Introduction
Abdominal aortic aneurysm (AAA) is a disease with a
high mortality rate, which manifests as permanent abdominal aortic
dilation with potential rupture and substantial hemorrhage, which
eventually leads to patients succumbing to mortality (1,2).
AAA ranks as the highest among all types of aneurysm in terms of
morbidity rates. The disease is characterized by local and
permanent dilation of the abdominal aortic wall, with the tumor
body diameter being higher than normal; this leads to a weakened
abdominal aortic wall, which is life-threatening once the tumor
body ruptures (1,2).
AAA is currently considered to be associated with
multiple factors, including heredity factors, biochemistry,
immunity, inflammation and hemodynamics (3). The AAA continues to dilate until
rupture when it occurs, and it is associated with a poor prognosis
(3). Nuclear factor (NF)-κB p65
is a type of endonuclear transcription factor. The NF-κB p65
signaling pathway is important in regulating inflammatory, immune
and cell apoptotic responses, the abnormal activation of which in
the aorta promotes the expression of inflammatory factors and
matrix mellatoproteinase (MMP)-hydrolyzed proteins, and becomes one
of the factors inducing aneurysm genesis (4). Tumor necrosis factor (TNF)-α is an
important pro-inflammatory cytokine, which is most closely
associated with the genesis and development of AAA inflammation
(5). It has been suggested that
the level of TNF-α in the synovial fluid of patients with
rheumatoid arthritis is positively correlated with the severity of
AAA. Inhibiting the overexpression of TNF-α can prevent AAA,
whereas antibody therapy targeting TNF-α can effectively alleviate
AAA symptoms in patients and delay the progression of inflammation
(6). TNF-α has been identified in
in vitro experiments to induce the cell production of
multiple inflammatory mediators, including interleukin (IL)-6,
IL-8, prostaglandin E2, collagenase and metalloproteinase,
promoting AAA inflammation and leading to articular damage
(6).
MicroRNAs (miRNAs) are a type of highly conserved,
small, non-coding RNA, which is distributed extensively in
eukaryotes (7). They are also
novel gene expression regulatory factors, which exert their
function mainly through inhibiting target gene protein translation
or degrading target mRNA (7). The
majority of miRNAs in animals are considered to exert their
functions through inhibiting post-transcriptional translation,
whereas they function mainly through degrading target mRNAs in
plants (8). miRNAs are involved
in cell differentiation, proliferation and apoptotic processes;
therefore, they are closely associated with multiple diseases,
including tumors, cardiovascular diseases, fibrotic diseases and
viral infections (9). Multiple
miRNAs regulating extracellular matrix degradation and smooth
muscle cell apoptosis have been identified in studies investigating
the genesis and development of AAA in previous years. They are
reported to potentially be involved in regulation (10). Zampetaki et al showed that
miR-195 may contribute to the pathogenesis of AAA (11).
Vascular endothelial growth factor (VEGF) can
promote endothelial formation and angiogenesis; however, it cannot
enhance the proliferation of other cell types, which is an
important characteristic of VEGF. In addition, VEGF can promote
plasmin activity, prevent thrombosis, and increase capillary
permeability and vasodilation. Therefore, VEGF is of significance
in the repair of vascular injury and prevention of restenosis
(12). It is known that the
phosphoinositide 3-kinase (PI3K)/AKT signaling pathway is one of
the important downstream signaling pathways of VEGF, which is
involved in numerous processes, including tumor proliferation and
metastasis (13). The PI3K/AKT
signaling pathway is extensively distributed in cells, and is a
signal transduction pathway involved in cell growth, proliferation,
differentiation, cell survival, adhesion, migration and
anti-apoptosis (14). Signaling
pathways can regulate expression at the transcription level,
promoting tumor angiogenesis. The aim of the present study was to
identify the function of miR-195 on AAA and its possible
mechanism.
Materials and methods
Ethics statement and patients
All experiments were approved by the Ethics
Committee of the General Hospital of People's Liberation Army
(Beijing, China). Whole blood samples from patients with AAA (n=6,
61.5±8.5 years old, male) and normal volunteers (n=6, 55.5±9.5 year
age, male) were collected from General Hospital of People's
Liberation Army (March and May 2015) and centrifuged at 1,000 × g
for 10 min at 4°C. Serum was stored at −70°C for subsequent
experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
RNA was isolated from serum samples using TRIzol
according to the manufacturer's protocol (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The RNA samples were first
reverse transcribed with miRNA-specific primers, using a TaqMan
miRNA reverse transcription kit (Invitrogen; Thermo Fisher
Scientific, Inc.) in an iQ5 Real-Time PCR detection system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The StepOne Plus Real-Time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) was
used to perform RT-qPCR using SYBR Green Supermix (Bio-Rad
Laboratories, Inc.) according to the manufacturer's protocol. The
following primers were used for the PCR: miR-195 forward,
5-GGGGAGCCAAAAGGGTCATCATCT-3′ and reverse,
5′-GAGGGGCCATCCACAGTCTTCT-3′; U6 forward, CTCGCTTCGGCAGCACA, and
reverse, 5′-AACGCTTCACGAATTTGCGT-3′. The following thermocycling
conditions were used for PCR: Initial at 95 °C for 5 min; 40 cycles
of 95°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec. The data
were analyzed using the 2−∆∆Cq method (15).
Culture cell and transfection
Primary human umbilical vein endothelial cells
(HUVECs; PromoCell GmbH, Heidelberg, Germany) were cultured in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with
penicillin/streptomycin (100 U/ml and 100 μg/ml),
L-glutamine (2 mM) and FBS (10%, Thermo Fisher Scientific, Inc.) at
37°C in humidified air containing 5% CO2. The miR-195
mimics and negative mimics were obtained from iGene Biotechnology,
Inc. (Shanghai, China). The cells were transfected with miR-195
mimics and negative mimics using Lipofectamine 2000 transfection
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) for 24 h.
Subsequently, the cells (1×105 cell/well) were seeded in
6-well-plates and treated with 120 nM angiotensin II
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 20 h at 37°C,
prior to treatment with 100 nM of lipopolysaccharide (LPS, Beyotime
Institute of Biotechnology, Nanjing, China) for 4 h at 37°C.
ELISA
The supernatants of the cells were collected and the
concentrations of IL-1β ELISA kit (cat. no. PI305) and IL-6 ELISA
kit (cat. no. PI330) were assayed (both Beyotime Institute of
Biotechnology). Absorbance detection was performed using a
microplate reader (Bio-Rad Laboratories, Inc.) at 450 nm.
Western blot analysis
Total cellular lysates were prepared with
radioimmunoprecipitation assay buffer, according to the
manufacturer's protocol (Beyotime Institute of Biotechnology). The
protein concentrations were determined using the Bicinchoninic Acid
assay (Thermo Fisher Scientific, Inc.). Subsequently, 40 μg
of the protein samples were separated by 8–12% SDS-PAGE and
transferred onto a polyvinylidene fluoride membrane. The membrane
was blocked with 5%- skim milk powder-TBST and western blot
analysis was performed with the following primary antibodies: MMP-2
(cat. no. 40994, 1:2,000), MMP-9 (cat. no. 13667, 1:2,000), TNF-α
(cat. no. 11948, 1:2,000), NF-κB (cat. no. 8242, 1:2,000), VEGF
(cat. no. 9698, 1:2,000), PI3K (cat. no. PI3K, 1:2,000), p-Akt
(cat. no. 4060, 1:1,000) and GAPDH (cat. no. 5174, 1:5,000, Cell
Signaling Technology, Inc., Danvers, MA, USA) at 4°C overnight.
Horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G
(cat. no. 7076, 1:5,000; Cell Signaling Technology, Inc.) was used
as a secondary antibody at 37°C for 1 h and visualized using
enhanced chemiluminescence detection (EMD Millipore, Billerica, MA,
USA).
Statistical analysis
The results are expressed as the mean ± standard
deviation and analyzed using SPSS 19.0 (IBM Corp., Armonk, NY,
USA). Statistical analysis was performed using one-way analysis of
variance followed by Bonferroni's post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression levels of miR-195 in patients
with AAA
With the aim of examining the miRNAs involved in the
bone regeneration process of AAA, the present study analyzed the
expression levels of miR-195 in patients with AAA and normal
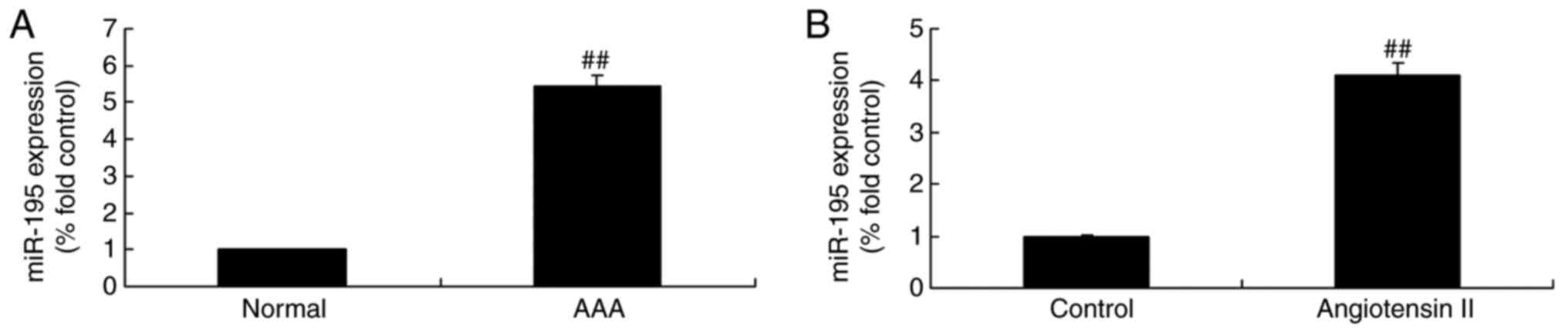
volunteers. As shown in Fig. 1A,
the expression levels of miR-195 in patients with AAA were higher,
compared with those in the normal volunteers. In the angiotensin
II-induced cell model, the expression level of miR-195 was also
increased, compared with that in the control group (Fig. 1B).
miR-195 promotes the levels of IL-1β and
IL-6 in angiotensin II-vascular smooth muscle cells
In order to determine whether miR-195 affects
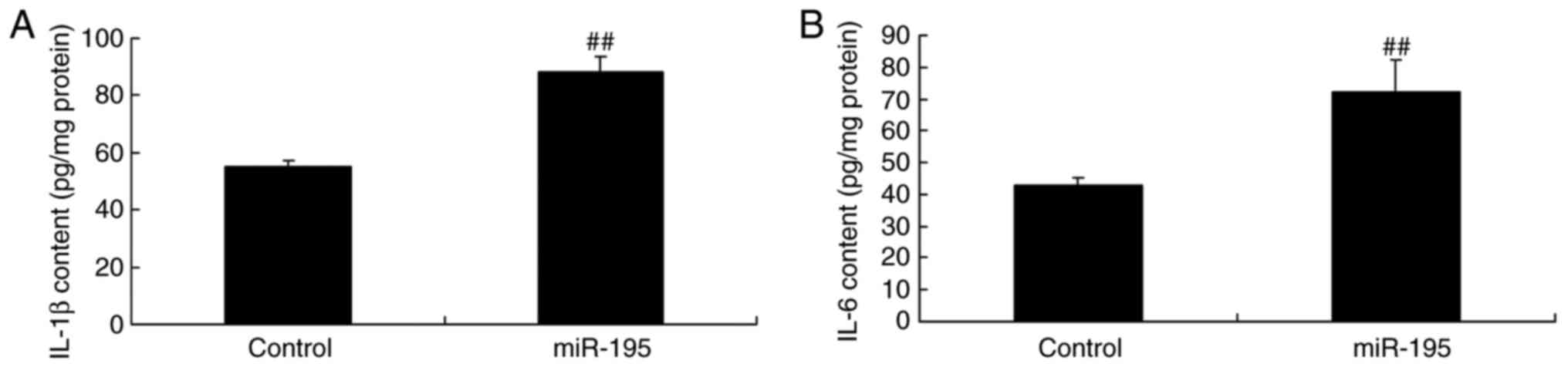
inflammation in angiotensin II-vascular smooth muscle cells, the
levels of IL-1β and IL-6 were examined for the array. As shown in
Fig. 2A and B, miR-195
effectively increased the levels of IL-1β and IL-6 in the
angiotensin II-vascular smooth muscle cells.
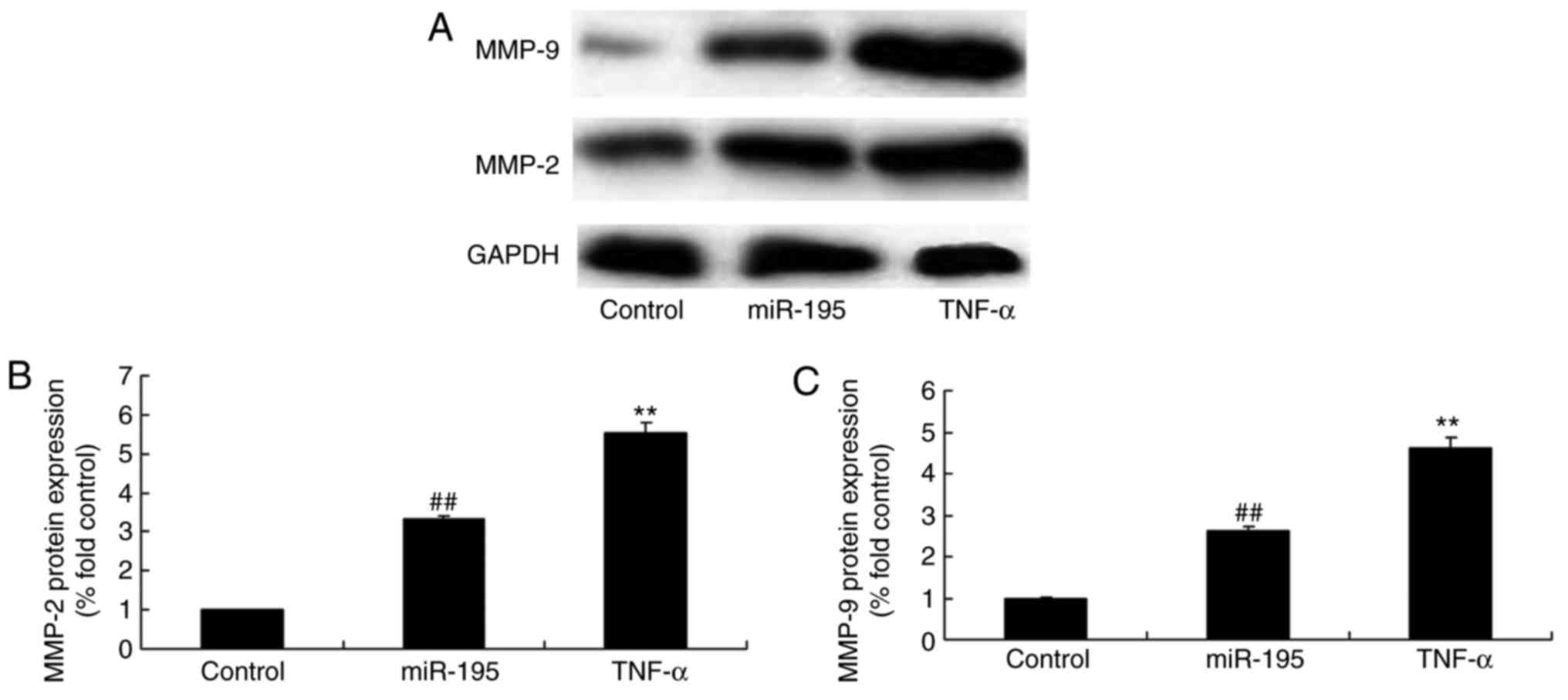
miR-195 promotes the protein expression
of MMP-2 and MMP-9 in angiotensin II-vascular smooth muscle
cells
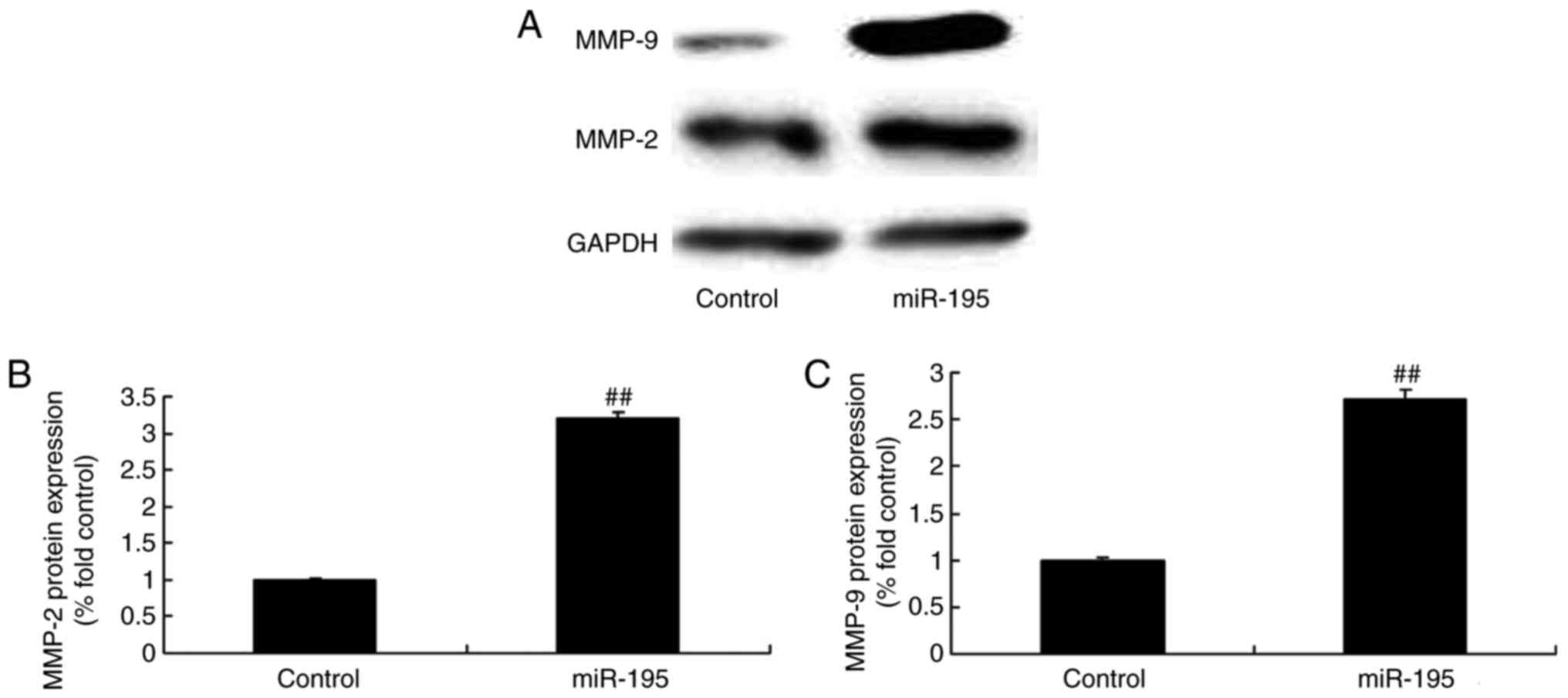
To confirm the protein expression of MMP-2 and MMP-9
following induction of the overexpression of miR-195, the protein
expression levels of MMP-2 and MMP-9 in the angiotensin II-vascular
smooth muscle cells were measured. As shown in Fig. 3A–C miR-195 effectively promoted
the protein expression levels of MMP-2 and MMP-9 in the angiotensin
II-vascular smooth muscle cells.
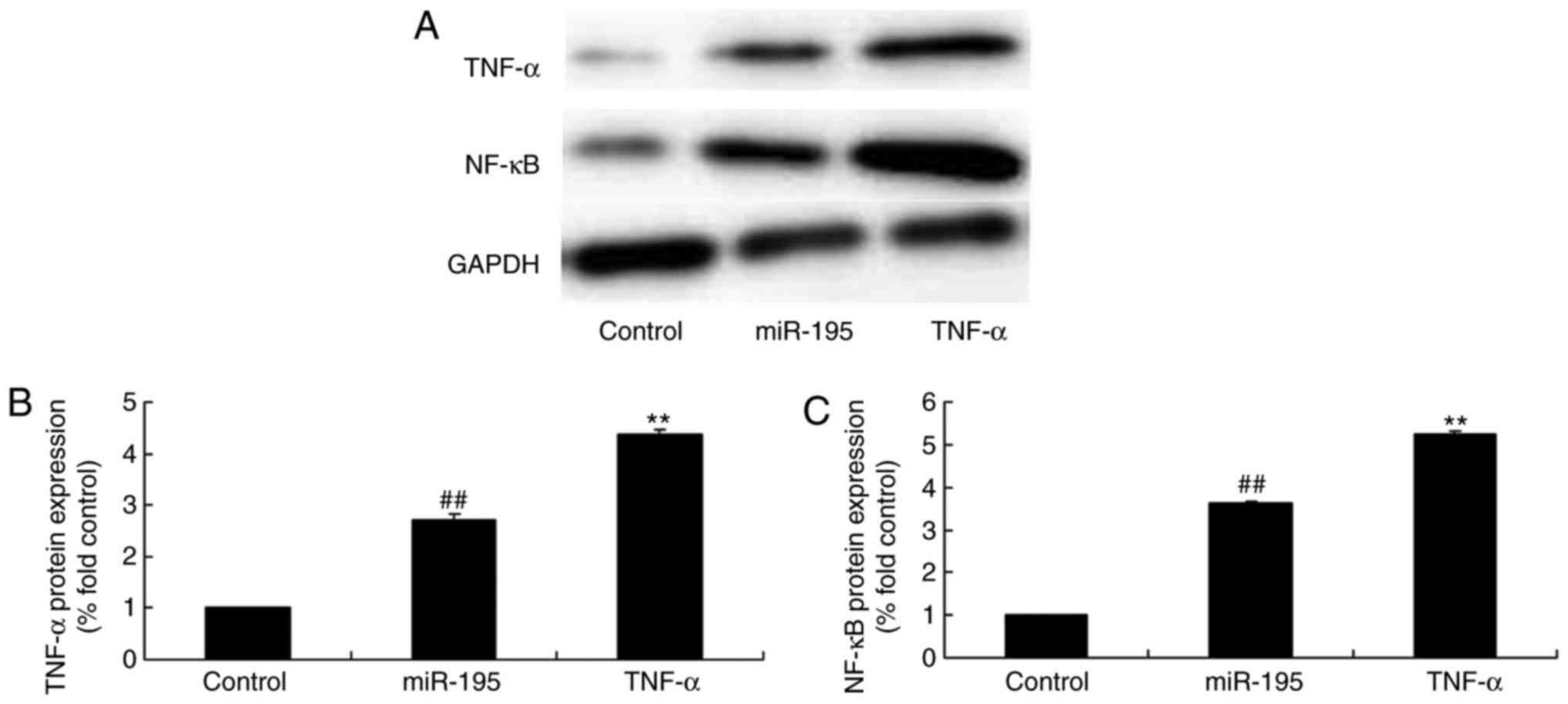
miR-195 upregulates the protein
expression of TNF-α and NF-κB in angiotensin II-vascular smooth
muscle cells
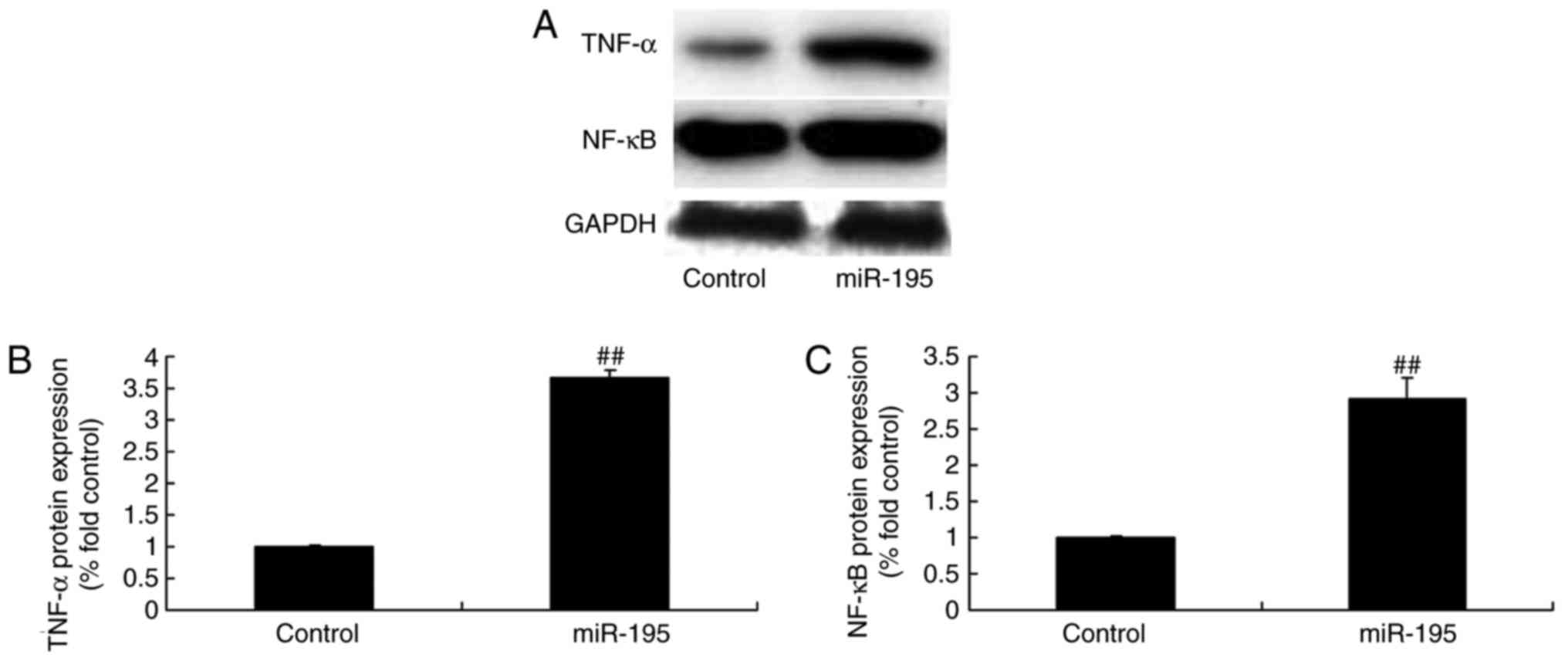
To confirm the expression of inflammatory proteins
in the AAA model, the protein expression levels of TNF-α and NF-κB
were determined following the overexpression of miR-195. As shown
in Fig. 4A–C, miR-195
significantly upregulated the protein expression of TNF-α and NF-κB
in the angiotensin II-vascular smooth muscle cells.
miR-195 suppresses the protein expression
of VEGF, PI3K and p-Akt in angiotensin II-vascular smooth muscle
cells
To further elucidate the effect of miR-195 during
AAA, the protein expression levels of VEGF, PI3K and p-Akt were
determined in angiotensin II-vascular smooth muscle cells following
the overexpression of miR-195. The protein expression levels of
VEGF, PI3K and p-Akt in the angiotensin II-vascular smooth muscle
cells were significantly downregulated by miR-195 (Fig. 5A–D).
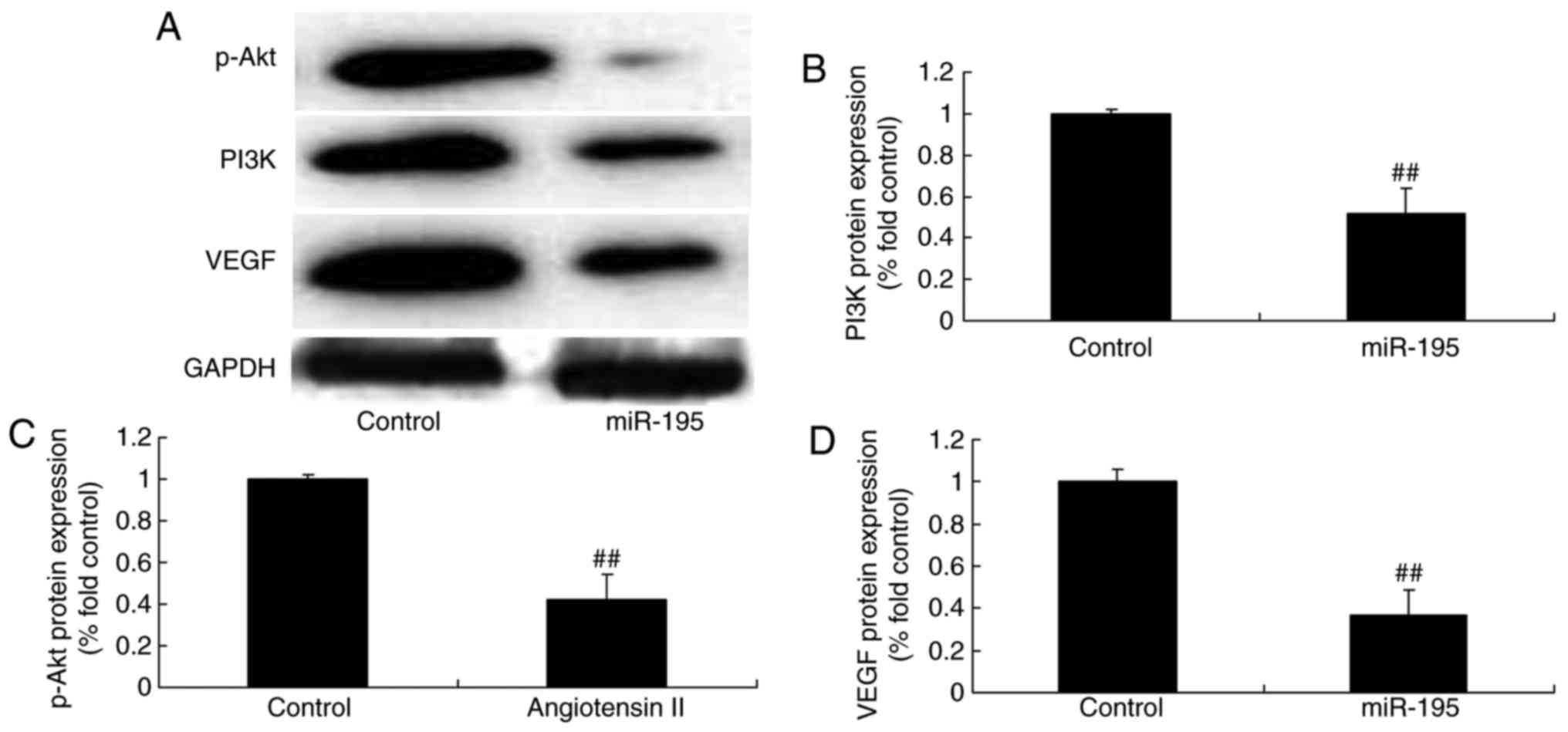 | Figure 5miR-195 suppresses the protein
expression of VEGF, PI3K and p-Akt in angiotensin II-vascular
smooth muscle cells. miR-195 suppressed the protein expression of
VEGF, PI3K and p-Akt in angiotensin II-vascular smooth muscle
cells, determined using (A) western blot analysis with statistical
analysis of (B) PI3K, (C) p-Akt and (C) VEGF.
##P<0.01, vs. control group. Control, negative
control group; miR, microRNA; miR-195, miR-195 mimics group; VEGF,
vascular endothelial growth factor; PI3K, phosphoinositide
3-kinase. |
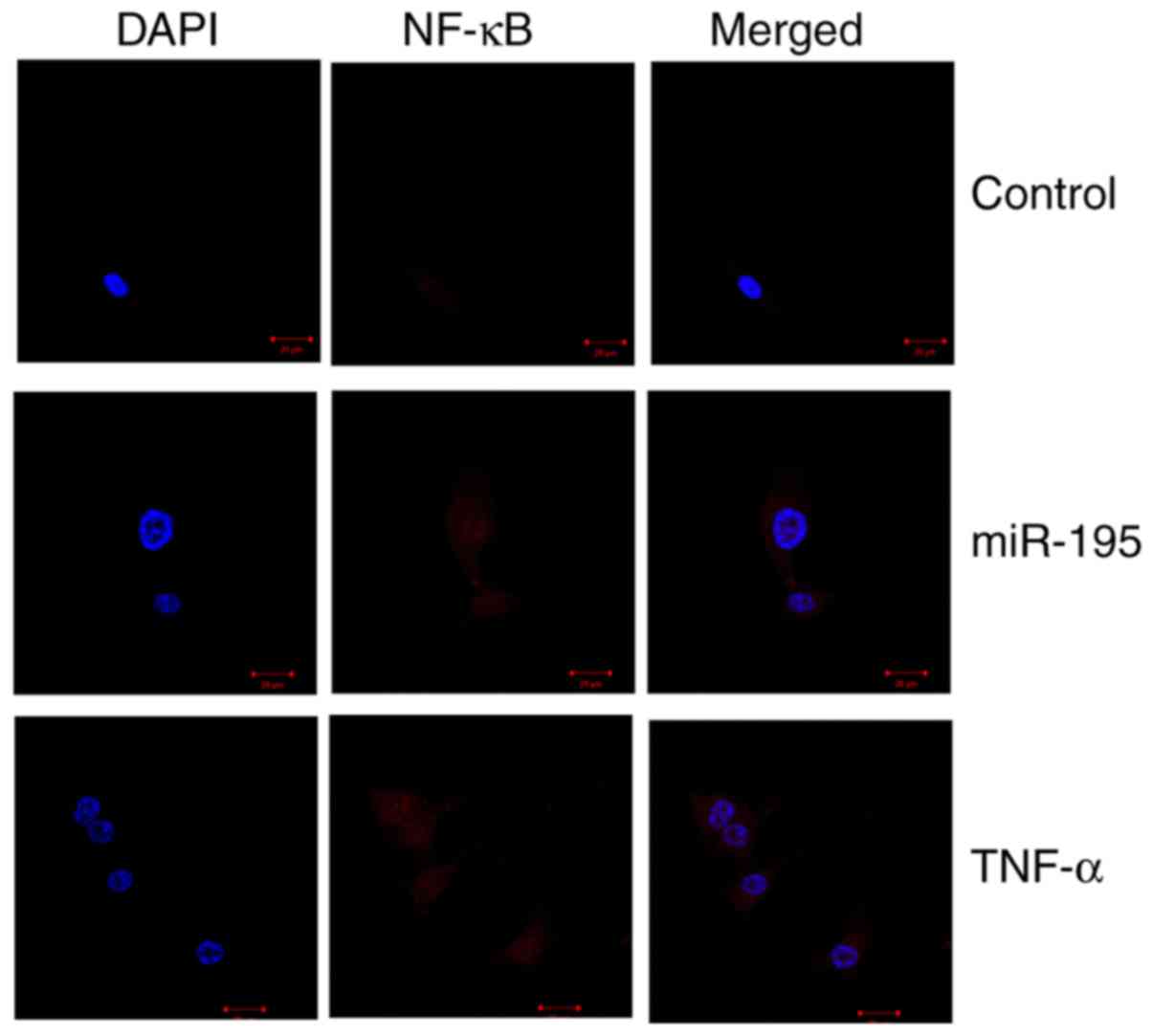
TNF-α promotes the protein expression of
TNF-α and NF-κB by miR-195
To characterize the effect of TNF-α on the function
of miR-195 in AAA, the present study investigated the protein
expression of TNF-α and NF-κB following treatment with miR-195 and
TNF-α protein. As shown in Fig.
6A–C, the TNF-α recombinant protein significantly promoted the
protein expression of TNF-α and NF-κB in the angiotensin
II-vascular smooth muscle cells by miR-195. In addition,
immunofluorescence was used to observe the protein expression of
NF-κB in angiotensin II-vascular smooth muscle cells overexpressing
miR-195. As shown in Fig. 7, the
protein expression of NF-κB in the miR-195-overexpressing group was
higher, compared with that in the control group, and the
combination of miR-195 and TNF-α recombinant protein significantly
increased the protein expression of NF-κB in the angiotensin
II-vascular smooth muscle cells, compared with that in the miR-195
group.
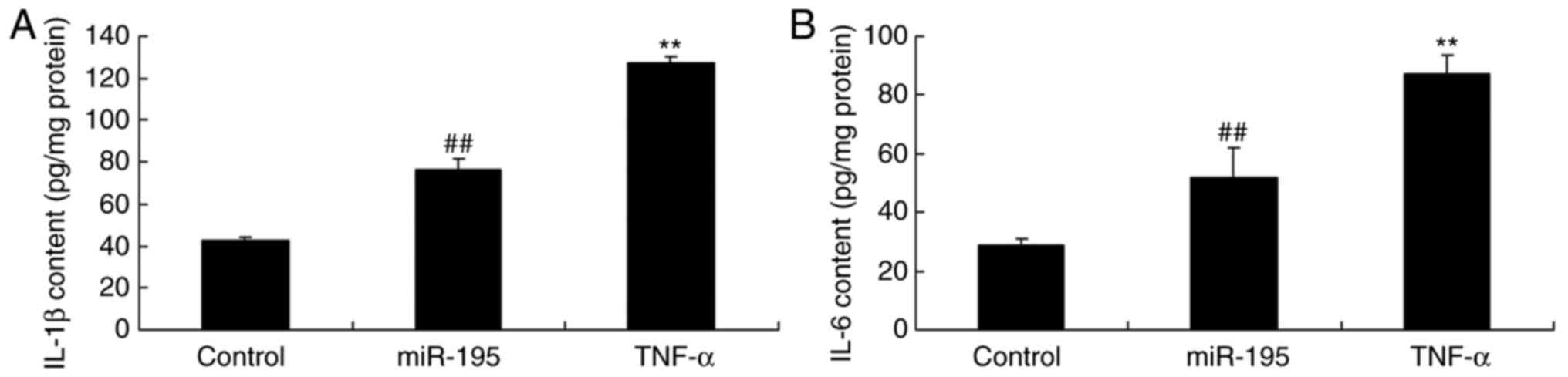
TNF-α promotes the generation of IL-1β
and IL-6 by miR-195
To confirm whether the levels of IL-1β and IL-6
induced by miR-195 were affected by TNF-α in AAA, the levels of
IL-1β and IL-6 were quantified using ELISA kits. The increased
levels of IL-1β and IL-6 in the angiotensin II-vascular smooth
muscle cells induced by miR-195 were significantly promoted by the
TNF-α recombinant protein (Fig. 8A
and B).
TNF-α promotes the protein expression of
MMP-2 and MMP-9 by miR-195
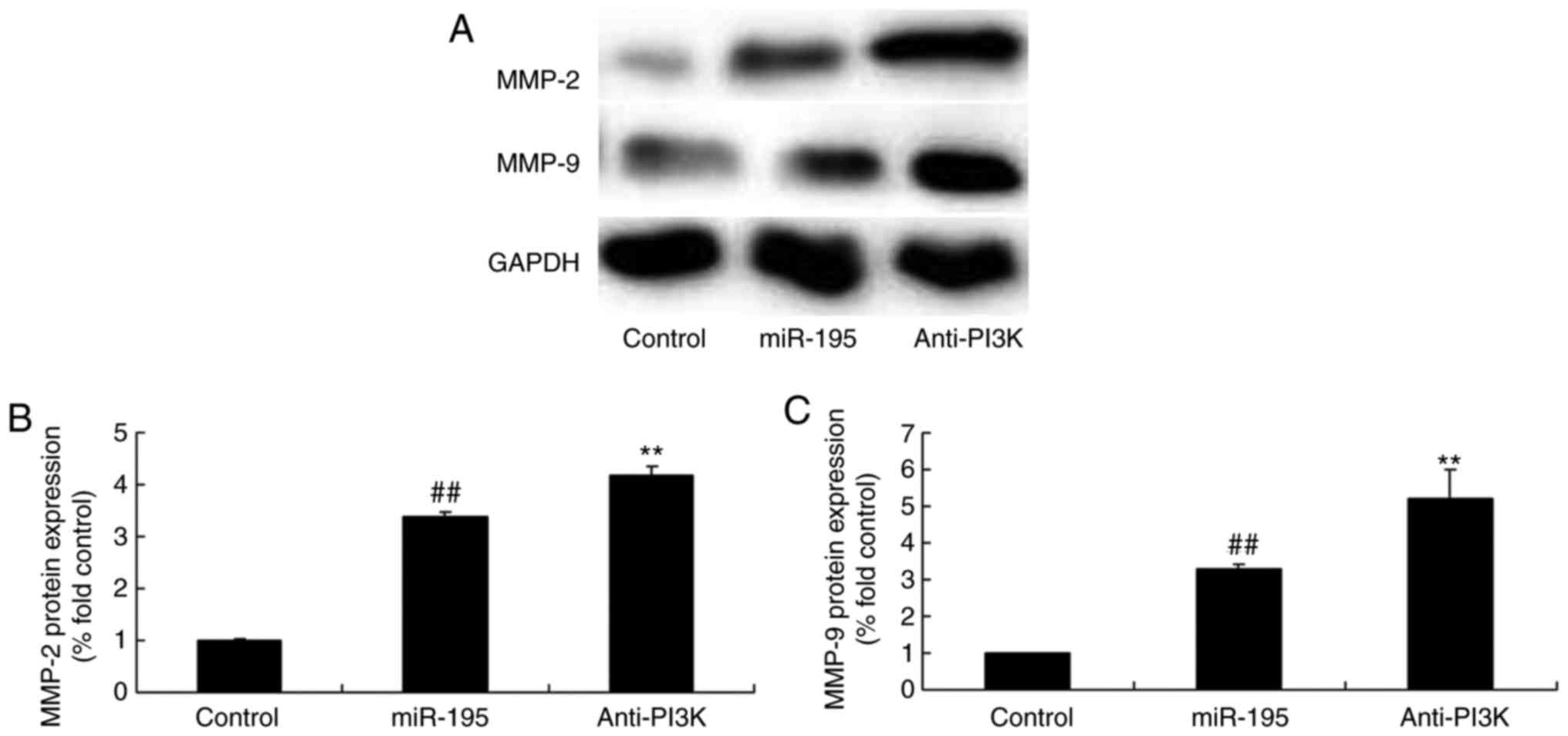
It was also found that TNF-α significantly promoted
the protein expression of MMP-2 and MMP-9 by miR-195 in the
angiotensin II-vascular smooth muscle cells (Fig. 9A–C). These data showed that
miR-195/TNF-α affected inflammation in the development of AAA.
PI3K affects the protein expression
levels of VEGF, PI3K and p-Akt by miR-195
The present study subsequently investigated whether
PI3K was a potential target for miR-195 in the development of AAA.
The PI3K inhibitor, LY294002, suppressed the PI3K signaling pathway
in angiotensin II-vascular smooth muscle cells; the protein
expression levels of VEGF, PI3K and p-Akt in the angiotensin
II-vascular smooth muscle cells decreased by miR-195 were
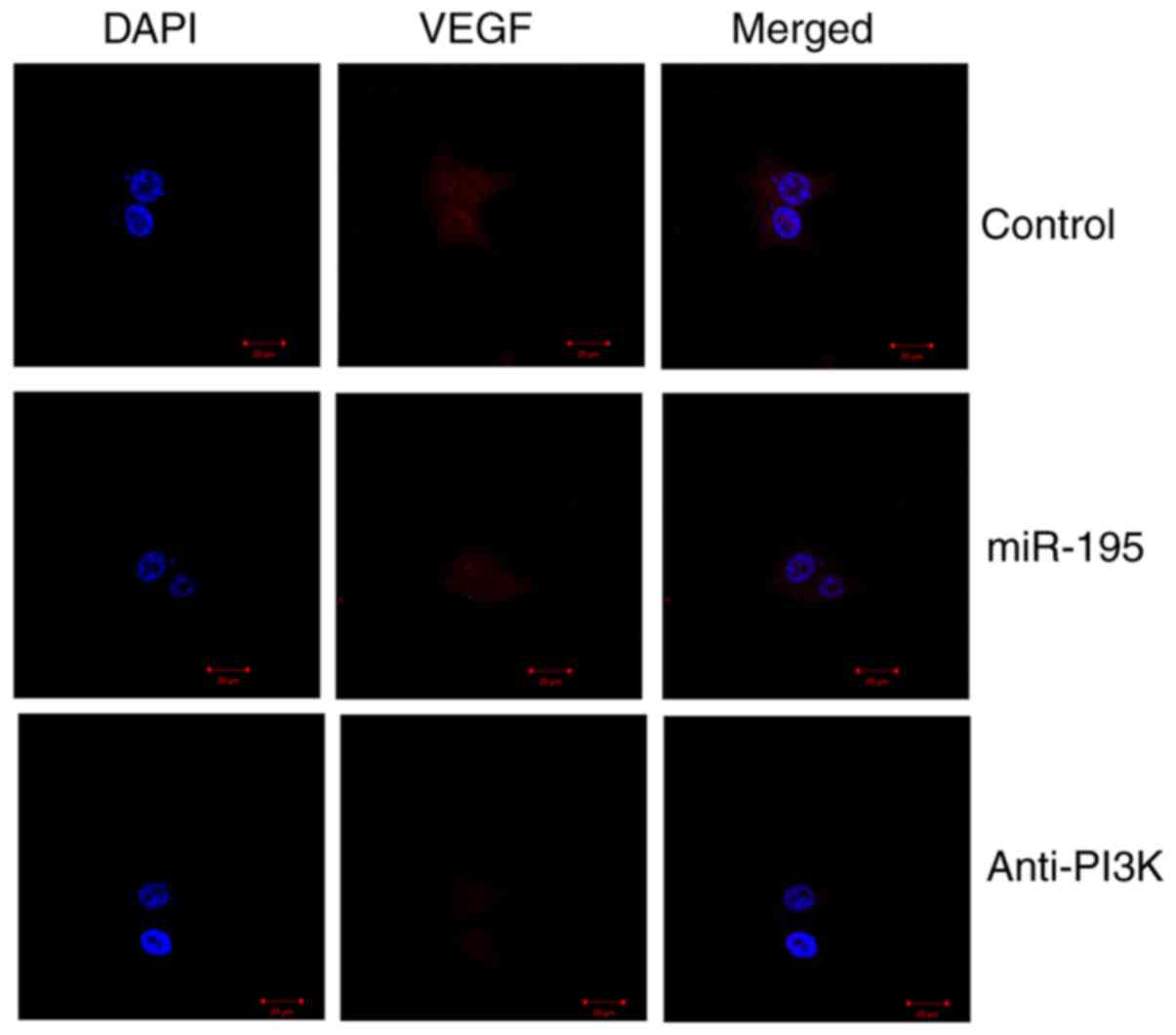
suppressed significantly by the PI3K inhibitor (Fig. 10A–D). It was found that miR-195
significantly suppressed the protein expression of VEGF in the
angiotensin II-vascular smooth muscle cells, compared with that in
the control group. The PI3K inhibitor led to significant
suppression of the protein expression of VEGF in the angiotensin
II-vascular smooth muscle cells with miR-195, compared with that in
the miR-195 group without the inhibitor (Fig. 11).
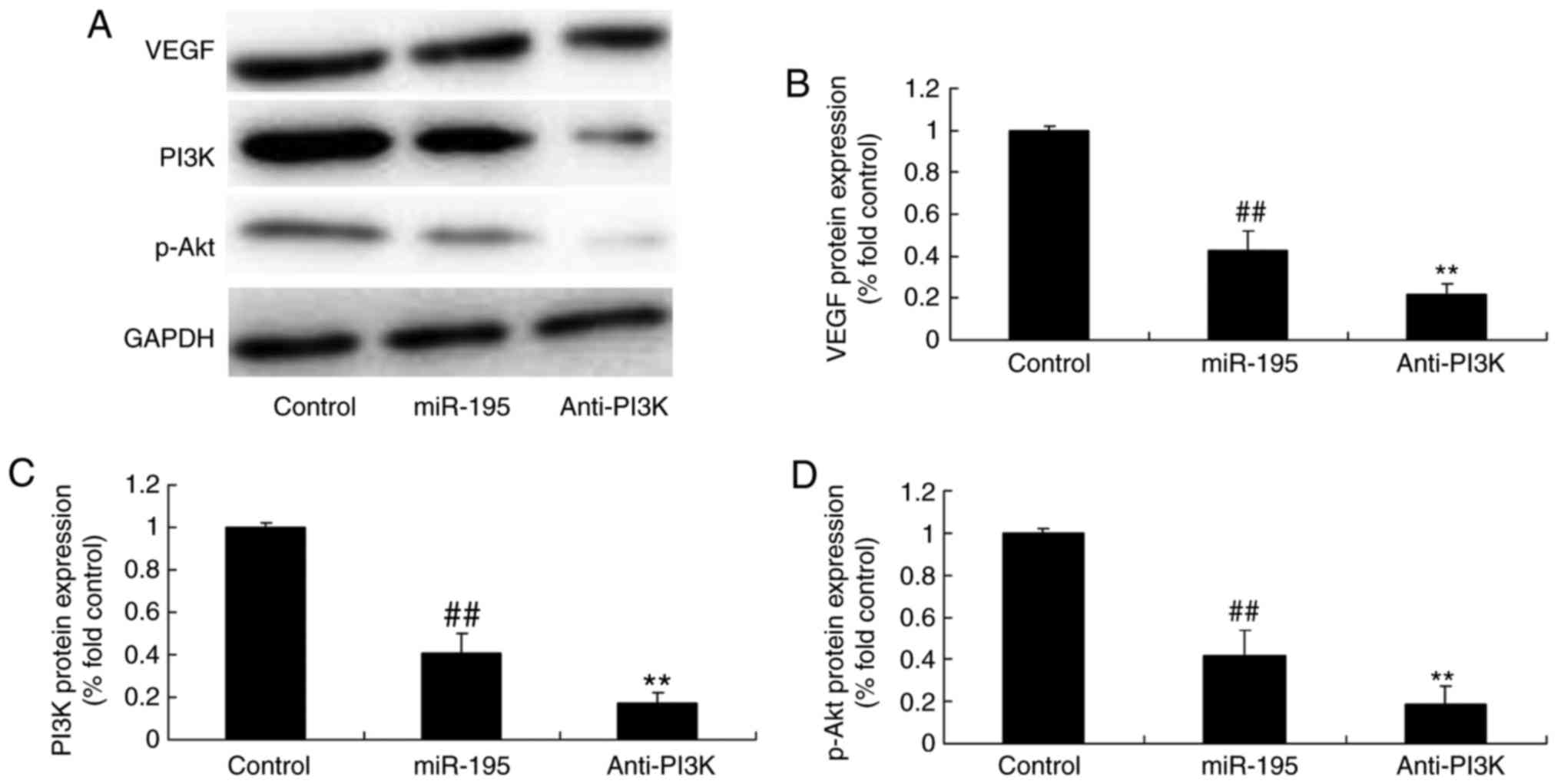 | Figure 10PI3K affects the generation of VEGF,
PI3K and p-Akt proteins by miR-195. PI3K affected the protein
levels of VEGF, PI3K and p-Akt by miR-195, determined using (A)
western blot analysis with statistical analysis of (B) VEGF, (C)
PI3K and (D) p-Akt. ##P<0.01, vs. control group;
**P<0.01, vs. miR-195 group. Control, negative
control group; miR, microRNA; VEGF, vascular endothelial growth
factor; PI3K, phosphoinositide 3-kinase; p-Akt, phosphorylated Akt;
miR-195, miR-195 mimics group; Anti-PI3K, miR-195 mimics+PI3K
inhibitor group. |
PI3K affects the protein expression of
MMP-2 and MMP-9 by miR-195
To determine whether miR-195 directly binds PI3K in
the development of AAA, the protein expression levels of MMP-2 and
MMP-9 were examined in angiotensin II-vascular smooth muscle cells.
The PI3K inhibitor significantly induced the protein expression of
MMP-2 and MMP-9 in the angiotensin II-vascular smooth muscle cells
with miR-195, compared with that in the miR-195 group without the
inhibitor (Fig. 12A–C).
Discussion
AAA is a serious, life-threatening vascular disease,
which predominantly affects older men (15). Its morbidity rate has increased
gradually, and it has become one of the top 10 causes of mortality
in the elderly worldwide (15).
Its risk factors include old age, being male, smoking, family
history, central obesity, low high-density lipoprotein
cholesterolemia and hypertension (15). AAA includes the following major
pathogeneses: First is extracellular matrix degradation;
extracellular matrix of the aorta is composed of collagen, elastin,
fibronectin and laminin, which is the major component for
maintaining the structural integrity and elasticity of vascular
wall (15). Extracellular matrix
degradation is mainly mediated by MMPs, among which, MMP-2 and
MMP-9 are the most important and have been investigated extensively
(2). The enhanced expression of
MMP or reduced expression of its specific inhibitor induces
increased MMP activity, which results in extracellular matrix
degradation, loss of integrity and reduced elasticity of the
vascular wall, eventually leading to aortectasia or aortic aneurysm
formation (16). Second is smooth
muscle cell apoptosis; it has been shown in human AAA tissue
specimens that loss of smooth muscle cells in the tunica media is
associated with smooth muscle cell apoptosis, and extracellular
matrix degradation can lead to anoikis of smooth muscle cells
(17). Third is inflammation, and
fourth is angiogenesis. Angiogenesis is closely associated with the
inflammatory response, and it the former is commonly considered an
important factor promoting aortic rupture, whereas inflammation can
regulate the genesis and development of AAA through stimulating
angiogenesis (18). These
important pathogeneses are closely associated, among which
extracellular matrix degradation is the most important. In the
present study, the expression levels of miR-195 were we analyzed in
patients with AAA and were found to be higher, compared with those
in normal volunteers. miR-195 effectively promoted the protein
expression of MMP-2 and MMP-9 in angiotensin II-vascular smooth
muscle cells. Cai et al also reported that miR-195 inhibited
the tumor progression of prostate cancer via MMP-9 and VEGF
(19).
miRNAs are a novel type of gene expression
regulatory factor, which inhibits the translation process of mRNAs
encoding proteins through binding with the target mRNA
3′untranslated region and inducing regulatory effects; it is also
important in cell differentiation, proliferation, apoptosis and
metabolism (20). Previously, it
was found that multiple miRNAs are associated with the genesis and
development of AAA, having regulatory effects on extracellular
matrix degradation, vascular smooth muscle cell apoptosis,
inflammation and angiogenesis (21). In the present study, it was found
that miR-195 effectively increased the levels of IL-1β and IL-6 in
angiotensin II-vascular smooth muscle cells. Chen et al
reported that miR-195 suppressed ulcerative colitis-induced
inflammation through targeting small mothers against
decapentaplegic 7 (22).
NF-κB p65 predominantly exists in the cytoplasm in
the form of an inactive precursor at rest. It can induce the
expression of target genes once it is activated, including
inflammatory mediators IL-1 and IL-6, and MMPs (23). Of these, the transcriptional
activation of target MMP-2 and MMP-9 can lead to the degradation
and destruction of abdominal aortic extracellular matrix (24). NF-κB inhibitor has been shown in
animal experiments to significantly inhibit the formation of AAA
(24). In addition, the present
study demonstrated that miR-195 significantly upregulated the
protein expression of TNF-α and NF-κB in angiotensin II-vascular
smooth muscle cells. TNF-α promoted the pre-inflammatory effect of
miR-195 on the protein expression of TNF-α and NF-κB, the levels of
IL-1β and IL-6, and the protein expression of MMP-2 and MMP-9 in
angiotensin II-vascular smooth muscle cells. Ding et al
showed that miR-195 suppresses cancer cell proliferation and
migration in hepatocellular carcinoma through the TNF-α/NF-κB
pathway (25).
Angiogenesis refers to the process of growing new
blood vessels from endothelial cells in original blood vessels
through budding, migration and proliferation (12). VEGF can increase number of
vesicles in endothelial cells as the most specific key
precipitating factor of angiogenesis, which increases vascular
permeability (12). VEGF is an
agent with the highest selectivity in promoting endothelial cell
mitosis; it can promote the proliferation of smooth muscle cells,
epithelial cells and fibroblasts, and induce angiogenesis. It also
stimulates endothelial cells to produce nitric oxide, thus exerting
a function of vascular maintenance (26). VEGF binds with its receptor,
releases multiple growth factors and cytokines, induces the
proliferation and migration of endothelial cells, and eventually
promotes angiogenesis (13). The
present study demonstrated that miR-195 significantly downregulated
the protein expression of VEGF in angiotensin II-vascular smooth
muscle cells. Almeida et al suggested that miR-195 regulates
important mechanisms for bone regeneration through the expression
of VEGF (27).
PI3K and its downstream AKT constitute an important
signaling pathway, which is termed the PI3K/AKT signaling pathway
and is vital for the survival, differentiation, proliferation and
apoptosis of cells (14). The
association between PI3K and tumors is supported in numerous
studies, and an imbalance of PI3K/AKT is involved in multiple human
tumor diseases, including lung cancer, nasopharyngeal carcinoma,
liver cancer, gastrointestinal cancer, breast cancer, ovarian
cancer, renal carcinoma, prostate cancer, lymphoma, malignant
glioma and medulloblastoma (28).
The correlation between PI3K/AKT and non-tumor diseases, including
hepatic fibrosis, Alzheimer's disease, diabetes and cardiovascular
diseases, has gradually attracted attention (29). The present study found that
miR-195 significantly downregulated the protein expression of PI3K
and p-AKT in angiotensin II-vascular smooth muscle cells. The
suppression of PI3K promoted the pre-inflammatory effect of miR-195
on the protein expression of PI3K, p-Akt and VEGF, levels of IL-1β
and IL-6, and protein expression of MMP-2 and MMP-9 in angiotensin
II-vascular smooth muscle cells. Sun et al indicated that
miR-195 has a tumor suppressive effect in ACHN cells through the
PI3K/Akt signaling pathways (30).
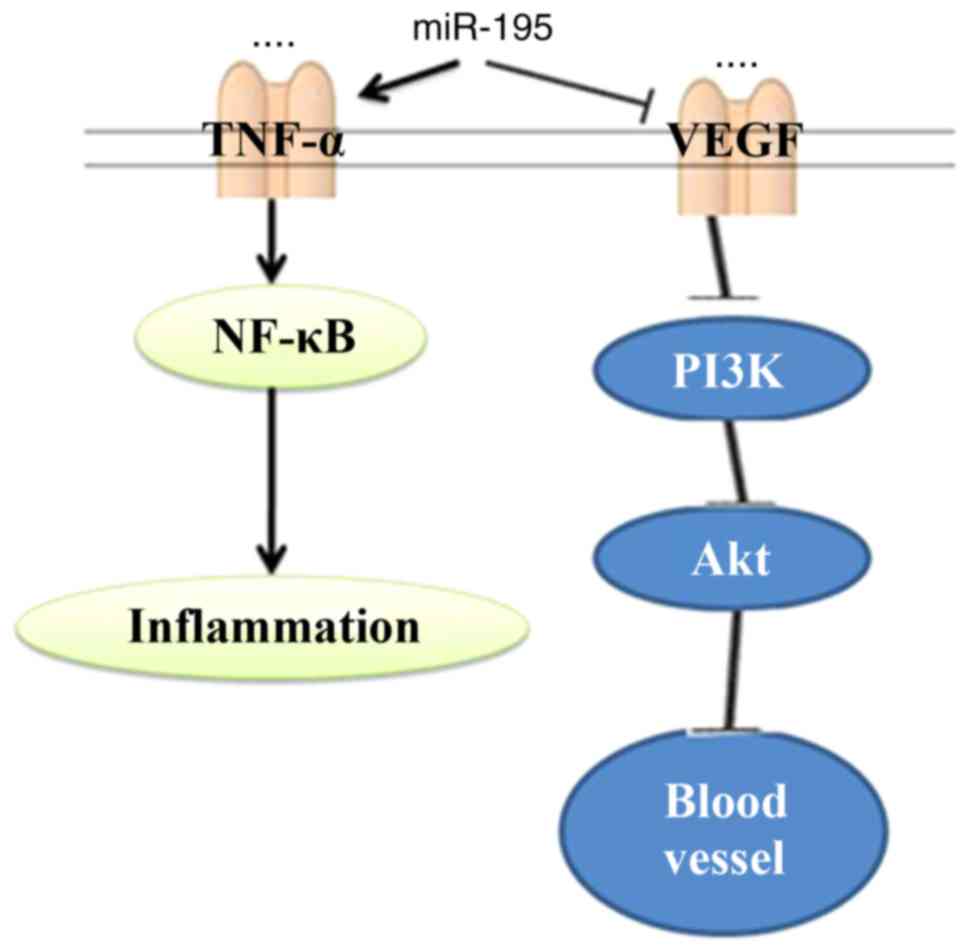
In conclusion, the present study demonstrated that
miR-195 suppressed AAA through the TNF-α/NF-κB and VEGF/PI3K/Akt
pathways (Fig. 13). Taken
together, these observations revealed that miR-195 functioned as an
anti-inflammatory in AAA through the TNF-α/NF-κB and VEGF/PI3K/Akt
pathways.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu J, Zuo SW, Li Y, Jia X, Jia SH, Zhang
T, Song YX, Wei YQ, Xiong J, Hu YH and Guo W: Hyperhomocysteinaemia
is an independent risk factor of abdominal aortic aneurysm in a
Chinese Han population. Sci Rep. 6:179662016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lindeman JH, Abdul-Hussien H, van Bockel
JH, Wolterbeek R and Kleemann R: Clinical trial of doxycycline for
matrix metalloproteinase-9 inhibition in patients with an abdominal
aneurysm: Doxycycline selectively depletes aortic wall neutrophils
and cytotoxic T cells. Circulation. 119:2209–2216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lindberg S, Zarrouk M, Holst J and
Gottsater A: Inflammatory markers associated with abdominal aortic
aneurysm. Eur Cytokine Netw. 27:75–80. 2016.PubMed/NCBI
|
|
4
|
Ciavarella C, Alviano F, Gallitto E, Ricci
F, Buzzi M, Velati C, Stella A, Freyrie A and Pasquinelli G: Human
vascular wall mesenchymal stromal cells contribute to abdominal
aortic aneurysm pathogenesis through an impaired immunomodulatory
activity and increased levels of matrix metalloproteinase-9. Circ
J. 79:1460–1469. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y, Ait-Oufella H, Herbin O, Bonnin P,
Ramkhelawon B, Taleb S, Huang J, Offenstadt G, Combadière C, Rénia
L, et al: TGF-beta activity protects against inflammatory aortic
aneurysm progression and complications in angiotensin II-infused
mice. J Clin Invest. 120:422–432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang L, Wang B, Li H, Lu H, Qiu F, Xiong
L, Xu Y, Wang G, Liu X, Wu H and Jing H: Quercetin, a flavonoid
with anti-inflammatory activity, suppresses the development of
abdominal aortic aneurysms in mice. Eur J Pharmacol. 690:133–141.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Biros E, Moran CS, Wang Y, Walker PJ,
Cardinal J and Golledge J: microRNA profiling in patients with
abdominal aortic aneurysms: The significance of miR-155. Clin Sci
(Lond). 126:795–803. 2014. View Article : Google Scholar
|
|
8
|
Maegdefessel L, Azuma J and Tsao PS:
MicroRNA-29b regulation of abdominal aortic aneurysm development.
Trends Cardiovasc Med. 24:1–6. 2014. View Article : Google Scholar
|
|
9
|
Carvalho LS: Can microRNAs improve
prediction of abdominal aortic aneurysm growth? Atherosclerosis.
256:131–133. 2017. View Article : Google Scholar
|
|
10
|
Spin JM and Tsao PS: Battle of the bulge:
miR-195 versus miR-29b in aortic aneurysm. Circ Res. 115:812–813.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zampetaki A, Attia R, Mayr U, Gomes RS,
Phinikaridou A, Yin X, Langley SR, Willeit P, Lu R, Fanshawe B, et
al: Role of miR-195 in aortic aneurysmal disease. Circ Res.
115:857–866. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaneko H, Anzai T, Takahashi T, Kohno T,
Shimoda M, Sasaki A, Shimizu H, Nagai T, Maekawa Y, Yoshimura K, et
al: Role of vascular endothelial growth factor-A in development of
abdominal aortic aneurysm. Cardiovasc Res. 91:358–367. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wolanska M, Bankowska-Guszczyn E,
Sobolewski K and Kowalewski R: Expression of VEGFs and its
receptors in abdominal aortic aneurysm. Int Angiol. 34:520–528.
2015.PubMed/NCBI
|
|
14
|
Yu B, Liu L, Sun H and Chen Y: Long
noncoding RNA AK056155 involved in the development of Loeys-Dietz
syndrome through AKT/PI3K signaling pathway. Int J Clin Exp Pathol.
8:10768–10775. 2015.PubMed/NCBI
|
|
15
|
Walsh SR, Sadat U, Boyle JR, Tang TY,
Lapsley M, Norden AG and Gaunt ME: Remote ischemic preconditioning
for renal protection during elective open infrarenal abdominal
aortic aneurysm repair: Randomized controlled trial. Vasc
Endovascular Surg. 44:334–340. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Karlsson L, Bergqvist D, Lindbäck J and
Pärsson H: Expansion of small-diameter abdominal aortic aneurysms
is not reflected by the release of inflammatory mediators IL-6,
MMP-9 and CRP in plasma. Eur J Vasc Endovasc Surg. 37:420–424.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bruegger D, Bauer A, Rehm M, Niklas M,
Jacob M, Irlbeck M, Becker BF and Christ F: Effect of hypertonic
saline dextran on acid-base balance in patients undergoing surgery
of abdominal aortic aneurysm. Crit Care Med. 33:556–563. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muehling BM, Ortlieb L, Oberhuber A and
Orend KH: Fast track management reduces the systemic inflammatory
response and organ failure following elective infrarenal aortic
aneurysm repair. Interact Cardiovasc Thorac Surg. 12:784–788. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cai C, Chen QB, Han ZD, Zhang YQ, He HC,
Chen JH, Chen YR, Yang SB, Wu YD, Zeng YR, et al: miR-195 inhibits
tumor progression by targeting RPS6KB1 in human prostate cancer.
Clin Cancer Res. 21:4922–4934. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wanhainen A, Mani K, Vorkapic E, De Basso
R, Björck M, Länne T and Wågsäter D: Screening of circulating
microRNA biomarkers for prevalence of abdominal aortic aneurysm and
aneurysm growth. Atherosclerosis. 256:82–88. 2017. View Article : Google Scholar
|
|
21
|
Davis FM, Rateri DL and Daugherty A:
Abdominal aortic aneurysm: Novel mechanisms and therapies. Curr
Opin Cardiol. 30:566–573. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen G, Cao S, Liu F and Liu Y: miR-195
plays a role in steroid resistance of ulcerative colitis by
targeting Smad7. Biochem J. 471:357–367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mi T, Nie B, Zhang C and Zhou H: The
elevated expression of osteopontin and NF-kappaB in human aortic
aneurysms and its implication. J Huazhong Univ Sci Technolog Med
Sci. 31:602–607. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang F, Banker G, Liu X, Suwanabol PA,
Lengfeld J, Yamanouchi D, Kent KC and Liu B: The novel function of
advanced glycation end products in regulation of MMP-9 production.
J Surg Res. 171:871–876. 2011. View Article : Google Scholar
|
|
25
|
Ding J, Huang S, Wang Y, Tian Q, Zha R,
Shi H, Wang Q, Ge C, Chen T, Zhao Y, et al: Genome-wide screening
reveals that miR-195 targets the TNF-alpha/NF-kappaB pathway by
down-regulating IkappaB kinase alpha and TAB3 in hepatocellular
carcinoma. Hepatology. 58:654–666. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nishibe T, Dardik A, Kondo Y, Kudo F, Muto
A, Nishi M, Nishibe M and Shigematsu H: Expression and localization
of vascular endothelial growth factor in normal abdominal aorta and
abdominal aortic aneurysm. Int Angiol. 29:260–265. 2010.PubMed/NCBI
|
|
27
|
Almeida MI, Silva AM, Vasconcelos DM,
Almeida CR, Caires H, Pinto MT, Calin GA, Santos SG and Barbosa MA:
miR-195 in human primary mesenchymal stromal/stem cells regulates
proliferation, osteogenesis and paracrine effect on angiogenesis.
Oncotarget. 7:7–22. 2016. View Article : Google Scholar :
|
|
28
|
Zhang S, Kan X, Li Y, Li P, Zhang C, Li G,
Du J and You B: Deficiency of γδT cells protects against abdominal
aortic aneurysms by regulating phosphoinositide 3-kinase/AKT
signaling. J Vasc Surg. S0741–S5214(16): 31854–7. 2016.
|
|
29
|
Keppler-Noreuil KM, Parker VE, Darling TN
and Martinez-Agosto JA: Somatic overgrowth disorders of the
PI3K/AKT/mTOR pathway & therapeutic strategies. Am J Med Genet
C Semin Med Genet. 172:402–421. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun P, Wang L, Lu Y, Liu Y, Li L, Yin L,
Zhang C, Zhao W, Shen B and Xu W: MicroRNA-195 targets VEGFR2 and
has a tumor suppressive role in ACHN cells via PI3K/Akt and
Raf/MEK/ERK signaling pathways. Int J Oncol. 49:1155–1163. 2016.
View Article : Google Scholar : PubMed/NCBI
|