Introduction
Ketamine, which is an ionotropic glutamatergic
N-methyl-D-aspartate (NMDA) receptor antagonist, can produce a
fast-acting antidepressant response in patients with major
depressive disorder (1-3). Ketamine is used clinically for
pediatric anesthesia and sedation, as an anesthetic in the
treatment of asthma, and in pain management (4,5).
In addition, ketamine, in liquid or powdered form, is a widely used
recreational drug that can be injected, ingested or added to
materials for smoking (6,7). The Drug Enforcement Administration
of Taiwan categorizes ketamine as a Schedule III substance due to
its illegal abuse.
Although ketamine rarely produces serious withdrawal
symptoms, it does exert dose-dependent psychedelic and psychotic
effects (8,9). In addition to its neurotoxic
effects, ketamine affects other cell lineages due to its role as an
NMDA receptor antagonist (10).
For example, ketamine abuse can induce cystitis and cause serious
damage to the urinary tract (11,12). Ketamine abusers may experience
urinary frequency, urgency, dysuria, urge incontinence, hemorrhagic
cystitis and hydronephrosis (13-15). In long-term ketamine abusers,
bladder capacity may decrease substantially to one-tenth of the
primary capacity (13,16,17). Major surgical operations,
including augmentation ileocystoplasty, have been reported to
efficaciously relieve refractory ketamine-associated bladder pain
and to reduce urinary tract symptoms (7,18).
In addition to surgery, chondroitin sulfate therapy can relieve
symptoms of severe ketamine-induced contracted bladder (19). In addition, transformation of the
bladder wall and increased interstitial fibrosis in the bladder
tissue have been observed in rats following long-term ketamine
administration (7,20,21).
The mechanism underlying bladder contracture, a
serious ketamine-induced side effect, remains unclear. Previous
studies have suggested that clinical manifestations induced by
ketamine may be mediated through a specific neurogenic mechanism or
possible hypersensitivity (18,22). Furthermore, clinical data have
suggested the involvement of chronic inflammation and epithelial
repair in this lower urinary tract symptom (22-24). The side effects of ketamine abuse
have emerged as a novel challenge for urologists (16).
Given the previous clinical findings, it may be
hypothesized that pathological alterations in smooth muscle cells
(SMCs) of the urinary bladder serve a crucial role in the mechanism
underlying ketamine-induced cystitis. The present study used two
lineages of SMCs, one from differentiated foreskin-derived
fibroblast-like stromal cells (FDSCs) and the other from cultured
normal aortic SMCs, to investigate ketamine-induced molecular
alterations. Immunocytochemical staining was used to examine the
effects of ketamine on differentiated FDSCs and cell viability. To
determine the effects of ketamine on oxidative stress (25), polymerase chain reaction (PCR)
array-based expression profiling was conducted. The
ketamine-induced molecular alterations were subsequently validated
using quantitative (q)PCR or western blot analysis. An
understanding of the molecular alterations involved in
ketamine-associated bladder dysfunction may contribute to the
development of adjuvant therapy for ketamine abusers.
Materials and methods
Differentiation of human FDSCs to SMCs
and cultiva- tion of normal aortic SMCs
SMCs from two different lineages, FDSC-derived SMCs
and cultured human normal aortic SMCs, were used to evaluate the
effects of ketamine. Initially, FDSCs were harvested from foreskin
tissues. This protocol was approved by the institutional review
board of Cathay General Hospital (CGH-P100084). To differentiate
isolated stromal cells into SMCs, FDSCs were cultivated in complete
Dulbecco's modified Eagle's medium (#12100046; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) on fibronectin-coated culture
plates, supplemented with two growth factors, transforming growth
factor (TGF)-β1 (2.5 ng/ml) (#240-B; R&D Systems, Inc.,
Minneapolis, MN, USA) and platelet-derived growth factor-BB
(PDGF-BB, 5 ng/ml) (#220-BB; R&D Systems, Inc.) in a humidified
incubator at 37°C and 5% CO2, for 5-10 passages within 4
weeks (26-28). In addition, human normal aortic
SMCs [American Type Culture Collection (ATCC) CRL-1999] were
cultured in ATCC-formulated F-12K medium (ATCC #30-2004; both ATCC,
Manassas, VA, USA) at 37°C in an incubator containing 5%
CO2 with extra components [0.05 mg/ml ascorbic acid;
0.01 mg/ml insulin; 0.01 mg/ml transferrin; 10 ng/ml sodium
selenite; 0.03 mg/ml endothelial cell growth supplement; 10% fetal
bovine serum; 10 mM(4-(2-hydroxyethyl)-1-piperazineethanesulfonic
acid); 10 mM TES] suggested by the manufacturer.
Treatment of SMCs with ketamine and
cyclophosphamide (CTX)
To determine the effects of ketamine on normal SMCs,
the concentration of residual ketamine (7.744 µg/ml) in the
urinary bladder was used as a reference concentration in the direct
treatment of FDSC-derived SMCs and human normal aortic SMCs
(7). FDSC-derived SMCs were
subjected to cyclic ketamine treatment. The prescription license
for the use of ketamine (Ketalar Injection; Pfizer Ltd., Surrey,
UK) in the present study was permitted by the Food and Drug
Administration of the Ministry of Health and Welfare in Taiwan
(announcement no. 1000088118; Taipei, Taiwan, R.O.C.) and by Cathay
General Hospital (announcement no. CRM09500000101; Taipei, Taiwan,
R.O.C.). One cycle of ketamine treatment comprised incubation of
SMCs in a growth factor-containing medium with various doses of
ketamine (0, 1 and 10 µg/ml) for 1 day, followed by a 1 day
culture without ketamine, both at 37°C. In an additional
experiment, cultured human normal aortic SMCs were incubated with
the chemo-therapeutic agent CTX (10 µM) (Endoxan Injection;
Baxter Deutschland GmbH, Unterschleißheim, Germany) for 5 h
following 1-day ketamine (10 µg/ml) treatment at 37°C
(29).
Immunodetection of smooth muscle actin
(SMA), collagens, interleukin (IL)-6 and inducible nitric oxide
synthase (iNOS)
For a comprehensive study, immunocytochemical and
immunofluorescent (IF) staining, and western blot analysis were
performed on FDSC-derived SMCs and normal aortic SMCs treated with
various concentrations of ketamine. Briefly, cells on glass slides
were fixed with 4% paraformaldehyde in phosphate-buffered saline
for 5 min and were then permeabilized with 0.1% Triton X-100 for 20
min at room temperature. To ensure the differentiation efficacy of
the FDSCs, SMA was detected by incubating the slides with undiluted
anti-SMA antibody (#IR611) for 30 min at room temperature, and
staining was developed using a Dako REAL detection system (#K4065)
(both Agilent Technologies, Inc., Santa Clara, CA, USA) according
to the manufacturer's protocols. IF staining was used to detect
type II collagen (COL2), IL-6 and iNOS expression. Briefly, COL2
was detected by incubating the FDSC-derived ketamine-treated SMCs
with anti-COL2 (MAB1330, 1:5) for 12 h at 4°C and fluorescein
isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin (Ig)G
(AP192F, 1:200) for 1 h at 25°C (both EMD Millipore, Billerica, MA,
USA).
To identify alterations in IL-6 and iNOS expression
following CTX (0 or 10 µM) and ketamine (0 or 10
µg/ml) treatment, normal aortic SMCs were subjected to IF
immunostaining. Briefly, SMA was detected using anti-SMA (sc-1616,
1:50; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and
FITC-conjugated anti-goat IgG (#02-13-06, 1:200; KPL, Inc.,
Gaithersburg, MD, USA). Anti-IL-6 (ab6672, 1:500) and anti-iNOS
(ab3523, 1:20) (both Abcam, Cambridge, UK) were used to colocalize
SMA with IL-6 and iNOS, respectively, and Cy3-conjugated
anti-rabbit IgG was used as a secondary anti-body (AP182C, 1:200;
EMD Millipore). The incubation time for all primary antibodies was
12 h at 4°C and for secondary antibodies was 1 h at 25°C. Finally,
the nuclei were identified by counterstaining with
4′,6-diamidino-2-phenylindole. The stained samples were dehydrated,
mounted and analyzed using a Nikon Eclipse 80i fluorescence
microscope (Nikon Instruments Europe BV, Amsterdam, The
Netherlands).
Western blot analysis was performed to detect COL1
expression in FDSC-derived SMCs that underwent various numbers of
ketamine treatment cycles according to the standard protocol.
Briefly, cell lysates were prepared using a
radioimmunoprecipitation assay buffer (Intron Biotechnology, Inc.,
Seongnam, South Korea) and quantified using Bio-Rad Protein Assay
kit II (#5000002; Bio-Rad Laboratories, Inc., Hercules, CA, USA),
according to the manufacturers' proto-cols. The protein aliquot (30
µg) of each lysate was separated by 12% SDS-PAGE and was
transferred to a polyvinylidene fluoride membrane using a TE70
semidry transfer unit (GE Healthcare Life Sciences, Little
Chalfont, UK). The membrane was blocked in 3% bovine serum albumin
(#A3803; Merck KGaA, Darmstadt, Germany) for 1 h at 25°C, and COL1
was immunoblotted using anti-COL1 (ab292, 1:5,000; Abcam) for 1 h
at 25°C. Anti-GAPDH (AM4300, 1:4,000; Thermo Fisher Scientific,
Inc.) was used as a protein-loading control to ensure equal protein
levels in all samples (incubation time, 1 h at 25°C). The secondary
antibodies used were as follows: Biotinylated anti-rabbit IgG
(BA-1000, 1:1,000; Vector Laboratories, Inc., Burlingame, CA, USA)
for COL1 (30 min at 25°C) and horseradish peroxidase-conjugated
anti-mouse IgG for GAPDH (1 h at 25°C) (AP192P, 1:5,000; EMD
Millipore). The bands were visualized using a VECTASTAIN ABC-AmP
Chemiluminescence Detection kit (AK-6601; Vector Laboratories,
Inc.) for COL1 (10 min at 25°C) and Western Lightning Plus-ECL
(#NEL103001EA; PerkinElmer, Inc., Waltham, MA, USA) for GAPDH,
according to the manufacturers' protocols. Finally, images were
captured using an Alpha Innotech FluorChem FC2 Imager
(ProteinSimple, San Jose, CA, USA). Each protein band was recorded
using UVP Bioimaging systems (UVP, LLC, Upland, CA, USA). The
relative protein expression levels of COL1 normalized to GAPDH were
identified by densitometry using ImageQuant, version 5.2 (Molecular
Dynamics Inc, Chatsworth, CA, USA) and are expressed as the mean ±
standard deviation.
Molecular alterations induced by ketamine
in FDSC-derived SMCs
Total RNA was isolated from FDSC-derived SMCs
treated with or without ketamine, using TRI Reagent (T9424;
Sigma-Aldrich; Merck KGaA). RNA was reverse transcribed to cDNA
using oligo(dT) primer and a PowerScript® Reverse
Transcriptase kit (Invitrogen; Thermo Fisher Scientific, Inc.)
following appropriate DNA digestion (AM2222; Thermo Fisher
Scientific, Inc.), according to the manufacturers' proto-cols.
The inflammatory response and oxidative stress are
believed to be involved in ketamine-associated cystitis. The
present study examined the effects of ketamine on the differential
expression of inflammation and oxidative stress markers in
FDSC-derived SMCs with or without ketamine treatment (10
µg/ml) by comparing their expression levels. The relative
mRNA expression levels of specific inflammatory markers were
quantified using a gene expression assay with universal TaqMan
probes in a LightCycler 1.5 system (both Roche Diagnostics, Basel,
Switzerland). Primer sequences are listed in Table I. The thermocycling conditions
were as follows: IL-6, TGF-β1, COX-2, and GAPDH were pre-incubated
(95°C for 5 min) and amplified (95°C for 10sec, 60°C for 30 sec)
for 55 cycles. To detect ketamine-induced oxidative stress, an
RT2 Profiler™ PCR array (Human Oxidative Stress Plus,
PAHS-065Y; Qiagen N.V., Venlo, The Netherlands) was used with an
Applied Biosystems 7300 Real-Time PCR system (Thermo Fisher
Scientific, Inc.), according to the manufacturers' protocols.
 | Table IProbes and primer sequences for
quantification of IL-6, TGF-β1 and COX-2. |
Table I
Probes and primer sequences for
quantification of IL-6, TGF-β1 and COX-2.
| Gene name | Accession no. | Sequence
(5′-3′) | Probe no. |
|---|
| IL-6 | NM_000600 | F:
gatgagtacaaaagtcctgatcca | 40 |
| | R:
ctgcagccactggttctgt | |
| TGF-β1 | NM_000660 | F:
tggacacgcagtacagcaa | 11 |
| | R:
cttgcggcccacgtagta | |
| COX-2 | AY462100 | F:
gctttatgctgaagccctatga | 2 |
| | R:
tccaactctgcagacatttcc | |
| GAPDH | NM_002046 | F:
ctctgctcctcctgttcgac | 60 |
| | R:
acgaccaaatccgttgactc | |
The data were analyzed using software supplied by
Qiagen (RT2 Profiler™ PCR Array Data Analysis, version
3.5) and were normalized to the expression of five internal
housekeeping genes: β-actin, NM_001101; β-2-microglobulin,
NM_004048; GAPDH, NM_002046 and the comparative
2−ΔΔCq method was used to analyze the relative changes
in gene expression; hypoxanthine phosphoribosyltransferase 1,
NM_000194; and ribosomal protein lateral stalk subunit P0,
NM_001002. The significant targets [code numbers for titin
(TTN): PPH06931F and iNOS: PPH00173F] were validated
using a specific RT2 qPCR primer assay (Qiagen N.V.)
with the Applied Biosystems 7300 Real-Time PCR system. Dissociation
curves were analyzed after each reaction to assess the
quantification specificity in the RT2 qPCR primer assay.
Each quantification was calculated relative to the level of an
endogenous control, GAPDH (NM_002046). Those showing changes
in specific genes >2-fold were considered significant
candidates.
Statistical analysis
The Student's t-test was used to compare the
significant differences in gene expression between different
groups. All statistical analyses were performed using SPSS software
(v. 22.0; IBM Corp., Armonk, NY, USA). The data shown here are
representative of at least three experiments with similar results,
and P-values <0.05 are considered significant.
Results
Deformation of FCSC-derived SMCs by
ketamine
The effects of ketamine on human SMCs were studied
using FDSC-derived SMCs and normal aortic SMCs. Differentiation of
FDSCs into SMCs was confirmed by the positive signals observed by
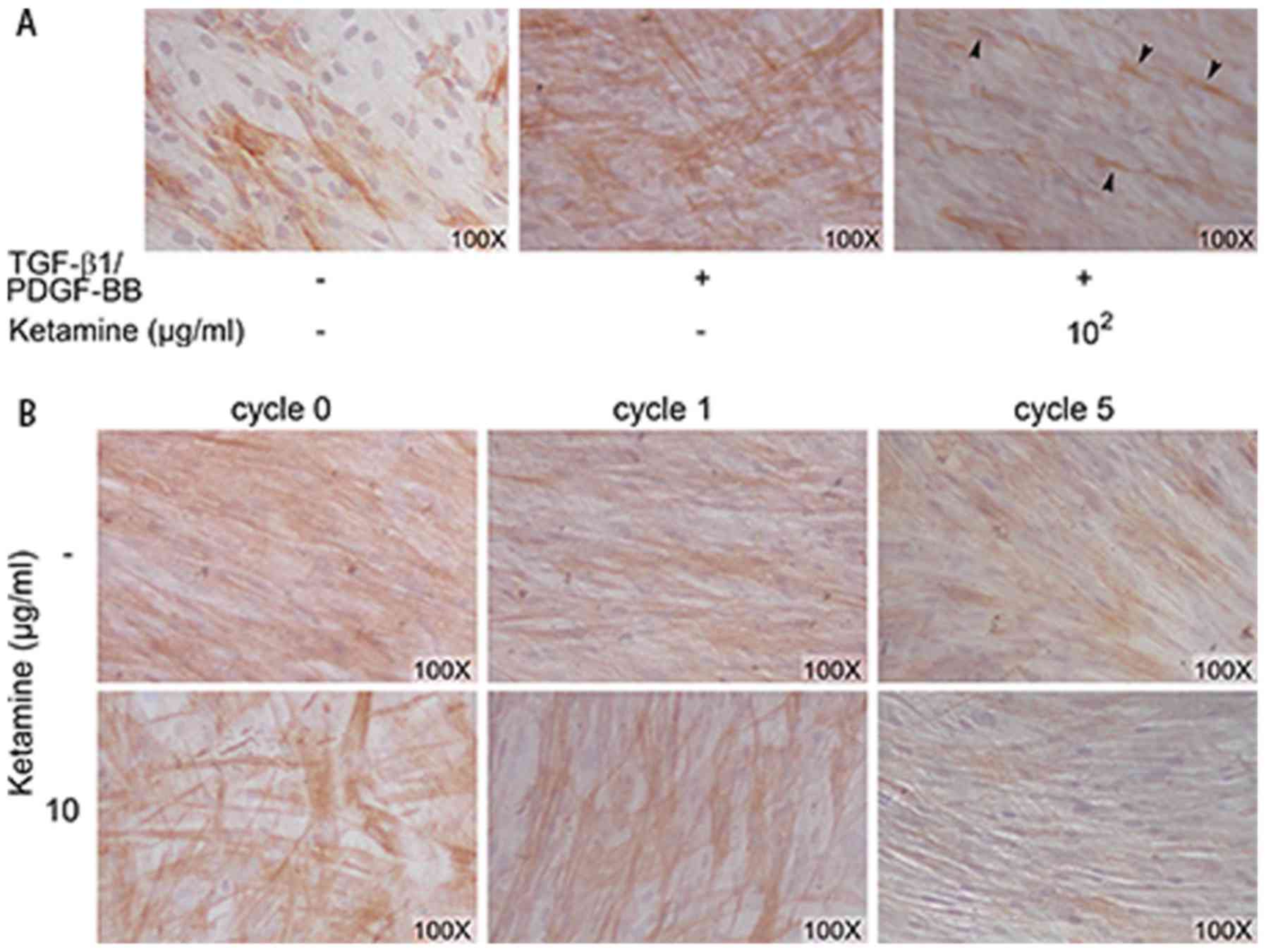
immunostaining with an anti-SMA antibody (Fig. 1A). The FDSC-derived SMCs shrank
and deformed into a spindle shape when treated with 102
µg/ml ketamine. Based on our observations of the molecular
effects of ketamine on the urinary bladder (7) and the marginal concentration of
ketamine detected in the urine of ketamine abusers, 10 µg/ml
was selected as the concentration for cyclic treatment of SMCs
(30,31). Compared with untreated
FDSC-derived SMCs, cells grew to achieve a spindle-like morphology
with additional cycles of ketamine treatment (Fig. 1B).
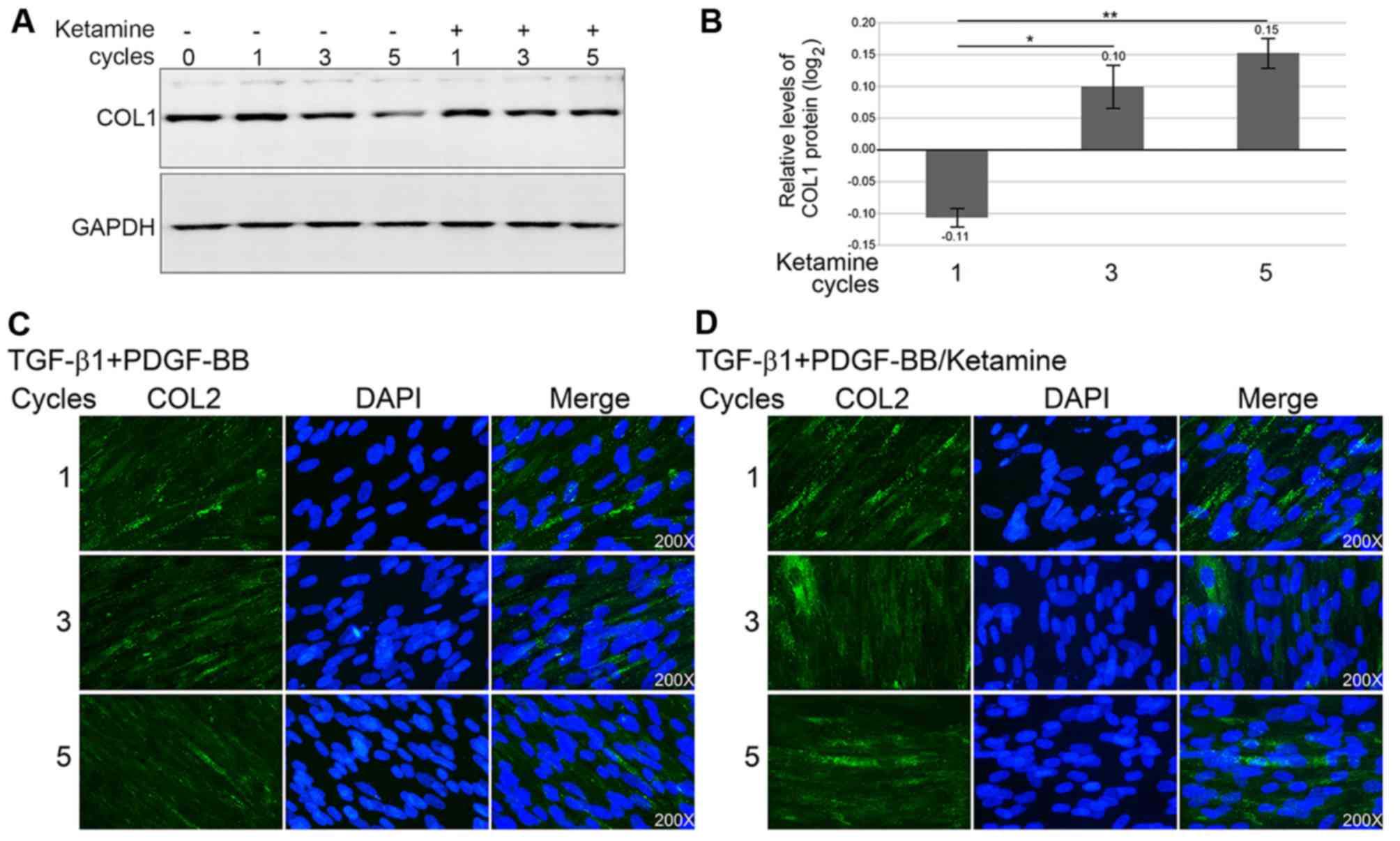
Collagen expression and deposition is
increased in FDSC-derived SMCs following ketamine treatment
To investigate whether fibrosis is induced in
FDSC-derived ketamine-treated SMCs, the protein expression levels
of frequently studied collagens, COL1 and COL2, were detected.
Western blot analysis demonstrated that COL1, which is associated
with fibrosis, was decreased in FDSC-derived SMCs (Fig. 2A). This decrease was prevented in
cells exposed to cyclic ketamine treatment. Densitometric analysis
was used to semi-quantify the relative expression levels of COL1;
the protein expression levels of COL1 were increased with
increasing cycles (Fig. 2B). COL2
deposition around cell nuclei in the cytosol of FDSC-derived SMCs
was identified after 5 cycles of ketamine treatment. Obvious COL2
deposition appears increased in ketamine-treated cells (Fig. 2C), not in cells treated with
TGF-β1 and PDGF-BB only (Fig.
2D).
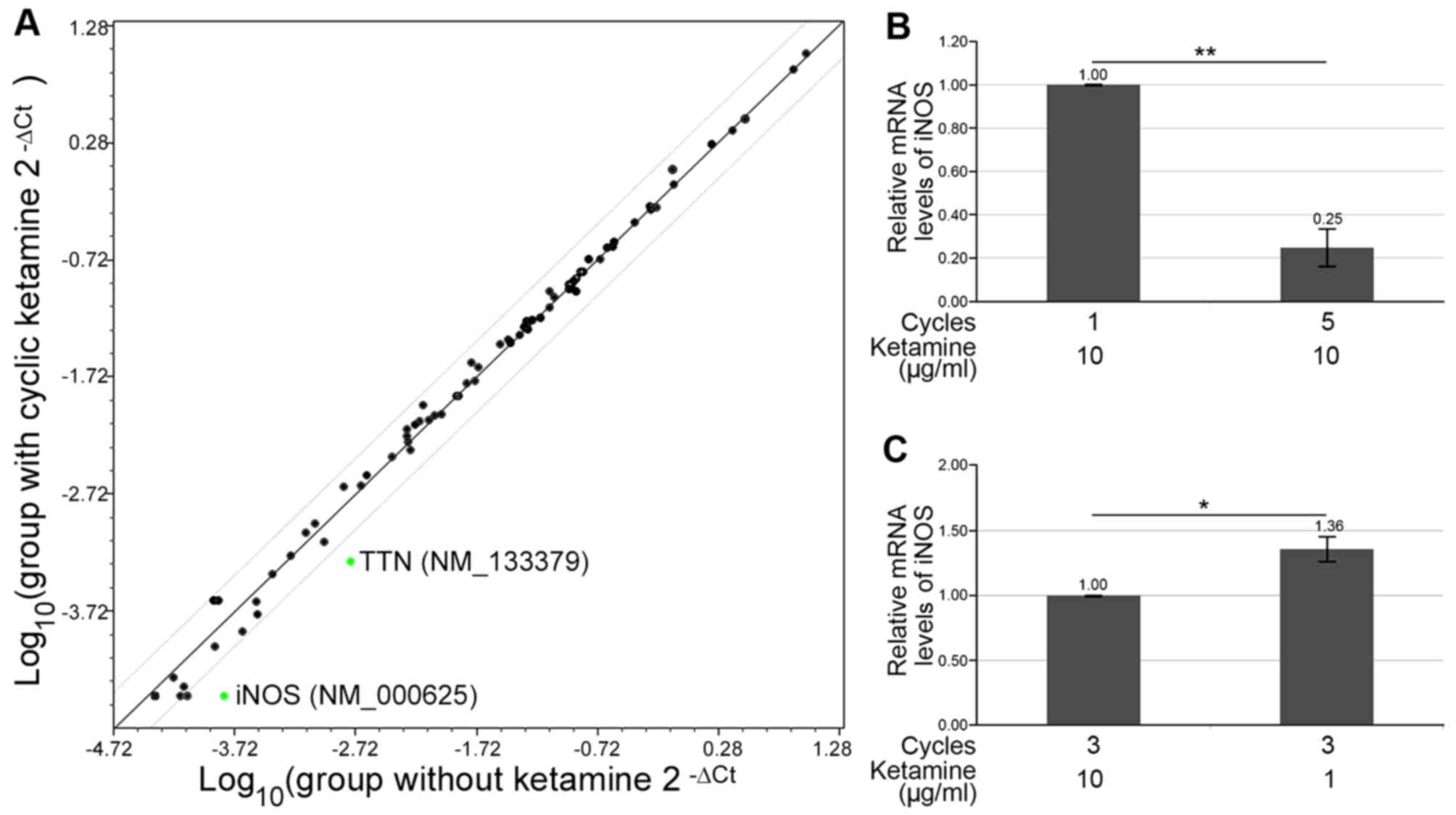
Detection of altered mRNA expression in
FDSC-derived SMCs following ketamine treatment
Molecular alterations were detected in cells
following cyclic ketamine treatment. Cyclooxygenase-2 levels were
increased and IL-6 levels were decreased, whereas
TGF-β1 levels were not markedly altered after 5 cycles of
ketamine treatment in FDSC-derived SMCs (Fig. 3A). The mRNA expression levels of
IL-6 were increased when the concentration of ketamine was
lowered from 10 to 1 µg/ml during cyclic ketamine treatment
(Fig. 3B). A pathway-focused PCR
array was used to examine oxidative stress; the results indicated
that the expression levels of two genes associated with oxidative
stress, TTN (NM_003319) and iNOS (NM_000625), were
significantly reduced, with fold-changes of 0.41 and 0.29,
respectively, in FDSC-derived SMCs following 8 cycles of ketamine
treatment (Fig. 4A). All other
genes exhibited fold-changes within the range of 0.5 and 2.0.
However, only iNOS was downregulated (0.25-fold) after
repeated ketamine treatment, as determined by specific qPCR
validation in FDSC-derived SMCs (Fig.
4B). The mRNA expression levels of iNOS were restored
when the concentration of ketamine was decreased from 10 to 1
µg/ml during cyclic ketamine treatment (Fig. 4C).
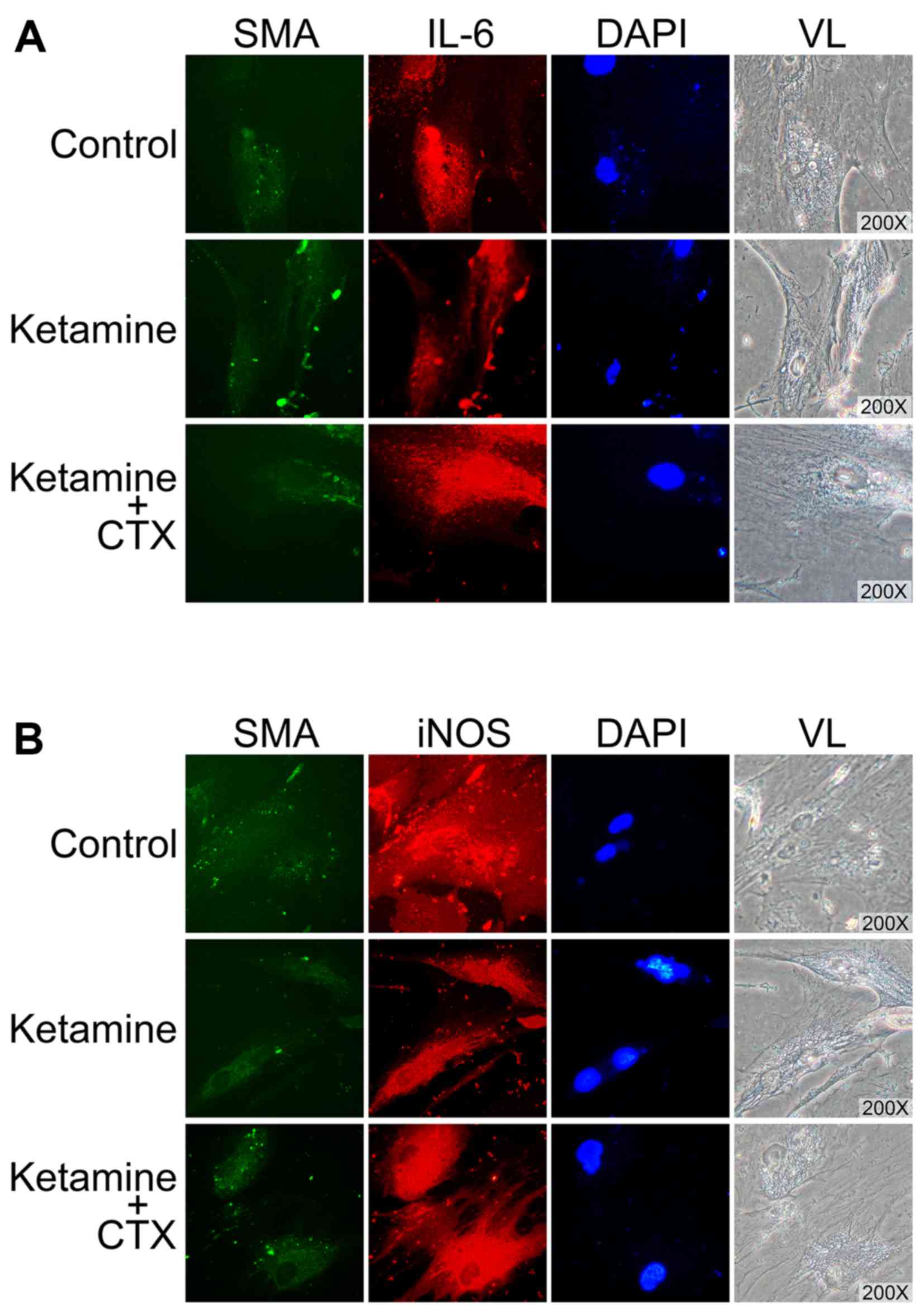
Restoration of protein levels of IL-6 and
iNOS by CTX in ketamine-treated normal aortic SMCs
Similar to the results presented in Fig. 1B, normal aortic SMCs also became
deformed and exhibited a spindle-like morphology following
treatment with 10 µg/ml ketamine for 24 h (Fig. 5). All SMCs expressed consistent
levels of SMA (labeled green with FITC in the figure); however,
reduced levels of IL-6 and iNOS (both labeled red with Cy3) were
detected following ketamine treatment (Fig. 5). Conversely, the levels of IL-6
and iNOS were restored following 10 µM CTX treatment
(Fig. 5).
Discussion
Ketamine abusers exhibit numerous clinical symptoms
(32). Typically, long-term
(>2 years) ketamine abuse results in bilateral lower ureteral
stricture and a contracted bladder with severely reduced bladder
capacity (<55 ml) (33,34).
Clinical observations include urinary frequency (average, >31
times/day) and nocturia (12.5 times/day) in ketamine abusers due to
their reduced bladder capacity (7). In addition, genetic alterations have
been observed in ketamine-induced cystitis (35,36). However, to the best of our
knowledge, no translational study has indicated any potential
therapy for these pathological and molecular alterations in the
ketamine-injured human bladder.
Although ketamine-induced cystitis can cause bladder
pain, which is mediated through a specific neurogenic mechanism on
the urothelium, we proposed that the main muscle component of the
urinary bladder wall, the detrusor muscle, is also damaged by
ketamine (22,37,38). However, dynamic genetic
alterations involving irreversible bladder contracture in the human
detrusor muscle are difficult to analyze in ketamine abusers.
Therefore, two types of cultured normal human SMCs, FDSC-derived
SMCs and aortic SMCs, were used in the present study to evaluate
in vitro ketamine-induced molecular alterations.
Ketamine and its active metabolites (e.g.,
norketamine) are believed to exert a direct toxic effect on the
bladder mucosa (17,35). In the cultured normal SMCs used in
the present study, ketamine, but not norketamine, induced a
cytotoxic effect on cell growth (data not shown). Similarly,
ketamine has been reported to exert toxicity in chondrocyte cell
cultures and induce cytoskeleton interruption (39,40). Collectively, these results
suggested that ketamine may dose-dependently damage the urinary
bladder and affect SMC stability. This is consistent with the
findings of Shahani et al who reported that severe
ulcerative cystitis results from chronic ketamine use (13). Since 1998, mouse models have been
used to demonstrate that accelerated fibrosis and collagen
deposition coincide in the renal interstitium during ureteral
obstruction (41). Furthermore, a
rat model has been used to indicate that ketamine increases
interstitial fibrosis in urinary bladder tissues (20,42,43). Increased fibrosis can be examined
by measuring the deposition of various types of collagen (44), and excessive synthesis of COL1 and
COL2 indicates fibrosis in various organs (45,46). The excessive COL1 and COL2 levels
detected in response to ketamine in the present study suggested
that fibrosis occurs in ketamine-treated normal human SMCs.
The expression of IL-6, which is an IL with
pleiotropic functions as a proinflammatory cytokine and an
anti-inflammatory myokine (47),
was reduced following cyclic ketamine treatment in the present
study. However, some inflammatory markers were upregulated in our
previous model of ketamine-induced cystitis (35,42). Given the present data and those of
previous studies, it may be concluded that inflammatory markers are
expressed differently between various types of ketamine-treated
cells (48). Numerous studies
regarding antidepressants and neural pathways have indicated that
ketamine is associated with the downregulation of proinflammatory
cytokines, including IL-6 (36,49). In addition, the expression of IL-6
was positively correlated with the migration of vascular SMCs
(50). Therefore, reduced IL-6
expression in ketamine-treated SMCs may be associated with the
function of IL-6 as an anti-inflammatory myokine, thus suggesting
that reduced IL-6 levels may impair muscle contraction (1). Conversely, the proinflammatory
effects of IL-6 may increase the migratory rate of SMCs (51). This indicates that the
ketamine-caused decreasing IL-6 is negatively correlated with the
bladder stretching. Clinical observations have indicated that
patients feel better and that their symptoms improve when they stop
or reduce ketamine use. The observation that IL-6 expression was
restored in the present study when ketamine treatment was removed
or its concentration was reduced is consistent with these clinical
observations. Therefore, ketamine abusers are strongly advised to
terminate ketamine use. In addition, ketamine induced COL1 and COL2
deposition, and reduced IL-6 expression in SMCs; these effects may
be associated with muscle contraction in the urinary bladder. In
other words, the increased fibrosis and decreased myokine
additionally cause the bladder dysfunction.
The ketamine-induced molecular alterations detected
in SMCs may also include the inhibition of iNOS (48). There is evidence to suggest that
reactive oxygen species are crucial to the pathogenesis of various
diseases (52,53). In a rat model, subanesthetic doses
of ketamine increased oxidative stress and induced a
schizophrenia-like condition (25). The effects of iNOS in response to
oxidative stress were observed in epithelial cells (54). The findings of the present study,
and those of other studies, suggested that the ketamine-induced
cystitis may also result from a reduction in iNOS levels following
treatment of SMCs with cyclic ketamine. However, in a previous
study, selective inhibition of NOS could increase muscle thickness
(55). The reduction in nitric
oxide levels due to reduced iNOS may also increase smooth muscle
contraction (e.g., frequency or strength) (56). Therefore, in ketamine abusers,
cyclic ketamine treatment may lead to continuous muscle contraction
and to the development of small bladder capacity due to iNOS
downregulation. These findings indicated that reversing the effects
of oxidative stress is a potential therapeutic strategy in addition
to surgery.
CTX, which belongs to the group of
oxazaphosphorines, exhibits strong activity against several
experimental tumors and is widely used in the treatment of various
clinical conditions, including lymphoproliferative disorders, small
cell lung cancer, breast cancer and other severe forms of rheumatic
diseases, such as lupus erythematosus, systemic sclerosis and
vasculitis (57,58). However, CTX and its isomeric
analogue ifosfamide (IFS) can dose-dependently induce hemorrhagic
cystitis, a urological disorder (59,60). Following administration of CTX,
iNOS bladder activity increases, which is associated with
histopathological alterations (61). Ozguven et al reported that
appropriate ketamine pretreatment attenuated experimental
IFS-induced hemorrhagic cystitis in a rat model (62). This result suggested that IFS (or
CTX) and ketamine have opposing effects on the urinary bladder,
probably due to their different effects on iNOS activities. In the
present study, the results observed in the ketamine-treated SMCs
are consistent with the results of previous studies, which
suggested that the production of proinflammatory mediators, such as
IL-6 and free oxygen radicals (nitric oxide), are reduced in SMCs
following ketamine treatment (60,62).
It seems likely that ketamine-induced cystitis is
propagated via an accumulation of molecular alterations in the
urinary bladder, particularly in SMCs. These alterations may result
in the clinical symptoms observed in ketamine abusers. A specific
therapeutic agent, CTX, may counteract oxidative damage and reverse
ketamine-induced aberrations (Fig.
6).
In conclusion, the findings of the present study
provided a novel insight into the molecular mechanisms underlying
ketamine-induced cystitis and may suggest novel clinical
treatments.
Acknowledgments
The present study was supported by grants from the
Cathay General Hospital (grant nos. CGH-MR-10022 and CGH-MR-A10316)
to Y.-C.W.
Abbreviations:
|
SMCs
|
smooth muscle cells
|
|
FDSCs
|
foreskin-derived fibroblast-like
stromal cells
|
|
NMDA
|
N-methyl-D-aspartate
|
|
PCR
|
polymerase chain reaction
|
|
qPCR
|
quantitative PCR
|
|
TGF-β1
|
transforming growth factor-β1
|
|
PDGF-BB
|
platelet-derived growth factor-BB
|
|
CTX
|
cyclophosphamide
|
|
IF
|
immunofluorescent
|
|
COL1
|
type I collagen
|
|
COL2
|
type II collagen
|
|
IL-6
|
interleukin-6
|
|
iNOS
|
inducible nitric oxide synthase
|
|
FITC
|
fluorescein isothiocyanate
|
|
TTN
|
titin
|
References
|
1
|
Zarate C, Duman RS, Liu G, Sartori S,
Quiroz J and Murck H: New paradigms for treatment-resistant
depression. Ann NY Acad Sci. 1292:21–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Naughton M, Clarke G, O'Leary OF, Cryan JF
and Dinan TG: A review of ketamine in affective disorders: Current
evidence of clinical efficacy, limitations of use and pre-clinical
evidence on proposed mechanisms of action. J Affect Disord.
156:24–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kavalali ET and Monteggia LM: How does
ketamine elicit a rapid antidepressant response. Curr Opin
Pharmacol. 20:35–39. 2015. View Article : Google Scholar
|
|
4
|
Jha AK, Bhardwaj N, Yaddanapudi S, Sharma
RK and Mahajan JK: A randomized study of surgical site infiltration
with bupivacaine or ketamine for pain relief in children following
cleft palate repair. Paediatr Anaesth. 23:401–406. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tawfic QA: A review of the use of ketamine
in pain management. J Opioid Manag. 9:379–388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ricaurte GA and McCann UD: Recognition and
management of complications of new recreational drug use. Lancet.
365:2137–2145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang YC, Chen SK and Lin CM: Breaking the
drug addiction cycle is not easy in ketamine abusers. Int J Urol.
17:496author reply 497. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jansen KL and Darracot-Cankovic R: The
nonmedical use of ketamine, part two: A review of problem use and
dependence. J Psychoactive Drugs. 33:151–158. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lim DK: Ketamine associated psychedelic
effects and dependence. Singapore Med J. 44:31–34. 2003.PubMed/NCBI
|
|
10
|
Slikker W Jr, Liu F, Rainosek SW,
Patterson TA, Sadovova N, Hanig JP, Paule MG and Wang C:
Ketamine-induced toxicity in neurons differentiated from neural
stem cells. Mol Neurobiol. 52:959–969. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gray T and Dass M: Ketamine cystitis: An
emerging diagnostic and therapeutic challenge. Br J Hosp Med
(Lond). 73:576–579. 2012. View Article : Google Scholar
|
|
12
|
Gu D, Huang J, Yin Y, Shan Z, Zheng S and
Wu P: Long-term ketamine abuse induces cystitis in rats by
impairing the bladder epithelial barrier. Mol Biol Rep.
41:7313–7322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shahani R, Streutker C, Dickson B and
Stewart RJ: Ketamine-associated ulcerative cystitis: A new clinical
entity. Urology. 69:810–812. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chu PS, Ma WK, Wong SC, Chu RW, Cheng CH,
Wong S, Tse JM, Lau FL, Yiu MK and Man CW: The destruction of the
lower urinary tract by ketamine abuse: A new syndrome. BJU Int.
102:1616–1622. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Colebunders B and Van Erps P: Cystitis due
to the use of ketamine as a recreational drug: A case report. J Med
Case Reports. 2:2192008. View Article : Google Scholar :
|
|
16
|
Chu PS, Kwok SC, Lam KM, Chu TY, Chan SW,
Man CW, Ma WK, Chui KL, Yiu MK, Chan YC, et al: 'Street
ketamine'-associated bladder dysfunction: A report of ten cases.
Hong Kong Med J. 13:311–313. 2007.PubMed/NCBI
|
|
17
|
Chen CH, Lee MH, Chen YC and Lin MF:
Ketamine-snorting associated cystitis. J Formos Med Assoc.
110:787–791. 2011. View Article : Google Scholar
|
|
18
|
Cheung RY, Lee JH, Chan SS, Liu DW and
Choy KW: A pilot study of urine cytokines in ketamine-associated
lower urinary tract symptoms. Int Urogynecol J Pelvic Floor
Dysfunct. 25:1715–1719. 2014. View Article : Google Scholar
|
|
19
|
Smart C, Kabir M and Pati J: Treatment of
ketamine-associated cystitis with chondroitin sulphate. Br J Nurs.
22:S4S6S8–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chuang SM, Liu KM, Li YL, Jang MY, Lee HH,
Wu WJ, Chang WC, Levin RM and Juan YS: Dual involvements of
cyclooxygenase and nitric oxide synthase expressions in
ketamine-induced ulcerative cystitis in rat bladder. Neurourol
Urodyn. 32:1137–1143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gu D, Huang J, Shan Z, Yin Y, Zheng S and
Wu P: Effects of long-term ketamine administration on rat bladder
protein levels: A proteomic investigation using two-dimensional
difference gel electrophoresis system. Int J Urol. 20:1024–1031.
2013.PubMed/NCBI
|
|
22
|
Baker SC, Stahlschmidt J, Oxley J, Hinley
J, Eardley I, Marsh F, Gillatt D, Fulford S and Southgate J: Nerve
hyperplasia: A unique feature of ketamine cystitis. Acta
Neuropathol Commun. 1:642013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jhang JF, Hsu YH, Jiang YH and Kuo HC:
Elevated serum IgE may be associated with development of ketamine
cystitis. J Urol. 192:1249–1256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mami I, Tavernier Q, Bouvier N, Aboukamis
R, Desbuissons G, Rabant M, Poindessous V, Laurent-Puig P, Beaune
P, Tharaux PL, et al: A novel extrinsic pathway for the unfolded
protein response in the kidney. J Am Soc Nephrol. 27:2670–2683.
2016.PubMed/NCBI
|
|
25
|
de Oliveira L, Spiazzi CM, Bortolin T,
Canever L, Petronilho F, Mina FG, Dal-Pizzol F, Quevedo J and Zugno
AI: Different sub-anesthetic doses of ketamine increase oxidative
stress in the brain of rats. Prog Neuropsychopharmacol Biol
Psychiatry. 33:1003–1008. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Toma JG, Akhavan M, Fernandes KJ,
Barnabé-Heider F, Sadikot A, Kaplan DR and Miller FD: Isolation of
multipotent adult stem cells from the dermis of mammalian skin. Nat
Cell Biol. 3:778–784. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fernandes KJ, McKenzie IA, Mill P, Smith
KM, Akhavan M, Barnabé-Heider F, Biernaskie J, Junek A, Kobayashi
NR, Toma JG, et al: A dermal niche for multipotent adult
skin-derived precursor cells. Nat Cell Biol. 6:1082–1093. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang HI, Chen SK, Ling QD, Chien CC, Liu
HT and Chan SH: Multilineage differentiation potential of
fibroblast-like stromal cells derived from human skin. Tissue Eng
Part A. 16:1491–1501. 2010. View Article : Google Scholar
|
|
29
|
Wang CC, Weng TI, Wu ET, Wu MH, Yang RS
and Liu SH: Involvement of interleukin-6-regulated nitric oxide
synthase in hemorrhagic cystitis and impaired bladder contractions
in young rats induced by acrolein, a urinary metabolite of
cyclophosphamide. Toxicol Sci. 131:302–310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moore KA, Sklerov J, Levine B and Jacobs
AJ: Urine concentrations of ketamine and norketamine following
illegal consumption. J Anal Toxicol. 25:583–588. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ho YH, Wang CC, Hsiao YT, Ko WK and Wu SM:
Analysis of ten abused drugs in urine by large volume sample
stacking-sweeping capillary electrophoresis with an experimental
design strategy. J Chromatogr A. 1295:136–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tam YH, Ng CF, Pang KK, Yee CH, Chu WC,
Leung VY, Wong GL, Wong VW, Chan HL and Lai PB: One-stop clinic for
ketamine-associated uropathy: Report on service delivery model,
patients' characteristics and non-invasive investigations at
baseline by a cross-sectional study in a prospective cohort of 318
teenagers and young adults. BJU Int. 114:754–760. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ng CM, Ma WK, To KC and Yiu MK: The
Chinese version of the pelvic pain and urgency/frequency symptom
scale: A useful assessment tool for street-ketamine abusers with
lower urinary tract symptoms. Hong Kong Med J. 18:123–130.
2012.PubMed/NCBI
|
|
34
|
Chung SD, Wang CC and Kuo HC: Augmentation
enterocystoplasty is effective in relieving refractory
ketamine-related bladder pain. Neurourol Urodyn. 33:1207–1211.
2014. View Article : Google Scholar
|
|
35
|
Lin HC, Lee HS, Chiueh TS, Lin YC, Lin HA,
Lin YC, Cha TL and Meng E: Histopathological assessment of
inflammation and expression of inflammatory markers in patients
with ketamine-induced cystitis. Mol Med Rep. 11:2421–2428. 2015.
View Article : Google Scholar :
|
|
36
|
Shen CH, Wang ST, Lee YR, Liu SY, Li YZ,
Wu JD, Chen YJ and Liu YW: Biological effect of ketamine in
urothelial cell lines and global gene expression analysis in the
bladders of ketamine injected mice. Mol Med Rep. 11:887–895. 2015.
View Article : Google Scholar
|
|
37
|
Andersson KE and Arner A: Urinary bladder
contraction and relaxation: Physiology and pathophysiology. Physiol
Rev. 84:935–986. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ibeawuchi CU, Ajayi OI and Ebeigbe AB:
Vascular effect of ketamine in isolated rabbit aortic smooth
muscle. Niger J Physiol Sci. 23:85–88. 2008.
|
|
39
|
Ozturk AM, Ergun MA, Demir T, Gungor I,
Yilmaz A and Kaya K: Ketamine is toxic to chondrocyte cell
cultures. Bone Joint J. 96-B:989–994. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chang HC, Chen TL and Chen RM:
Cytoskeleton interruption in human hepatoma HepG2 cells induced by
ketamine occurs possibly through suppression of calcium
mobilization and mitochondrial function. Drug Metab Dispos.
37:24–31. 2009. View Article : Google Scholar
|
|
41
|
Ma J, Nishimura H, Fogo A, Kon V, Inagami
T and Ichikawa I: Accelerated fibrosis and collagen deposition
develop in the renal interstitium of angiotensin type 2 receptor
null mutant mice during ureteral obstruction. Kidney Int.
53:937–944. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Juan YS, Lee YL, Long CY, Wong JH, Jang
MY, Lu JH, Wu WJ, Huang YS, Chang WC and Chuang SM: Translocation
of NF-κB and expression of cyclooxygenase-2 are enhanced by
ketamine-induced ulcerative cystitis in rat bladder. Am J Pathol.
185:2269–2285. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Song M, Yu HY, Chun JY, Shin DM, Song SH,
Choo MS and Song YS: The fibrosis of ketamine, a noncompetitive
N-methyl-d-aspartic acid receptor antagonist dose-dependent change
in a ketamine-induced cystitis rat model. Drug Chem Toxicol.
39:206–212. 2016. View Article : Google Scholar
|
|
44
|
Chen CZ and Raghunath M: Focus on
collagen: In vitro systems to study fibrogenesis and antifibrosis
state of the art. Fibrogenesis Tissue Repair. 2:72009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Borg BB, Seetharam A, Subramanian V, Basha
HI, Lisker-Melman M, Korenblat K, Anderson CD, Shenoy S, Chapman
WC, Crippin JS, et al: Immune response to extra-cellular matrix
collagen in chronic hepatitis C-induced liver fibrosis. Liver
Transpl. 17:814–823. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Challa AA, Vukmirovic M, Blackmon J and
Stefanovic B: Withaferin-A reduces type I collagen expression in
vitro and inhibits development of myocardial fibrosis in vivo. PLoS
One. 7:e429892012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Muñoz-Cánoves P, Scheele C, Pedersen BK
and Serrano AL: Interleukin-6 myokine signaling in skeletal muscle:
A double-edged sword. FEBS J. 280:4131–4148. 2013. View Article : Google Scholar
|
|
48
|
Yang C, Jiang RY, Shen J, Hong T, Liu N,
Ding LC, Wang DM, Chen LJ, Xu B and Zhu B: Ketamine attenuates the
lipopolysaccharide-induced inflammatory response in cultured N2a
cells. Mol Med Rep. 8:217–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tang SH, Yu JG, Li JJ and Sun JY:
Neuroprotective effect of ketamine on acute spinal cord injury in
rats. Genet Mol Res. 14:3551–3556. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lee GL, Wu JY, Yeh CC and Kuo CC: TLR4
induces CREB-mediated IL-6 production via upregulation of F-spondin
to promote vascular smooth muscle cell migration. Biochem Biophys
Res Commun. 473:1205–1210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hiram R, Rizcallah E, Marouan S, Sirois C,
Sirois M, Morin C, Fortin S and Rousseau E: Resolvin E1 normalizes
contractility, Ca2+ sensitivity and smooth muscle cell
migration rate in TNF-α-and IL-6-pretreated human pulmonary
arteries. Am J Physiol Lung Cell Mol Physiol. 309:L776–L788.
2015.PubMed/NCBI
|
|
52
|
Cascella R, Ragazzo M, Strafella C,
Missiroli F, Borgiani P, Angelucci F, Marsella LT, Cusumano A,
Novelli G, Ricci F, et al: Age-related macular degeneration:
Insights into inflammatory genes. J Ophthalmol.
582842:2014.PubMed/NCBI
|
|
53
|
Ye ZW, Zhang J, Townsend DM and Tew KD:
Oxidative stress, redox regulation and diseases of cellular
differentiation. Biochim Biophys Acta. 1850:1607–1621. 2015.
View Article : Google Scholar :
|
|
54
|
Dijkstra G, Blokzijl H, Bok L, Homan M,
van Goor H, Faber KN, Jansen PL and Moshage H: Opposite effect of
oxidative stress on inducible nitric oxide synthase and haem
oxygenase-1 expression in intestinal inflammation:
Anti-inflammatory effect of carbon monoxide. J Pathol. 204:296–303.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Soyer T, Boybeyi Ö and Atasoy P: Selective
inhibition of nitric oxide synthase causes increased muscle
thickness in rat esophagus. J Pediatr Surg. 50:1112–1114. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Al-Azemi M, Refaat B, Amer S, Ola B,
Chapman N and Ledger W: The expression of inducible nitric oxide
synthase in the human fallopian tube during the menstrual cycle and
in ectopic pregnancy. Fertil Steril. 94:833–840. 2010. View Article : Google Scholar
|
|
57
|
Dobrek Ł and Thor PJ: Bladder urotoxicity
pathophysiology induced by the oxazaphosphorine alkylating agents
and its chemoprevention. Postepy Hig Med Dosw Online. 66:592–602.
2012. View Article : Google Scholar
|
|
58
|
Yilmaz N, Emmungil H, Gucenmez S, Ozen G,
Yildiz F, Balkarli A, Kimyon G, Coskun BN, Dogan I, Pamuk ON, et
al: Incidence of cyclophosphamide-induced urotoxicity and
protective effect of mesna in rheumatic diseases. J Rheumatol.
42:1661–1666. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gray KJ, Engelmann UH, Johnson EH and
Fishman IJ: Evaluation of misoprostol cytoprotection of the bladder
with cyclophosphamide (Cytoxan) therapy. J Urol. 136:497–500. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang N, Yu HY, Shen XF, Gao ZQ, Yang C,
Yang JJ and Zhang GF: The rapid antidepressant effect of ketamine
in rats is associated with downregulation of pro-inflammatory
cytokines in the hippocampus. Ups J Med Sci. 120:241–248. 2015.
View Article : Google Scholar
|
|
61
|
Souza-Fiho MV, Lima MV, Pompeu MM, Ballejo
G, Cunha FQ and Ribeiro RdeA: Involvement of nitric oxide in the
pathogenesis of cyclophosphamide-induced hemorrhagic cystitis. Am J
Pathol. 150:247–256. 1997.PubMed/NCBI
|
|
62
|
Ozguven AA, Yılmaz O, Taneli F, Ulman C,
Vatansever S and Onag A: Protective effect of ketamine against
hemorrhagic cystitis in rats receiving ifosfamide. Indian J
Pharmacol. 46:147–151. 2014. View Article : Google Scholar : PubMed/NCBI
|