Introduction
Retinal ganglion cells (RGCs) are important neurons
in the retina that transmit visual signals from the retina to the
brain (1,2). RGCs are particularly susceptible to
acute, transient and mild systemic hypoxic stress that induces
their apoptosis or necrosis (3).
The loss of RGCs in a number of ocular pathologies, such as retinal
detachment, leads to irreversible visual injury (1,2).
Hypoxia is the critical pathological factor for several optic
neuropathies, and hypoxia-induced apoptosis of RGCs results in
progressive vision loss (4–6).
Detachment of the retina causes retinal ischemia/reperfusion and
hypoxia, resulting in apoptosis of RGCs (7), which is irreversible; thus,
protecting RGCs against hypoxia-evoked cell apoptosis is crucial
for treating hypoxia-induced retinal diseases.
The Notch signaling pathway plays an important role
in various cellular processes, including cell proliferation and
apoptosis, and is involved in numerous pathological processes
(8,9). There are four Notch receptors
(Notch1-4) and five Notch ligands, including Jagged1, Jagged2,
Delta-like 1 (Dll1), Dll3 and Dll4 (10). The binding of Notch receptors and
ligands results in cleavage of the Notch intracellular domain
(NICD) that translocates into the nucleus to activate the
transcription of Notch target genes, such as Hes1 and Hey1
(11,12). The Notch signaling pathway plays
an important role in the development of the liver, kidney, eye,
heart and skeleton (13). Notch
signaling has been implicated in the regulation of an increasing
number of stem cells in several different tissues (14). Aberrant activation of the Notch
signaling pathway has been observed in various cancers (15). The Notch signaling pathway is
extensively involved in regulating cell proliferation, apoptosis
and differentiation through crosstalk with other signaling pathways
(16). Recent studies indicated
that Notch signaling participates in several hypoxia-induced
pathological processes (17,18). Notch signaling has also been shown
to protect the myocardium (19),
liver (20) and cerebrum
(21) from ischemia/reperfusion-
and hypoxia-induced injury. However, the role of Notch signaling in
hypoxia-induced cell apoptosis of RGCs has not been extensively
investigated.
A group of small non-coding RNAs, referred to as
microRNAs (miRNAs), have drawn significant attention over the past
few decades, due to their negative regulatory effects on gene
expression. miRNAs consist of ~22 nucleotides and suppress gene
expression by targeting the 3′-untranslated region (3′-UTR),
leading to translation inhibition (22–24). Thus, miRNAs are involved in the
regulation of a wide range of cellular processes, such as stress
response, cell survival and apoptosis (22). Several studies have demonstrated
that targeting specific miRNAs may be a novel strategy for
inhibiting apoptosis of RGCs (25–27). However, the precise mechanisms of
miRNAs in regulating RGC apoptosis have not been fully
elucidated.
miR-137 is a neuron-associated miR that is
abundantly expressed in the brain and regulates neural
differentiation and maturation (28–30). Recently, miR-137 was reported to
be a hypoxia-responsive miRNA in mouse brain cells exposed to
hypoxic conditions (31).
However, whether miR-137 plays a role in RGCs exposed to hypoxia
remains unknown. In the present study, the role of miR-137 and the
involvement of Notch signaling in hypoxia-induced apoptosis of RGCs
was investigated.
Materials and methods
Cell culture
The rat retinal ganglion cell line RGC-5 and human
embryonic kidney 293 (HEK-293) cells were obtained from the
American Type Culture Collection (Manassas, VA, USA) and were
cultured in Dulbecco's modified Eagle's medium (DMEM) containing
10% fetal calf serum (both from Gibco-BRL; Thermo Fisher
Scientific, Rockville, MD, USA) and 1/100 streptomycin/penicillin
(Sigma-Aldrich; Merck KGaA, St. Louis, MO, USA). Cells were
routinely maintained in a culture chamber containing 5%
CO2 at 37°C.
Hypoxia treatment
Cells were cultured in a multi-gas incubator (Thermo
Fisher Scientific, Shanghai, China) containing 5% O2,
90% N2 and 5% CO2, and incubated at 37°C.
After washing twice with deoxygenated serum-free DMEM, cells from
each treatment were incubated in the hypoxic incubator for 24, 48
and 72 h. Cells cultured under normoxic conditions were used as
control.
Quantitative polymerase chain reaction
(qPCR) analysis
Briefly, total RNA was extracted using the miRNeasy
mini kit (Qiagen, Dusseldorf, Germany) according to the recommended
protocols. cDNA was synthesized using M-MLV reverse transcriptase
(BioTeke, Beijing, China) or miScript reverse transcription kit
(Qiagen). qPCR was performed using SYBR-Green PCR Master Mix (for
mRNA amplification) or TaqMan miRNA reverse transcription kit (for
miRNA amplification) (Applied Biosystems, Carlsbad, CA, USA). The
primers used were as follows: miR-137 forward,
5′-gcgcgcttattgcttaagaatac-3′ and reverse, 5′-gtgcagggtccgaggt-3;
U6 snRNA forward, 5′-ctcgcttcggcagcaca-3′ and reverse,
5′-aacgcttcacgaatttgcgt-3′; Notch1 forward,
5′-atgactgcccaggaaacaac-3′ and reverse, 5′-gtccagccattgacacacac-3′;
Hes-1 forward, 5′-agccaactgaaaacacctgatt-3′ and reverse,
5′-ggactttatgattagcagtgg-3′; Hey1 forward,
5′-ccgcttcgtgttcgcctggt-3′ and reverse, 5′-tgctgcctgtgagggtgtcg-3′;
and β-actin forward, 5′-gtggggcgccccaggcacca-3′ and reverse,
5′-cttccttaatgtcacgcacgatttc-3′. β-actin (for mRNA) and U6 snRNA
(for miRNA) were used as internal control genes. Relative gene
expression was calculated using the 2−ΔΔCq method
(32).
Cell transfection
The miR-137 mimics
(5′-uuauugcuuaagaauacgcguagtt-3′), miR-137 inhibitor (anti-miR-137,
5′-cuacgcguauucuuaagcaauaa-3′ with 2′-O-methyl modification) and
their negative controls (miR-NC or anti-miR-NC) were synthesized by
GenePharma (Shanghai, China). The miRNAs and Notch1 siRNA (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) were transfected
into cells using Lipofectamine 2000 reagent (Invitrogen, Carlsbad,
CA, USA) according to the manufacture's instruction. The
transfections were incubated for 24 h and then subjected to hypoxic
conditions for another 24 h.
Cell viability
The
3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and lactate dehydrogenase (LDH) assays were used to assess cell
viability. For the MTT assay, cells were seeded into 96-well plates
(5×103 cells/well) overnight. Thereafter, the cells were
transfected with miR-137 mimics, anti-miR-137 or Notch1 siRNA for
24 h under normoxic conditions, followed by 24 h under hypoxic
conditions. Following incubation, 20 µl of MTT stock
solution (Sigma-Aldrich; Merck KGaA) were added to each well and
incubated for 4 h. The medium was discarded and the formazan
crystals were dissolved by addition of dimethyl sulfoxide (200
µl/well; Sigma-Aldrich; Merck KGaA). After gently shaking
for 15 min, the optical density (OD) value at 490 nm was measured
using a microplate reader (Bio-Tek Instruments; Winooski, VT, USA).
The LDH assay was performed using a cytotoxicity LDH assay kit
(BioVision, Milpitas, CA, USA). Briefly, cells were seeded into
96-well plates with treatments as described above. Subsequently,
0.2% Triton X-100 was added to lyse the cells, followed by
centrifugation at 250 × g for 2 min at 4°C. A total of 100
µl of the supernatant from each well were added to a new
96-well plate; then, 100 µl of working solution was added to
each well and incubated for 30 min in the dark. The reaction was
stopped by adding 50 µl of stop solution. The OD value at
490 nm was determined using a microplate reader. The lysis ratio
was calculated as follows: (Experimental release-spontaneous
release)/(maximum release-spontaneous release) ×100%.
Apoptosis assay
Cell apoptosis was detected with the caspase-3
activity assay using a commercial kit (BioVision). Briefly, after
treatment, cells were harvested, lysed in ice-cold cell lysis
buffer and centrifuged at 10,000 × g for 1 min. Protein
concentration was measured in the resultant supernatant.
Subsequently, 50 µl of cell lysis buffer was added to 100
µg protein and incubated with 50 µl of reaction
buffer and 5 µl of 4 mM DEVD-pNA substrate at 37°C for 2 h.
The OD value at 405 nm was determined using a microplate
reader.
Western blot analysis
Proteins were extracted from the cells using a
protein extraction kit (Applygen Technologies, Beijing, China). The
protein concentration was measured using the Bio-Rad Protein Assay
kit (Bio-Rad, Hercules, CA, USA). A total of 25 µg proteins
from different groups were separated by 12.5% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis. The proteins were then
transferred to a polyvinylidene difluoride (PVDF) membrane
(Millipore, Temecula, CA, USA). The membranes were then blocked by
3% non-fat milk at 37°C for 1 h. The membranes were incubated at
4°C overnight with primary antibodies. The membranes were washed
thrice with TBST and then incubated with goat anti-rabbit
horseradish peroxidase-labeled secondary antibodies (sc-2004;
1:5,000; Santa Cruz Biotechnology, Inc.) at 37°C for 1 h. The bands
were visualized using an enhanced chemiluminescence kit (Pierce,
Rockford, IL, USA) and intensity was quantitatively analyzed using
Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD,
USA). Rabbit polyclonal primary antibodies including anti-Notch1
(sc-9170; 1:200) anti-PTEN (sc-9145; 1:250), anti-Akt (sc-8312;
1:500), anti-pAkt (sc-135650; 1:200) and anti-β-actin (sc-7210;
1:500) were purchased from Santa Cruz Biotechnology, Inc. Rabbit
monoclonal anti-NICD (no. 3608; 1:1,000) was purchased from Cell
Signaling Technology (Danvers, MA, USA).
Dual-luciferase reporter assay
The 3′-UTR of Notch1, containing the miR-137 binding
site, was amplified and then cloned into pmirGLO Dual-Luciferase
miRNA Target Expression Vectors (Promega, Madison, WI, USA). For
reporter assays, cells were seeded into 24-well plates and
co-transfected with miR-137 mimics and pmirGLO-Notch1 3′-UTR
constructs using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific). After a 48-h incubation, cells were lysed and firefly
and Renilla luciferase activity was detected using the
Dual-Luciferase Assay system (Promega).
Statistical analysis
Quantitative data are presented as mean ± standard
deviation. Statistical analyses were performed by Student's t-test
or one-way analysis of variance using SPSS software, version 11.5
(SPSS, Inc., Chicago, IL, USA). A P-value <0.05 was defined as
statistically significant.
Results
miR-137 affects the survival of RGC-5
cells exposed to hypoxic conditions
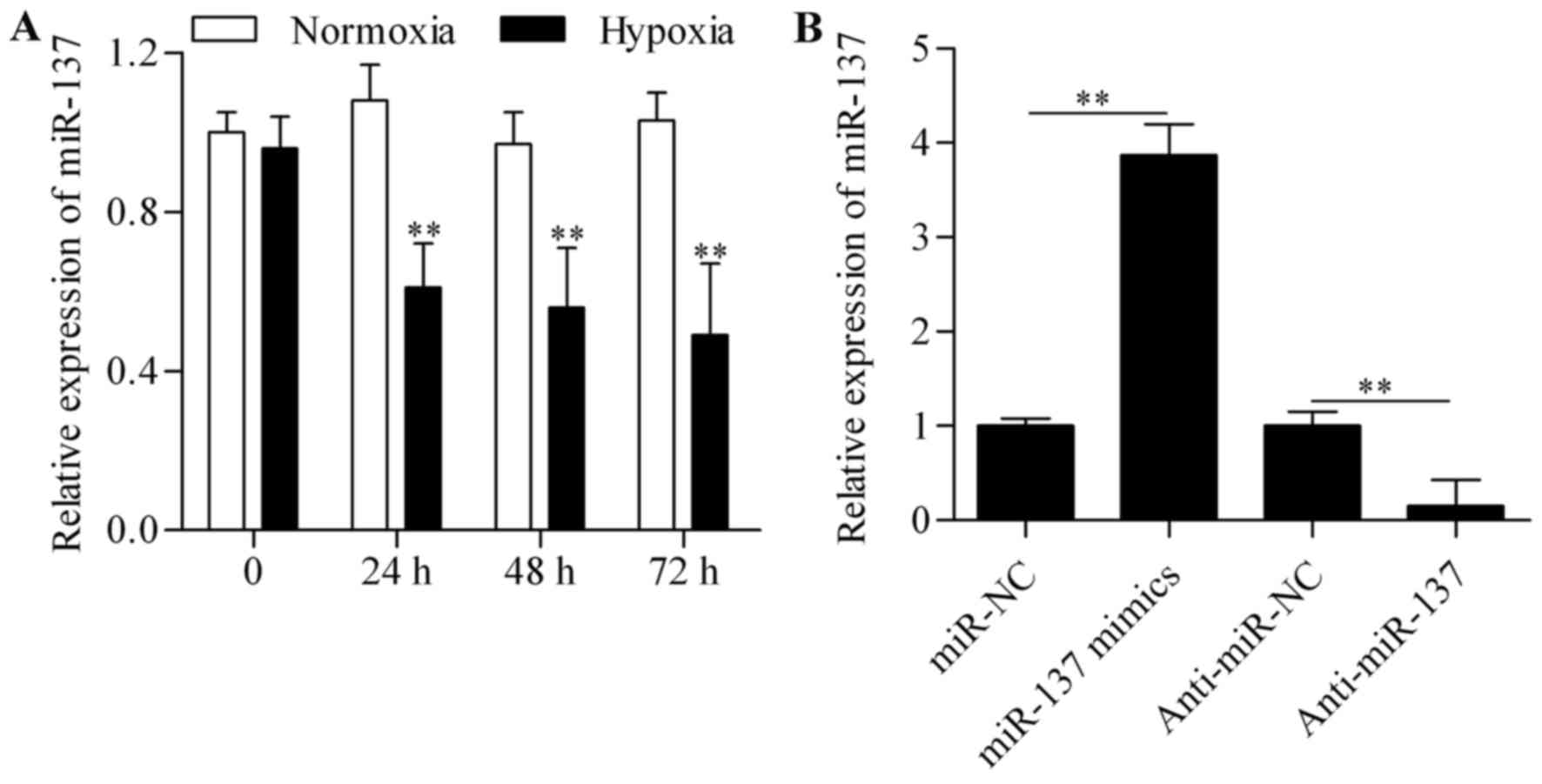
To investigate whether miR-137 is a
hypoxia-responsive miRNA in RGC-5 cells, the expression levels of
miR-137 were measured by qPCR. The results revealed that the
expression of miR-137 was significantly suppressed in RGC-5 cells
subjected to hypoxic conditions (Fig.
1A), indicating that miR-137 may play a role in RGC-5 cells
following hypoxia. To further investigate this role, gain- and
loss-of-function experiments were performed. RGC-5 cells were
transfected with miR-137 mimics or anti-miR-137 to overexpress or
silence miR-137, respectively. On qPCR analysis, miR-137 expression
was found to be markedly increased in cells transfected with
miR-137 mimics and significantly decreased by anti-miR-137
transfection (Fig. 1B). The
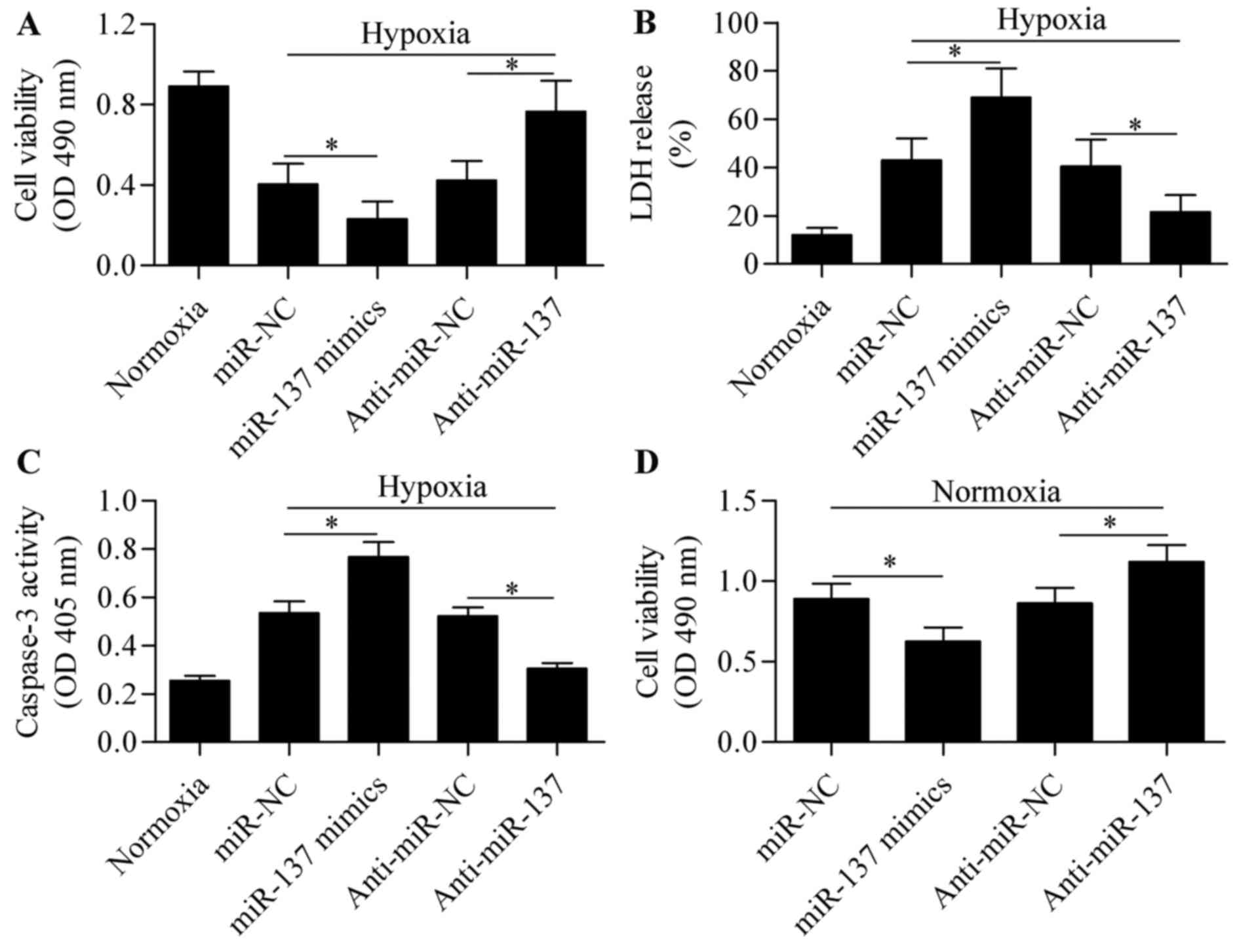
effect of miR-137 overexpression or silencing on hypoxia-induced
cell injury was next analyzed. Results from the MTT and LDH assays
revealed that miR-137 overexpression significantly aggravated
hypoxia-induced cell injury, whereas suppression of miR-137
markedly reversed the inhibitory effect of hypoxia on cell
viability (Fig. 2A and B). To
verify the role of miR-137 in RGC-5 cell survival following
hypoxia, hypoxia-induced apoptosis was analyzed using a caspase-3
assay. RGC-5 cells subjected to hypoxia exhibited higher level of
caspase-3 activity, indicating hypoxia-induced cell apoptosis
(Fig. 2C). As expected, the
caspase-3 activity was further upregulated by miR-137
overexpression, but was significantly downregulated by miR-137
silencing (Fig. 2C). In addition,
the role of miR-137 on cell viability of RGCs was further examined
under normal conditions. The results demonstrated that miR-137
overexpression significantly inhibited cell viability, while
miR-137 suppression promoted viability of RGCs (Fig. 2D). These results suggest that
suppression of miR-137 is conducive to sustaining viability of
RGC-5 cells under hypoxic conditions.
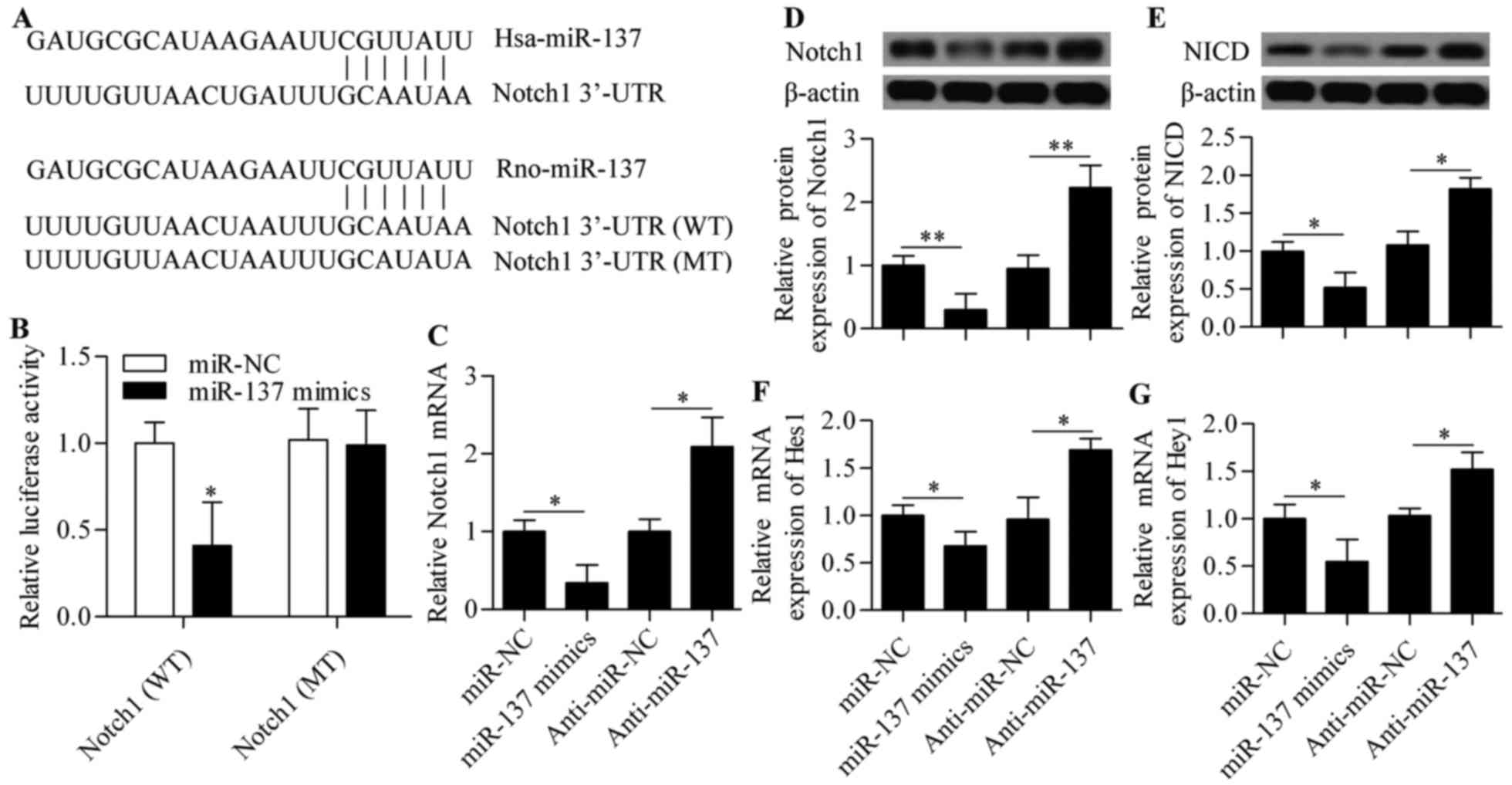
Notch1 is a direct target of miR-137
To elucidate the molecular mechanism of miR-137 in
regulating RGC-5 cell survival, bioinformatics analyses were
conducted to identify genes that contain putative target sites in
their 3′-UTR sequences. Interestingly, Notch1 was found to be a
potential target of miR-137 and the binding sites were conserved
from human to rats (Fig. 3A). To
validate this prediction, the direct interaction between miR-137
and Notch1 3′-UTR was examined using a dual-luciferase reporter
assay. The results revealed that miR-137 mimics significantly
decreased the luciferase activity of the pmirGLO reporter vector
containing the wild-type 3′-UTR of Notch1 (WT) (Fig. 3B). This inhibitory effect was not
observed in the pmirGLO reporter vector carrying Notch1 with
mutated 3′-UTR target sites (MT) (Fig. 3B), confirming the specificity of
miR-137 for Notch.
The effects of miR-137 on the Notch signaling
pathway were detected by qPCR and western blot analysis.
Overexpression of miR-137 resulted in a significant decrease of
Notch1 mRNA and protein expression, whereas anti-miR-137
transfection caused a marked increase of Notch1 mRNA and protein
expression (Fig. 3C and D).
Subsequently, the expression levels of NICD, Hes1 and Hey1 were
also affected, in that the protein expression of NICD and the mRNA
expression of Hes1 and Hey1 were significantly decreased by miR-137
overexpression and increased by miR-137 inhibition (Fig. 3E–G). These results indicate that
miR-137 regulates the Notch signaling pathway by targeting
Notch1.
miR-137 regulates hypoxia-induced
apoptosis through Notch1
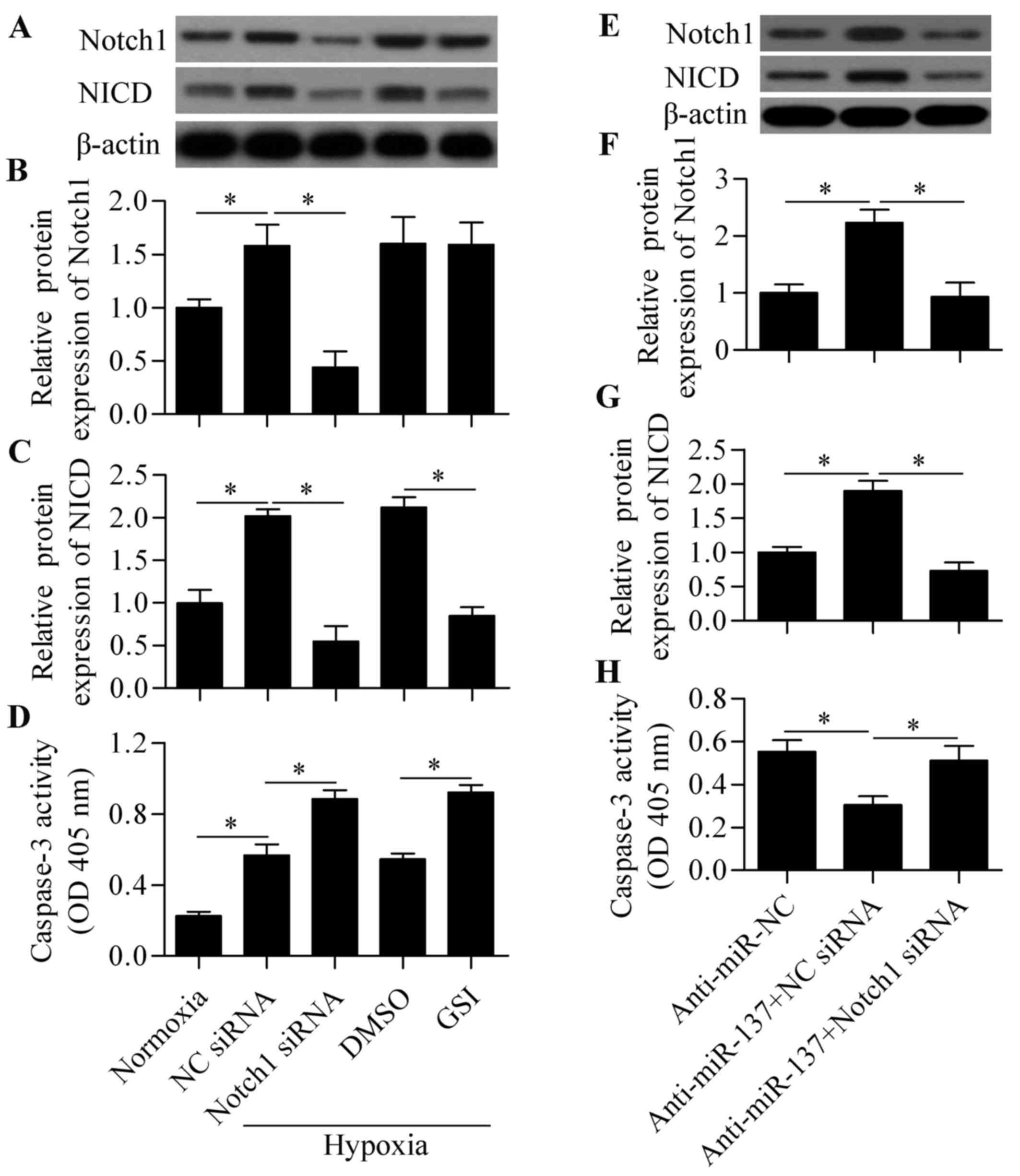
To verify whether the Notch1-mediated signaling
pathway was involved in response to hypoxia in RGC-5 cells, the
effect of blocking Notch signaling on cell survival under hypoxia
was investigated. Compared with normoxia, hypoxic conditions
significantly induced the expression of Notch1 and NICD expression
(Fig. 4A–C), indicating that
hypoxia activated Notch signaling in RGC-5 cells. Next, Notch
signaling was blocked by Notch1 siRNA or γ-secretase inhibitors
(GSI) to investigate the role of Notch signaling in hypoxia-induced
cell apoptosis. The results revealed that both Notch siRNA and GSI
treatment significantly decreased the expression of NICD,
indicating a suppressive effect on Notch signaling (Fig. 4A–C). We also demonstrated that
blocking Notch signaling significantly aggravated cell apoptosis
induced by hypoxia (Fig. 4D).
These results indicated that the Notch signaling pathway plays an
important role in regulating RGC-5 cell survival in response to
hypoxia. To investigate whether miR-137 acts through Notch1, the
effect of Notch1 siRNA on anti-miR-137-mediated cell survival was
determined. The results demonstrated that knockdown of Notch1
significantly reversed the protective effect of anti-miR-137 on
hypoxia-induced cell apoptosis (Fig.
4E–H). Taken together, these results indicate that miR-137
regulates hypoxia-induced apoptosis in RGC-5 cells through
Notch1.
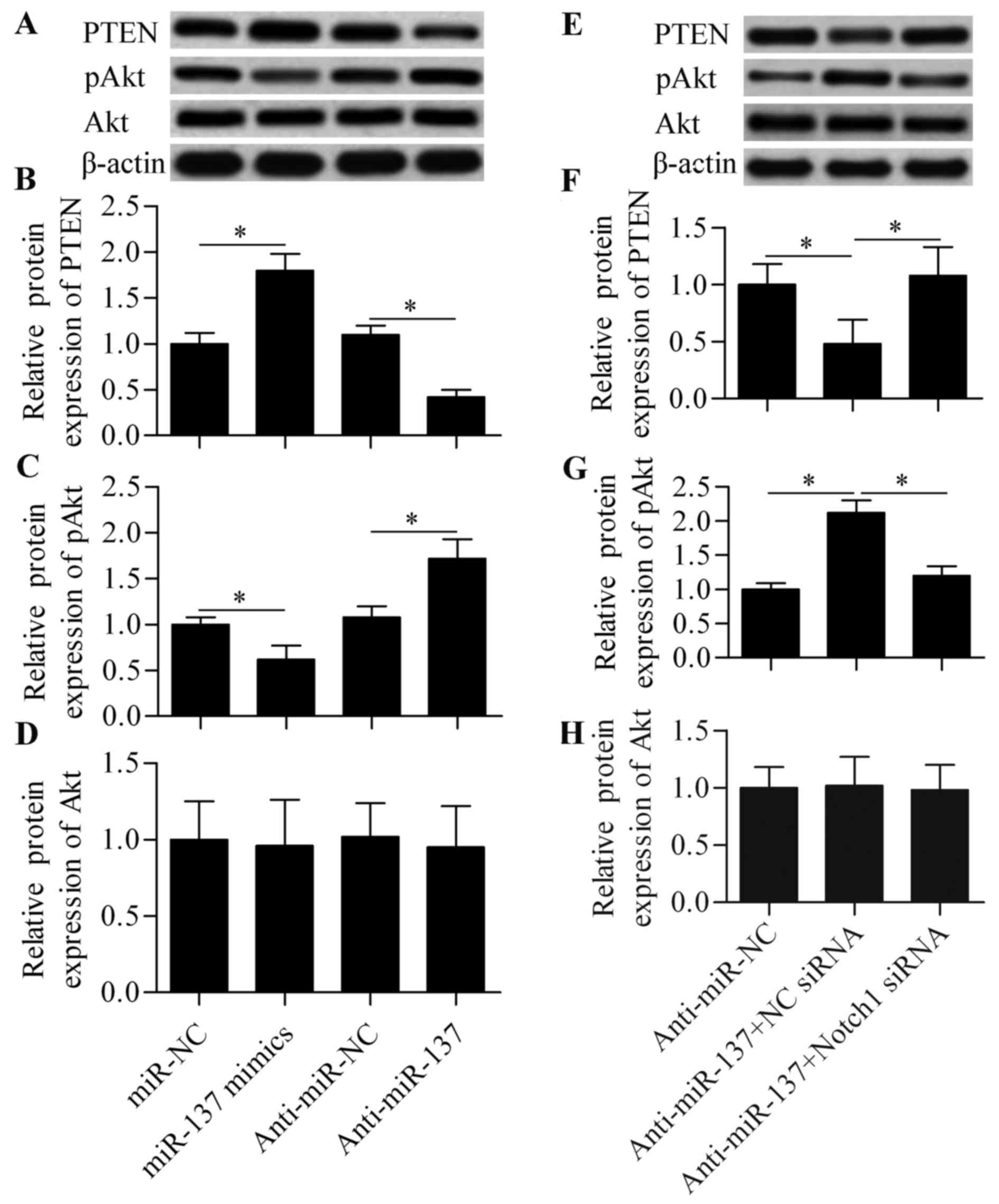
PTEN/Akt survival signaling is involved
in miR-137-mediated Notch signaling
To further investigate the molecular mechanism by
which miR-137 regulates cell survival in response to hypoxia, the
effect of miR-137 on PTEN/Akt survival signaling was analyzed. Our
results demonstrated that miR-137 overexpression significantly
increased the expression of PTEN and decreased the expression of
pAkt, whereas anti-miR-137 exerted the opposite effects (Fig. 5A–C). Moreover, the effect of
anti-miR-137 was significantly blocked by Notch1 siRNA (Fig. 5E–G). Conversely, these treatments
exerted no obvious effect on total Akt expression (Fig. 5D and H). Based on these results,
it may be concluded that miR-137 regulates PTEN/Akt survival
signaling through Notch1-mediated signaling. Downregulation of
miR-137 increases Noth1 expression and activates the expression of
Hes1, a negative regulator of PTEN. Then, Hes1 binds to the PTEN
promoter and inhibits PTEN expression (33), thus leading to the upregulation of
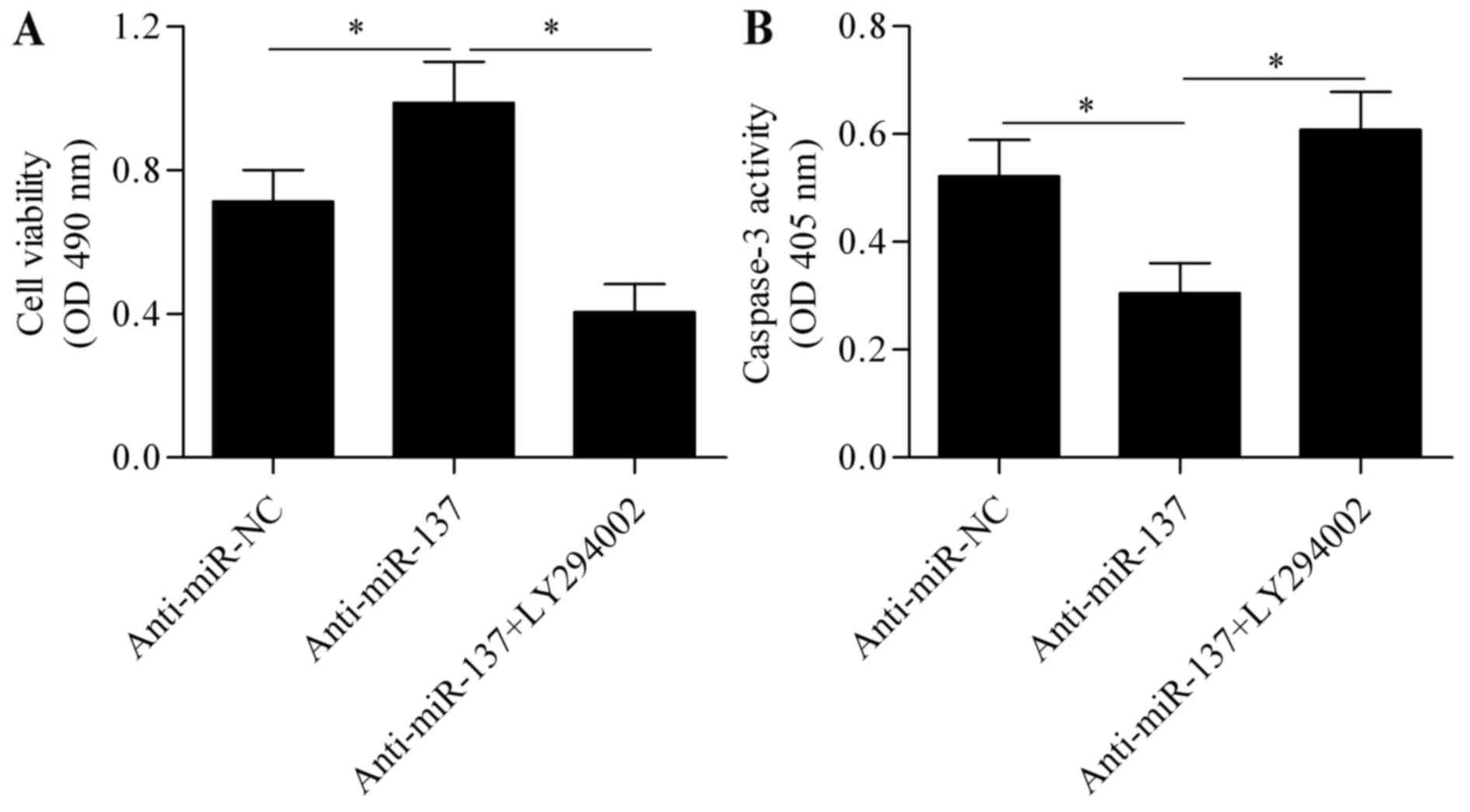
pAkt. To further verify that Akt signaling is involved in the
mechanism underlying miR-137 regulation of hypoxia-induced
apoptosis, Akt signaling was blocked using the specific inhibitor
LY294002 (Beyotime Institute of Biotechnology, Haimen, China),
followed by assessment of the effect of anti-miR-137 on cell
apoptosis. The results revealed that the protective effect of
anti-miR-137 on hypoxia-induced cell injury was significantly
abrogated by blocking Akt signaling (Fig. 6A and B). Taken together, these
results indicate that PTEN/Akt survival signaling is involved in
miR-137-mediated Notch signaling.
Discussion
The present study demonstrated that miR-137 is
significantly decreased in RGCs under hypoxic conditions,
suggesting that miR-137 is a hypoxia-responsive miR. The
downregulation of miR-137 may protect RGCs against hypoxia-induced
apoptosis, whereas overexpression of miR-137 may aggravate cell
apoptosis. These results suggest that miR-137 may serve as a
potential target for inhibiting RGC apoptosis. Notch1 was
identified as a novel target gene of miR-137. The underlying
mechanism was associated with Notch signaling, which was shown to
participate in hypoxia-induced cell apoptosis. These data indicate
that miR-137 may serve as a promising molecular target for the
treatment of optic neuropathy.
Various studies have demonstrated that miR-137
induces cell apoptosis and inhibits cell proliferation in a number
of cancer cells (34–36). The overexpression of miR-137
significantly improves the inhibition rate and apoptosis rate in
multiple myeloma cells treated with dexamethasone (37). Downregulation of miR-137 inhibits
oxidative stress-induced cardiomyocyte apoptosis (38). Similarly, downregulation of
miR-137 may protect cells against hypoxia-induced apoptosis
(31). These findings suggest
that miR-137 is a positive regulator of cell apoptosis and may
function as a critical regulator in various pathological processes.
The role of miR-137 in neurons has been widely investigated.
miR-137 is expressed in the brain and regulates neural stem cell
proliferation and differentiation (28), as well as neuronal maturation
(30). Downregulation of miR-137
has also been found in Alzheimer's disease (39). However, whether miR-137 is
involved in regulating RGC apoptosis is currently unknown. We
herein demonstrated that miR-137 was downregulated in RGCs under
hypoxic conditions, and suppression of miR-137 was able to protect
RGCs against hypoxia-induced apoptosis. Our results further support
the hypothesis that miR-137 is a hypoxia-responsive miR. Li et
al (31) demonstrated that
the downregulation of miR-137 under hypoxic conditions increased
the expression of mitophagy receptors FUNDC1 and NIX, protecting
cells from hypoxia-induced apoptosis by activating mitophagy
(31). Therefore, the
downregulation of miR-137 in response to hypoxia may be a
self-protective mechanism of cells adapting to these
conditions.
It was recently suggested that the Notch signaling
pathway is important in the response to extracellular stress,
particularly ischemic or hypoxic stresses. Yu and Song (40) reported that Notch1 signaling
suppressed hypoxia-induced cardiomyocyte apoptosis by inhibiting
the cell apoptosis pathway. Pei et al (41) demonstrated that Notch1 signaling
protected cardiomyocytes against ischemia/reperfusion injury by
reducing oxidative/nitrative stress. Ischemic preconditioning and
postconditioning activates Notch signaling, promoting
cardioprotection (42,43). Certain drugs, such as relaxin and
TNF-α inhibitor, protect cardiomyocytes from ischemic or hypoxic
damage by activating Notch signaling (44,45). Electroacupuncture pretreatment and
isoflurane preconditioning attenuated cerebral ischemia-reperfusion
injury through activation of the Notch signaling pathway (21,46). Furthermore, Notch signaling is
also involved in hepatic and intestinal ischemia/reperfusion
(20,47). Overall, these findings indicated
that Notch signaling activation was protective for cells against
ischemic or hypoxic conditions; however, the role of Notch
signaling in RGCs exposed to hypoxia remained unknown. In the
present study, we demonstrated that Notch signaling was activated
in response to hypoxia in RGCs and that blocking Notch signaling
with Notch1 siRNA or Notch inhibitor significantly aggravated cell
apoptosis induced by hypoxia. These findings confirm a prosurvival
signaling role for Notch in response to extracellular insults.
Of note, Notch1 has been found to be the target gene
of miR-137. Luciferase reporter assays demonstrated that miR-137
directly targets the 3′-UTR of Notch1. Overexpression of miR-137
inhibits Notch1 expression, whereas suppression of miR-137
increases expression and activates Notch signaling. Therefore, we
hypothesized that the downregulation of miR-137 by hypoxia may
contribute to activation of Notch signaling to protect cells. Our
data demonstrated that knockdown of Notch1 significantly blocked
the protective effect of anti-miR-137, suggesting that miR-137
acted through targeting Notch1. Therefore, targeting miR-137 is a
feasible method for modulating Notch signaling. Regulation of Notch
signaling by miRNAs is becoming more widely investigated. In fact,
several miRNAs, including miR-34a/c (48–50), miR-139-5p (51) and miR-200b (52), have been reported to be capable of
modulating Notch signaling by targeting Notch1. To the best of our
knowledge, this study is the first to report a direct asociation
between miR-137 and Notch signaling, providing a novel molecular
target for modulating Notch signaling.
Akt signaling is another important prosurvival
signaling pathway for survival in RGCs (53). In the present study, activation of
Notch signaling by downregulation of miR-137 was found to inhibit
the expression of PTEN, the suppressor of Akt signaling, and thus
increased the protein expression of pAkt. It has been demonstrated
that the target gene of Notch signaling, Hes1, may bind to the PTEN
promoter and inhibit PTEN expression (33). The activation of Akt signaling may
be a major mechanism through which Notch signaling exerts
prosurvival effect. In agreement with our findings, a recent study
reported that miR-137 inhibited the phosphorylation of AKT and
promoted cell apoptosis in response to dexamethasone in multiple
myeloma cells (37). In addition,
Cheng et al revealed that miR-137 functioned as a tumor
suppressor through targeting cyclooxygenase-2, which subsequently
suppressed the activation of the Akt signaling pathway in gastric
cancer cells in vitro and in vivo (54). In the present study, we found that
miR-137 regulated the Akt signaling pathway through modulating
Notch1. Of note, Li et al demonstrated that miR-137
regulated cell apoptosis through regulation of FUNDC1 and
NIX-mediated mitophagy (31).
However, all these findings support the hypothesis that miR-137 is
an important regulator of cell apoptosis. However, miR-137 may have
diverse function targets in different cell types in response to
different stimuli.
The role of miRNAs in regulating RGC apoptosis
remains poorly understood. Kong et al reported that
suppression of miR-100 protected RGCs against oxidative
stress-induced apoptosis by targeting the insulin-like growth
factor-1 receptor (IGF1R) (25).
Upregulation of miR-96 reduces RGC apoptosis by targeting caspase-2
(55). Suppression of miR-134 was
also found to protect RGCs against oxidative stress-induced
apoptosis (26). Most recently,
Kang et al (27) revealed
that overexpression of miR-26a protected RGCs against oxidative
stress-induced apoptosis by inhibiting PTEN and activating Akt
signaling. These studies indicated that miRNAs may be novel tools
for preventing RGC apoptosis. The present study demonstrated that
miR-137 regulates RGC apoptosis in response to hypoxia through
targeting Notch1 and Notch signaling, suggesting that a
miR-137/Notch1 axis has the potential to be used as a molecular
target for treatment of hypoxia-induced retinal diseases.
Glossary
Abbreviations
Abbreviations:
|
RGCs
|
retinal ganglion cells
|
|
miRNAs
|
microRNAs
|
|
NICD
|
Notch intracellular domain
|
|
3′-UTR
|
3′-untranslated region
|
|
GSI
|
γ-secretase inhibitors
|
Acknowledgments
The present study was supported by Hospital Fund
Project 2010A-2.
References
|
1
|
Isenmann S, Kretz A and Cellerino A:
Molecular determinants of retinal ganglion cell development,
survival, and regeneration. Prog Retin Eye Res. 22:483–543. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lo AC, Woo TT, Wong RL and Wong D:
Apoptosis and other cell death mechanisms after retinal detachment:
Implications for photoreceptor rescue. Ophthalmologica. 226(Suppl
1): 10–17. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kergoat H, Hérard ME and Lemay M: RGC
sensitivity to mild systemic hypoxia. Invest Ophthalmol Vis Sci.
47:5423–5427. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Osborne NN, Casson RJ, Wood JP, Chidlow G,
Graham M and Melena J: Retinal ischemia: Mechanisms of damage and
potential therapeutic strategies. Prog Retin Eye Res. 23:91–147.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen YN, Yamada H, Mao W, Matsuyama S,
Aihara M and Araie M: Hypoxia-induced retinal ganglion cell death
and the neuroprotective effects of beta-adrenergic antagonists.
Brain Res. 1148:28–37. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaur C, Foulds WS and Ling EA:
Hypoxia-ischemia and retinal ganglion cell damage. Clin Ophthalmol.
2:879–889. 2008. View Article : Google Scholar
|
|
7
|
Garweg JG, Tappeiner C and Halberstadt M:
Pathophysiology of proliferative vitreoretinopathy in retinal
detachment. Surv Ophthalmol. 58:321–329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Joutel A and Tournier-Lasserve E: Notch
signalling pathway and human diseases. Semin Cell Dev Biol.
9:619–625. 1998. View Article : Google Scholar
|
|
9
|
Marignol L, Rivera-Figueroa K, Lynch T and
Hollywood D: Hypoxia, notch signalling, and prostate cancer. Nat
Rev Urol. 10:405–413. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Samon JB, Champhekar A, Minter LM, Telfer
JC, Miele L, Fauq A, Das P, Golde TE and Osborne BA: Notch1 and
TGFbeta1 cooperatively regulate Foxp3 expression and the
maintenance of peripheral regulatory T cells. Blood. 112:1813–1821.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McCright B: Notch signaling in kidney
development. Curr Opin Nephrol Hypertens. 12:5–10. 2003. View Article : Google Scholar
|
|
12
|
Hayward P, Kalmar T and Arias AM:
Wnt/Notch signalling and information processing during development.
Development. 135:411–424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fiúza UM and Arias AM: Cell and molecular
biology of Notch. J Endocrinol. 194:459–474. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J, Sato C, Cerletti M and Wagers A:
Notch signaling in the regulation of stem cell self-renewal and
differentiation. Curr Top Dev Biol. 92:367–409. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan X, Wu H, Xu H, Xiong H, Chu Q, Yu S,
Wu GS and Wu K: Notch signaling: An emerging therapeutic target for
cancer treatment. Cancer Lett. 369:20–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hurlbut GD, Kankel MW, Lake RJ and
Artavanis-Tsakonas S: Crossing paths with Notch in the
hyper-network. Curr Opin Cell Biol. 19:166–175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pear WS and Simon MC: Lasting longer
without oxygen: The influence of hypoxia on Notch signaling. Cancer
Cell. 8:435–437. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Al Haj Zen A and Madeddu P: Notch
signalling in ischaemia-induced angiogenesis. Biochem Soc Trans.
37:1221–1227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu L, Li F, Zhao G, Yang Y, Jin Z, Zhai M,
Yu W, Zhao L, Chen W, Duan W, et al: Protective effect of berberine
against myocardial ischemia reperfusion injury: Role of
Notch1/Hes1-PTEN/Akt signaling. Apoptosis. 20:796–810. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu HC, Qin HY, He F, Wang L, Fu W, Liu D,
Guo FC, Liang L, Dou KF and Han H: Canonical notch pathway protects
hepatocytes from ischemia/reperfusion injury in mice by repressing
reactive oxygen species production through JAK2/STAT3 signaling.
Hepatology. 54:979–988. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang HP, Sun YY, Chen XM, Yuan LB, Su BX,
Ma R, Zhao RN, Dong HL and Xiong L: The neuroprotective effects of
isoflurane preconditioning in a murine transient global cerebral
ischemia-reperfusion model: The role of the Notch signaling
pathway. Neuromolecular Med. 16:191–204. 2014. View Article : Google Scholar
|
|
22
|
Mendell JT and Olson EN: MicroRNAs in
stress signaling and human disease. Cell. 148:1172–1187. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: microRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kong N, Lu X and Li B: Downregulation of
microRNA-100 protects apoptosis and promotes neuronal growth in
retinal ganglion cells. BMC Mol Biol. 15:252014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shao Y, Yu Y, Zhou Q, Li C, Yang L and Pei
CG: Inhibition of miR-134 protects against hydrogen
peroxide-induced apoptosis in retinal ganglion cells. J Mol
Neurosci. 56:461–471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kang Y, Jia P, Zhao H, Hu C and Yang X:
MicroRNA-26a overexpression protects RGC-5 cells against
H2O2-induced apoptosis. Biochem Biophys Res Commun. 460:164–169.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun G, Ye P, Murai K, Lang MF, Li S, Zhang
H, Li W, Fu C, Yin J, Wang A, et al: miR-137 forms a regulatory
loop with nuclear receptor TLX and LSD1 in neural stem cells. Nat
Commun. 2:5292011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen L, Wang X, Wang H, Li Y, Yan W, Han
L, Zhang K, Zhang J, Wang Y, Feng Y, et al: miR-137 is frequently
down-regulated in glioblastoma and is a negative regulator of
Cox-2. Eur J Cancer. 48:3104–3111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Smrt RD, Szulwach KE, Pfeiffer RL, Li X,
Guo W, Pathania M, Teng ZQ, Luo Y, Peng J, Bordey A, et al:
MicroRNA miR-137 regulates neuronal maturation by targeting
ubiquitin ligase mind bomb-1. Stem Cells. 28:1060–1070. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li W, Zhang X, Zhuang H, Chen HG, Chen Y,
Tian W, Wu W, Li Y, Wang S, Zhang L, et al: MicroRNA-137 is a novel
hypoxia-responsive microRNA that inhibits mitophagy via regulation
of two mitophagy receptors FUNDC1 and NIX. J Biol Chem.
289:10691–10701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Palomero T, Dominguez M and Ferrando AA:
The role of the PTEN/AKT Pathway in NOTCH1-induced leukemia. Cell
Cycle. 7:965–970. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang Y, Li F, Saha MN, Abdi J, Qiu L and
Chang H: miR-137 and miR-197 Induce Apoptosis and Suppress
Tumorigenicity by Targeting MCL-1 in Multiple Myeloma. Clin Cancer
Res. 21:2399–2411. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng X, Dong J, Gong T, Zhang Z, Wang Y,
Li Y, Shang Y, Li K, Ren G, Feng B, et al: MicroRNA library-based
functional screening identified miR-137 as a suppresser of gastric
cancer cell proliferation. J Cancer Res Clin Oncol. 141:785–795.
2015. View Article : Google Scholar
|
|
36
|
Deng D, Xue L, Shao N, Qu H, Wang Q, Wang
S, Xia X, Yang Y and Zhi F: miR-137 acts as a tumor suppressor in
astrocytoma by targeting RASGRF1. Tumour Biol. 37:3331–3340. 2016.
View Article : Google Scholar
|
|
37
|
Zhang B, Ma L, Wei J, Hu J, Zhao Z, Wang
Y, Chen Y and Zhao F: miR-137 suppresses the phosphorylation of AKT
and improves the dexamethasone sensitivity in multiple myeloma
cells via targeting MITF. Curr Cancer Drug Targets. 16:12016.
View Article : Google Scholar
|
|
38
|
Wang J, Xu R, Wu J and Li Z: MicroRNA-137
negatively regulates H2O2-induced
cardiomyocyte apoptosis through CDC42. Med Sci Monit. 21:3498–3504.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Geekiyanage H and Chan C:
MicroRNA-137/181c regulates serine palmitoyltransferase and in turn
amyloid β, novel targets in sporadic Alzheimer's disease. J
Neurosci. 31:14820–14830. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu B and Song B: Notch1 signalling
inhibits cardiomyocyte apoptosis in ischaemic postconditioning.
Heart Lung Circ. 23:152–158. 2014. View Article : Google Scholar
|
|
41
|
Pei H, Yu Q, Xue Q, Guo Y, Sun L, Hong Z,
Han H, Gao E, Qu Y and Tao L: Notch1 cardioprotection in myocardial
ischemia/reperfusion involves reduction of oxidative/nitrative
stress. Basic Res Cardiol. 108:3732013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou XL, Wan L, Xu QR, Zhao Y and Liu JC:
Notch signaling activation contributes to cardioprotection provided
by ischemic preconditioning and postconditioning. J Transl Med.
11:2512013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhou XL, Wan L and Liu JC: Activated
Notch1 reduces myocardial ischemia reperfusion injury in vitro
during ischemic postconditioning by crosstalk with the RISK
signaling pathway. Chin Med J (Engl). 126:4545–4551. 2013.
|
|
44
|
Boccalini G, Sassoli C, Formigli L, Bani D
and Nistri S: Relaxin protects cardiac muscle cells from
hypoxia/reoxygenation injury: Involvement of the Notch-1 pathway.
FASEB J. 29:239–249. 2015. View Article : Google Scholar
|
|
45
|
Pei H, Song X, Peng C, Tan Y, Li Y, Li X,
Ma S, Wang Q, Huang R, Yang D, et al: TNF-α inhibitor protects
against myocardial ischemia/reperfusion injury via Notch1-mediated
suppression of oxidative/nitrative stress. Free Radic Biol Med.
82:114–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhao Y, Chen X, Ma L, Zuo Z, Zhu Z, Zhu X,
Wang Q, He E, Xiong L, Pei J, et al: Electroacupuncture
pretreatment induces tolerance against focal cerebral ischemia
through activation of canonical Notch pathway. BMC Neurosci.
13:1112012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen G, Zhang Z, Cheng Y, Xiao W, Qiu Y,
Yu M, Sun L, Wang W, Du G, Gu Y, et al: The canonical Notch
signaling was involved in the regulation of intestinal epithelial
cells apoptosis after intestinal ischemia/reperfusion injury. Int J
Mol Sci. 15:7883–7896. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kang L, Mao J, Tao Y, Song B, Ma W, Lu Y,
Zhao L, Li J, Yang B and Li L: MicroRNA-34a suppresses the breast
cancer stem cell-like characteristics by downregulating Notch1
pathway. Cancer Sci. 106:700–708. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Park EY, Chang E, Lee EJ, Lee HW, Kang HG,
Chun KH, Woo YM, Kong HK, Ko JY, Suzuki H, et al: Targeting of
miR34a-NOTCH1 axis reduced breast cancer stemness and
chemoresistance. Cancer Res. 74:7573–7582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu XD, Zhang LY, Zhu TC, Zhang RF, Wang
SL and Bao Y: Overexpression of miR-34c inhibits high
glucose-induced apoptosis in podocytes by targeting Notch signaling
pathways. Int J Clin Exp Pathol. 8:4525–4534. 2015.PubMed/NCBI
|
|
51
|
Zhang HD, Sun DW, Mao L, Zhang J, Jiang
LH, Li J, Wu Y, Ji H, Chen W, Wang J, et al: MiR-139-5p inhibits
the biological function of breast cancer cells by targeting Notch1
and mediates chemosensitivity to docetaxel. Biochem Biophys Res
Commun. 465:702–713. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang X, Ni W and Lei K: miR-200b
suppresses cell growth, migration and invasion by targeting Notch1
in nasopharyngeal carcinoma. Cell Physiol Biochem. 32:1288–1298.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang X, Wei A, Liu Y, He G, Zhou Z and Yu
Z: IGF-1 protects retinal ganglion cells from hypoxia-induced
apoptosis by activating the Erk-1/2 and Akt pathways. Mol Vis.
19:1901–1912. 2013.PubMed/NCBI
|
|
54
|
Cheng Y, Li Y, Liu D, Zhang R and Zhang J:
miR-137 effects on gastric carcinogenesis are mediated by targeting
Cox-2-activated PI3K/AKT signaling pathway. FEBS Lett.
588:3274–3281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang S and Li K: MicroRNA-96 regulates
RGC-5 cell growth through caspase-dependent apoptosis. Int J Clin
Exp Med. 7:3694–3702. 2014.PubMed/NCBI
|