Introduction
Extensive application of herbicides not only
pollutes the environment, but also endangers people's health.
Herbicides are amongst the most harmful types of water-polluting
agents, particularly the triazine derivative herbicides used
worldwide as residual non-selective herbicides to control
broad-leaved weeds and annual grasses (1). Owing to its high potency and the
broad spectrum of weeds it kills, atrizine has been replaced
gradually by simazine
(6-chloro-N,N′-diethyl-1,3,5-triazine-2,4-diamine)
since the 1960s (2) in
agricultural and non-agricultural scenarios. Simazine, which is
detectable in both surface and ground water (3), has multiple exposure pathways,
including water and air, and particularly affects the food chain
(4–7). The diversity of the means of
exposure increases the risk to human health.
Previous studies on simazine have mainly focused on
its mutagenicity, reproductive developmental toxicity and
immunotoxicity (8–14). Lengthy exposure to a low dose of
simazine has been demonstrated to influence the development of
early life stages in mammals, where it acts as a neural endocrine
disruptor and has an immunotoxic effect (14,15).
Dopamine (DA) is an important neurotransmitter in
the mammalian brain and regulates movement, emotional, cognitive,
memory and other physiological functions of the central nervous
system. Dysfunction of the DA system may lead to Parkinson's
disease (PD), schizophrenia, depression and other diseases
(16–19). The aim of the present study was to
investigate the effect of simazine on the DA system of rats that
were exposed to simazine during the prepubertal period. The
expression levels of the main metabolic factors in the dopaminergic
system and the contents of the monoamines and their main
metabolites were determined in order to evaluate the effects of
simazine on DA metabolism.
Materials and methods
Materials
Simazine (98% pure) was obtained from Zhejiang
Zhongshan Chemical Industry Group Co., Ltd. (Zhejiang, China). The
solutions of simazine used for treatment at various levels (12.5,
50 and 200 mg/kg body weight) were prepared by dissolving simazine
in 2% (w/v) starch. All standard materials used in this study were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Animals and treatment
All animal procedures in the present study were
approved by the Medical Ethics Committee of Harbin Medical
University (Harbin, China) and were conducted in accordance with
the guidelines for animal experimentation issued as standards for
laboratory animal research by the National Institutes of Health
(Bethesda, MD, USA). A group of 60 male Sprague-Dawley (SD) rats
(21 days old; weighing 72–81 g) purchased from Vital River
Laboratories Co., Ltd. (Beijing, China) was divided randomly into
four groups (n=15) and body weight was taken into account when
assigning the rats to each group.
All animals were kept in a feeding room under
controlled environmental conditions (photoperiod, 12-h light/dark
cycle; temperature, 22±2̊C; relative humidity, 50±15%) and supplied
with a standard laboratory diet and purified water ad
libitum.
The rats were allowed to acclimatize to these
conditions for 1 week and no anesthetic was administered to avoid
any interference with biochemical values. The four groups were fed
separately in standard cages. A dosage of 0 (control group), 12.5,
50 or 200 mg/kg simazine (in 2% starch solution) was administered
to the rats at the same time each morning by oral gavage for 40
days. The brains were then removed rapidly and placed into iced
physiological saline. The striatum and midbrain from the whole
brain were dissociated carefully and tissues were stored frozen at
−80°C.
Total RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA separated from the midbrain tissues was
isolated using TRIzol® reagent (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) following the manufacturer's
protocol. The concentration of RNA was measured with an ND-2000c
spectrophotometer (NanoDrop; Thermo Fisher Scientific, Inc.,
Wilmington, DE, USA). PrimeScript® RT with a gDNA Eraser
reagent kit (Takara Biotechnology Co., Ltd., Dalian, China) was
used to synthesize cDNA from 1 µg total RNA according to the
manufacturer's protocol. The PCR primers were designed and
synthesized by Generay Biotech Co., Ltd. (Shanghai, China) and the
sequences are presented in Table
I.
 | Table IPrimers used for polymerase chain
reaction amplification. |
Table I
Primers used for polymerase chain
reaction amplification.
| Targets | Primer
sequences | Size (bp) | Accession no.
(GenBank) |
|---|
| DAT | F:
5′-tcaccaataactgctatagagacgc-3′ | 83 | NM_012694.2 |
| R:
5′-gaagacgacgaagccagaggag-3′ | | |
| TH | F:
5′-agcctgtgtactttgtgtccgaga-3′ | 138 | NM_012740.3 |
| R:
5′-tgtgagggctgtccagtacgtc-3′ | | |
| Nurr1 | F:
5′-ccaatccggcaatgaccag-3′ | 129 | NM_019328.3 |
| R:
5′-tgatgatctccatagagccagtcag-3′ | | |
| VMAT2 | F:
5′-actcttccaggagggcagtcac-3′ | 174 | NM_013031.1 |
| R:
5′-tatgaatgggttagtgaggagctgg-3′ | | |
| AADC | F:
5′-attccttcagatggcaactactc-3′ | 123 | NM_001270853.1 |
| R:
5′-gcagcaagatgtggttccta-3′ | | |
| MAO | F:
5′-ttcgccagccagtaggtaggat-3′ | 116 | NM_033653.1 |
| R:
5′-attcaacacctctctagctgctcg-3′ | | |
| COMT | F:
5′-ctgacttcctggcgtatgtgagag-3′ | 104 | NM_012531.2 |
| R:
5′-gattgccttctccaagccgtc-3′ | | |
| β-actin | F:
5′-ccgtaaagacctctatgccaaca-3′ | 102 | NM_031144.2 |
| R:
5′-ctaggagccagggcagtaatctc-3′ | | |
The cDNA was amplified using the SYBR-Green method
(SYBR® Premix Ex Taq™ II; Takara Biotechnology Co.,
Ltd.) in an ABI 7500 Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The cycling conditions comprised
dena turation at 95̊C for 5 sec, and 40 cycles of annealing at 58̊C
for 34 sec with extension at 72̊C for 30 sec. The cycle at which
sample fluorescence reached the threshold was defined as the
threshold cycle, also known as quantification cycle
(Cq). The results are expressed as the relative
expression ratio calculated upon the basis of the qPCR efficiency
(E) and ∆Cq. The ∆Cq value for
each gene (target or reference) was calculated by subtracting the
Cq value of the target sample from that of the
control sample. As shown in equation
1, the ratio of target gene expression in treatment versus
control samples was derived from the ratio between target gene
efficiency (E target) to the power of target ∆Cq
(the ∆Cq target value) and reference gene
efficiency (E reference) to the power of the reference
∆Cq (∆Cq reference) (20).
Ratio=(Etarget)ΔCqtarget(Ereference)ΔCqreference
Immunoblotting
Protein was extracted from the midbrain tissues
using radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China) on ice for 2 h and then centrifuged
at 12,000 × g for 15 min at 4°C. The supernatant was collected and
a BCA protein assay kit (Beyotime Institute of Biotechnology) was
used to measure the protein concentration. Equal amounts of total
protein (60 µg) were subjected to 10% SDS-PAGE and transferred to
polyvinylidene fluoride membranes. The membranes were blocked with
0.5% (w/v) bovine serum albumin (Promega Corporation, Madison, WI,
USA) for 0.5 h at room temperature. The membranes were then
stripped and incubated overnight at 4°C with rabbit polyclonal
orphan nuclear hormone (Nurr1) antibody (1:500 dilution in blocking
buffer; sc-990; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-tyrosine hydroxylase (TH) antibody (1:1,000 dilution in
blocking buffer; AB152; EMD Millipore, Billerica, MA, USA), anti-DA
transporter (DAT) antibody (1:500 dilution in blocking buffer;
sc-14002), anti-catechol-O-methyltransferase (COMT) antibody (1:500
dilution in blocking buffer; sc-25844), anti-monoamine oxidase
(MAO) antibody (1:500 dilution in blocking buffer; sc-18401),
anti-AADC antibody (1:200 dilution in blocking buffer; sc-46909)
and anti-vesicular monoamine transporter 2 (VMAT2) antibody (1:500
dilution in blocking buffer; sc-15314) (all from Santa Cruz
Biotechnology, Inc.), and β-actin (1:500 dilution in blocking
buffer; YT0099; ImmunoWay Biotechnology Co., Plano, TX, USA). After
washing three times with Tris-buffered saline containing 0.1% (v/v)
Tween-20 at room temperature, membranes were incubated at room
temperature for 1.5 h with alkaline phosphatase (ALP) goat
anti-rabbit secondary antibody (AP-1000; Vector Labs, Burlingame,
CA, USA) at a 1:1,000 dilution in blocking buffer. Membranes were
washed with Tris-buffered saline three times at room temperature
and then incubated with Western Blue® Stabilized
Substrate for ALP (Promega Corporation) for 3 min at room
temperature (26–28°C). Relative expression (%) was calculated based
upon protein density using the ChemiQ3650 Analysis System
(Bioshine, Shanghai, China). The density was calculated using
Quantity One v4.6.2 software. Sample blot density was normalized to
β-actin.
Quantification of monoamines and their
main metabolites
The concentrations of monoamines and their main
metabolites in the striatum were assessed using high-performance
liquid chromatography with a fluorescence detector (HPLC-FLD). The
corpus striatum tissues of the rats in each group were homogenized
in 0.1 M perchloric acid and centrifuged at 12,000 x g for 15 min
at 4°C, then filtered through a 0.2-µm cellulose membrane. The
supernatants were collected to measure the contents of monoamines
and main metabolites. Three samples per group were injected into a
Waters chromatograph equipped with a fluorescence detector (G1321C,
1260 FLD; Agilent Technologies, Inc., Santa Clara, CA, USA) and a
Cosmosil C18 column (5 µm, 4.6x250 mm; Nacalai Tesque, Inc., Kyoto,
Japan).
The mobile phase consisted of 20 mM trisodium
citrate and 50 mM sodium hydrogen phosphate. Samples were separated
at 28°C and a flow rate of 1.2 ml/min. The detector was set at an
excitation wavelength of 285 nm and an emission wavelength of 333
nm. The data were quantified using the area under the peak
technique and external standards. Quantification was verified using
calibration curves obtained from individual monoamine
standards.
Statistical analysis
Experimental data were analyzed using SPSS
statistical analysis software, version 18.0 (SPSS, Inc., Chicago,
IL, USA) and expressed the data as mean ± standard error of the
mean. Intergroup differences in body weight and food consumption
were analyzed using one-way analysis of variance (ANOVA) and
significant differences among groups were detected using
Bonferroni's multiple comparison tests when significant differences
among the groups were identified by ANOVA. P<0.05 was considered
to indicate a statistically significant difference.
Results
Body weight and food consumption
No statistically significant difference in body
weight or food consumption was detected among the groups during the
40 days of treatment (data not shown).
Changes in monoamine and main metabolite
contents
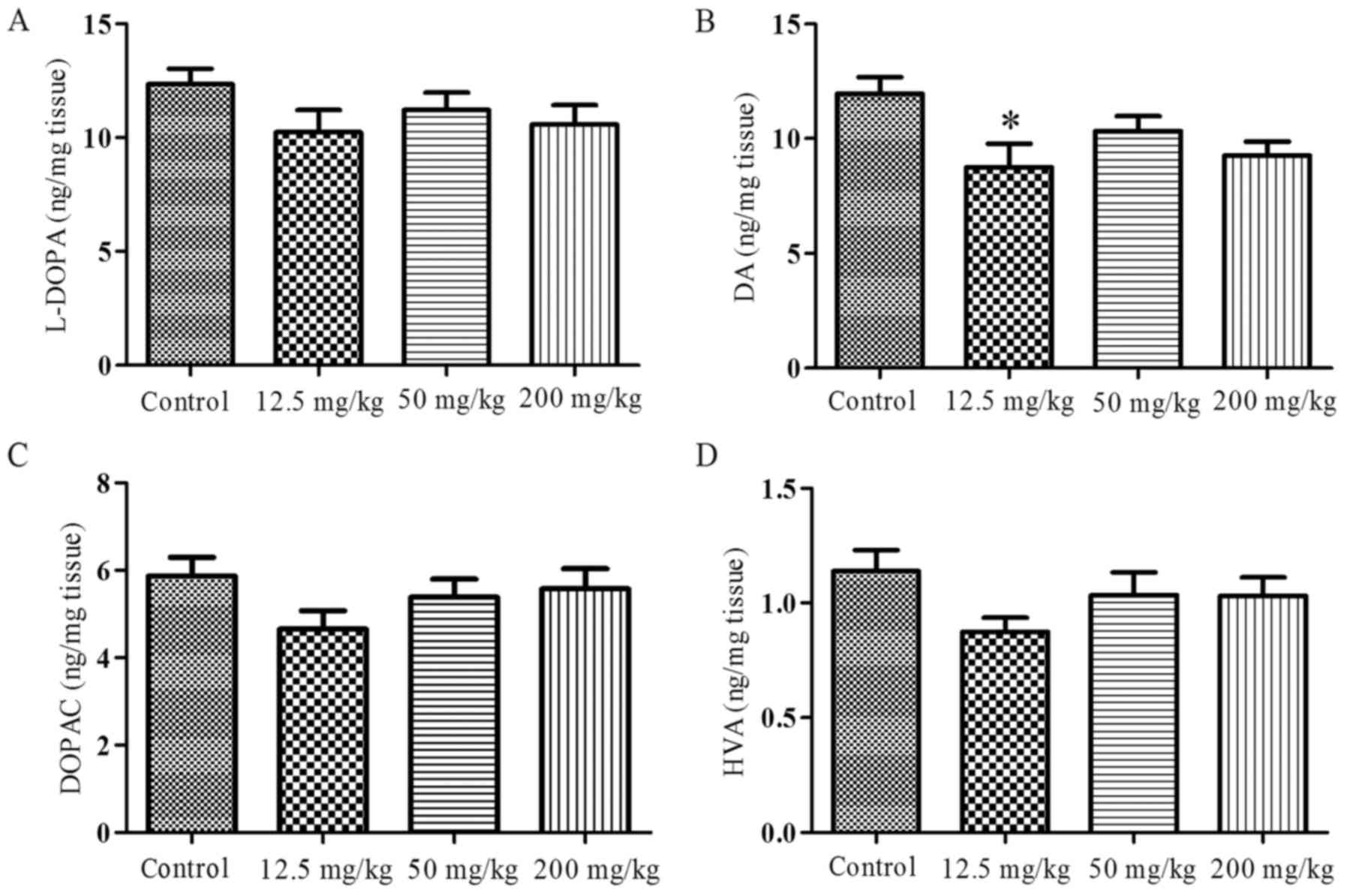
The contents of levodopa (L-DOPA), DA,
dihydroxy-phenyl-acetic acid (DOPAC) and homovanillic acid (HVA) in
the striatum were measured following 40 days exposure to simazine
and are presented in Fig. 1. The
levels of L-DOPA (Fig. 1A), DOPAC
(Fig. 1C) and HVA (Fig. 1D) exhibited a slight reduction in
the simazine treatment groups compared with the control group;
however, the difference was not significant (P>0.05). The levels
of DA (Fig. 1B) in the simazine
treatment groups were lower compared with that in the control, and
the DA level in the 12.5 mg/kg group was decreased significantly
compared with the control (P<0.05).
Changes in the levels of Nurr1, DAT,
VMAT2 and TH mRNA following exposure to simazine
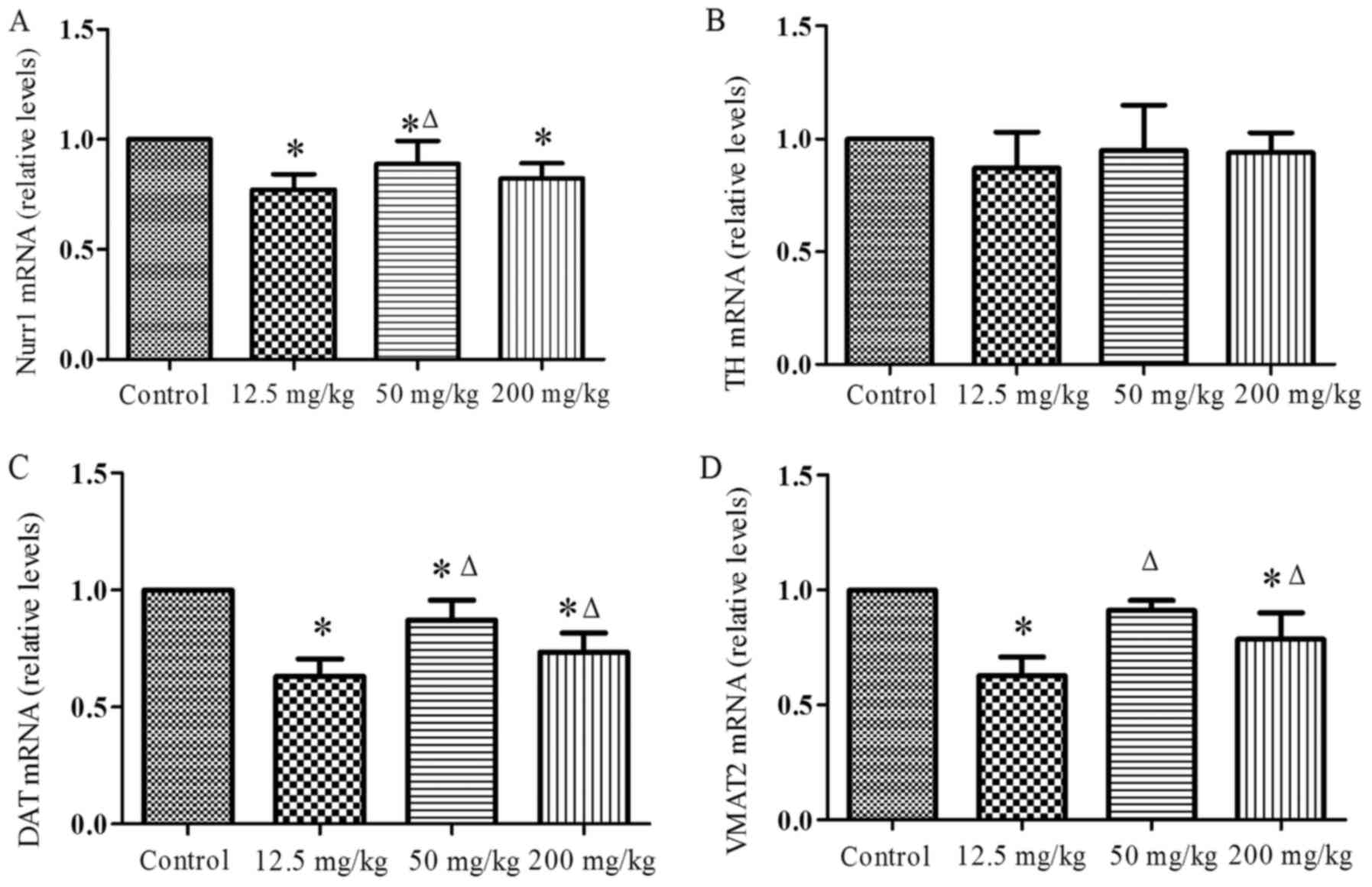
Fig. 2 shows the
changes in the levels of Nurr1, DAT, VMAT2 and TH mRNA induced by
exposure to simazine. The Nurr1 (Fig.
2A) mRNA levels of the simazine treatment groups were all lower
compared with that of the control and the differences were
statistically significant (P<0.05). In addition, the Nurr1 mRNA
level of 50 mg/kg group was increased significantly compared with
that of the 12.5 mg/kg group (P<0.05). The levels of TH mRNA
(Fig. 2B) in the simazine
treatment groups were all slightly lower compared with that of the
control group, but the differences were not significant
(P>0.05). DAT mRNA levels (Fig.
2C) of the simazine treatment groups were all significantly
lower compared with that of the control group (P<0.05). The
VMAT2 (Fig. 2D) mRNA levels of
the 12.5 and 200 mg/kg groups were significantly lower compared
with that of the control group (P<0.05). However, the DAT and
VMAT2 mRNA levels of the 50 and 200 mg/kg groups were significantly
increased compared with that of the 12.5 mg/kg group
(P<0.05).
Changes in the levels of MAO, COMT and
aromatic amino acid decarboxylase (AADC) mRNA induced by exposure
to simazine
MAO (Fig. 3A) mRNA
levels of the simazine treatment groups exhibited an upward trend,
and the MAO mRNA level of the 12.5 mg/kg group was significantly
decreased compared with that of the control group (P<0.05),
while the levels of the 50 and 200 mg/kg groups were increased
compared with that of the 12.5 mg/kg group and the differences were
significant statistically (P<0.05). The COMT (Fig. 3B) mRNA levels of the simazine
treatment groups also exhibited an uptrend, but only the level of
the 500 mg/kg group was increased significantly compared with those
of the control and 12.5 mg/kg groups (P<0.05). The AADC
(Fig. 3C) levels of the 12.5 and
200 mg/kg groups were significant decreased compared with that of
the control group (P<0.05), while the levels of the 50 and 200
mg/kg groups were increased compared with that of the 12.5 mg/kg
group and the differences were statistically significant
(P<0.05).
Changes in the expression levels of
Nurr1, TH, DAT and VMAT2 protein induced by simazine
The expression levels of Nurr1, TH, DAT and VMAT2
proteins induced by exposure to simazine are shown in Fig. 4. The Nurr1 protein level (Fig. 4A) was decreased in all simazine
treatment groups, but the levels in the 12.5 and 200 mg/kg groups
were significantly different compared with that in the control
group (P<0.05). The expression levels of TH protein (Fig. 4B) in all simazine treatment groups
were slightly lower compared with that of the control group, but
the differences were not significant (P>0.05). The expression
levels of DAT protein (Fig. 4C)
of all simazine treatment groups were lower compared with that of
the control and the differences were statistically significant
(P<0.05). The VMAT2 protein level (Fig. 4D) was decreased in all dose
groups; however, only the 12.5 mg/kg group exhibited a significant
reduction in VMAT2 expression compared with the control group
(P<0.05).
Changes in the expression levels of MAO,
COMT and AADC protein induced by simazine
The expression levels of MAO, COMT and AADC protein
induced by exposure to simazine are shown in Fig. 5. The MAO (Fig. 5A) and COMT (Fig. 5B) protein levels in all simazine
treatment groups exhibited an upward trend, but the differences
were not significant (P>0.05). The AADC (Fig. 5C) protein levels in all simazine
treatment groups were lower compared with that in the control and
the differences were statistically significant (P<0.05). In
addition, the expression levels of AADC in the 50 and 200 mg/kg
groups were increased significantly compared with that in the 12.5
mg/ kg group (P<0.05). Representative western blotting results
for the MAO, COMT and AADC proteins are presented in Fig. 6.
 | Figure 6Representative images of protein
expression detected via western blotting. Lane 1, control group;
lane 2, 12.5 mg/kg simazine group; lane 3, 50 mg/kg simazine group;
and lane 4, 200 mg/kg simazine group. Notably, lanes 2–4 for MAO
and COMT appeared darker than lane 1. Nurr1, orphan nuclear
hormone; TH, tyrosine hydroxylase; DAT, dopamine transporter;
VMAT2, vesicular monoamine transporter 2; MAO, monoamine oxidase;
COMT, catechol-O-methyltransferase. |
Discussion
Due to its widespread use, simazine has been
detected in samples of soil and ground water and the toxicity of
simazine to mammals urgently requires investigation. The present
study attempted to research the effects of simazine on the
metabolism of dopaminergic neurons. DA synthesis and transfer
disorders may lead to the onset of PD, Alzheimer's disease or other
common neurological disorders (21–23). Since puberty is a crucial period
of dopaminergic neuron development (24), 60 male SD rats were treated with
different doses of simazine throughout puberty in order to detect
changes of the main factors involved in the process of DA synthesis
and metabolism.
The process of DA metabolism in the brain includes
synthesis, storage, release, reuptake and inactivation. The
tyrosine in catecholamine neurons is converted to L-DOPA under the
catalytic action of TH. The L-DOPA is then converted to DA by AADC
(25). Following the synthesis of
DA in dopaminergic neurons, DA is transported to and stored in
vesicles by VMAT2. When an action potential reaches a presynaptic
terminal, DA is released to the synaptic cleft and functions
through binding with the postsynaptic receptors. Reuptake of DA
includes two steps. In the first step, DA is transported from the
synaptic cleft back into the presynaptic membranes by DAT. In the
second step, DA is stored in vesicles by VMAT2 (26,27). DA in the synaptic cleft is
transformed to the final product, HVA, under the enzymolysis of
COMT and MAO, while intracellular DA is transformed to DOPAC by MAO
(Fig. 6). In normal
circumstances, each factor acts to maintain the steady-state
condition of the DA system. When any of the steps or factors is
affected, it may disrupt the balance of the DA system and cause a
series of physiological effects (28,29).
TH is a key rate-limiting enzyme, and TH+
neurons are able to produce either L-DOPA or DA in target areas of
ventral midbrain dopaminergic neurons. In addition, Keber et
al (29) suggested that
striatal TH+ cells may synthesize DA cooperatively and
subsequently contribute to supraphysiological concentrations of
synaptic DA. In the present study, the mRNA and protein expression
levels of TH in the ventral mesencephalon were detected following
exposure to simazine for 40 days during the prepubertal period, and
the results demonstrated that TH exhibited no significant changes.
Since TH is required for the synthesis of L-DOPA, the content of DA
is not likely to change via this mechanism. Expression of the TH
gene may involve interaction with numerous other genes,
particularly Nurr1, and previously reported data indicate that mRNA
and protein expression of TH exhibited a high consistency with that
of Nurr1 (30,31).
Nurr1, which is crucial for homeostasis and the
development of DA neurons, is an essential transcription factor for
the differentiation, maturation and maintenance of midbrain
dopaminergic neurons (32,33).
Disruption of Nurr1 function contributes to dopaminergic neuron
dysfunction as indicated by considerable clinical and experimental
data (34–37). Studies have shown that while the
midbrain structure is complete in Nurr1-deficient mice, these mice
have a lack of dopaminergic neurons and the expression of certain
genes, including AADC, VMAT and DAT, which are associated with
dopaminergic synthesis, transport, storage and release (35,38,39). Therefore, during dopaminergic
neuron development, Nurr1 influences the expression of a number of
other genes, including TH, DAT and VMAT2 (40). In the present study,
simazine-induced reductions in Nurr1 mRNA and protein expression in
the ventral mesencephalon were observed in all dose groups. The
reduction of Nurr1 gene expression influences the expression of DAT
and VMAT2, and affects the content of DA as a result (41,42).
DAT is synthesized and expressed by the soma,
dendrites and axons of dopaminergic neurons and is distributed on
the membranes of dendrites and axons. The main function of DAT is
to take up DA in the synaptic cleft. Due to its specificity for DA,
the content of DAT reflects dopaminergic system function.
Therefore, numerous studies have evaluated the presynaptic function
of dopaminergic neurons via DAT, and some have reported the complex
regulatory effects of DAT and its effects on the regulation of
other proteins (43–47). The main function of VMAT2 is to
store DA in vesicles. The inhibition of VMAT2 contributes to
dopaminergic neuron death and recent evidence suggests that the
vesicular storage of DA may contribute to the demise of the nigral
neurons in PD (49). Therefore,
VMAT2 serves a key role in the storage of DA in vesicles to avoid
its degradation by MAO. Approximately 80% of monoamine
neurotransmitters are reabsorbed by nerve terminals and reuptake is
the main method of suspending the physiological effects of
monoamine neurotransmitters; therefore, DAT and VMAT2 are crucial
(48–50). The present study demonstrated that
exposure to simazine during the prepubertal period decreased DAT
and VMAT2 expression in the ventral mesencephalon. A previous study
revealed that the mRNA and protein levels of DAT in the blood
leukocytes of patients with PD were significantly reduced (51). Lower expression levels of DAT and
VMAT2 is likely to reduce the transport and storage capacity of DA
and inhibit the reuptake of DA into vesicles. This may increase the
accumulation of DA in the cytoplasm and induce oxidative stress and
toxicity leading to the death of dopaminergic neurons. Thus, it may
be speculated that affecting the transport and reuptake of DA by
influencing Nurr1, DAT and VMAT2 expression is one pathway by which
the content and activity of DA are decreased by simazine.
AADC is a homodimeric pyridoxal phosphate-dependent
enzyme responsible for the synthesis of DA. AADC converts L-DOPA to
DA and is considered the rate-limiting enzyme for the synthesis of
biogenic amines. Studies have shown that AADC deficiency is a rare
pediatric neurometabolic disease in children and indicate that
defects in the AADC gene result in neurotransmitter deficiencies
(52–56). COMT and MAO are important
DA-degrading enzymes. The COMT gene has attracted strong
neuroscientific interest due to its implication in dopaminergic
neurotransmission (57). These
two genes have been observed to have an association with cognitive
function (58,59). MAO and COMT inhibitors are the
present optimal PD treatments and they maintain the monoamine
balance (60). The effects of
COMT and MAO on DA differ according to their site of action
(Fig. 7). In the present study,
it was observed that the expression of COMT and MAO mRNA tended to
increase following exposure to simazine, and the expression levels
in the 50 and 200 mg/kg groups for the MAO gene, and the 200 mg/kg
group for the COMT gene were significantly higher compared with
those of the 12.5 mg/kg group. However, no significant difference
in the corresponding protein expression was observed. The mRNA and
protein expression of AADC in the simazine treatment groups was
lower than that in the control group, but exhibited a rising trend
as the simazine dose increased. The reduced expression of AADC is
likely to lead to a reduction in the concentration of DA, while the
increasing levels of COMT and MAO would accelerate the degradation
of DA, but cause no significant changes in its metabolic products,
DOPAC and HVA. Therefore, it may be speculated that affecting the
synthesis and metabolism of DA by influencing AADC, MAO and COMT
expression is another pathway involved in the reduction of the
content and activity of DA by simazine.
 | Figure 7Effects on DA synthesis and
metabolism induced by exposed to simazine. Δ represents a certain
amount of DA; ↑ indicates upregulation; ↓ indicates downregulation;
the black circles which contain dopamine indicate vesicles.
Analysis indicated that MAO and COMT were upregulated and other
main genes in the pathway were downregulated. These changes
affected the content of DA in the midbrain. AADC, aromatic amino
acid decarboxylase; COMT, catechol-O-methyltransferase; DA,
dopamine; DAT, dopamine transporter; DOPAC, dihydroxy-phenyl-acetic
acid; HVA, homovanillic acid; MAO, monoamine oxidase; 3-MT,
3-methoxytyramine; TH, tyrosine hydroxylase; VMAT, vesicular
monoamine transporter 2. |
In conclusion, the present study observed the
neurotoxic effect of simazine on the dopaminergic metabolism
system. Simazine affected the synthesis, transport and metabolism
of DA and led to dysfunction in the natural balance of the brain
dopaminergic system. One possible underlying mechanism is a
reduction in the levels of Nurr1, DAT and VMAT2 impacting upon the
transport of DA; another is the decreased level of AADC and
increased levels of MAO and COMT impacting upon the synthesis and
metabolism of DA. The above factors resulted in a reduction in the
level of DA and may lead to dopaminergic system-associated
neurological disorders. The understanding of the neurotoxicity of
simazine remains incomplete and further research is required to
elucidate it further.
Acknowledgments
The present study was supported by the National
Nature Science Foundation of China (grant no. 81072332).
References
|
1
|
Elbashir AA and Aboul-Enein HY: Separation
and analysis of triazine herbcide residues by capillary
electrophoresis. Biomed Chromatogr. 29:835–842. 2015. View Article : Google Scholar
|
|
2
|
Silva M and Iyer P: Toxicity endpoint
selections for a simazine risk assessment. Birth Defects Res B Dev
Reprod Toxicol. 101:308–324. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salvestrini S, Canzano S, Iovino P, Leone
V and Capasso S: Modelling the biphasic sorption of simazine,
imidacloprid, and boscalid in water/soil systems. J Environ Sci
Health B. 49:578–590. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brevini TA, Zanetto SB and Cillo F:
Effects of endocrine disruptors on developmental and reproductive
functions. Curr Drug Targets Immune Endocr Metabol Disord. 5:1–10.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sannino F, Marocco A, Garrone E, Esposito
S and Pansini M: Adsorption of simazine on zeolite H-Y and sol-gel
technique manufactured porous silica: a comparative study in model
and natural waters. J Environ Sci Health B. 50:777–787. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wan R, Yang Y, Sun W, Wang Z and Xie S:
Simazine biodegradation and community structures of
ammonia-oxidizing microorganisms in bioaugmented soil: impact of
ammonia and nitrate nitrogen sources. Environ Sci Pollut Res Int.
21:3175–3181. 2014. View Article : Google Scholar
|
|
7
|
Wan R, Wang Z and Xie S: Dynamics of
communities of bacteria and ammonia-oxidizing microorganisms in
response to simazine attenuation in agricultural soil. Sci Total
Environ. 472:502–508. 2014. View Article : Google Scholar
|
|
8
|
Velisek J, Stara A, Machova J, Dvorak P,
Zuskova E and Svobodova Z: Effects of low-concentrations of
simazine on early life stages of common carp (Cyprinus carpio L.).
Neuro Endocrinol Lett. 33(Suppl 3): 90–95. 2012.
|
|
9
|
Park S, Kim S, Jin H, Lee K and Bae J:
Impaired development of female mouse offspring maternally exposed
to simazine. Environ Toxicol Pharmacol. 38:845–851. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zorrilla LM, Gibson EK and Stoker TE: The
effects of simazine, a chlorotriazine herbicide, on pubertal
development in the female Wistar rat. Reprod Toxicol. 29:393–400.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bogdanffy MS, O'Connor JC, Hansen JF,
Gaddamidi V, Van Pelt CS, Green JW and Cook JC: Chronic toxicity
and oncogenicity bioassay in rats with the chloro-s-triazine
herbicide cyanazine. J Toxicol Environ Health A. 60:567–586. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim KR, Son EW, Hee-Um S, Kim BO, Rhee DK
and Pyo S: Immune alterations in mice exposed to the herbicide
simazine. J Toxicol Environ Health A. 66:1159–1173. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim KR, Son EW, Rhee DK and Pyo S: The
immunomodulatory effects of the herbicide simazine on murine
macrophage functions in vitro. Toxicol In Vitro. 16:517–523. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ren R, Sun DJ, Yan H, Wu YP and Zhang Y:
Oral exposure to the herbicide simazine induces mouse spleen
immunotoxicity and immune cell apoptosis. Toxicol Pathol. 41:63–72.
2013. View Article : Google Scholar
|
|
15
|
Sai L, Liu Y, Qu B, Yu G, Guo Q, Bo C, Xie
L, Jia Q, Li Y, Li X, et al: The effects of simazine, a
chlorotriazine herbicide, on the expression of genes in developing
male Xenopus laevis. Bull Environ Contam Toxicol. 95:157–163. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu J, Li X, Yang J, Wu Y and Li B: Effects
of simazine exposure on neuronal development-related factors in
MN9D cells. Med Sci Monit. 22:2831–2838. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamaguchi Y, Lee YA and Goto Y: Dopamine
in socioecological and evolutionary perspectives: implications for
psychiatric disorders. Front Neurosci. 9:2192015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krabbe S, Duda J, Schiemann J, Poetschke
C, Schneider G, Kandel ER, Liss B, Roeper J and Simpson EH:
Increased dopamine D2 receptor activity in the striatum alters the
firing pattern of dopamine neurons in the ventral tegmental area.
Proc Natl Acad Sci USA. 112:E1498–E1506. 2015.PubMed/NCBI
|
|
19
|
Chastain LG, Qu H, Bourke CH, Iuvone PM,
Dobner PR, Nemeroff CB and Kinkead B: Striatal dopamine receptor
plasticity in neurotensin deficient mice. Behav Brain Res.
280:160–171. 2015. View Article : Google Scholar :
|
|
20
|
Yuan JS, Reed A, Chen F and Stewart CN Jr:
Statistical analysis of real-time PCR data. BMC Bioinformatics.
7:852006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ojha S, Javed H, Azimullah S, Abul Khair
SB and Haque ME: Neuroprotective potential of ferulic acid in the
rotenone model of Parkinson's disease. Drug Des Devel Ther.
9:5499–5510. 2015.PubMed/NCBI
|
|
22
|
Tsai EM, Wang YC, Lee TT, Tsai CF, Chen
HS, Lai FJ, Yokoyama KK, Hsieh TH, Wu RM and Lee JN: Dynamic Trk
and G protein signalings regulate dopaminergic neurodifferentiation
in human trophoblast stem cells. PLoS One. 10:e01438522015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kitao Y, Ageta-Ishihara N, Takahashi R,
Kinoshita M and Hori O: Transgenic supplementation of SIRT1 fails
to alleviate acute loss of nigrostriatal dopamine neurons and
gliosis in a mouse model of MPTP-induced parkinsonism. F1000Res.
4:1302015.PubMed/NCBI
|
|
24
|
Gomes FV, Guimarães FS and Grace AA:
Effects of pubertal cannabinoid administration on attentional
set-shifting and dopaminergic hyper-responsivity in a developmental
disruption model of schizophrenia. Int J Neuropsychopharmacol.
18:2014.PubMed/NCBI
|
|
25
|
Koblinger K, Füzesi T, Ejdrygiewicz J,
Krajacic A, Bains JS and Whelan PJ: Characterization of A11 neurons
projecting to the spinal cord of mice. PloS One. 9:e1096362014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Del Pino J, Moyano P, Ruiz M, Anadón MJ,
Diaz MJ, García JM, Labajo-González E and Frejo MT: Amitraz changes
NE, DA and 5-HT biosynthesis and metabolism mediated by alterations
in estradiol content in CNS of male rats. Chemosphere. 181:518–529.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maasz G, Zrinyi Z, Reglodi D, Petrovics D,
Rivnyak A, Kiss T, Jungling A, Tamas A and Pirger Z: Pituitary
adenylate cyclase-activating polypeptide (PACAP) has a
neuroprotective function in dopamine-based neurodegeneration in rat
and snail parkinsonian models. Dis Model Mech. 10:127–139. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang M: Two-step production of monoamines
in monoenzymatic cells in the spinal cord: a different control
strategy of neurotransmitter supply? Neural Regen Res.
11:1904–1909. 2016. View Article : Google Scholar
|
|
29
|
Keber U, Klietz M, Carlsson T, Oertel WH,
Weihe E, Schäfer MK, Höglinger GU and Depboylu C: Striatal tyrosine
hydroxylase-positive neurons are associated with L-DOPA-induced
dyskinesia in hemiparkinsonian mice. Neuroscience. 298:302–317.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sathiakumar N, MacLennan PA, Mandel J and
Delzell E: A review of epidemiologic studies of triazine herbicides
and cancer. Crit Rev Toxicol. 41(Suppl 1): 1–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y, Sun Y, Yang J, Wu Y, Yu J and Li B:
The long-term effects of the herbicide atrazine on the dopaminergic
system following exposure during pubertal development. Mutat Res
Genet Toxicol Environ Mutagen. 763:23–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rodríguez-Traver E, Solís O, Díaz-Guerra
E, Ortiz Ó, Vergaño-Vera E, Méndez-Gómez HR, García-Sanz P,
Moratalla R and Vicario-Abejón C: Role of Nurr1 in the generation
and differentiation of dopaminergic neurons from stem cells.
Neurotox Res. 30:14–31. 2016. View Article : Google Scholar
|
|
33
|
Hammond SL, Safe S and Tjalkens RB: A
novel synthetic activator of Nurr1 induces dopaminergic gene
expression and protects against 6-hydroxydopamine neurotoxicity in
vitro. Neurosci Lett. 607:83–89. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smidt MP, Smits SM and Burbach JP:
Molecular mechanisms underlying midbrain dopamine neuron
development and function. Eur J Pharmacol. 480:75–88. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Decressac M, Volakakis N, Björklund A and
Perlmann T: NURR1 in Parkinson disease - from pathogenesis to
therapeutic potential. Nat Rev Neurol. 9:629–636. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hwang DY, Hong S, Jeong JW, Choi S, Kim H,
Kim J and Kim KS: Vesicular monoamine transporter 2 and dopamine
transporter are molecular targets of Pitx3 in the ventral midbrain
dopamine neurons. J Neurochem. 111:1202–1212. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang C, Wan X, He Y, Pan T, Jankovic J
and Le W: Age-dependent dopaminergic dysfunction in Nurr1 knockout
mice. Exp Neurol. 191:154–162. 2005. View Article : Google Scholar
|
|
38
|
Semchuk KM, Love EJ and Lee RG:
Parkinson's disease and exposure to agricultural work and pesticide
chemicals. Neurology. 42:1328–1335. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Priyadarshi A, Khuder SA, Schaub EA and
Shrivastava S: A meta-analysis of Parkinson's disease and exposure
to pesticides. Neurotoxicology. 21:435–440. 2000.PubMed/NCBI
|
|
40
|
Sun Y, Li YS, Yang JW, Yu J, Wu YP and Li
BX: Exposure to atrazine during gestation and lactation periods:
toxicity effects on dopaminergic neurons in offspring by
downregulation of Nurr1 and VMAT2. Int J Mol Sci. 15:2811–2825.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hermanson E, Joseph B, Castro D, Lindqvist
E, Aarnisalo P, Wallén A, Benoit G, Hengerer B, Olson L and
Perlmann T: Nurr1 regulates dopamine synthesis and storage in MN9D
dopamine cells. Exp Cell Res. 288:324–334. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Smits SM, Ponnio T, Conneely OM, Burbach
JP and Smidt MP: Involvement of Nurr1 in specifying the
neurotransmitter identity of ventral midbrain dopaminergic neurons.
Eur J Neurosci. 18:1731–1738. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Miller GW, Erickson JD, Perez JT, Penland
SN, Mash DC, Rye DB and Levey AI: Immunochemical analysis of
vesicular monoamine transporter (VMAT2) protein in Parkinson's
disease. Exp Neurol. 156:138–148. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lu W and Wolf ME: Expression of dopamine
transporter and vesicular monoamine transporter 2 mRNAs in rat
midbrain after repeated amphetamine administration. Brain Res Mol
Brain Res. 49:137–148. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fornai F, Battaglia G, Gesi M, Giorgi FS,
Orzi F, Nicoletti F and Ruggieri S: Time-course and dose-response
study on the effects of chronic L-DOPA administration on striatal
dopamine levels and dopamine transporter following MPTP toxicity.
Brain Res. 887:110–117. 2000. View Article : Google Scholar
|
|
46
|
Miller GW, Staley JK, Heilman CJ, Perez
JT, Mash DC, Rye DB and Levey AI: Immunochemical analysis of
dopamine transporter protein in Parkinson's disease. Ann Neurol.
41:530–539. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jaakkola E, Joutsa J and Kaasinen V:
Predictors of normal and abnormal outcome in clinical brain
dopamine transporter imaging. J Neural Transm (Vienna).
123:205–209. 2016. View Article : Google Scholar
|
|
48
|
Choi WS, Kim HW and Xia Z: JNK inhibition
of VMAT2 contributes to rotenone-induced oxidative stress and
dopamine neuron death. Toxicology. 328:75–81. 2015. View Article : Google Scholar :
|
|
49
|
Lohr KM and Miller GW: VMAT2 and
Parkinson's disease: harnessing the dopamine vesicle. Expert Rev
Neurother. 14:1115–1117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hall FS, Itokawa K, Schmitt A, Moessner R,
Sora I, Lesch KP and Uhl GR: Decreased vesicular monoamine
transporter 2 (VMAT2) and dopamine transporter (DAT) function in
knockout mice affects aging of dopaminergic systems.
Neuropharmacology. 76(Pt A): 146–155. 2014. View Article : Google Scholar
|
|
51
|
Caronti B, Antonini G, Calderaro C,
Ruggieri S, Palladini G, Pontieri FE and Colosimo C: Dopamine
transporter immunore-activity in peripheral blood lymphocytes in
Parkinson's disease. J Neural Transm Vienna. 108:803–807. 2001.
View Article : Google Scholar
|
|
52
|
Helman G, Pappa MB and Pearl PL: Widening
phenotypic spectrum of AADC deficiency, a disorder of dopamine and
serotonin synthesis. JIMD Rep. 17:23–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Helman G, Pappa MB and Pearl PL: Erratum
to: widening phenotypic spectrum of AADC deficiency, a disorder of
dopamine and serotonin synthesis. JIMD Rep. 17:972014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hwu WL, Lee NC, Chien YH, Muramatsu S and
Ichinose H: AADC deficiency: occurring in humans, modeled in
rodents. Adv Pharmacol. 68:273–284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shih DF, Hsiao CD, Min MY, Lai WS, Yang
CW, Lee WT and Lee SJ: Aromatic L-amino acid decarboxylase (AADC)
is crucial for brain development and motor functions. PLoS One.
8:e717412013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Duan CL, Su Y, Zhao CL, Lu LL, Xu QY and
Yang H: The assays of activities and function of TH, AADC, and GCH1
and their potential use in ex vivo gene therapy of PD. Brain Res
Brain Res Protoc. 16:37–43. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Witte AV and Flöel A: Effects of COMT
polymorphisms on brain function and behavior in health and disease.
Brain Res Bull. 88:418–428. 2012. View Article : Google Scholar
|
|
58
|
Twamley EW, Hua JP, Burton CZ, Vella L,
Chinh K, Bilder RM and Kelsoe JR: Effects of COMT genotype on
cognitive ability and functional capacity in individuals with
schizophrenia. Schizophr Res. 159:114–117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
van Amelsvoort T, Zinkstok J, Figee M,
Daly E, Morris R, Owen MJ, Murphy KC, De Haan L, Linszen DH, Glaser
B, et al: Effects of a functional COMT polymorphism on brain
anatomy and cognitive function in adults with velo-cardio-facial
syndrome. Psychol Med. 38:89–100. 2008. View Article : Google Scholar
|
|
60
|
Dorszewska J, Prendecki M, Oczkowska A,
Rozycka A, Lianeri M and Kozubski W: Polymorphism of the COMT, MAO,
DAT, NET and 5-HTT genes, and biogenic amines in Parkinson's
disease. Curr Genomics. 14:518–533. 2013. View Article : Google Scholar
|