Introduction
Nasopharyngeal carcinoma (NPC), a tumor arising from
nasopharyngeal epithelial cells, is one of the most common head and
neck cancers in Southeast Asia and Taiwan. Lymph node and distant
organ metastasis is a major challenge in NPC, as it is often
responsible for treatment failure (1,2).
Although radiotherapy, alone or combined with chemotherapy, has
been traditionally used as the treatment of choice for NPC, a
significant number of patients with advanced NPC develop metastasis
and local recurrence. Therefore, in order to establish more
effectively targeted therapies, the mechanisms underlying
resistance development and metastasis of NPC must be
elucidated.
Epithelial cell adhesions may allow survival of
cancer cells and facilitate tumor growth. However, some tumor cells
develop properties that enable them to survive under
anchorage-independent conditions and continue to proliferate
(3,4). These cells are referred to as being
resistant to anoikis, and they are able to invade easily and
migrate to distant metastatic sites (5). The mechanism underlying tumor cells
acquiring anoikis resistance and enhanced migratory ability remains
largely unexplored. However, it is crucial to elucidate why NPC
cells gain metastatic potential following resistance to
anoikis.
Signal transducer and activator of transcription 3
(STAT3) is an oncogenic transcription factor that mediates cellular
response to several growth factors and cytokines. Increased
expression of STAT3 is involved in the control of cell growth and
has been associated with inflammation, survival, proliferation and
angiogenesis (6–8). In addition, activation of STAT3 was
detected in the development and progression of various tumors, and
this pathway was found to be a potential target for cancer
treatment (9). Phosphorylation of
STAT3 enhances the expression of a variety of proliferation and
survival genes, such as cell survival-related genes (cyclin D1 and
survivin), and anti-apoptotic proteins (Bcl-2 and Bcl-xL) (10,11). It has been reported that
activation of STAT3 may directly contribute to the invasiveness of
NPC cells (12,13). However, the association between
STAT3 expression and anoikis resistance, which may result in
increased NPC aggressiveness, has not been clearly determined. The
aim of the present study was to evaluate the potential role of
STAT3 in anoikis resistance, and investigate whether the activation
of STAT3 may lead to tumor progression and invasion in NPC.
Materials and methods
Cell culture
Two human NPC cell lines, TW01 and TW06, were
cultured in 10-cm2 dishes with Dulbecco's modified
Eagle's medium (DMEM; Gibco/Thermo Fisher Scientific, Carlsbad, CA,
USA) and 10% fetal bovine serum (FBS; Bioind, Kibbutz Beit Haemek,
Israel), 1% sodium pyruvate (Bioind), 1% penicillin, streptomycin,
amphotericin (PSA; Bioind), and 1% non-essential amino acids (NEAA;
Bioind). The cells were incubated at 37°C in a humidified
atmosphere of 95% air and 5% CO2.
Migration and invasion assay
The cells were incubated under either
anchorage-dependent or -independent conditions for 48 h, and were
then seeded in 6-well plates and grown to confluence. Analysis of
cell migration was performed by the wound healing method. Cell
monolayers were scraped with a 10-μl tip and photographed at
the beginning of the assay (0 h) and at 24 h under the respective
conditions. The invasion assay was performed by the Transwell
system with a polycarbonate filter membrane (24-well insert, pore
size 8 μm; Corning Costar, Corning, NY, USA). Each well was
coated with Matrigel (60 μg; BD Biosciences, San Diego, CA,
USA) immediately prior to the invasion assay. After a 48-h
incubation under adherent or suspension conditions, the cells were
transferred to the upper chamber in a medium without serum or
growth factors, and the medium supplemented with serum was used as
a chemoattractant in the lower chamber. The cells were incubated
for 48 h, and the cells on the lower surface of the membrane were
fixed with methanol and stained with crystal violet. The cells
invading the membrane were counted under a light microscope
(magnification, ×100, 5 random fields/well).
Measurement of cells resistance to
anoikis
TW01 and TW06 cells in the culture plate were
detached and made into a single-cell suspension in serum-free
DMEM/F12, and then seeded into a poly-HEMA-coated 6-well plates at
a density of 1.5×105 cells/ml for 48 h. The cells were
harvested and apoptosis was evaluated by flow cytometry using an
Annexin V-FITC̸propidium iodide kit (BD Pharmingen, San Diego, CA,
USA). Anoikis resistance was calculated as percentage compared with
control.
Antibodies and reagents
Antibodies against STAT3 (cat. no. S5933),
phosphorylated STAT3 (tyrosine 705; cat. no. S4933), E-cadherin
(cat. no. U3254), N-cadherin (cat. no. SAB2702400) and vimentin
(cat. no. V6389), were purchased from Sigma-Aldrich (St. Louis, MO,
USA). Antibodies against Bcl-2 (cat. no. sc-492) and survivin (cat.
no. sc-17779) were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). The STAT3 inhibitor Stattic was obtained
from Selleck Chemicals (Boston, MA, USA). Stock solutions were
prepared and stored according to the manufacturer's guidelines.
STAT3 blockade with shRNA
transfection
Two STAT3 shRNAs with green fluorescent protein
(GFP) obtained from Qiagen (Valencia, CA, USA) (cat. no. 336311)
were used to knock down STAT3 as shRNA1 and shRNA2. Transfections
in TW01 and TW06 cells were performed using PolyJet™ reagent
(SL100688; SignaGen® Laboratories, Rockville, MD, USA)
according to the manufacturer's protocol. After 24 h of
transfection, the cells were processed for anoikis assay, invasion
assay or western blotting, as described above. Prior to the
experiment, the extent of silencing was tested by western
blotting.
Western blot analysis
TW01 cells were incubated in either anchorage or
suspension culture, with or without any treatment, for 48 h. The
cells were then collected and lysed using RIPA buffer (Thermo
Fisher Scientific Inc., Rockford, IL, USA) containing protease
inhibitors. Samples with 20 μg of protein were subjected to
sodium dodecyl sulfate-polyacrylamide gel electrophoresis,
transferred onto a polyvinylidene difluoride membrane (Millipore,
Bedford, MA, USA), followed by blocking with 5% milk in
Tris-buffered saline (TBS). After blocking, the membranes were
immunoblotted with the specific antibodies and incubated in TBS at
4°C overnight. Primary antibodies against STAT3 (rabbit
polyclonal), phosphorylated STAT3 (tyrosine 705; rabbit
polyclonal), E-cadherin (rat monoclonal), N-cadherin (rabbit
polyclonal) and vimentin (mouse monoclonal) were used at a dilution
of 1:1,000. Primary antibodies against Bcl-2 (rabbit polyclonal)
and survivin (mouse monoclonal) were used at a dilution of 1:200. A
rabbit polyclonal anti-GAPDH (cat. no. G9545; Sigma-Aldrich) was
used as sample-loading control at a dilution of 1:1,000. The
membranes were then washed three times with TBST (1X TBS, 0.1%
Tween-20), and then incubated with goat anti-rabbit (cat. no.
ab205718, Abcam, Cambridge, UK), goat anti-mouse (cat. no.
ab205719, Abcam), or goat anti-rat (cat. no. ab97057, Abcam)
secondary antibodies conjugated with horseradish peroxidase at a
dilution of 1:5,000 in TBS for 1 h at room temperature. The
membranes were washed again in TBST three times at room
temperature. Immunoreactive protein bands were detected with the
enhanced chemiluminescence detection system (GE Healthcare, Life
Sciences, Pittsburgh, PA, USA).
Flow cytometry for anti-CD44 antibody
analysis
The presence of CD44 on the cell surface was
detected using anti-CD44-FITC (BD Biosciences) antibody. The
dissociated cells were stained with anti-human CD44 and were then
incubated at 4°C for 15 min in the dark. Following incubation, the
cells were washed once with cold FACS buffer. The labeled cells
were analyzed by Gallious Flow Cytometry (Beckman Coulter, Brea,
CA, USA) and the data were analyzed with FlowJo software (Tree Star
Inc., Ashland, OR, USA).
Results
A considerable number of NPC cells
exhibit anoikis resistance
Anoikis resistance was evaluated in TW01 and TW06
NPC cells, which were cultured under anchorage-independent
conditions for 48 h. Cell survival was assessed by flow cytometry
and compared with the cells simultaneously cultured under adherent
conditions. Although anoikis was induced to a certain extent in
tumor cells under anchorage-independent conditions, a significant
percentage of NPC cells resisted anoikis. A total of ~46% of TW01
cells resisted anoikis, whereas 67% of TW06 cells resisted anoikis
when cultured under anchorage-independent conditions (Fig. 1A).
Anoikis-resistant NPC cells exhibit a
high capability of migration and invasion
Migration and invasion assays were performed to
compare the metastatic potential of anoikis-resistant cells with
that of adherent cells. The wound healing assay was used to
evaluate cell migration in TW01 and TW06 cells. The cells were
incubated under anchorage or anchorage-independent conditions for
48 h and then transferred to 6-well plates, and a wound was created
when the cells were attached and started to grow. The results
demonstrated that TW01 and TW06 cells that resisted anoikis healed
the wound at a more rapid rate compared with adherent cells
(Fig. 1B).
The invasion capacity of NPC cells was evaluated
with the Transwell invasion assay. TW01 and TW06 cells that
resisted anoikis were also found to be highly invasive compared
with adherent cells (Fig. 1C).
Anoikis-resistant TW01 cells exhibited a 58% increased rate of
invasion, whereas TW06 cells exhibited a 74% increased rate of
invasion compared with their respective adherent cells (Fig. 1D). These results suggested that
anoikis-resistant cells acquire enhanced migratory and invasive
properties.
Overexpression of STAT3 in
anoikis-resistant NPC cells
To better understand the molecular changes that are
associated with anoikis resistance and a highly migratory and
invasive phenotype in NPC cells, the expression levels of proteins,
including STAT3, phosphorylated STAT3 (p-STAT3), B-cell lymphoma-2
(Bcl-2) and survivin, was examined in TW01 anoikis-resistant and
adherent cells by western blot analysis. The results demonstrated a
significant increase in STAT3 and p-STAT3 in the cells that
resisted anoikis compared with adherent cells. In addition, in
anoikis-resistant cells, a significant increase was observed in the
expression of the anti-apoptotic proteins Bcl-2 and survivin, which
are regulated by STAT3 (Fig.
2).
STAT3 inhibitors overcome anoikis
resistance in NPC cells
To determine whether STAT3 plays a significant role
in anoikis resistance as well as in increased migratory and
invasive potential leading to metastasis, the effect of the STAT3
inhibitor Stattic on anoikis resistance was evaluated in NPC cells.
Following treatment with 0, 5, 10 and 15 μM Stattic under
suspension conditions for 48 h, TW01 and TW06 cells were
re-cultured on adherent plates. A significant reduction was
observed in anoikis resistance following Stattic treatment in these
NPC cells. Treatment with 5 μM Stattic reduced anoikis
resistance by 43% in TW01 and by 32% in TW06 cells. The growth
inhibition was increased by 78 and 69% following Stattic treatment
at 5 and 10 μM, respectively (Fig. 3). Both cell lines exhibited a
>90% reduction in anoikis following treatment with 15 μM
Stattic.
Interference with STAT3 expression blocks
anoikis resistance in NPC cells
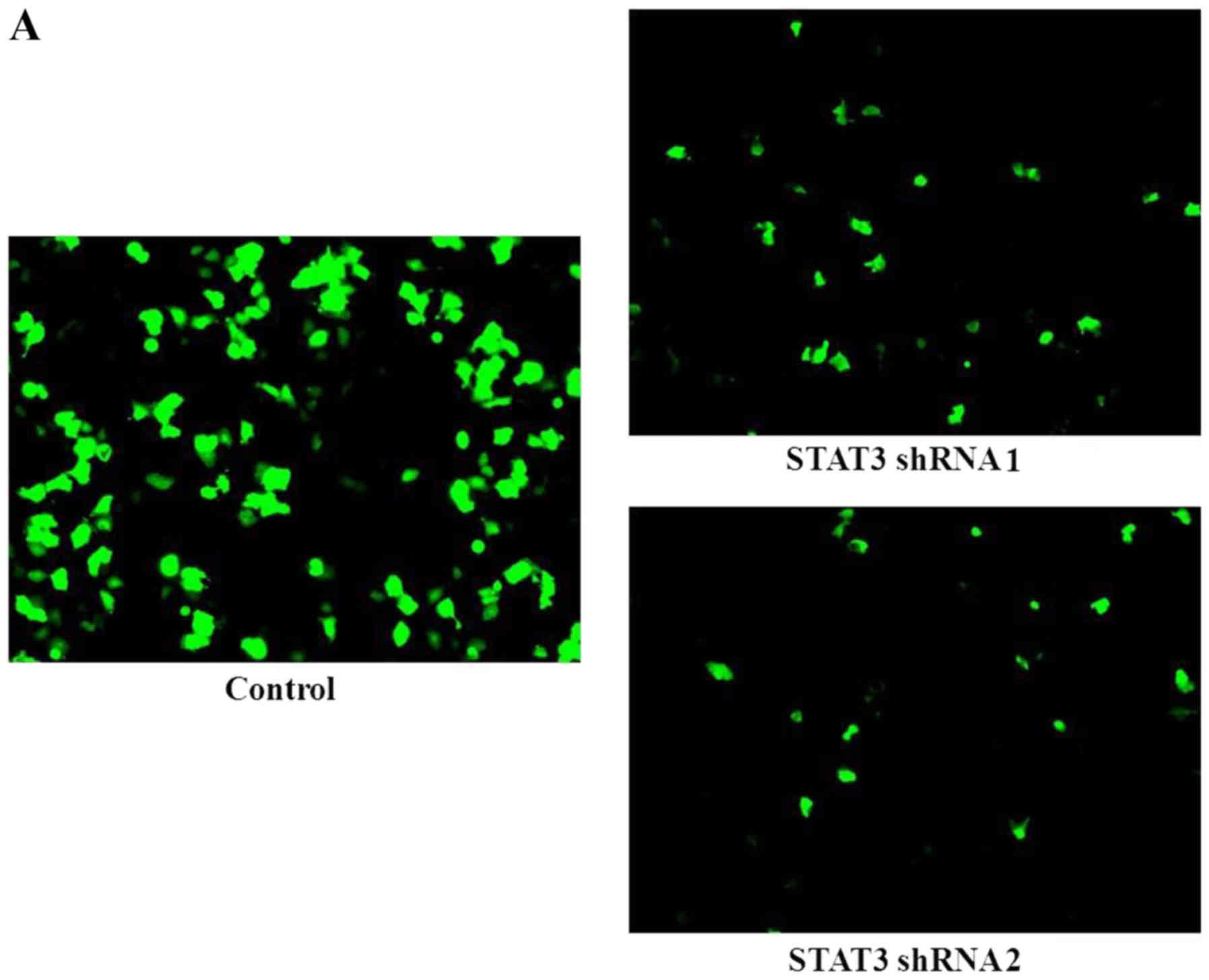
shRNA-mediated knockdown of STAT3 in TW01 and TW06
cells was further performed to establish the role of STAT3 in
anoikis-resistant NPC cells. Two STAT3 shRNAs (shRNA1 and shRNA2)
and scrambled shRNA as control were used for STAT3 knockdown. The
STAT3-transfected cells expressing GFP were processed for
fluorescence microscopy (Fig. 4A)
and constitutively stable cells were used in the following
experiments. The sensitivity to anoikis was further calculated and
the result demonstrated a significant decrease in anoikis
resistance as a result of STAT3 knockdown (Fig. 4B). Knockdown of STAT3 expression
confers a 55–75% reduction of anoikis resistance in TW01 cells and
a 40–65% reduction in TW06 cells. Our results demonstrated that the
percentage of anoikis-resistant cells was highly correlated with
the level of STAT3 expression.
Silencing STAT3 diminishes the
invasiveness of anoikis-resistant NPC cells
Since the results demonstrated that anoikis
resistance was associated with enhanced invasiveness, the Transwell
invasion assay was performed in TW01 and TW06 cells after silencing
STAT3. There was a significant decrease in invasion after STAT3
silencing in these tumor cells, and the extent of decreased
invasion was correlated with the level of STAT3 expression
(Fig. 4C). STAT3 silencing
contributed to a 75–85% decrease in the rate of invasion of TW01
cells, and there was a ~65–80% decrease in the rate of invasion of
TW06 cells after STAT3 silencing. Therefore, inhibition of STAT3
expression in TW01 and TW06 cells that have acquired anoikis
resistance not only increased sensitivity to anoikis, but also
interfered with their invasion capacity.
Silenced STAT3 decreases
epithelial-to-mesenchymal transition (EMT) markers and CD44
expression
As changes in invasive properties were significantly
correlated with STAT3 expression of anoikis-resistant NPC cells,
the effect of STAT3 knockdown on the expression of migration and
invasion regulatory proteins was further examined by western blot
analysis and CD44 expression by flow cytometry. The levels of
expression of EMT-related proteins, including E-cadherin,
N-cadherin and vimentin, was analyzed by western blot analysis and
CD44 expression was detected by flow cytometry in anoikis-resistant
TW01 cells with and without STAT3 silencing. Our data indicated
that knockdown of STAT3 significantly increased E-cadherin, but
decreased N-cadherin and vimentin expression, as evaluated by
western blot analysis (Fig. 5A).
In addition, the results of flow cytometry demonstrated that CD44
expression was significantly decreased in STAT3 shRNA-transfected
TW01 cells compared with non-specific shRNA-transfected TW01 cells
(Fig. 5B).
Discussion
NPC is associated with a high incidence of
recurrence and frequent initial dissemination to regional lymph
nodes, representing a major challenge in clinical practice.
Although radiotherapy and chemotherapeutic agents have been used in
the management of NPC, these tumors usually recur and metastasize
following treatment (14,15). Anoikis is apoptosis that occurs in
anchorage-dependent cells when they detach from the surrounding
extracellular matrix, and it has been suggested that cancer cells
develop different strategies for overcoming anoikis. Resistance to
anoikis may enable tumor cells to metastasize and facilitate tumor
formation in other sites (16,17). However, the mechanisms underlying
anoikis resistance in NPC cells, which may lead to a high rate of
metastasis, have not been fully elucidated. In the present study, a
considerable number of NPC cells were found to resist anoikis when
grown under anchorage-independent conditions. The cells that
resisted anoikis exhibited higher migratory and invasive properties
compared with the cells that were cultured under
anchorage-dependent condition. These highly migratory NPC cells may
survive from anchorage-independent cell cultures and exhibit
anoikis resistance compared with the poorly migratory NPC cell
line. These results suggest that anoikis resistance is an important
characteristic of more aggressive NPC cells.
Previous studies have demonstrated the involvement
of STAT3 in cellular growth signaling and its important role in
cell survival, proliferation and invasion (18,19). STAT3 has attracted significant
attention, as it is involved in malignant progression, and
constitutes an attractive therapeutic target. In addition, several
studies have demonstrated that STAT3 may induce anoikis resistance,
promoting tumor cell survival in certain cancers (20–22). Our results also revealed that
anoikis-resistant NPC cells exhibited significantly increased
expression of STAT3 with enhanced expression of Bcl-2 and survivin
compared with adherent cells.
The present study proposed the hypothesis that the
activation of STAT3 induces anoikis resistance in NPC cells and
promotes metastasis. We examined the expression of STAT3 in
anoikis-resistant tumor cells, and assessed whether inhibition of
STAT3 leads to sensitization of NPC cells to anoikis. Following
Stattic treatment, significant reduction in anoikis resistance was
observed in TW01 and TW06 cells. Moreover, a remarkable reduction
in anoikis resistance was observed following STAT3 silencing. The
level of STAT3 silencing was significantly correlated with
non-adherent cell survival, indicating that anoikis resistance of
NPC cells was dependent on STAT3. Notably, STAT3 silencing also
reduced the invasiveness and migratory ability of anoikis-resistant
NPC cells. The reduction in the rate of invasion was also found to
be significantly correlated with the level of STAT3 silencing by
shRNAs. These results revealed that the effect of STAT3 on anoikis
resistance was correlated with invasion in NPC. In support of the
abovementioned findings, there was a significant change in the
expression of EMT-related proteins, including N-cadherin and
vimentin, which was significantly inhibited after silencing STAT3.
Furthermore, knockdown of STAT3 significantly increased E-cadherin
expression, as evaluated by western blot analysis. A switch from
N-cadherin to E-cadherin expression indicates that STAT3 silencing
reverses EMT, and may exert a significant inhibitory effect on the
progression of NPC. Decreased CD44 expression was detected by flow
cytometry in anoikis-resistant NPC cells following STAT3 knockdown.
Previous studies demonstrated that CD44 promotes EMT and is
associated with cancer invasion through several mechanisms
(23,24). Our results further revealed that
STAT3 represents a link between anoikis suppression and EMT, both
of which may contribute to metastasis. Since increased EMT-related
characteristics have been found to be associated with worse
outcomes in patients with NPC (25,26) these data support that the
constitutive activation of STAT3 is predictive of poor prognosis.
These findings are in agreement with previous studies investigating
the role of STAT3 in the aggressiveness of various tumors (27,28). The important role of STAT3 in
conferring resistance to anoikis and promoting metastasis in NPC
was further investigated. In conclusion, the present study
elucidated the association of STAT3-mediated anoikis resistance
with enhanced cell migration and invasion in NPC. The findings
suggest that targeting STAT3 may be an important therapeutic
strategy for preventing or inhibiting anoikis sensitization and
cellular invasiveness in NPC.
Acknowledgments
This study was supported by a grant from the Taipei
City Hospital (TPCH-105-025) and the Department of Health, Taipei
City Government, Taipei, Taiwan.
References
|
1
|
Yeh SA, Tang Y, Lui CC, Huang YJ and Huang
EY: Treatment outcomes and late complications of 849 patients with
nasopharyngeal carcinoma treated with radiotherapy alone. Int J
Radiat Oncol Biol Phys. 62:672–679. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun JD, Chen CZ, Chen JZ, Li DS, Chen ZJ,
Zhou MZ and Li DR: Long term outcomes and prognostic factors of n0
stage nasopharyngeal carcinoma: A single institutional experience
with 610 patients. Asian Pac J Cancer Prev. 13:2101–2107. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagaprashantha LD, Vatsyayan R, Lelsani
PC, Awasthi S and Singhal SS: The sensors and regulators of
cell-matrix surveillance in anoikis resistance of tumors. Int J
Cancer. 128:743–752. 2011. View Article : Google Scholar
|
|
4
|
Zhong X and Rescorla FJ: Cell surface
adhesion molecules and adhesion-initiated signaling: Understanding
of anoikis resistance mechanisms and therapeutic opportunities.
Cell Signal. 24:393–401. 2012. View Article : Google Scholar
|
|
5
|
Morimoto-Tomita M, Ohashi Y, Matsubara A,
Tsuiji M and Irimura T: Mouse colon carcinoma cells established for
high incidence of experimental hepatic metastasis exhibit
accelerated and anchorage-independent growth. Clin Exp Metastasis.
22:513–521. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Egwuagu CE: STAT3 in CD4+ T
helper cell differentiation and inflammatory diseases. Cytokine.
47:149–156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Konnikova L, Simeone MC, Kruger MM,
Kotecki M and Cochran BH: Signal transducer and activator of
transcription 3 (STAT3) regulates human telomerase reverse
transcriptase (hTERT) expression in human cancer and primary cells.
Cancer Res. 65:6516–6520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Z and Han ZC: STAT3: A critical
transcription activator in angiogenesis. Med Res Rev. 28:185–200.
2008. View Article : Google Scholar
|
|
9
|
Peyser ND and Grandis JR: Critical
analysis of the potential for targeting STAT3 in human malignancy.
Onco Targets Ther. 6:999–1010. 2013.PubMed/NCBI
|
|
10
|
Banerjee K and Resat H: Constitutive
activation of STAT3 in breast cancer cells: A review. Int J Cancer.
138:2570–2578. 2016. View Article : Google Scholar :
|
|
11
|
Weerasinghe P, Garcia GE, Zhu Q, Yuan P,
Feng L, Mao L and Jing N: Inhibition of Stat3 activation and tumor
growth suppression of non-small cell lung cancer by G-quartet
oligonucleotides. Int J Oncol. 31:129–136. 2007.PubMed/NCBI
|
|
12
|
Lui VW, Wong EY, Ho Y, Hong B, Wong SC,
Tao Q, Choi GC, Au TC, Ho K, Yau DM, et al: STAT3 activation
contributes directly to Epstein-Barr virus-mediated invasiveness of
nasopharyngeal cancer cells in vitro. Int J Cancer. 125:1884–1893.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Z, Luo F, Li L, Yang L, Hu D, Ma X,
Lu Z, Sun L and Cao Y: STAT3 activation induced by Epstein-Barr
virus latent membrane protein1 causes vascular endothelial growth
factor expression and cellular invasiveness via JAK3 And ERK
signaling. Eur J Cancer. 46:2996–3006. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bensouda Y, Kaikani W, Ahbeddou N, Rahhali
R, Jabri M, Mrabti H, Boussen H and Errihani H: Treatment for
metastatic nasopharyngeal carcinoma. Eur Ann Otorhinolaryngol Head
Neck Dis. 128:79–85. 2011. View Article : Google Scholar
|
|
15
|
Pan CC, Lu J, Yu JR, Chen P, Li W, Huang
ZL, Zhao M, Huang ZM, Xia YF, Wu YH, et al: Challenges in the
modification of the M1 stage of the TNM staging system for
nasopharyngeal carcinoma: A study of 1027 cases and review of the
literature. Exp Ther Med. 4:334–338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tan K, Goldstein D, Crowe P and Yang JL:
Uncovering a key to the process of metastasis in human cancers: A
review of critical regulators of anoikis. J Cancer Res Clin Oncol.
139:1795–1805. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paoli P, Giannoni E and Chiarugi P:
Anoikis molecular pathways and its role in cancer progression.
Biochim Biophys Acta. 1833:3481–3498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Raptis L, Arulanandam R, Vultur A, Geletu
M, Chevalier S and Feracci H: Beyond structure, to survival:
Activation of Stat3 by cadherin engagement. Biochem Cell Biol.
87:835–843. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fitzgerald JS, Poehlmann TG, Schleussner E
and Markert UR: Trophoblast invasion: The role of intracellular
cytokine signalling via signal transducer and activator of
transcription 3 (STAT3). Hum Reprod Update. 14:335–344. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang W, Zong CS, Hermanto U,
Lopez-Bergami P, Ronai Z and Wang LH: RACK1 recruits STAT3
specifically to insulin and insulin-like growth factor 1 receptors
for activation, which is important for regulating
anchorage-independent growth. Mol Cell Biol. 26:413–424. 2006.
View Article : Google Scholar :
|
|
21
|
Cheng HL, Su SJ, Huang LW, Hsieh BS, Hu
YC, Hung TC and Chang KL: Arecoline induces HA22T/VGH hepatoma
cells to undergo anoikis - involvement of STAT3 and RhoA
activation. Mol Cancer. 9:1262010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu Y, Chen H, Duan C, Liu D, Qian L, Yang
Z, Guo L, Song L, Yu M, Hu M, et al: Deficiency of Erbin induces
resistance of cervical cancer cells to anoikis in a STAT3-dependent
manner. Oncogenesis. 2:e522013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao Y, Ruan B, Liu W, Wang J, Yang X,
Zhang Z, Li X, Duan J, Zhang F, Ding R, et al: Knockdown of CD44
inhibits the invasion and metastasis of hepatocellular carcinoma
both in vitro and in vivo by reversing epithelial-mesenchymal
transition. Oncotarget. 6:7828–7837. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin CH, Hung PH and Chen YJ: CD44 is
associated with the aggressive phenotype of nasopharyngeal
carcinoma through redox regulation. Int J Mol Sci. 14:13266–13281.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo W and Yao K: Molecular
characterization and clinical implications of spindle cells in
nasopharyngeal carcinoma: A novel molecule-morphology model of
tumor progression proposed. PLoS One. 8:e831352013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin JC, Liao SK, Lee EH, Hung MS, Sayion
Y, Chen HC, Kang CC, Huang LS and Cherng JM: Molecular events
associated with epithelial to mesenchymal transition of
nasopharyngeal carcinoma cells in the absence of Epstein-Barr virus
genome. J Biomed Sci. 16:1052009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kamran MZ, Patil P and Gude RP: Role of
STAT3 in cancer metastasis and translational advances. BioMed Res
Int. 2013:4218212013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Devarajan E and Huang S: STAT3 as a
central regulator of tumor metastases. Curr Mol Med. 9:626–633.
2009. View Article : Google Scholar : PubMed/NCBI
|