Introduction
Pulmonary fibrosis (PF) is a specific form of
chronic fibrosing interstitial pneumonia limited to the lungs,
which is characterized by disordered lung function (1,2).
The etiology of PF is so complex that no accordance has been
achieved. Of the diverse etiologies of PF, there is a common
characteristic, i.e., the irregular deposition of extracellular
matrix (ECM) that plays a key role in maintaining the normal lung
tissue structure (3). One of the
major sources of ECM are myofibroblasts, which have been identified
as the intermediate between bone smooth muscle cells and
fibroblasts, and are characterized by the expression of α-smooth
muscle actin (α-SMA). Previous studies have proven that the
differentiation of fibroblasts into myofibroblasts is modulated by
transforming growth factor-β1 (TGF-β1). The molecule is capable of
regulating the expression of α-SMA in fibroblasts as well (4,5).
Based on these overwhelming findings, TGF-β1 has already been
regarded as a potential target for the amelioration of PF. Current
treatments of PF involving the targeting of TGF-β1 are based on
neutralizing antibodies, antisense TGF-β1 oligo deoxynucleotides
and specific inhibitors to TβR kinases. However, TGF-β1 plays
important roles in regulating inflammation and acts as a tumor
suppressor in some contexts; thus, arbitrarily reducing the
systemic level of this cytokine may have some unexpected
side-effects. The development of novel but non-invasive schemes
regulating TGF-β1 is therefore critical for the improvement of the
outcome and survival of patients with PF.
Recent evidence that embryonic stem cells (ESCs) or
adult stem cells are capable of repairing and regenerating the
injured or diseased tissues (6)
has inspired extensive investigation of the stem cell-based
therapies in treating devastating and incurable lung diseases.
Considering ethical or safety issues associated with the
application of ESCs, most studies focused on stem cell therapies
are performed based on adult stem cells, such as induced
pluripotent stem cells (iPSCs) (7). iPSC is a multi-lineage
differentiation cell population which can be conveniently induced
from certain cell lines through reprograming by specifically
transcription factor transduction (8,9),
and can provide a resource for stem cell-based therapies.
Treatments of endotoxin-induced acute lung injury using iPSCs have
already achieved considerable outcomes; the therapy stimulates the
production of paracrine mediators and regulates neutrophil
activities in response to endotoxins and the inflammatory response
(8,10). Moreover, these reports have also
proven the low tumorigenic risk of the use of iPSCs scheme as an
advantage over antibody-based therapies (8,9).
However, it remains unclear as to whether iPSC-based treatment has
the capability to regulate the dysexpression of TGF-β1, inhibit the
differentiation of fibroblasts into myofibroblasts and attenuate
the damage due to PF.
Thus, in the present study, we attempted to evaluate
the protective effects of iPSC-conditioned medium (iPSC-CM) against
PF through the TGF-β1-related pathway in a mouse model of
bleomycin-induced PF. The effects of iPSC-CM on pulmonary
morphology were determined by hematoxylin and eosin (H&E)
staining and Masson's staining. The regulatory function of iPSC-CM
on TGF-β1 and other molecules related to PF was evaluated using
enzyme-linked immunosorbent assay (ELISA), reverse
transcription-quantitative PCR (RT-qPCR) and western blot
analysis.
Materials and methods
Chemicals, animals and cell culture
Antibodies against proliferating cell nuclear
antigen [proliferating cell nuclear antigen (PCNA); cat. no.
bs-2006R), p-Smad-2 (cat. no. bs-5618R), Smad-2 (cat. no.
bs-0718R), p-Smad-3 (cat. no. bs-5459R) and Smad-3 (cat. no.
bs-3484R) were all purchased from Bioss, Inc. (Woburn, MA, USA).
Antibodies against α-smooth muscle actin (α-SMA; cat. no. BM0002)
and collagen I (cat. no. BA0325), were purchased from Wuhan Boster
Biological Technology, Ltd. (Wuhan, China). Antibody against
β-actin (cat. no. sc-47778) was purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Male C57BL/6 mice (8–10
weeks old; weighing 24 to 45 g) were purchased from the
Experimental Animal Center of China Medical University. All the
animals were maintained at 20–25°C with a constant humidity of
55±5% with free access to food and water. All animal experiments
were conducted in accordance with the Institutional Animal Ethics
Committee and Animal Care Guidelines of Shengjing Hospital of China
Medical University which governed the use of the animals.
Previously preserved human lung fibroblasts (HFL1 cell line; cat.
no. GNHu28; Cell Bank of Chinese Academy of Sciences, Shanghai,
China) were cultured in dulbecco's modified Eagle's medium
(DMEM/F-12) medium [10% (v/v) fetal bovine serum (FBS) and 1% (v/v)
antibiotics mixture] in 95% air and 5% CO2 at 37°C.
Generation of iPSCs by mouse 3-gene
transfection, identification and cell culture
Mouse 3-gene iPSCs were established via the
transfection of Oct4/Sox2/Klf4 into C57BL/6 mice as previously
described (7,11). Mouse embryonic fibroblasts (MEFs)
were isolated from the placentas of 10 C57 pregnant mice (10 weeks
old, at 2 weeks of gestation, purchased from Charles River Lab,
Beijing, China). Briefly, the placentas were washed with PBS twice.
The outer membrane of the placenta was removed carefully with
ophthalmic scissors and the left tissues were washed with PBS again
and cut into 1–3 mm3 sections. The tissues were
incubated in FBS in an atmosphere consisting of 5% CO2
and 95% air at 37°C for 12 h and transferred to DMEM (supplemented
with 10% FBS) and cultured for 5 days. The cells were then
transferred to wells of a 12-well plate and cultured for a further
10–15 days. Afterwards, the supernatant of the cultures was
discarded and the cells were incubated with 0.25% pancreatin until
the cells turned into spheroidal shape. The cells were then
collected by centrifuging at 1,000 rpm for 5 min and preserved at
37°C for subsequent assays. ESCs (cat. no. SCSP-226) were purchased
from the Cell Bank of Chinese Academy of Sciences. Briefly, the
MEFs were incubated in one well of the 6-well plates at a density
of 8×105/well one day prior to transfection with
lentivirus vectors encoding Oct4/Sox2/Klf4 mouse complementary DNA.
For transfection, equal amounts of the supernatants containing each
of the three vectors were mixed and transferred to the MEF dishes
and incubated overnight. The cells were then replated in fresh
medium and incubated overnight before the medium was replaced with
DMEM supplemented with 10% FBS. At 48 h after transfection,
positive colonies were selected using DMEM supplemented with 15%
FBS, 2 mM L-glutamine, 1×10−4 M non-essential amino acid
and 1×10−4 M M2-mercaptoethanol. The total RNA in
undifferentiated iPSCs, MEFs and ESCs were extracted using the RNA
simple total RNA kit according to the manufacturer's instructions
(no. DP419; Tiangen, Beijing, China) for RT-qPCR validation as
described below. Thereafter, the osteogenic differentiation and
adipogenic differentiation potential of the iPSCs, MEF and ESCs
were detected using the Alizarin Red S and Oil Red O staining
methods, respectively as previously described (12). Following the identification of the
iPSCs, the undifferentiated iPSCs were routinely cultured at 37°C
in an atmosphere of 95% air and 5% CO2.
Osteogenic and adipogenic induction
Osteogenic induction was performed by incubating the
cells in medium consisting of DMEM/F12 (cat. no. SH30023.01B;
HyClone, Logan, UT, USA) supplemented with 10% FBS (cat. no.
SH30084.03; HyClone), 0.25 mmol/l ASA (cat. no. A8960-5G;
Sigma-Aldrich, St. Louis, MO, USA), 10 mmol/l β-glycerolphosphate
(cat. no. 201205053; Biosharp, St. Louis, MO, USA), and
10−7 mol/l dexamethasone (cat. no. D1756;
Sigma-Aldrich).
Adipogenic induction was performed by incubating the
cells in medium consisting of DMEM/F12 (cat. no. SH30023.01B;
HyClone) supplemented with 10−7 mol/l dexamethasone
(cat. no. D1756; Sigma-Aldrich), 100 nmol/l insulin (Fosun Pharma,
Shanghai, China), 0.2 mmol/l indomethacin (cat. no. 17378-5G), and
5% FBS (cat. no. SH30084.03; HyClone).
Detection of osteogenic and adipogenic
potential
For Alizarin Red staining, the cells were fixed with
4% paraformaldehyde for 15 min and stained with 0.1% Alizarin Red
(cat. no. A5533-25G; Sigma-Aldrich) for 40 min at room temperature.
The results were detected under a microscope at ×400 magnification.
For Oil Red O staining, cells were fixed with 4% paraformaldehyde
for 30 min and stained with 0.6% Oil Red O (cat. no. O0625-25G;
Sigma-Aldrich) for 1 h at room temperature. The results were
detected under a microscope at ×400 magnification.
Preparation of the iPSC-CM
The iPSCs were culture routinely for 24 h before
being transferred to serum-free DMEM [containing 2 mM L-glutamine
(cat. no. 59202C) and 1×10−4 M non-essential amino acid
(cat. no. H7145) (both from Sigma-Aldrich), 1×10−4 M
M2-mercapto ethanol (cat. no. 21985-023; Gibio)] and cultured for a
further 48 h. The supernatants (supernatant 1) of the iPSC medium
were collected and centrifuged for 10 min at 1,500 rpm to separate
the supernatants (supernatant 2) from precipitation. For subsequent
experiments, the supernatant was employed as iPSC-CM.
Treatment of HFL1 cells with iPSC-CM and
TGF-β1
To evaluate the inhibitory effect of iPSC-CM on the
proliferation of fibroblasts, HLF1 cells were treated with various
combinations of iPSC-CM and TGF-β1 as follows: i) the control
group, normal HFL1 cells; ii) the TGF-β1 group, HFL1 cells
incubated with TGF-β1 (5 ng/ml) for 24 h to induce differentiation
into myofibroblasts; iii) the 30% iPSC-CM group, HFL1 cells
incubated with 30% iPSC-CM and TGF-β1 (5 ng/ml) for 24 h; iv) the
50% iPSC-CM group, HLF1 cells incubated with 50% iPSC-CM and TGF-β1
(5 ng/ml) for 24 h; v) the 100% iPSC-CM group, HLF1 cells incubated
with 100% iPSC-CM and TGF-β1 (5 ng/ml) for 24 h. Each treatment was
represented by at least 3 replicates. Moreover, the cytotoxicity of
iPSC-CM was assessed and no impairment on the cell normal
biological processes was detected (Figs. 1 and 2).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Upon completion of the 24 h of incubation, MTT assay
was performed to determine the viability of the HLF1 cells in the
different groups. Briefly, 50 μl exponentially growing cells
(2×105 cells/ml) were seeded into a 96-well plate in
triplicate. Subsequently, 5 mg/ml MTT were added to each well
followed by incubation for 4 h at 37°C. The optical density (OD)
values in different wells were recorded using a mircoplate reader
(ELX-800; BioTek Instruments, Inc., Winooski, VT, USA) at 490 nm.
The survival rates (%) of the different treatment groups were
calculated as follows: (OD value in treatment group - OD value in
blank control group)/(OD value in negative control group - OD value
in blank control group) ×100%.
RT-qPCR
The RNA samples were reverse transcribed into cDNA
using Super M-MLV reverse transcriptase (no. RP6502; Bioteke,
Beijing, China), and the final reaction mixture of volume 20
μl contained 10 μl of SYBR-Green Mastermix, 0.5
μl of each of the following primers: Nanog forward,
5′-CAGGGCTATCTGGTGAACG-3′ and reverse, 5′-CGAA GTTATGGAGCGGAGC-3′;
octamer-binding transcription factor 4 (OCT4) forward,
5′-CCCAACGAGAAGAGTATGAGG-3′ and reverse, 5′-GAGCAGTGACGGGAACAGA-3′;
SOX2 forward, 5′-GCACAGATGCAACCGATGC-3′ and reverse,
5′-TCGGACTTGACCACAGAGCC-3′; Kruppel-like factor 4 (Klf4) forward,
5′-CCTACTTATCTGCCTTGCTGATTGTC-3′ and reverse,
5′-CCCCCAGATTGCCCGAGAT-3′; Fbxo15 (Fbx15) forward,
5′-GGGATAAAGAAGATGGATACTGG-3′ and reverse,
5′-GATTGTCCAACCTAAGCCAGA-3′; TGF-β1 forward,
5′-GCAACAATTCCTGGCGTTACCT-3′ and reverse,
5′-GAAAGCCCTGTATTCCGTCTCC-3′; α-SMA forward,
5′-TCCCTTGAGAAGAGTTACGAGTT-3′ and reverse,
5′-ATGATGCTGTTGTAGGTGGTT-3′; collagen I forward,
5′-AGGTGTTGTGCGATGACGTGAT-3′ and reverse,
5′-TGGTTTCTTGGTCGGTGGGTGA-3′; murine β-actin forward,
5′-CTGTGCCCATCTACGAGGGCTAT-3′ and reverse,
5′-TTTGATGTCACGCACGATTTCC-3′; Homo sapiens β-actin forward,
5′-CTTAGTTGCGTTACACCCTTTCTTG-3′ and reverse,
5′-CTGTCACCTTCACCGTTCCAGTTT-3′, 1 μl of the cDNA template,
and 8 μl ddH2O. Thermal cycling parameters for
the amplification were as follows: a denaturation step at 95°C for
10 min, followed by 40 cycles at 95°C for 10 sec, 60°C for 20 sec
and 72°C for 30 sec. Relative gene expression was evaluated with
Exicyler™ 96 (Bioneer, Daejeon, Korea). The relative expression
levels of different genets were determined according to the
2−ΔΔCt method.
Western blot analysis
Total proteins from the different groups were
extracted using the total protein extraction kit according to the
manufacturer's instructions (cat. no. WLA019; Wanleibio, Shenyang,
China) and the concentration of each sample was determined using
the BCA kit (cat. no. WLA004, Wanleibio). β-actin was used as
internal reference protein. All the extracts were boiled in loading
buffer for 5 min and 20 μg of protein was subject to a 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE). Targeted proteins were then transferred onto
polyvinylidene difluoride (PVDF) membranes. The membranes were
washed with TBST for 5 min and then transferred into blocking
buffer for overnight incubation at 4°C. Following 3 cycles of 5 min
washes with TBST, primary antibodies against different proteins
[PCNA (1:500), α-SMA (1:400), collagen I (1:400), p-Smad-2 (1:500),
Smad-2 (1:500), p-Smad-3 (1:500), Smad-3 (1:500) and β-actin
(1:1,000)] were incubated with the membranes for 1 h at room
temperature. After an additional 3 washes, secondary IgG-HRP
antibodies [1:5,000; goat anti-rabbit antibody (cat. no. WLA023;
Wanleibio), goat anti-mouse IgG-HRP antibody (cat. no. A0216;
Beyotime Biotechnology, Shanghai, China)] were added and incubated
with the membranes for 40 min. Following a final 3 washes using
TBST, the blots were developed using Beyo ECL Plus reagent and the
results were detected in the gel imaging system. The relative
expression levels of different proteins were calculated using
Bio-Rad Quantity One software (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Detection of the effects of iPSC-CM on
the TGF-β1 mediated differentiation of HFL cells into
myofibroblasts
The HFL1 cells were treated as described above only
with the incubation course changed to 48 h. The mRNA and protein
expression levels of α-SMA and collagen I were determined using
RT-qPCR and western blot analysis as described above.
Detection of the effects of iPSC-CM on
the TGF-β1 signal transduction pathway in HFL1 cells
The HFL1 cells were primarily incubated with various
percentages of iPSC-CM as mentioned above for 24 h and were then
treated with 5 ng/ml TGF-β1 for a further 30 min. The expression of
Smad-2, p-Smad-2, Smad-3 and p-Smad-3 was detected using western
blot analysis.
Establishment of mouse models of PF
Male C57BL/6 mice were randomly divided into 3
groups (6 in each group) as follows: i) the control group: mice
were intratracheally injected with normal saline under anesthesia
via an intraperitoneal injection of 80 mg/kg pentobarbital sodium
for 24 h; ii) the PF group: mice were intratracheally injected with
5 mg/kg bleomycin sulfate (Merck Millipore, darmstadt, Germany)
under anesthesia for 24 h to induce PF before being intravenously
injected with 200 μl normal saline; iii) the PF + iPSC-CM
group: after induction of PF, mice in this group were intravenously
injected with 200 μl iPSC-CM. At 21 days after the
establishment of the model, the mice in different groups were
sacrificed to collect bronchoalveolar lavage fluid (BALF) and lung
tissues. The main bronchus of right lung of each mouse was ligated
and a catheter was inserted into the right lung at the same time.
The lung was washed with normal saline using a catheter 3 times
(0.5 ml each time) and the BALF was collected. The mice were then
sacrificed and the lungs were collected. The right lung was cut
into sections and fixed with 4% paraformaldehyde, and the tissues
of the left lung were preserved at −80°C.
H&E staining
Each lung tissue sample was fixed in 4%
paraformaldehyde, dehydrated with a graded ethanol series, embedded
in paraffin blocks, cut into 3-μm-thick sections, and
stained with H&E following standard histologic techniques. The
results of the staining were observed using a microscope (DP73;
Olympus, Tokyo, Japan) at ×200 magnification.
Analysis of collagen accumulation
To assess the effects of iPSC-CM on collagen
accumulation due to PF, Masson's trichrome staining [Aniline blue
(cat. no. 229661000), Ponceau (cat. no. p8330) and acid fuchsin
(cat. no. 71019360); Sinopharm Group, Beijing, China)] was utilized
to demonstrate the changes in the tissue samples which were
associated with the formation of collagen according to the method
of Flint and Lyons (13). ELISA
for collagen I was also conducted using an ELISA kit (StressXpress;
Assay designs/Stressgen Bioreagents) according to the
manufacturer's instructions. Moreover, the content of
hydroxyproline was determined using a previously described method
(13). The expression of α-SMA
was determined by western blot analysis as described above.
Determination of the effects iPSC-CM on
the TGF-β1 signal transduction pathway in C57BL/6 mice
The content of TGF-β1 in BALF was determined by
ELISA. The expression of TGF-β1 in different lung tissue samples
was quantified by RT-qPCR. The production of TGF-β1, Smad-2,
p-Smad-2, Smad-3 and p-Smad-3 was assessed by western blot
analysis.
Statistical analysis
All the data are expressed as the means ± SD. ANOVA
and post hoc multiple comparisons were conducted using the LSD
method with a general liner model with a significance level of
0.05. All the statistical analyses and graph manipulation were
conducted using S R language version 3.2.1 (R Foundation for
Statistical Computing).
Results
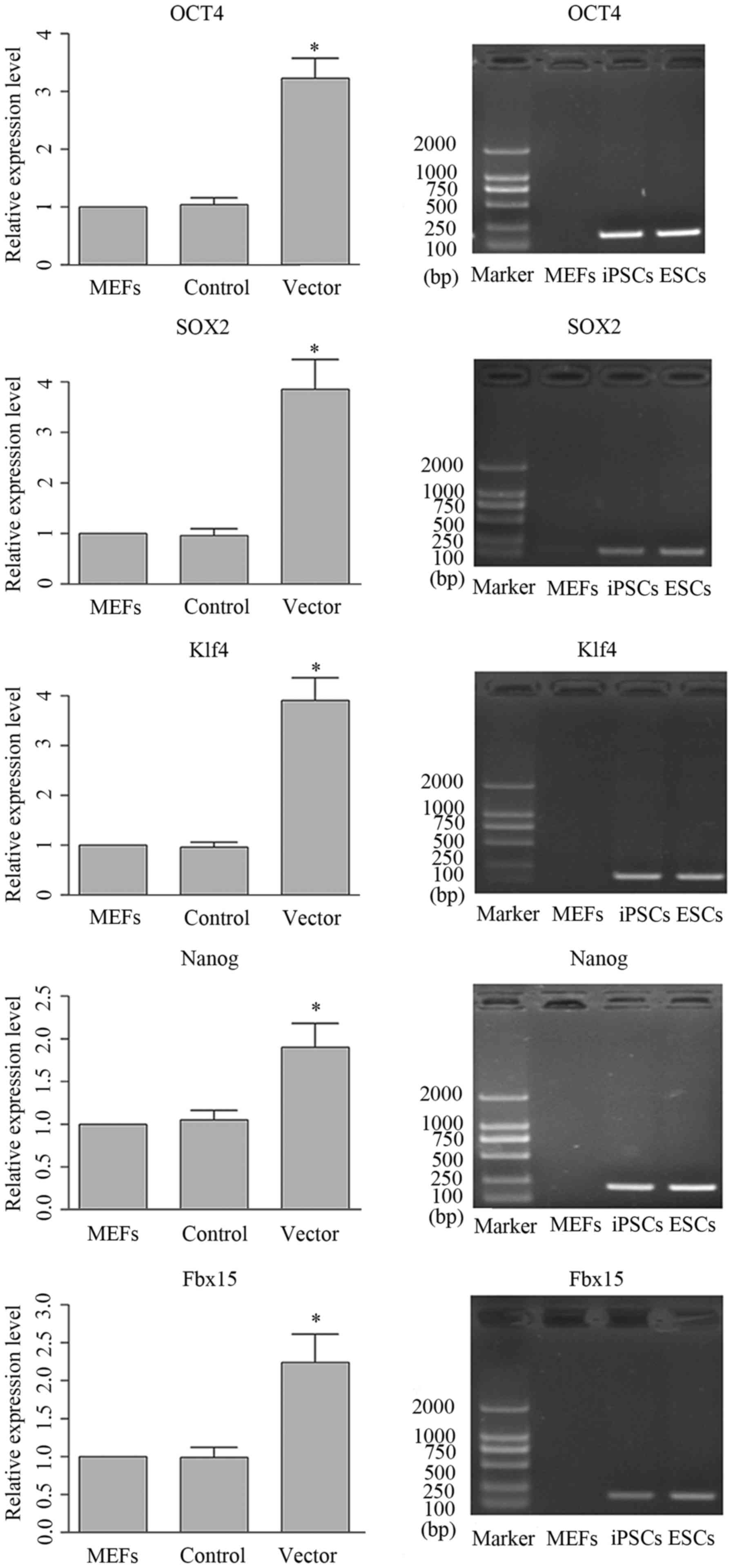
Generation of iPSCs using mouse 3-gene
transfection
iPSCs were generated by MEFs using mouse 3-gene
transfection method. Positive clones were selected and the
expression patterns of the genes which were the signature of mouse
ESCs were validated by RT-qPCR. The representative image and
quantitative analysis of RT-qPCR are shown in Fig. 3. It was illustrated that following
transfection, the expression levels of the target genes were all
upregulated. The expression patterns in the iPSCs and ESCs were
identical, and the differences between iPSCs and MEFs was
statistically significant, which indicated the successful
generation of IPSCs with the present method. Furthermore, the
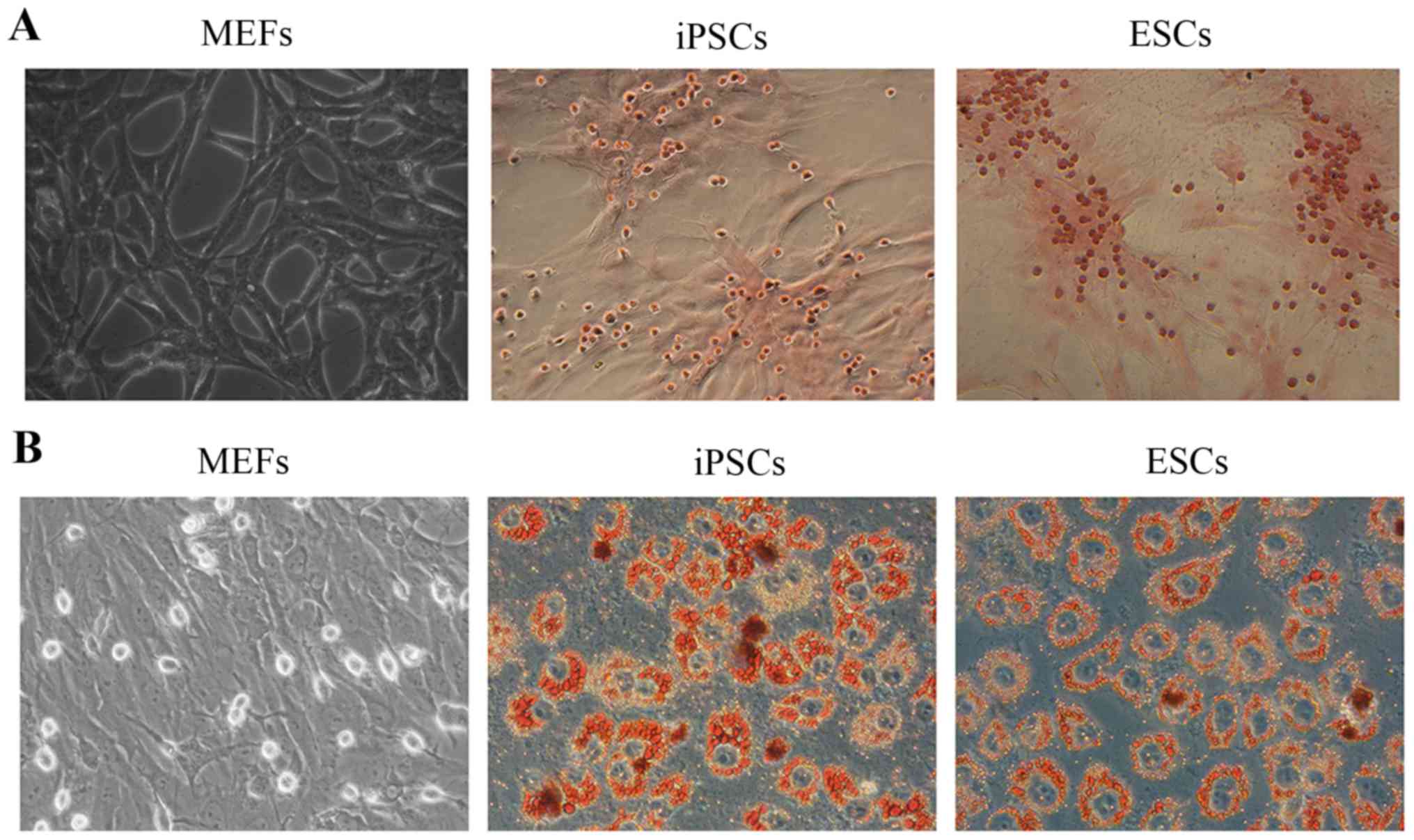
differentiation of the mouse 3-gene-transfected iPSCs towards the
osteogenic and adipogenic lineages were detected using the Alizarin
Red S and Oil Red O staining methods, respectively. As shown in
Fig. 4A, the deposition of
Alizarin Red could be detected in the iPSCs and ESCs, while the
MEFs showed no reaction with Alizarin Red, representing the
osteogenic differentiation potential of iPSCs which was identical
to that of the ESCs. Similarly, in Oil Red O assays, the deposition
of Oil Red O could only be detected in the iPSCs and ESCs (Fig. 4B). The above-mentioned resutls
indicate the successful generation of iPSCs using the mouse 3-gene
transfection method.
The administration of iPSC-CM inhibits
the proliferation of HFL1 cells
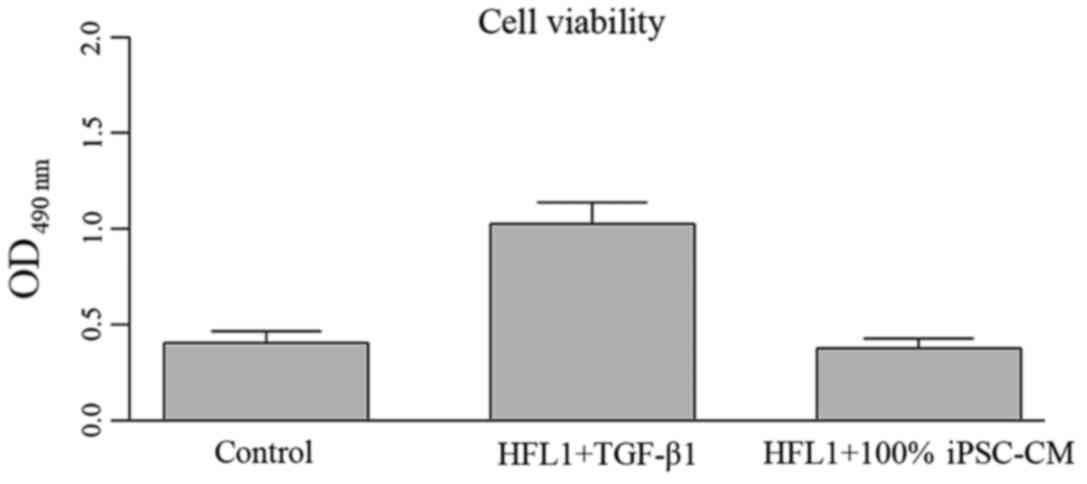
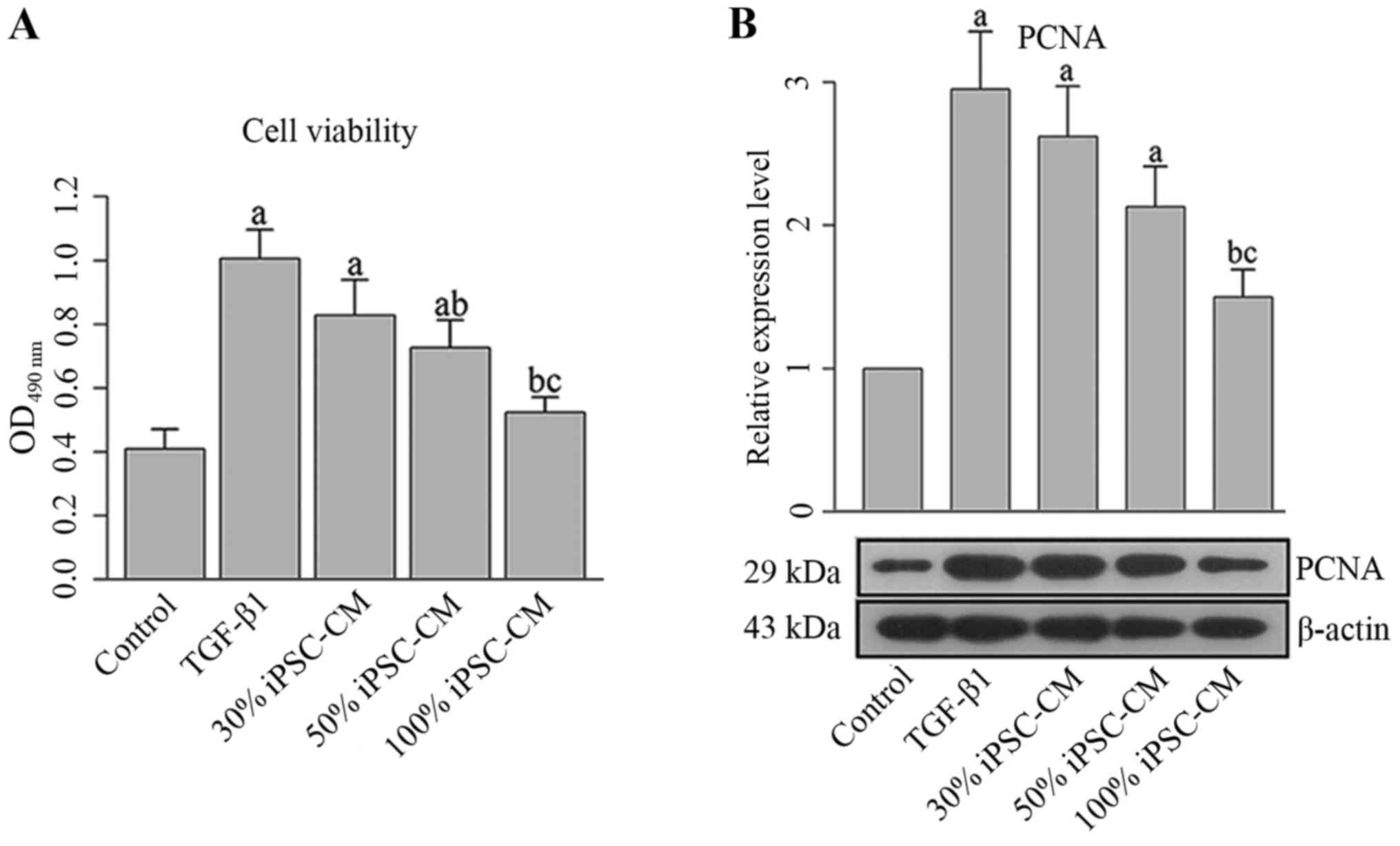
The growth of HFL1 cells was directly assessed by
MTT assay and indirectly determined using PCNA western blot
analysis. As illustrated in Fig.
5A, proliferative ability of the HFL1 cells was enhanced
following incubation with TGF-β1 for 24 h. Following treatment with
iPSC-CM, the viability of the HFL1 cells was significantly
inhibited. The differences between the TGF-β1 group and the 50%
iPSC-CM or 100% iPSC-CM groups were statistically significant
(P<0.05; Fig. 5A). Moreover,
with the increasing iPSC-CM concentration, the inhibitory effect of
the iPSC-CM was significantly enhanced, with the proliferation of
the HLF1 cells in the 100% iPSC-CM group being comparable to that
of the cells in the control group, representing a dose-dependent
regulatory effect of iPSC-CM on the viability of HFL1 cells
(Fig. 5A). A similar pattern with
the prodcution of PCNA was also recorded by western blot analysis,
confirming the inhibitory effect of iPSC-CM on the TGF-β1-induced
proliferation of HLF1 cells (Fig.
5B).
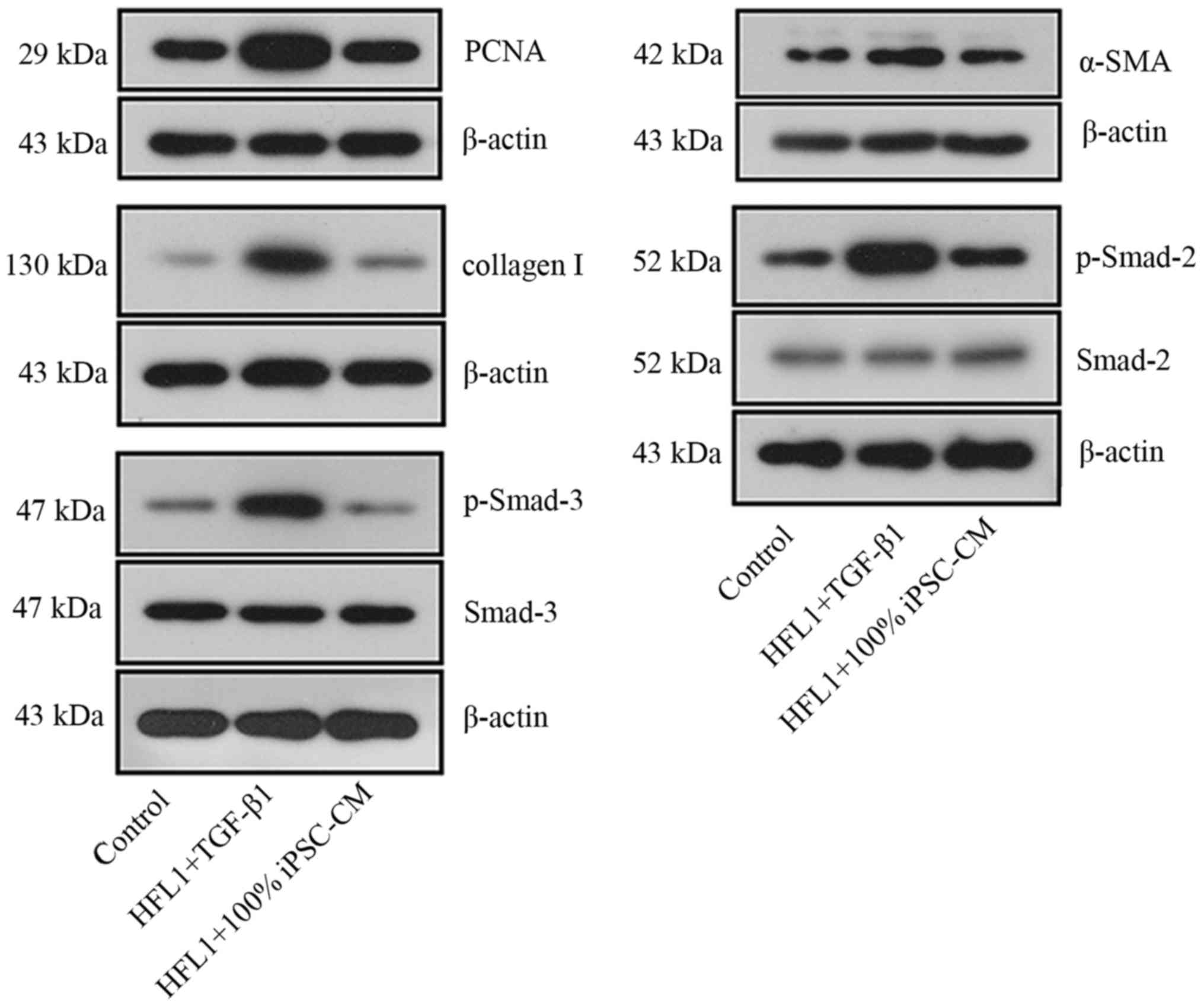
iPSC-CM inhibits the TGF-β1-induced
differentiation of HFL1 cells into myofibroblasts via the
Smad-mediated signal transduction pathway
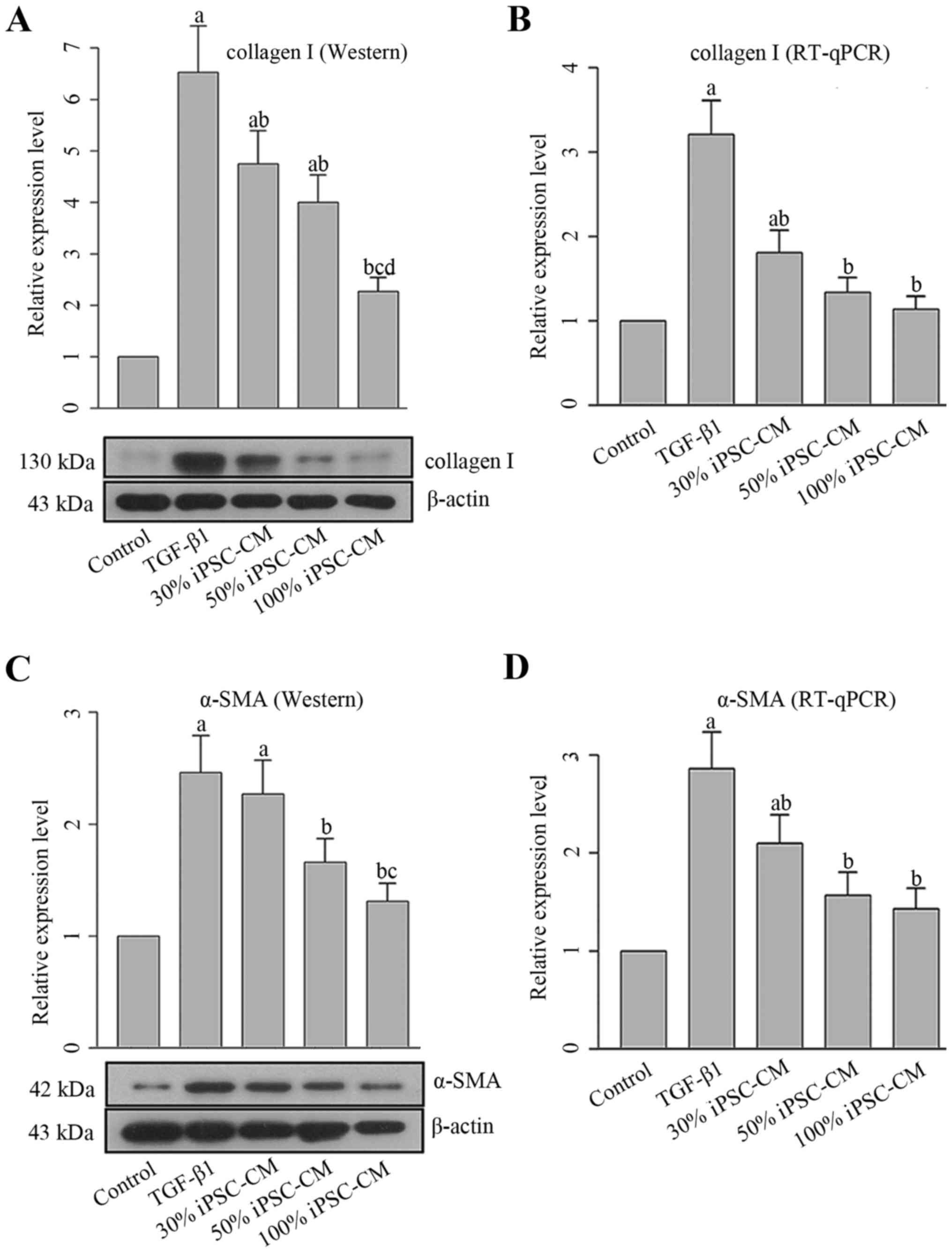
PF is characterized by the activation of collagen,
and myofibroblasts are characterized by the expression of α-SMA.
Therefore, the levels of collagen I and α-SMA were both determined
at the mRNA and protein level. As shown in Fig. 6, incubation with TGF-β1 increased
the expression levels of both molecules compared with the control
HFL1 cells. Similar to the results of MTT assay and PCNA content,
iPSC-CM reverse the effects induced by TGF-β1, which further
resulted in the inhibition of the differentiation of HFL1 cells
into myoblasts. These effects were also exerted in a dose-dependent
manner.
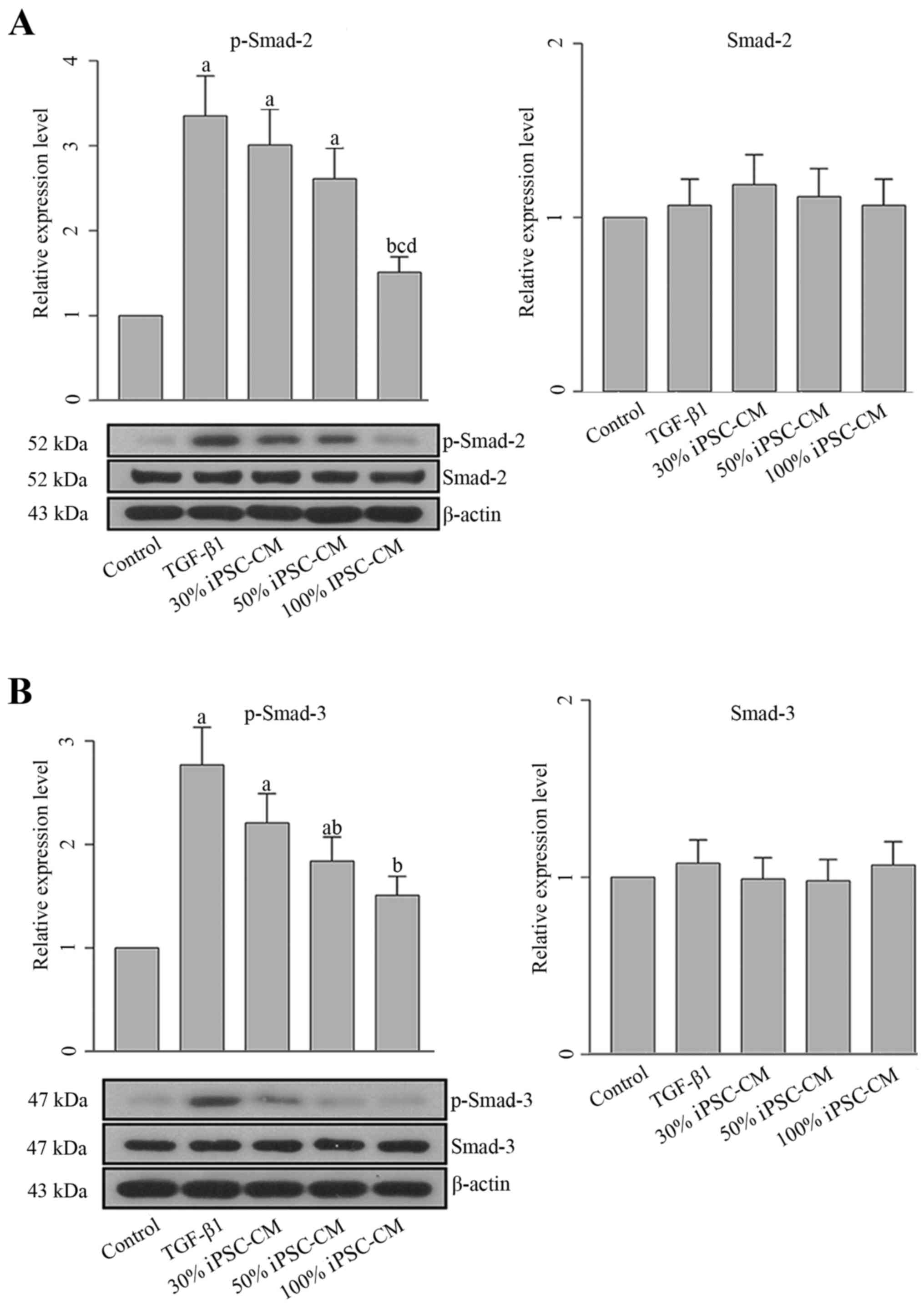
To determine whether the effects of iPSC-CM on PF
are exerted through the TGF-β1-mediated signal transduction
pathway, the production and activation of Smad-2 and Smad-3 in HFL1
cells were also detected. The overexpression of p-Smad-2 and
p-Smad-3 in the HLF1 cells was observed following incubation with
TGF-β1 (Fig. 7). No significant
changes were observed in the levels of total Smad-2 and Smad-3. The
activation of Smad-2 and Smad-3 was associated with all collagen
types, and these results confirmed that the TGF-β1-induced
differentiation of fibroblasts into myofibroblasts was regulated by
Smad proteins. However, following treatment with iPSC-CM, the
phosphorylation of Smad-2 and Smad-3 was decreased (Fig. 7), which further blocked the
effects of TGF-β1 on HFL1 cells.
The administration of iPSC-CM attenuates
bleomycin-induced lung injury in mice with PF
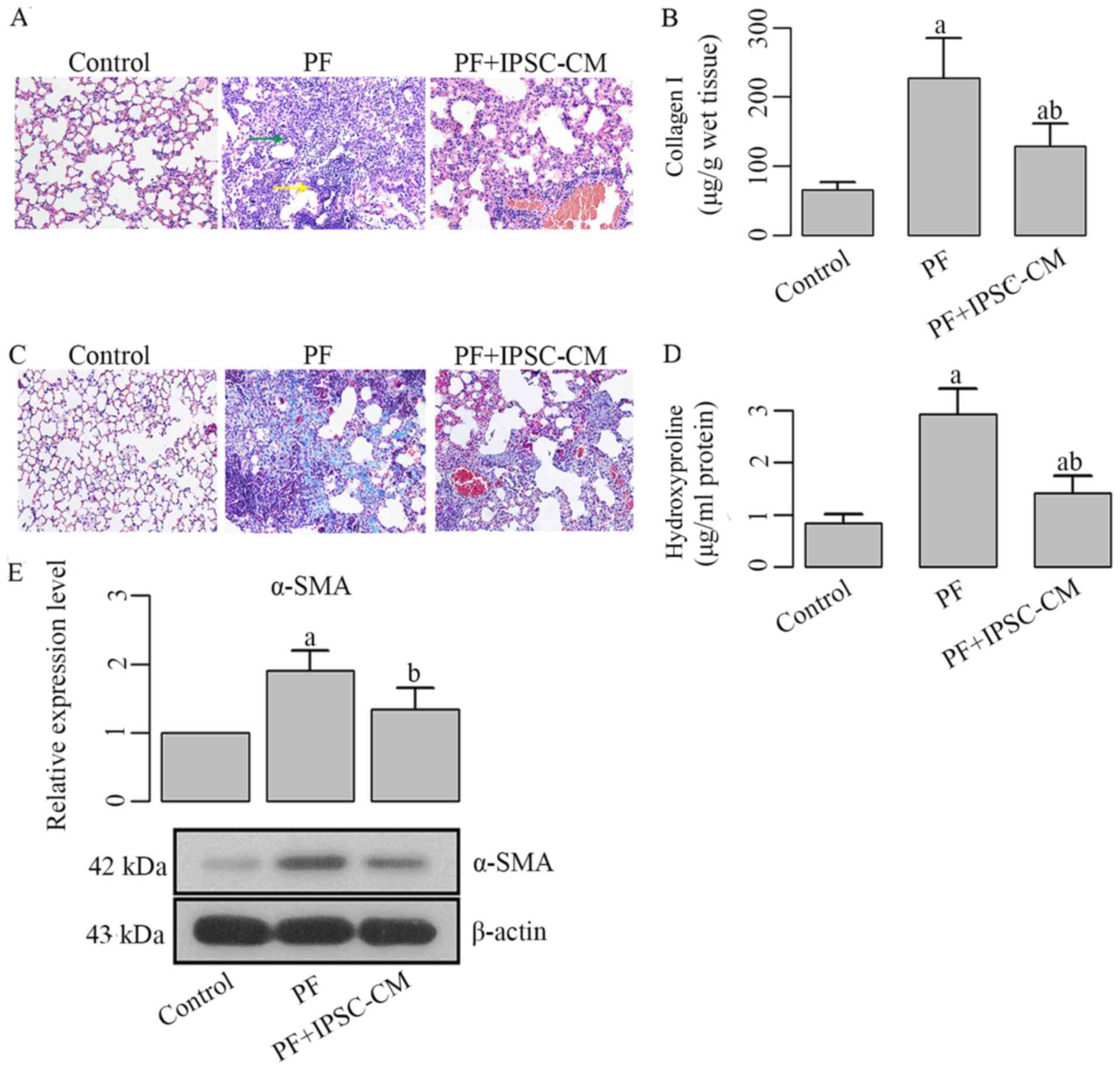
The injection of bleomycin led to severe lung injury
in mice, as illustrated in the representative image of H&E
staining, in which the injured tissues were characterized by
neutrophilic alveolitis and patched areas (Fig. 8A). The administration of iPSC-CM
markdly reduced the number of infiltrative neutrophils and injured
areas. Moreover, the bleomycin-induced interstitial thickening,
inflammation and distortion of cell architecture were all
attenuated following treatment with iPSC-CM (Fig. 8A).
Collagen accumulation is attenuated by
treatment with iPSC-CM
The production and distribution of collagen I in
different lung samples was determined using ELISA and Masson's
staining, respectively. Marked differences in collagen I production
were observed between the PF group and PF + iPSC-CM group (Fig. 8B and C), as illustrated by the
quantitative results of ELISA and representative images of Masson's
trichrome staining (collagen I was stained blue). To further assess
the tissue collagen content, the levels of hydroxyproline were
measured. The values of the hydroxyproline level were lower in the
PF + iPSC-CM group compared with the PF group (Fig. 8d), the difference being
statistically significant (P<0.05). In addition to the
above-mentioned detections, the production of α-SMA was also
decreased by treatment with iPSC-CM (Fig. 8E). These findings all indicated
the suppression of bleomycin-induced collagen synthesis in mice
treated with iPSC-CM.
iPSC-CM exerts inhibitory effects on the
differentiation of fibroblasts into myofibroblasts in mice with PF
via the blocking of the TGF-β1-mediated pathway
The promoting effect of TGF-β1 on myofibroblast
differentiation was evaluated in an in vitro system. The
results clearly demonstrated the important role of TGF-β1 in
activating the expression of collagen I and α-SMA, and the
phosphorylation of Smad-2 and Smad-3. To verify the role of TGF-β1
in PF, the content of TGF-β1 in our model mice was quantified as
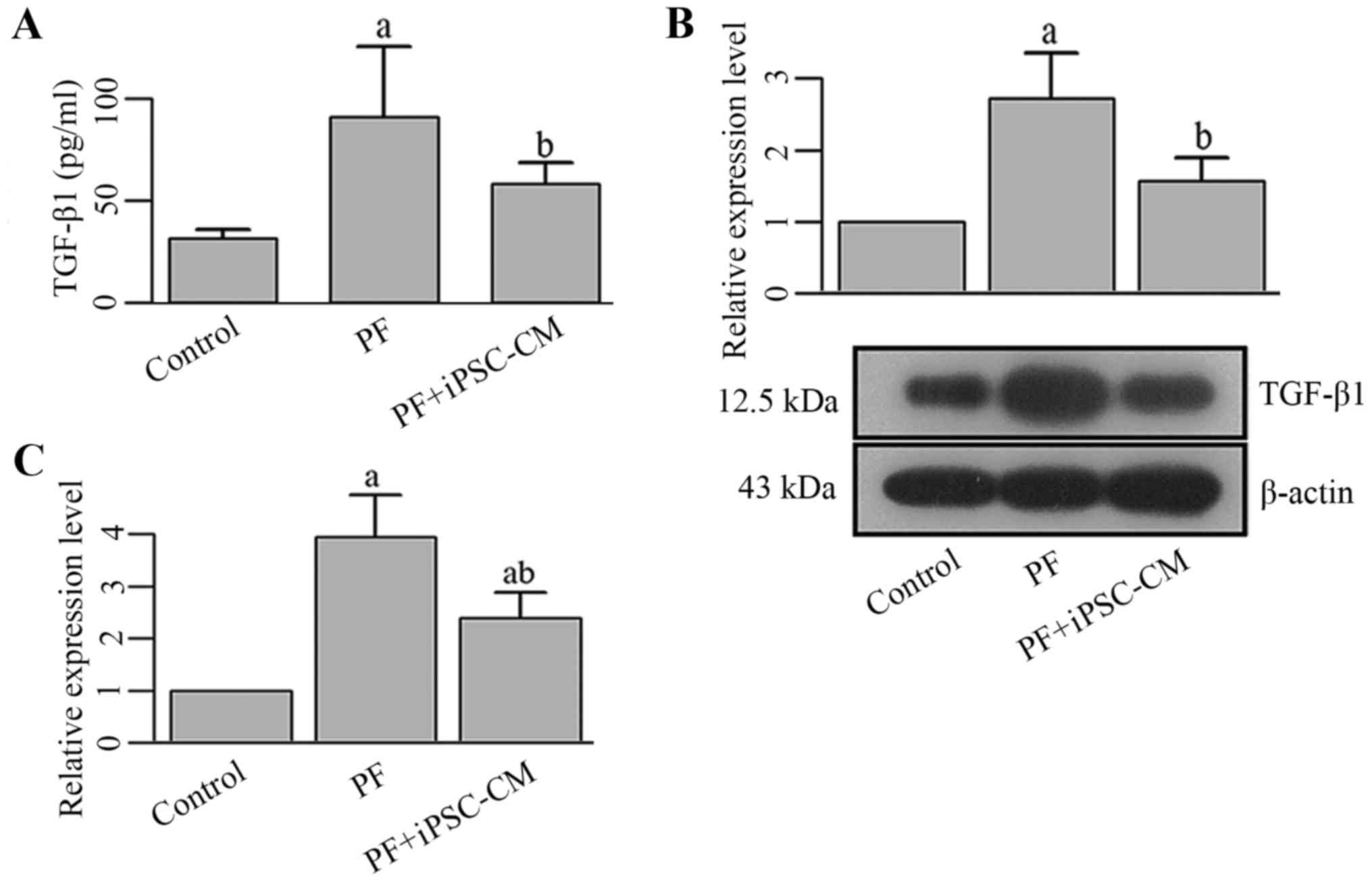
well. It was found that the synthesis and transcription of TGF-β1
was enhanced in the BALF and lung tissues from the mice in the PF
group (Fig. 9). Moreover, the
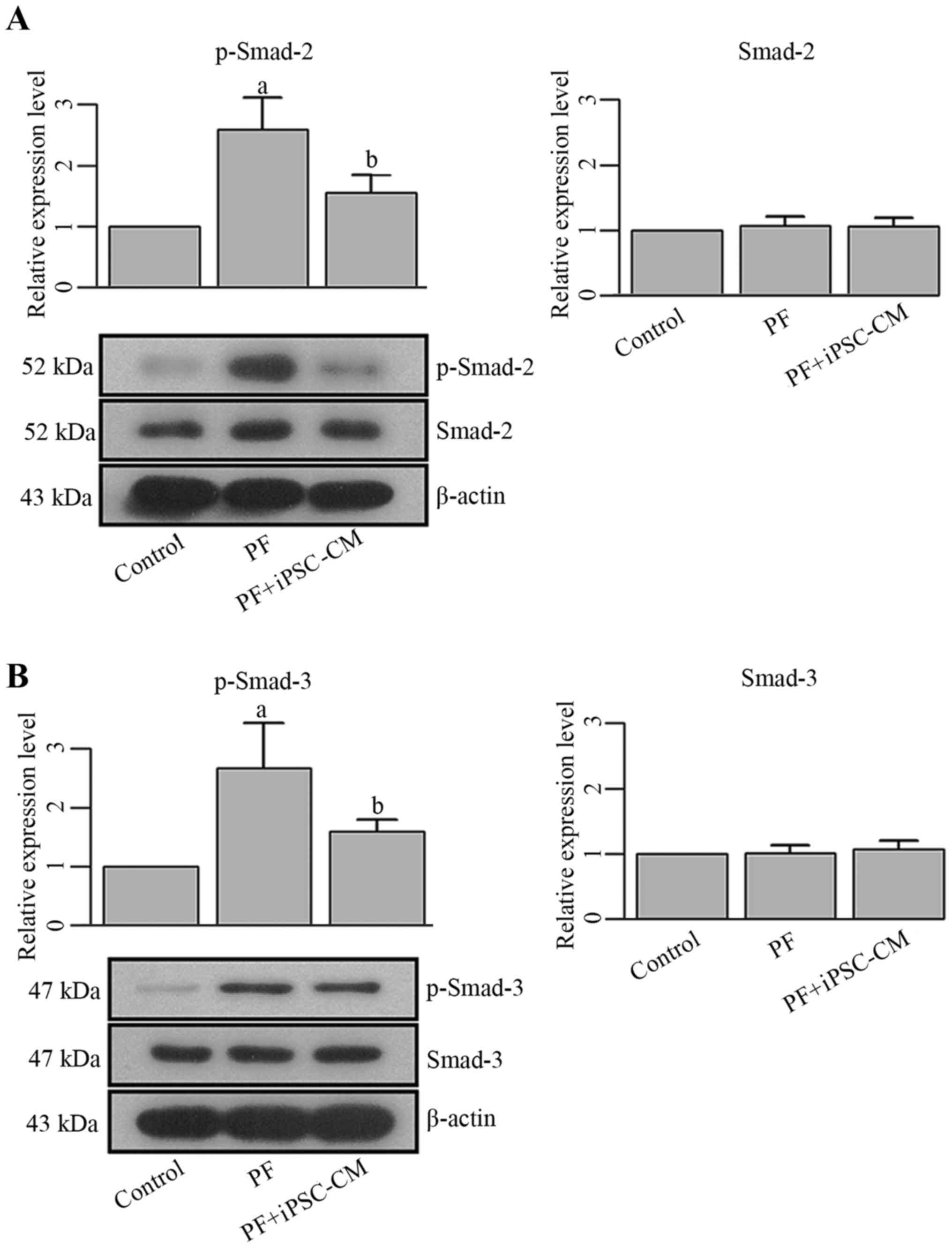
phosphorylation levels of Smad-2 and Smad-3 were also upregulated
in the PF group, as also observed the in vitro experiments
(Fig. 10). Following the
administration of iPSC-CM, the expression levels of these molecules
were reversed to a relatively regular level. No significant changes
were observed in the levels of total Smad-2 and Smad-3. The
downregulation of these molecules was accompanied by an improved
lung structure in mice, inferring that the administration of
iPSC-CM not only inhibited the differentiation of PFs into
myofibroblasts, but was also able to attenuate the injury induced
by PF.
Discussion
Traditional interventions of PF rely on the specific
interference of TGF-β1, a cytokine mediating the differentiation of
fibroblasts into myofibroblasts, and the accumulation of collagens
in PF. Whereas these schemes have had some achievements, they also
lead to certain unexpected side-effects in that TGF-β1 is a key
factor involved in multiple biological processes. The arbitrary
inhibition of TGF-β1 will certainly result in abnormalities, such
as tumors. Fortunately, previous studies have highlighted the
therapeutic potential of stem cell-based therapies in improving the
outcome of bleomycin-induced PF in animal models (14–17).
Compared with the traditional means of using
TGF-β1-specific antibodies, stem-based therapies have the advantage
of a high treatment efficicay and low tumorigenic risk (7). In the present study, attention was
paid to the potential of CM of iPSCs in reducing bleomycin-induced
lung injury instead of previously reported mesenchymal stem cells
(14,15,18–21). The administration of iPSC-CM
markedly attenuated the proliferation of HFL1 human fibroblasts and
reduced the collagen accumulation in these cells. In adidtion, the
lung collagen content and pulmonary structure in the model mice
were significantly improved by iPSC-CM treatment. Remarkably,
iPSC-CM treatment influenced the TGF-β1/Smad pathway both in
vivo and in vitro, which preliminarily explained the
mechanism through which iPSC-CM inhibited the differentiation of
fibroblasts into myofibroblasts and alleviated PF.
As the intermediate between normal fibroblasts and
smooth muscle cells, myofibrolasts have the capability of
synthesizing interstitial collagens and the expression α-SMA at the
same time. The de novo appearance of myofibroblasts at sites
undergoing active extracellular matrix deposition suggests that
these cells are closely associated with the genesis of fibrotic
lesions (22). Although the
origin of myofibroblasts is controversial, it is generally accepted
that the differentiation of fibroblasts into myofibroblast is
activated by TGF-β1 (4,23). In our in vitro experiments,
the HFL1 human fibroblasts were exposed to TGF-β1 to form
myofibroblast-like cells. It was clearly demonstrated that the
TGF-β1-exposed HFL1 cells had a significantly higher proliferative
ability compared with the normal HFL1 cells. In vivo, wound
fibroblasts are thought to be removed by apoptosis following
maturation; however, the activation of TGF-β1 results in the
formation of granulation tissue in which α-SMA-expressing
myofibroblasts are abundant, indicating the high survival ability
of myofibroblast-like cells (24). Thus, reducing the viability of
myofibroblasts will certainly reduce the accumulation of collagen
and improve the condition of PF. In the present study, the
administration with iPSC-CM markedly reduced the proliferation of
TGF-β1-exposed HFL1 cells. Moreover, the synthesis of α-SMA was
significantly down-regulated both in vitro and in
vivo, which indicated the decreased amount of
myofibroblast-like cells as well. As a result of the reduced number
of myofibroblast-like cells, the suppression of collagen
accumulation was illustrated by the downregulated levels of
collagen I and hydroxyproline. Based on the results of H&E
staining, the inhibitory effects of iPSC-CM on fibroblast
differentiation into myofibroblasts also improved the lung
structure of the model mice; although dysregular structure could be
still observed, substantial cells retained their normal structure.
All these findings confirmed the potential of iPSC-CM in
attenuating lung tissue injury due PF.
To further explore the mechanisms responsible for
these treatment processes, we quantified the expression and
activation of molecules involved in the TGF-β1-induced
myofibroblast differentiation in vitro, and verified the
results in mice iwth bleomycin-induced PF. As expected, the cells
or mice treated with iPSC-CM had a significantly lower expression
of the active form of TGF-β1, Smad-2 and Smad-3 compared with the
untreated animals with PF. The Smad-dependent TGF-β1-induced
production of ECM in PF has been previously reported (23). Evans et al confirmed that
TGF-β1-dependent cell proliferation required both Smad-2 and Smad-3
(4). Although in our study the
roles of Smad-2 and Smad-3 seemed to similar previous studies have
doubted this conclusion (25–27). It was previously reported by
Phanish et al that it may be the endogenous ratio of Smad-2
and Smad-3 that contributed to the determination of the function of
Smad-3 (28). Additionally,
TGF-β1 is capable of exerting its function through other
Smad-independent pathways, including the p38, mitogen activated
protein kinase (MAPK) and extracellular signal-regulated kinase
(ERK) pathways. Although in the present study we revealed the key
role of the TGF-β1/Smad pathway in the effects of iPSC-CM
administration on PF, it should be stated that the underlying
mechanisms responsible for the attenuating effects of iPSC-CM on PF
require further investigation beyond the findings of the present
study.
In conclusion, our study revealed the potential of
iPSC-CM as a promising therapy against PF. The administration of
iPSC-CM inhibited the differentiation of fibroblasts into
myofibroblasts by inhibiting the activation of the TGF-β1/Smad
pathway. iPSCs induced from MEFs is a convenient method with which
to obtain cells with low tumorigenic potential based on previous
studies (29,30). Although our study attempted to
provide an explanation about the protective effects of iPSC-CM
against PF, the underlying mechanisms responsible for the
attenuating effects of iPSC-CM on PF have only been partially
revealed. In order to facilitate the practical application of iPSCs
or iPSC-CM, further comprehensive studies are warranted in the
future.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (no. 81400042) and the Science
and Technology Project of Department of Education, Liaoning
Province (no. L2013299).
References
|
1
|
Ware LB and Matthay MA: The acute
respiratory distress syndrome. N Engl J Med. 342:1334–1349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Perez A, Rogers RM and Dauber JH: The
prognosis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol
Biol. 29(Suppl 3): S19–S26. 2003.PubMed/NCBI
|
|
3
|
Phan SH: The myofibroblast in pulmonary
fibrosis. Chest. 122(Suppl 6): 286S–289S. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Evans RA, Tian YC, Steadman R and Phillips
AO: TGF-β1-mediated fibroblast-myofibroblast terminal
differentiation-the role of Smad proteins. Exp Cell Res.
282:90–100. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sime PJ, Xing Z, Graham FL, Csaky KG and
Gauldie J: Adeno-vector-mediated gene transfer of active
transforming growth factor-beta1 induces prolonged severe fibrosis
in rat lung. J Clin Invest. 100:768–776. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hawkins F and Kotton DN: Embryonic and
induced pluripotent stem cells for lung regeneration. Ann Am Thorac
Soc. 12(Suppl 1): S50–S53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
How CK, Chien Y, Yang KY, Shih HC, Juan
CC, Yang YP, Chiou GY, Huang PI, Chang YL, Chen LK, et al: Induced
pluripotent stem cells mediate the release of interferon
gamma-induced protein 10 and alleviate bleomycin-induced lung
inflammation and fibrosis. Shock. 39:261–270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang KY, Shih HC, How CK, Chen CY, Hsu HS,
Yang CW, Lee YC, Perng RP, Peng CH, Li HY, et al: IV delivery of
induced pluripotent stem cells attenuates endotoxin-induced acute
lung injury in mice. Chest. 140:1243–1253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li HY, Chien Y, Chen YJ, Chen SF, Chang
YL, Chiang CH, Jeng SY, Chang CM, Wang ML, Chen LK, et al:
Reprogramming induced pluripotent stem cells in the absence of
c-Myc for differentiation into hepatocyte-like cells. Biomaterials.
32:5994–6005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Su VY, Chiou SH, Lin CS, Chen WC, Yu WK,
Chen YW, Chen CY and Yang KY: Induced pluripotent stem cells reduce
neutrophil chemotaxis via activating GRK2 in endotoxin-induced
acute lung injury. Respirology. 22:1156–1164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li F and Niyibizi C: Cells derived from
murine induced pluripotent stem cells (iPSC) by treatment with
members of TGF-beta family give rise to osteoblasts differentiation
and form bone in vivo. BMC Cell Biol. 13:352012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Flint MH and Lyons MF: The effect of
heating and denaturation on the staining of collagen by the Masson
trichrome procedure. Histochem J. 7:547–555. 1975. View Article : Google Scholar
|
|
14
|
Munger JS, Huang X, Kawakatsu H, Griffiths
MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et
al: The integrin alpha v beta 6 binds and activates latent TGF beta
1: a mechanism for regulating pulmonary inflammation and fibrosis.
Cell. 96:319–328. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ortiz LA, Gambelli F, McBride C, Gaupp D,
Baddoo M, Kaminski N and Phinney DG: Mesenchymal stem cell
engraftment in lung is enhanced in response to bleomycin exposure
and ameliorates its fibrotic effects. Proc natl Acad Sci USA.
100:8407–8411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rojas M, Xu J, Woods CR, Mora AL, Spears
W, Roman J and Brigham KL: Bone marrow-derived mesenchymal stem
cells in repair of the injured lung. Am J Respir Cell Mol Biol.
33:145–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen AS, Haslett C, Feldsien DC, Henson PM
and Cherniack RM: The intensity of chronic lung inflammation and
fibrosis after bleomycin is directly related to the severity of
acute injury. Am Rev Respir Dis. 137:564–571. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakagawa M, Koyanagi M, Tanabe K,
Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N
and Yamanaka S: Generation of induced pluripotent stem cells
without Myc from mouse and human fibroblasts. Nat Biotechnol.
26:101–106. 2008. View
Article : Google Scholar
|
|
19
|
Moodley Y, Ilancheran S, Samuel C,
Vaghjiani V, Atienza D, Williams ED, Jenkin G, Wallace E, Trounson
A and Manuelpillai U: Human amnion epithelial cell transplantation
abrogates lung fibrosis and augments repair. Am J Respir Crit Care
Med. 182:643–651. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumamoto M, Nishiwaki T, Matsuo N, Kimura
H and Matsushima K: Minimally cultured bone marrow mesenchymal stem
cells ameliorate fibrotic lung injury. Eur Respir J. 34:740–748.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ortiz LA, Dutreil M, Fattman C, Pandey AC,
Torres G, Go K and Phinney DG: Interleukin 1 receptor antagonist
mediates the antiinflammatory and antifibrotic effect of
mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA.
104:11002–11007. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee SH, Jang AS, Kim YE, Cha JY, Kim TH,
Jung S, Park SK, Lee YK, Won JH, Kim YH, et al: Modulation of
cytokine and nitric oxide by mesenchymal stem cell transfer in lung
injury/fibrosis. Respir Res. 11:162010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sappino AP, Schürch W and Gabbiani G:
Differentiation repertoire of fibroblastic cells: expression of
cytoskeletal proteins as marker of phenotypic modulations. Lab
Invest. 63:144–161. 1990.PubMed/NCBI
|
|
24
|
Zhang HY and Phan SH: Inhibition of
myofibroblast apoptosis by transforming growth factor β1. Am J
Respir Cell Mol Biol. 21:658–665. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Midgley AC, Rogers M, Hallett MB, Clayton
A, Bowen T, Phillips AO and Steadman R: Transforming growth
factor-β1 (TGF-β1)-stimulated fibroblast to myofibroblast
differentiation is mediated by hyaluronan (HA)-facilitated
epidermal growth factor receptor (EGFR) and CD44 co-localization in
lipid rafts. J Biol Chem. 288:14824–14838. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Piek E, Ju WJ, Heyer J, Escalante-Alcalde
D, Stewart CL, Weinstein M, Deng C, Kucherlapati R, Bottinger EP
and Roberts AB: Functional characterization of transforming growth
factor β signaling in Smad2- and Smad3-deficient fibroblasts. J
Biol Chem. 276:19945–19953. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang YC, Piek E, Zavadil J, Liang D, Xie
D, Heyer J, Pavlidis P, Kucherlapati R, Roberts AB and Böttinger
EP: Hierarchical model of gene regulation by transforming growth
factor beta. Proc Natl Acad Sci USA. 100:10269–10274. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Phanish MK, Wahab NA, Colville-Nash P,
Hendry BM and Dockrell ME: The differential role of Smad2 and Smad3
in the regulation of pro-fibrotic TGFbeta1 responses in human
proximal-tubule epithelial cells. Biochem J. 393:601–607. 2006.
View Article : Google Scholar
|
|
29
|
Ohnuki M and Takahashi K: Present and
future challenges of induced pluripotent stem cells. Philos Trans R
Soc Lond B Biol Sci. 370:201403672015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Harding J and Mirochnitchenko O:
Preclinical studies for induced pluripotent stem cell-based
therapeutics. J Biol Chem. 289:4585–4593. 2014. View Article : Google Scholar :
|