Introduction
Bronchopulmonary dysplasia (BPD) is a common chronic
lung disease in premature infants. With the introduction of
prenatal steroid use, progressive ventilator strategies, and
improved survival of very low birth weight (VLBW) infants, the
incidence of BPD has increased to as high as 68% among infants born
at 22–28 weeks of gestation (1).
These infants require re-admission to the hospital within the first
2 years after birth, and even as adolescents for lung function
abnormalities consistent with increased incidence of asthma, chest
deformities and pulmonary hypertension. Additionally, delayed
neurodevelopment, particularly cerebral palsy, cognitive and motor
function abnormalitites, are common in BPD infants; however, there
is yet no effective and safe therapy for this condition (2).
'Old BPD', associated with prominent
fibroproliferation, which was first characterized by Northway et
al in 1967, is currently less striking. The more modern concept
is that BPD is a disruption of distal lung growth, with prominent
impaired alveolar and vascular growth, which has been defined as
'new BPD' (2). Mechanical
ventilation and oxygen supplementation are major contributors to
BPD (3). Unlike 'old BPD',
patients with 'new BPD' may have been exposed to low inspired
oxygen concentrations (2).
Hyperoxia-induced inflammation, oxidative stress, apoptosis and
cell death lead to disruption of lung development (4).
Epithelial-to-mesenchymal transition (EMT) is
generally known as the process during which epithelial cells lose
their polarity and junctions, and acquire the characteristics of
mesenchymal cells (5). It was
previously confirmed that hyperoxia induced type II cells in the
lung to differentiate into fibroblasts through EMT, thus affecting
alveolar development (6). This is
associated with downregulation of RUNX3, a gene that is crucial for
lung development. It was also demonstrated that the EMT occurring
in the BPD model is associated with epigenetic modifications.
Histone methyltransferase enhancer of zeste homolog 2 (EZH2), a key
epigenetic modification regulator, was upregulated in type II cells
in the model group and silenced the expression of RUNX3 through
trimethylated H3K27 (7). SOX4,
which belongs to the SRY-related HMG box gene family (8), is a master regulator during
transforming growth factor (TGF)-β-induced EMT (9). More recently, selected studies have
elucidated the role of the SOX4/EZH2 axis in epigenetic
modifications during EMT (10,11). The aim of the present study was to
determine whether dysregulated expression of SOX4 in the
hyperoxia-exposed lung may contribute to EMT in BPD through
EZH2.
Materials and methods
Animal model
All animal procedures were reviewed and approved by
the Experimental Animal Ethics Committee of China Medical
University. A total of 20 pregnant Sprague-Dawley rats (age, 8–10
weeks; weight, 200–250 g) were purchased from the Experimental
Animal Center of China Medical University, and the BPD model was
constructed as previously described. Briefly, newborn rats were
randomly divided into two groups within 12 h of birth: The BPD
model rats were exposed to 80–85% oxygen in a sealed Plexiglas tank
for 1–21 days, while the control group rats were exposed to room
air (21% oxygen). During this time, the oxygen concentration was
continuously monitored with an oxygen analyzer and maternal rats
were switched between the model and control groups every 24 h to
avoid oxygen toxicity.
Lung tissue specimens
On postnatal days 1, 3, 7, 14 and 21, 8 pups in each
group were anesthetized by intraperitoneal injection with 10%
chloral hydrate, and the lungs were inflated with
phosphate-buffered saline (PBS) at 20 cm H2O pressure;
the lung tissues were then collected. The right middle lung lobes
were fixed in 4% paraformaldehyde (PFA) for hematoxylin and eosin
(H&E) and immunohistochemical staining; the remaining lung
lobes were stored at −80°C for subsequent quantitative PCR (qPCR)
and western blot analysis.
Lung histology
PFA-fixed lung tissues were dehydrated through
graded alcohol and xylene, and embedded in paraffin. Subsequently,
4-μm sections were prepared for H&E staining for
morphometric analysis. The radial alveolar count (RAC) provides a
simple and relatively accurate assessment of lung development.
According to the radial count method proposed by Emery and Mithal
(12), alveoli transected along
the perpendicular line from the center of the respiratory
bronchiole to the nearest connective tissue septum or pleura, are
enumerated. Image-Pro Plus 6.0 software (Media Cybernetics,
Rockville, MD, USA) was used to measure the mean alveolar diameter
(MAD) and alveolar septal thickness (AST). At least 5 fields were
captured per section, and at least 20 alveoli were measured per
field.
Double immunofluorescence
Following drying in a 60°C oven overnight, the
tissue sections were deparaffinized in xylene and rehydrated in a
gradient ethanol series. For antigen retrieval, the sections were
microwaved in 10 mM citrate buffer (pH 6.0) for 20 min. The
sections were then incubated in 5% bovine serum for 1 h at 37°C. A
combination of two primary antibodies, rabbit polyclonal anti-SOX4
(SAB2108306; 1:100 dilution; Sigma-Aldrich Merck KGaA, St. Louis,
MO, USA) and mouse monoclonal anti-p180 (ab24751; 1:50 dilution;
Abcam, Cambridge, UK), was used for incubating the sections
overnight at 4°C, while for negative controls PBS was used in the
place of the antibody. The sections were then incubated with a
mixture of two secondary antibodies, Alexa Fluor-594 donkey
anti-rabbit IgG (ab150076; 1:500 dilution; Abcam) and Alexa
Fluor-488 donkey anti-mouse IgG (ab150105; 1:500 dilution; Abcam)
for 4 h at room temperature, followed by DAPI incubation for
nuclear staining. Double immuofluoresence images were acquired
using a confocal laser-scanning microscope (C1; Nikon, Tokyo,
Japan).
Immunohistochemistry
Following deparaffinization and microwave heating,
as previously described, the sections were treated sequentially
with 3% H2O2 for 20 min to block endogenous
peroxidase activity, and goat serum for 1 h at 37°C to block
non-specific binding. The sections were incubated overnight at 4°C
with SOX4 antibody (SAB2108306; 1:1500 dilution; Sigma-Aldrich) or
EZH2 antibody (612666; 1:200 dilution; BD Transduction
Laboratories, San Jose, CA, USA), then incubated with secondary
antibody and streptavidin-horseradish peroxidase for 20 min.
Finally, the sections were developed with 3,3′-diaminobenzidine and
counterstained with hematoxylin. Antigen expression was assessed
using an image analyzer (Image-Pro Plus 6.0; Media Cybernetics,
Rockville, MD, USA).
Western blotting
Western blotting was performed using standard
protocols. In brief, total protein was extracted from frozen lung
tissue samples and mixed with loading buffer. Equal amounts of
protein were separated by 10% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDA-PAGE) (100 V for 3 h) and transferred to
polyacryl-amide difluoride (PVDF) membranes (100 V for 50 min).
Membranes were then blocked in 5% skimmed milk for 2 h at room
temperature. Membranes incubated with rabbit anti-SOX4 (SAB2108306;
1:800 dilution; Sigma-Aldrich Merck KGaA) or mouse anti-EZH2
(612666; 1:1,000 dilution; BD Transduction Laboratories, San Jose,
CA, USA) were shaken overnight at 4°C. The next day, the membranes
were incubated with horseradish peroxidase-conjugated goat
anti-rabbit or anti-mouse secondary antibody (1:5,000 dilution;
Proteintech, Rosemont, IL, USA) for 2 h after washing three times
in Tris-buffered saline and Polysorbate 20, and developed using
enhanced chemiluminescence reagents (Thermo Scientific Pierce;
Thermo Fisher Scientific, Waltham, MA, USA). Densitometry values
were detected for all bands and standardized relative to
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) for each
sample.
qPCR
Total RNA was extracted from the right lung lobes
using TRIzol reagent (Takara Biotechnology Co., Kyoto, Japan)
according to the manufacturer's instructions. Following
purification according to the kit instructions, cDNA was
reverse-transcribed from 1 μg RNA from each sample using
Prime Script RT reagent kit with gDNA Eraser (Takara Biotechnology
Co.). The primers were designed as follows: SOX4 forward,
5′-ATGTCCCTGGGCAGTTTCAG-3′ and reverse, 5′-TGCAATAGTCCGGGAACTCG-3′;
GAPDH forward, 5′-AGACAGCCGCATCTTCTTGT-3′ and reverse,
5′-CTTGCCGTGGGTAGAGTCAT-3′. qPCR was performed using SYBR Premix Ex
Tag (Takara Biotechnology Co.) on LightCycler (Thermo Fischer
Scientific, Carlsbad, CA, USA); the amplification program was 95°C
for 30 sec, 40 cycles of 95°C for 30 sec and 60°C for 34 sec.
Relative mRNA expression of SOX4 normalized to GAPDH was determined
by the 2−ΔΔCq method.
Statistical analysis
Statistical analysis was performed using SPSS 21.0
software (IBM, Armonk, NY, USA). Any significant difference between
two groups was compared using an unpaired t-test, and correlation
analysis was performed via Pearson's tests. All data are presented
as mean ± standard deviation. P<0.05 was considered to indicate
statistically significant differences.
Results
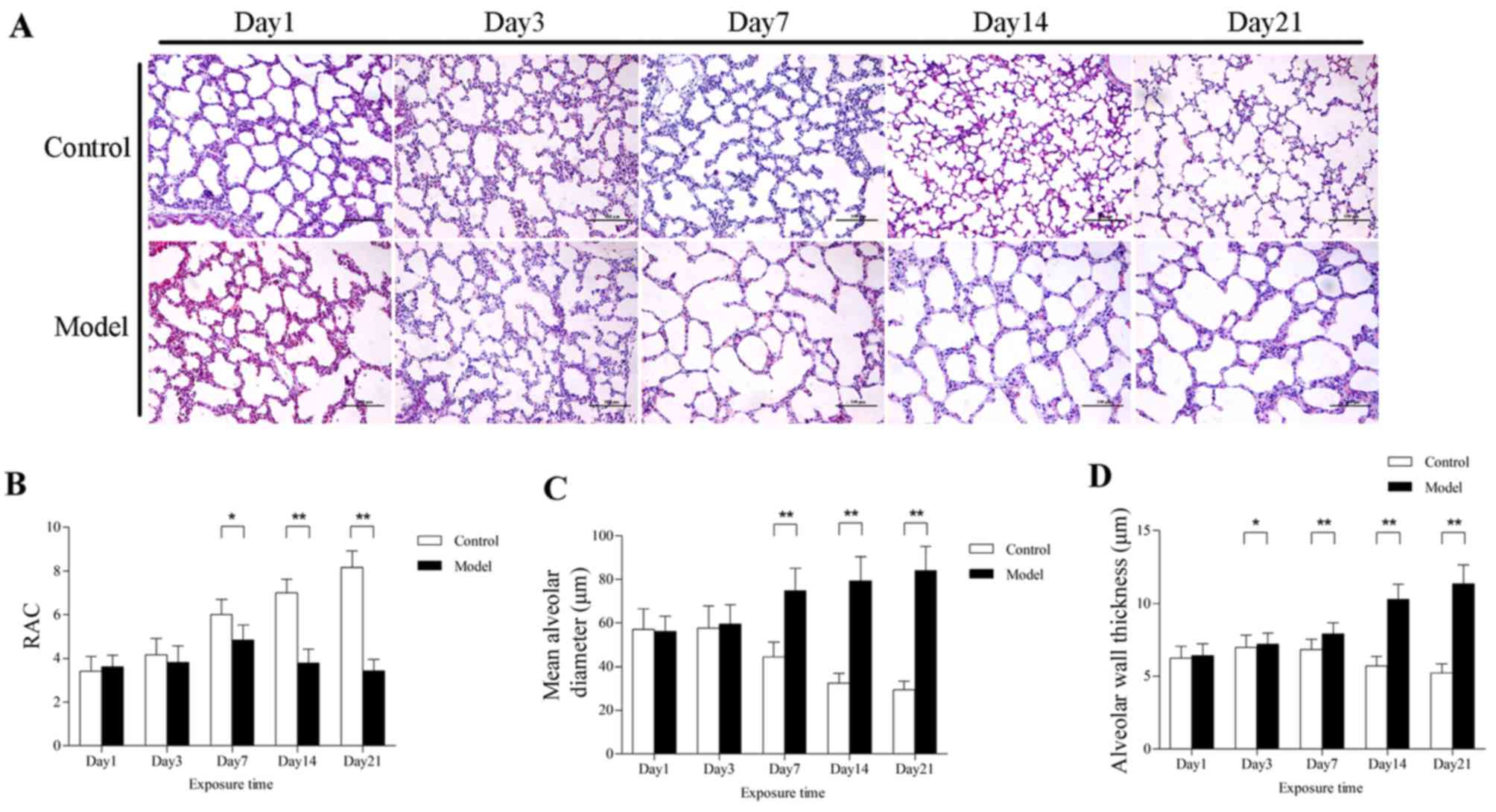
Prolonged hyperoxia disrupts lung
development
Compared with the control group, the survival rate
of rat pups exposed to hyperoxia was significantly decreased. As an
important index of lung development, the RACs of the control group
increased gradually, beginning on the day of birth. In pups exposed
to hyperoxia for days, the RACs were significantly lower compared
with those of the control group, particularly on days 7, 14 and 21
(Fig. 1B). Additionally,
prolonged hyperoxia caused substantial morphological alteration of
lung tissues. There was an increase in alveolar size in the model
group, as quantified by an increase in MAD and AST, and the
difference between the two groups was more pronounced with the
prolongation of the hyperoxia exposure time (Fig. 1C and D). By contrast, no
significant histological difference was found on days 1 and 3.
Taken together, these results demonstrate that prolonged exposure
to hyperoxia interrupts alveolarization, as demonstrated by a
decreased number of alveoli and increased diameter and septal
thicknesses of the alveoli.
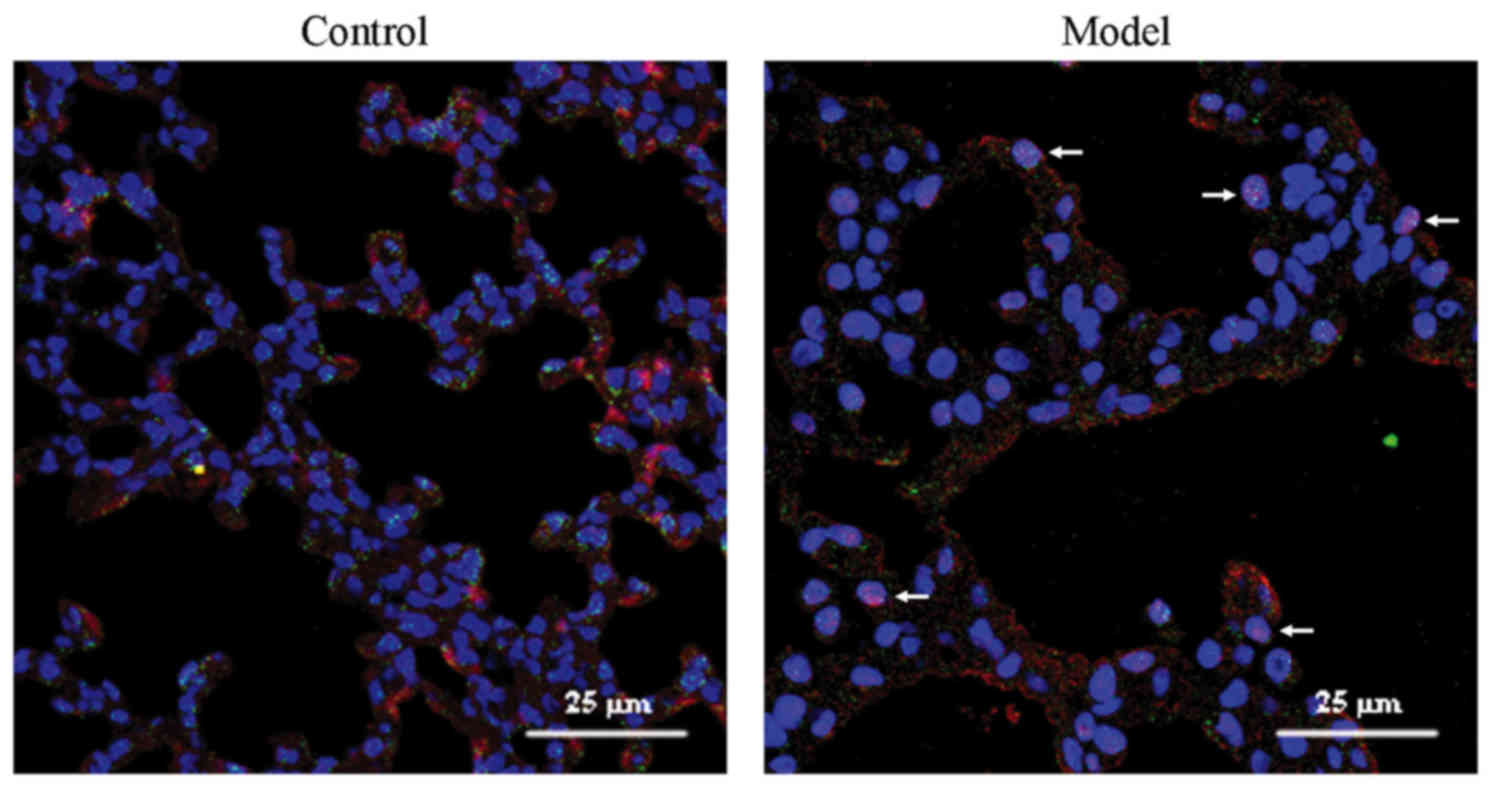
SOX4 expression in alveolar type II cells
exposed to hyperoxia
Double immunofluorescence was used to determine SOX4
localization. p180 is a lamellar body protein that is highly
expressed in the lung, and plays an important role in the formation
of pulmonary surfactant. Green p180 fluorescence represented type
II cells. Co-expression of p180 and red SOX4 fluorescence appeared
as orange. In the control group, only few cells co-expressed red
SOX4 and green p180 fluorescence. By contrast, cells with red SOX4
fluorescence in the nuclei and green p180 fluorescence or orange
co-expressed fluorescence in the cytoplasm were observed in the
model group (Fig. 2).
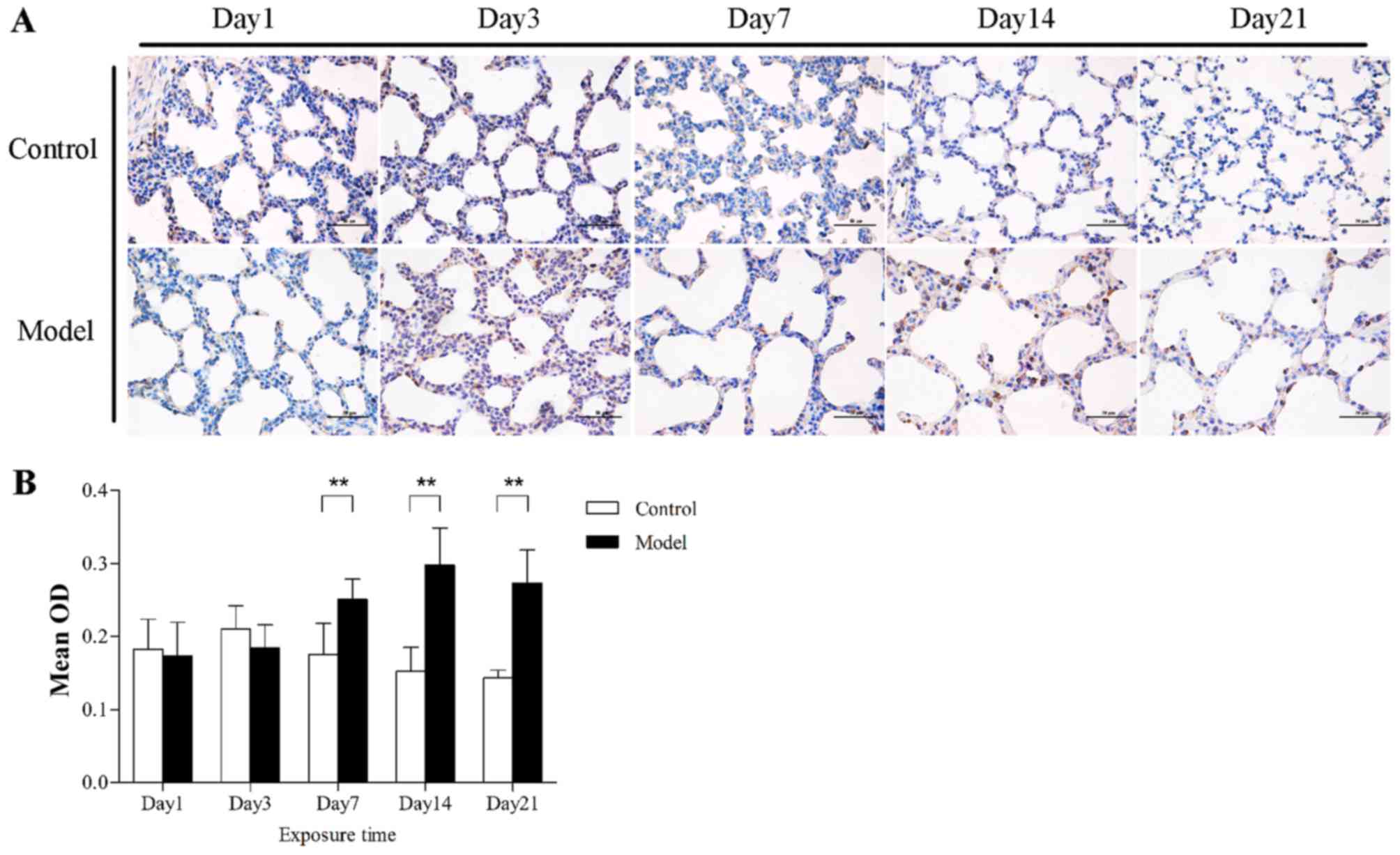
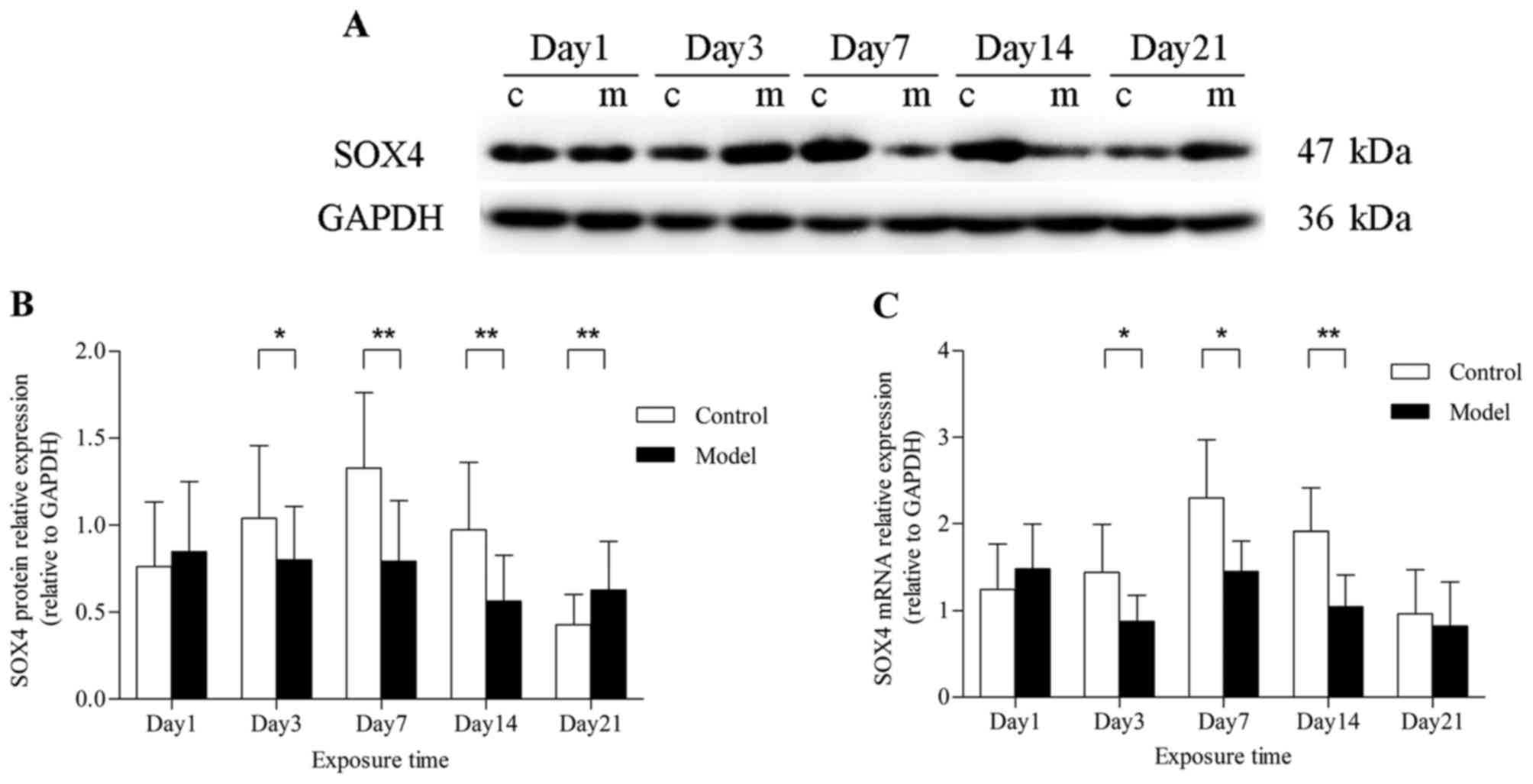
SOX4 expression in rat lungs exposed to
hyperoxia
To investigate whether hyperoxia altered the
expression of SOX4 in rat lungs, immunohistochemistry, western blot
analysis and qPCR were performed. After hyperoxia exposure for ≥7
days, SOX4 was localized to the nucleus as well as the cytoplasm of
alveolar epithelial cells in the model group, but was poorly
expressed in the control group (Fig.
3). The results differed, however, in total lung tissues. On
day 1, the level of SOX4 expression was not different between the
model and control groups, at both the mRNA and protein level.
Furthermore, SOX4 protein expression was found to be downregulated
after hyperoxia exposure for 3 days (P<0.05) and decreased
markedly at 7 and 14 days (P<0.01). When hyperoxia exposure was
prolonged to 21 days, the SOX4 protein expression in the model
group, rather than decreasing, it was higher compared with that
under normoxic conditions (P<0.01) (Fig. 4A and B). A similar effect on SOX4
mRNA expression was verified by qPCR analysis, but there was no
significant difference at 21 days (Fig. 4C).
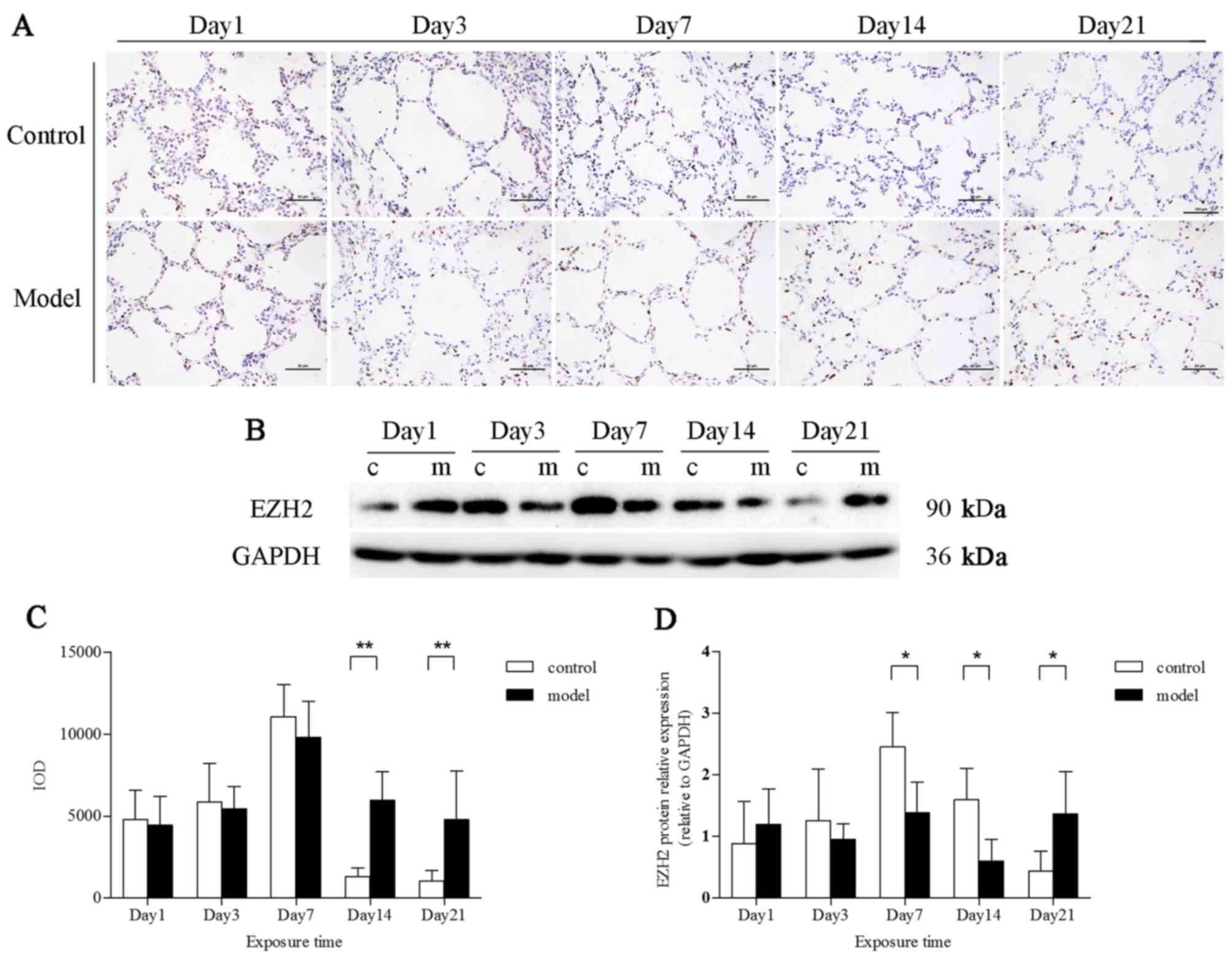
EZH2 expression in rat lungs exposed to
hyperoxia
EZH2 was expressed in the nuclei of alveolar
epithelial cells. After exposure to hyperoxia for 14 and 21 days,
EZH2 expression was markedly upregulated (P<0.01) (Fig. 5A and C). However, the EZH2 protein
levels in total lung tissues were downregulated in the model group
at 7 and 14 days, as demonstrated by western blot analysis
(P<0.01). EZH2 protein expression in the model group, compared
with that in the control group, was increased at 21 days (Fig. 5B and D).
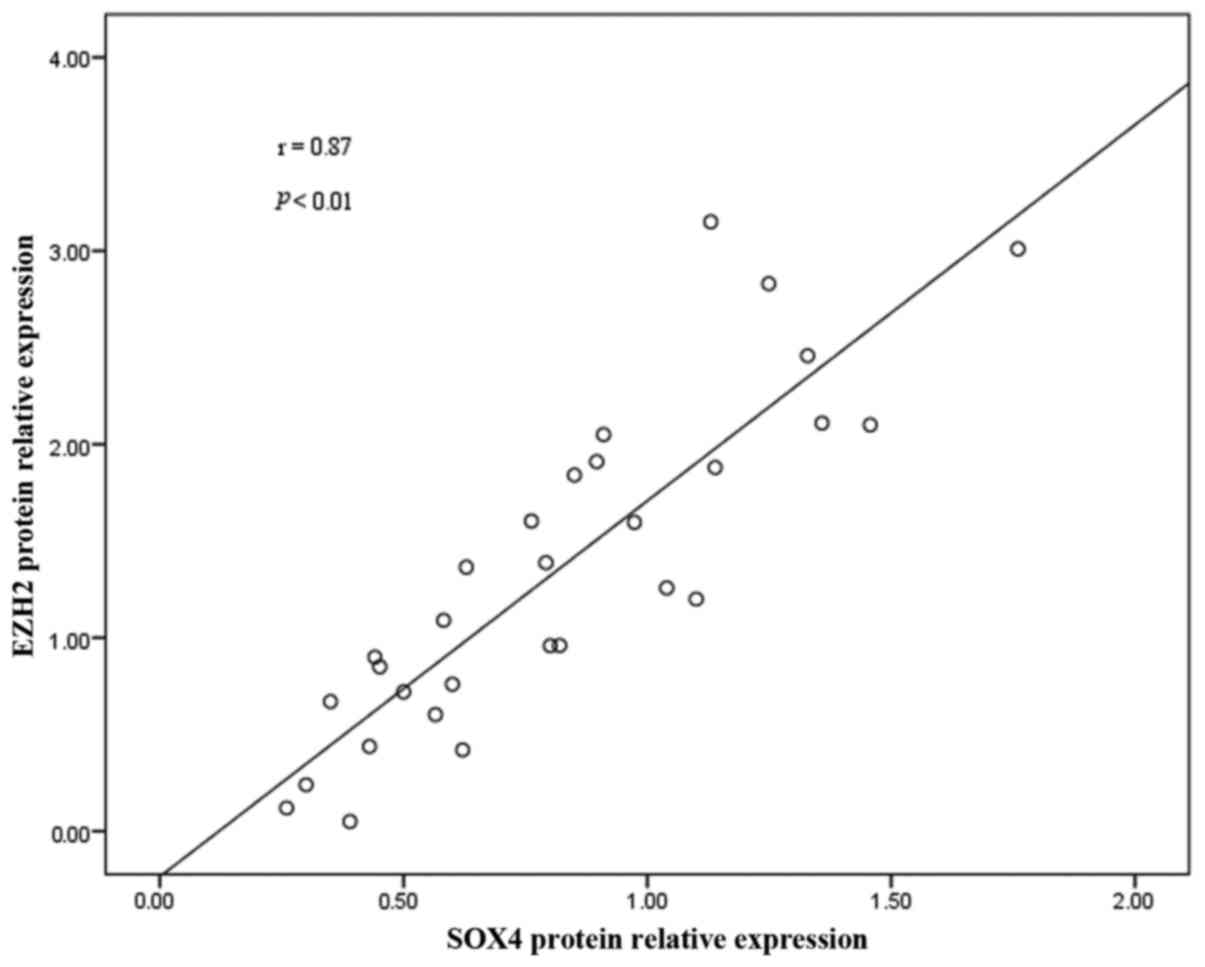
Correlation between SOX4 expression and
EZH2 in rat lungs
To confirm the correlation between SOX4 protein
expression and EZH2, correlation analyses were performed. EZH2
protein expression was found to be positively correlated with that
of SOX4 (r=0.87; P<0.01) (Fig.
6).
Discussion
The etiology of BPD is multifactorial, and long-term
mechanical ventilation is one of the primary risk factors
increasing the incidence of BPD in VLBW infants, although it may
decrease mortality in this patient population (13). In the present study, lower oxygen
concentrations were used (80–85%) compared with those in previous
studies (90–95%), and it was demonstrated that prolonged hyperoxia
inhibited pulmonary development. There was a distinct morphological
change: The alveoli decreased in number and were larger, more
simplified, and displaying thickened alveolar septa in the model
group, consistently with the pathological characteristics of 'new
BPD'. The underlying mechanisms, however, remain largely unknown
(2).
The alveolar epithelial surface is composed of
alveolar type I and II cells. Type II cells comprise 16% of total
lung cells and 60% of alveolar epithelial cells, which only cover
5% of the alveolar surface (14).
Regeneration of the alveolar epithelium following lung injury is
one of the major functions attributed to alveolar type II cells.
Upon exposure to hyperoxia, type I cells are more susceptible to
injury, and type II cells appear to increasingly proliferate and
transdifferentiate into type I cells, which are resistant to
oxidative damage (15). However,
previous studies confirmed that hyperoxia inhibits activation of
type II cells (16,17). A number of studies on the
pathological foundation of BPD have focused on type II cell injury,
with respect to proliferation, apoptosis and transdifferentiation
into type I cells or mesenchymal cells (EMT).
EMT is accompanied by apparent changes in cell
morphology and motility. As a physiological phenomenon, EMT is
involved in embryogenesis, tumorigenesis and wound healing through
cell signaling pathways, such as TGF-β/Smad and Wnt/β-catenin
(18). It was previously
demonstrated that alveolar type II cells underwent EMT in
hyperoxia-induced BPD in newborn rats. Ultrastructural changes
occurred in type II cells that differentiated into fibroblasts,
followed by altered expression of E-cadherin, N-cadherin and
α-smooth muscle actin (6). RUNX3
is a runt-domain transcription factor that plays a crucial role in
lung development. In RUNX3 null mouse lungs, EMT markers are
abnormally expressed, along with low levels of E-cadherin and high
fibronectin expression (19). Our
previous study confirmed that RUNX3 is downregulated in the
hyperoxia-induced BPD model, which is attributable to
EZH2-catalyzed histone methylation (7). EZH2, the histone methyltransferase,
is a component of polycomb-repressive complex 2 (20). EZH2 epigenetically silences gene
expression through the trimethylation of histone 3 lysine 27
(H3K27me3), and has been implicated in several types of cancer
(11). However, the mechanism of
EZH2 expression regulation in BPD is entirely unknown.
SOX4, a member of the group C subfamily of SOX
transcription factors, plays an important role in embryogenesis and
cell fate determination (21).
SOX4 is important for lung development. Its expression is
widespread in both the epithelium and the mesenchyme of the
developing lung (22), with its
expression in the bronchial duct epithelium being more abundant
compared with that in surrounding lung tissues (23). During the canalicular period, SOX4
participates in transcription during differentiation of the
conducting airway epithelium and affects lung morphogenesis
(24). Its abnormal expression
may cause various lung diseases. In non-small-cell lung cancer,
SOX4 is overexpressed through 6p gene amplification and plays an
important role in cancer progression and prognosis (25,26). The present study demonstrated that
SOX4 expression increased during the early postnatal stages and
decreased in the later stages in the control group, with lower
expression in type II cells and higher expression in total lung
tissues compared with the BPD group. The low proportion of type II
cells in rat lung tissues, of which 46% and 30% are capillary
endothelial and interstitial cells, respectively (14), may account for this result. This
suggests that SOX4 dysregulation may be one of the causes of
BPD.
SOX4 is a master regulator during TGF-β-induced
EMT(9). In breast cancer, SOX4 is
overexpressed and triggers EMT to promote tumor progression and
metastasis (27). Ablation of
SOX4 expression results in reduced expression of EMT inducers, such
as Snail, Twist and Zeb (28). In
the present study, SOX4 was upregulated in type II cells and may be
associated with the occurrence of EMT in the BPD model. More
recently, certain studies elucidated the importance of the
SOX4/EZH2 axis in epigenetic modification during EMT (10,11). SOX4 directly regulates the
expression of EZH2, which is involved in gene silencing via
H3K27me3 modification in the promoters of target genes such as the
epithelial marker E-cadherin (10,29). This study also revealed that EZH2
was upregulated in type II cells exposed to hyperoxia, and
increased at a later time compared with SOX4. EZH2 expression
increased, beginning 14 days after hyperoxia inhalation, while SOX4
increased after 7 days. Correlation analysis between SOX4 and EZH2
protein expression revealed a positive correlation. According to
these findings, we hypothesized that upregulation of SOX4 in type
II cells may promote EMT by modulating EZH2 expression, thereby
disrupting alveolarization and lung development in BPD.
As lower expression of SOX4 in total lung tissues
was observed in the model group, SOX4 may act through a different
mechanism in other lung cells, such as fibroblasts. It was
previously demonstrated that prolonged hyperoxia results in high
BAX expression in fibroblasts and promotes apoptosis (30). Another study demonstrated that
downregulated SOX4 expression induces apoptosis in lung cancer
(31). Hur et al reported
that the HMG box of SOX4 interacts with p53 and reduces p53
transcriptional activity on the BAX promoter, resulting in
inhibition of p53-mediated apoptosis (32). Therefore, reduced SOX4 expression
in lung tissues may relieve its inhibition of p53-mediated
apoptosis and upregulate BAX expression, thereby promoting
apoptosis of lung fibroblasts in the model group. Additionally, it
has been confirmed that repressing EZH2 inhibits proliferation and
induces apoptosis in cancer cells (33). Consistent with SOX4 expression,
the expression of EZH2 in total lungs in the model group was also
reduced; however, this reduction occurred later compared with that
of SOX4, indicating that SOX4 may promote fibroblast apoptosis by
regulating EZH2.
As EMT generates fibroblasts for wound healing and
tissue regeneration (17),
upregulated expression of SOX4 in type II cells may trigger
epithelial cells to transdifferentiate into fibroblasts during
oxidative epithelial injury repair. Downregulation of SOX4 in the
early stages of hyperoxia in fibroblasts appeared to restrain the
process of EMT against excessive fibroblast proliferation, and
increased SOX4 expression in the later stages may indicate severe
EMT that may lead to fibrosis, thus adversely affecting long-term
prognosis. This hypothesis, which is consistent with lineage
tracing studies showing that the number of fibroblasts
transdifferentiated from epithelial cells is small, despite
co-expression of epithelial and mesenchymal markers during human
idiopathic pulmonary fibrosis (34), may be closer to the definition of
the 'new BPD'. Otherwise, EMT of type II cells may interfere with
their transdifferentiation into type I cells, leading to abnormal
epithelial injury repair and, thus, affecting alveolarization. A
study recently reported that SOX4 induction may be inhibited by
caffeine, which has been used for BPD management (35). Thus, SOX4 may be a novel
therapeutic target for BPD. Using caffeine during the early stages
of BPD may downregulate SOX4 expression in the later stages,
improving long-term prognosis.
In conclusion, the present study confirmed that the
expression of the transcription factor SOX4 is increased in type II
cells in BPD, but is reduced in total lung tissues. Dysregulation
of SOX4 disrupts lung development in BPD by controlling EZH2
expression. However, further studies are required to determine the
precise mechanism of action of SOX4 in BPD.
Acknowledgments
The present study was supported by grants from the
Natural Science Foundation of China (nos. 81571479 and
81471489).
References
|
1
|
Baker CD and Abman SH: Impaired pulmonary
vascular development in bronchopulmonary dysplasia. Neonatology.
107:344–351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kinsella JP, Greenough A and Abman SH:
Bronchopulmonary dysplasia. Lancet. 367:1421–1431. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baraldi E and Filippone M: Chronic lung
disease after premature birth. N Engl J Med. 357:1946–1955. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bhandari V: Hyperoxia-derived lung damage
in preterm infants. Semin Fetal Neonatal Med. 15:223–229. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang H, Fu J, Xue X, Yao L, Qiao L, Hou A,
Jin L and Xing Y: Epithelial-mesenchymal transitions in
bronchopulmonary dysplasia of newborn rats. Pediatr Pulmonol.
49:1112–1123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu Y, Fu J, Yang H, Pan Y, Yao L and Xue
X: Hyperoxia-induced methylation decreases RUNX3 in a newborn rat
model of bronchopulmonary dysplasia. Respir Res. 16:752015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wegner M: From head to toes: The multiple
facets of Sox proteins. Nucleic Acids Res. 27:1409–1420. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vervoort SJ, van Boxtel R and Coffer PJ:
The role of SRY-related HMG box transcription factor 4 (SOX4) in
tumorigenesis and metastasis: Friend or foe? Oncogene.
32:3397–3409. 2013. View Article : Google Scholar
|
|
10
|
Tiwari N, Tiwari VK, Waldmeier L, Balwierz
PJ, Arnold P, Pachkov M, Meyer-Schaller N, Schübeler D, van
Nimwegen E and Christofori G: Sox4 is a master regulator of
epithelial-mesenchymal transition by controlling Ezh2 expression
and epigenetic reprogramming. Cancer Cell. 23:768–783. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hasegawa S, Nagano H, Konno M, Eguchi H,
Tomokuni A, Tomimaru Y, Asaoka T, Wada H, Hama N, Kawamoto K, et
al: A crucial epithelial to mesenchymal transition regulator,
Sox4/Ezh2 axis is closely related to the clinical outcome in
pancreatic cancer patients. Int J Oncol. 48:145–152. 2016.
View Article : Google Scholar
|
|
12
|
Emery JL and Mithal A: The number of
alveoli in the terminal respiratory unit of man during late
intrauterine life and childhood. Arch Dis Child. 35:544–547. 1960.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jobe AH: The new bronchopulmonary
dysplasia. Curr Opin Pediatr. 23:167–172. 2011. View Article : Google Scholar
|
|
14
|
Crapo JD, Barry BE, Gehr P, Bachofen M and
Weibel ER: Cell number and cell characteristics of the normal human
lung. Am Rev Respir Dis. 126:332–337. 1982.PubMed/NCBI
|
|
15
|
Castranova V, Rabovsky J, Tucker JH and
Miles PR: The alveolar type II epithelial cell: A multifunctional
pneumocyte. Toxicol Appl Pharmacol. 93:472–483. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hou A, Fu J, Yang H, Zhu Y, Pan Y, Xu S
and Xue X: Hyperoxia stimulates the transdifferentiation of type II
alveolar epithelial cells in newborn rats. Am J Physiol Lung Cell
Mol Physiol. 308:L861–L872. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu W, Xu B, Zhao Y, Yang N, Liu C, Wen G
and Zhang B: Wnt5a reverses the inhibitory effect of hyperoxia on
transdifferentiation of alveolar epithelial type II cells to type I
cells. J Physiol Biochem. 71:823–838. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pain M, Bermudez O, Lacoste P, Royer PJ,
Botturi K, Tissot A, Brouard S, Eickelberg O and Magnan A: Tissue
remodelling in chronic bronchial diseases: from the epithelial to
mesenchymal phenotype. Eur Respir Rev. 23:118–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JM, Shin JO, Cho KW, Hosoya A, Cho SW,
Lee YS, Ryoo HM, Bae SC and Jung HS: Runx3 is a crucial regulator
of alveolar differentiation and lung tumorigenesis in mice.
Differentiation. 81:261–268. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang C, Liu X, Chen Z, Huang H, Jin Y,
Kolokythas A, Wang A, Dai Y, Wong DT and Zhou X: Polycomb group
protein EZH2-mediated E-cadherin repression promotes metastasis of
oral tongue squamous cell carcinoma. Mol Carcinog. 52:229–236.
2013. View
Article : Google Scholar
|
|
21
|
Penzo-Méndez AI: Critical roles for SoxC
transcription factors in development and cancer. Int J Biochem Cell
Biol. 42:425–428. 2010. View Article : Google Scholar :
|
|
22
|
Dy P, Penzo-Méndez A, Wang H, Pedraza CE,
Macklin WB and Lefebvre V: The three SoxC proteins - Sox4, Sox11
and Sox12 - exhibit overlapping expression patterns and molecular
properties. Nucleic Acids Res. 36:3101–3117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hoser M, Potzner MR, Koch JM, Bösl MR,
Wegner M and Sock E: Sox12 deletion in the mouse reveals
nonreciprocal redundancy with the related Sox4 and Sox11
transcription factors. Mol Cell Biol. 28:4675–4687. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maeda Y, Davé V and Whitsett JA:
Transcriptional control of lung morphogenesis. Physiol Rev.
87:219–244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang D, Hao T, Pan Y, Qian X and Zhou D:
Increased expression of SOX4 is a biomarker for malignant status
and poor prognosis in patients with non-small cell lung cancer. Mol
Cell Biochem. 402:75–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Medina PP, Castillo SD, Blanco S,
Sanz-Garcia M, Largo C, Alvarez S, Yokota J, Gonzalez-Neira A,
Benitez J, Clevers HC, et al: The SRY-HMG box gene, SOX4, is a
target of gene amplification at chromosome 6p in lung cancer. Hum
Mol Genet. 18:1343–1352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vervoort SJ, Lourenço AR, van Boxtel R and
Coffer PJ: SOX4 mediates TGF-β-induced expression of mesenchymal
markers during mammary cell epithelial to mesenchymal transition.
PLoS One. 8:e532382013. View Article : Google Scholar
|
|
28
|
Parvani JG and Schiemann WP: Sox4, EMT
programs, and the metastatic progression of breast cancers:
Mastering the masters of EMT. Breast Cancer Res. 15:R722013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao Q, Yu J, Dhanasekaran SM, Kim JH, Mani
RS, Tomlins SA, Mehra R, Laxman B, Cao X, Yu J, et al: Repression
of E-cadherin by the polycomb group protein EZH2 in cancer.
Oncogene. 27:7274–7284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu Y, Liu X, Zhang H, Fu J and Xue X:
dynamic changes of Bax/Bcl-2 expression in lung tissue and
fibroblasts of neonatal rats after inhaling high concentration
oxygen. J Appl Clin Pediatr. 26:589–592. 2011.
|
|
31
|
Zhou Y, Wang X, Huang Y, Chen Y, Zhao G,
Yao Q, Jin C, Huang Y, Liu X and Li G: Downregulated SOX4
expression suppresses cell proliferation, metastasis and induces
apoptosis in Xuanwei female lung cancer patients. J Cell Biochem.
116:1007–1018. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hur W, Rhim H, Jung CK, Kim JD, Bae SH,
Jang JW, Yang JM, Oh ST, Kim DG, Wang HJ, et al: SOX4
overexpression regulates the p53-mediated apoptosis in
hepatocellular carcinoma: Clinical implication and functional
analysis in vitro. Carcinogenesis. 31:1298–1307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie L, Zhang Z, Tan Z, He R, Zeng X, Xie
Y, Li S, Tang G, Tang H and He X: MicroRNA-124 inhibits
proliferation and induces apoptosis by directly repressing EZH2 in
gastric cancer. Mol Cell Biochem. 392:153–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kage H and Borok Z: EMT and interstitial
lung disease: A mysterious relationship. Curr Opin Pulm Med.
18:517–523. 2012.PubMed/NCBI
|
|
35
|
Pan X, Zhao J, Zhang WN, Li HY, Mu R, Zhou
T, Zhang HY, Gong WL, Yu M, Man JH, et al: Induction of SOX4 by DNA
damage is critical for p53 stabilization and function. Proc Natl
Acad Sci USA. 106:3788–3793. 2009. View Article : Google Scholar : PubMed/NCBI
|