Introduction
Abnormal left ventricular (LV) diastolic function is
closely associated with increased cardiovascular morbidity and
mortality, and is a precursor of heart failure (HF) with preserved
ejection fraction (HFpEF). HFpEF accounts for 30–50% of cases of HF
and is becoming increasingly prevalent; in addition, nearly all
patients with HF symptoms, including those with reduced EF, have
some component of HFpEF (1). The
abnormal deposition of fibrillar collagen, and other extracellular
matrix components, may account for this pathological state, which
leads to increased stiffness and decreased compliance of the LV
wall. In recent years, research has focused on diastolic
dysfunction as an early marker of target organ damage in
hypertension (2–4). Clinical data and the results of
experiments using animal models have indicated that alterations in
diastole precede the development of HF in hypertensive heart
disease. Spontaneously hypertensive rat (SHR) is a well-established
animal model, which resembles numerous aspects of human essential
hypertension and has been regarded as a useful tool for studying
transitions and the underlying mechanisms of LV diastolic
dysfunction and HFpEF. Slama et al (5) detected normal LV systolic function,
which was associated with impaired LV relaxation and compliance in
SHRs; this was present early in life and progressively increased
over the lifetime of SHRs. In order to study the molecular
mechanisms underlying the development of LV hypertrophy and its
transition to diastolic HF, Rysä et al (6) analyzed global alterations in gene
expression in SHRs of different age brackets, and detected
increased expression of hypertrophy-associated genes during the
transition from LV hypertrophy to HFpEF.
MicroRNAs (miRNAs/miRs) are a group of small
non-coding RNAs that regulate gene expression at the
post-transcriptional level and serve as key regulators in
cardiovascular diseases, including hypertension, cardiac
hypertrophy and HF. To the best of our knowledge, no previous study
has reported the use of high-throughput small RNA sequencing in
identifying miRNA profile alterations between young and old SHRs.
Since advanced aging serves an important role in the process of
diastolic dysfunction, the determination of alterations in miRNA
profiles that may affect LV hypertrophy and to what extent they
differ between young and aging SHRs is of great value. The present
study aimed to analyze miRNA gene profiles in myocardial tissues
between young and aging SHRs, in order to provide novel insights
into potential therapeutic targets for the prevention and treatment
of HFpEF resulting from primary hypertension.
Materials and methods
Experimental animals
A total of 6 male SHRs weighing about 400 g were
purchased from Shanghai Laboratory Animal centre, chinese Academy
of Sciences (Shanghai, china), among which 3 rats were 3 months
old, and the remaining 3 rats were 12 months old. All rats were
housed in a room at 23°C, 60% humidity under a 12-h light/dark
cycle. Food and water were supplied ad libitum. All
experiments and procedures were performed in compliance with the
Guide for the Care and Use of Laboratory Animals published by the
US National Institutes of Health (NIH; publication no. 85–23,
revised 1996). The present study was approved by the Animal Care
Committee in Zhongshan Hospital, Fudan University (Shanghai,
China).
Experimental protocol
Body weight (BW) was determined using an electronic
scale. Systolic blood pressure (SBP) was measured according to the
tail-cuff method (BP-2000 Blood Pressure Analysis System; Visitech
Systems, Apex, NC, USA). Subsequently, rats were subjected to
transthoracic high frequency echocardiography. BW, SBP and
echocardiography were assessed in one day just before
euthanization. Once echocardiography was completed, all rats were
euthanized and the LV was rapidly excised and weighed, in order to
calculate LV mass index (LVMI), which is expressed as a ratio of LV
weight (mg)/total BW (g). In addition, venous blood samples were
collected for further study. The middle section of the LV at the
papillary level was immediately cut into 2-mm transverse sections,
some of which were immersed in 10% neutral buffered
paraformaldehyde for histological analysis. Other transverse
sections were snap frozen in Tissue-Tek® optimum cutting
temperature compound (Sakura Finetek USA, Inc., Torrance, CA, USA)
and were stored at −80°C for dihydroethidium (DHE) staining. The
remaining tissue was quickly treated with TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) at −80°C for RNA preparation.
Echocardiography
After anesthesia, the precordial region of the rats
was shaved and they were placed in a supine position on the warmed
stage of a Vevo 770 High Resolution Echocardiography system
(VisualSonics, Inc., Toronto, ON, Canada). Transthoracic
echocardiographic measurements were performed using a 17.5 MHz
scanhead. Parasternal long- and short-axis two-chamber M-mode views
were obtained at midpapillary level, in order to determine LV
end-diastolic diameter (LVEDD), LV end-systolic diameter (LVESD),
LV posterior wall thickness (LVPW) and interventricular septum
thickness in diastole (IVS). LV ejection fraction (LVEF) and
fractional shortening (LVFS) were measured directly from the
long-axis image. Transmitral flow velocity was assessed from a
four-chamber apical view using pulsed-wave Doppler imaging. Peak
velocities of early (E) and late (A) mitral inflow waves,
isovolumic relaxation time (IVRT) and E-wave deceleration time (DT)
were measured, and the E/A ratio was calculated. Lateral mitral
annulus velocity was also assessed using tissue Doppler imaging.
Peak early (E′) and late (A′) diastolic annular velocities were
recorded, and the E′/A′, E/E′ were calculated. All measurements
were averaged from four consecutive cardiac cycles and were
conducted by an experienced echocardiographer blinded to the
protocol.
Plasma N-terminal pro-natriuretic peptide
(NT-proBNP) assay
Venous blood was collected into EDTA tubes
immediately after sacrifice. Samples were centrifuged at 4°c,
at3500 × g for 10 min and the plasma samples were collected and
stored at −80°C for subsequent analysis. Plasma NT-proBNP
concentration was quantified using an ELISA kit (SEA485Ra;
Cloud-Clone Corp., Katy, TX, USA) according to the manufacturer's
protocol. Briefly, standard or sample, detection reagent, substrate
solution and stop solution were sequentially added to the wells and
incubated, with repeated washing as appropriate. Absorbance was
measured using a microplate reader at a wavelength of 450 nm, and
sample values were calculated from the standard curve.
Histopathological analysis and DHE
staining
Transverse LV specimens were fixed in 10% buffered
formalin for 24 h at room temperature, embedded in paraffin and cut
into 4-μm sections. Hematoxylin and eosin staining at room
temperature was used to assess cardiomyocyte cross-sectional area;
an average of ≥100 myocytes taken from five randomly selected
high-powered fields were analyzed (magnification, ×400) for each
specimen. Masson trichrome staining was used to evaluate the extent
of fibrosis, which was obtained by dividing the sum of the fibrotic
area by total tissue area under five randomly selected high-powered
fields (magnification, ×200). Microvessel density was assessed by
cluster of differentiation (CD)31 immunohistochemical staining.
Following deparaffinization by gradient xylene and ethanol,
inactivation of endogenous enzyme with hydrogen peroxide,
heat-induced antigen retrieval and sealing with goat serum, slides
were incubated with rabbit anti-CD31 monoclonal antibody (1:500;
ab182981; Abcam, Cambridge, UK) for 12 h at 4°c, followed by
incubation with biotinylated goat anti-rabbit immunoglobulin G and
streptavidin-biotin-peroxidase complex (1:100; SA2002; Wuhan Boster
Biological Technology, Ltd., Wuhan, China) for 20 min each at room
temperature. Subsequently, the slides were treated with
3′-diaminobenzidine; brown staining was considered positive.
Microvessel density was expressed as the number of CD31+
endothelial cells (including single cells, cluster or tubular
structures) in five randomly selected high-powered fields
(magnification, ×400) for each section. Digital images were
captured under a microscope (Leica Microsystems GmbH, Wetzlar,
Germany) and analyzed using a high-resolution digital image
analysis system (Qwin V3; Leica Microsystems GmbH). DHE staining
was used to quantify reactive oxygen species (ROS) production in
heart samples. Unfixed frozen sections (8 μm) of myocardium
were incubated with DHE (5 μmol/l, 100 μl/section;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C in a
humidified chamber protected from light for 30 min. Digital images
were captured using a fluorescence microscope (Leica Microsystems
GmbH) and were analyzed by ImageJ software (version 1.44; NIH,
Bethesda, MD, USA). Images of five randomly selected fields
(magnification, ×200) of each sample were captured with the in
situ fluorescence intensity calculated and averaged.
RNA extraction, small RNA sequencing and
data processing
Small RNA sequencing was commercially conducted by
Guangzhou RiboBio Co., Ltd (Guangzhou, China). Total RNA was
isolated from tissues using the TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
RNA purity was assessed using the ND-1000 Nanodrop (NanoDrop;
Thermo Fisher Scientific, Inc., Wilmington, DE, USA). Each RNA
sample had an A260:A280 ratio >1.8 and an A260:A230 ratio
>2.0. RNA integrity was evaluated using the Agilent 2200
TapeStation (Agilent Technologies, Inc., Santa Clara, CA, USA) and
each sample had a RINe >7.0. For cDNA library
construction, 1.0 μg RNA was ligated with 3′ RNA adapter
(AAGATCGGAAGAGCACACGTCT), followed by 5′ adapter ligation
(GUUCAGAGUUCUACAGUCCGACGAUC). Subsequently, the adapter-ligated
RNAs were subjected to reverse transcription-polymerase chain
reaction (RT-PCR) and were amplified for 12 cycles. Subsequently,
the PCR products were size-selected by PAGE, according to the
instructions of the TruSeq® Small RNA Sample Prep kit
(Illumina, Inc., San Diego, CA, USA). The purified library products
were evaluated using the Agilent 2200 TapeStation (Agilent
Technologies, Inc.) and were diluted to 10 pM for cluster
generation in situ on the HiSeq 2500 single-end flow cell,
followed by sequencing (1×50 bp) on HiSeq 2500 (Illumina,
Inc.).
After both 3′ and 5′ adaptor sequences were trimmed,
reads containing the adapter and poly-N regions, and those of low
quality were filtered according to base quality value, including a
phred quality mean score >30 and reads between 18 and 40 nt in
length. Clean reads were aligned against Rat Nov. 2014 (rn6)
assembly, by means of Burrows-Wheeler Aligner (https://sourceforge.net/projects/bio-bwa/), allowing
seven mismatches and a gap length of 7 bp. The functional
annotations of small RNA variants were then generated. The
predicted miRNAs were annotated and identified using miRBase v.21.0
(http://www.mirbase.org/). In each sample, miRNA
expression levels were scaled and normalized as transcripts per
million of total aligned miRNA reads. Read counts of 3-month-old
and 12-month-old groups were compared and
log2-transformed. Candidate miRNAs with fold-change
>2.0 and P<0.05 were subjected to clustering analysis using
Cluster 3.0 and Java Treeview software (http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm).
Validation of miRNA expression and
inflammation-associated mRNA detection
RT-quantitative PCR (RT-qPCR) was conducted to
verify eight significantly altered and remodeling-associated
miRNAs, which were revealed by small RNA sequencing. In addition,
the mRNA expression levels of tumor necrosis factor-α (TNF-α),
intercellular cell adhesion molecule-1 (ICAM-1) and vascular cell
adhesion molecule-1 (VCAM-1) were detected by RT-qPCR. Firstly,
total RNA was isolated from LV tissue samples using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). cDNA was synthesized in a 20 μl reaction volume using
PrimeScript™ RT reagent kit (Takara Bio, Inc. Otsu, Japan)
according to the manufacturer's protocol. qPCR was performed using
the SYBR® Premix Ex Taq™ kit (Takara Bio, Inc.)
in a 10 μl reaction volume containing cDNA template,
SYBR® Premix Ex Taq™, PCR forward primer, PCR
reverse primer and RNase-free water on a CFX96 Touch™ Real-Time PCR
Detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
miRNA-specific stem-loop RT and PCR primers were designed and
provided by Guangzhou RiboBio Co., Ltd., whereas mRNA primers were
provided by Sangon Biotech Co., Ltd. (Shanghai, China) (Table I). PCR thermal cycling involved a
denaturing step at 95°C for 30 sec, followed by 40 cycles of
annealing at 95°C for 5 sec and extension at 60°C for 34 sec; each
sample was analyzed in triplicate. Quantification of relative miRNA
expression was normalized to RNU6 as an endogenous control and mRNA
expression was normalized to β-actin using the standard
22−ΔΔCq method (7).
 | Table IPrimers used for reverse
transcription-quantitative polymerase chain reaction quantification
of differentially expressed miRNAs and inflammation-associated
mRNAs. |
Table I
Primers used for reverse
transcription-quantitative polymerase chain reaction quantification
of differentially expressed miRNAs and inflammation-associated
mRNAs.
| miRNA/mRNA | Primer
sequence |
|---|
| rno-miR-132-3p | |
| RT primer |
5′-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACCGACCAT-3 |
| Forward |
5′-GCGTAACAGTCTACAGCCAT-3′ |
| Reverse |
5′-CAGTGCGTGTCGTGGAGT-3′ |
| rno-miR-182 | |
| RT primer |
5′-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACCGGTGTG-3′ |
| Forward |
5′-GGTTTGGCAATGGTAGAACTCA-3′ |
| Reverse |
5′-CAGTGCGTGTCGTGGAGT-3′ |
|
rno-miR-208b-3p | |
| RT primer |
5′-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACACCTTTT-3′ |
| Forward |
5′-GGCCGGGATAAGACGAACAA-3′ |
| Reverse |
5′-CAGTGCGTGTCGTGGAGT-3′ |
| rno-miR-212-3p | |
| RT primer |
5′-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACTGGCCGT-3′ |
| Forward |
5′-TAACAGTCTCCAGUCACGGCCA-3′ |
| Reverse |
5′-CAGTGCGTGTCGTGGAGT-3′ |
| rno-miR-214-3p | |
| RT primer |
5′-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACCTGCCTG-3′ |
| Forward |
5′-CGACAGCAGGCACAGACA-3′ |
| Reverse |
5′-CAGTGCGTGTCGTGGAGT-3′ |
|
rno-miR-218a-5p | |
| RT primer |
5′-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACACATGGT-3′ |
| Forward |
5′-GCGGTTGTGCTTGATCTAAC-3′ |
| Reverse |
5′-CAGTGCGTGTCGTGGAGT-3′ |
| rno-miR-221-3p | |
| RT primer |
5′-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACGAAACCC-3′ |
| Forward |
5′-GGAGCTACATTGTCTGCTGG-3′ |
| Reverse |
5′-CAGTGCGTGTCGTGGAGT-3′ |
| rno-miR-222-3p | |
| RT primer |
5′-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACACCCAGT-3′ |
| Forward |
5′-AGCUTACATCTGGCTACTGGGT-3′ |
| Reverse |
5′-CAGTGCGTGTCGTGGAGT-3′ |
| U6 | |
| RT primer |
5′-AACGCTTCACGAATTTGCGT-3′ |
| Forward |
5′-CTCGCTTCGGCAGCACA-3′ |
| Reverse |
5′-AACGCTTCACGAATTTGCGT-3′ |
| TNF-α | |
| Forward |
5′-ATGGGCTCCCTCTCATCAGT-3′ |
| Reverse |
5′-GCTTGGTGGTTTGCTACGAC-3′ |
| ICAM-1 | |
| Forward |
5′-ACCTGGACAAGAAGGACTGC-3′ |
| Reverse |
5′-GGTCAGATTAGGGGCTGGAT-3′ |
| VCAM-1 | |
| Forward |
5′-TGGGAAACTGGAAAGAGGAA-3′ |
| Reverse |
5′-CATCAGGAGCCAAACACTTG-3′ |
| β-actin | |
| Forward |
5′-GAAGTGTGACGTTGACATCCG-3′ |
| Reverse |
5′-TGCTGATCCACATCTGCTGGA-3′ |
miRNA targets prediction, Gene Ontology
(GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway analysis
As aforementioned, for the eight differentially
expressed and remodeling-associated miRNAs, target genes were
predicted using the miRDB-miRanda algorithm and were then subjected
to GO enrichment analysis using the Blast2GO program (https://www.blast2go.com/). All target genes were
mapped to each term of the GO database (http://www.geneonology.org) to calculate the number of
genes corresponding to each GO term. The significantly enriched GO
terms (P<0.05) in biological process, cellular component and
molecular function were identified by hypergeometric distribution.
As a major public pathway-associated database, KEGG (http://www.genome.jp/kegg/) identifies significantly
enriched signaling pathways in target gene candidates compared with
the entire reference gene background. Significantly enriched KEGG
pathways (P<0.05) of the predicted target genes were identified
using KOBAS software version 2.0 (KOBAS, Surrey, UK).
Statistical analysis
Experiments were repeated 3 times and all values are
expressed as the means ± standard deviation. Group differences were
evaluated using Student's t-test. P<0.05 was considered to
indicate a statistically significant difference. All analyses were
conducted using SPSS 18.0 for Windows (SPSS Inc., Chicago, IL,
USA). Data processing for miRNAs screening and target gene
functional annotation was performed by Guangzhou RiboBio Co.,
Ltd.
Results
SEP, LVMI and plasma NT-proBNP
SBP (187.3±5.5 mmHg vs. 162.3±9.6 mmHg, P<0.05)
and LVMI (2.89±0.27 mg/g vs. 2.07±0.26 mg/g, P<0.05) were
significantly increased in 12-month-old SHRs compared with in
3-month-old SHRs. Plasma NT-proBNP concentration was also elevated
from 259.93±24.74 pg/ml at 3 months to 419.10±28.11 pg/ml at 12
months (P<0.01).
Echocardiographic parameters for 3- and
12-month-old SHRs
Compared with at 3 months of age, LV geometry of
SHRs at 12 months exhibited eccentric hypertrophy and a tendency
towards LV dilation, which was associated with increased IVS, LVPW
and LVEDD. Conversely, LVESD, LVFS and LVEF remained unchanged
between 3 and 12 months of age, thus indicating that LV systolic
function was unimpaired in 12-month-old SHRs. Diastolic function
was assessed by transmitral pulsed-wave Doppler imaging and tissue
Doppler imaging of lateral mitral annulus. E/A ratio and E′/A′
ratio were significantly decreased, whereas E/E′ was increased in
12-month-old SHRs compared with in 3-month-old SHRs (Fig. 1A). In addition, at 12 months of
age, SHRs exhibited prolonged IVRT and DT (Table II). Collectively, these data
suggested that aging SHRs developed HF with a preserved LVEF,
marked LV hypertrophy, and abnormal LV diastolic properties, as
characterized by delayed LV relaxation and shortened LV filling
time.
 | Figure 1Typical images for echocardiography
and histological analysis. (A) Transmitral pulsed-wave Doppler
imaging and tissue Doppler imaging at the level of lateral mitral
annulus obtained from apical four-chamber view. Peak flow velocity
of the E/A waves and peak tissue velocity of E′/A′ diastolic waves
were assessed. A decreased E/A ratio and E′/A′ ratio could be
observed in 12-month-old SHRs compared with in 3-month-old SHRs.
(B) H&E staining (magnification, ×400), Masson's trichrome
staining (magnification, ×200), CD31 staining (magnification, ×400)
and DHE staining (magnification, ×200), for the calculation of
cardiomyocyte CSA, relative fibrosis area (%), microvessel density
and reactive oxygen species production, respectively. Five randomly
selected fields from each left ventricular section were measured.
*P<0.05 and **P<0.01 vs. 3-months-old
SHRs. A, late; CD31, cluster of differentiation 31; CSA,
cross-sectional area; DHE, dihydroethidium; E, early; H&E,
hematoxylin and eosin; SHRs, spontaneously hypertensive rats. |
 | Table IIComparison of echocardiographic data
between 3- and 12-month-old spontaneously hypertensive rats. |
Table II
Comparison of echocardiographic data
between 3- and 12-month-old spontaneously hypertensive rats.
| Variable | 3-month-old | 12-month-old | P-value |
|---|
| LVEDD (mm) | 5.23±0.25 | 6.27±0.55 | 0.042 |
| LVESD (mm) | 2.90±0.17 | 3.27±0.25 | 0.106 |
| IVS (mm) | 1.83±0.25 | 2.47±0.17 | 0.033 |
| LVPW (mm) | 1.87±0.15 | 2.93±0.25 | 0.003 |
| LVEF (%) | 82.6±2.2 | 82.1±2.4 | 0.778 |
| LVFS (%) | 44.6±1.4 | 47.7±5.4 | 0.391 |
| DT (ms) | 20.3±1.5 | 24.7±1.5 | 0.025 |
| IVRT (ms) | 34.7±1.5 | 38.3±1.2 | 0.029 |
| E/A | 2.25±0.07 | 1.72±0.23 | 0.018 |
| E′/A′ | 1.64±0.26 | 0.78±0.02 | 0.004 |
| E/E′ | 26.0±2.5 | 38.2±2.0 | 0.003 |
Histological alterations and ROS
production in LV myocardial tissues
Histological analysis demonstrated that LV
myocardium developed significant hypertrophy and fibrosis in SHRs
at 12 months old compared with at 3 months old, as presented by
markedly increased myocyte cross-sectional area (P<0.01) and
percentage of fibrosis area (P<0.05). Progressive myocardial
hypertrophy and interstitial fibrosis may account for LV stiffness
and diastolic dysfunction in aging SHRs. The results of CD31
immunohistochemical staining demonstrated that microvessel density
was significantly reduced in 12-month-old SHRs (P<0.01), thus
indicating that capillary rarefaction is present in the myocardial
tissue of aging SHRs. In addition, DHE fluorescence intensity was
significantly increased in 12-month-old SHRs compared with in
3-month-old SHRs (P<0.05), thus suggesting that ROS production
was elevated in aging SHRs (Fig.
1B).
Deep small RNA sequencing data analysis
and verification
To identity miRNAs associated with the progression
of spontaneous hypertension, six small RNA differential expression
libraries were constructed and sequenced using the high-throughput
HiSeq 2500 platform. After trimming the 3′ and 5′ adapter
sequences, and removing low quality reads, as well as reads
containing poly N tags and reads <18 nt or >40 nt, an average
of 14,295,600 and 13,165,714 clean reads were obtained from the
3-month-old and 12-month-old SHR groups, respectively. The
sequenced clean small RNAs included various categories of transfer
RNA, ribosomal RNA, small nuclear RNA, small nucleolar RNA,
piwi-interacting RNA, miRNA and other unannotated reads, of which
miRNA tags accounted for 7,432,397 (51.99%) and 6,495,689 (49.34%)
for these two groups, respectively (Fig. 2A and B). The results of
high-throughput sequencing indicated that 385 miRNAs were
coexpressed in the 3- and 12-month-old SHR groups; whereas 14 and
25 were preferentially expressed in 3- and 12-month-old SHRs,
respectively (Fig. 2C). Of the
385 coexpressed miRNAs, 82 miRNAs were up- or downregulated between
the 3-month-old and 12-month-old SHRs with a fold-change >2.0
(Fig. 2D), among which 26 mature
miRNAs were differentially expressed with a fold-change >2.0 and
P<0.05, including those expressed from the 5′-arm and 3′-arm.
Compared with 3-month-old SHRs, 21 miRNAs were upregulated and five
miRNAs were downregulated in 12-month-old SHRs (Table III). A heat map was constructed
to visualize the results of hierarchical clustering of the
differentially expressed miRNAs between the 3-month-old and
12-month-old SHR samples (Fig.
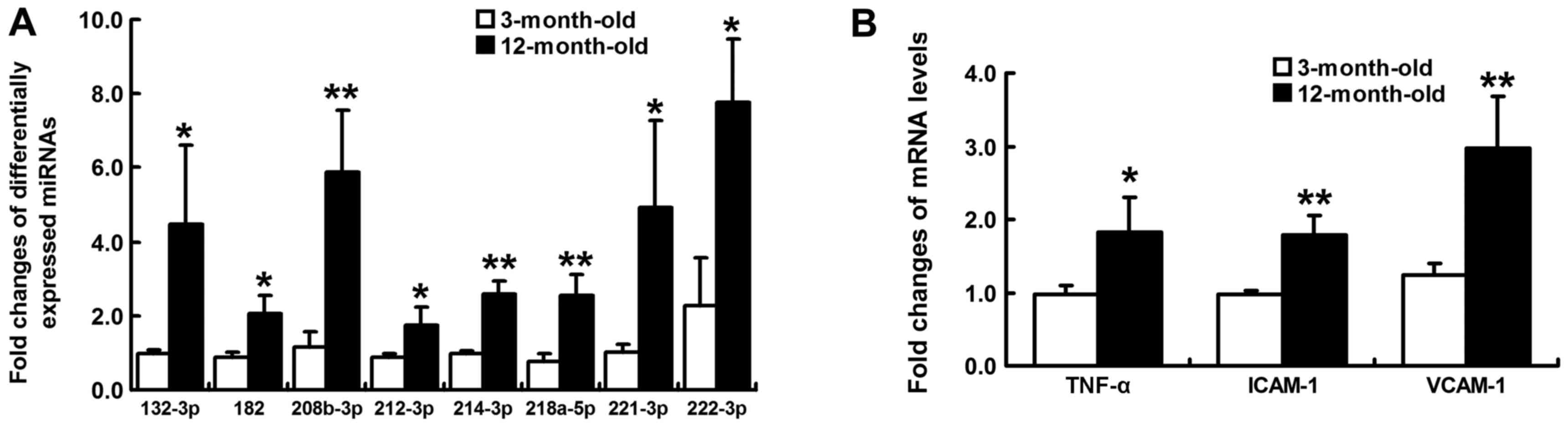
3). Notably, the majority of those closely associated with
myocardial remodeling were upregulated, including rno-miR-132-3p,
rno-miR-182, rno-miR-208b-3p, rno-miR-212-3p, rno-miR-214-3p,
rno-miR-218a-5p, rno-miR-221-3p and rno-miR-222-3p. The expression
levels of these miRNAs were validated by RT-qPCR, the results of
which identified a similar pattern of upregulation consistent with
small RNA sequencing data (Fig.
4A).
 | Figure 2High-throughput small RNA sequencing
analysis in 3- and 12-month-old SHRs. Pie-charts representing the
distribution of different classes of small RNA reads in (A) 3- and
(B) 12-month-old SHR groups, in which miRNAs constituted 51.99 and
49.34% of all small RNA reads. Data were averaged from three
samples from each group. (C) Venn diagram indicating the
distribution of 424 unique miRNAs between 3- and 12-month-old SHRs.
The overlapping section contains 385 coexpressed miRNAs, among
which 26 miRNAs were significantly differentially expressed
(P<0.05). (D) Differences in the miRNA profiles from 3- and
12-month old SHRs, presented as a scatter plot with normalized
values (number of reads per million clean tags). A total of 57
miRNAs were upregulated (red) and 25 miRNAs were downregulated
(green) with a fold-change >2 irrespective of P-value. The
remaining 303 miRNAs were equally expressed (blue) with a
fold-change <2. miRNA, microRNA; piRNA, piwi-interacting RNA;
rRNA, ribosomal RNA; SHRs, spontaneously hypertensive rats; snRNA,
small nuclear RNA; snoRNA, small nucleolar RNA; tRNA, transfer
RNA. |
 | Table IIISignificantly up- and downregulated
miRNAs between 3- and 12-month-old spontaneously hypertensive rats
(including those expressed from the 5′-arm and 3′-arm). |
Table III
Significantly up- and downregulated
miRNAs between 3- and 12-month-old spontaneously hypertensive rats
(including those expressed from the 5′-arm and 3′-arm).
| miRNA | Normalized read no.
| Fold
(log2) | P-value |
|---|
| 3 m | 12 m |
|---|
| Upregulated | | | | |
|
rno-miR-132-3p | 7.31 | 16.31 | 1.16 | <0.001 |
|
rno-miR-132-5p | 2.07 | 6.37 | 1.62 | 0.03 |
| rno-miR-182 | 6.43 | 15.16 | 1.24 | 0.01 |
|
rno-miR-208b-3p | 64.66 | 250.22 | 1.95 | <0.001 |
|
rno-miR-212-3p | 0.95 | 4.03 | 2.08 | 0.04 |
|
rno-miR-214-3p | 68.72 | 153.49 | 1.16 | <0.001 |
|
rno-miR-218a-5p | 69.29 | 140.69 | 1.02 | <0.001 |
|
rno-miR-221-3p | 116.83 | 290.16 | 1.31 | <0.001 |
|
rno-miR-221-5p | 5.99 | 12.20 | 1.03 | 0.03 |
|
rno-miR-222-3p | 43.55 | 109.15 | 1.33 | <0.001 |
|
rno-miR-375-3p | 0.92 | 33.60 | 5.19 | <0.001 |
|
rno-miR-129-5p | 1.18 | 5.56 | 2.24 | 0.01 |
| rno-miR-9a-3p | 2.65 | 8.30 | 1.65 | 0.04 |
|
rno-miR-31a-5p | 4.30 | 19.85 | 2.21 | <0.001 |
|
rno-miR-183-5p | 3.01 | 7.91 | 1.39 | 0.02 |
|
rno-miR-224-5p | 70.84 | 152.60 | 1.11 | <0.001 |
| rno-miR-31b | 0.90 | 5.81 | 2.70 | <0.001 |
| rno-miR-147 | 3.23 | 8.40 | 1.38 | 0.03 |
|
rno-miR-708-5p | 3.86 | 8.89 | 1.20 | 0.04 |
|
rno-miR-92b-3p | 1.34 | 4.73 | 1.82 | 0.05 |
|
rno-miR-511-3p | 2.30 | 6.63 | 1.53 | 0.03 |
| Downregulated | | | | |
|
rno-miR-208a-5p | 20.33 | 9.16 | −1.15 | <0.001 |
|
rno-miR-208a-3p | 521.89 | 187.50 | −1.48 | <0.001 |
|
rno-miR-181c-3p | 12.05 | 5.79 | −1.06 | 0.01 |
|
rno-miR-547-3p | 9.81 | 4.5236 | −1.12 | 0.02 |
|
rno-miR-208b-5p | 9.38 | 4.1846 | −1.17 | 0.03 |
Inflammation-associated mRNA
expression
In order to investigate myocardial inflammatory
reaction, the mRNA expression levels of TNF-α, ICAM-1 and VCAM-1
were compared between young and aging SHRs. As a result, these
proinflammatory cytokines were significantly upregulated in
12-month-old SHRs (P<0.05; Fig.
4B).
Target gene prediction and functional
annotation
To further elucidate the biological functions and
signaling pathways associated with the eight aforementioned miRNAs,
target gene prediction was performed using the miRDB-miRanda
algorithm; a total of 1,306 genes were identified. Subsequently,
the target genes were analyzed using Blast2GO program and KOBAS 2.0
software for GO annotation and KEGG pathway analysis, respectively,
in order to understand the molecular interaction and reaction
networks. In the GO annotation analysis, 590 biological process
terms, 40 cellular component terms and 58 molecular function terms
were significantly enriched (P<0.05). Notably, protein kinase
regulator activity, regulation of peptidyl-tyrosine
phosphorylation, membrane raft, voltage-gated potassium channel
complex and activity, and regulation of protein serine/threonine
kinase activity were among the most significantly enriched GO
terms, thus indicating that the target genes participated largely
in cellular signal transduction (Fig.
5A). KEGG pathway analysis identified 39 significantly enriched
pathways associated with the target genes. Adrenergic signaling in
cardiomyocytes, ErbB signaling pathway, mTOR signaling pathway,
FoxO signaling pathway, Ras signaling pathway, insulin secretion,
adipocytokine signaling pathway, HIF-1 signaling pathway, Rap1
signaling pathway, VEGF signaling pathway, chemokine signaling
pathway and TNF signaling pathway were among the most enriched
categories (Fig. 5B).
Discussion
The present study detected increased LV hypertrophy,
fibrosis and stiffness in aging SHRs, leading to diastolic HF;
these findings were consistent with those of a previous study
(2). Notably, the present study
identified a markedly different myocardial miRNA expression profile
in 3-month-old SHRs compared with in 12-month-old SHRs, thus
suggesting that miRNAs may exert important roles during the
transition from hypertension to the onset of diastolic dysfunction
and HFpEF in SHRs. A total of 21 miRNAs were upregulated in
12-month-old SHRs, whereas five miRNAs were downregulated. Several
of the upregulated miRNAs were involved in cardiac hypertrophy and
fibrosis, cardiomyocyte autophagy, angiogenesis, insulin signaling,
glucose metabolism and the inflammatory response, and therefore may
lead to adverse cardiac remodeling. Bioinformatics analysis
revealed that numerous signaling pathways targeting myocardial
hypertrophy, autophagy, insulin metabolism, angiogenesis and
inflammatory response were involved during the progression of
diastolic dysfunction.
Although cardiac hypertrophy is a compensatory
mechanism for the maintenance of cardiac output, prolonged
pathological hypertrophy has adverse consequences associated with
HF and sudden death. Conversely, activation of autophagy during
stress conditions is generally considered an adaptive response that
compensates for energy loss and rescues spontaneous cardiac
hypertrophy. It has been reported that overexpression of the
miR-212/132 family directly targets the antihypertrophic and
pro-autophagic forkhead box (FoxO)3 transcription factor, which
leads to hyperactivation of prohypertrophic calcineurin/nuclear
factor of activated T-cells signaling and an attenuated autophagic
response (8). In vivo
miR-214 overexpression has been reported to induce cardiac
hypertrophy by reducing the mRNA expression levels of enhancer of
zeste homolog 2 (9). miR-221 has
been reported to inhibit autophagy, induce re-expression of fetal
genes, and promote cardiac hypertrophy and remodeling by modulating
the p27/cyclin-dependent kinase 2/mammalian target of rapamycin
(mTOR) axis (10,11). All of these miRNAs were
upregulated in 12-month-old SHRs in the present study, and may be
associated with the accelerated cardiac hypertrophy detected in
aging SHRs. Considering that miR-208b-5p has been defined as the
released or destroyed strand, upregulated miR-208b and
downregulated miR-208a expression was observed in aging SHRs in the
present study. In rodents, miR-208b has been reported to be largely
expressed in fetal hearts, whereas its expression is reduced in
adult rodents. Conversely, miR-208a exhibits lower expression
levels during heart development, becoming predominant in adulthood
(12). In addition, it is well
known that cardiac hypertrophy is associated with the reappearance
of a pattern of gene expression that is similar to that in fetal
and newborn rat hearts. The results of the present study are
therefore in agreement with the concept of re-expression of fetal
genes in cardiac hypertrophy.
Insulin resistance has also been confirmed to be
associated with LV diastolic dysfunction, independent of overt
diabetes. In addition, adiponectin signaling has been reported to
exert protective effects on glucose metabolism and insulin
sensitization (13), whereas
impaired adiponectin signaling may be associated with increased
progression of LV hypertrophy in patients presenting with
hypertension (14). Chen et
al reported that miR-221 was able to inhibit adiponectin
receptor (AdipoR)1 translation by binding to its 3′-untranslated
region (15). miR 218 has also
been revealed to target AdipoR2 mRNA and inhibit
adiponectin-induced AMP-activated protein kinase activation and
glucose uptake (16). In
addition, overexpression of miR-375 has been reported to suppress
glucose-induced insulin secretion, in which myotrophin was
predicted to be a target gene and the mechanism was correlated with
a direct effect on insulin exocytosis (17). All of these upregulated miRNAs may
impair adiponectin signaling, insulin signaling and glucose
metabolism, thus promoting deterioration of cardiac diastolic
function.
Angiogenesis serves an important role in cardiac
hypertrophy. Hypertrophic stimuli initially promote angiogenesis in
the heart, allowing cardiac growth and angiogenesis to be
coordinated in the adaptive phase of hypertrophy. It has previously
been reported that upregulation of miR-182 is associated with
angiogenesis-induced hypertrophic response (18). However, capillary rarefaction,
resulting from incoordination between cardiomyocyte growth and
angiogenesis in the heart, promotes the transition from adaptive
cardiac hypertrophy to HF (19).
A previous study indicated that aberrant overexpression of miR-214
may reduce cardiac angiogenesis by targeting X-box binding
protein-1 in the maladaptive phase of cardiac hypertrophy (20). The miR-221/222 cluster possesses
strictly antiangiogenic properties in mature endothelial cells, and
stimulates vascular smooth muscle cell dedifferentiation and a
switch from the contractile to synthetic phenotype, thus causing
arterial wall thickening (21).
Furthermore, increased levels of miR-92a/b in the heart has been
suggested to inhibit angiogenesis and contribute to the remodeling
process in a previous study (22). In the present study, obvious
myocardial capillary rarefaction developed in aging SHRs; such
microvascular impairment and cardiac endothelial cell remodeling
has a causative role in the onset and progression of HFpEF.
It has been reported that the miR-221/222 cluster
has an important role in the regulation of vascular inflammation
(15,21). Upregulation of miR-221/222 in
senescent endothelial cells may be a consequence of age-associated
endothelial dysfunction, and the stimulatory effects of low-grade
inflammation commonly persisted in the wall of aged vessels.
Furthermore, miR-221 may promote the expression of adhesion
molecules and enhance the inflammatory response by inhibiting
adiponectin signaling (15). The
present study detected significantly higher myocardial mRNA
expression levels of proinflammatory cytokines, including TNF-α,
ICAM-1 and VCAM-1, in 12-month-old SHRs compared with in
3-month-old SHRs. Furthermore, ROS overproduction was observed in
aging SHRs, thus inducing oxidative stress and triggering cardiac
remodeling, which serves a key role in the development of
hypertension and cardiac hypertrophy (23).
Several of the signaling pathways enriched with the
miRNA targets in the present study were associated with cardiac
hypertrophy and autophagy pathways (i.e., adrenergic signaling in
cardiomyocytes, ErbB signaling pathway, mTOR signaling pathway,
FoxO signaling pathway, Ras signaling pathway) (24–30), insulin and glucose metabolism
pathways (i.e., insulin secretion, adipocytokine signaling pathway)
(31), angiogenesis pathways
(i.e., HIF-1 signaling pathway, Rap1 signaling pathway, VEGF
signaling pathway) (32), and the
inflammatory response (i.e., chemokine signaling pathway, TNF
signaling pathway). These results verified the critical role of the
aforementioned upregulated miRNAs in regulating cardiac
hypertrophy, autophagy, insulin signaling, angiogenesis and
inflammation, which are associated with the progressive diastolic
dysfunction in aging SHRs. However, biological signaling pathways
comprise a complex network and no signaling pathway exerts
completely positive or negative effects. For example, ErbB2
overexpression causes activation of prohypertrophic gene pathways,
including the phosphoinositide 3-kinase/protein kinase B pathway,
whereas disabled ErbB signaling results in a loss of
cardioprotection in cardiac hypertrophy and contributes to the
development of early failure (25,26). Similarly, basal autophagy serves
dual (beneficial and detrimental) roles in cardiac myocyte
function; either too little or too much autophagy elicits
maladaptive and untoward effects. Therefore, the progression from
mere hypertension to HFpEF is associated with a complex spectrum of
pathophysiological conditions, including aberrant cardiac
homeostasis, instead of simply one-dimensional mechanisms.
Notably, abnormal diastolic filling is closely
associated with hypertension and advanced age. Hypertension is much
more common in the elderly, and cardiac aging is a specific
pathophysiological process, which independently contributes to
deterioration of diastolic function. Palao et al (33) reported that the maturation of WKY
and SHR vessels exhibited considerable overlap in terms of miRNA
expression profiles. Upregulated miR-222 expression was detected in
both SHRs and WKY rats when comparing mature rats with young rats,
whereas upregulated miR-132 expression was specific for the
maturation of SHR vessels. Zhang et al (34) detected increased circulating
miR-92a, miR-222 and miR-375 expression during the aging process.
In addition, Rippe et al (35) indicated that compared with
early-passage arterial endothelial cells, miR-221/222 were
upregulated and miR-214 was downregulated in late-passage senescent
endothelial cells. Therefore, these findings indicated that the
miR-132/212 family may be involved in the development of HFpEF.
Since miR-214 and miR-208b expression is usually reduced during the
aging process, the upregulation of these miRNAs in aging SHRs in
the present study may be associated with HFpEF pathology. However,
upregulated miR-92a, miR-221/222 and miR-375 may be associated with
the normal aging process. In addition, hypertension and the aging
process should not be distinctly separated when referring to the
pathogenesis of HFpEF, since hypertension prevalence also increases
with aging. The upregulated miRNAs in the present study may
elucidate the regulatory mechanisms of HFpEF in aging SHRs.
In conclusion, the present study identified
different miRNA expression profiles in aging SHRs compared with in
young SHRs, thus indicating the critical role of miRNAs in the
process of HFpEF. Notwithstanding lack of transgenic experiments to
verify these findings, GO category and KEGG pathway enrichment
analyses suggested that the target genes of these differentially
expressed miRNAs were involved in signaling pathways associated
with cardiac hypertrophy, autophagy, insulin metabolism,
angiogenesis and inflammation. Further genetic manipulations are
required to identify detailed miRNA-targets for the generation of
novel therapeutic strategies for the treatment of HFpEF.
Acknowledgments
The present study was supported by grants from the
National Basic Research Program of China (973 Program; grant no.
2012cB518605) and the National Natural Science Foundation of China
(grant no. 81370199). The study was presented as an abstract at the
28th Great Wall International Congress of Cardiology.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Owan TE, Hodge DO, Herges RM, Jacobsen SJ,
Roger VL and Redfield MM: Trends in prevalence and outcome of heart
failure with preserved ejection fraction. N Engl J Med.
355:251–259. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cingolani OH, Yang XP, Cavasin MA and
Carretero OA: Increased systolic performance with diastolic
dysfunction in adult spontaneously hypertensive rats. Hypertension.
41:249–254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Messerli FH, Rimoldi SF and Bangalore S:
The transition from hypertension to heart failure: Contemporary
update. JACC Heart Fail. 5:543–551. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gu H, Li Y, Fok H, Simpson J, Kentish JC,
Shah AM and Chowienczyk PJ: Reduced first-phase ejection fraction
and sustained myocardial wall stress in hyptensive patients with
diastolic dysfunction: A manifestation of impaired shortening
deactivation that links systolic to diastolic dysfunction and
preserves systolic ejection fraction. Hypertesion. 69:633–640.
2017. View Article : Google Scholar
|
|
5
|
Slama M, Ahn J, Varagic J, Susic D and
Frohlich ED: Long-term left ventricular echocardiographic follow-up
of SHR and WKY rats: Effects of hypertension and age. Am J Physiol
Heart Circ Physiol. 286:H181–H185. 2004. View Article : Google Scholar
|
|
6
|
Rysä J, Leskinen H, Ilves M and Ruskoaho
H: Distinct upregulation of extracellular matrix genes in
transition from hypertrophy to hypertensive heart failure.
Hypertension. 45:927–933. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta c(T)). Method Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
8
|
Ucar A, Gupta SK, Fiedler J, Erikci E,
Kardasinski M, Batkai S, Dangwal S, Kumarswamy R, Bang C, Holzmann
A, et al: The miRNA-212/132 family regulates both cardiac
hypertrophy and cardiomyocyte autophagy. Nat Commun. 3:10782012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang T, Gu H, Chen X, Fu S, Wang C, Xu H,
Feng Q and Ni Y: Cardiac hypertrophy and dysfunction induced by
overexpression of miR-21. in vivo J Surg Res. 192:317–325. 2014.
View Article : Google Scholar
|
|
10
|
Su M, Wang J, Wang C, Wang X, Dong W, Qiu
W, Wang Y, Zhao X, Zou Y, Song L, et al: MicroRNA-221 inhibits
autophagy and promotes heart failure by modulating the
p27/CDK2/mTOR axis. Cell Death Differ. 22:986–999. 2015. View Article : Google Scholar :
|
|
11
|
Wang C, Wang S, Zhao P, Wang X, Wang J,
Wang Y, Song L, Zou Y and Hui R: MiR-221 promotes cardiac
hypertrophy in vitro through the modulation of p27 expression. J
Cell Biochem. 113:2040–2046. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oliveira-Carvalho V, Carvalho VO and
Bocchi EA: The emerging role of miR-208a in the heart. DNA Cell
Biol. 32:8–12. 2013. View Article : Google Scholar
|
|
13
|
Kadowaki T, Yamauchi T, Kubota N, Hara K,
Ueki K and Tobe K: Adiponectin and adiponectin receptors in insulin
resistance, diabetes, and the metabolic syndrome. J Clin Invest.
116:1784–1792. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hong SJ, Park CG, Seo HS, Oh DJ and Ro YM:
Associations among plasma adiponectin, hypertension, left
ventricular diastolic function and left ventricular mass index.
Blood Press. 13:236–242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen CF, Huang J, Li H, Zhang C, Huang X,
Tong G and Xu YZ: MicroRNA-221 regulates endothelial nitric oxide
production and inflammatory response by targeting adiponectin
receptor 1. Gene. 565:246–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du H, Fu Z, He G, Wang Y, Xia G, Fang M
and Zhang T: MicroRNA-218 targets adiponectin receptor 2 to
regulate adiponectin signaling. Mol Med Rep. 11:4701–4705. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Poy MN, Eliasson L, Krutzfeldt J, Kuwajima
S, Ma X, Macdonald PE, Pfeffer S, Tuschl T, Rajewsky N, Rorsman P
and Stoffel M: A pancreatic islet-specific microRNA regulates
insulin secretion. Nature. 432:226–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li N, Hwangbo C, Jaba IM, Zhang J,
Papangeli I, Han J, Mikush N, Larrivée B, Eichmann A, Chun HJ, et
al: miR-182 modulates myocardial Hypertrophic response induced by
angiogenesis in heart. Sci Rep. 6:212282016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shimizu I and Minamino T: Physiological
and pathological cardiac hypertrophy. J Mol cell cardiol.
97:245–262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duan Q, Yang L, Gong W, Chaugai S, Wang F,
Chen C, Wang P, Zou MH and Wang DW: MicroRNA-214 is upregulated in
heart failure patients and suppresses XBP1-mediated endothelial
cells angiogenesis. J Cell Physiol. 230:1964–1973. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chistiakov DA, Sobenin IA, Orekhov AN and
Bobryshev YV: Human miR-221/222 in physiological and
atherosclerotic vascular remodeling. BioMed Res Int.
2015:3545172015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goren Y, Kushnir M, Zafrir B, Tabak S,
Lewis BS and Amir O: Serum levels of microRNAs in patients with
heart failure. Eur J Heart Fail. 14:147–154. 2012. View Article : Google Scholar
|
|
23
|
Bertagnolli M, Schenkel PC, Campos C,
Mostarda CT, Casarini DE, Belló-Klein A, Irigoyen MC and Rigatto K:
Exercise training reduces sympathetic modulation on cardiovascular
system and cardiac oxidative stress in spontaneously hypertensive
rats. Am J Hypertens. 21:1188–1193. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barki-Harrington L, Perrino C and Rockman
HA: Network integration of the adrenergic system in cardiac
hypertrophy. Cardiovasc Res. 63:391–402. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sysa-Shah P, Xu Y, Guo X, Belmonte F, Kang
B, Bedja D, Pin S, Tsuchiya N and Gabrielson K: Cardiac-specific
over-expression of epidermal growth factor receptor 2 (ErbB2)
induces pro-survival pathways and hypertrophic cardiomyopathy in
mice. PLoS One. 7:e428052012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rohrbach S, Yan X, Weinberg EO, Hasan F,
Bartunek J, Marchionni MA and Lorell BH: Neuregulin in cardiac
hypertrophy in rats with aortic stenosis. Differential expression
of erbB2 and erbB4 receptors. Circulation. 100:407–412. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hua Y, Zhang Y, Ceylan-Isik AF, Wold LE,
Nunn JM and Ren J: Chronic Akt activation accentuates aging-induced
cardiac hypertrophy and myocardial contractile dysfunction: Role of
autophagy. Basic Res Cardiol. 106:1173–1191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ni YG, Berenji K, Wang N, Oh M, Sachan N,
Dey A, Cheng J, Lu G, Morris DJ, Castrillon DH, et al: Foxo
transcription factors blunt cardiac hypertrophy by inhibiting
calcineurin signaling. Circulation. 114:1159–1168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sengupta A, Molkentin JD and Yutzey KE:
FoxO transcription factors promote autophagy in cardiomyocytes. J
Biol Chem. 284:28319–28331. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gelb BD and Tartaglia M: RAS signaling
pathway mutations and hypertrophic cardiomyopathy: Getting into and
out of the thick of it. J Clin Invest. 121:844–847. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shibata R, Ouchi N, Ito M, Kihara S,
Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K,
et al: Adiponectin-mediated modulation of hypertrophic signals in
the heart. Nat Med. 10:1384–1389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carmona G, Göttig S, Orlandi A, Scheele J,
Bäuerle T, Jugold M, Kiessling F, Henschler R, Zeiher AM, Dimmeler
S and Chavakis E: Role of the small GTPase Rap1 for integrin
activity regulation in endothelial cells and angiogenesis. Blood.
113:488–497. 2009. View Article : Google Scholar
|
|
33
|
Palao T, Swärd K, Jongejan A, Moerland PD,
de Vos J, van Weert A, Arribas SM, Groma G, vanBavel E and Bakker
EN: Gene expression and microRNA expression analysis in small
arteries of spontaneously hypertensive rats. Evidence for ER
stress. PLoS One. 10:e01370272015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang H, Yang H, Zhang C, Jing Y, Wang C,
Liu C, Zhang R, Wang J, Zhang J, Zen K, et al: Investigation of
microRNA expression in human serum during the aging process. J
Gerontol A Biol Sci Med Sci. 70:102–109. 2015. View Article : Google Scholar
|
|
35
|
Rippe C, Blimline M, Magerko KA, Lawson
BR, LaRocca TJ, Donato AJ and Seals DR: MicroRNA changes in human
arterial endothelial cells with senescence: Relation to apoptosis,
eNOS and inflammation. Exp Gerontol. 47:45–51. 2012. View Article : Google Scholar
|