Introduction
The organ dysfunction induced by sepsis is largely
due to systemic inflammation, which results in dysfunction of the
microvasculature, particularly microvascular endothelial cells
(1,2). Impaired barrier function in the
vascular wall, neutrophil influx into organs, impaired distribution
of blood flow in microvascular beds and microvascular thromboses
are the most common forms of dysfunction of microvasculature
(3). The dysfunction of
microvasculature is important clinically, as it has been documented
early in the course of sepsis in humans and is associated with
increased mortality rates, particularly when it persists over time
(4,5).
Endothelial dysfunction is an early stage in several
vascular diseases and inflammation is involved in the pathological
process of organ dysfunction, primarily by motivating endothelial
dysfunction. One of the most common results of inflammation is the
activation of endothelial cell apoptosis (6-9).
The lungs are important organs and the systemic
inflammatory response caused by sepsis is an important risk factor
for pulmonary microvascular dysfunction (10–12). Multiple mechanisms promote septic
pulmonary microvascular dysfunction, including activation by
mechanical interaction with activated leukocytes, inflammatory
cytokines, and exposure to harmful leukocyte-derived molecules,
including oxidants (13). These
factors result in pulmonary microvascular endothelial cell
abnormalities, including disruption of inter-pulmonary
micro-vascular endothelial cell junctions and cytoskeleton-driven
retraction. In addition, the locally produced inflammatory factors
in target organs may have a direct and important effect in the
pathological process.
2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside (TSG;
Fig. 1) is one of the major
bioactive constituents extracted from Polygonum multiflorum
Thunb, and exhibits various pharmacologic activities, including
anti-inflammatory, antioxidant, and anti-atherosclerotic effects,
improvement of memory and learning ability, neuroprotection,
anti-aging, attenuation of human platelet aggregation and promotion
of hair growth (14–19). However, the protective effect of
TSG on sepsis-induced inflammatory injury remains to be fully
elucidated.
Sepsis can induce the expression of several
inflammatory factors (20,21)
and the anti-inflammatory effect of TSG is well-documented
(22–25). However, the pro-inflammatory
mechanisms in sepsis and the mechanism underlying the
anti-inflammatory effect of TSG induced by septic serum remain to
be elucidated. The present study provided evidence that
inflammatory factors were produced by activated endothelial cells
via the reactive oxygen species (ROS)-mitogen-activated protein
kinase (MAPK)-nuclear factor (NF)-κB signaling pathway. TSG exerted
a protective effect via interfering with this signaling
pathway.
Materials and methods
Reagents
TSG was obtained from the National Food and Drug
Testing Institute (Beijing, China). Tissue culture medium 199
(M199) was purchased from GE Healthcare Life Sciences (South Logan,
UT, USA) and fetal bovine serum (FBS) were acquired from Gibco;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Endothelial cell
growth factor (ECGF) was purchased from Roche Diagnostics (Basel,
Switzerland). TRIzol and a One-Step RT-PCR kit were from
Invitrogen; Thermo Fisher Scientific, Inc. Extracellular
signal-regulated kinase (ERK) 1/2 inhibitor PD98059, p38 inhibitor
SB203580, c-Jun N-terminal kinase (JNK) inhibitor SP600125,
antioxidant N-acetylcysteine (NAC), and NF-κB inhibitor pyrrolidine
dithiocarbamate (PDTC) were from Sigma-Aldrich; Merck Millipore
(Darmstadt, Germany). Rabbit NF-κB antibody (cat. no. ab207297) and
rabbit CD31 antibody (cat. no. ab228364) were provided by Abcam
(Cambridge, MA, USA). Rabbit monoclonal β-actin antibody (cat. no.
NC011) was obtained from Zhuangzhi Biotech (Xi'an, China). ERK1/2
(cat. no. AF1051) and phosphorylated (phospho)-ERK1/2 (cat. no.
AM071) antibodies, and 2′,7′-dichlorodihydrofluoro-rescein
diacetate (H2DCF-DA) were obtained from Beyotime
Institute of Biotechnology (Jiangsu, China). Phospho-p38 (cat. no.
8632S) and p38 (cat. no. 8690T) antibodies were from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Goat anti-rabbit IgG, HRP
conjugated (cat. no. CW0103) or Goat anti-mouse IgG, HRP conjugated
(cat. no. CW0110S) secondary antibodies were provided by CW Biotech
Co., Ltd. (Beijing, China). Enzyme-linked immunosorbent assay
(ELISA) kits for detecting IL-1β, IL-6 and C-reactive protein (CRP)
were from West Tang Biotechnology (Shanghai, China). All other
materials, except where indicated were from Sigma-Aldrich; Merck
KGaA, and were of analytical grade.
Primary culture of pulmonary aortic
endothelial cells (PAECs)
The rat PAECs were isolated from the pulmonary
aortae of male Sprague-Dawley (SD) rats (6 weeks old) based on
previously described methods (26) and identified using
immunofluorescence staining with CD31 antibody (Fig. 2). Before the experiment, all rats
had free access to food and water, and were maintained in a
constant environment with a conventional 12/12 h light/dark cycle.
The cells were cultured in M199 media supplemented with 20% FBS,
100 U/ml streptomycin, 100 U/ml penicillin, 95 µg/ml heparin
and 20 µg/ml ECGF. The cells were maintained in a 5%
humidified air CO2 atmosphere at 37°C. Cells at passages
3–8 at 80–90% confluence were used in the present study. All
experimental procedures were performed in strict accordance with
the international, national and institutional rules, and approved
by the Institutional Animal Care Committee of Sun Yat-Sen
University (Guangzhou, China).
Preparation of septic serum
The male SD rats (n=16, weight: 160–180 g) were
randomly assigned into two groups. Septic model rats were
established according to the experiment procedure described by Niwa
et al (27) as follows: A
laparotomy was performed through a midline incision of each animal
under ether anesthesia. The cecum was filled with feces by milking
stools back from the ascending colon, following which the distal
one third of the cecum was tied off. The ligated region of the
cecum was punctured twice with an 18-gauge needle, following which
the bowel was replaced in the peritoneal cavity and the abdomen
closed. In the control group, the rats were treated in the same
manner as the septic rat models, but without ligation of the cecum
and puncturing. At 7 h post-surgery, serum samples were collected
via the abdominal aorta.
Cell viability assay
The CCK-8 method was used to detect the cell
viability of PAECs. Approximately 1-1.5×106 cells were
incubated with septic serum for 12 h, incubated with different
concentrations of TSG for 12 h or subjected to septic serum
treatment in the absence/presence of TSG in 96-well plates at 37°C.
The experimental protocol was as follows: 10 µl CCK-8
solution was added to each well, followed by incubation for 4 h at
37°C. The optical density was measured at 450 nm using a microplate
reader (Molecular Devices LLC, Sunnyvale, CA, USA). The mean
optical density of six wells was used to calculate the cell
viability percentage.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The PAECs were cultured in 6-well plates at a
density of 1×105 cells per well. Following the indicated
treatments, the cells were washed twice with ice-cold PBS and the
total mRNA was extracted using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The concentration of RNA was determined by measuring the absorbance
at 260 nm. The total RNA was then reverse transcribed to cDNA using
high capacity Reverse Transcriptase and 1 µM oligo(dT) from
Takara Bio, Inc. (Tokyo, Japan). The primer pair sequences were
specific to rat IL-1β, forward, 5′-CCC AAC TGG TAC ATC AGC ACC TCT
C-3′ and reverse, 5′-CTA TGT CCC GAC CAT TGC TG-3′); IL-6, forward,
5′-GAT TGT ATG AAC AGC GAT GAT GC-3′ and reverse, 5′-AGA AAC GGA
ACT CCA GAA GAC C-3′); CRP, forward, 5′-TTG GTG GGA GAC ATT GGA
GA-3′ and reverse, 5′-AAC ATT GGG GCT GAA TAC CCT AC-3′; and GAPDH,
forward, 5′-TGG AGT CTA CTG GCG TCT T-3′ and reverse, 5′-TGT CAT
ATT TCT CGT GGT TCA-3′. cDNA (100 ng) was amplified using the above
primer pairs (0.25 mM for forward and reverse primers) and
normalized to the level of GAPDH. SYBR premix Ex taq (cat. no.
DRR081A; Takara, Bio, Inc.) was used in the present study and the
reaction was performed with the Mx3000P quantitative PCR system
(Stratagene, La Jolla, CA, Inc.). The basic protocol for the qPCR
was as follows: Initial incubation at 94°C for 55 sec, followed by
40 cycles of 94°C for 15 sec, 60°C for 30 sec, and 72°C for 30 sec.
All samples were run in triplicate, and analyzed using the
2−ΔΔCq method as previously described (28)
ELISA analysis
The PAECs (1-1.5×106) were cultured in a
96-well plate and stimulated with septic serum for 12 h or
pretreated with TSG for 2 h. The supernatant was then collected,
and the levels of IL-1β, IL-6 and CRP in the supernatant were
assayed using ELISA kits specific for rat IL-1β, IL-6 and CRP.
Immunofluorescence staining
The PAECs were incubated with the primary rabbit
anti-cd31 antibody (1:300 dilution) overnight at 4°C and were then
incubated with the secondary Alexa fluor 488-conjugated antibody
(1:500 dilution) for 3 h at room temperature. Subsequently, the
cells were stained in 2-(4-amidinophenyl)-6-indolecarbamidine
dihydrochloride solution. Each of these steps was followed by
washing with ice-cold PBS three times. Finally, the cells on
coverslips were preserved in anti-fade mounting medium and the
expression of CD31 was observed under a fluorescent microscope.
Western blot analysis
Following the indicated treatments, the cells were
washed twice with ice-cold PBS (pH 7.4) and lysed in lysis buffer
supplemented with protease inhibitor cocktail and phosphatase
inhibitors (Roche Diagnostics; 100 µl per well of a 6-well
plate). The concentration of protein was measured using a BCA
protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Equal quantities of the protein (30 µg) were loaded and
separated by 10% SDS-PAGE, and blotted onto a PVDF membrane (0.45
µm; GE Healthcare Life Sciences). The membranes were
incubated with anti-β-actin (1:2,000), anti-p38 (1:1,000) or
anti-phospho-p38 (1:500), anti-ERK1/2 (1:1,000) or
anti-phospho-ERK1/2 (1:800) antibodies at 4°C overnight. Following
washing with PBS three times, the membranes were incubated with the
goat anti-rabbit IgG HRP conjugated or goat anti-mouse IgG HRP
conjugated secondary antibodies (1:2,500) for 3 h. The immune
complexes were enhanced using chemiluminescence and band
intensities were measured and analyzed by Image-Pro Plus software
(Version 10; Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
The results are represented as the mean ± standard
error of the mean. Statistical analysis was performed using the
Steer-Dwass or Mann-Whitney multiple comparison test. Analysis was
performed using GraphPad prism 5 (GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
TSG increases the cell viability of
septic serum-induced PAECs
As shown in Fig.
3A, the septic serum significantly decreased the cell viability
of the PAECs following incubation for 12, 24 or 48 h. Treatment
with TSG alone did not affect the viability of the PAECs (Fig. 3B). However, pretreatment of the
cells with TSG at a concentration of 80 µmol/l for 2 h,
followed by co-incubation with septic serum for another 12 h
increased the viability of the PAECs, in a concentration-dependent
manner, compared with the viability in the septic serum group
(Fig. 3C).
TSG decreases septic serum-induced
inflammatory cytokine expression in PAECs
The mRNA levels of IL-1β, IL-6 and CRP were
determined using RT-qPCR analysis. As shown in Fig. 4A–C, the mRNA levels of IL-1β, IL-6
and CRP in the PAECs were significantly increased following
exposure to septic serum for 12 h (P<0.01 vs. control). By
contrast, pretreatment with TSG at a concentration 40 µmol/l
for 2 h, followed by co-incubation with septic serum significantly
decreased the expression of mRNA in a concentration-dependent
manner.
 | Figure 4TSG decreases mRNA and protein
expression levels of IL-1β, IL-6 and CRP in pulmonary aortic
endothelial cells. The cells were subjected to SS treatment in the
absence or presence of TSG for 12 h. mRNA and protein expression
levels of IL-1β, IL-6 and CRP were determined using RT-qPCR and
ELISA analysis, respectively. mRNA expression levels of (A) IL-1β,
(B) IL-6 and (C) CRP. Protein expression levels of (D) IL-1β, (E)
IL-6 and (F) CRP. Results were from six independent experiments for
ELISA and three independent experiments for RT-qPCR analysis, and
are expressed as the mean ± standard error of the mean.
##P<0.01 vs. control; *P<0.05 and
**P<0.01 vs. SS alone. TSG,
2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside; IL,
interleukin; CRP, C-reactive protein; SS, septic serum; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction;
ELISA, enzyme-linked immunosorbent assay. |
The results from the ELISA analysis indicated that
the protein expression levels of IL-1β, IL-6 and CRP in the PAECs
were significantly increased following exposure to septic serum for
12 h (P<0.01 vs. septic serum only). However, pretreatment with
TSG at a concentration of 40 µmol/l for 2 h, followed by
co-incubation with septic serum, decreased the levels of IL-1β and
IL-6 in the supernatant in a concentration-dependent manner
(Fig. 4D and E). Pretreatment
with TSG at a concentration of 80 µmol/l for 2 h, followed
by co-incubation with septic serum notably decreased the level of
CRP in the supernatant in a concentration-dependent manner
(Fig. 4F).
TSG decreases septic serum-induced ROS
generation in PAECs
As shown in Fig. 5
septic serum increased the generation of intracellular ROS in
PAECs, whereas pretreatment with TSG for 2 h significantly
eliminated the septic serum-induced intracellular expression of ROS
(Fig. 5).
TSG decreases septic serum-induced
protein expression levels of phosphorylated ERK1/2, p38 and
JNK
As it is reported that MAPK is important in the
expression of several inflammatory cytokines, the upregulated
expression of inflammatory cytokines by septic serum in PAECs may
be associated with MAPK signaling. The results of the present study
showed that the protein levels of phospho-ERK1/2, phospho-P38 and
phospho-JNK in the PAECs were increased following stimulation with
septic serum (Fig. 6A–C).
Pretreatment with TSG for 2 h significantly reduced the septic
serum-induced protein expression of phospho-ERK1/2, phospho-P38 and
phospho-JNK in the PAECs. Subsequent experiments indicated that
pretreatment with the antioxidant NAC for 1.5 h, alone or in
combination with TSG decreased the protein expression levels of
phospho-ERK1/2, phospho-P38 and phospho-JNK. These results
indicated that the MAPK pathway may be involved in septic
serum-induced inflammatory cytokine expression, whereas TSG
eliminated the septic serum-induced expression of inflammatory
cytokines in the PAECs. Taken together, these results showed that
TSG inhibited septic serum-induced inflammatory injury via
interfering with the ROS-MAPK signaling pathway in PAECs.
 | Figure 6Effect of TSG on SS-activated (A)
ERK1/2, (B) p38 and (C) JNK phosphorylation in PAECs. Following
pretreatment with TSG for 2 h, the PAECs were stimulated with SS
for 1.5 h. The phosphorylation of ERK1/2, p38 and JNK was detected
using western blot analysis. The results from three independent
experiments are expressed as the mean ± standard error of the mean.
*P<0.05 and **P<0.01 vs. SS alone;
##P<0.01 vs. control. TSG, 2,3,5,4′-tetrahydroxystilb
ene-2-O-β-D-glucoside; SS, septic serum; PAECs,
pulmonary arterial endothelial cells; ERK, extracellular
signal-regulated kinase; JNK, c-Jun N-terminal kinase; p-,
phosphorylated; NAC, N-acetylcysteine. |
TSG decreases septic serum-induced levels
of NF-κB in PAECs
MAPK and NF-κB are involved in the expression of
several inflammatory cytokines, and septic serum-induced inf
lammation in PAECs may be associated with ROS-MAPK-NF-κB signaling.
The results of the present study showed that the nuclear expression
of NF-κB in PAECs was increased following stimulation with septic
serum for 12 h. Pretreatment of the cells with NAC (antioxidant),
PD98059 (ERK1/2 inhibitor), SB203580 (p38 MAPK inhibitor), SP600125
(JNK inhibitor) or PDTC (NF-κB inhibitor) for 1.5 h significantly
reduced the septic serum-induced nuclear expression of NF-κB
(Fig. 7). These results also
indicated that ROS was involved in the septic serum-induced nuclear
expression of NF-κB in PAECs.
TSG decreases septic serum induced
inflammation via the ROS-MAPK-NF-κB signaling pathway in PAECs
As shown in Fig.
4, the protein levels of IL-1β, IL-6 and CRP in the cells were
significantly decreased following pretreatment with TSG for 1 h
(P<0.05 or P<0.01 vs. control). As a positive control, the
cells pretreated with PD98059 (ERK1/2 inhibitor), SB203580 (p38
MAPK inhibitor), SP600125 (JNK inhibitor) or antioxidant NAC for 1
h showed similar effects (Fig.
8). These results indicated that the ROS-MAPK-NF-κB signaling
pathway was involved in septic serum-induced inflammation in the
PAECs.
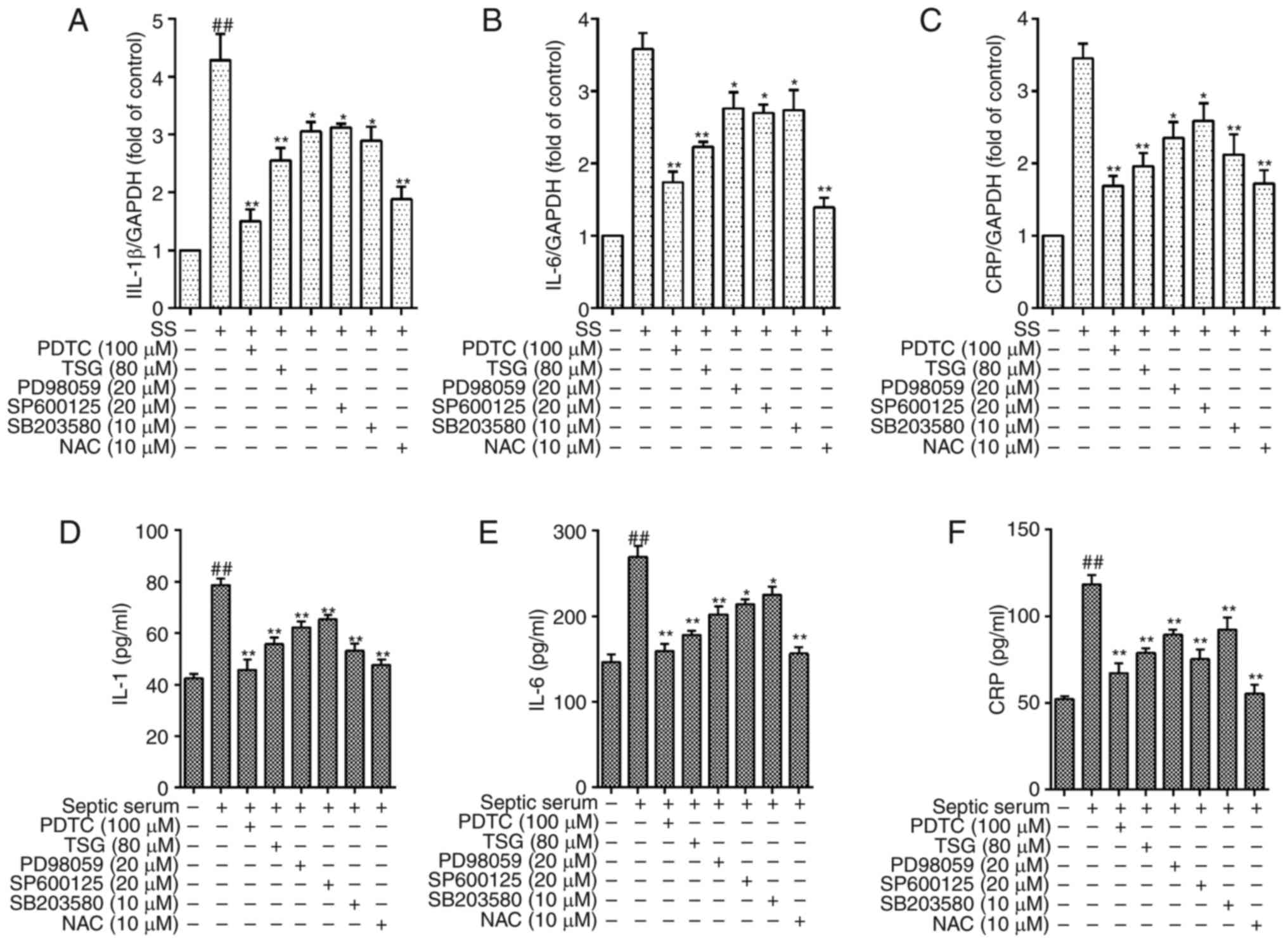 | Figure 8TSG decreases protein expression
levels of IL-1β, IL-6 and CRP via reactive oxygen
species-mitogen-activated protein kinase-nuclear factor-κB in
PAECs. The PAECs were subjected to SS treatment in the absence or
presence of TSG for 12 h. The mRNA and protein expression levels of
IL-1β, IL-6 and CRP were then identified using RT-qPCR analysis and
ELISA, respectively. mRNA expression of (A) IL-1β, (B) IL-6 and (C)
CRP. Protein expression of (D) IL-1β, (E) IL-6 and (F) CRP. Results
were from six independent experiments for ELISA and three
independent experiments for quantitative RT-qPCR, and expressed as
the mean ± standard error of the mean. ##P<0.01 vs.
control; *P<0.05 and **P<0.01 vs. SS
alone. TSG, 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glu
coside; SS, septic serum; PAECs, pulmonary arterial endothelial
cells; PDTC, pyrrolidine dithiocarbamate; NAC, N-acetylcysteine;
CRP, C-reactive protein; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; ELISA,
enzyme-linked immunosorbent assay. |
Discussion
In the present report, in vitro conditions of
septicemia were established to examine the protective effect of TSG
on septic serum-induced inflammatory injury in PAECs. The results
confirmed that the stimulation of PAECs with septic serum led to
upregulation in the protein expression levels of IL-1β, IL-6 and
CRP, whereas pretreatment with TSG significantly reduced the
expression of these inflammatory cytokines in PAECs, in a
concentration-dependent manner. Subsequent experiments indicated
that the protective effect of TSG was predominantly through
interfering with the ROS-MAPK-NF-κB signaling pathway.
In sepsis, multiple organ dysfunction is mainly due
to systemic inflammatory injury of the microvasculature,
particularly microvascular endothelial cells (29-32). Numerous clinical and laboratory
investigations have indicated that, for microvascular and
microvascular endothelial cells, dysfunction or injury are the
initial stages of organ dysfunction (33). In addition, increased numbers of
circulating endothelial cells and soluble markers of endothelial
cell damage correlate with increased severity of sepsis and higher
mortality rates (34,35). In addition, this septic
microvascular dysfunction is clinically relevant, as the presence
of microvascular dysfunction in human sepsis is associated with
more severe sepsis, organ dysfunction, and increased mortality
rates (36,37). The clinical outcomes, including
survival rates, have been reported to be particularly poor if
septic microvascular dysfunction persists over time despite usual
clinical management (13,27,38–40). In the present study, a rat model
of sepsis was established, and rat septic serum was used to
investigate the pro-inflammatory effect of septic serum and the
protective effect of TSG. The results showed that septic serum
significantly induced the expression levels of IL-1β, IL-6 and CRP,
whereas TSG eliminated the pro-inflammatory effects of septic serum
in the endothelial cells.
The activation of an inflammatory effect occurs via
multiple pathways, and data from clinical and experimental
investigations have shown that oxidative damage to endothelial
cells is severe in sepsis (41–43). ROS are important secondary
messengers and are directly involved in oxidative stress. The
present study showed that ROS were important in the septic
serum-induced expression of inflammatory factors. Pre-treatment
with antioxidant NAC 10−2 M significantly inhibited the
protein expression levels of IL-1β, IL-6 and CRP in PAECs, whereas
cells co-cultivated with TSG reduced septic serum-induced
superoxide anion generation in PAECs.
MAPK and NF-κ B signaling are pivotal in
inflammation (44–49). The activation of NF-κB is
responsible for the expression of several inflammatory cytokines.
The results of the present study showed that p38 MAPK and NF-κB
were involved in the protein expression of IL-1β, IL-6 and CRP
induced by septic serum; the selective p38 MAPK and NF-κB
inhibitor, PDTC, significantly inhibited the protein expression of
IL-1β, IL-6 and CRP in PAECs, and TSG had a similar effect to the
specific inhibitor.
In conclusion, the present study demonstrated that
septic serum induced the protein expression of IL-1β, IL-6 and CRP
in PAECs, and that TSG inhibited the septic serum-induced
inflammatory injury via interfering with the ROS-p38MAPK-NF-κB
signaling pathway. These results provide novel evidence supporting
the potential inflammatory effect of septic serum and the
anti-inflammatory effect of TSG.
References
|
1
|
Churpek MM, Snyder A, Han X, Sokol S,
Pettit N, Howell MD and Edelson DP: Quick sepsis-related organ
failure assessment, systemic inflammatory response syndrome, and
early warning scores for detecting clinical deterioration in
infected patients outside the intensive care unit. Am J Respir Crit
Care Med. 195:906–911. 2017. View Article : Google Scholar
|
|
2
|
Colbert JF, Schmidt EP, Faubel S and Ginde
AA: Severe sepsis outcomes among hospitalizations with inflammatory
bowel disease. Shock. 47:128–131. 2017. View Article : Google Scholar
|
|
3
|
Kanashiro A, Sônego F, Ferreira RG,
Castanheira FV, Leite CA, Borges VF, Nascimento DC, Cólon DF,
Alves-Filho JC, Ulloa L and Cunha FQ: Therapeutic potential and
limitations of cholinergic anti-inflammatory pathway in sepsis.
Pharmacol Res. 117:1–8. 2017. View Article : Google Scholar
|
|
4
|
Martin JB and Badeaux JE: Interpreting
laboratory tests in infection: Making sense of biomarkers in sepsis
and systemic inflammatory response syndrome for intensive care unit
patients. Crit Care Nurs Clin North Am. 29:119–130. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Md Ralib A, Mat Nor MB and Pickering JW:
Plasma neutrophil gelatinase-associated lipocalin diagnosed acute
kidney injury in patients with systemic inflammatory disease and
sepsis. Nephrology (Carlton). 22:412–419. 2017. View Article : Google Scholar
|
|
6
|
Arcêncio L and Evora PR: The lack of
clinical applications would be the cause of low interest in an
endothelial dysfunction classification. Arq Bras Cardiol.
108:97–99. 2017.In English, Portuguese. PubMed/NCBI
|
|
7
|
Downey RM, Liao P, Millson EC, Quyyumi AA,
Sher S and Park J: Endothelial dysfunction correlates with
exaggerated exercise pressor response during whole body maximal
exercise in chronic kidney disease. Am J Physiol Renal Physiol.
312:F917–F924. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Graw JA, Yu B, Rezoagli E, Warren HS, Buys
ES, Bloch DB and Zapol WM: Endothelial dysfunction inhibits the
ability of haptoglobin to prevent hemoglobin-induced hypertension.
Am J Physiol Heart Circ Physiol. 312:H1120–H1127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hashemi M, Heshmat-Ghahdarijani K, Zarean
E, Baktash F and Mortazavi ZS: Evaluation of the effect of
high-dose folic acid on endothelial dysfunction in pre-eclamptic
patients: A randomized clinical trial. J Res Med Sci. 21:1142016.
View Article : Google Scholar
|
|
10
|
Rothenbach PA, Dahl B, Schwartz JJ,
O'Keefe GE, Yamamoto M, Lee WM, Horton JW, Yin HL and Turnage RH:
Recombinant plasma gelsolin infusion attenuates burn-induced
pulmonary microvascular dysfunction. J Appl Physiol (1985).
96:25–31. 2004. View Article : Google Scholar
|
|
11
|
Tsukamoto M, Tampo Y, Sawada M and Yonaha
M: Paraquat-induced membrane dysfunction in pulmonary microvascular
endothelial cells. Pharmacol Toxicol. 86:102–109. 2000. View Article : Google Scholar
|
|
12
|
Tsukamoto M, Tampo Y, Sawada M and Yonaha
M: Paraquat-induced oxidative stress and dysfunction of the
glutathione redox cycle in pulmonary microvascular endothelial
cells. Toxicol Appl Pharmacol. 178:82–92. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Farley KS, Wang LF, Law C and Mehta S:
Alveolar macrophage inducible nitric oxide synthase-dependent
pulmonary micro-vascular endothelial cell septic barrier
dysfunction. Microvasc Res. 76:208–216. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang MJ, Xiao JH, Wang Y, Yan YL, Yang J
and Wang JL: 2,3,5,4′-Tetrahydroxystilbene-2-O-beta-D-glucoside
improves gastrointestinal motility disorders in STZ-induced
diabetic mice. PLoS One. 7:e502912012. View Article : Google Scholar
|
|
15
|
He H, Wang S, Tian J, Chen L, Zhang W,
Zhao J, Tang H, Zhang X and Chen J: Protective effects of
2,3,5,4′-tetrahydro xystilbene-2-O-β-D-glucoside in the
MPTP-induced mouse model of Parkinson's disease: Involvement of
reactive oxygen species-mediated JNK, P38 and mitochondrial
pathways. Eur J Pharmacol. 767:175–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin CL, Hsieh SL, Leung W, Jeng JH, Huang
GC, Lee CT and Wu CC: 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-gluc
oside suppresses human colorectal cancer cell metastasis through
inhibiting NF-κB activation. Int J Oncol. 49:629–638. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ling S, Duan J, Ni R and Xu JW:
2,3,5,4′-Tetrahydroxystilbene -2-O-β-D-glucoside promotes
expression of the longevity gene klotho. Oxid Med Cell Longev.
2016:31282352016. View Article : Google Scholar
|
|
18
|
Ling S and Xu JW: Biological Activities of
2,3,5,4′-tetrah ydroxystilbene-2-O-β-D-glucoside in antiaging and
anti-aging-related disease treatments. Oxid Med Cell Longev.
2016:49732392016. View Article : Google Scholar
|
|
19
|
Peng Y, Zeng Y, Xu J, Huang XL, Zhang W
and Xu XL: PPAR-gamma is involved in the protective effect of
2,3,4′,5-tetra-hydroxystilbene-2-O-beta-D-glucoside against cardiac
fibrosis in pressure-overloaded rats. Eur J Pharmacol. 791:105–114.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lambertucci F, Motiño O, Villar S, Rigalli
JP, de Luján Alvarez M, Catania VA, Martín-Sanz P, Carnovale CE,
Quiroga AD, Francés DE and Ronco MT: Benznidazole, the trypanocidal
drug used for chagas disease, induces hepatic NRF2 activation and
attenuates the inflammatory response in a murine model of sepsis.
Toxicol Appl Pharmacol. 315:12–22. 2017. View Article : Google Scholar
|
|
21
|
Li HR, Liu J, Zhang SL, Luo T, Wu F, Dong
JH, Guo YJ and Zhao L: Corilagin ameliorates the extreme
inflammatory status in sepsis through TLR4 signaling pathways. BMC
Complement Altern Med. 17:182017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park SY, Jin ML, Wang Z, Park G and Choi
YW: 2,3,4′,5-tetrahyd roxystilbene-2-O-β-d-glucoside exerts
anti-inflammatory effects on lipopolysaccharide-stimulated
microglia by inhibiting NF-κB and activating AMPK/Nrf2 pathways.
Food Chem Toxicol. 97:159–167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang M, Yu LM, Zhao H, Zhou XX, Yang Q,
Song F, Yan L, Zhai ME, Li BY, Zhang B, et al:
2,3,5,4′-Tetrahydroxystilbe ne-2-O-β-D-glucoside protects murine
hearts against ischemia/reperfusion injury by activating
Notch1/Hes1 signaling and attenuating endoplasmic reticulum stress.
Acta Pharmacol Sin. 38:317–330. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang W, Chen XF, Huang YJ, Chen QQ, Bao
YJ and Zhu W: 2,3,4′,5-Tetrahydroxystilbene-2-O-β-D-glucoside
inhibits angiotensin II-induced cardiac fibroblast proliferation
via suppression of the reactive oxygen species-extracellular
signal-regulated kinase 1/2 pathway. Clin Exp Pharmacol Physiol.
39:429–437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao J, Xu S, Song F, Nian L, Zhou X and
Wang S: 2,3,5,4′-tetr ahydroxystilbene-2-O-β-D-glucoside protects
human umbilical vein endothelial cells against
lysophosphatidylcholine-induced apoptosis by upregulating
superoxide dismutase and glutathione peroxidase. IUBMB Life.
66:711–722. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peng G, Wen X, Shi Y, Jiang Y, Hu G, Zhou
Y and Ran P: Development of a new method for the isolation and
culture of pulmonary arterial endothelial cells from rat pulmonary
arteries. J Vasc Res. 50:468–477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Niwa H, Ogawa Y, Kido Y, Abe Y, Kobayashi
M, Mori T and Tanaka T: The rate of lipid oxidation in septic rat
models. Jpn J Surg. 19:439–445. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Cepinskas G and Wilson JX: Inflammatory
response in micro-vascular endothelium in sepsis: Role of oxidants.
J Clin Biochem Nutr. 42:175–184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Garrean S, Gao XP, Brovkovych V, Shimizu
J, Zhao YY and Vogel SM: Caveolin-1 regulates NF-kappaB activation
and lung inflammatory response to sepsis induced by
lipopolysaccharide. J Immunol. 177:4853–4860. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McCuskey RS, Nishida J, McDonnell D, Baker
GL, Urbaschek R and Urbaschek B: Effect of immunoglobulin G on the
hepatic microvascular inflammatory response during sepsis. Shock.
5:28–33. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Orfanos SE, Kotanidou A, Glynos C,
Athanasiou C, Tsigkos S, Dimopoulou I, Sotiropoulou C, Zakynthinos
S, Armaganidis A, Papapetropoulos A and Roussos C: Angiopoietin-2
is increased in severe sepsis: Correlation with inflammatory
mediators. Crit Care Med. 35:199–206. 2007. View Article : Google Scholar
|
|
33
|
Biegańska-Hensoldt S and Rosolowska-Huszcz
D: Polyphenols in preventing endothelial dysfunction. Postepy Hig
Med Dosw (Online). 71:227–235. 2017. View Article : Google Scholar
|
|
34
|
Moussa MD, Santonocito C, Fagnoul D,
Donadello K, Pradier O, Gaussem P, De Backer D and Vincent JL:
Evaluation of endothelial damage in sepsis-related ARDS using
circulating endothelial cells. Intensive Care Med. 41:231–238.
2015. View Article : Google Scholar
|
|
35
|
Schlichting DE, Waxman AB, O'Brien LA,
Wang T, Naum CC, Rubeiz GJ, Um SL, Williams M and Yan SC:
Circulating endothelial and endothelial progenitor cells in
patients with severe sepsis. Microvasc Res. 81:216–221. 2011.
View Article : Google Scholar
|
|
36
|
Lush CW and Kvietys PR: Microvascular
dysfunction in sepsis. Microcirculation. 7:83–101. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vincent JL and De Backer D: Microvascular
dysfunction as a cause of organ dysfunction in severe sepsis. Crit
Care. 9(Suppl 4): S9–S12. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nishida J, Ekataksin W, McDonnell D,
Urbaschek R, Urbaschek B and McCuskey RS: Ethanol exacerbates
hepatic microvascular dysfunction, endotoxemia, and lethality in
septic mice. Shock. 1:413–418. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Armour J, Tyml K, Lidington D and Wilson
JX: Ascorbate prevents microvascular dysfunction in the skeletal
muscle of the septic rat. J Appl Physiol (1985). 90:795–803. 2001.
View Article : Google Scholar
|
|
40
|
De Blasi RA, Palmisani S, Alampi D,
Mercieri M, Romano R, Collini S and Pinto G: Microvascular
dysfunction and skeletal muscle oxygenation assessed by
phase-modulation near-infrared spectroscopy in patients with septic
shock. Intensive Care Med. 31:1661–1668. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Constantino L, Goncalves RC, Giombelli VR,
Tomasi CD, Vuolo F, Kist LW, de Oliveira GM, Pasquali MA, Bogo MR,
Mauad T, et al: Regulation of lung oxidative damage by endogenous
superoxide dismutase in sepsis. Intensive Care Med Exp. 2:172014.
View Article : Google Scholar
|
|
42
|
Schwalm MT, Pasquali M, Miguel SP, Dos
Santos JP, Vuolo F, Comim CM, Petronilho F, Quevedo J, Gelain DP,
Moreira JC, et al: Acute brain inflammation and oxidative damage
are related to long-term cognitive deficits and markers of
neurodegeneration in sepsis-survivor rats. Mol Neurobiol.
49:380–385. 2014. View Article : Google Scholar
|
|
43
|
Taner G, Aydin S, Bacanli M, Sarıgöl Z,
Sahin T, Başaran AA and Başaran N: Modulating effects of
pycnogenol® on oxidative stress and DNA damage induced
by sepsis in rats. Phytother Res. 28:1692–1700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
O'Sullivan AW, Wang JH and Redmond HP:
NF-kappaB and p38 MAPK inhibition improve survival in endotoxin
shock and in a cecal ligation and puncture model of sepsis in
combination with antibiotic therapy. J Surg Res. 152:46–53. 2009.
View Article : Google Scholar
|
|
45
|
Ronco MT, Manarin R, Francés D, Serra E,
Revelli S and Carnovale C: Benznidazole treatment attenuates liver
NF-κB activity and MAPK in a cecal ligation and puncture model of
sepsis. Mol Immunol. 48:867–873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Song GY, Chung CS, Chaudry IH and Ayala A:
Immune suppression in polymicrobial sepsis: differential regulation
of Th1 and Th2 responses by p38 MAPK. J Surg Res. 91:141–146. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Song GY, Chung CS, Jarrar D, Chaudry IH
and Ayala A: Evolution of an immune suppressive macrophage
phenotype as a product of P38 MAPK activation in polymicrobial
sepsis. Shock. 15:42–48. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Song GY, Chung CS, Jarrar D, Cioffi WG and
Ayala A: Mechanism of immune dysfunction in sepsis: Inducible
nitric oxide-meditated alterations in p38 MAPK activation. J
Trauma. 53:276–2832. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sun Y, Li YH, Wu XX, Zheng W, Guo ZH, Li
Y, Chen T, Hua ZC and Xu Q: Ethanol extract from Artemisia vestita,
a traditional Tibetan medicine, exerts anti-sepsis action through
down-regulating the MAPK and NF-kappaB pathways. Int J Mol Med.
17:957–962. 2006.PubMed/NCBI
|






















