Introduction
Globally, gastric cancer (GC) has become the second
leading cause of cancer-associated mortality in recent centuries
(1). Since the majority of
patients with GC are diagnosed at the late stages of the disease,
which are associated with metastasis, treatment has little effect
on survival. For these patients, chemotherapy is the standard
treatment; however, patients undergoing chemotherapy often exhibit
poor efficacy due to multidrug resistance (MDR), which leads to the
failure of chemotherapeutic approaches (2). In general, drug resistance and
metastasis/invasion are the two major obstacles to the success of
cancer treatment. Furthermore, it has previously been reported that
drug-resistant cancer cells possess enhanced invasive ability
(3). Despite the fact that
numerous mechanisms underlying drug resistance have been
extensively investigated, including drug delivery and drug-induced
cell apoptosis, the predominant mechanism that induces drug
resistance remains largely obscure. Therefore, investigating the
mechanisms of regulation that underlie drug resistance-mediated
metastasis is the key for the effective diagnosis and treatment of
GC.
MicroRNAs (miRNAs/miRs) are noncoding RNAs that
consist of 19–24 nucleotides. miRNAs regulate gene expression by
targeting the 3′-untranslated regions of mRNAs. Emerging evidence
has indicated that miRNAs serve an important role in the drug
resistance and metastasis of various types of cancer, including GC
(4). For example, the
miR-200bc/429 cluster sensitizes human cancer cell lines to
anticancer drugs by targeting B-cell lymphoma-2, which is a
well-known anti-apoptotic gene (5). In addition, our previous experiments
revealed that miR-1284 has the potential to reverse drug resistance
and inhibit metastasis of GC cells by downregulating eukaryotic
translation initiation factor 4A1 (6). Furthermore, Rawlings-Goss et
al reported that miR-647 may be associated with the most
extensive cancers (breast, testicular, colon, germ cell and gastric
cancer) and may be considered a biomarker for GC (7). Meanwhile, our previous study also
suggested that miR-647 exerts powerful anti-tumorigenic effects
in vitro and in vivo, and may represent a promising
therapeutic agent against GC (8).
A recent study reported that overexpression of miR-647 results in
improved prognosis of Taxol-resistant ovarian cancer cells
(9). However, the underlying role
of miR-647 in drug resistance and metastasis of GC remains to be
elucidated.
The present study aimed to determine the effects of
miR-647 on drug resistance and metastasis of GC in vitro and
in vivo, and to explore its functional mechanisms.
Materials and methods
Ethics statement
The present study was approved by the ethical board
of the First Affiliated Hospital of Guangxi Medical University
(Guangxi, China), and complied with the Declaration of Helsinki.
All animal procedures conducted in the present study followed the
provisions of the Ethics Committee of the First Affiliated Hospital
of Guangxi Medical University. Written informed consent was
obtained from all patients.
Human tissue samples and cell lines
Sixteen pairs of GC tissues and adjacent nontumor
tissues (located 5 cm away from the tumor) were collected during
surgery at the First Affiliated Hospital of Guangxi Medical
University between 2013 and 2015 (age of patients, 47–75 years old;
male, 10 cases; female, 6 cases). Tissue samples were preserved in
liquid nitrogen. Vincristine-resistant SGC7901 (SGC7901/VCR) cells
were obtained from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). SGC7901/VCR and GES-1 (obtained from the Cell
Center of Xiangya Medical University, Changsha, China) cells were
cultured in RPMI-1640 (Hyclone; GE Healthcare Life Sciences, Logan,
UT, USA), supplemented with 50 mg/ml penicillin, 100 mg/ml
streptomycin and 10% fetal bovine serum (Sijiqing Biotech, Co.,
Ltd, Hangzhou, China). Cells were cultured at 37.8°C in an
atmosphere containing 5.0% CO2. Additionally, the groups
treated with VCR were supplemented with 0.8 μg/ml
vincristine, while the groups treated without VCR were not.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted from SGC7901/VCR cells using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). According to the manufacturer's protocol, cDNA
was reverse transcribed from 1,000 ng RNA using the PrimeScript™ RT
Reagent kit (Takara Bio, Inc., Otsu, Japan). The mRNA and miRNA
expression levels were calculated using GAPDH (mRNA) or U6 (miRNA)
as a reference gene. RT-qPCR was conducted using SYBR®
Premix Ex Taq™ II (Tli RNaseH Plus) and a ROX Plus Reagent kit
(Takara Bio, Inc.) according to the manufacturer's protocol. The
thermocycling conditions were as follows: pre-denaturation, 95°C
for 30 sec; amplified reaction, 95°C for 5 sec, 60°C for 20 sec, 40
cycles; dissociation curve, 95°C for 60 sec, 55°C for 30 sec, 95°C
for 30 sec. The mRNA and miRNA expression levels were analyzed
using the 2−ΔΔCq method (10). The primer
sequences used are listed as Table І.
Antibodies
Rabbit anti-human antibodies specific to focal
adhesion kinase (FAK; ab40794), matrix metalloproteinase (MMP)2
(ab92536), MMP12 (ab52897), cluster of differentiation (CD)44
(ab51037), snail family transcriptional repressor 1 (SNAIL1;
ab82846) and GAPDH (ab9485); and mouse anti-human antibodies
specific to ankyrin-B (ANK2; ab131419) were obtained from Abcam
(Cambridge, UK). Infrared-labeled IRDye 800 goat anti-rabbit (P/N
926-32211) and donkey anti-mouse (P/N 926-32212) secondary
antibodies were obtained from LI-COR Biosciences (Lincoln, NE,
USA).
Bioinformatics analysis
The target genes of miR-647 were analyzed by
TargetScan (http://www.targetscan.org) and
microRNA.org (http://www.microrna.org/microrna/home.do).
Establishment of stable cell lines
The miR-647 overexpression vector [LV-miR-647-green
fluorescent protein (GFP)] and null vector (LV-GFP) were purchased
from Shanghai GeneChem Co., Ltd. (Shanghai, China). SGC7901/VCR
cells were seeded in 6-well plates (3×104/well) with
antibiotic-free medium. After seeding into 6-well plates for 24 h,
cells were infected with the indicated viral supernatants at a
multiplicity of infection (MOI) of 100 PFU/cell (MOI=100) with
antibiotic-free medium. To obtain stable infection, the cells were
incubated in the presence of 700 mg/ml G418 (Invitrogen; Thermo
Fisher Scientific, Inc.) for 2–3 weeks at 37°C and were then
collected. The cells were divided into three groups: The control
group, which consisted of untreated SGC7901/VCR cells; the LV-GFP
group, which consisted of cells infected with the LV-GFP negative
control lentiviral vector; and the LV-miR-647 group, which
consisted of cells infected with the LV-miR-647-GFP recombinant
lentiviral vector. miR-647 expression in the infected cells was
detected by RT-qPCR.
Cytotoxicity assay
Cells (2×103 cells/well) were seeded into
96-well plates. After 24 h, vincristine at six concentrations (0,
0.2, 0.4, 0.8, 1.6 and 3.2 mg/ml) was added into the common medium.
After 48 h (37°C), 10 μl Cell Counting kit-8 reagent (CCK-8;
Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added
and the cells were incubated for 1 h at 37°C in an atmosphere
containing 5% CO2. Subsequently, absorbance was detected
at a wavelength of 450 nm. The half maximal inhibitory
concentration (IC50) was calculated according to the
relative survival curve. Each experiment was processed in
quadruplicate.
Cell cycle analysis
Cells were washed twice with PBS and fixed with 70%
ethanol for 12 h at 4°C. The cells were then incubated in a
solution containing RNase (200 ng/ml; RT405-12; Tiangen, Beijing,
China) and propidium iodide (0.05 mg/ml) at room temperature for 30
min. Results were then analyzed by flow cytometry (BD Biosciences,
San Jose, CA, USA). The data was analyzed with the CellQuest™
software (BD Biosciences).
Apoptosis assay
Apoptosis was determined by flow cytometry using the
Apoptosis Detection kit (BD Biosciences), according to the
manufacturer's protocol. Briefly, the cells were incubated in a
solution containing Annexin V-phycoerythrin (5 μl/ml) and
7-amino-actinomycin D (5 μl/ml) at 4°C in the dark for 30
min. Results were then analyzed by flow cytometry. The data was
analyzed with the CellQuest™ software (BD Biosciences).
Wound healing assay
In order to inhibit cell proliferation, cells were
cultured with mitomycin C (10 μg/ml; Sigma, St. Louis, MO,
USA) in 6-well plates (3×106/well). Subsequently, a
straight wound was generated using a 200 μl sterilized pipet
tip and the cells were washed twice with PBS to remove nonadherent
cells. Wound healing was observed at 0, 24, 48 and 72 h under a
microscope (Nikon TS100; Nikon, Tokyo, Japan), and relative
motility was calculated according to the following formula:
Relative motility = (initial distance-a time-point
distance)/initial distance × 100%. A time-point distance could be
24, 48, 72 h (Fig. 2C).
Cell invasion assay
Serum-free RPMI-1640 (75 μl) containing 1
μg/ml Matrigel (BD Biosciences) was added to the upper
chamber of Transwell apparatus (6.5 mm; Corning Inc., Corning, NY,
USA). Cells (5×104) were seeded into the upper chamber
alongside serum-free 200 μl RPMI-1640, whereas 700 μl
RPMI-1640 containing 5% fetal bovine serum was added to the lower
chamber. After 24 h (37°C), the cells that had invaded through the
membranes were stained with Giemsa, and the number of visible cells
was counted in six random views at ×200 magnification using a
microscope (Nikon TS100; Nikon).
Western blot analysis
Proteins were extracted from cells using cell lysis
buffer (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China). Subsequently, proteins were separated by 10%
SDS-PAGE and were transferred onto nitrocellulose membranes.
Protein concentration was measured by BCA Protein Assay kit
(Beyotime Institute of Biotechnology, Shanghai, China). The protein
samples (100 μg) were loaded onto the gel. The membranes
were incubated in blocking buffer for 1 h at room temperature. The
membranes were then incubated with primary antibodies (1:1,000)
overnight at 4°C and were washed with Tris-buffered saline
containing 0.1% Tween. The membranes were then incubated with
infrared-labeled secondary antibodies (1:10,000) for 1 h at room
temperature and LI-COR Odyssey Imaging System (LI-COR Biosciences)
was used to analyze densitometry. GAPDH (1:1,000) was used as an
internal control.
Effects of miR-647 on GC cells in
vivo
BALBC/c nude mice (age, 4–5 weeks) were provided by
the Guangxi Animal Center (Nanning, China). All animal experimental
studies were implemented in the Guangxi Animal Center. Mice were
maintained in specific-pathogen-free surroundings, and feeding
schedules conformed to the guidelines of the Ethics Committee of
Guangxi Medical University (temperature, 20–25°C; humidity, 40–70%;
light and dark 12 h respectively; having access to sterilized food
and water). All the mice lived under these conditions since they
were born. Tumors were implanted by an injection of
4×107 SGC-7901/VCR cells suspended in 100 μl PBS
(Beyotime Institute of Biotechnology) into the armpit region of
nude mice. After 4 days, ~5 mm-diameter tumors were achieved. The
mice were randomly divided into six groups (n=6 mice/group):
Control-nonVCR group, LV-GFP-nonVCR group, LV-miR-647-nonVCR group,
control group, LV-GFP group and LV-miR-647 group. An intratumoral
injection of LV-miR-647-GFP or LV-GFP (5×106 TU) in 100
μl PBS was directed into the tumors of LV-miR-647-nonVCR and
LV-miR-647 groups, or LV-GFP-nonVCR and LV-GFP groups,
respectively; a similar volume of PBS was injected into the
control-nonVCR and control groups. VCR was additionally
administered by intraperitoneal injection (200 ng/kg) into the
control, LV-GFP and LV-miR-647 groups. Following the first
operation (first intratumoral injection), the mice received the
same treatment every 2 days. Tumor size was calculated every 4 days
using a vernier caliper. The length (a) and the width (b) were
measured, and tumor volume was determined as follows: Tumor volume
= a x b2/2. Relative tumor volume (RTV) was estimated
according to the following equation: RTV =
Vt/V0 where V0 refers to the tumor
volume at the time of intraperitoneal injection; and Vt
refers to the tumor volume at the next measurement. After 20 days
of feeding, the mice were sacrificed and tumors were evaluated.
Tumors were immersed in 4% formaldehyde, and were embedded in
paraffin following dehydration with an ethanol series. The tumors
were fixated at room temperature for 24 h. Subsequently, tumor
segments were dewaxed, rehydrated and stained with hematoxylin and
eosin (H&E). Sections were then observed under a middle power
field (×200) (Nikon TS100; Nikon).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical analyses were conducted using SPSS 13.0
(SPSS, Inc., Chicago, IL, USA). Data were analyzed using Student's
t-test, one-way analysis of variance (ANOVA) or χ2 test.
The results between 3 groups were compared by ANOVA and the results
between 2 groups were compared by t-test. P<0.05 was considered
to indicate a statistically significant difference.
Results
miR-647 is decreased in GC tissue
specimens and drug-resistant GC cells
To ascertain whether miR-647 was associated with the
generation of GC metastasis and drug resistance, the present study
detected miR-647 expression in GC tissue specimens from patients
with distant metastasis and compared them to adjacent nontumor
tissues. In 11 out of 16 GC cases, miR-647 expression was
downregulated compared with in the adjacent nontumor tissues, as
determined by RT-qPCR (P<0.05; Fig. 1A). Furthermore, miR-647 expression
was detected in SGC7901/VCR and SGC7901 cells, and in the human
gastric epithelial cell line GES-1. The expression levels of
miR-647 in GES-1 cells were 3.4- and 7.3-fold higher compared with
in SGC7901 and SGC7901/VCR cells, respectively (P<0.05); and
miR-647 expression was 2.1-fold higher in SGC7901 cells compared
with in SGC7901/VCR cells (P<0.05; Fig. 1B). These results indicated that
miR-647 may be associated with distant metastasis of GC and
drug-resistant GC cells.
miR-647 recombinant lentiviral vectors
increase miR-647 expression
To test the hypothesis that miR-647 recombinant
lentiviral vectors could increase the expression levels of miR-647
in SGC7901/VCR cells, these cells were infected with LV-miR-647-GFP
(LV-miR-647 group) and LV-GFP (LV-GFP group). The expression levels
of miR-647 in cells from the LV-miR-647 group were 8.7- and
8.3-fold higher compared with in the LV-GFP and control groups,
respectively (P<0.05; Fig.
1C). These observations suggested that LV-miR-647-GFP may
upregulate miR-647 expression in SGC7901/VCR cells.
miR-647 is able to overcome GC drug
resistance in vitro
SGC7901/VCR cells were selected from SGC7901 cells
using the anticancer drug vincristine, and the expression levels of
miR-647 were observed to be downregulated in SGC7901/VCR cells
compared with in SGC7901 and GES-1 cells (Fig. 1B). The present study hypothesized
that miR-647 could regulate drug resistance in GC cells; therefore,
the effects of miR-647 overexpression on the drug sensitivity of
SGC7901/VCR cells was determined using a CCK-8 assay. The results
demonstrated that the IC50 value for vincristine in the
LV-miR-647 group was 1.69±0.03 μg/ml, which was
significantly lower than that in the LV-GFP (2.46±0.10
μg/ml) and control groups (2.50±0.02 μg/ml)
(P<0.05; Fig. 1D). These
findings indicated that miR-647 may markedly enhance the
sensitivity of GC to vincristine.
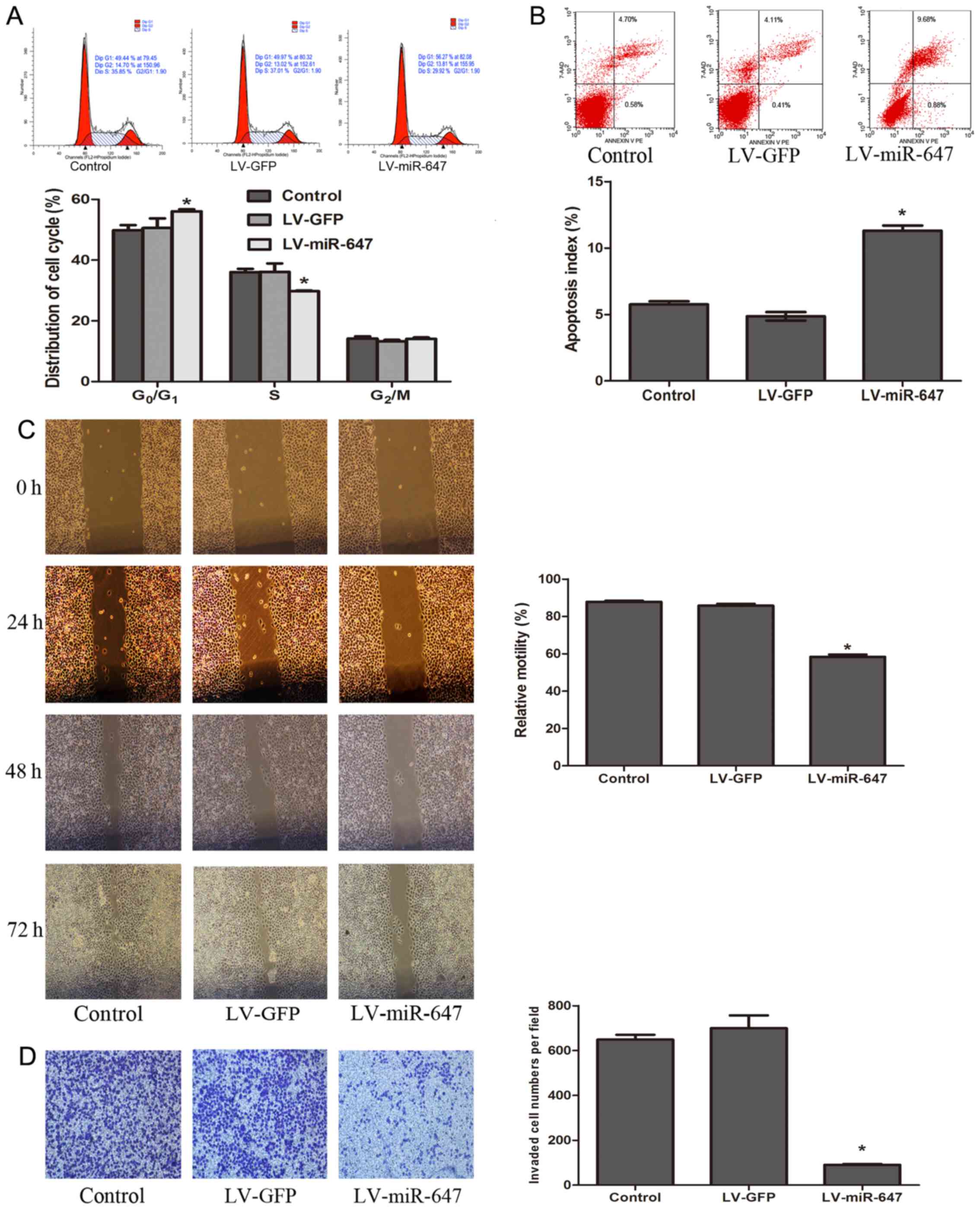
miR-647 promotes cell cycle arrest at the
G0/G1 phase
To explore whether miR-647 overexpression-induced
drug sensitivity was mediated by an alteration in a specific phase
of the cell cycle, flow cytometry was used to detect the number of
cells in each phase of the cell cycle in each group. The results
indicated that the number of cells in G0/G1
phase was markedly increased, whereas the number of cells in S
phase was decreased in the LV-miR-647 group (P<0.05; Fig. 2A). There was no statistical
significance in the groups without vincristine treatment (data not
shown). These findings indicated that reversal of drug resistance
is associated with miR-647, which may prevent cells from entering S
phase and leads to a reduction in the number of cells.
miR-647 accelerates drug-induced
apoptosis
Enhancing drug-induced apoptosis is a key mechanism
for reversing drug resistance. The present results verified that
following incubation with vincristine, the apoptotic rate was
markedly increased in the LV-miR-647 group compared with in the
LV-GFP and control groups (P<0.05; Fig. 2B). There was no statistical
significance in the groups without vincristine treatment (data not
shown). These results indicated that miR-647 increased drug-induced
apoptosis of GC cells, thereby reversing drug resistance.
miR-647 decreases migration and invasion
of GC cells
Since miR-647 was downregulated in tissues from
patients with GC and distant metastasis, it was hypothesized that
miR-647 may be correlated with the migration and invasion of cells.
To test this hypothesis, the migratory and invasive ability of each
group was determined using wound healing and Transwell assays.
After 72 h, the LV-miR-647 group exhibited reduced migratory
ability compared with in the LV-GFP and control groups. The
relative motility rates of cells in the LV-miR-647, LV-GFP and
control groups were 58.43±2.08, 85.79±0.96 and 87.77±1.06%,
respectively (P<0.05; Fig.
2C). With regards to cell invasion, the Transwell assay
indicated that the number of cells that invaded through the
Matrigel membrane in the LV-miR-647 group was reduced compared with
in the LV-GFP and control groups; the number of cells in the LV-GFP
and control groups that invaded through the membrane was 7.7- and
7.1-fold greater compared with in the LV-miR-647 group,
respectively (P<0.05; Fig.
2D). These results suggested that miR-647 may decrease the
migration and invasion of GC cells.
miR-647 modulates drug resistance by
reducing the expression levels of ANK2, FAK, MMP2, MMP12, CD44 and
SNAIL2
The data generated using TargetScan 6.2 and
microRNA.org databases revealed that ANK2 is an
underlying target of miR-647 (Fig.
3A). In the present study, the mRNA and protein expression
levels of ANK2, FAK, MMP2, MMP12, CD44 and SNAIL1 were
significantly reduced in the LV-miR-647 group compared with in the
LV-GFP and control groups (P<0.05; Fig. 3B and C), thus providing a
mechanistic basis for the reversal of drug resistance in
drug-resistant GC cells upon miR-647 overexpression.
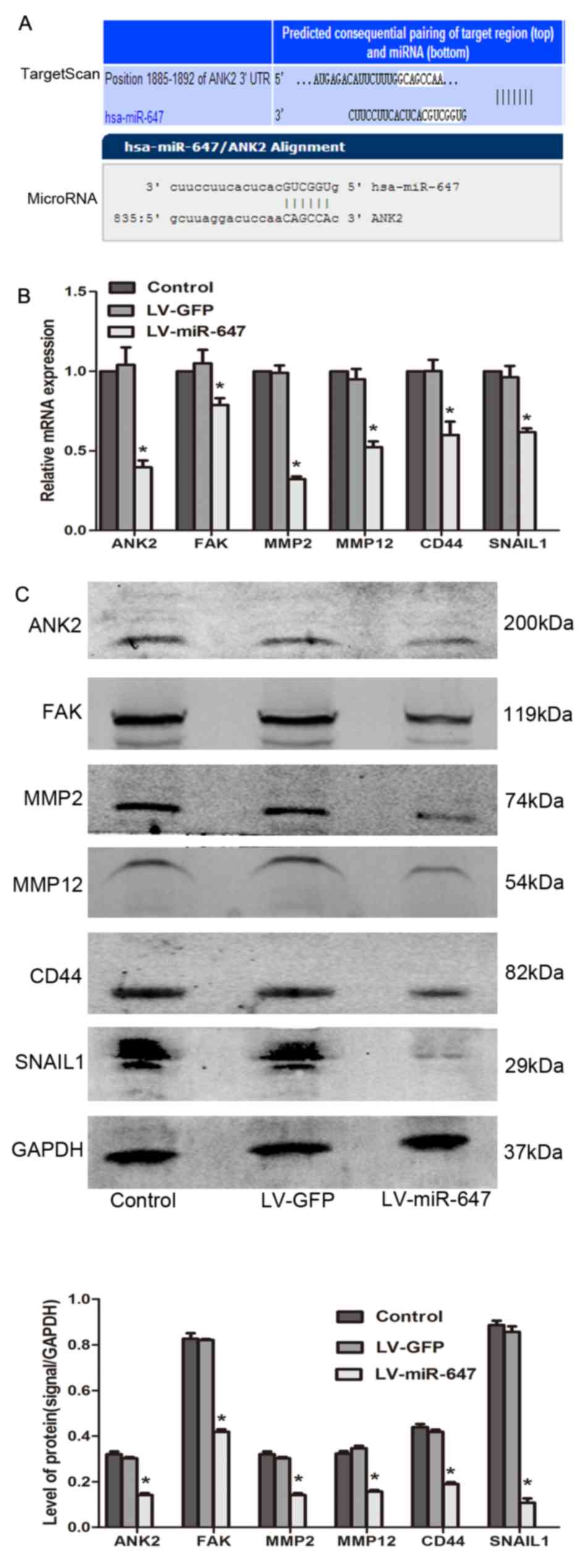 | Figure 3miR-647 modulates drug resistance by
reducing ANK2, FAK, MMP2, MMP12, CD44 and SNAIL1 expression. (A)
TargetScan and microRNA.org were used to predict
the target gene of miR-647. (B) ANK2, FAK, MMP2, MMP12, CD44 and
SNAIL1 mRNA expression was detected by reverse
transcription-quantitative polymerase chain reaction (n=4). (C)
ANK2, FAK, MMP2, MMP12, CD44 and SNAIL1 protein expression was
detected using western blotting (n=3). *P<0.05 vs.
the LV-GFP and control groups. Data are presented as the means ±
standard error of the mean. ANK2, ankyrin-B; CD44, cluster of
differentiation 44; FAK, focal adhesion kinase; GFP, green
fluorescent protein; miR-647, microRNA-647; MMP, matrix
metalloproteinase; SNAIL1, snail family transcriptional repressor
1. |
miR-647 reverses drug resistance in GC in
vivo
To further support the in vitro observations
in the present study, the effects of miR-647 were determined on the
growth of SGC7901/VCR cells in vivo; LV-miR-647-GFP and
LV-GFP were intratumorally injected into SGC7901/VCR tumors that
were transplanted subcutaneously into nude mice. A total of 20 days
after intratumoral injection, the RTV of the LV-miR-647 group was
2.04±0.10, which was significantly reduced compared with in the
LV-GFP group (2.94±0.40) and the control group (2.82±0.46)
(P<0.05; Fig. 4A). However,
there was no statistical significance among the Control-nonVCR,
LV-GFP-nonVCR and LV-miR-647-nonVCR groups. RT-qPCR confirmed that
miR-647 expression was increased in xenografts from the LV-miR-647
or LV-miR-647-nonVCR groups compared with in the LV-GFP or
LV-GFP-nonVCR groups and the control or control-nonVCR groups,
respectively (P<0.05; Fig.
4B). Images of the xenografts from each group are presented in
Fig. 4C, and were confirmed by
H&E staining analysis (Fig.
4D). H&E staining confirmed that the lumps were
transplantation tumors. These data suggested that miR-647 may
reverse drug resistance in GC in vivo.
Discussion
Due to the absence of efficacious methods for early
diagnosis and management of tumor metastases, the majority of
patients with GC are often unable to be treated by surgery.
Chemotherapy is considered to be the preferable therapeutic option
for these patients; however, the occurrence of MDR has become a
vital factor associated with the failure of routine chemotherapy,
resulting in metastasis of the most malignant tumors (11). Drug resistance and tumor
metastasis/invasion have been identified as the classical hallmarks
of cancer malignancy, and are major causes of poor clinical outcome
in patients with cancer (12–15). Therefore, discovery of novel
molecular biomarkers and targets for the diagnosis and treatment of
GC is imperative.
Accumulating evidence has suggested that miRNAs are
involved in the initial development of drug resistance and
metastasis in patients with GC (16). Our previous study demonstrated
that miR-1284 functions as a novel regulator to reduce drug
resistance and metastasis of GC (6). Notably, Rawlings-Goss et al
(7) reported that miR-647 is
associated with malignant cancer phenotypes and is often used as a
biomarker for GC. Furthermore, dysregulation of miR-647 has been
reported to be associated with Taxol resistance in ovarian cancer
(9). However, the detailed
association between miR-647 and GC tumorigenesis remains unclear.
The mechanism underlying the involvement of miR-647 in drug
resistance and metastasis of GC has yet to be elucidated.
The present study indicated that the expression
levels of miR-647 in GC tissues and SGC7901/VCR cells were reduced
compared with in the controls. Overexpression of miR-647 reversed
vincristine resistance in SGC7901/VCR cells, prevented cells from
entering S phase of the cell cycle and induced cell apoptosis.
Furthermore, overexpression of miR-647 downregulated migration and
invasion of SGC7901/VCR cells, and sensitized tumors to
chemotherapy in vivo, as demonstrated by a decrease in RTV
in nude mice. To the best of our knowledge, the present data is the
first to demonstrate that miR-647 may function as a novel regulator
of MDR in GC.
The exact mechanism underlying the functions of
miR-647 in GC MDR remains unclear. The present study provided
evidence to suggest that miR-647 overexpression regulates
SGC7901/VCR cell drug resistance by reducing ANK2, FAK, MMP2,
MMP12, CD44 and SNAIL1 expression. Previous studies have reported
that ANK2 encodes one of three ankyrins: ankyrin-R (ANK1),
ankyrin-B (ANK2) and ankyrin-G (ANK3), which belong to a
cytoskeletal protein family (17,18). This particular family is known to
be associated with various integral membrane proteins (17,18). Overexpression of ankyrins has been
observed in various cancer cells, including prostate, breast, and
ovarian cancers (19–21), and they are known to serve crucial
roles in cell growth, membrane transportation, metastasis and
migration of cancer cells (18,19). The present study demonstrated that
miR-647 can regulate ANK2 activity and suppress ANK2 expression,
thus indicating that miR-647 may produce biological functions via
downregulating ANK2. In addition, a previous study reported that
ANK2 is overexpressed in pancreatic tumors, whereas downregulation
of ANK2 attenuated growth and invasion of pancreatic cancer
(22). In addition, Savas et
al revealed that drug resistance in cancer cells is associated
with ANK2 (23). However, there
is currently insufficient evidence to confirm the exact role of
ANK2 in drug resistance and metastasis of GC. To the best of our
knowledge, the present findings that miR-647 reverses drug
resistance in GC by regulating ANK2 are the first to provide
evidence regarding the relationship between miR-647 and ANK2, and
their detailed function in drug resistance and metastasis.
Ankyrins, including ANK1, ANK2 and ANK3, which link
various transmembrane proteins to the actin network, also bind
domains in CD44 (24).
Furthermore, inhibiting CD44 may decrease the expression of SNAIL1
and the invasive ability of pancreatic cancer cells (25). These previous findings were
consistent with those of the present study, which indicated that
overexpression of miR-647 reversed drug resistance, decreased
invasion of SGC7901/VCR cells, and attenuated ANK2, CD44 and SNAIL1
activation. CD44 is a member of the hyaluronan receptor family,
which has been reported to be associated with drug resistance
(26). Furthermore, there is a
strong evidence to suggest that small interfering RNA against CD44
may reduce drug resistance through a decrease in transport
efficacy, which can in turn enhance cytoplasmic drug concentration
(27). In addition, in head and
neck squamous cell carcinoma, the deletion of SNAIL1 has been
reported to contribute to the inhibition of migration and invasion,
and MDR reversal, which further supports the present findings
(28). In the present study,
miR-647 expression was revealed to decrease the expression of
ANK2/CD44/SNAIL1 signaling pathways, directly or indirectly, which
may be responsible for overcoming drug resistance in GC cells in
vitro and in vivo.
Previous studies have reported that ANK2 may have an
effect on signal transduction mediated by FAK activity.
Furthermore, FAK activity has a pivotal role in the secretion of
MMPs (22,29). In the present study, miR-647
overexpression reversed drug resistance and inhibited metastasis of
SGC7901/VCR cells (invasion/migration of the cells), which was
accompanied by reduced ANK2 activation and decreased FAK, MMP2 and
MMP12 expression. The present study indicated that the
miR-647/ANK2/FAK/MMP2/MMP12 signaling pathway is an uncommon
integrated network that may mediate the metastasis of GC. As a
potential predictor for tumor metastasis, decreased FAK is involved
in the inhibition of tumor metastasis and invasion in GC (30). In addition, MMP2 and MMP12 belong
to the MMP family, which are well known for their essential roles
in tumor metastasis and invasiveness (31,32). Previous studies have also reported
that inhibiting MMP2 and MMP12 suppresses the invasion of gastric
and lung cancers (33,34). Notably, primary tumors and
metastasis exhibit varying levels of drug resistance; metastasis is
generally associated with more severe drug-resistance (35). Therefore, inhibiting metastasis
may provide a key strategy to prevent drug resistance.
In conclusion, the present study is the first, to
the best of our knowledge, to provide information regarding the
association between miR-647 and ANK2 in the drug resistance, thus
indicating the potential role of miR-647 in the regulation of drug
resistance and metastasis. Therefore, miR-647 may be considered a
biomarker of GC, and more studies are required to confirm that
targeting this gene may aid in the diagnosis and treatment of
GC.
Abbreviations:
|
MDR
|
multidrug resistance
|
|
GC
|
gastric cancer
|
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81660511,
30860273 and 81060201), the Natural Science Foundation of Guangxi
(grant no. 2015GXNSFDA227001), the Key Health Science Foundation of
Guangxi (grant no. 14124004-1-9), and the Innovation Project of
Guangxi Graduate Education.
Notes
[1] Competing
interests
The authors declare there is no competing
interest.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fan D, Zhang X, Chen X, Mou Z, Hu J, Zhou
S, Ding J and Wu K: Bird's-eye view on gastric cancer research of
the past 25 years. J Gastroenterol Hepatol. 20:360–365. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu L, Zhou D, Jiang X, Song K, Li K and
Ding W: Loss of E-cadherin in multidrug resistant breast cancer
cell line MCF-7/Adr: Possible implication in the enhanced invasive
ability. Eur Rev Med Pharmacol Sci. 16:1271–1279. 2012.PubMed/NCBI
|
|
4
|
Chen CZ: MicroRNAs as oncogenes and tumor
suppressors. N Engl J Med. 353:1768–1771. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu W, Xu H, Zhu D, Zhi H, Wang T, Wang J,
Jiang B, Shu Y and Liu P: miR-200bc/429 cluster modulates multidrug
resistance of human cancer cell lines by targeting BCL2 and XIAP.
Cancer Chemother Pharmacol. 69:723–731. 2012. View Article : Google Scholar
|
|
6
|
Cao W, Wei W, Zhan Z, Xie Y and Xiao Q:
miR-1284 modulates multidrug resistance of gastric cancer cells by
targeting EIF4A1. Oncol Rep. 35:2583–2591. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rawlings-Goss RA, Campbell MC and Tishkoff
SA: Global population-specific variation in miRNA associated with
cancer risk and clinical biomarkers. BMC Med Genomics. 7:532014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao WL, Wei WY, Zhan ZX, Xie DY, Xie YB
and Xiao Q: The role of miR-647 in human gastric cancer
suppression. Oncol Rep. 37:1401–1411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim YW, Kim EY, Jeon D, Liu JL, Kim HS,
Choi JW and Ahn WS: Differential microRNA expression signatures and
cell type-specific association with Taxol resistance in ovarian
cancer cells. Drug Des Devel Ther. 8:293–314. 2014.
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
11
|
An Y, Zhang Z, Shang Y, Jiang X, Dong J,
Yu P, Nie Y and Zhao Q: miR-23b 3p regulates the chemoresistance of
gastric cancer cells by targeting ATG12 and HMGB2. Cell Death Dis.
6:e17662015. View Article : Google Scholar
|
|
12
|
Gisel A, Valvano M, El Idrissi IG,
Nardulli P, Azzariti A, Carrieri A, Contino M and Colabufo NA:
miRNAs for the detection of multidrug resistance: Overview and
perspectives. Molecules. 19:5611–5623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pérez-Tomás R: Multidrug resistance:
Retrospect and prospects in anti-cancer drug treatment. Curr Med
Chem. 13:1859–1876. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nishida T, Egashira Y, Akutagawa H, Fujii
M, Uchiyama K, Shibayama Y and Hirose Y: Predictors of lymph node
metastasis in T1 colorectal carcinoma: An immunophenotypic analysis
of 265 patients. Dis Colon Rectum. 57:905–915. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Díaz-López A, Moreno-Bueno G and Cano A:
Role of microRNA in epithelial to mesenchymal transition and
metastasis and clinical perspectives. Cancer Manag Res. 6:205–216.
2014.PubMed/NCBI
|
|
16
|
Sui H, Cai GX, Pan SF, Deng WL, Wang YW,
Chen ZS, Cai SJ, Zhu HR and Li Q: miR200c attenuates P-gp-mediated
MDR and metastasis by targeting JNK2/c-Jun signaling pathway in
colorectal cancer. Mol Cancer Ther. 13:3137–3151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lambert S and Bennett V: Postmitotic
expression of ankyrinR and beta R-spectrin in discrete neuronal
populations of the rat brain. J Neurosci. 13:3725–3735.
1993.PubMed/NCBI
|
|
18
|
De Matteis MA and Morrow JS: The role of
ankyrin and spectrin in membrane transport and domain formation.
Curr Opin Cell Biol. 10:542–549. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu D and Bourguignon LY: Interaction
between CD44 and the repeat domain of ankyrin promotes hyaluronic
acid-mediated ovarian tumor cell migration. J Cell Physiol.
183:182–195. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bourguignon LY, Zhu H, Shao L and Chen YW:
Ankyrin-Tiam1 interaction promotes Rac1 signaling and metastatic
breast tumor cell invasion and migration. J Cell Biol. 150:177–191.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu D and Bourguignon LY: The
ankyrin-binding domain of CD44s is involved in regulating
hyaluronic acid-mediated functions and prostate tumor cell
transformation. Cell Motil Cytoskeleton. 39:209–222. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Y, Löhr M and Jesnowski R: Inhibition
of ankyrin-B expression reduces growth and invasion of human
pancreatic ductal adenocarcinoma. Pancreatology. 10:586–596. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Savas S, Azorsa DO, Jarjanazi H,
Ibrahim-Zada I, Gonzales IM, Arora S, Henderson MC, Choi YH,
Briollais L, Ozcelik H, et al: NCI60 cancer cell line panel data
and RNAi analysis help identify EAF2 as a modulator of simvastatin
and lovastatin response in HCT-116 cells. PLoS One. 6:e183062011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lokeshwar VB, Fregien N and Bourguignon
LY: Ankyrin-binding domain of CD44(GP85) is required for the
expression of hyaluronic acid-mediated adhesion function. J Cell
Biol. 126:1099–1109. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang W, Zhang Y, Kane KT, Collins MA,
Simeone DM, di Magliano MP and Nguyen KT: CD44 regulates pancreatic
cancer invasion through MT1-MMP. Mol Cancer Res. 13:9–15. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miletti-González KE, Chen S, Muthukumaran
N, Saglimbeni GN, Wu X, Yang J, Apolito K, Shih WJ, Hait WN and
Rodríguez-Rodríguez L: The CD44 receptor interacts with
P-glycoprotein to promote cell migration and invasion in cancer.
Cancer Res. 65:6660–6667. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang X, Iyer AK, Singh A, Choy E, Hornicek
FJ, Amiji MM and Duan Z: MDR1 siRNA loaded hyaluronic acid-based
CD44 targeted nanoparticle systems circumvent paclitaxel resistance
in ovarian cancer. Sci Rep. 5:85092015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nieh S, Jao SW, Yang CY, Lin YS, Tseng YH,
Liu CL, Lee TY, Liu TY, Chu YH and Chen SF: Regulation of tumor
progression via the Snail-RKIP signaling pathway by nicotine
exposure in head and neck squamous cell carcinoma. Head Neck.
37:1712–1721. 2015. View Article : Google Scholar
|
|
29
|
Sein TT, Thant AA, Hiraiwa Y, Amin AR,
Sohara Y, Liu Y, Matsuda S, Yamamoto T and Hamaguchi M: A role for
FAK in the Concanavalin A-dependent secretion of matrix
metalloproteinase-2 and -9. Oncogene. 19:5539–5542. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
You T, Gao W, Wei J, Jin X, Zhao Z, Wang C
and Li Y: Overexpression of LIMK1 promotes tumor growth and
metastasis in gastric cancer. Biomed Pharmacother. 69:96–101. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu L, Tanimoto A, Murata Y, Sasaguri T,
Fan J, Sasaguri Y and Watanabe T: Matrix metalloproteinase-12 gene
expression in human vascular smooth muscle cells. Genes Cells.
8:225–234. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie S, Issa R, Sukkar MB, Oltmanns U,
Bhavsar PK, Papi A, Caramori G, Adcock I and Chung KF: Induction
and regulation of matrix metalloproteinase-12 in human airway
smooth muscle cells. Respir Res. 6:1482005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang B, Xu YF, He BS, Pan YQ, Zhang LR,
Zhu C, Qu LL and Wang SK: RNAi-mediated silencing of CD147 inhibits
tumor cell proliferation, invasion and increases chemosensitivity
to cisplatin in SGC7901 cells in vitro. J Exp Clin Cancer Res.
29:612010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lv FZ, Wang JL, Wu Y, Chen HF and Shen XY:
Knockdown of MMP12 inhibits the growth and invasion of lung
adenocarcinoma cells. Int J Immunopathol Pharmacol. 28:77–84. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
O'Shaughnessy J: Extending survival with
chemotherapy in metastatic breast cancer. Oncologist. 10(Suppl 3):
20–29. 2005. View Article : Google Scholar : PubMed/NCBI
|