | Forward:
TCTCTCCGTAATGGAAGACC | | |
| Reverse:
GCATTATGAGACATCCCCAC | 55 | 474 |
| C/EBPA | Forward:
CCAAGAAGTCGGTGGACAAGAA | | |
| Reverse:
TCATTGTCACTGGTCAGCTCCA | 62 | 145 |
| Osterix | Forward:
TAATGGGCTCCTTTCACCTG | | |
| Reverse:
CACTGGGCAGACAGTCAGAA | 60 | 161 |
|
Osteopontin | Forward:
GAGACCCTTCCAAGTAAGTCCA | | |
| Reverse:
GATGTCCTCGTCTGTAGCATCA | 62 | 354 |
| Type I
collagen | Forward:
CACAGAGGTTTCAGTGGTTTGG | | |
| Reverse:
GCACCAGTAGCACCATCATTTC | 62 | 191 |
| AP2 | Forward:
AAGAAGTAGGAGTGGGCTTTGC | | |
| Reverse:
CCACCACCAGTTTATCATCCTC | 62 | 381 |
Small interfering RNA (siRNA) gene
silencing
Specific knockdown of gene expression was performed
using siRNA (Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
specific for DLX5. Briefly, 2×105 MSCs were transfected
with 10 µM of negative control or targeted siRNA according
to the manufacturer's protocol. Following incubation for 7 h at
37°C and 5% CO2, normal growth medium was added. After
one day, medium was replaced with fresh normal growth medium. The
effect of gene knockdown by siRNA was evaluated by RT-PCR assay.
MSCs were treated with DLX5-siRNA when medium was replaced
for the entire induction period.
Cell viability test
The viability of chemically treated cells was
analyzed by the trypan blue exclusion method (Invitrogen). Briefly,
cells were seeded at a density of 5×104 cells in 12-well
plates (Nunc, Roskilde, Denmark). The next day, 3, 6, 12, 24 or 48
µM of tanshinone IIA was added to the cells. After 3 days,
the cells were harvested and trypan blue-stained cells were
counted.
Analysis of calcium concentration
Following osteogenic induction, the calcium content
of cells was determined using a Calcium (CPC) LiquiColor Test
(Stanbio Laboratory, Boerne, TX, USA) according to the
manufacturer's instructions. Briefly, the cells were washed with
phosphate-buffered saline (PBS; Invitrogen) and 0.5 N HCl was added
to the cells. The cells were harvested and transferred to a new
tube. After shaking for 3 h with an orbital shaker, the supernatant
was transferred to a new tube for analysis. Color and base reagents
were added to the supernatant, and then absorbances were detected
at 550 nm. The cells cultured in DMEM were used as the control.
Statistical analysis
Quantitative data are expressed as the means ±
standard deviation (SD). Statistical comparisons were performed by
a Student's t-test and one-way analysis of variance (ANOVA) with
post-hoc Bonferroni corrections. The differences were considered
statistically significant at P<0.05.
Results
Characterization of bone marrow- and cord
blood-derived MSCs
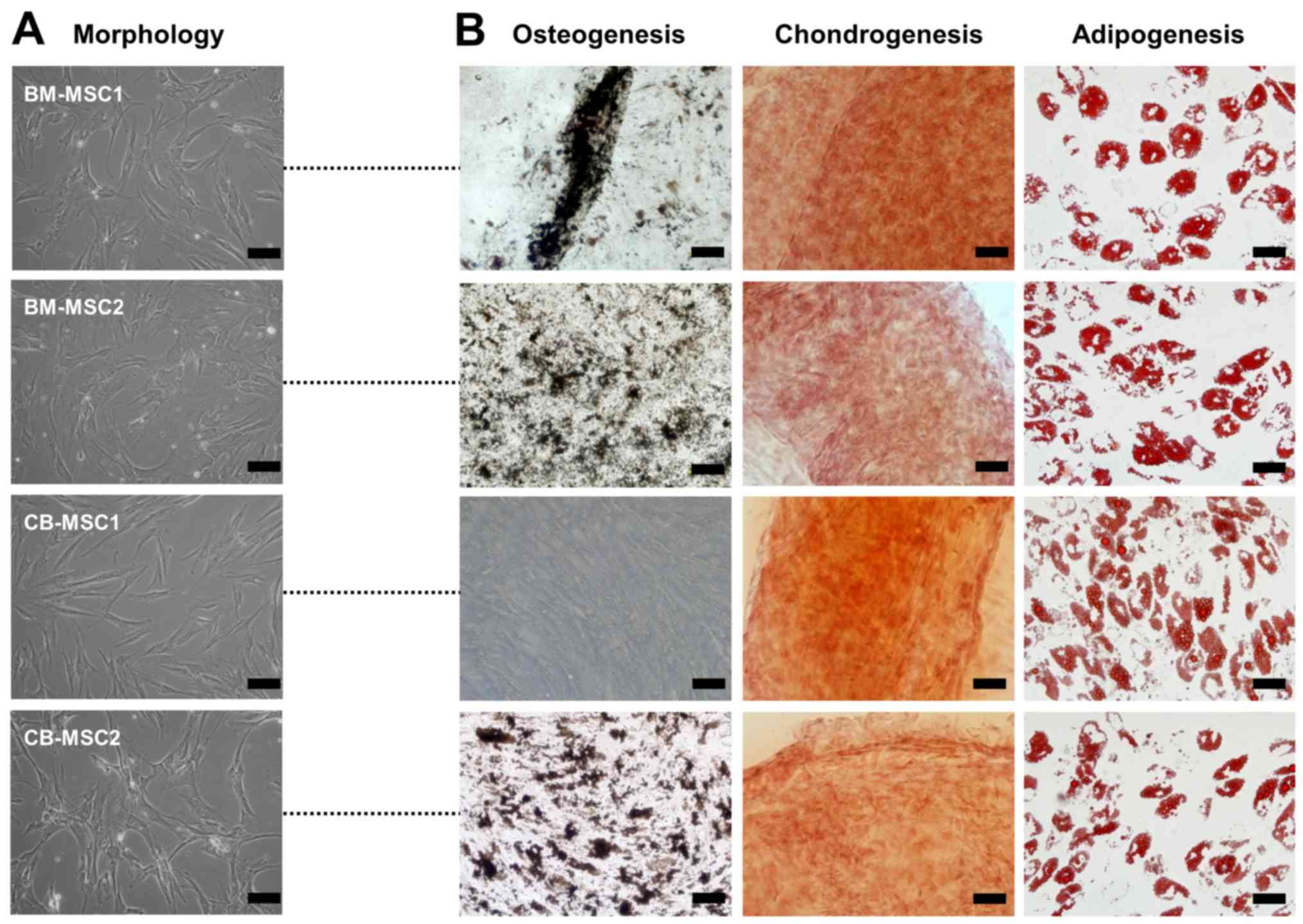
All MSCs derived from bone marrow and cord blood
showed a similar spindle-shaped morphology (Fig. 1A). Surface markers of the MSCs
were analyzed, and the results showed that all cells exhibited
similar immunophenotypic patterns. The cells were positive for
CD29, CD44, CD73, CD90 and CD105, all known markers of MSCs,
whereas the MSCs were negative for markers of endothelial and
hematopoietic cells such as CD14, CD31, CD34, CD45 and CD106 (data
not shown). These results confirmed that the cultured cells
expressed typical MSC surface markers. To determine their
differentiation capacity, the cells were induced to display
osteogenic, chondrogenic, or adipogenic phenotypes. Of the MSCs
derived from bone marrow and cord blood, one sample of cord blood
MSCs (CB-MSC1) did not differentiate into osteoblasts despite a
sufficient induction period, whereas the other MSCs exhibited
tri-lineage differentiation potential, developing into osteoblasts,
chondrocytes and adipocytes (Fig.
1B). Together, these data indicate that not all MSCs with
fibroblast-like morphologies and MSC surface proteins have
tri-lineage differentiation capacities.
Osteogenesis and DLX5 expression of
MSCs
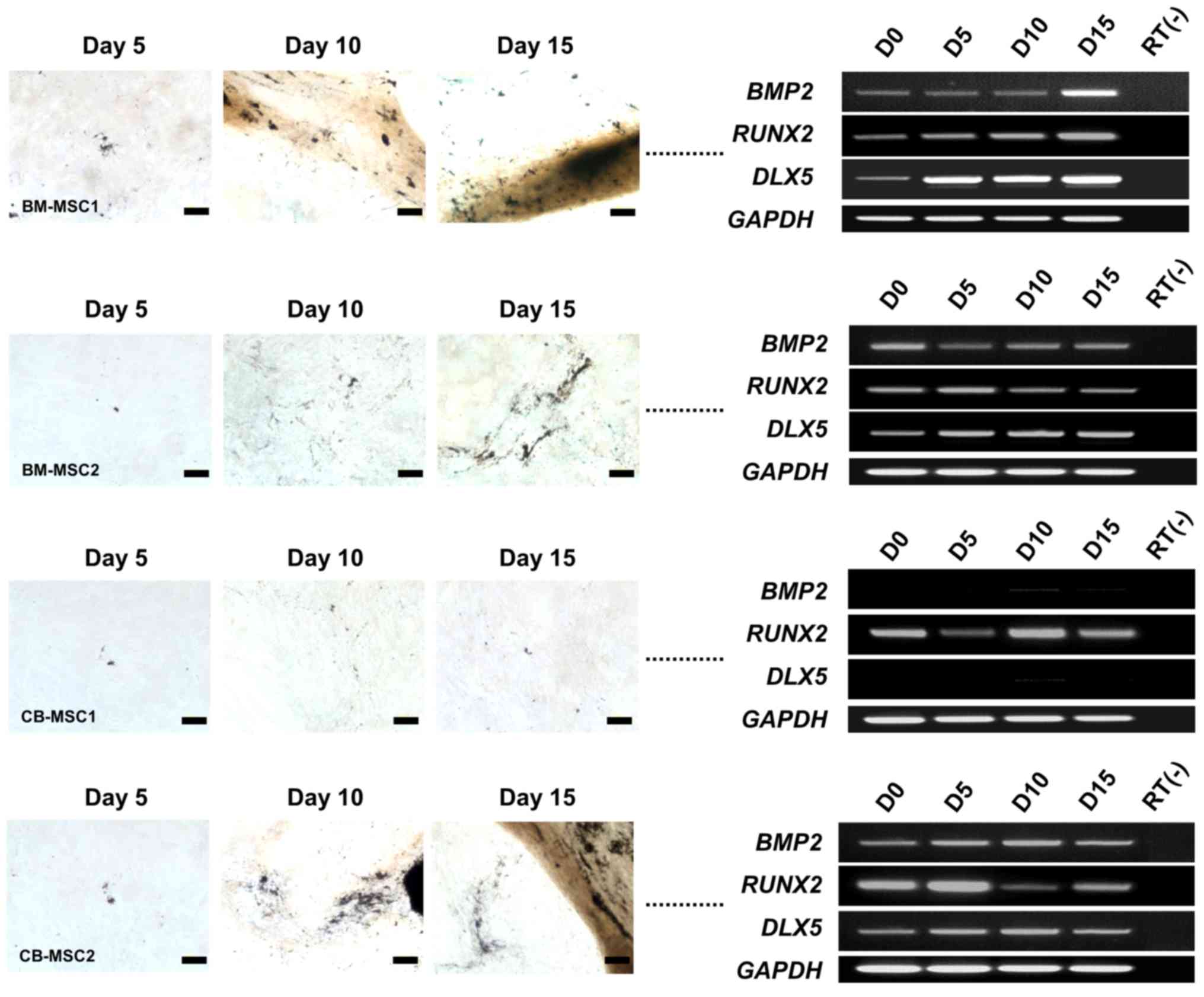
To investigate osteogenic molecular profiles
associated with morphological changes, we performed an RT-PCR
analysis of specific osteogenesis markers, namely BMP2,
RUNX2 and DLX5, during the induction of bone marrow-
and cord blood-derived MSCs from different donors. BMP2,
RUNX2 and DLX5 were expressed in all MSCs that
differentiated into osteoblasts, regardless of the induction period
(Fig. 2). However, CB-MSC1, which
did not differentiate into an osteogenic phenotype, did not express
BMP2 and DLX5 at any time in the induction
environment. Interestingly, RUNX2 was independently
expressed in all MSCs, regardless of their osteogenic potential
(Fig. 2). These results coincide
with previous data, confirming DLX5 as a marker for the
osteogenic potential of MSCs (3).
Based on these results, we noted that DLX5 with BMP2
signaling may be the only critical factors for osteogenesis of
MSCs.
Effect of DLX5 knockdown on the
differentiation potential of MSCs
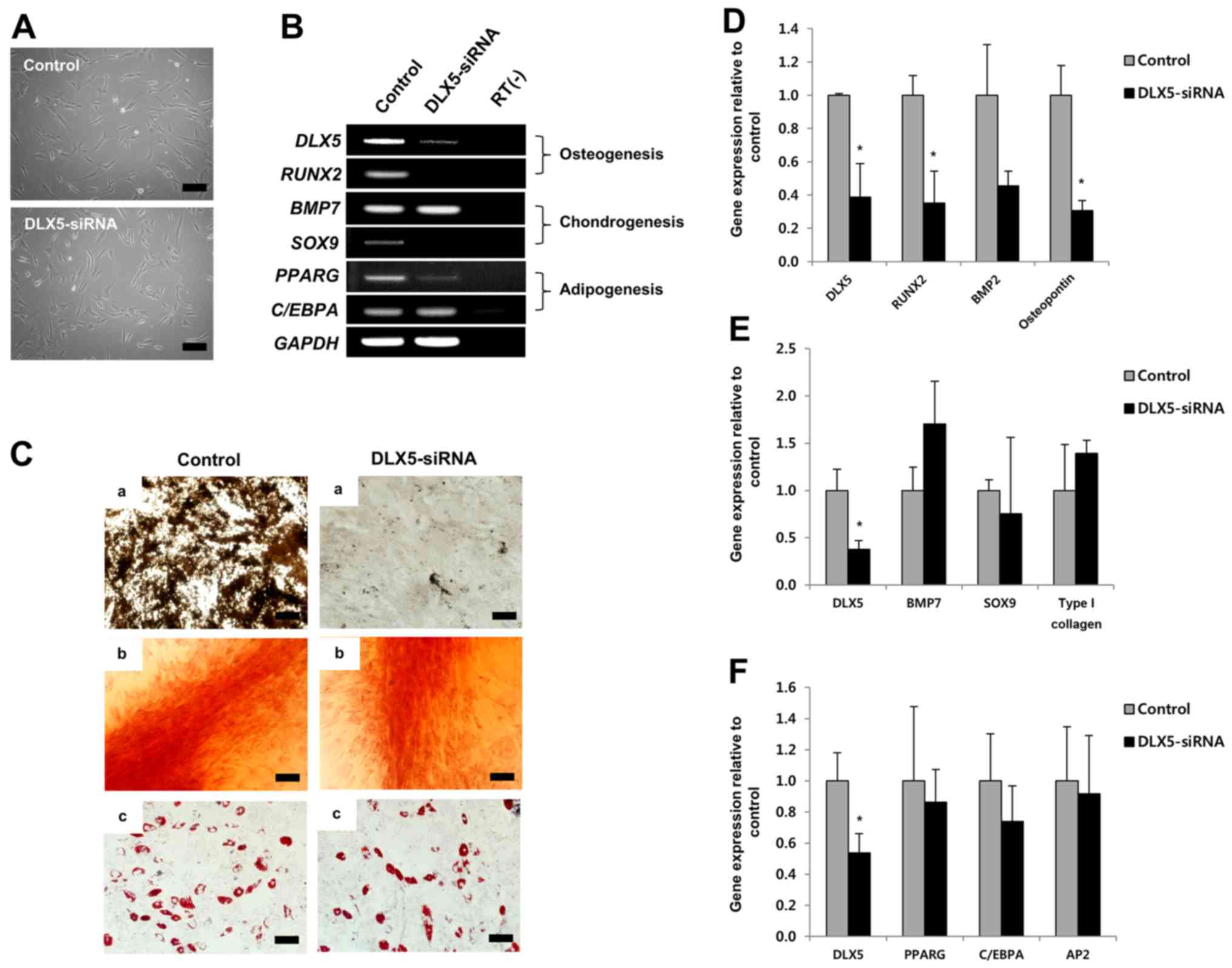
To examine the role of DLX5 in the
tri-lineage differentiation of MSCs, we employed siRNA-mediated
knockdown of DLX5, using DLX5-expressing cells. The
morphologies of cultured MSCs before induction were unaffected,
compared to those of the control, by short-term treatment with
DLX5-siRNA (Fig. 3A).
RT-PCR results showed that DLX5-siRNA substantially
decreased expression of the DLX5 gene and completely
silenced the osteogenic marker gene RUNX2 and chondrogenic
marker gene SOX9. C/EBPA of the adipogenic marker
genes was unaffected by DLX5-siRNA treatment, while
PPARG expression was slightly decreased (Fig. 3B). These results indicate that
osteogenesis of MSCs can be markedly affected by DLX5-siRNA
knockdown.
We next performed a differentiation assay in the
presence of DLX5-siRNA. Surprisingly, the osteogenic
capacity of MSCs treated with DLX5-siRNA was significantly
decreased, whereas chondrogenic and adipogenic capacities were
similar, relative to that of the control, although MSCs did not
express the SOX9 gene following DLX5-siRNA treatment
(Fig. 3C). We then analyzed gene
expression levels related to tri-lineage differentiation by RT-PCR
after induction. Expression of the following genes was evaluated:
DLX5, RUNX2, BMP2 and osteopontin for
osteogenesis; DLX5, BMP7, SOX9 and type I
collagen for chondrogenesis; and DLX5, PPARG,
C/EBPA and AP2 for adipogenesis. The levels of
RUNX2 and osteopontin gene expression were
significantly decreased relative to the control by inhibition of
DLX5 (Fig. 3D), whereas no
significant differences were detected in the expression of
chondrogenesis- (Fig. 3E) or
adipogenesis- (Fig. 3F) related
genes. Relative gene expression was normalized to that of
GAPDH, the internal control. These results strongly suggest
that DLX5 is the most powerful and specific transcription
factor for osteogenic differentiation.
Tanshinone IIA induces DLX5 through BMP2
signaling in MSCs
Tanshinone IIA, a major active phytochemical, is
involved in bone metabolism. It has a wide range of biological
activities, including anti-inflammation and anti-oxidation
(25-27). Moreover, tanshinone IIA is known
to enhance BMP-2 stimulation of cells to differentiate into
osteoblasts (23). Ultimately,
stimulation by tanshinone IIA induces osteogenesis via regulation
of osteogenic factors, including BMP2 and DLX5.
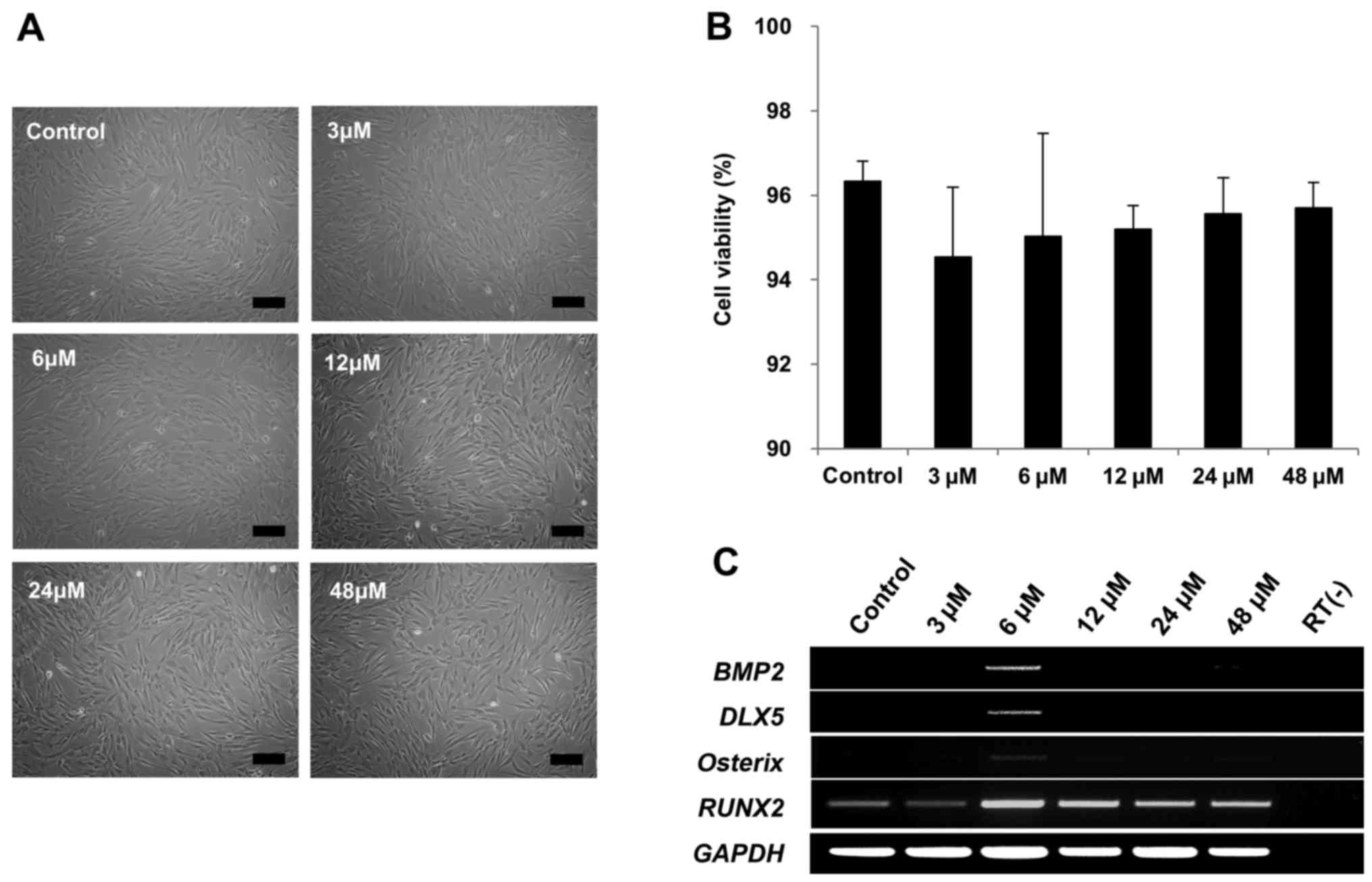
Therefore, we tested whether tanshinone IIA could
induce DLX5 in DLX5 not-expressing MSCs. Cell
morphologies were not affected by 3, 6, 12, 24 or 48 µM of
tanshinone IIA treatment (Fig.
4A). In addition, cell viability of >90% was maintained with
tanshinone IIA treatment (Fig.
4B). Remarkably, BMP2, DLX5 and osterix
genes were only induced in response to 6 µM of tanshinone
IIA treatment, as early as after 3 days of cultivation, indicating
the activation of DLX5 by the BMP2 pathway. However,
RUNX2 was similarly expressed (Fig. 4C). Taken together, these results
show that tanshinone IIA can induce DLX5, as well as the most
prominent factors of osteogenesis.
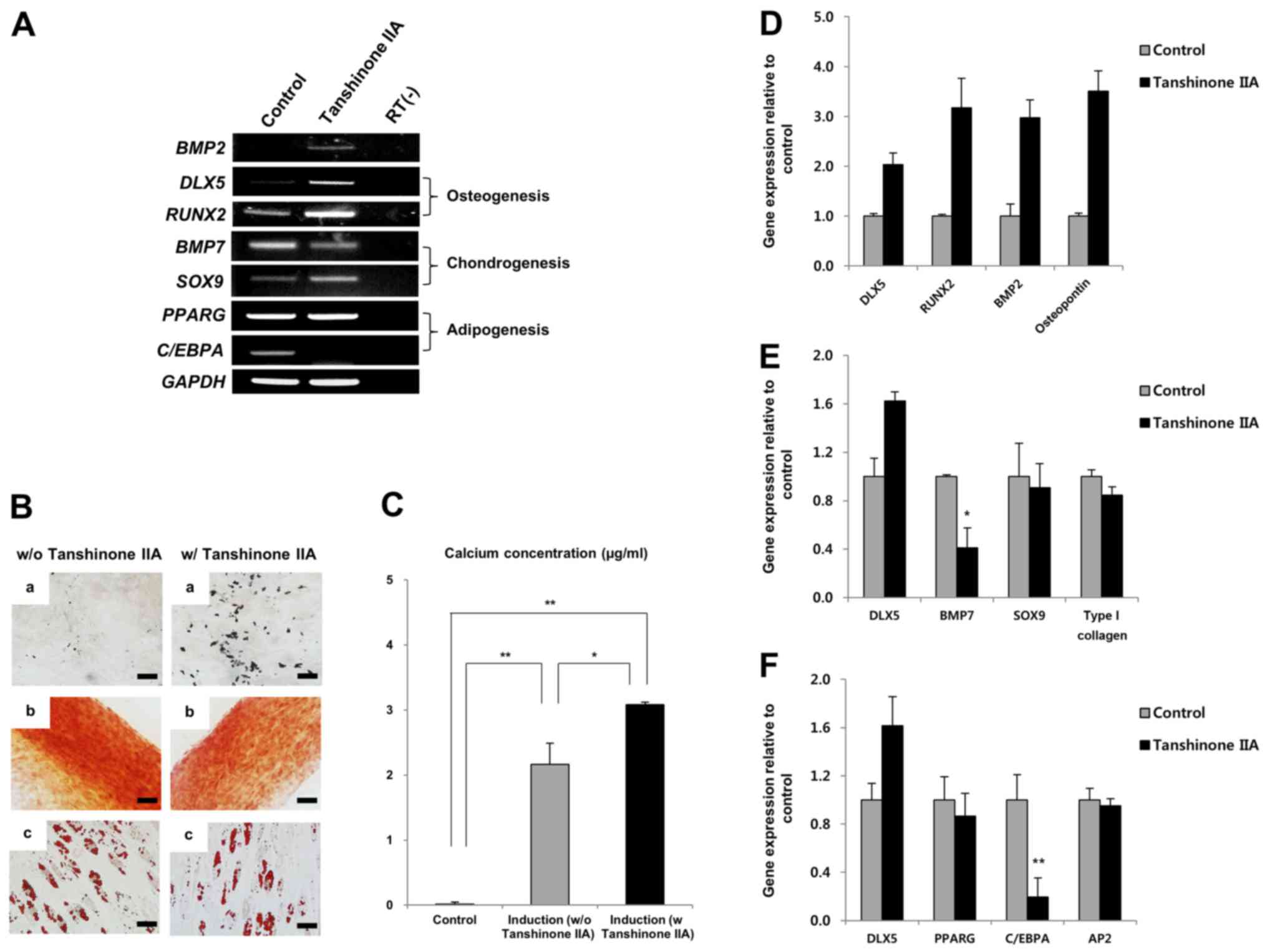
Tanshinone IIA enhances osteogenesis of
MSCs by inducing DLX5 with BMP2
We next treated DLX5 not-expressing MSCs with
6 µM tanshinone IIA to induce osteogenesis. After tanshinone
IIA treatment, we did not find any differences in our morphological
investigation. Subsequently, the effect of tanshinone IIA on the
osteogenic potential of MSCs was evaluated by analyzing the
expression levels of genes associated with osteogenic
differentiation and by visualizing the staining of induced cells.
Tanshinone IIA significantly induced BMP2 and DLX5,
as well as upregulated RUNX2 genes involved in osteogenesis.
The expression of SOX9, which is involved in chondrogenesis,
was similarly upregulated, compared to that of the control, despite
tanshinone IIA treatment, whereas BMP7 expression was
decreased (Fig. 5A).
Interestingly, expression of C/EBPA, a transcription factor
for adipogenesis, was completely inhibited by tanshinone IIA
(Fig. 5A). The differentiation
assay revealed that tanshinone IIA specifically enhanced
osteogenesis of MSCs (Fig. 5B).
This finding was confirmed by calcium deposition assay, as shown in
Fig. 5C. Furthermore, tanshinone
IIA-mediated enhancement of DLX5 through the induction of
BMP2 upregulated mRNA expression of RUNX2 and
osteopontin during osteogenic differentiation of MSCs
(Fig. 5D). As shown in Fig. 5A, we again confirmed that the
expression of BMP7 was decreased significantly during
chondrogenesis (Fig. 5E), and
that C/EBPA expression was suppressed by tanshinone IIA
during adipogenic differentiation (Fig. 5F). Relative gene expression in the
differentiated MSCs was normalized to GAPDH, the internal
control. Surprisingly, tanshinone IIA-treated MSCs differentiated
into chondrocytes and adipocytes despite the suppression of
BMP7 and C/EBPA genes (Fig. 5B). Taken together, these results
indicate that tanshinone IIA induces osteogenesis in DLX5
not-expressing MSCs by activating DLX5 through BMP2
expression.
Discussion
MSCs derived from various tissues have become a
preferred cell type in the field of regenerative medicine due to
their plastic and immunosuppressive properties (28). Although stem cells hold great
promise for future therapeutic applications, clinical applications
using these cells have been stymied by an insufficient
understanding of stem cell biology, including the complex genetic
processes in these cells. Therefore, further characterization of
stem cells via diverse approaches such as genomics and proteomics
will be critical for a better understanding and utilization of stem
cells.
Previous research on MSCs from different sources has
documented their variable differentiation potential and has shown
that this variation in osteogenic potential depends on DLX5
gene expression (3). Consistent
with the results of a previous study, DLX5 expression was
not detected in MSCs that did not have the capacity to
differentiate into osteoblasts. In addition, BMP2 gene
expression was not observed in this study when DLX5
not-expressing MSCs were maintained in an osteogenic environment.
Differentiation of stem cells is a complex process governed by
various genetic networks, and the biological functions of genes
associated with MSC differentiation remain unclear. In the present
study, we aimed to investigate the precise role of the DLX5
gene during the osteogenesis of MSCs, including whether the
DLX5 gene is important for initiating osteogenesis and
whether the gene is sufficient to completely drive osteogenesis
from MSCs.
DLX5, a member of the DLX family of homeobox
genes, is known to be a key regulator of differentiation involved
in developing skeletal elements and of osteogenesis and
chondrogenesis in the formation of hard tissues (29). Several studies suggest that
DLX5 acts as a modulator of osteogenesis in various cell
types (18,30). However, the mechanism underlying
osteogenic differentiation, including the role of DLX5, is
still controversial, especially in MSCs. In this study, we used
MSCs derived from bone marrow and cord blood, less than 5 passages,
and expressing and/or not expressing the DLX5 gene to
identify the effects, including the effects on genes activated and
inactivated by DLX5 in the course of differentiation. In
order to investigate the role of DLX5, we profiled
morphological and gene expression changes associated with
osteogenesis of MSCs. As mentioned above, DLX5
not-expressing MSCs without BMP2 expression failed to
differentiate into osteoblasts. However, RUNX2 was
consistently expressed during osteogenic induction, irrespective of
the expression of BMP2 and DLX5. To further examine
the effect of DLX5 on osteogenesis, siRNA, which targets the
DLX5 gene, was used to inhibit endogenous DLX5
expression in MSCs. Knockdown of DLX5 using siRNA did not
alter the morphology and the proliferation rate of the cells.
Seventy-two hours after siRNA transfection, RUNX2 and
SOX9 genes specific for osteogenesis and chondrogenesis,
respectively, were inhibited in the cultured cells. In addition,
osteogenic differentiation of MSCs was significantly suppressed by
DLX5-siRNA, with a decrease in osteopontin gene
expression compared to the control. In contrast, the chondrogenic
and adipogenic potential of these cells was unaffected by
DLX5-siRNA, as proteoglycans for chondrogenesis and neutral
lipids for adipogenesis were similarly detected by
immunohistochemical staining in control cells exposed to inductive
conditions. These results indicate that DLX5 drives the
osteogenic differentiation program in MSCs.
Tanshinone IIA is a major active phytochemical that
is isolated from the roots of S. miltiorrhiza and enhances
BMP2-stimulated differentiation of myoblasts into osteoblasts
(23). However, there is little
research on the effects of tanshinone IIA on the osteogenic
differentiation of MSCs. To examine the effects of tanshinone IIA
on MSCs, DLX5 not-expressing cells were cultured with
tanshinone IIA. Our data showed that DLX5 induced by
tanshinone IIA activated osteogenic marker genes, including
osterix, RUNX2 and osteopontin, in cooperation
with BMP2, with cell morphologies that remained similar to
control cells, whereas tanshinone IIA suppressed BMP7 gene
(chondrogenesis) and C/EBPA gene (adipogenesis) expression.
These results are in line with previous studies showing that
DLX5 plays a role in BMP2-induced osteogenesis
through upregulation of the RUNX2 gene, and that it
functions as part of the BMP signaling pathway (21,31). In addition, these results suggest
that tanshinone IIA is involved in the BMP2 signaling
pathway and DLX5-induced osteogenic differentiation.
Functional validation with tanshinone IIA was carried out by
differentiation assays and PCR analysis. MSCs with strongly
upregulated DLX5, RUNX2, BMP2 and
osteopontin genes following tanshinone IIA treatment
differentiated into osteoblasts and showed significantly increased
calcium deposition compared to DLX5 not-expressing cells.
However, a higher concentration of tanshinone IIA (6 µM)
decreased the osteogenic capacity of MSCs, indicating that
osteogenic differentiation following DLX5 induction in
treated cells is tanshinone IIA concentration-dependent (data not
shown). Additionally, MSCs treated with tanshinone IIA
differentiated into chondrocytes and adipocytes despite inhibition
of BMP7 and C/EBPA, indicating that these genes may
not be essential factors for differentiation. Furthermore,
DLX5 may play a role as an osteogenesis determinant through
the upregulation of RUNX2 and the downregulation of
BMP7 and C/EBPA.
Here, we showed that DLX5 is a specific
target of BMP2-induced osteogenesis of MSCs, demonstrating
that DLX5 and BMP2 can contribute to
RUNX2-independent regulation of osteogenesis. This indicates
that RUNX2 induction is not mediated by BMP2 and
DLX5 in MSCs as previously reported (32). Additionally, we confirmed that
RUNX2 is not essential for the induction of an osteogenic
lineage of MSCs, indicating that RUNX2 may function in
concert with DLX5 to induce osteogenic differentiation by
regulating the expression of osteogenesis-specific markers such as
osteopontin. These findings are in agreement with previous
results showing that DLX5 plays an important role in the
activation of osteogenesis by regulating BMP-induced RUNX2
(22). Moreover, we showed that
tanshinone IIA is capable of stimulating DLX5 expression
with BMP2, resulting in osteogenic differentiation of MSCs.
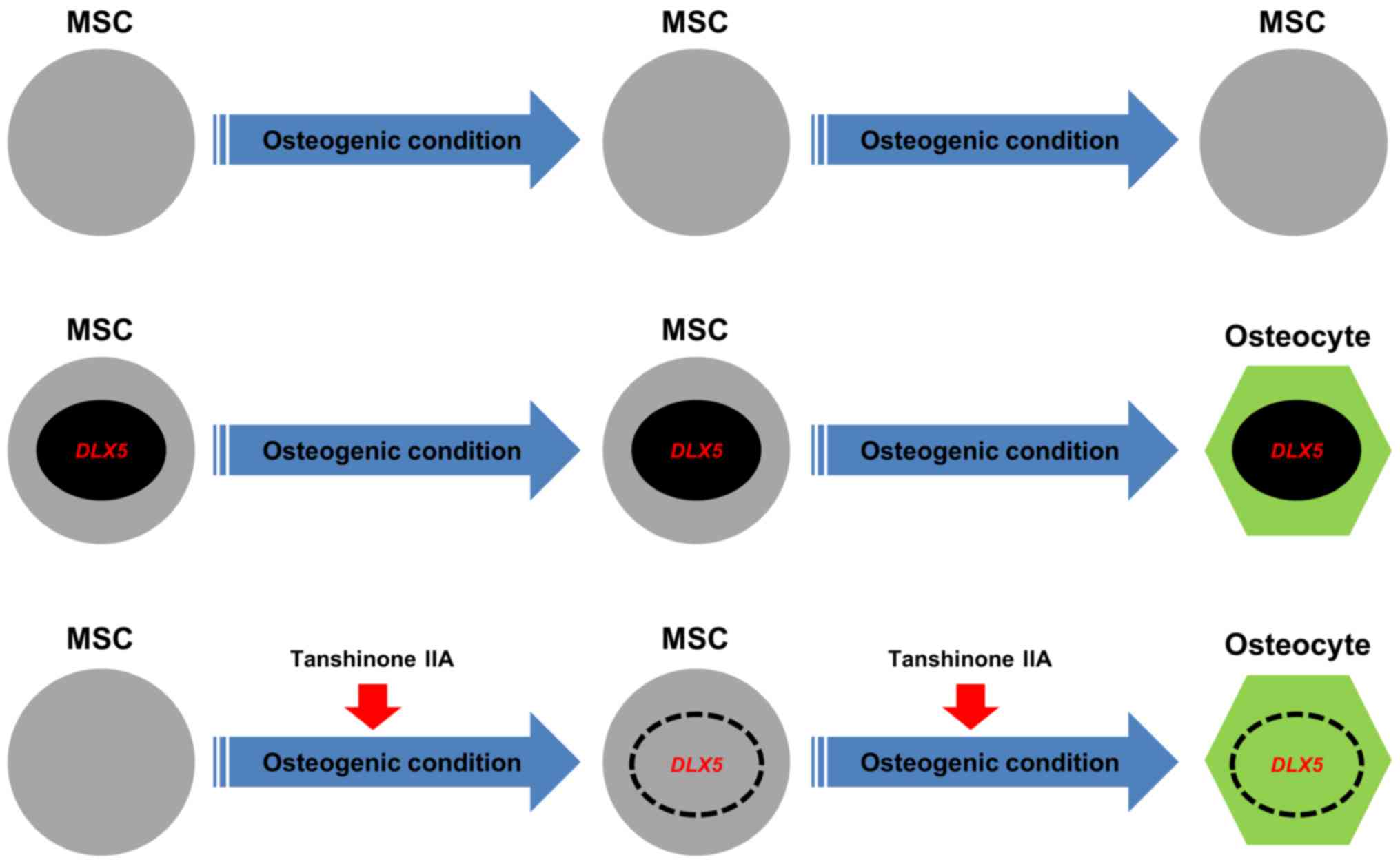
To the best of our knowledge, we showed for the first time that
tanshinone IIA can be used in place of DLX5 to induce
differentiate of MSCs into osteoblasts. Fig. 6 shows a schematic model that
summarizes the osteogenesis of MSCs by the induction of the
DLX5 gene using tanshinone IIA. Our findings contribute to
the development of effective bone regeneration therapies for the
treatment of bone diseases. Furthermore, tanshinone IIA is a
chemical compound that may be used for the treatment of bone
diseases; however, our in vitro results require in
vivo validation. Additional investigations are required for a
deeper understanding of the upstream and downstream signaling
pathways related to other osteogenesis-related factors.
In conclusion, our data showed that DLX5
plays a role as a master transcription factor in osteogenic
differentiation, and that tanshinone IIA, coincident with the
induction of BMP2, synergistically induces osteogenesis by
targeting DLX5.
Acknowledgments
The present study was supported by Grant HI15C0942
from the Korea Health Technology R&D Project through the Korea
Health Industry Development Institute (KHIDI), funded by the
Ministry of Health and Welfare, Republic of Korea.
References
|
1
|
Koç ON and Lazarus HM: Mesenchymal stem
cells: Heading into the clinic. Bone Marrow Transplant. 27:235–239.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prockop DJ, Gregory CA and Spees JL: One
strategy for cell and gene therapy: Harnessing the power of adult
stem cells to repair tissues. Proc Natl Acad Sci USA. 100(Suppl 1):
11917–11923. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heo JS, Choi Y, Kim HS and Kim HO:
Comparison of molecular profiles of human mesenchymal stem cells
derived from bone marrow, umbilical cord blood, placenta and
adipose tissue. Int J Mol Med. 37:115–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meijer GJ, de Bruijn JD, Koole R and van
Blitterswijk CA: Cell-based bone tissue engineering. PLoS Med.
4:e92007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Komori T: Regulation of osteoblast
differentiation by transcription factors. J Cell Biochem.
99:1233–1239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wagner ER, Luther G, Zhu G, Luo Q, Shi Q,
Kim SH, Gao JL, Huang E, Gao Y, Yang K, et al: Defective osteogenic
differentiation in the development of osteosarcoma. Sarcoma.
2011:3252382011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamaguchi A, Komori T and Suda T:
Regulation of osteoblast differentiation mediated by bone
morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev.
21:393–411. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen X, Li X, Wang W and Lufkin T: Dlx5
and Dlx6: An evolutionary conserved pair of murine homeobox genes
expressed in the embryonic skeleton. Ann NY Acad Sci. 785:38–47.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Depew MJ, Lufkin T and Rubenstein JL:
Specification of jaw subdivisions by Dlx genes. Science.
298:381–385. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Robledo RF, Rajan L, Li X and Lufkin T:
The Dlx5 and Dlx6 homeobox genes are essential for craniofacial,
axial, and appendicular skeletal development. Genes Dev.
16:1089–1101. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferrari D, Sumoy L, Gannon J, Sun H, Brown
AM, Upholt WB and Kosher RA: The expression pattern of the
Distal-less homeobox-containing gene Dlx-5 in the developing chick
limb bud suggests its involvement in apical ectodermal ridge
activity, pattern formation, and cartilage differentiation. Mech
Dev. 52:257–264. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Newberry EP, Latifi T and Towler DA:
Reciprocal regulation of osteocalcin transcription by the
homeodomain proteins Msx2 and Dlx5. Biochemistry. 37:16360–16368.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ryoo HM, Hoffmann HM, Beumer T, Frenkel B,
Towler DA, Stein GS, Stein JL, van Wijnen AJ and Lian JB:
Stage-specific expression of Dlx-5 during osteoblast
differentiation: Involvement in regulation of osteocalcin gene
expression. Mol Endocrinol. 11:1681–1694. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weiss KM, Ruddle FH and Bollekens J: Dlx
and other homeobox genes in the morphological development of the
dentition. Connect Tissue Res. 32:35–40. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Acampora D, Merlo GR, Paleari L, Zerega B,
Postiglione MP, Mantero S, Bober E, Barbieri O, Simeone A and Levi
G: Craniofacial, vestibular and bone defects in mice lacking the
Distal-less-related gene Dlx5. Development. 126:3795–3809.
1999.PubMed/NCBI
|
|
16
|
Depew MJ, Liu JK, Long JE, Presley R,
Meneses JJ, Pedersen RA and Rubenstein JL: Dlx5 regulates regional
development of the branchial arches and sensory capsules.
Development. 126:3831–3846. 1999.PubMed/NCBI
|
|
17
|
Miyama K, Yamada G, Yamamoto TS, Takagi C,
Miyado K, Sakai M, Ueno N and Shibuya H: A BMP-inducible gene,
dlx5, regulates osteoblast differentiation and mesoderm induction.
Dev Biol. 208:123–133. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tadic T, Dodig M, Erceg I, Marijanovic I,
Mina M, Kalajzic Z, Velonis D, Kronenberg MS, Kosher RA, Ferrari D,
et al: Overexpression of Dlx5 in chicken calvarial cells
accelerates osteoblastic differentiation. J Bone Miner Res.
17:1008–1014. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maeno T, Moriishi T, Yoshida CA, Komori H,
Kanatani N, Izumi S, Takaoka K and Komori T: Early onset of Runx2
expression caused craniosynostosis, ectopic bone formation, and
limb defects. Bone. 49:673–682. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rosen V: BMP2 signaling in bone
development and repair. Cytokine Growth Factor Rev. 20:475–480.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Holleville N, Quilhac A, Bontoux M and
Monsoro-Burq AH: BMP signals regulate Dlx5 during early avian skull
development. Dev Biol. 257:177–189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee MH, Kim YJ, Kim HJ, Park HD, Kang AR,
Kyung HM, Sung JH, Wozney JM, Kim HJ and Ryoo HM: BMP-2-induced
Runx2 expression is mediated by Dlx5, and TGF-beta 1 opposes the
BMP-2-induced osteoblast differentiation by suppression of Dlx5
expression. J Biol Chem. 278:34387–34394. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim HJ and Kim SH: Tanshinone IIA enhances
BMP-2-stimulated commitment of C2C12 cells into osteoblasts via p38
activation. Amino Acids. 39:1217–1226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sohn HS, Heo JS, Kim HS, Choi Y and Kim
HO: Duration of in vitro storage affects the key stem cell features
of human bone marrow-derived mesenchymal stromal cells for clinical
transplantation. Cytotherapy. 15:460–466. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jang SI, Jeong SI, Kim KJ, Kim HJ, Yu HH,
Park R, Kim HM and You YO: Tanshinone IIA from Salvia miltiorrhiza
inhibits inducible nitric oxide synthase expression and production
of TNF-alpha, IL-1beta and IL-6 in activated RAW 264.7 cells.
Planta Med. 69:1057–1059. 2003. View Article : Google Scholar
|
|
26
|
Lee SY, Choi DY and Woo ER: Inhibition of
osteoclast differentiation by tanshinones from the root of Salvia
miltiorrhiza Bunge. Arch Pharm Res. 28:909–913. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X, Wei Y, Yuan S, Liu G, Lu Y, Zhang
J and Wang W: Potential anticancer activity of tanshinone IIA
against human breast cancer. Int J Cancer. 116:799–807. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ménard C and Tarte K: Immunoregulatory
properties of clinical grade mesenchymal stromal cells: Evidence,
uncertainties, and clinical application. Stem Cell Res Ther.
4:642013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Simeone A, Acampora D, Pannese M,
D'Esposito M, Stornaiuolo A, Gulisano M, Mallamaci A, Kastury K,
Druck T and Huebner K: Cloning and characterization of two members
of the vertebrate Dlx gene family. Proc Natl Acad Sci USA.
91:2250–2254. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Erceg I, Tadić T, Kronenberg MS,
Marijanović I and Lichtler AC: Dlx5 regulation of mouse osteoblast
differentiation mediated by avian retrovirus vector. Croat Med J.
44:407–411. 2003.PubMed/NCBI
|
|
31
|
Lee MH, Kim YJ, Yoon WJ, Kim JI, Kim BG,
Hwang YS, Wozney JM, Chi XZ, Bae SC, Choi KY, et al: Dlx5
specifically regulates Runx2 type II expression by binding to
homeodomain-response elements in the Runx2 distal promoter. J Biol
Chem. 280:35579–35587. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Z, Song Y, Zhang X, Tang J, Chen J
and Chen Y: Msx1/Bmp4 genetic pathway regulates mammalian alveolar
bone formation via induction of Dlx5 and Cbfa1. Mech Dev.
120:1469–1479. 2003. View Article : Google Scholar : PubMed/NCBI
|