Introduction
Polycyclic aromatic hydrocarbons (PAHs) are
well-known air pollutants released from the incomplete combustion
of wood, coal, diesel, fat and tobacco from natural as well as
anthropogenic sources (1,2). Long-term exposure to these chemicals
has toxic effects on humans and animals, including developmental
changes, abnormal immune response and carcinogenesis (3–6).
The main mechanism responsible for the toxicity of PAHs is
considered to be their ability to activate the aryl hydrocarbon
receptor (AhR)-mediated pathway (7). Dimerization of the PAHs/AhR complex
with the AhR nuclear translocator causes it to bind to its cognate
DNA-binding site, the xenobiotic response element, which leads to
the transcriptional activation of xenobiotic-metabolizing enzymes
(XMEs) such as cytochrome P450 (CYP), epoxide hydrolase (EH),
glutathione S-transferase, UDP-glucuronosyltransferase,
sulfotransferase, NAD(P)H quinone oxidoreductase, and aldo-keto
reductase (8,9). Activated XMEs play a major role, not
only in the detoxification of PAHs, but also in the conversion of
PAHs into active metabolites. For example, the CYP1A1 and 1B1
enzymes, in cooperation with EH, play major roles in activating
PAHs to highly reactive diol-epoxides that are considered to be
carcinogenic metabolites (10).
Exposure to xenobiotics, including PAHs, may produce
reactive metabolites and cause imbalance between endogenous and
exogenous oxidants. This may subsequently induce a decrease in
antioxidant defenses and allow oxidative tissue damage. It has been
previously reported that the blood level of phenanthrene, an
aromatic hydrocarbon, is positively correlated with blood
malondialdehyde levels in human samples (11). Moreover, exposure of zebrafish to
PAHs induced oxidative stress involved in the regulation of Nrf2
(12). In lymphoma cells, PAHs
depleted intracellular levels of glutathione (GSH), contributing to
cell injury (13). Moreover,
chronic exposure to benzo(a)pyrene produced reactive oxygen
species, which was accompanied by caspase-3 activation and
apoptotic cell death (14). Taken
together, the findings of those studies suggest that the toxic
effect of PAHs likely occurs through the oxidative stress
pathway.
In the present study, metabolite profiling by global
metabolomics revealed that cells chronically exposed to
3-methylcholanthrene (3MC) have low concentrations of GSH, which is
a powerful endogenous antioxidant. While previous studies have
demonstrated that oxidative stress plays a critical role in the
AhR-dependent toxic response, the effect of PAHs on GSH
biosynthesis has not been extensively investigated. GSH is a
tripeptide synthesized as the final product in the transsulfuration
pathway. GSH is the most abundant molecule among endogenous
antioxidants and it plays a central role in sulfhydryl homeostasis,
acting as a major cellular antioxidant. The concentration of GSH in
cells is tightly regulated by de novo synthesis, utilization
and export. However, it has been demonstrated that intracellular
GSH decreases under conditions of oxidative stress, or through
generation of reactive metabolites from xenobiotics (15). Reduced levels of GSH are also
observed in various pathophysiological conditions, such as sepsis,
acute Wilson's disease, and inherited deficiencies in GSH synthesis
(15,16).
The objective of the present study was to determine
the effect of chronic exposure to 3MC, a major PAH, on the
transsulfuration pathway using targeted metabolomics analysis,
aiming to provide novel insights into the mechanism through which
3MC affects intracellular GSH levels and renders cells vulnerable
to a second insult of oxidative stress.
Materials and methods
Reagents and antibodies
3MC,
3-(4,5-dimethylthiazol)-2,5-di-phenyltetrazolium bromide (MTT),
S-adenosylmethionine (SAM), S-adenosylhomocysteine
(SAH), L-amino acids analytical standard, hypotaurine, taurine,
o-phthaldiadehyde, 7-benzo-2-oxa-1,3-diazole-4-sulfonic acid
(SBD-F), tris(2-carboxyethyl)phosphine hydrochloride (TCEP),
N-(2-mercaptopropionyl)glycine (MPG), GSH, tert-butyl hydroperoxide
(t-BHP) and crystal violet were purchased from Sigma
Aldrich; Merck KGaA (St. Louis, MO, USA). Anti-cysteine dioxygenase
(CDO) antibody was obtained from Abcam (Cambridge, MA, USA).
Anti-γ-glutamylcysteine ligase catalytic subunit (GCL) antibody and
anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Peroxidase-conjugated antibodies against mouse or rabbit IgG
were obtained from Thermo Fisher Scientific (Waltham, MA, USA).
Cell culture
The non-tumorigenic mouse liver cell line BNL CL.2
(designated as CL2) and 3MC-transformed BNL 1MEA. 7R.1 cells
(designated as 1MEA), which were derived from CL2 cells chronically
treated with 3MC, were obtained from the Korea Research Institute
of Bioscience and Biotechnology (Daejeon, Korea). Both cell lines
were established and characterized by Gold et al (17). The cells were grown in Dulbecco's
modified Eagle's medium containing 10% fetal bovine serum, 100 U/ml
penicillin and 100 µg/ml streptomycin (GenDEPOT, Barker, TX,
USA) at 37°C in a humidified incubator with 5% CO2.
Metabolomic analysis using nuclear
magnetic resonance (NMR) spectroscopy
A cell pellet of 25 mg was transferred to a 4-mm NMR
nanotube (Agilent Technologies, Santa Clara, CA, USA).
D2O (25 µl) containing 2 mM 3-(trimethylsilyl)
propionic-2,2,3,3-d4 acid sodium salt
(TSP-d4) was then transferred to a separate NMR
nanotube. Samples were measured using high-resolution magic angle
spinning (HR-MAS) NMR spectroscopy. All spectra were acquired with
a 600.167-MHz Agilent NMR spectrometer equipped with a 4-mm gHX
NanoProbe (Agilent Technologies) and a spinning rate of 2,050 Hz.
The Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence was used due to
the suppression of water and the presence of high molecular mass
compounds. The acquisition time was 1.703 msec, the relaxation
delay was 1 sec, and 128 transients were collected. All spectra
were processed and assigned using Chenomx NMR Suite 7.1
professional with the Chenomx 600 MHz library database (Chenomx,
Edmonton, AB, Canada). Spectra were binned from 0.5 to 10 ppm for
multivariate statistical analysis. The binning size was 0.001 ppm
and water peak (4.5–4.9 ppm) and spinning side band peak were
excluded (1.15–1.2, 3.61–2.69, 6.56–7.16 and 8.0–8.4 ppm). The
binning data were normalized to total area. Principal component
analysis (PCA) was conducted with the SIMCA-P+ software
package (version 12.0; Umetrics, Umeå, Sweden). R2
parameter indicates the explained variation in the data and
goodness of fit and Q2 parameter represents the
predictive power of the model (18).
Validation of metabolites in the
transsulfuration pathway
Washed cells in 100-mm culture plates were harvested
24 h after a medium change by scraping in either 5% perchloric acid
to determine SAM, SAH, cysteine and GSH, or in ice-cold methanol to
determine the levels of methionine, glutamate, glycine, hypotaurine
and taurine. The protein pellet was dissolved in 0.1 M NaOH
solution, and the protein concentration was determined using a
bicinchoninic acid (BCA) protein assay kit (Thermo Fisher
Scientific).
SAM and SAH were analyzed by high performance liquid
chromatography (HPLC) (Ultimate 3000™; Thermo Fisher Scientific)
equipped with a UV detector (UV-3000; 254 nm; Thermo Fisher
Scientific), using the modified method of She et al
(19). Separation was
accomplished using a Hector T-C18 column (5 µm × 4.6 mm ×
100 mm) (Chiral Technology Korea, Daejeon, Korea).
Methione, glutamate, glycine, hypotaurine and
taurine were derivatized with
o-phthalaldehyde/2-mercaptoethanol and quantified using HPLC
with a fluorescence detector (FLD-3100; excitation wavelength, 338
nm; and emission wavelength, 425 nm; Thermo Fisher Scientific)
(20,21). They were separated using a Hector
T-C18 column (3 µm × 4.6 mm × 100 mm).
Total cysteine and GSH were analyzed using the SBD-F
sample derivatization method (22). MPG (50 µl), as an internal
standard, was added to the samples (50 µl) and briefly mixed
by vortex. Following the addition of 10 µl of a 10% (w/v)
TCEP solution, the tubes were incubated at room temperature for 30
min. Subsequently, 90 µl of a 10% (w/v) trichloroacetic acid
solution with 1 mM ethylenediaminetetraacetic acid (EDTA) was added
to each sample, briefly mixed by vortex and centrifuged (13,000 × g
for 10 min). The supernatant (50 µl) was then added to
another tube containing 10 µl of 1.55 M NaOH, 125 µl
of 0.125 M borate buffer (pH 9.5) with 4 mM EDTA, and 50 µl
of 0.1% (w/v) SBD-F in borate buffer (0.125 M with 4 mM EDTA). The
samples were incubated at 60°C for 1 h, and a 20 µl aliquot
was then injected into the HPLC equipped with a fluorescence
detector (excitation wavelength, 385 nm; and emission wavelength,
515 nm). The chromatographic separation was achieved using a Hector
M-C18 column (3 µm × 4.6 mm × 150 mm).
Immunoblot analysis
Cells were lysed with ice-cold PRO-PREP™ protein
extract solution (Intron, Seongnam, Korea), and the protein
concentration was quantified using the BCA procedure (Thermo Fisher
Scientific). Equal amounts of protein samples were separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a
10% polyacrylamide gel, and then transferred onto a polyvinylidene
difluoride membrane (Millipore, Billerica, MA, USA). The membrane
was blocked with 5% skimmed milk in 100 mM Tris-HCl (pH 7.5), 150
mM NaCl and 0.2% Tween-20 (TBST) for 1 h at room temperature. The
blots were incubated overnight with primary antibodies diluted in
TBST containing 5% milk at 4°C. After three washes with TBST, the
blot was incubated with the appropriate horseradish
peroxidase-conjugated secondary antibodies. The antigen was
detected using a Western Bright ECL HRP substrate kit (Advansta,
Menlo Park, CA, USA).
Determination of cell viability
Cells were plated in a 96-well plate and cell
viability was determined 24 h after t-BHP treatment by the
MTT assay, according to the manufacturer's instructions. Briefly,
after incubation with MTT (0.5 mg/ml) for 4 h at 37°C, formazan
precipitates formed by mitochondrial dehydrogenases in viable cells
were extracted with dimethyl sulfoxide. The absorbance of the
converted dye was measured at 540 nm using the Multiskan GO reader
(Thermo Fisher Scientific), and the results were expressed as a
percentage (%) compared with the vehicle treatment.
Fluorescence-activated cell sorting
(FACS) analysis
Apoptotic and live cells were determined by FACS
analysis using Annexin V-FITC staining. The cells were treated with
t-BHP for 24 h, and were subsequently harvested,
trypsinized, washed once in cold phosphate-buffered saline and
suspended in 1X binding buffer. The counted cells were stained in
prop-idium iodide and Annexin V-FITC solution (Annexin V-FITC
apoptosis detection kit; BD Biosciences, Bedford, MA, USA) at room
temperature for 15 min in the dark. The stained cells were analyzed
by flow cytometry within 1 h. Apoptotic and live cells were
analyzed by Becton Dickinson FACSscan flow cytometer and BD
FACSDiva software (BD Biosciences).
Statistical analysis
All the results are expressed as mean ± standard
deviation (n=5) and were analyzed by a two-tailed Student's t-test.
The acceptable level of statistical significance was set at
P<0.05.
Results
Metabolite profiling and PCA of
1H NMR spectroscopy in CL2 and 1MEA cells
Upon 1H NMR analysis, pattern recognition
using PCA of the NMR spectra in metabolite profiling revealed clear
clustering between CL2 and 1MEA cells (Fig. 1A). The PCA loading plots revealed
that several metabolites, such as acetate, alanine, creatine,
glutamate, glutamine, glycine, sn-glycero-3-phosphocholine, GSH,
myoinositol, lactate and o-phosphocholine, differed
significantly between the two cell types (Fig. 1B). In detailed spectra analysis,
it was identified that acetate (×0.21), choline (×0.72), formate
(×0.51), GSH (×0.59), glycerol (×0.38), isoleucine (×0.61), leucine
(×0.62), niacinamide (×0.44), o-phosphocholine (×0.06),
phenylalanine (×0.63), serine (×0.71), succinate (×0.56), tyrosine
(×0.68), uridine (×0.42), valine (×0.70) and
sn-glycero-3-phospho-choline (×0.31) were significantly lower in
the 1MEA cells compared with the CL2 cells (P<0.05).
Alternatively, glutamate (×1.61), glutamine (×6.17), glycine
(×1.45), proline (×2.02) and myo-inositol (×1.32) were
significantly higher in 1MEA cells compared with CL2 cells
(P<0.05; Fig. 1C).
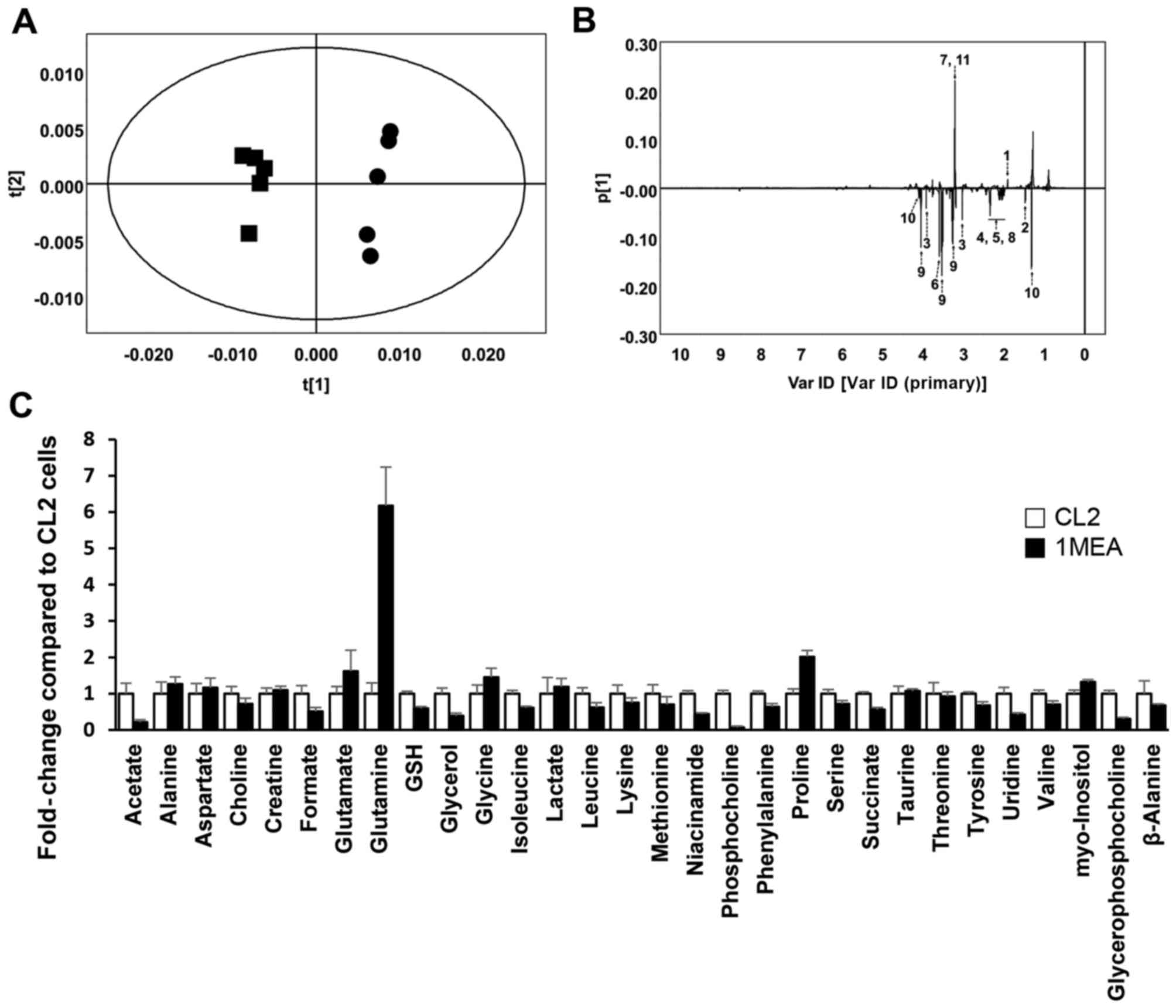 | Figure 1Results of 1H nuclear
magnetic resonance (NMR) spectroscopy in CL2 and 1MEA cells.
(R2X= 0.873, Q2=0.682) (A) Principal
component analysis (PCA) score plot. Circles, CL2; squares, 1MEA.
(B) PCA loading plot of CL2 vs. 1MEA. 1, Acetate; 2, alanine; 3,
creatine; 4, glutamate; 5, glutamine; 6, glycine; 7,
glycerophosphocholine; 8, GSH; 9, inositol; 10, lactate; 11,
phosphocholine. (C) Fold changes of quantified metabolites from
1H NMR spectra. Each value represents the mean ±
standard deviation. GSH, glutathione. |
1MEA cells exhibit aberrant changes in
metabolites of the transsulfuration pathway
GSH plays a critical role in removing reactive
metabolites from the cell, and a disturbance in its synthesis
reduces a cell's resistance to oxidative stress. Since there are
limited data available showing that 3MC causes oxidative stress,
attention was paid to the significantly lower level of
intracellular GSH in 1MEA cells compared with that in CL2 cells.
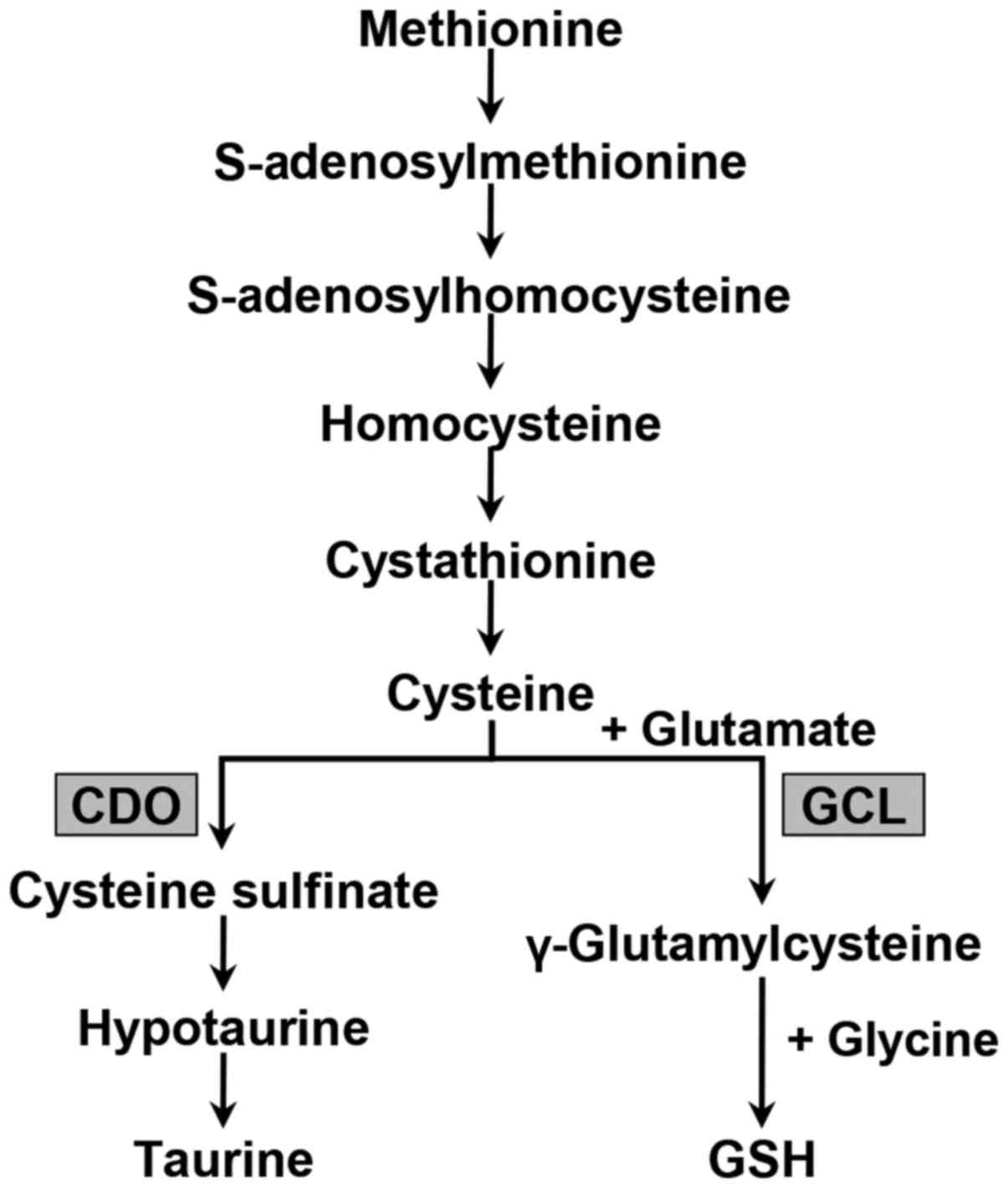
Specifically, we focused on the transsulfuration pathway that
produces GSH and taurine from the essential amino acid methionine
(Fig. 2). To investigate and
validate all the major metabolites in the transsulfuation pathway,
HPLC with a UV or fluorescence detector was used. The cellular
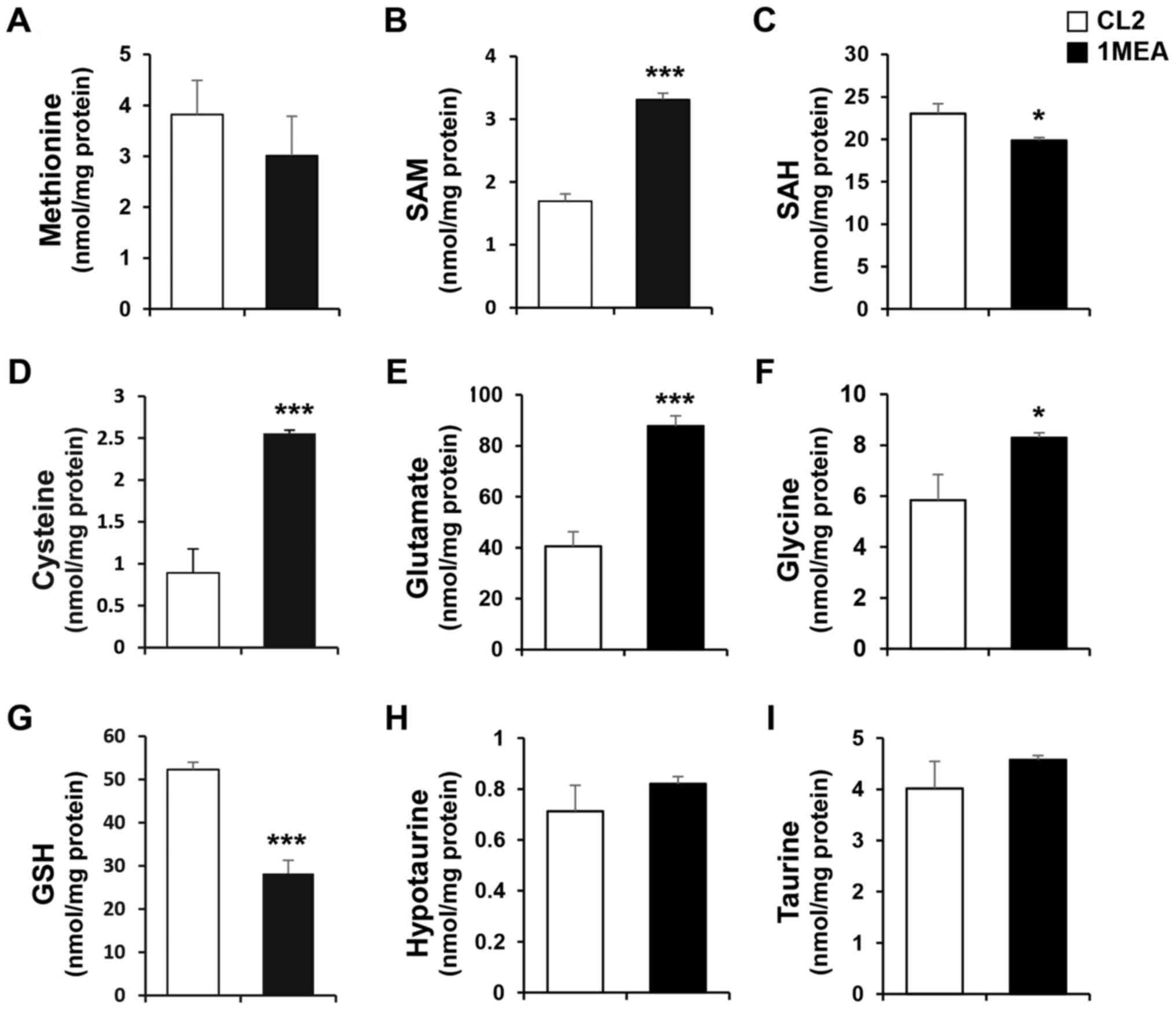
levels of SAM, cysteine, glutamate and glycine were signifi-cantly
increased 2.0-, 2.7-, 2.1- and 1.4-fold, respectively, in 1MEA
cells compared with CL2 cells (Fig.
3B, D–F). On the contrary, the concentration of SAH was
slightly decreased in 1MEA cells (Fig. 3C). The levels of methionine,
hypotaurine and taurine did not change (Fig. 3A, H and I), but the GSH levels in
1MEA cells were significantly reduced compared with those in CL2
cells (Fig. 3G). Cysteine is the
precursor metabolite used to synthesize both GSH and taurine, with
GSH being a tripeptide composed of glutamate, cysteine and glycine.
Considering the increased levels of cysteine, glutamate and glycine
in 1MEA cells, the decreased levels of GSH cannot be fully
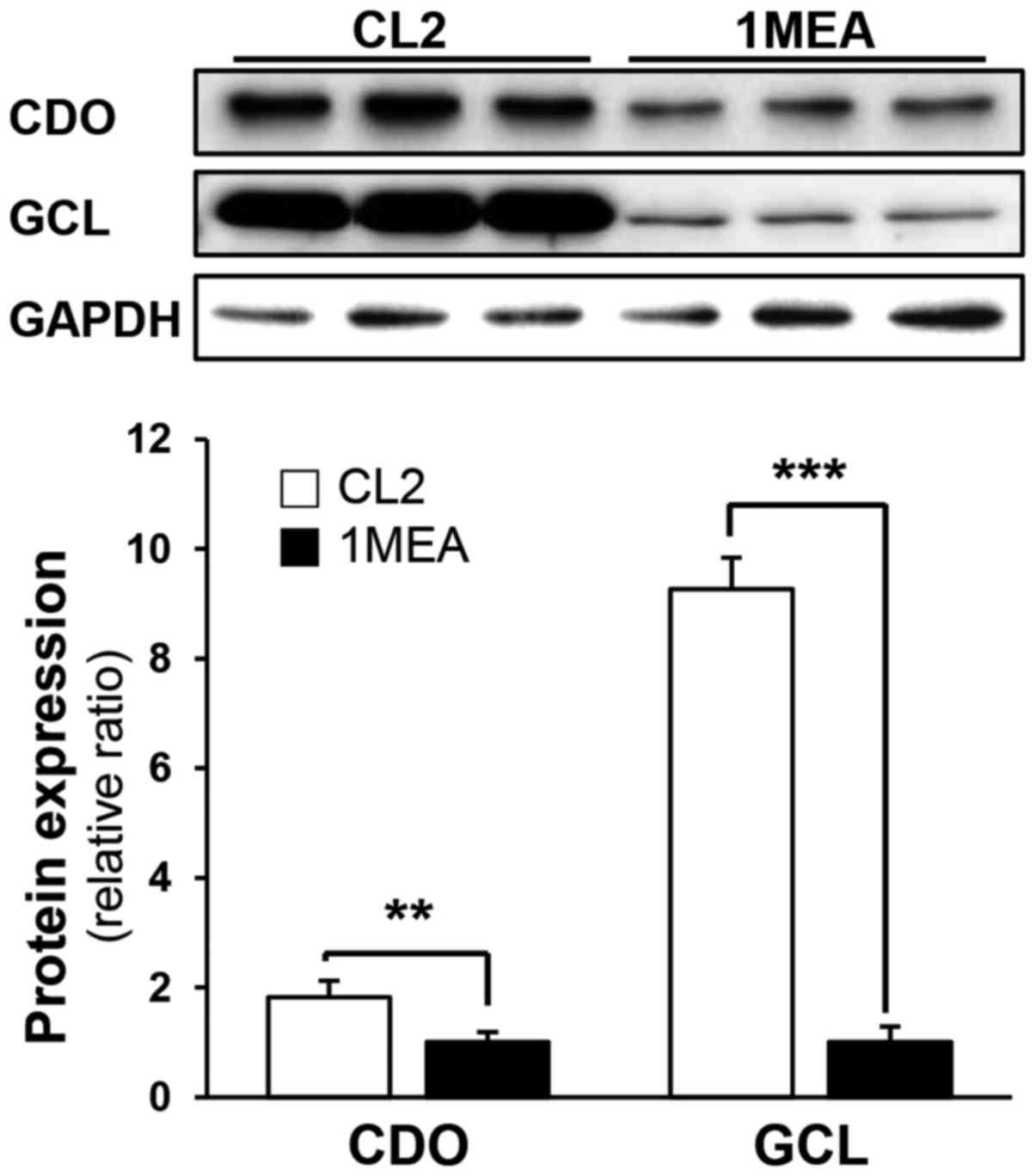
explained. To investigate why the GSH levels decreased in 1MEA
cells, the expression of CDO and GCL, which are the rate-limiting
enzymes in the production of taurine and GSH, respectively, was
subsequently investigated. In 1MEA cells, CDO expression decreased
to 60% of that in CL2 cells, whereas GCL expression decreased to
11% of that in CL2 cells (Fig.
4). Thus, it is suggested that reduced GCL protein expression
contributes to decreased GSH synthesis.
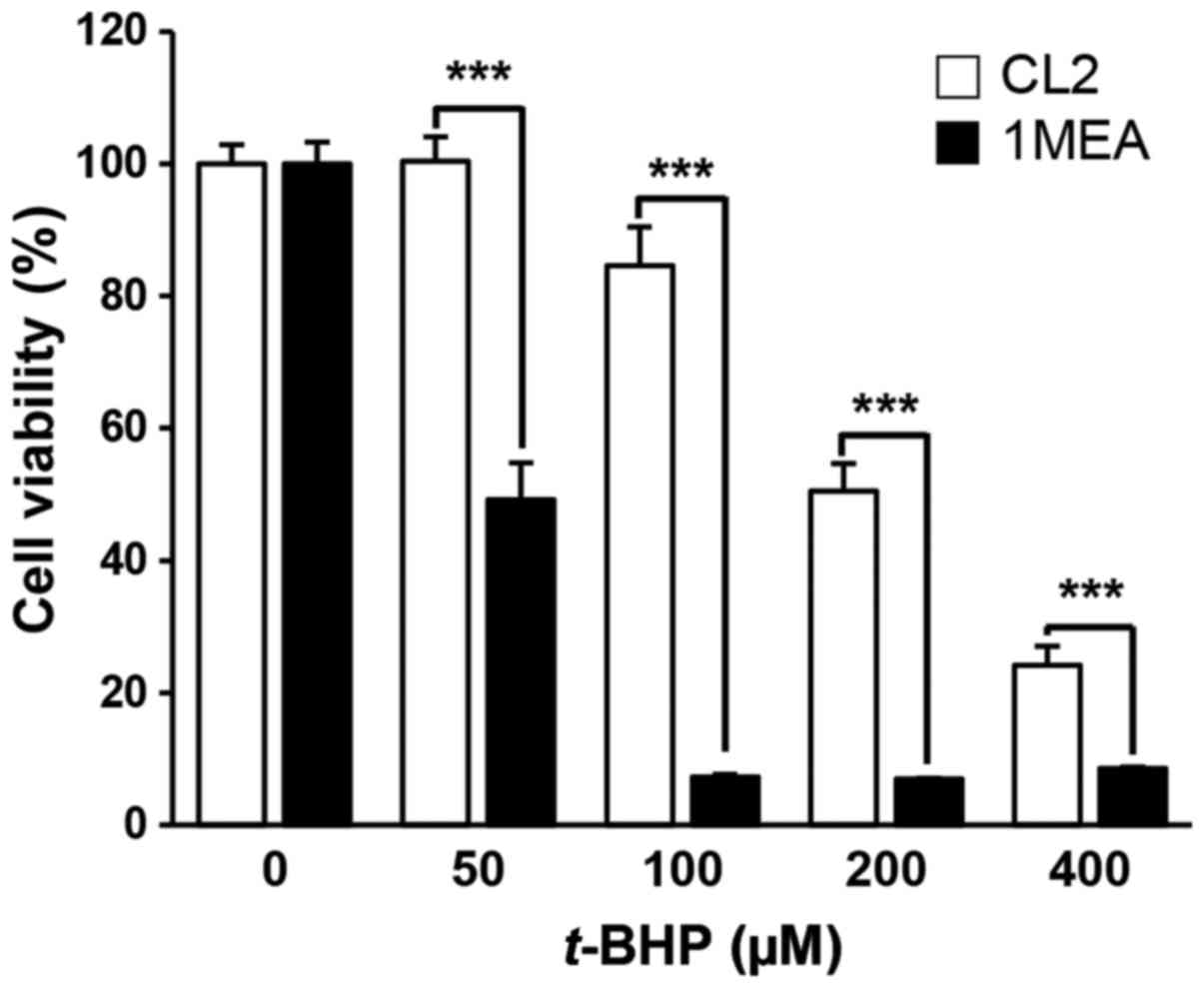
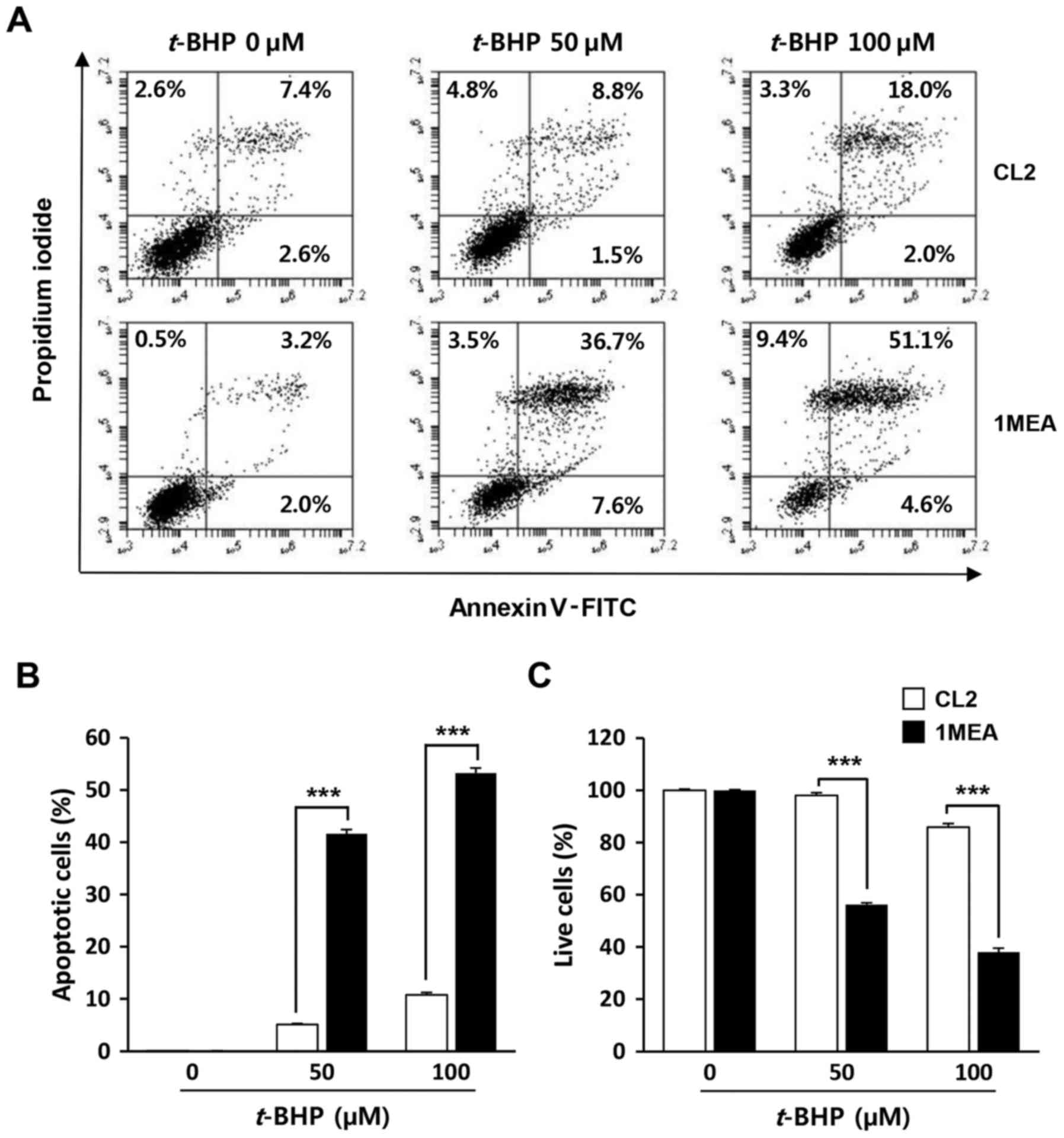
1MEA cells are more vulnerable to cell
death incuced by t-BHP treatment compared with CL2 cells
To investigate whether low concentrations of GSH in
1MEA cells affected the rate of cell death by exogenous oxidative
stress, the cells were exposed to t-BHP, a producer of
reactive oxygen species, with cell viability being examined 24 h
post-treatment (Fig. 5). As
expected, 1MEA cells were more sensitive to t-BHP treatment,
with a 50 µM t-BHP treatment leading to 50% 1MEA cell
death rate, while the survival of CL2 cells was not affected. To
determine oxidative stress-induced apoptotic cell death, flow
cytometry analysis was performed. As shown in Fig. 6, increase of early apoptosis
(lower right quadrant) and late apoptosis (upper right quadrant)
were clearly observed in t-BHP-treated 1MEA cells compared
with CL2 cells.
Discussion
There is increasing evidence that GSH is reduced in
several human diseases, and this contributes to the deterioration
of the condition (23). In a
number of of these diseases, GSH depletion, which is often caused
by decreases in the expression of GSH synthesizing enzymes, is
considered to play a major role in oxidative tissue damage
(24). In the present study, 1MEA
cells, transformed by chronically exposing CL2 cells to 3MC, were
found to be more susceptible to oxidative stress compared with CL2
cells, due to the decreased synthesis of GSH. Although the main
concern regarding PAH toxicity is its ability to activate the
AhR-mediated pathway and lead to cancer development, these results
indicate that the deleterious effect of 3MC may be mediated by a
reduction in the antioxidant capacity of the cells.
To understand the altered metabolism and identify
abnormal pathways in response to chronic 3MC exposure, we have
introduced the technique of global metabolite profiling followed by
targeted metabolomic analysis. Specifically, HR-MAS NMR
spectroscopy was used to perform non-destructive metabolite
profiling of cells. Thus, changes in the metabolites involved in
the synthesis of sulfur-containing amino acids, which are
associated with a cellular redox state, were observed.
Sulfur-containing amino acid metabolism occurs
primarily via the transsulfuration pathway, which results in the
transfer of the methionine sulfur to serine to form cysteine
(Fig. 2). Subsequently, cysteine
is irreversibly metabolized to yield taurine or GSH. CDO catalyzes
the oxidation of cysteine to cysteine sulfinate, which is converted
primarily to taurine via hypotaurine and the enzyme cysteine
sulfinate decarboxylase. Alternatively, the synthesis of GSH from
its constituent amino acids involves two ATP-requiring enzymatic
steps: The first step of GSH biosynthesis is rate-limiting and
catalyzed by GCL to make γ-glutamylcysteine from glutamate and
cysteine; the second step in GSH synthesis is catalyzed by GSH
synthetase to form GSH from γ-glutamylcysteine and glycine
(25).
The increase in the amount of amino acids that
constitute GSH, and the seemingly contradictory decrease in the
concentration of GSH in 1MEA cells, as compared to CL2 cells, were
of particular interest in the present study. Synthesis of GSH is
largely limited by two factors: The availability of cysteine and
the activity of GCL (26).
Cysteine concentrations are regulated by a balance between the
rates of its synthesis through the transsulfuration pathway and its
metabolism to GSH, inorganic sulfate, or taurine (27,28). Previous studies have suggested
that cysteine availability is a major determining factor for the
partitioning of cysteine sulfur to either GSH, taurine, or
inorganic sulfates in rat liver (29,30). Specifically, low cysteine
availability would favor its utilization for the synthesis of GSH,
and high cysteine availability enhances its catabolism to inorganic
sulfate and taurine. Although CDO expression in 1MEA cells was
lower compared with that in CL2 cells, it did not lead to any
difference in hypotaurine and taurine concentrations between
groups, thereby indicating that a sufficient amount of cysteine is
supplied to synthesize taurine. However, GCL expression in 1MEA
cells was markedly reduced, which may account for the increased
cysteine concentrations, as well as the reduced GSH levels. These
results suggest that blocking GSH synthesis may have a major effect
on the partitioning of cysteine sulfur to taurine synthesis.
In conclusion, the present results indicate that
cells transformed by chronic exposure to 3MC exhibited abnormal
changes in the transsulfuration pathway, accompanied by increased
availability of cysteine to taurine rather than GSH synthesis.
Inhibition of cysteine catabolism to GSH by 3MC may play an
important role in the preservation of intracellular taurine.
Finally, the potentiation of t-BHP-induced death in 1MEA
cells appears to be associated with a blockage in GSH synthesis. It
has been reported that long-term exposure to PAHs, including 3MC,
is the cause of serious health problems by various cellular
effects. Further studies to determine the biological effect of 3MC
on the dysregulation of the transsulfuration pathway are currently
underway in this laboratory.
Acknowledgments
This study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Korean government
(MSIP) (no. 2009-0083538). The study was also supported by the
Basic Science Research Program through the National Research
Foundation of Korea (NRF) funded by the Ministry of Science, ICT
and Future Planning (grant no. NRF-2014R1A 1A1005435).
References
|
1
|
Van Metre PC and Mahler BJ: Trends in
hydrophobic organic contaminants in urban and reference lake
sediments across the United States, 1970–2001. Environ Sci Technol.
39:5567–5574. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weisman D, Alkio M and Colón-Carmona A:
Transcriptional responses to polycyclic aromatic
hydrocarbon-induced stress in Arabidopsis thaliana reveal the
involvement of hormone and defense signaling pathways. BMC Plant
Biol. 10:592010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim KH, Jahan SA, Kabir E and Brown RJ: A
review of airborne polycyclic aromatic hydrocarbons (PAHs) and
their human health effects. Environ Int. 60:71–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pelclová D, Fenclová Z, Dlasková Z, Urban
P, Lukás E, Procházka B, Rappe C, Preiss J, Kocan A and Vejlupková
J: Biochemical, neuropsychological, and neurological abnormalities
following 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure. Arch
Environ Health. 56:493–500. 2001. View Article : Google Scholar
|
|
5
|
Baccarelli A, Mocarelli P, Patterson DG
Jr, Bonzini M, Pesatori AC, Caporaso N and Landi MT: Immunologic
effects of dioxin: New results from Seveso and comparison with
other studies. Environ Health Perspect. 110:1169–1173. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pesatori AC, Consonni D, Bachetti S,
Zocchetti C, Bonzini M, Baccarelli A and Bertazzi PA: Short- and
long-term morbidity and mortality in the population exposed to
dioxin after the 'Seveso accident'. Ind Health. 41:127–138. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mimura J and Fujii-Kuriyama Y: Functional
role of AhR in the expression of toxic effects by TCDD. Biochim
Biophys Acta. 1619:263–268. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Denison MS and Nagy SR: Activation of the
aryl hydrocarbon receptor by structurally diverse exogenous and
endogenous chemicals. Annu Rev Pharmacol Toxicol. 43:309–334. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nebert DW, Dalton TP, Okey AB and Gonzalez
FJ: Role of aryl hydrocarbon receptor-mediated induction of the
CYP1 enzymes in environmental toxicity and cancer. J Biol Chem.
279:23847–23850. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shimada T: Xenobiotic-metabolizing enzymes
involved in activation and detoxification of carcinogenic
polycyclic aromatic hydrocarbons. Drug Metab Pharmacokinet.
21:257–276. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suresh R, Shally A, Mahdi AA, Patel DK,
Singh VK and Rita M: Assessment of association of exposure to
polycyclic aromatic hydrocarbons with bronchial asthma and
oxidative stress in children: A case control study. Indian J Occup
Environ Med. 13:33–37. 2009. View Article : Google Scholar
|
|
12
|
Van Tiem LA and Di Giulio RT: AHR2
knockdown prevents PAH-mediated cardiac toxicity and XRE- and
ARE-associated gene induction in zebrafish (Danio rerio). Toxicol
Appl Pharmacol. 254:280–287. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuan JW, Krieger JA, Maples KR, Born JL
and Burchiel SW: Polycyclic aromatic hydrocarbons decrease
intracellular glutathione levels in the A20.1 murine B cell
lymphoma. Fundam Appl Toxicol. 23:336–341. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ranjit S, Midde NM, Sinha N, Patters BJ,
Rahman MA, Cory TJ, Rao PS and Kumar S: Effect of polyaryl
hydrocarbons on cyto-toxicity in monocytic cells: Potential role of
cytochromes P450 and oxidative stress pathways. PLoS One.
11:e01638272016. View Article : Google Scholar
|
|
15
|
Lu SC: Glutathione synthesis. Biochim
Biophys Acta. 1830:3143–3153. 2013. View Article : Google Scholar :
|
|
16
|
Summer KH and Eisenburg J: Low content of
hepatic reduced glutathione in patients with Wilson's disease.
Biochem Med. 34:107–111. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gold LS, Slone TH, Manley NB and Bernstein
L: Target organs in chronic bioassays of 533 chemical carcinogens.
Environ Health Perspect. 93:233–246. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wheelock AM and Wheelock CE: Trials and
tribulations of 'omics data analysis: Assessing quality of
SIMCA-based multivariate models using examples from pulmonary
medicine. Mol Biosyst. 9:2589–2596. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
She QB, Nagao I, Hayakawa T and Tsuge H: A
simple HPLC method for the determination of S-adenosylmethionine
and S-adenosylhomocysteine in rat tissues: The effect of vitamin B6
deficiency on these concentrations in rat liver. Biochem Biophys
Res Commun. 205:1748–1754. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jung YS, Kim SJ, Kwon DY and Kim YC:
Comparison of the effects of buthioninesulfoximine and phorone on
the metabolism of sulfur-containing amino acids in rat liver.
Biochem Biophys Res Commun. 368:913–918. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ide T: Simple high-performance liquid
chromatographic method for assaying cysteinesulfinic acid
decarboxylase activity in rat tissue. J Chromatogr B Biomed Sci
Appl. 694:325–332. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nolin TD, McMenamin ME and Himmelfarb J:
Simultaneous determination of total homocysteine, cysteine,
cysteinylglycine, and glutathione in human plasma by
high-performance liquid chromatography: Application to studies of
oxidative stress. J Chromatogr B Analyt Technol Biomed Life Sci.
852:554–561. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ballatori N, Krance SM, Notenboom S, Shi
S, Tieu K and Hammond CL: Glutathione dysregulation and the
etiology and progression of human diseases. Biol Chem. 390:191–214.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu SC: Regulation of glutathione
synthesis. Mol Aspects Med. 30:42–59. 2009. View Article : Google Scholar :
|
|
25
|
Stipanuk MH: Sulfur amino acid metabolism:
Pathways for production and removal of homocysteine and cysteine.
Annu Rev Nutr. 24:539–577. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meister A and Anderson ME: Glutathione.
Annu Rev Biochem. 52:711–760. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Coloso RM, Drake MR and Stipanuk MH:
Effect of bathocuproine disulfonate, a copper chelator, on
cyst(e)ine metabolism by freshly isolated rat hepatocytes. Am J
Physiol. 259:E443–E450. 1990.PubMed/NCBI
|
|
28
|
Garcia RA and Stipanuk MH: The splanchnic
organs, liver and kidney have unique roles in the metabolism of
sulfur amino acids and their metabolites in rats. J Nutr.
122:1693–1701. 1992.PubMed/NCBI
|
|
29
|
Kwon YH and Stipanuk MH: Cysteine
regulates expression of cysteine dioxygenase and
gamma-glutamylcysteine synthetase in cultured rat hepatocytes. Am J
Physiol Endocrinol Metab. 280:E804–E815. 2001.PubMed/NCBI
|
|
30
|
Stipanuk MH, Coloso RM, Garcia RA and
Banks MF: Cysteine concentration regulates cysteine metabolism to
glutathione, sulfate and taurine in rat hepatocytes. J Nutr.
122:420–427. 1992.PubMed/NCBI
|