Introduction
Osteoporosis (OP) is a systemic metabolic bone
disease characterized by an increase in bone fragility and
susceptibility to bone fracture. It not only reduces the quality of
life of elderly individuals, but also causes significant financial
burden to patients' families and society. Despite the fact that OP
has developed into a serious social issue that has gained extensive
attention worldwide (1), the
pathogenesis of OP remains unclear.
Blood vessels are an important factor in the process
of bone formation, and angiogenesis plays an important role in
maintaining the dynamic balance between bone regeneration and
resorption (2). Exogenous
vascular endothelial growth factor (VEGF) has been shown to enhance
fracture repair in a mouse model of femoral fracture by promoting
angiogenesis, ossification, and bone turnover (3). Similarly, angiogenesis is an
important feature of fracture repair, where an adequate blood
supply is the basis of successful bone regeneration (4). Furthermore, inhibition of
angiogenesis has been shown to inhibit fracture repair (5). The incidence of postmenopausal OP is
associated with a reduction in bone marrow microvessels, and the
promotion of angiogenesis exhibits anti-osteoporotic effects
(6). Therefore, in view of the
importance of angiogenesis in the growth and development of bone,
it may be closely associated with OP (7–10).
Vascular endothelial cells (VECs) are simple
squamous cells that exhibit selective permeability, regulate
vascular tone, promote blood capillary formation, and exhibit other
important physiological functions. Several studies have shown that
apoptosis in VECs is closely associated with endoplasmic reticulum
(ER) stress and mitochondrial depolarization, and that inhibition
of apoptosis can protect the vascular endothelium (11,12). In addition, VECs secrete various
vasoactive substances that regulate vascular function such as
endothelin (ET) and nitric oxide (NO), which can further affect
bone metabolism. Prispy et al (13) found that reduction in metaphyseal
bone mass is associated with decreased bone marrow blood flow in
elderly patients, and is induced by inhibition of
endothelium-dependent vasodilation. Furthermore, physical
exercise-induced increase in bone mass was shown to be associated
with enhanced endothelium-dependent vasodilation via the NO
synthase signaling pathway (14).
Naringin, a flavonoid compound, is the main active
ingredient in the traditional Chinese medicinal plant
Drynaria, and exerts various biological and pharmacological
effects (15). Drynaria is
widely used in the treatment of OP, although its mechanism remains
unclear (9). Studies have shown
that naringin promotes the differentiation and proliferation of
various types of cells (16–18), and was found to improve bone mass
in an osteoporotic rat model (19). Naringin also promoted osteoclast
apoptosis through the mitochondrial-mediated apoptotic pathway,
thereby inhibiting bone loss in an ovariectomized (OVX) rat model
of OP and exhibiting anti-osteoporotic pharmacological activity
(20). However, its effects on
VECs and angiogenesis remain unclear. The present study explored
the effects of naringin on the function, proliferation, and
apoptosis of VECs, as well as on angiogenesis and its mechanisms,
to provide a theoretical basis for the clinical application of
naringin in the treatment of OP.
Materials and methods
In vitro experiments
Culture of rat VECs
All protocols were approved by the Renji Hospital
Animal Care and Ethics Committee. Lung tissue was aseptically
excised from 1-day-old newborn Sprague-Dawley (SD) rats and rinsed
with phosphate-buffered saline (PBS) to remove the pleura. Tissue
was trimmed into 1-mm3 tissue blocks, cultured in
Dulbecco's modified Eagle's medium (DMEM) low-glucose medium
supplemented with 10% serum in 6-cm culture dishes, and incubated
at 37°C and 5% CO2 for about 3–5 days until cells
emerged from the tissue edges. Cells were digested with 0.25%
trypsin, filtered to remove tissue, and further cultured for 1
week. After that, cells were digested and sub-cultured until the
fifth passage before use.
MTT cell proliferation assay
MTT assay was performed as described previously
(18). Briefly, VECs were seeded
at a density of 1×104 cells/well into a 96-well plate.
After 12 h of culture, cells were treated with 10% fetal bovine
serum (FBS) containing 0, 1, 10, 50, 100, or 200 μg/ml
naringin (Sigma-Aldrich, St. Louis, MO, USA) for 12, 24, 36, 48,
60, 72, or 96 h. Next, cells were collected and an MTT assay was
performed according to the manufacturer's instructions (Beyotime
Institute of Biotechnology, Shanghai, China).
Hoechst 33258 staining
VECs were randomly divided into four groups: control
(10% FBS), serum starvation (0% FBS), low-concentration treatment
(0% FBS + 10 μg/ml naringin), and high-concentration
treatment (0% FBS + 100 μg/ml naringin). Serum-free
starvation culture conditions were established by growing cells to
80–90% confluence before discarding the medium, washing cells twice
with PBS, and culturing in FBS-free DMEM. This cell preparation
procedure was repeated for subsequent cytological assays.
For Hoechst 33258 staining, cells were seeded at
1×105 cells/well into a 6-well plate. Cells were treated
for 24 h, and cellular morphological changes were observed by
inverted phase contrast microscopy. Culture medium was removed and
Hoechst 33258 staining solution (Beyotime Institute of
Biotechnology) was added to the cells. Morphological nuclear
changes were observed under fluorescence microscopy.
Detection of apoptosis by flow
cytometry
Cells were seeded at 2×105 cells/well
into 6-cm culture dishes and treated as described above for 24 or
48 h prior to digestion with 0.05% trypsin-EDTA to harvest the
cells. Cells were then subjected to Annexin V/propidium iodide (PI)
staining and flow cytometry was performed according to the
manufacturer's instructions (BD Pharmingen, San Diego, CA, USA).
Apoptotic cells were counted and represented as a percentage of the
total cell count.
Detection of proteins associated with
apoptosis by western blotting
Cells were seeded at 5×105 cells/well
into 6-cm culture dishes and treated for 24 h as described above,
after which 100 μl 2X SDS cell lysis solution was added per
dish. Cellular proteins were then harvested and stored at −70°C. A
mitochondrial and cytosolic protein preparation kit (Applygen
Technologies, Inc., Beijing, China) was used to extract cytoplasmic
proteins for the detection of cytochrome c (Cyt.c). After
measurement of protein concentrations, 40 μg of protein per
sample was subjected to electrophoresis and subsequently
transferred to a nitrocellulose membrane (Bio-Rad, Berkeley, CA,
USA). The membrane was blocked using skimmed milk and incubated
overnight at 4°C with primary antibodies for GRP78 (sc-13968), CHOP
(sc-7351), caspase-12 (sc-5627), or Cyt.c (sc-13561) (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) at 1:100 dilution, or
with antibody for GAPDH (sc-47724; Santa Cruz Biotechnology, Inc.)
at a 1:750 dilution. Next, the membrane was washed and incubated at
room temperature for 2 h with secondary antibodies (cat. no. A0192;
Beyotime Institute of Biotechnology), followed by enhanced
chemiluminescence (ECL) autoradiography. Band intensity was
measured using an ImageMaster™ VDS video documentation system
(Amersham Biosciences Inc., Piscataway, NJ) and represented as
fold-change relative to the GAPDH control.
Detection of changes in mitochondrial
membrane potential by JC-1 staining
Cells were seeded at 1×105 cells/well
into 6-well plates and treated for 24 h. JC-1 probe (Beyotime
Institute of Biotechnology) was added to detect changes in
mitochondrial membrane potential as described in our previous study
(21).
Detection of caspase-3, -8, and -9
activities
Cells were seeded at 5×105 cells/well
into 6-cm culture dishes, and harvested after 24 h of treatment.
Caspase-3, -8, and -9 activities were detected using a caspase-3,
-8, and -9 activity assay kit (Beyotime Institute of
Biotechnology), and OD at 405 nm was measured using a microplate
spectrophotometer (BioTek, Winooski, VT, USA). The caspase activity
of cells in each treatment group was represented as fold-change in
absorbance relative to control.
Detection of ET and NO in culture
medium
Cells were seeded at 5×105 cells/well
into 6-cm culture dishes and treated for 24 or 48 h before 100
μl of the supernatant was collected from each well and
centrifuged to remove impurities and cell debris. ET was detected
by ELISA. A standard curve was obtained via serial dilution of
standards included in the assay kit (Beyotime Institute of
Biotechnology). Each sample was aliquoted into a microtiter plate
and OD at 450 nm was measured. NO was detected using a Griess assay
(Beyotime Institute of Biotechnology), carried out in accordance
with the manufacturer's instructions and with OD measured at 540 nm
using a microplate spectrophotometer.
In vivo experiments
Postmenopausal osteoporotic rat model
grouping and drug treatment
Six-month-old female SD rats (body weight, 220±8 g)
were provided by the Experimental Animal Center of Renji Hospital.
The experimental animals received humane care, and the study
protocol conformed to the guidelines of the Renji Hospital Ethics
Committee. Forty-eight rats were randomly divided into four groups
(n=12 per group): OVX, sham, high-concentration naringin treatment
(200 mg/kg), and low-concentration naringin treatment (100 mg/kg).
After rats were anesthetized via an intraperitoneal injection of
ketamine (75 mg/kg), bilateral incisions were made at the lower
back into the abdominal cavity. The incision wounds were washed and
sutured after removing both ovaries. For the sham group, fat of
equivalent weight to an ovary was excised from the vicinity of each
ovary. On the third day after ovariectomy, rats in the high- and
low-concentration treatment groups were administered naringin
solution by daily oral gavage. Rats in the OVX and sham groups were
administered physiological saline solution (6 ml/kg daily) by oral
gavage. After 3 months of treatment, rats were sacrificed by
anesthesia with an intra-peritoneal injection of sodium
pentobarbital (40 mg/kg).
Bone densitometry
Dual-energy X-ray absorptiometry (DXA; GE
Healthcare, Piscataway, NJ, USA) was used to measure bone mineral
density (BMD) of the fourth lumbar vertebra (L4), and data analyses
were carried out using pre-installed software.
Detection of ET and NO in serum
Blood was collected from the abdominal aorta of rats
prior to sacrifice and centrifuged. Changes in ET and NO expression
were detected in 100 μl aliquots of the resulting serum
using the method described above.
Bone marrow angiogenesis assay
Distal femur specimens from rats were routinely
decalcified and embedded in paraffin. Immunohistochemical staining
of VECs for CD34 was carried out according to the manufacturer's
instructions (R&D Systems, Inc., Minneapolis, MN, USA).
Specimens were observed under a microscope at ×100 magnification
and microvessels in three randomly selected fields of view were
counted to obtain an average value (22).
Statistical analysis
The results of each measurement are represented as
mean ± SD and data processing was performed using SPSS v18.0
software (SPSS Inc., Chicago, IL, USA). One-way analysis of
variance (ANOVA) was used for comparisons between groups, and
P<0.05 was considered statistically significant.
Results
Effect of naringin on VEC
proliferation
The MTT assay results showed that each group treated
with a naringin dose of 1-100 μg/ml had a significantly
higher cell proliferation rate than the control group, and that the
proliferation-promoting effect increased significantly in a
concentration-dependent manner up to 100 μg/ml. However, the
proliferation-promoting effect ceased at 200 μg/ml, an
effect that may be related to drug toxicity. These results revealed
that naringin promoted the proliferation of VECs in a specific
time- and dose-dependent manner (Fig.
1).
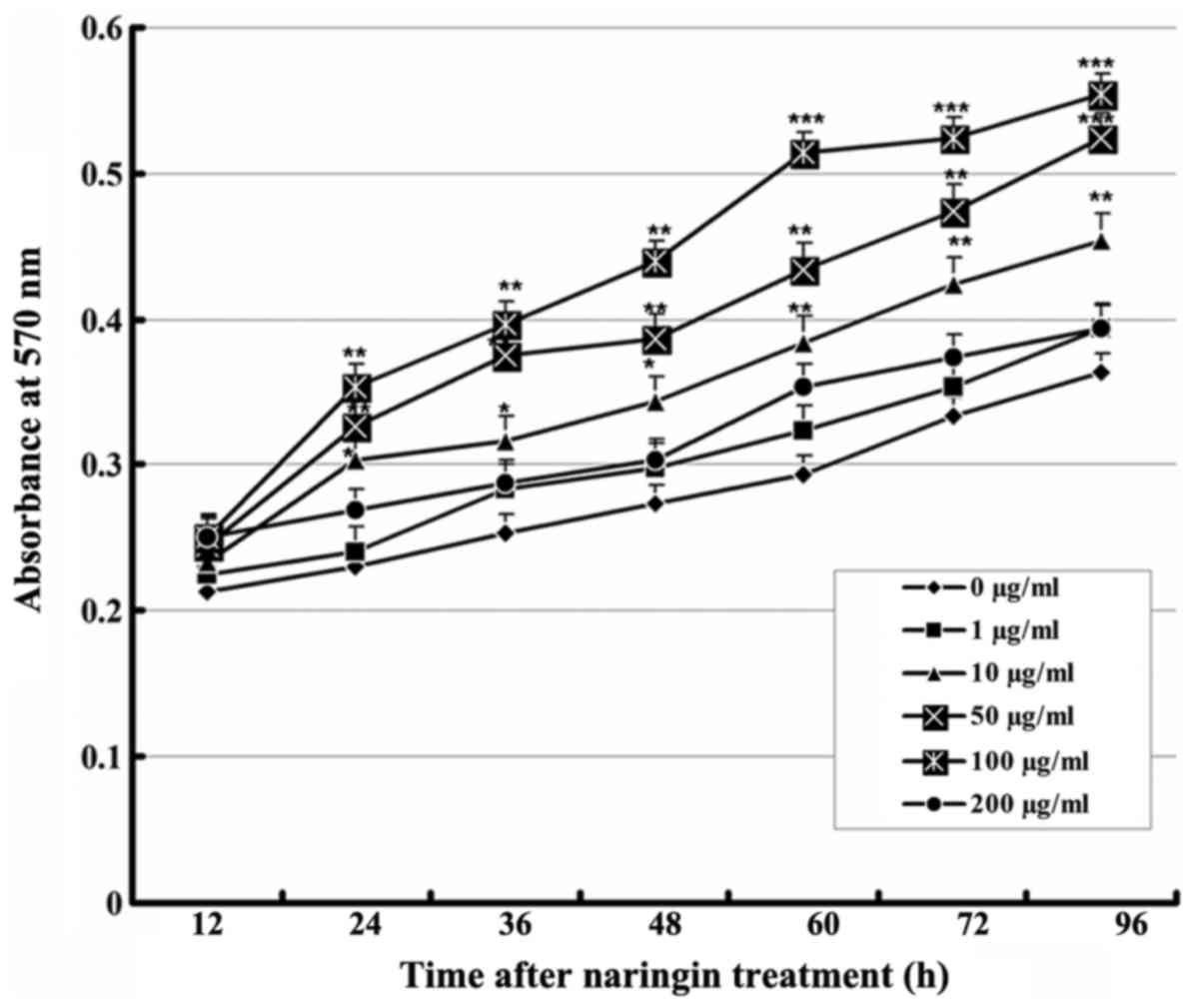 | Figure 1Effect of naringin on the
proliferation of VECs. Cells were cultured with vehicle or various
concentrations of naringin. At 12, 24, 36, 48, 60, 72, and 96 h
after treatment, absorbance at 570 nm was measured (n=9).
Comparison with the control group: *P<0.05,
**P<0.01, ***P<0.001. VECs, vascular
endothelial cells. |
Effects of naringin on serum
starvation-induced apoptosis in VECs
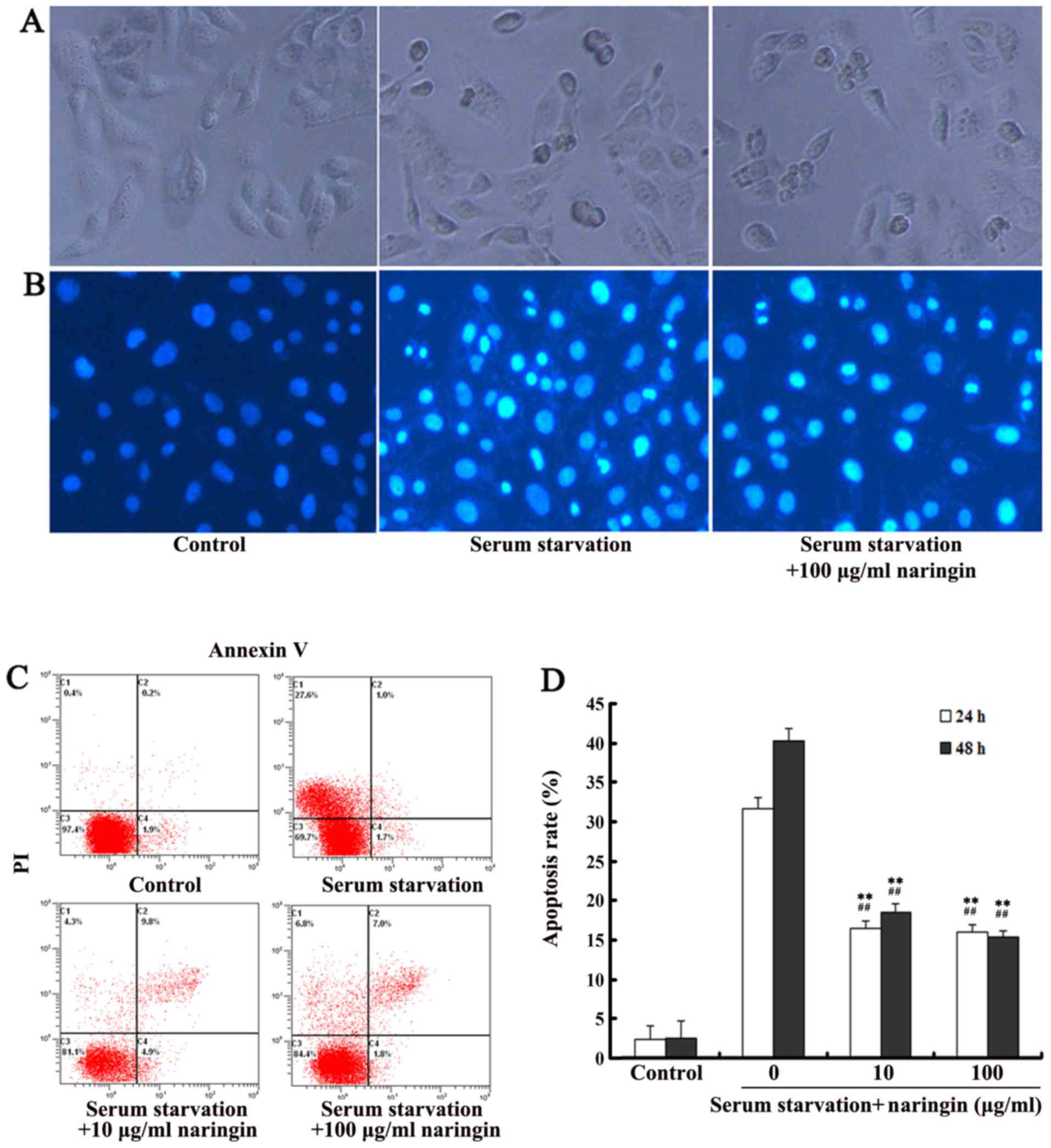
After 24 h, cells in the serum starvation group
showed apoptotic morphology including cell rounding and shrinkage,
vacuolization, and detachment from the plate (Fig. 2A); Hoechst 33258 staining showed
increased chromatin condensation and nuclear fragmentation.
However, treatment with a high concentration of naringin
effectively suppressed these phenomena (Fig. 2B). After cells were starved for 24
and 48 h, flow cytometry showed that the average apoptosis ratio in
the control VECs was only 2.3±0.8%, but was significantly increased
up to 31.1±1.6 and 39.6±1.7%, respectively. The apoptosis ratio in
both the high- and low-concentration naringin treatment groups was
significantly decreased compared to that in the serum starvation
group (P<0.01; Fig. 2C and
D).
Effects of naringin on the expression of
proteins associated with ER stress-induced apoptosis
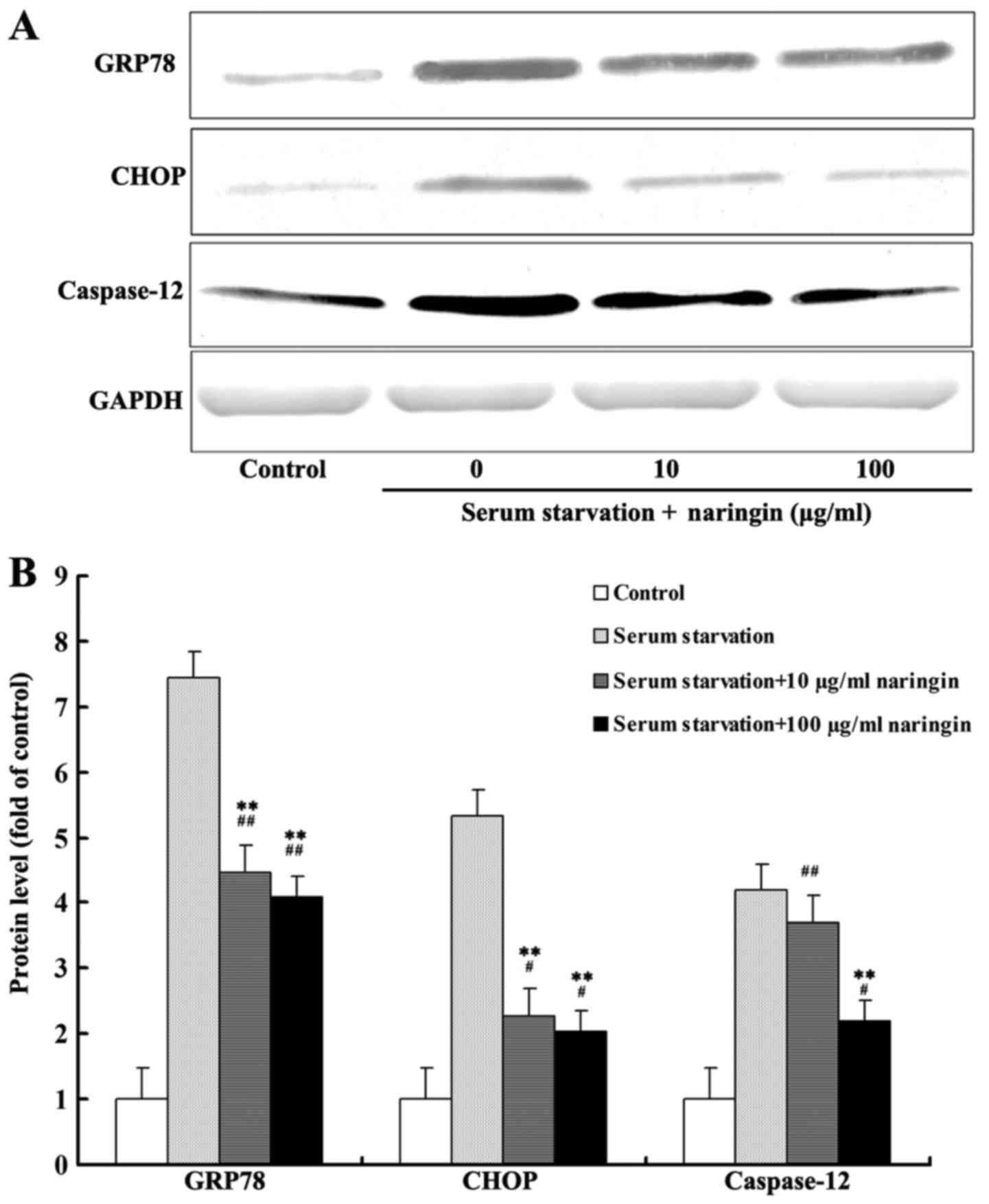
After 24 h of serum starvation, protein expression
of GRP78, CHOP, and caspase-12 was increased significantly compared
with that noted in the control group. The expression of GRP78 and
CHOP in both the high- and low-concentration naringin treatment
groups was significantly decreased compared to that in the serum
starvation group. Furthermore, caspase-12 expression in the
high-concentration treatment group was significantly decreased
compared to that in the serum starvation group. Although the
expression of caspase-12 was also decreased in the
low-concentration treatment group, the difference was statistically
insignificant compared with the serum starvation group (Fig. 3A and B). According to our results,
the inhibition of these three proteins by naringin did not reach
control levels, suggesting that naringin partially inhibits the
expression of proteins associated with starvation-induced ER
stress.
Effects of naringin on the
mitochondrial-mediated apoptotic pathway
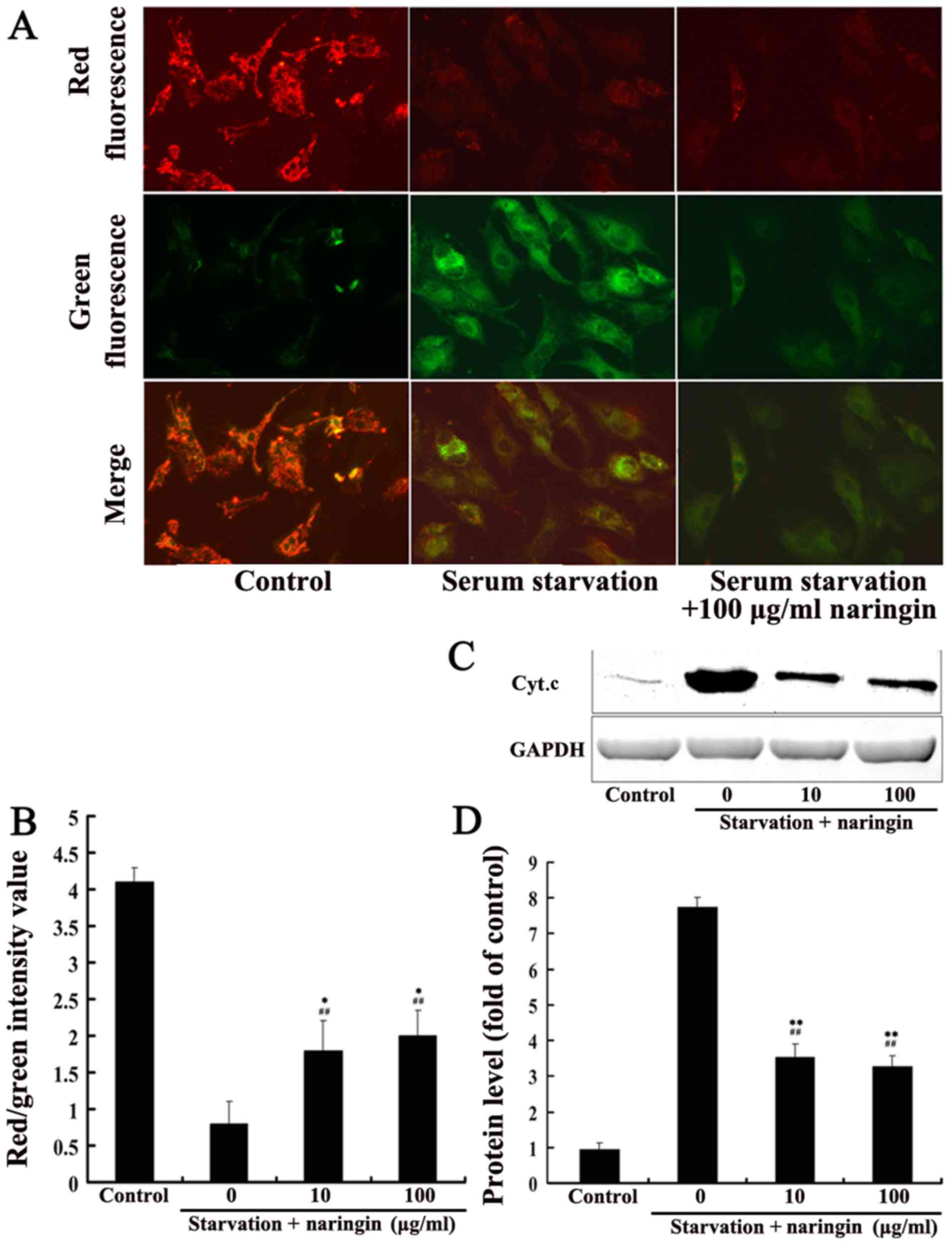
After 24 h of serum starvation, the mitochondrial
membrane potential of the serum starvation group was significantly
decreased compared with that of the control group (average value of
red/green fluorescence intensity, 0.6±0.2 vs. 3.6±0.3, P<0.01).
The mitochondrial membrane potential in both the high- and
low-concentration naringin treatment groups (1.5±0.3 and 1.8±0.4,
respectively) was significantly increased compared to that in the
serum starvation group (3.6±0.3) but did not reach control levels,
suggesting that naringin partially inhibits the mitochondrial
membrane depolarization induced by starvation (Fig. 4A and B). After 24 h of starvation,
Cyt.c protein expression in the cytoplasm was significantly
increased, and both the low and high concentrations of naringin
significantly inhibited the release of Cyt.c protein into the
cytoplasm (P<0.01) (Fig. 4C and
D).
Effects of naringin on caspase-3, -8, and
-9 activities
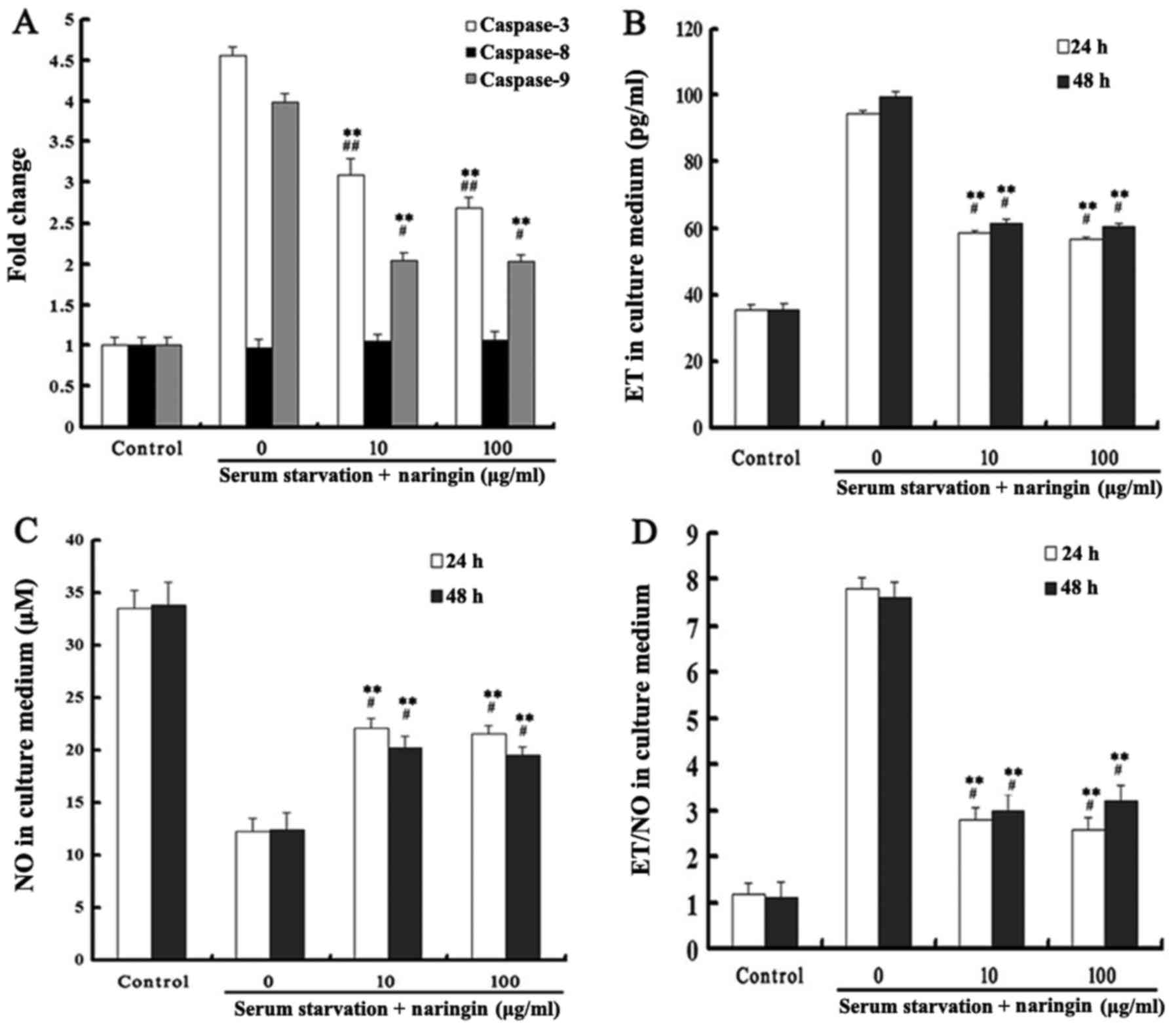
Caspase-3 is an important protein for apoptosis
execution, while the activation of caspase-9 plays a key role in
the mitochondrial-mediated apoptotic pathway. Our results showed
that caspase-3 and -9 activities were significantly increased after
24 h of starvation, and that both high and low concentrations of
naringin significantly inhibited caspase-3 and -9 activities
(P<0.05). Caspase-8 activity in each treatment group did not
change (Fig. 5A).
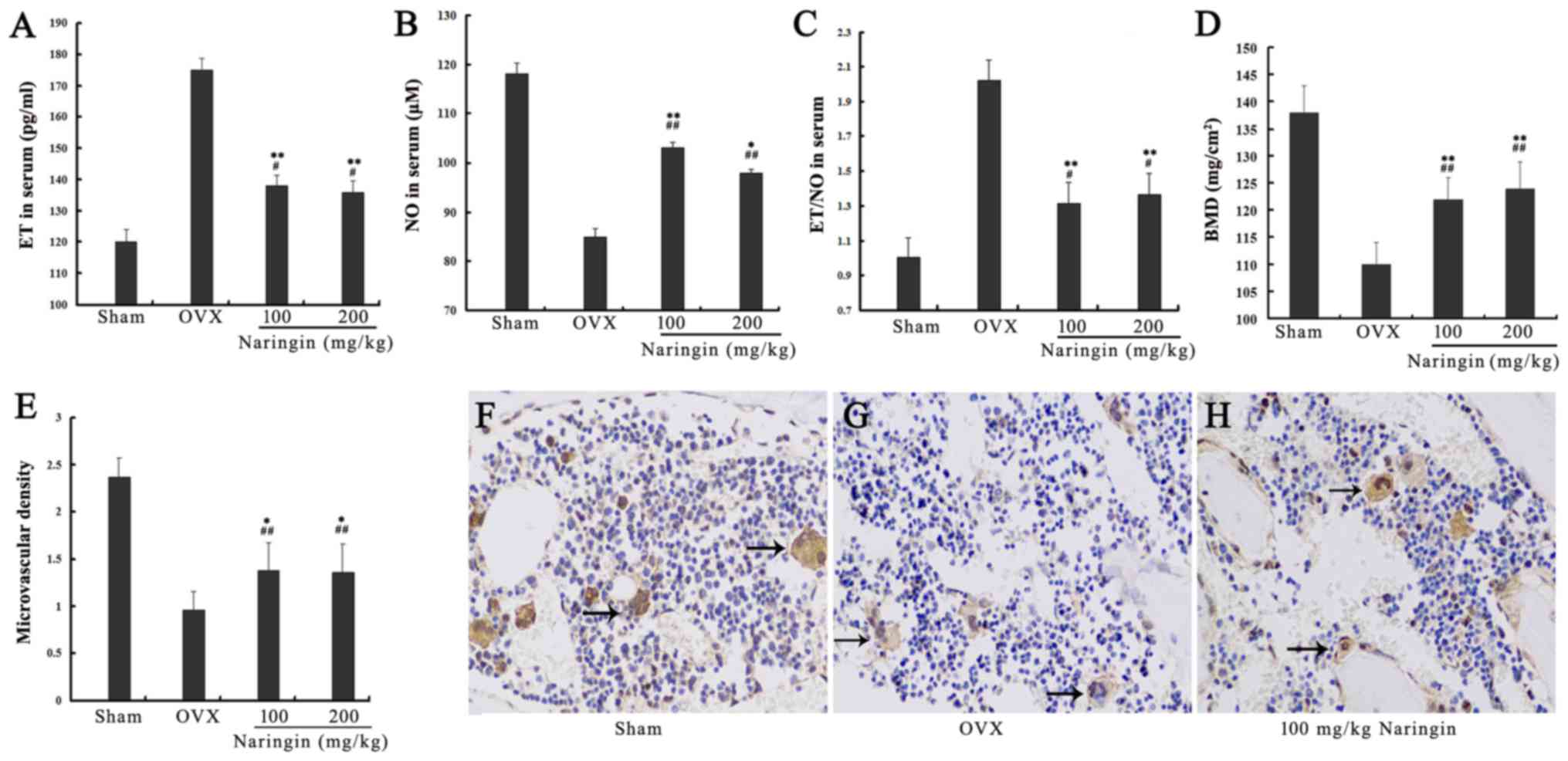
Effects of naringin on ET and NO
expression
After serum starvation, the expression of ET was
significantly increased while the expression of NO was decreased in
the serum starvation group. Both high and low concentrations of
naringin significantly reduced the ratio of ET to NO by inhibiting
the expression of ET while enhancing the expression of NO.
Differences in ET and NO levels between 24 and 48 h of starvation
and between high and low concentrations of naringin were not
statistically significant (P>0.05). These results indicated that
naringin achieved drug efficacy at a low concentration at 24 h, and
that a further increase in drug concentration and exposure time did
not enhance the effectiveness of the drug (Fig. 5B–D). After OVX rats were
administered high and low concentrations of naringin for 3 months,
the expression of ET in serum was significantly decreased
(P<0.05) while the expression of NO was significantly increased
compared with the OVX group (P<0.05). However, the expression
levels of both ET and NO did not decrease to the levels observed in
the sham group (P<0.05) (Fig.
6A–C).
Effects of naringin on BMD
BMD of the OVX group was significantly decreased
compared to that of the sham group, indicating that the model
design was successful. The BMD of the fourth lumbar vertebra (L4)
in both the high- and low-concentration naringin treatment groups
was significantly increased compared to that of the OVX group after
surgery (P<0.05), but did not increase to the BMD levels of the
sham group (Fig. 6D).
Effects of naringin on microvascular
angiogenesis
The results of microvascular density analysis
(number of microvessels/×100 magnification) showed that
microvascular density in both the high- and low-concentration
naringin groups was increased significantly compared to that in the
OVX group (P<0.05), although it was lower than the sham group
microvascular density (Fig.
6E–H).
Discussion
VECs and new blood vessels are important factors in
the process of bone formation (2). Evidence has shown that
postmenopausal OP may be associated with the apoptosis and
dysfunction of VECs and a decrease in bone marrow microvessels, and
that the promotion of angiogenesis exhibits anti-osteoporotic
effects (6,7,9).
Naringin, the main active ingredient of the traditional Chinese
medicinal fern Drynaria, is characterized by estrogen-like
properties that exhibit therapeutic effects on OP by a mechanism
that has not been elucidated (16,20). Numerous studies have shown that
naringin promotes cell proliferation and inhibits cell apoptosis
(16–18,20,23,24). However, its effects on VEC
apoptosis and function, as well as on angiogenesis, have not been
reported. This study showed, for the first time, that naringin
exhibits a significant proliferation-promoting effect on VECs in a
time- and dose-dependent manner. Previous studies of the induction
of endothelial cell apoptosis in vitro mostly used exogenous
apoptosis-inducing agents (such as hydrogen peroxide) to construct
an apoptosis model (25), a
method which may not accurately reflect in vivo conditions.
Therefore, in this study, we employed a serum starvation-induced
model of apoptosis to simulate an in vivo-like pathological
state of nutritional deficiency in VECs (26,27). Our results showed that we
successfully induced apoptotic cell death by serum starvation,
according to the cellular and nuclear morphological changes
observed in VECs, similar to previous studies (26,27). Previous studies found that
naringin inhibited apoptosis in human neuroblastoma cells and
neurons (23,24), further supporting our findings
that the apoptosis ratio was significantly decreased in both the
high- and low-concentration naringin treatment groups compared to
that in the serum starvation group. These results indicated that
naringin reduced the serum starvation-induced apoptosis ratio in
VECs, but was incapable of completely inhibiting apoptosis.
There are two classical apoptotic pathways: the
death receptor pathway and the mitochondrial-mediated pathway, of
which the former recruits and activates primarily caspase-8 through
the binding of the Fas ligand on the cell membrane to its receptor,
leading to cell degradation. Mitochondrial permeability transition
results in the reduction in mitochondrial membrane potential and
release of Cyt.c into the cytoplasm, activating caspase-9 to cause
apoptosis via the mitochondrial-mediated pathway (28). In the newly discovered ER
stress-mediated apoptotic pathway, severe ER stress leads to the
upregulation of proteins such as GRP78, CHOP, and caspase-12,
leading to apoptosis (29).
Caspase-3 is the central component of apoptotic pathways. Our
results showed that the increase in apoptosis ratio induced by
serum starvation was correlated with increased expression of Cyt.c,
caspase-9, and proteins associated with ER stress (GRP78, CHOP, and
caspase-12), and decreased mitochondrial membrane potential. These
findings indicated that serum starvation induced apoptosis in VECs
via ER stress- and mitochondrial-mediated pathways. However, the
expression of caspase-8 was unaffected by this process, suggesting
that the death receptor-mediated pathway of apoptosis was not
involved. Intervention with both high and low concentrations of
naringin inhibited the expression of GRP78, CHOP, Cyt.c, and
caspases -3, -9, and -12 as well as decreased the mitochondrial
membrane potential, thereby reducing the apoptosis ratio. These
results preliminarily indicate that naringin inhibits
starvation-induced apoptosis in VECs by inhibiting the
mitochondrial- and ER stress-mediated apoptotic pathways. These
findings are similar to results reported by Kim et al
(23), who showed that naringin
inhibited apoptosis induced by rotenone in human neuroblastoma
cells by reducing caspase-3 and -9 activity. However, we found that
the inhibitory effect of naringin on apoptosis did not reach the
level of the control group, suggesting other apoptotic pathways may
participate in starvation-induced apoptosis in VECs in addition to
the ER stress- and mitochondrial-mediated pathways. Estrogen
receptor activation can inhibit apoptosis in VECs by regulating the
ER stress-mediated pathway (30),
and some studies have shown that naringin has estrogen-like effects
(16,31). Whether these effects play a role
in inhibiting apoptosis, however, requires verification by further
studies.
Vascular endothelium can produce and release various
vasoactive substances after injury, the most important of which are
ET and NO (32). ET exerts a
significant vasoconstrictive effect, and studies have found that
serum levels of ET in postmenopausal OP patients were significantly
decreased after hormone replacement therapy with estrogen,
suggesting that ET can affect bone metabolism (33,34). NO is an endothelium-derived
relaxing factor (EDRF) with multiple beneficial effects such as
vasodilation, inhibition of oxygen-derived free radicals, and
prevention of leukocyte adhesion to vessel walls. Studies have
shown that naringin can regulate the synthesis of NO, thus
improving Huntington's disease-like symptoms in rats (35) and post-stroke depression in mice
via NO modulation (36). Recent
studies have demonstrated that ET/NO are important
vasoconstrictory/vasodilatory factors and that disruption of their
balance leads to microcirculatory disturbances, affecting tissue
metabolism (32). Therefore, some
studies have suggested that ET/NO disruption directly affects bone
microcirculation, preventing blood nutrients from being transported
into bone tissue and thereby affecting bone metabolism,
contributing to the pathogenesis of OP (9). Our in vitro experiments
showed that both low and high concentrations of naringin reduced
the ET/NO ratio in culture medium by inhibiting the excretion of ET
by endothelial cells while enhancing NO expression. We further
tested naringin in the OVX rat model of OP and found that it exerts
a similar in vivo and in vitro regulatory function on
ET and NO, and is associated with BMD in rats. These results
indicate that naringin allows VECs to function normally by
regulating ET and NO expression and maintaining their balance in
vivo and in vitro, a mechanism by which naringin may
modulate vascular function to further affect bone metabolism.
Studies suggest that localized nutritional
deficiency in osteoblasts due to vascular dysfunction and reduced
neovascularization contributes to the pathogenesis of OP, and that
modulation of angiogenesis can be beneficial in patients with OP
(2,7,9).
Recent studies have found that OVX mice with OP have a
significantly reduced distribution of intraosseous blood vessels
compared with control mice, indicating that localized intraosseous
angiogenesis is significantly associated with OP (8). In addition, some researchers have
reviewed the correlation between OP and blood vessels from
different perspectives and have suggested that aging-induced
vascular dysfunction may be associated with OP; hence, the control
of angiogenesis can reduce risk factors for OP as well as incidence
and mortality rates for OP-related diseases (9,37).
The OVX model also promotes apoptosis and bone resorption while
inhibiting angiogenesis and bone development (38), findings that have indirectly
confirmed the correlation between localized angiogenesis and OP.
After we treated OVX rats with naringin for 3 months, MVD analysis
in the distal femur showed that the treatment groups had
significantly higher MVD than the OVX group, and were positively
correlated with BMD. Preliminary results indicate that the
promotion of angiogenesis may be one mechanism by which naringin
exerts its anti-osteoporotic effects.
We showed that naringin promoted VEC proliferation
and inhibited serum starvation-induced apoptosis in VECs by
inhibiting the mitochondrial- and ER stress-mediated apoptotic
pathways. Additionally, it regulated the function of endothelial
cells and promoted angiogenesis, thereby exhibiting an
anti-osteoporotic effect. This study provided experimental evidence
to clarify the mechanism of action of naringin in the treatment of
OP.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (81403443), Shanghai Municipal
Commission of Health and Family Planning (no. 2014Y039 and
'Xing-Lin New Star').
References
|
1
|
Lane NE: Epidemiology, etiology, and
diagnosis of osteoporosis. Am J Obstet Gynecol. 194(Suppl): S3–S11.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Almubarak S, Nethercott H, Freeberg M,
Beaudon C, Jha A, Jackson W, Marcucio R, Miclau T, Healy K and
Bahney C: Tissue engineering strategies for promoting vascularized
bone regeneration. Bone. 83:197–209. 2016. View Article : Google Scholar :
|
|
3
|
Street J, Bao M, deGuzman L, Bunting S,
Peale FV Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL,
Daugherty A, et al: Vascular endothelial growth factor stimulates
bone repair by promoting angiogenesis and bone turnover. Proc Natl
Acad Sci USA. 99:9656–9661. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shen X, Wan C, Ramaswamy G, Mavalli M,
Wang Y, Duvall CL, Deng LF, Guldberg RE, Eberhart A, Clemens TL, et
al: Prolyl hydroxylase inhibitors increase neoangiogenesis and
callus formation following femur fracture in mice. J Orthop Res.
27:1298–1305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song HJ, Lan BS, Cheng B, Zhang KF, Yan
HW, Wang WZ and Gao ZQ: Treatment of early avascular necrosis of
femoral head by small intestinal submucosal matrix with peripheral
blood stem cells. Transplant Proc. 43:2027–2032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu XD, Cai F, Liu L, Zhang Y and Yang AL:
MicroRNA-210 is involved in the regulation of postmenopausal
osteoporosis through promotion of VEGF expression and osteoblast
differentiation. Biol Chem. 396:339–347. 2015. View Article : Google Scholar
|
|
7
|
Saran U, Gemini Piperni S and Chatterjee
S: Role of angiogenesis in bone repair. Arch Biochem Biophys.
561:109–117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ding WG, Wei ZX and Liu JB: Reduced local
blood supply to the tibial metaphysis is associated with
ovariectomy-induced osteoporosis in mice. Connect Tissue Res.
52:25–29. 2011. View Article : Google Scholar
|
|
9
|
Alagiakrishnan K, Juby A, Hanley D,
Tymchak W and Sclater A: Role of vascular factors in osteoporosis.
J Gerontol A Biol Sci Med Sci. 58:362–366. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peng J, Hui K, Hao C, Peng Z, Gao QX, Jin
Q, Lei G, Min J, Qi Z, Bo C, et al: Low bone turnover and reduced
angiogenesis in streptozotocin-induced osteoporotic mice. Connect
Tissue Res. 57:277–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scull CM and Tabas I: Mechanisms of ER
stress-induced apoptosis in atherosclerosis. Arterioscler Thromb
Vasc Biol. 31:2792–2797. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smith MA and Schnellmann RG: Calpains,
mitochondria, and apoptosis. Cardiovasc Res. 96:32–37. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Prisby RD, Ramsey MW, Behnke BJ, Dominguez
JM 2nd, Donato AJ, Allen MR and Delp MD: Aging reduces skeletal
blood flow, endothelium-dependent vasodilation, and NO
bioavailability in rats. J Bone Miner Res. 22:1280–1288. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dominguez JM II, Prisby RD, Muller-Delp
JM, Allen MR and Delp MD: Increased nitric oxide-mediated
vasodilation of bone resistance arteries is associated with
increased trabecular bone volume after endurance training in rats.
Bone. 46:813–819. 2010. View Article : Google Scholar :
|
|
15
|
Bharti S, Rani N, Krishnamurthy B and Arya
DS: Preclinical evidence for the pharmacological actions of
naringin: A review. Planta Med. 80:437–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pang WY, Wang XL, Mok SK, Lai WP, Chow HK,
Leung PC, Yao XS and Wong MS: Naringin improves bone properties in
ovariectomized mice and exerts oestrogen-like activities in rat
osteoblast-like (UMR-106) cells. Br J Pharmacol. 159:1693–1703.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu M, Li Y and Yang ST: Effects of
naringin on the proliferation and osteogenic differentiation of
human amniotic fluid-derived stem cells. J Tissue Eng Regen Med
n/a. 2014.
|
|
18
|
Zhang P, Dai KR, Yan SG, Yan WQ, Zhang C,
Chen DQ, Xu B and Xu ZW: Effects of naringin on the proliferation
and osteogenic differentiation of human bone mesenchymal stem cell.
Eur J Pharmacol. 607:1–5. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei M, Yang Z, Li P, Zhang Y and Sse WC:
Anti-osteoporosis activity of naringin in the retinoic acid-induced
osteoporosis model. Am J Chin Med. 35:663–667. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li F, Sun X, Ma J, Ma X, Zhao B, Zhang Y,
Tian P, Li Y and Han Z: Naringin prevents ovariectomy-induced
osteoporosis and promotes osteoclasts apoptosis through the
mitochondria-mediated apoptosis pathway. Biochem Biophys Res
Commun. 452:629–635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang YH, Zhao CQ, Jiang LS and Dai LY:
Cyclic stretch-induced apoptosis in rat annulus fibrosus cells is
mediated in part by endoplasmic reticulum stress through nitric
oxide production. Eur Spine J. 20:1233–1243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Padró T, Ruiz S, Bieker R, Bürger H,
Steins M, Kienast J, Büchner T, Berdel WE and Mesters RM: Increased
angiogenesis in the bone marrow of patients with acute myeloid
leukemia. Blood. 95:2637–2644. 2000.
|
|
23
|
Kim HJ, Song JY, Park HJ, Park HK, Yun DH
and Chung JH: Naringin protects against rotenone-induced apoptosis
in human neuroblastoma SH-SY5Y cells. Korean J Physiol Pharmacol.
13:281–285. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rong W, Wang J, Liu X, Jiang L, Wei F, Hu
X, Han X and Liu Z: Naringin treatment improves functional recovery
by increasing BDNF and VEGF expression, inhibiting neuronal
apoptosis after spinal cord injury. Neurochem Res. 37:1615–1623.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu XX, Tang L, Ge R, Li JK, Kang Y, Zhu
MX, Li QS and Hao XL: iTRAQ-based quantitative proteomic analysis
of the anti-apoptotic effect of hyperin, which is mediated by Mcl-1
and Bid, in H2O2-injured EA.hy926 cells. Int
J Mol Med. 37:1083–1090. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Date T, Taniguchi I, Inada K, Matsuo S,
Miyanaga S, Yamane T, Abe Y, Sugimoto K and Mochizuki S: Nicorandil
inhibits serum starvation-induced apoptosis in vascular endothelial
cells. J Cardiovasc Pharmacol. 46:721–726. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hogg N, Browning J, Howard T, Winterford
C, Fitzpatrick D and Gobé G: Apoptosis in vascular endothelial
cells caused by serum deprivation, oxidative stress and
transforming growth factor-beta. Endothelium. 7:35–49. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Holcik M and Sonenberg N: Translational
control in stress and apoptosis. Nat Rev Mol Cell Biol. 6:318–327.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu J, Eto M, Akishita M, Okabe T and Ouchi
Y: A selective estrogen receptor modulator inhibits
TNF-alpha-induced apoptosis by activating ERK1/2 signaling pathway
in vascular endothelial cells. Vascul Pharmacol. 51:21–28. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wong RW and Rabie AB: Effect of naringin
collagen graft on bone formation. Biomaterials. 27:1824–1831. 2006.
View Article : Google Scholar
|
|
32
|
Uhlmann D, Uhlmann S and Spiegel HU:
Endothelin/nitric oxide balance influences hepatic
ischemia-reperfusion injury. J Cardiovasc Pharmacol. 36(Suppl 1):
S212–S214. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Haenggi W, Bersinger NA, Mueller MD and
Birkhaeuser MH: Decrease of serum endothelin levels with
postmenopausal hormone replacement therapy or tibolone. Gynecol
Endocrinol. 13:202–205. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gulhan I, Kebapcilar L, Alacacioglu A,
Bilgili S, Kume T, Aytac B and Gunaydin R: Postmenopausal women
with osteoporosis may be associated with high endothelin-1. Gynecol
Endocrinol. 25:674–678. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kumar P and Kumar A: Protective effect of
hesperidin and naringin against 3-nitropropionic acid induced
Huntington's like symptoms in rats: Possible role of nitric oxide.
Behav Brain Res. 206:38–46. 2010. View Article : Google Scholar
|
|
36
|
Aggarwal A, Gaur V and Kumar A: Nitric
oxide mechanism in the protective effect of naringin against
post-stroke depression (PSD) in mice. Life Sci. 86:928–935. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Byon CH and Chen Y: Molecular mechanisms
of vascular calcification in chronic kidney disease: The link
between bone and the vasculature. Curr Osteoporos Rep. 13:206–215.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Orlić I, Borovecki F, Simić P and
Vukicević S: Gene expression profiling in bone tissue of
osteoporotic mice. Arh Hig Rada Toksikol. 58:3–11. 2007. View Article : Google Scholar
|