Introduction
As a member of the small leucine-rich repeat
proteoglycan (SLRP) family (1,2),
periodontal ligament-associated protein-1 (PLAP-1) plays an
important role in maintaining the homeostasis of the periodontium
(3,4), and protects the periodontal ligament
from excessive osteogenesis by the negative regulation of the
osteoblastic differentiation of periodontal fibroblasts (5) and periodontal ligament stem cells
(PDLSCs) into mineralized tissue-forming cells (3,6).
Dental follicle stem cells (DFSCs) and PDLSCs are
potentially able to differentiate into the periodontal lineage
(7), and are therefore of value
in dental tissue engineering (8).
Bone marrow stromal cells (BMSCs) also have multilineage
differentiation potential (9).
Combined with biomaterials, BMSCs have been incorporated into
repair different bone defects, including periodontal bone defects,
in a number of studies (10–12). The majority of studies of tissue
engineering have focused on osteogenesis-promoting factors,
including osterix (Osx), bone morphogenetic protein-2 (BMP-2),
alkaline phosphatase (ALP), osteocalcin (OC) and bone sialoprotein
(BSP) (13–15), but our knowledge on
osteogenesis-inhibiting factors, such as PLAP-1, and their
molecular mechanisms is insufficient (16–18).
PLAP-1 is an important marker of the periodontal
ligament. PDL cells have been demonstrated to be multipotent, with
the microenvironment-dependent ability for differentiation into
osteoblasts or cementoblasts. As a negative regulator, PLAP-1
inhibits periodontal ligament mineralization. To investigate the
roles of PLAP-1 in the cytodifferentiation and mineralization of
BMSCs, rat BMSCs overexpressing PLAP-1 were established in our
previous study. The vector expressing the PLAP-1 gene was
transfected into BMSCs and stable transfectants that were
overexpressing PLAP-1 were established (19). The study showed that the
overexpression of the PLAP-1 gene inhibits the differentiation of
rat BMSCs (rBMSCs) into osteoblast-like cells in vitro
(19) and delays rat
critical-size skull defect repair in vivo (18). However, the molecular mechanisms
of PLAP-1 in the osteogenic differentiation of BMSCs and in
osteoclast activation in periodontal bone defect repair remain
unclear.
Osteoblasts are vital for bone formation and for the
maintenance of a dynamic equilibrium within bone tissues.
RANKL/RANK signaling regulates osteoclast differentiation and
activation in bone modeling and remodeling (20–22). OPG confers a protective effect
over bone, preventing excessive resorption by binding to RANKL and
impeding it from binding to RANK. Therefore, the OPG/RANKL ratio is
a fundamental determinant in bone (23).
In the present study, osteogenesis-associated
proteins, including ALP, BSP, runt-related transcription factor 2
(Runx2), Osx and OC, as indicators of rBMSC-induced osteogenesis
(22), were examined to assess
the role of PLAP-1 in the osteogenic differentiation of BMSCs.
Osteoclast number and the RANKL/OPG ratio were quantified to
analyze osteoclast activation. PLAP-1 is an important marker of the
periodontal ligament (24) and
studying its mechanism of action in the osteogenic differentiation
of BMSCs and in osteoclast activation may assist in furthering
dental tissue engineering (25).
Materials and methods
Animals
Male, 6-week-old, Wistar rats (n= 24; weight,
260–300 g; Laboratory Animal Center, Shandong University, Shandong,
China), which were acclimated for 1 week prior to the experiments,
were maintained on a normal hard food diet, with water ad
libitum. The animals were housed in cage racks, with a 12-h
light/12-h dark cycle (light on from 8:00 AM to 8:00 PM) at ambient
temperature (22–24°C) and 45% relative humidity. Experiments used
in this study were conducted according to the guidelines for Animal
Experimentation of Shandong University. The study was approved by
the Ethics Committee of the School of Stomatology, Shandong
University. Rats that received a periodontal bone defect of 5×2×1
mm according to a previously described procedure (26) were randomly allocated to 3 groups
according to differentially transfected-rBMSCs: PLAP-1 group
(collagen membranes with PLAP-1 lentivirus-transfected rBMSC were
transferred to the periodontal bone defects), vector group
(collagen membranes with empty vector lentivirus-transfected rBMSC
were transferred to the periodontal bone defects) and control group
(collagen membranes with normal rBMSC were transferred to the
periodontal bone defects). For statistical analysis, 8 animals were
present in each group.
Cell culture
Primary rBMSCs were harvested from 4-week-old Wistar
rats as previously described (9).
Briefly, the proximal end of the femora and the distal end of the
tibiae were excised. α-minimal essential medium (α-MEM; Gibco,
Grand Island, NY, USA), supplemented with 20% fetal bovine serum
(FBS; Gibco), 200 IU/ml penicillin and 200 mg/ml streptomycin
(Solarbio, Beijing, China) was used to flush the marrow gently from
the shafts with a 25-gauge needle. A single-cell suspension was
obtained by gently aspirating the cells sequentially through 20-
and 23-gauge needles. The bone marrow cells were then seeded into
culture flasks (Takara Bio, Inc., Otsu, Japan) at a cell density of
4.0×105 cells/cm2 and cultured using α-MEM
supplemented with 10% fetal bovine serum (FBS), 100 IU/ml
penicillin and 100 mg/ml streptomycin. The culture medium was
changed every 3 days, and the cells were subcultured 1:3 at
subconfluence. The adherent cells after one subculture were termed
rBMSCs. Cells (3-5 passages) were subsequently used for
experiments.
Overexpression of the PLAP-1 gene in
rBMSCs
The protocol of overexpression of the PLAP-1 gene in
rBMSCs was the same as previously described (18,27). rBMSCs were then plated in 6-well
plates (1×105 cells⁄well) and transfected with viral
stocks of pPBABE-hygro-PLAP-1, pPBABE-hygro or empty vector in the
presence of polybrene (6 µg⁄ml; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for 8 h. At 24 h post-transfection, the rBMSCs
were subjected to hygromycin B selection (50 µg⁄ml) for 2
weeks. The stably transduced rBMSCs were used for the following
experiments.
Cell seeding
Type I collagen membranes (Sigma-Aldrich; Merck
KGaA) were trimmed into 5×2×1-mm pieces and sterilized for usage as
previously described (21). The
differentially transfected-rBMSCs (1×104) were suspended
in 5-µl α-MEM, seeded on each surface of the collagen
scaffolds and cultured for 3 h in the incubator in 5%
CO2 at 37°C. After anesthetization by intraperitoneal
injection of 10% chloral hydrate (0.4 g/kg body weight), Bilateral
bone defects were created at the buccal aspect of the mandibular
molar. The signs of peritonitis were not observed following the
administration of 10% chloral hydrate. A defect of 5×2×1 mm was
made in the mandibular body using a dental drill driven at a low
speed with irrigation of 0.9% sodium chloride. Type I collagen
membranes and differentially transfected-rBMSCs were applied to
fill in the defect. The wounds were closed with nylon 4-0
sutures.
Tissue preparation
The rats were euthanized in order to minimize pain
and distress. The rats were sacrificed at 2, 4 and 6 weeks
post-surgery. Anesthetized by intraperitoneal injection of 10%
chloral hydrate (0.4 g/kg body weight), the rats were fixed with 4%
paraformaldehyde (PFA) via systemic circulation fixation for 30
min, and then the detached mandible was further fixed immediately
in 4% PFA for another 12 h at 4°C. The specimens were then
demineralized in 10% EDTA for 3 months. The demineralized tissues
were dehydrated by gradient ethanol, cleared with xylene and
embedded in paraffin. Serial sections of 5 µm in thickness
were sliced in the buccolingual direction. The specimens of
mandibular bone for the western blotting and RT-qPCR analyses were
not fixed. The defect areas were cut from the mandibular bone with
1-mm margins using bone cutting forceps and then rapidly frozen in
liquid nitrogen and stored at −80°C.
Micro-computed tomography (micro-CT)
imaging and analysis
For analysis of the alveolar bone loss, fixed
mandible samples were scanned using a PerkinElmer micro-CT
(PerkinElmer, Inc., Waltham, MA, USA) at 90 kV and 88 µA.
All scans were reoriented prior to analysis to uniformly align the
scan axes and anatomical positions. The specimens were scanned at a
resolution of 10 µm, ensuring that the defect areas were
encompassed. The three-dimensional (3D) volume viewer and analyzer
software (Analyze 12.0 and SimpleViewer version 5.1.2; PerkinElmer,
Inc.) were used for the visualization and quantification of
two-dimensional (2D) and 3D data on a personal computer output and
a standardized gray scale value was used to visualize only
mineralized tissues.
RT-PCR
Total RNA was extracted from the cells and bone
defect tissues with RNAiso Plus (Takara Bio, Inc.) according to the
manufacturer's instructions. A total of 1.0 mg RNA (in a 20-ml
reaction volume) was reverse transcribed using the PrimeScript RT
Reagent kit with gDNA Eraser (Takara Bio, Inc.). RT-PCR
amplifications labeled with SYBR Premix Ex Taq (Takara Bio, Inc.)
were performed in a Roche LightCycler 480 (Roche Diagnostics GmbH,
Mannheim, Germany) at 95°C for 30 sec, then at 95°C for 5 sec and
60°C for 30 sec for a total of 40 cycles. The primer sequences for
PLAP-1, ALP, BSP, Runx2, Osx and OC (19) were designed with Primer-BLAST
software from the National Center for Biotechnology Information
(Bethesda, MD, USA) nucleotide sequence database (Table I). Relative expression was
normalized to GAPDH using the 2−∆∆Cq method (28).
 | Table IPrimer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Genes | Upstream primer
(5′-3′) | Downstream primer
(3′-5′) |
|---|
| PLAP-1 |
CCTGGTAGGAGGGCTGGATT |
AGGGGTTCACTGGCTCTTTG |
| ALP |
GGAGATGGATGAGGCCATCG |
CGTCCACCACCTTGTAACCA |
| BSP |
GCCACATCTCAGGGGTAAC |
TGCATCTCCAGCCTTCTTGG |
| Runx2 |
CAGACACAATCCTCCCCACC |
GCCAGAGGCAGAAGTCAGAG |
| OSX |
GGATGGCGTCCTCTCTGCTTGAG |
AGGGAGCTGGGTAGGCGTCC |
| OC |
CAGGTGCAAAGCCCAGCGACT |
AGGGGATCTGGGTAGGGGGCT |
| GAPDH |
TGATGGGTGTGAACCACGAG |
CCCTTCCACGATGCCAAAGT |
Western blotting
The frozen samples were homogenized with hypotonic
lysis buffer (Solarbio). Protein concentrations were determined by
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology, Jiangsu, China), and the curves in the BSA protein
standard curves were used. Equal amounts of total proteins (20
µg per lane) were resolved by 10% SDS-polyacrylamide gel
electrophoresis and transferred to polyvinylidene difluoride
membranes (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The membranes was washed three times and blocked with 5%
skimmed milk (BD, Mr Ng Nanjing Biological, Nanjing, China) at room
temperature for 1 h. Next, the membranes were incubated with
antibodies against PLAP-1 (diluted 1:1,000; A3883-45C-AP; US
Biological, Salem, MA, USA), ALP (diluted 1:1,000; ab95462), BSP
(diluted 1:1,000; ab52128), Runx2 (diluted 1:1,000; ab23981), Osx
(diluted 1:1,000; ab209484), OC (diluted 1:1,000; ab13420) (all
Abcam, Cambridge, MA, USA), OPG (diluted 1:500; bs-0431R) or RANKL
(diluted 1:500; bs-0747R) (both Bioss, Beijing, China) overnight at
4°C. Secondary antibodies, horseradish peroxidase-linked goat
anti-rabbit IgG (diluted 1:5,000; CW0156S; CW Biotech, Beijing,
China), were then applied. The blots were visualized using enhanced
chemiluminescence reagents (EMD Millipore, Billerica, MA, USA), and
quantified by densitometric analysis [ImageJ (×64); 1.48u; National
Institutes of Health, Bethesda, MD, USA]. Equal protein loading was
shown by stripping and incubation with an anti-GAPDH antibody
(diluted 1:5,000; CW0100S; CW Biotech).
Tartrate-resistant acid phosphatase
(TRAP) staining
Sections were deparaffinized using xylene, hydrated
in gradient ethanols and gently washed twice with prewarmed,
filtered water (37°C). The sections were then fixed with stationary
liquid for 20 sec and stained with TRAP (Sigma-Aldrich; Merck KGaA)
for 60 min at 37°C. The TRAP-stained cells were then counterstained
with hematoxylin (Solarbio) at room temperature for 5 min, and
examined under a light microscope. TRAP+ multinucleated
cells containing three or more nuclei were counted as osteoclasts.
Osteoclasts were quantified by imaging five fields of view under
100-fold magnification and directly counting the number of
TRAP+ cells.
Statistical analysis
Statistically significant differences (P<0.05)
between the various groups were measured using one-way analysis of
variance and Student-Newman-Keuls test. All statistical analyses
were performed using the SPSS 17.0 statistical software package
(SPSS, Inc., Chicago, IL, USA). All data are expressed as the mean
± standard deviation.
Results
Micro-CT images
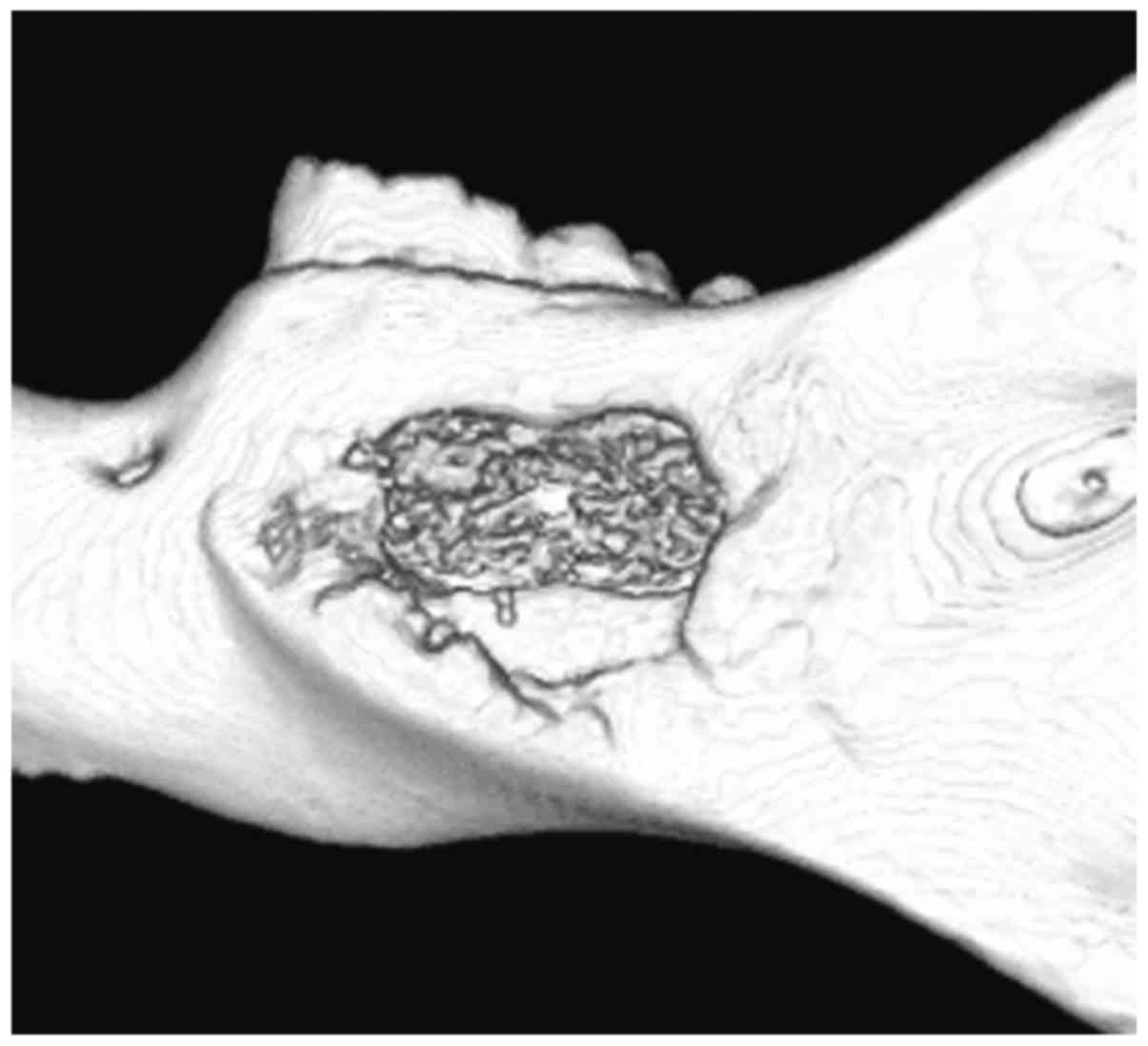
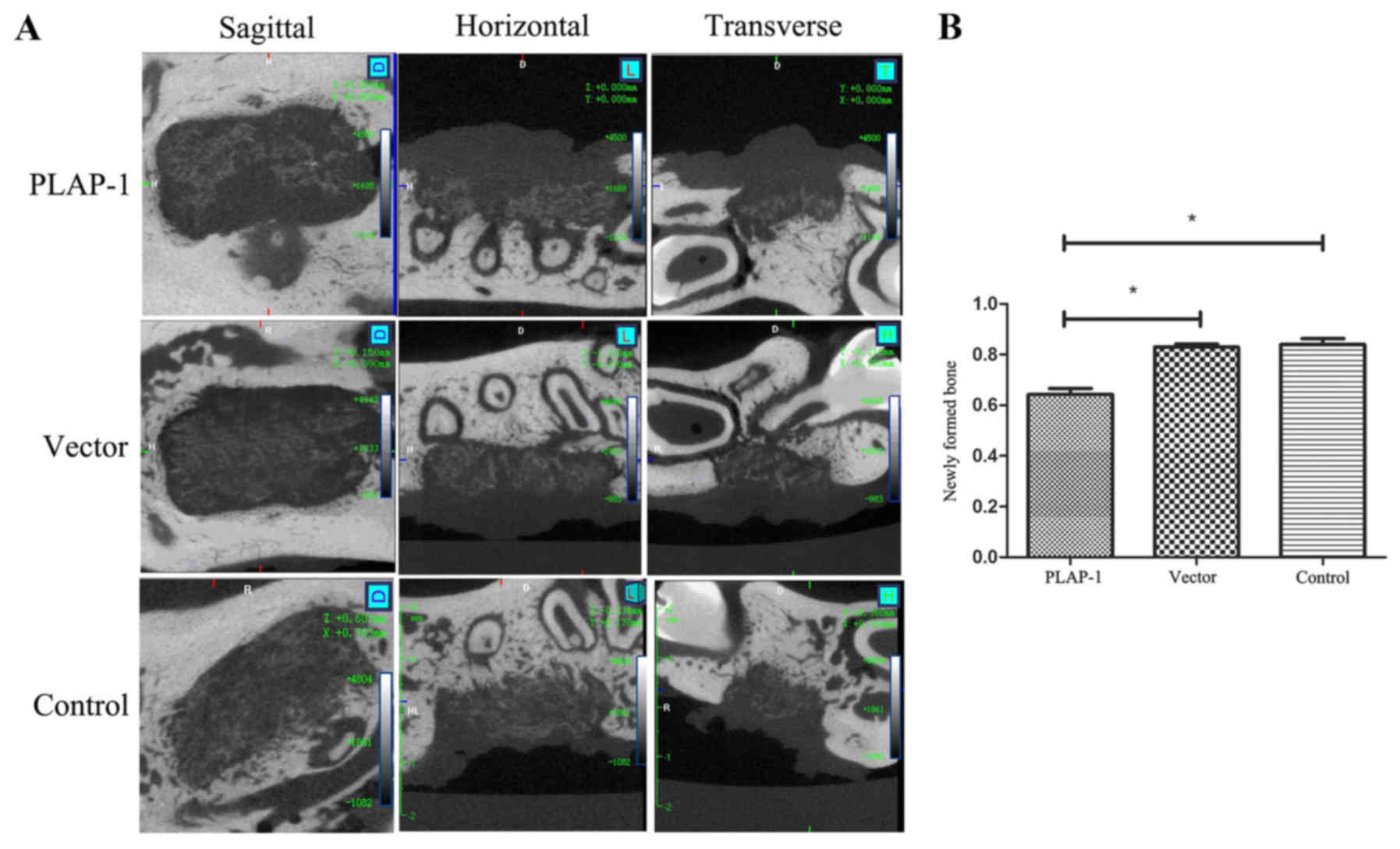
Newly formed mineralized bone could be found in the
defects at 4 weeks post-surgery (Fig.
1). The newly formed mineralized bone showed a trabecular
structure. The mineralization density of the new bone was lower
than that of normal bone (Fig.
2A). The new mineralized bone in the control and vector groups
was significantly greater in quantity than that in the PLAP-1 group
(P<0.05). There was no significant difference in the amount of
newly formed bone between the vector and control groups (Fig. 2B).
Histological observation
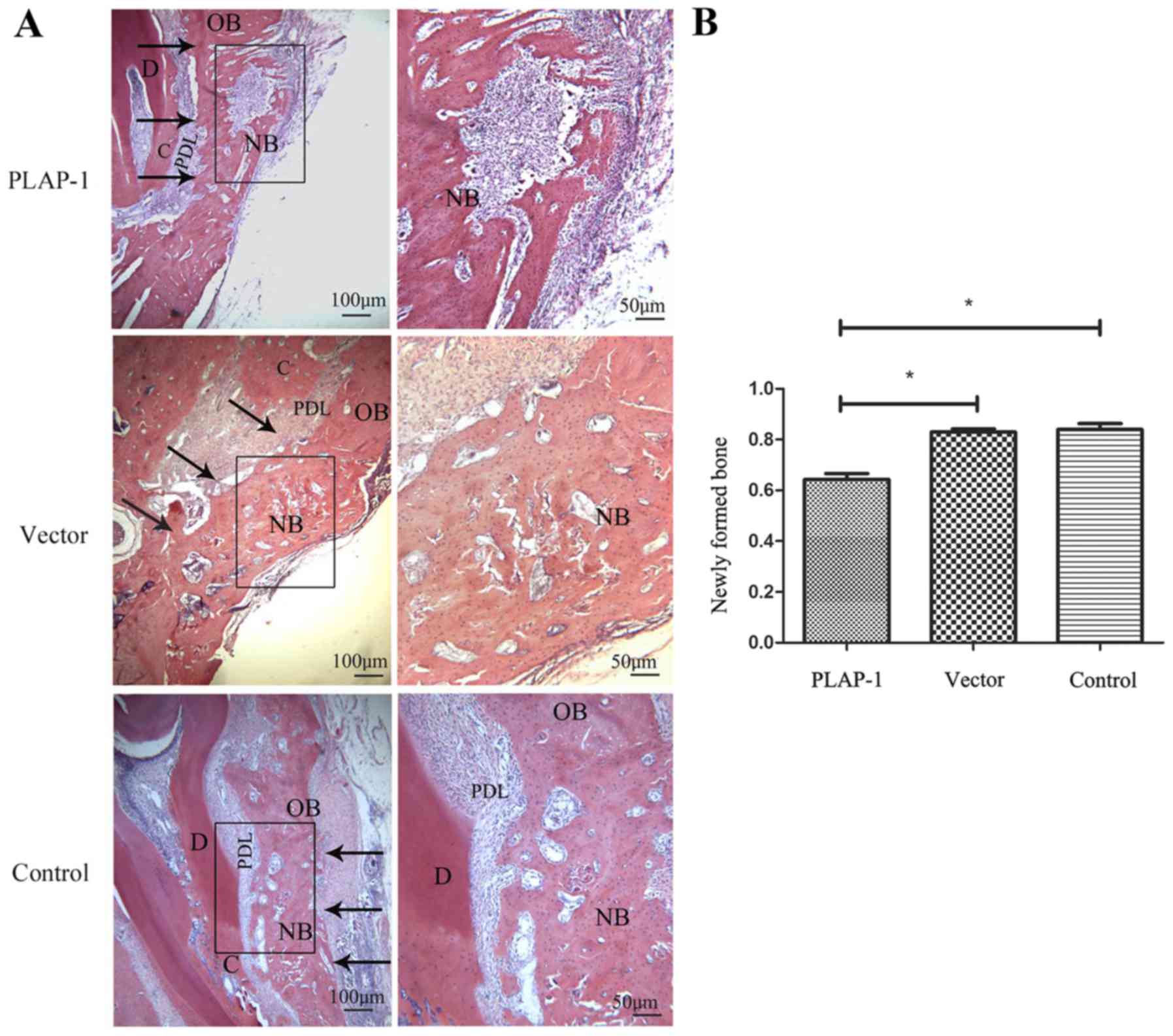
Histological observation showed that newly formed
bone had filled the majority of the defects of the PLAP-1 group at
6 weeks post-surgery. Newly formed bone trabeculae and lacunae were
visible in the new bone. Osteoblasts, multinucleated osteoclasts
and bone resorption pits could be viewed, which indicated that
osteogenesis and bone resorption occurred simultaneously in the
PLAP-1 group.
In the vector and control groups, newly formed bones
had almost filled the defects, but there were a lot of bone lacunae
at the new bone area. Osteoblasts could be observed at the edge of
the new bone. Few osteoclasts could be found in the two groups
(Fig. 3A). The newly formed bone
proportion was analyzed using Image-Pro Plus 6.0 (Media
Cybernetics, Silver Spring, MD, USA). The amount of new bone in the
PLAP-1 group was significantly less than that in the vector and
control groups (P<0.05). There was no significant difference in
the amount of newly formed bone between the vector and control
groups (Fig. 3B).
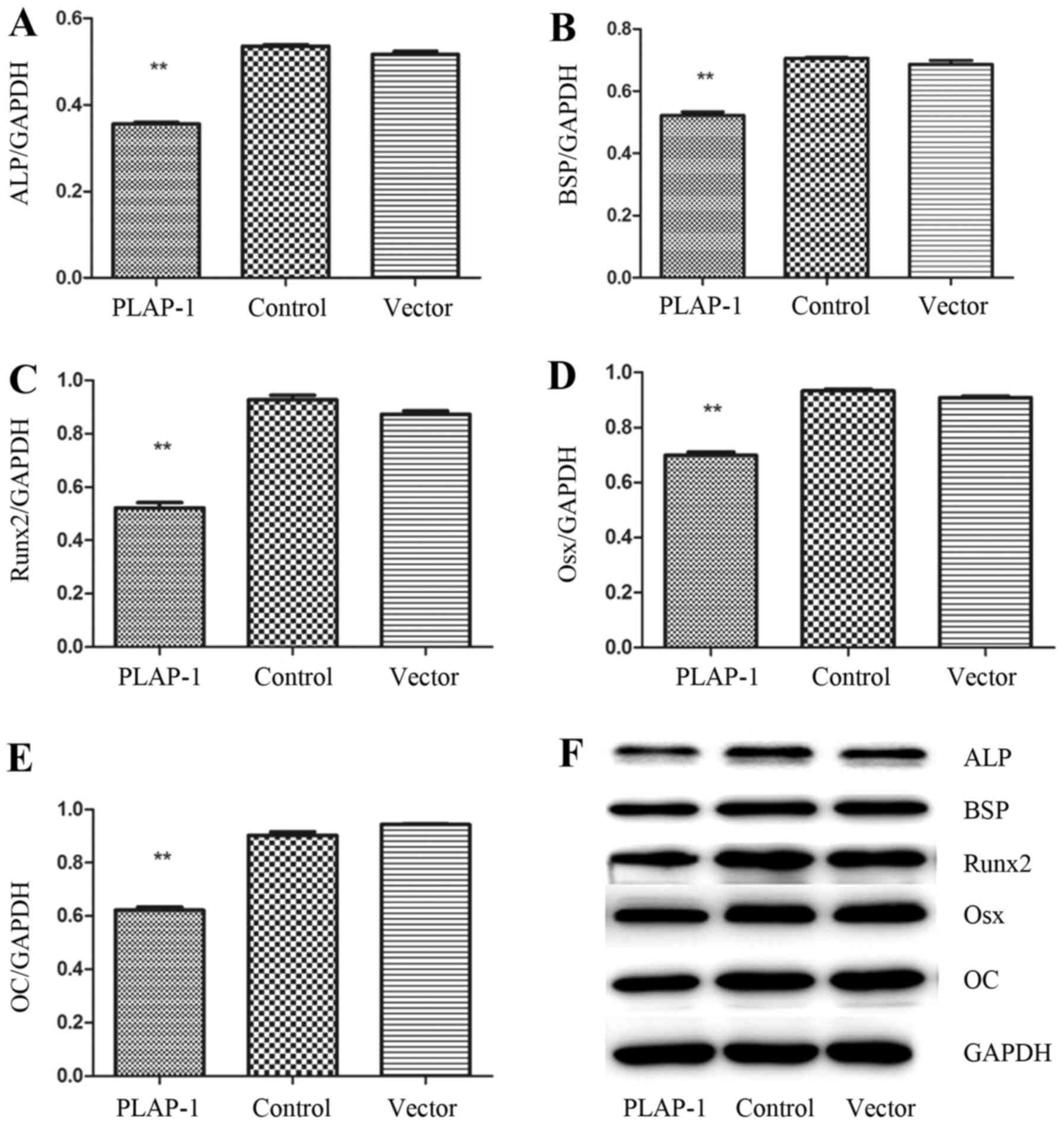
RT-qPCR
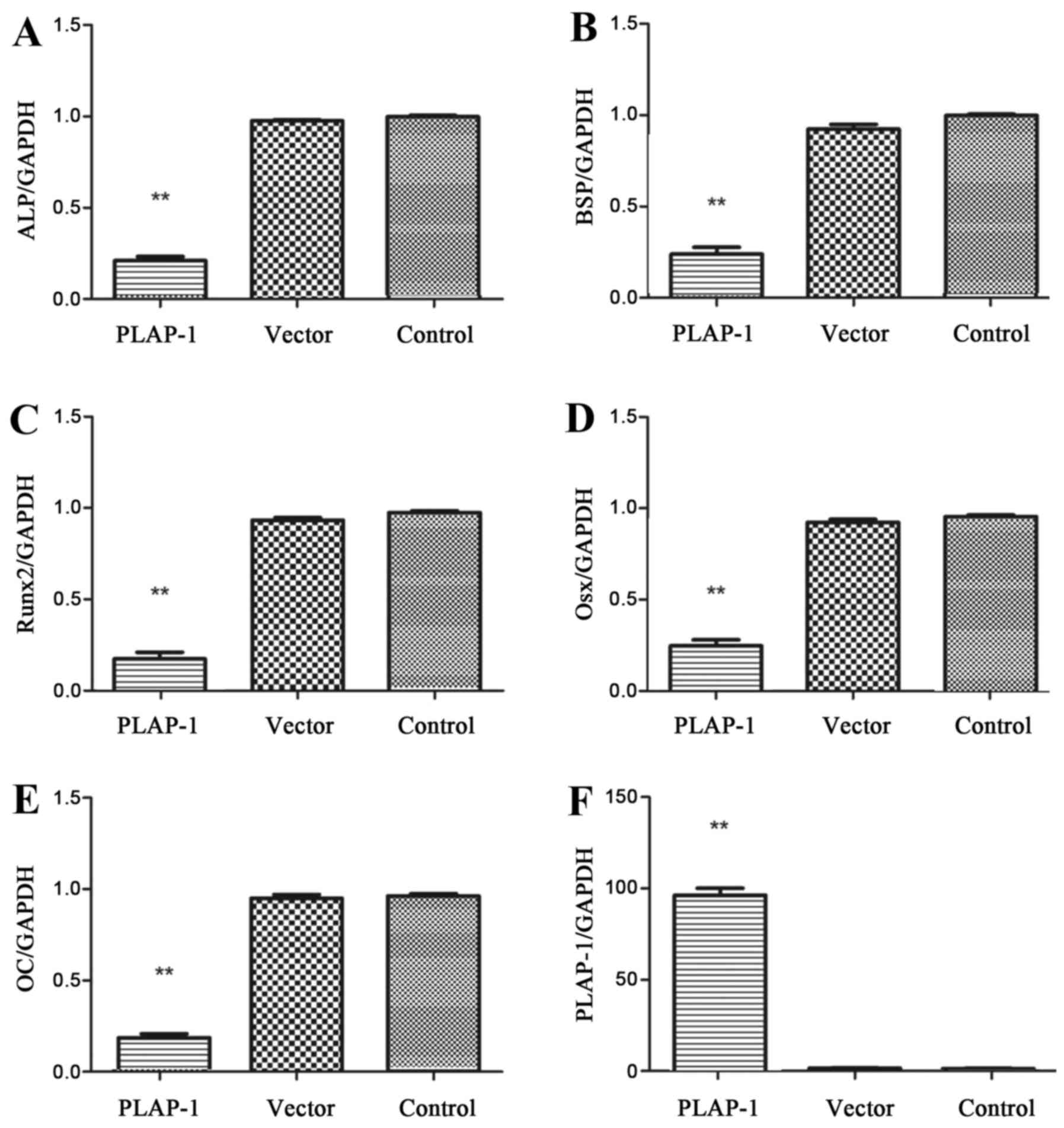
The mRNA expression of osteogenesis-associated
proteins in rat periodontal bone defects at 4 weeks post-surgery
was detected using RT-PCR. ALP, BSP, Runx2, Osx and OC mRNA
expression was deceased in the PLAP-1 group compared with that in
the vector and control groups (Fig.
4A–E) (P<0.01).
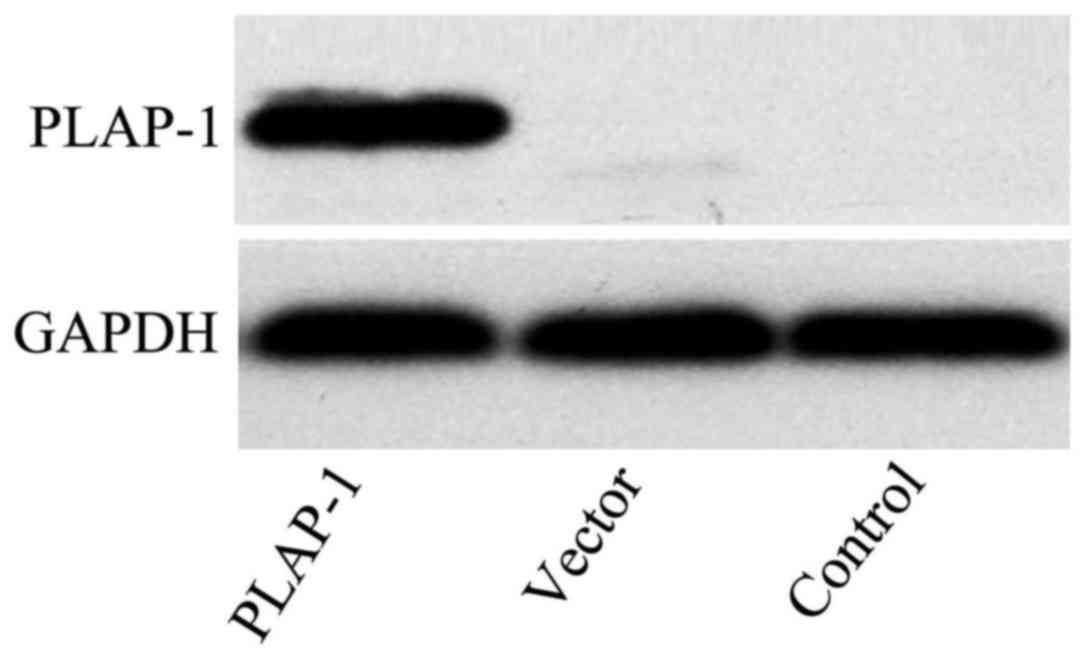
PLAP-1 expression in rBMSCs was elevated
significantly following transfection with pPBABE-hygro-PLAP-1
compared with that in rBMSCs transfected with empty vector and
normal rBMSCs (Figs. 4F and
5).
Western blotting
The expression of ALP, BSP, Runx2, Osx and OC in rat
periodontal bone defects at 4 weeks post-surgery was also detected
using western blotting. ALP, BSP, Runx2, Osx and OC expression was
deceased in the PLAP-1 group compared with that in the vector and
control groups (P<0.01) (Fig.
6A–F), which was similar to the mRNA expression results.
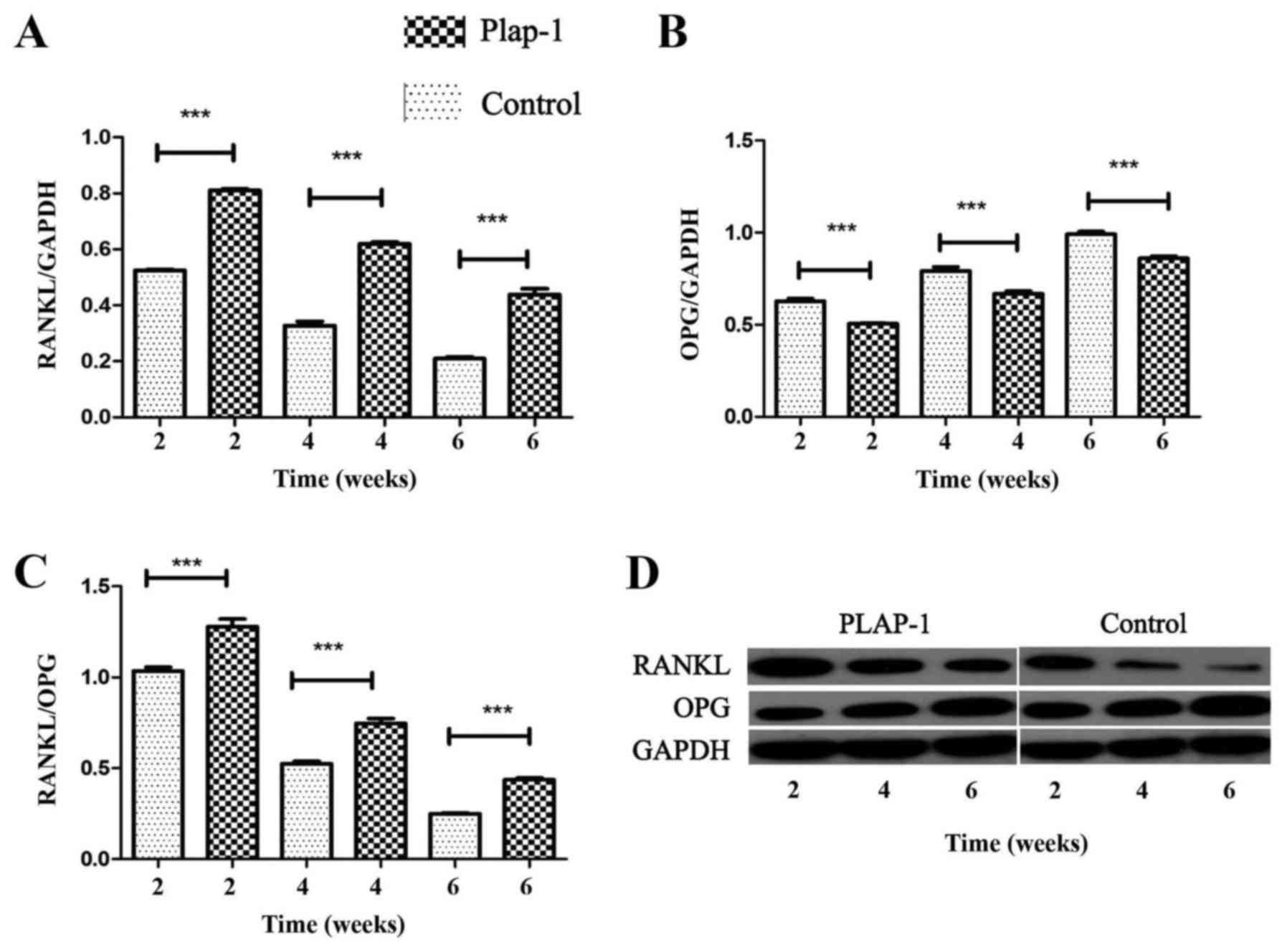
Expression of RANKL and OPG protein in rat periodontal bone defects
at 2, 4 and 6 weeks was detected by western blotting. RANKL protein
expression was upregulated during rat periodontal bone defect
repair, and higher expression was observed in the PLAP-1 group
compared with the control group (Fig.
7A). This trend was reversed for OPG; OPG was downregulated
compared with the higher expression of the control group from 2 to
6 weeks (Fig. 7B). The RANKL/OPG
ratio was upregulated in the PLAP-1 group compared with that in the
control group (P<0.01) (Fig. 7C
and D).
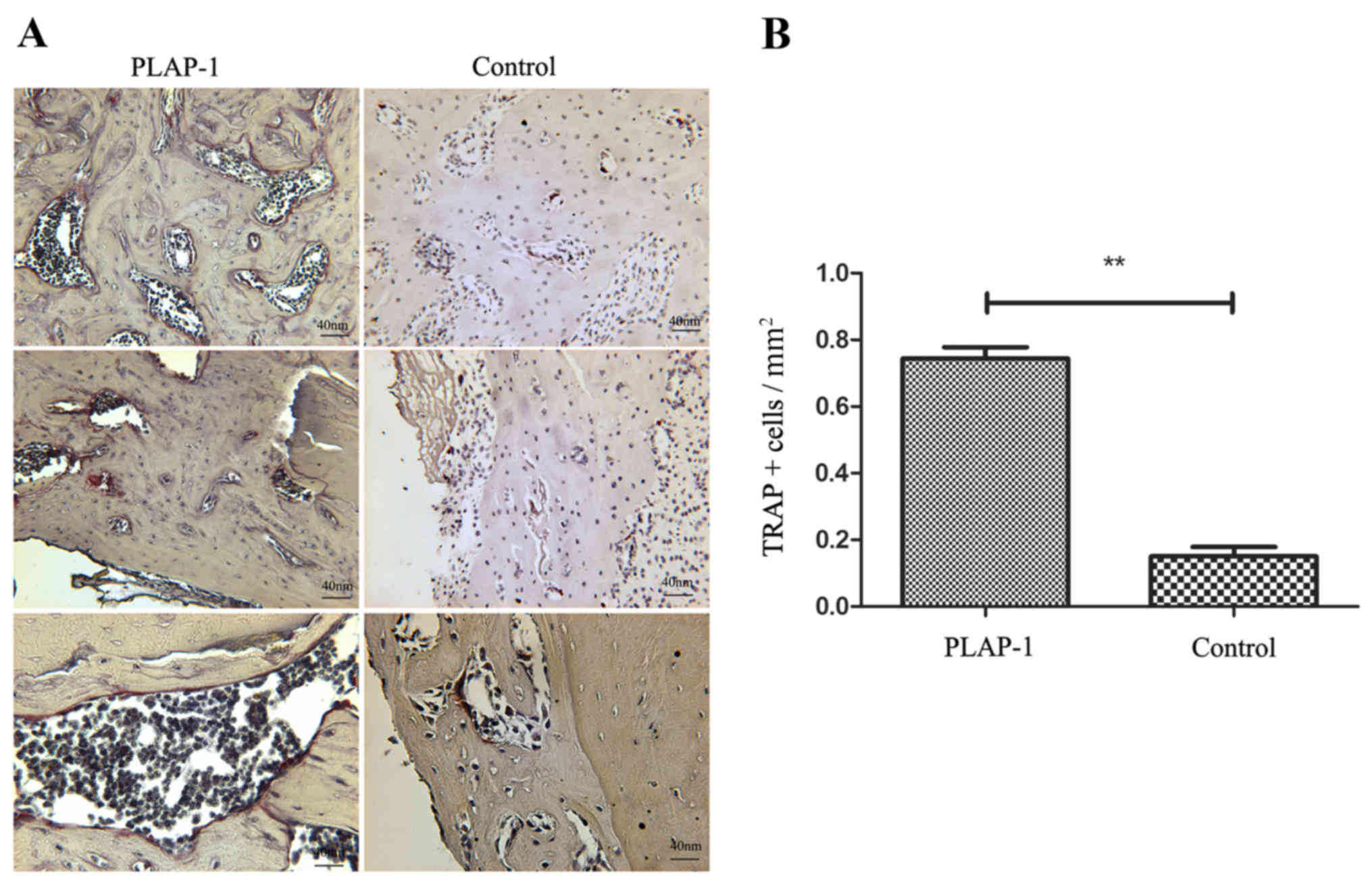
TRAP staining
TRAP staining of the periodontal defects was applied
at 6 weeks. TRAP is highly expressed by osteoclasts, which reflects
osteoclast activity. A number of TRAP+ multi-nucleated
cells were found in the PLAP-1 group. By contrast, TRAP+
cells were hardly detectable at 6 weeks in the control group
(Fig. 8A). The number of
TRAP+ cells in the periodontal defects of the PLAP-1
group was significantly higher than that in the control group
(P<0.01) (Fig. 8B).
Discussion
It is widely known that clinical periodontal tissue
regeneration in patients with serious periodontitis is difficult to
achieve (29). Besides the
conventional approach of anti-inflammatory therapy, dental tissue
engineering has been used to obtain periodontal tissue
regeneration. Seeding cells, including DFSCs, PDLSCs and BMSCs,
have been incorporated into the repair of periodontal bone defects
(30). BMSCs have a multilineage
differentiation potential, highly proliferative capacity and the
ability to differentiate into several cell lineages, including
muscle, bone, cartilage, epithelium, fat and neural progenitors
(31,32). BMSCs have been applied in studies
associated with osteoblast differentiation and bone regeneration.
In the present study, rBMSCs were applied as a cellular model to
investigate the functions of PLAP-1 in osteoblast differentiation
and osteoclast activation.
According to histological observations and micro-CT
examinations, PLAP-1 inhibited rat periodontal defect repair. The
formation and mineralization of new bone was less prominent in the
PLAP-1 group. The suppression of PLAP-1 would therefore be useful
for periodontal bone formation and regeneration. Hence, a better
understanding of the cellular and molecular mechanisms behind the
function of PLAP-1 is vital.
Osteoblast markers are well documented to primarily
include ALP, BSP, Runx2, Osx and OC. By observing the levels of
osteoblast markers, osteoblast differentiation from rBMSCs can be
speculated upon. The present RT-PCR and western blotting results
showed that PLAP-1 reduced the expression levels of these markers
at 4 weeks post-surgery compared with that in the vector and normal
groups; the osteogenic differentiation of rBMSCs was restrained
in vivo. The same effects in osteoblast differentiation have
been reported (19). PLAP-1
regulates periodontal ligament cell cytodifferentiation and
mineralization through BMP-2 activity. PLAP-1 inhibits the effect
of BMP by binding to BMP receptor, which indicates that PLAP-1
forms part of the negative feedback mechanism of BMP-2 (33).
Osteoclast formation, activation and survival is
regulated in normal bone modeling and remodeling by RANKL/RANK
signaling. Osteoclast number and activity can increase if there is
a change in the RANKL/OPG ratio (34). During the progression of rat
periodontal bone defect repair, osteoclast precursors are attracted
from the invading blood vessels close to newly formed bone
trabeculae. Multinucleated osteoclasts are formed by the fusion of
these precursors with each other, and the osteoclasts then resorb
the majority of the newly formed bone, leaving only a limited
number of trabeculae. The osteoblasts lay down new bone on certain
surface regions of the surviving trabeculae where there had
previously been osteoclastic resorption and a great deal of this
new bone is then resorbed by osteoclasts in a remodeling process
(35,36).
As aforementioned, there was no significant
difference between the vector and control groups in the present
study. So only the PLAP-1 and control groups were included in the
following experiments. RANKL and OPG expression in bone defect
tissues was detected at 2, 4 and 6 weeks during rat periodontal
bone defect repair. Higher RANKL/OPG ratio expression was observed
in the PLAP-1 group, that is, the PLAP-1 gene in the BMSCs led to
an increase in the RANKL/OPG ratio, which was further confirmed by
TRAP staining. A greater number of TRAP+ cells was
observed in the PLAP-1 group than in the control group even at the
late stage of defect repair. An extensive bone remodeling process
was observed in the PLAP-1 group. Overexpression of PLAP-1 promoted
osteoclast activation dependent on the upregulated RANKL/OPG
ratio.
Taken together, the present results showed that
PLAP-1 suppressed the differentiation of rBMSCs into osteoblasts
and promoted osteoclast activation in the rat periodontal bone
defects model. PLAP-1 exhibited positive effects on bone remodeling
by promoting osteoclastogenesis and reducing osteoblast
differentiation, leading to an inhibited repair effect.
The molecular mechanism of PLAP-1 in osteoblast
differentiation, osteoclastogenesis and bone remolding require
further investigation to promote bone functional regeneration. It
is of great importance to reveal the negative feedback regulation
between PLAP-1 and positive growth factors in mineralized tissues
under physiological and pathological conditions.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81271138), the Open
Foundation of Shandong Provincial Key Laboratory of Oral
Biomedicine (grant no. SDKQ201403) and the Shandong Province
Natural Science Foundation (grant no. ZR2015PH017).
References
|
1
|
Nakajima M, Kizawa H, Saitoh M, Kou I,
Miyazono K and Ikegawa S: Mechanisms for asporin function and
regulation in articular cartilage. J Biol Chem. 282:32185–32192.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ueda M, Goto T, Kuroishi KN, Gunjigake KK,
Ikeda E, Kataoka S, Nakatomi M, Toyono T, Seta Y and Kawamoto T:
Asporin in compressed periodontal ligament cells inhibits bone
formation. Arch Oral Biol. 62:86–92. 2016. View Article : Google Scholar
|
|
3
|
Yamada S, Ozawa Y, Tomoeda M, Matoba R,
Matsubara K and Murakami S: Regulation of PLAP-1 expression in
periodontal ligament cells. J Dent Res. 85:447–451. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamada S, Kitamura M and Murakami S:
PLAP-1: a novel molecule regulating homeostasis of periodontal
tissues. Jpn Dent Sci Rev. 44:137–144. 2008. View Article : Google Scholar
|
|
5
|
Chen YC, Ninomiya T, Hosoya A, Hiraga T,
Miyazawa H and Nakamura H: 1α,25-Dihydroxyvitamin D3 inhibits
osteoblastic differentiation of mouse periodontal fibroblasts. Arch
Oral Biol. 57:453–459. 2012. View Article : Google Scholar
|
|
6
|
Kajikawa T, Yamada S, Tauchi T, Awata T,
Yamaba S, Fujihara C and Murakami S: Inhibitory effects of
PLAP-1/asporin on periodontal ligament cells. J Dent Res.
93:400–405. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sowmya S, Chennazhi KP, Arzate H,
Jayachandran P, Nair SV and Jayakumar R: Periodontal specific
differentiation of dental follicle stem cells into osteoblast,
fibroblast, and cementoblast. Tissue Eng Part C Methods.
21:1044–1058. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ivanovski S, Vaquette C, Gronthos S,
Hutmacher DW and Bartold PM: Multiphasic scaffolds for periodontal
tissue engineering. J Dent Res. 93:1212–1221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li S, Tu Q, Zhang J, Stein G, Lian J, Yang
PS and Chen J: Systemically transplanted bone marrow stromal cells
contributing to bone tissue regeneration. J Cell Physiol.
215:204–209. 2008. View Article : Google Scholar
|
|
10
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kramer PR, Nares S, Kramer SF, Grogan D
and Kaiser M: Mesenchymal stem cells acquire characteristics of
cells in the periodontal ligament in vitro. J Dent Res. 83:27–34.
2004. View Article : Google Scholar
|
|
12
|
Nivedhitha Sundaram M, Sowmya S, Deepthi
S, Bumgardener JD and Jayakumar R: Bilayered construct for
simultaneous regeneration of alveolar bone and periodontal
ligament. J Biomed Mater Res B Appl Biomater. 104:761–770. 2016.
View Article : Google Scholar
|
|
13
|
Ohyama Y, Nifuji A, Maeda Y, Amagasa T and
Noda M: Spacio-temporal association and bone morphogenetic protein
regulation of sclerostin and osterix expression during embryonic
osteogenesis. Endocrinology. 145:4685–4692. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Parker AM and Katz AJ: Adipose-derived
stem cells for the regeneration of damaged tissues. Expert Opin
Biol Ther. 6:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tu Q, Zhang J, Paz J, Wade K, Yang P and
Chen J: Haplo-insufficiency of Runx2 results in bone formation
decrease and different BSP expression pattern changes in two
transgenic mouse models. Cell Physiol. 217:40–47. 2008. View Article : Google Scholar
|
|
16
|
Ikegawa S: Expression, regulation and
function of asporin, a susceptibility gene in common bone and joint
diseases. Curr Med Chem. 15:724–728. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kou I, Nakajima M and Ikegawa S:
Expression and regulation of the osteoarthritis-associated protein
asporin. J Biol Chem. 282:32193–32199. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu X, Sun J, Hu Y, Gao Y, Xiao C, Liu S
and Li S: Overexpression of PLAP-1 in bone marrow stromal cells
inhibits the rat critical-size skull defect repair. J Mol Histol.
46:251–261. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun J, Zhang T, Zhang P, Lv L, Wang Y,
Zhang J and Li S: Overexpression of the PLAP-1 gene inhibits the
differentiation of BMSCs into osteoblast-like cells. J Mol Histol.
45:599–608. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boyce BF and Xing L: Functions of
RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem
Biophys. 473:139–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu X, Lv L, Zhang J, Zhang T, Xiao C and
Li S: Expression of neuropeptides and bone remodeling-related
factors during periodontal tissue regeneration in denervated rats.
J Mol Histol. 46:195–203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Theoleyre S, Wittrant Y, Tat SK, Fortun Y,
Redini F and Heymann D: The molecular triad OPG/RANK/RANKL:
Involvement in the orchestration of pathophysiological bone
remodeling. Cytokine Growth Factor Rev. 15:457–475. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tu Q, Valverde P and Chen J: Osterix
enhances proliferation and osteogenic potential of bone marrow
stromal cells. Biochem Biophys Res Commun. 341:1257–1265. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tomoeda M, Yamada S, Shirai H, Ozawa Y,
Yanagita M and Murakami S: PLAP-1/asporin inhibits activation of
BMP receptor via its leucine-rich repeat motif. Biochem Biophys Res
Commun. 371:191–196. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Awata T, Yamada S, Tsushima K, Sakashita
H, Yamaba S, Kajikawa T, Yamashita M, Takedachi M, Yanagita M,
Kitamura M, et al: PLAP-1/asporin positively regulates FGF-2
activity. J Dent Res. 94:1417–1424. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lv L, Wang Y, Zhang J, Zhang T and Li S:
Healing of periodontal defects and calcitonin gene related peptide
expression following inferior alveolar nerve transection in rats. J
Mol Histol. 45:311–320. 2014. View Article : Google Scholar
|
|
27
|
Zhang PP, Li S, Yang PS and Sun J:
Construction and confirmation of a recombinant eukaryotic
expression plasmid pBABe-hygro-PLAP-1. Shanghai Kou Qiang Yi Xue.
19:635–640. 2010.In Chinese.
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta DeltaC(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Yamaba S, Yamada S, Kajikawa T, Awata T,
Sakashita H, Tsushima K, Fujihara C, Yanagita M and Murakami S:
PLAP-1/asporin regulates TLR2- and TLR4-induced inflammatory
responses. J Dent Res. 94:1706–1714. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liao W, Okada M, Sakamoto F, Okita N,
Inami K, Nishiura A, Hashimoto Y and Matsumoto N: In vitro human
periodontal ligament-like tissue formation with porous
poly-L-lactide matrix. Mater Sci Eng C. 33:3273–3280. 2013.
View Article : Google Scholar
|
|
31
|
Yamachika E, Tsujigiwa H, Matsubara M,
Hirata Y, Kita K, Takabatake K, Mizukawa N, Kaneda Y, Nagatsuka H
and Iida S: Basic fibroblast growth factor supports expansion of
mouse compact bone-derived mesenchymal stem cells (MSCs) and
regeneration of bone from MSC in vivo. J Mol Histol. 43:223–233.
2012. View Article : Google Scholar
|
|
32
|
Wu B, Ma X, Zhu D, Liu Y, Sun Z, Liu S,
Xue B, Du M and Yin X: Lentiviral delivery of biglycan promotes
proliferation and increases osteogenic potential of bone
marrow-derived mesenchymal stem cells in vitro. J Mol Histol.
44:423–431. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamada S, Tomoeda M, Ozawa Y, Yoneda S,
Terashima Y, Ikezawa K, Ikegawa S, Saito M, Toyosawa S and Murakami
S: PLAP-1/asporin, a novel negative regulator of periodontal
ligament mineralization. J Biol Chem. 282:23070–23080. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu XJ, Xiao CJ, Du YM, Liu S, Du Y and Li
S: Effect of hypoxia on the expression of RANKL/OPG in human
periodontal ligament cells in vitro. Int J Clin Exp Pathol.
8:12929–12935. 2015.
|
|
35
|
Yu X, Botchwey EA, Levine EM, Pollack SR
and Laurencin CT: Bioreactor-based bone tissue engineering: the
influence of dynamic flow on osteoblast phenotypic expression and
matrix mineralization. Proc Natl Acad Sci USA. 101:11203–11208.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li M, Hasegawa T, Hogo H, Tatsumi S, Liu
Z, Guo Y, Sasaki M, Tabata C, Yamamoto T, Ikeda K, et al:
Histological examination on osteoblastic activities in the alveolar
bone of transgenic mice with induced ablation of osteocytes. Histol
Histopathol. 28:327–335. 2013.PubMed/NCBI
|