Introduction
Acute pancreatitis (AP) is an acute inflammatory
disease, which is manifested predominantly as acinar cell injury,
oxidative stress and pancreatic inflammation. AP is frequently
caused by gallstone disease or excess alcohol ingestion and is the
primary contributor to morbidity and mortality rates worldwide
(1,2). The incidence of AP varies at 5-80
per 100,000 individuals throughout the world (3). AP in the majority of patients is in
a mild form, however, ~20% of patients develop a complicated
life-threatening disease, which requires intensive and prolonged
therapeutic intervention, and has a risk of organ failure and
carries a mortality rate of 7–15% (4). The treatment of mild AP is
supportive, however, severe AP characterized by pancreatic necrosis
may require surgery (5).
Laparotomy and debridement of the infected necrotic tissues have
been reported to be the gold standard treatment in previous decades
(6). However, the mortality rate
of surgical intervention exceeds 50% (7). Therefore, there is an urgent
requirement to identify novel diagnostic and therapeutic targets to
improve the survival rate of patients with AP.
Dual-specificity phosphatase-1 (DUSP1), also known
as mitogen-activated protein kinase (MAPK) phosphatase 1, is a
member of the MAPK phosphatase family and a potent negative
regulator of MAPK activity, with an increasingly recognized role in
tumor biology (8,9). DUSP1 is overexpressed in pancreatic
cancer cells in pancreatic ductal adenocarcinoma, where it
increases colony formation and promotes tumorigenicity (10). The DUSP1 gene is considered a
tumor suppressor and a regulator of cancer-associated inflammation
(11). DUSP1 is also important in
anti-inflammation effects (12).
MAPKs are considered evolutionarily well-conserved serine and
threonine protein enzymes, which are involved in signal
transduction pathways linking cell surface receptors with main
regulative nuclear and intracellular targets (13). In mammals, there are several MAPK
enzymes responsible for cell proliferation, apoptosis,
differentiation and survival (14). MAPK phosphatases, including DUSP1,
inhibit signal transduction and cytokine activation via MAPK
dephosphorylation or interference with effector molecules binding
to MAPKs, including extracellular signal-regulated kinase (ERK)
(15). MAPKs are considered to be
main signal transducers in the early stage of the development of AP
(16). Lentiviral vectors enable
the maintenance of the sustained long-term expression of transgenes
in various mammalian cells with a substantial packaging capacity
and extensive cell tropism, and their development produces a
system, which can be applied to decrease the expression of target
genes (17,18). The proinflammatory cytokines,
including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6),
not only promote inflammation but also have an effect on the
generation and duration of inflammatory pain by acting on sensory
nerve cells (19). Based on these
findings, it was hypothesized that there may be an effect of
silencing the DUSP1 gene by lentiviral vector-mediated small
interfering siRNA on the release of proinflammatory cytokines
through regulation of the MAPK signaling pathway in mice with
AP.
Materials and methods
Construction and detection of the
psiRNA-DUSP1 vector
Two DUSP1-siRNAs and one negative control (NC)
sequence were designed according to the mouse DUSP1 gene sequence
in the gene bank as follows: siRNA1: 5′-TAG CGT CAA GAC ATT TGC
TGA-3′; siRNA2: 5′-CTG TAC TAT CCT GTA AAT ATA-3′; and negative:
5′-AAC TGG ACT TCC AGA AGA ACA-3′. The siRNA and NC sequences were
synthe-sized by Shanghai Sangon Biological Technology Co., Ltd.
(Shanghai, China). According to the synthesized siRNA and NC
sequences, restriction sites BamHI and EcoRI were
added to both ends to synthesize oligo DNA for forming
double-stranded DNA post-annealing. Subsequently, the DNA was
inserted into a linear plasmid
pSIHl-H1-copGFP, which was enzyme-digested by
BamHI and EcoRI through the T4 DNA ligase
and was transformed into DH5α competent cells (CB10; Tiangen
Biotech Co., Ltd., Beijing, China). Subsequently, plasmids were
extracted and transfected with FuGENE6 (Roche Diagnostics,
Indianapolis, IN, USA) according to the manufacturers protocol.
There were four groups in the transfection experiments: Blank group
(transfected with empty plasmid pSIH), NC group (transfected with
pSIH-NC plasmid), siRNA1 group (transfected with pSIH-DUSP1-siRNA1
plasmid), and siRNA2 group (transfected with pSIH-DUSP1-siRNA2
plasmid). Following transfection for 48 h, the expression of DUSP1
in each group was detected using western blot analysis, as in the
subsequent animal experiment), and the silencing efficiency of
siRNA was determined using Gel-Pro Analyzer software (version 4.0;
Media Cybernetics, Inc., Rockville, MD, USA).
Lentivirus packaging and titer
determination
The pSIH-DUSP1-siRNA and pSIH-NC with high silencing
efficiency were selected to co-transfect 293T cells (purchased from
the Shanghai Institute of Cell Biology, Chinese Academy of
Sciences, Shanghai, China) with a packaging plasmid mixture
respectively using the lipofection method. The cells were divided
into three groups: Control group (cells infected with empty plasmid
lentivirus), scramble group (cells infected with NC lentivirus),
and siRNA group (cells infected with siRNA lentivirus). The method
was as follows: 12 h prior to transfection, the 293T were cells
seeded on a 6-well plate at 5×105 cells/well using
Dulbecco's modified Eagle's Medium (DMEM; Corning Incorporated,
Corning, NY, USA) with 5% fetal bovine serum (FBS; cat. no.
10100-147; Thermo Fisher Scientific, Inc., Waltham, MA, USA) as the
culture medium. The liposome Lipofectamine 2000 (20 µl) was added
to 500 µl of DMEM without serum and double antibody followed by
even mixing. To the corresponding 2 ml centrifuge tubes, 2 µg of
pSIH-DUSP1-siRNA, pSIH-NC, or empty plasmid and 10 µg of lentivirus
packaging plasmid was added, followed by mixing and incubating for
5 min at 37°C. The corresponding centrifuge tubes were mixed and
stood for 20 min, and then moved onto the 293T cell culture plate.
After 6 h, the culture medium was replaced. After 72 h, the
supernatant of the virus was collected for viral titer
determination. The 293T cells were incubated in a 96-well plate
with 2×108 cells/ml, and each well received 100 µl DMEM
containing 10% FBS overnight. In the viral titer determination
test, eight wells were assigned to each group, with the first
containing 10 µl of the virus to be tested. The remaining
wells were diluted at 10:1, with the final well serving as a blank
control. After 48 h, the number of green fluorescent cells was
observed under a fluorescence inverted microscope from a high
concentration to a low concentration. If the number of positive
cells in the first well was more than five, and the fluorescence
disappeared subsequent to this well, or if the fluorescence
disappeared in the later wells, and the number of positive cells in
this well was less than five, then the well was considered to be
metering well and recorded as 1 IU. The number of positive cells
was recorded as 'm', and the viral titer was calculated as follows:
Viral titer=m × (1 IU × dilution rate of metering well relative to
the first well)/volume of virus added to the first well (20).
Preparation and grouping of the AP mouse
model
A total of 105 male KM mice in healthy condition
(4–6 weeks old) with a weight range of 20±2 g at clean grade were
used. The animals were maintained at 50–60% humidity, 22–24°C and a
12 h day/night cycle. Animals were provided by the Experimental
animal Center of Sichuan University (Sichuan, China). Prior to the
experiment, the mice used for the AP model were fasted for 12 h for
gastrointestinal decompression in order to prevent the
overproduction of pancreatic juice; however, they all had free
access to water. The mice were intraperitoneally injected twice
with a 1 h interval with 20% L-arginine (cat. no. L101021;
Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) at 4 g/kg to
establish the AP mouse model. Using a Table of random numbers, the
mice were divided into seven groups with 15 mice in each group:
Control group (mice injected with the same dose of normal saline);
siRNA group (mice injected with 0.88 mg/kg DUSP1-siRNA
lenti-virus); AP group (AP mouse model); AP+PD98059 group [0.5 h
pre-induction and 1 h post-induction, the mice were
intraperitoneally injected with PD98059 at 10 mg/kg (cat. no.
HY-12028; MedChem Express; Monmouth Junction, NJ, USA); AP+siRNA
group (following successful model establishment, lentiviruses
containing 0.88 mg/kg DUSP1-siRNA were injected into mice
intraperitoneally); AP+scramble group (following successful model
establishment, 0.88 mg/kg scramble-siRNA lentiviral vectors were
injected intraperitoneally); AP+PD98059+siRNA group (0.5 h
pre-induction and 1 h post-induction, mice were intraperitoneally
injected with PD98059 at 10 mg/kg, and following successful model
establishment, lentiviruses containing 0.88 mg/kg DUSP1-siRNA were
injected into mice intraperitoneally). The mice in each group
received cardiac blood sampling (as blood specimen) at 12, 24 and
48 h post-modeling, respectively. After 48 h, the mice were
sacrificed and pancreatic tissue, liver tissue, lung tissue and
kidney tissue were immediately obtained. The present study was
approved by the experimental animal ethics committee of Qianfoshan
Hospital, Shandong University (Shandong, China), in accordance with
the principles of animal protection, animal welfare and ethics, and
in line with the relevant provisions of national experimental
animal welfare ethics.
Hematoxylin and eosin (HE) staining
The pancreatic, liver, kidney and lung tissues of
mice in each group were removed and fixed with 4% formaldehyde for
6 h, and immersed and embedded in paraffin. The paraffin-embedded
pancreatic tissues were cut into 3-µm sections, which were
heated at 60°C overnight. The sections were dewaxed in xylene I and
xylene II for 20 min each, soaked in 100, 95, 80 and 70% ethanol
for 5 min each, and then washed with distilled water. The sections
were dyed with hematoxylin staining for 10 min and rinsed with tap
water for 15 min until black-blue, followed by eosin staining for
30 sec, and washing with double distilled water for flushing red.
The sections were then dehydrated in ethanol, cleaned in xylene and
mounted by neutral gum. Histopathological examination of the HE
staining was performed using light microscopy (BX53; Olympus,
Tokyo, Japan). Using the morphological image analysis system
(I-Solutiontype; IMT i-solution Inc., Vancouver, BC, Canada),
images of the HE-stained sections of pancreatic, liver, kidney and
lung tissues in each group were captured at x400 magnification. The
experiment was repeated three times.
Enzyme-linked immunosorbent assay
(ELISA)
At 12, 24 and 48 h post-model establishment, five
mice in each group were selected for cardiac blood sampling. The
blood was reserved at room temperature for 30 min and then
centrifuged at 1,960 × g (4°C) for 15 min for separation of the
serum. Experimental procedures were performed in strict accordance
with the instructions of the TNF-α ELISA kit (cat. no. ab208348;
Abcam, Cambridge, MA, USA), IL-1β ELISA kit (cat. no. ab100704;
Abcam), IL-6 ELISA kit (cat. no. ab100713; Abcam), HMGB1 ELISA kit
(cat. no. E0399m; Beijing Huaxia Ocean Technology Co., Ltd.,
Beijing, China) and S100A12 ELISA kit (cat. no. hz-EL-M1036c;
Shanghai Huzhen Biological Technology Co., Ltd., Shanghai, China).
The ELISA kits were stood at room temperature for 20 min and
washing solution was prepared. Following dissolution, 100 µl
standard was added into the reaction plate to construct a standard
curve. A total of 100 µl of each sample was added to the
reaction plate for incubation at 37°C for 90 min, and the plate was
washed five times at intervals of 30 sec. Following washing, 100
µl of biotinylated antibody working fluid was added for
incubation at 37°C for 60 min, and the plate was washed five times
at intervals of 30 sec. Subsequently, 100 µl of the enzyme
binding agent (light-avoiding) working fluid was added for
incubation at 37°C for 30 min, and the plate was washed five times.
Finally, stop buffer was added to terminate the reaction and the
universal microplate reader (BioTek Synergy 2; BioTek Instruments,
Inc., Winooski, VT, USA) was used to measure the optical density
(OD) value of each well at 450 nm within 3 min. The standard curve
was drawn and expression levels of the proinflammatory factors
(TNF-α, IL-1β, IL-6 and HMGB1) and S100A12 were measured according
to the OD value.
Determination of serum amylase, lipase
and urinary trypsinogen-2 levels
The levels of amylase, lipase and urinary
trypsinogen-2 in serum were determined at 12, 24 and 48 h
post-model establishment in each group. An Olympus Au2700 system
(Olympus) was used to determine serum amylase levels. The lipase
was detected using a Roche modular P800 automatic biochemical
analyzer and its reagents (Roche Diagnostics GmbH, Mannheim,
Germany). Urine trypsinogen-2 was detected by ELISA with a Johnson
Bitros-350 automatic chemical analyzer (Johnson & Johnson, New
Brunswick, NJ, USA); the kits were purchased from Shanghai Westang
Bio-tech Co., Ltd. (Shanghai, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Based on the manufacturer's protocol of the TRIzol
kit (Invitrogen; Thermo Fisher Scientific, Inc.), total RNA from
the tissue specimens was extracted via the TRIzol one-step method.
RNA was dissolved in ultrapure water, which was treated with
diethylpyrocarbonate (Shanghai Sangon Biological Technology Co.,
Ltd., Shanghai, China). The ND-1000 UV/visible spectrophotometer
(Thermo Fisher Scientific, Inc.) was used to measure OD values at
260 and 280 nm, and the quality and concentration of total RNA were
identified and determined. The reverse transcription of the
extracted RNA was performed in two steps according to the protocol
of the kits (cat. no. RR037Q; Takara Biotechnology, Co., Ltd.,
Dalian, China). The reverse transcription system comprised 2
µl of 5X PrimeScript buffer (for real-time), 0.5 µl
of PrimeScript RT enzyme mix I, 0.5 µl of Oligo dT primer
(50 µm), 0.5 µl of random primers (100 µm) and
2 µg of total RNA, and RNase-free dH2O was added
to make the sample up to 20 µl. The reaction conditions were
as follows: 37°C for 15 min, 85°C for 5 sec and 4°C for 5 min. The
cDNA obtained by reverse transcription was stored temporarily for
further use in a refrigerator at −80°C. The RT-qPCR was performed
using the TaqMan probe method according to the kit protocol
(Fermentas; Thermo Fisher Scientific, Inc.). The primer sequences
are presented in Table I. The
reaction conditions were as follows: Pre-denaturation at 95°C for
30 sec; denaturation at 95°C for 10 sec, annealing at 60°C for 20
sec, and extension at 70°C for 10 sec, repeated for 40 cycles. The
reaction system was as follows: Premix Ex Taq or SYBR-Green mix
(12.5 µl), forward primer (1 µl), reverse primer (1
µl), cDNA (1–4 µl), ddH2O up to 25
µl. The RT-qPCR platform (Bio-Rad iQ5; Bio-Rad Laboratories,
Inc., Hercules, CA, USA) was used for detection. With β-actin as an
internal reference, the relative expression of each target gene was
calculated using the 2−ΔΔCq method (21). The experiment was repeated three
times and the mean value was obtained.
 | Table IPrimer sequences for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I
Primer sequences for reverse
transcription-quantitative polymerase chain reaction analysis.
| Factor | Primer
sequence |
|---|
| TNF-α | F:
5′-TGATCCGCGACGTGGAA-3′ |
| R:
5′-ACCGCCTGGAGTTCTGGAA-3′ |
| IL-1β | F:
5′-CTCCATGAGCTTTGTACAAGG-3′ |
| R:
5′-TGCTGATGTACCAGTTGGGG-3′ |
| IL-6 | F:
5′-CCAGAGATACAAAGAAATGATGG-3′ |
| R:
5′-ACTCCAGAAGACCAGAGGAAAT-3′ |
| DUSP1 | F:
5′-AACAGGGCAGAAGAGAAAGG-3′ |
| R:
5′-TCATCGGGAATGGTTAATACTG-3′ |
| HMGB1 | F:
5′-CTCAGAGAGGTGGAAGACCATGT-3′ |
| R:
5′-GGGATGTAGGTTTTCATTTCTCTTTC-3′ |
| S100A12 | F:
5′-CCATGCCCTCTACAAGAATGA-3′ |
| R:
5′-TATCACCATCGCAAGGAACTC-3′ |
| β-actin | F:
5′-GCACCACACCTTCTACAATG-3′ |
| R:
5′-TGCTTGCTGATCCACATCTG-3′ |
Western blot analysis
Pancreatic, liver, kidney and lung tissue samples
(0.3 g each) were extracted and cut into 0.5×0.5×0.5 mm sections on
ice with ophthalmic scissors following removal of blood and other
tissues with pre-cold 1X PBS solution. The prepared 3-ml lysate [7
mol/l urea, 2 mol/l thiourea, 5 ml/l IPG buffer (pH 3–10), 65
mmol/l DTT, 40 g/l CHAPS and 5 mg/l protease inhibitor] was added
to the sections and mixed, and the mixture was shattered on ice
using ultrasound. Subsequently, the sections were centrifuged at
12,000 g at 4°C for 30 min, and the supernatant was used as protein
extracts. The bicinchoninic acid method was used to determine the
protein concentration. Subsequently, 5X SDS lysate (cat. no.
P0013G; Beyotime Institute of Biotechnology, Beijing, China) was
added to inactivate proteins at 100°C for 5 min. A loading sample
(40 µg) was extracted for electrophoresis on a
polyacrylamide gel (5% concentrated gel and 12% separating gel).
Following transfer onto a nitrocellulose trans-membrane, the
membrane was sealed in TBS-T containing 5% bovine serum albumin
(BSA) (Guangzhou Tiancheng Medical Technology Co., Ltd., Guangzhou,
China) at room temperature for 1 h. The sealing liquid was
discarded and the membrane was placed in the plastic groove.
Subsequently, the membranes were incubated with the corresponding
concentration of primary rabbit anti-DUSP1 (cat. no. ab178883;
Abcam; 1:1,000), rabbit anti-ERK (cat. no. ab212206; Abcam;
1:1,000), rabbit anti-p-ERK (cat. no. ab214362; Abcam; 1:1,000),
rabbit anti- c-Jun N-terminal kinase (JNK; cat. no. ab199380;
Abcam; 1:2,500), rabbit anti-p-JNK (cat. no. ab76572; Abcam;
1:5,000), rabbit anti-p38 (cat. no. ab32142; Abcam; 1:1,000),
anti-p-p38 (cat. no. ab47363; Abcam; 1:1,000) and rabbit
anti-β-actin (cat. no. ab5694; Abcam 1:10,000). All the above
antibodies were prepared in 5% BSA. The transfer surface was upward
and the membranes were placed in a refrigerator at 4°C overnight.
The following day, TBST was used to rinse the membranes three times
(10 min per rinse). The diluted secondary antibody (goat
anti-rabbit; cat. no. ab6721; Abcam) was added for incubation at
4°C for 4–6 h, and the membranes were washed in TBST three times
for 15 min each. The chemiluminescence reagent A and B solutions
(Yan Hui Biological Co., Ltd., Shanghai, China; 1:1) were mixed and
evenly dripped on the membrane, and developer solution was used for
development. All bands were subjected to relative OD analysis. The
experiment was repeated three times with the mean value
calculated.
Statistical analysis
SPSS 21.0 statistical software (IBM SPSS, Armonk,
NY, USA) was used to process the data. The measurement data are
presented as the mean ± standard deviation. The comparisons among
multiple groups were performed using one-way analysis of variance,
followed by Fisher's least significant difference or Tamhane's T2
tests, and the comparisons between two groups were performed using
an independent t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
siRNA2 presents with higher silencing
efficiency
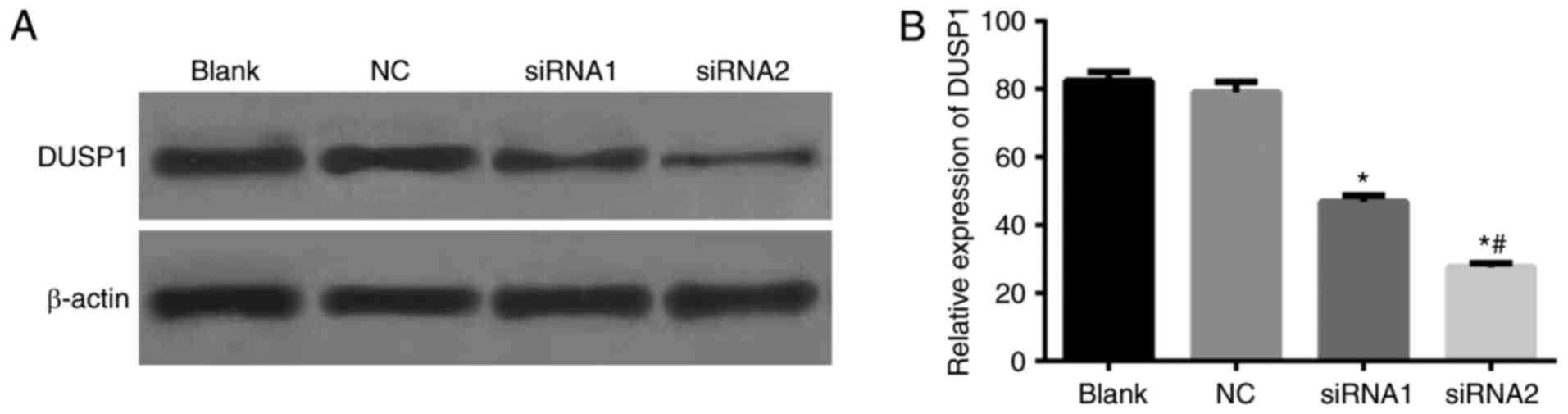
The results of western blot analysis (Fig. 1) demonstrated that, compared with
the blank group, no significant difference was identified in the
expression of DUSP1 in the NC group (P>0.05). Compared with the
blank group, the expression of DUSP1 in the siRNA1 group was
decreased (35.56%) and in the siRNA2 group was decreased (54.72%),
which indicated that the silencing efficiency of siRNA2 was higher.
Therefore, pSIH-DUSP1-siRNA2 was used to package with the
lentivirus.
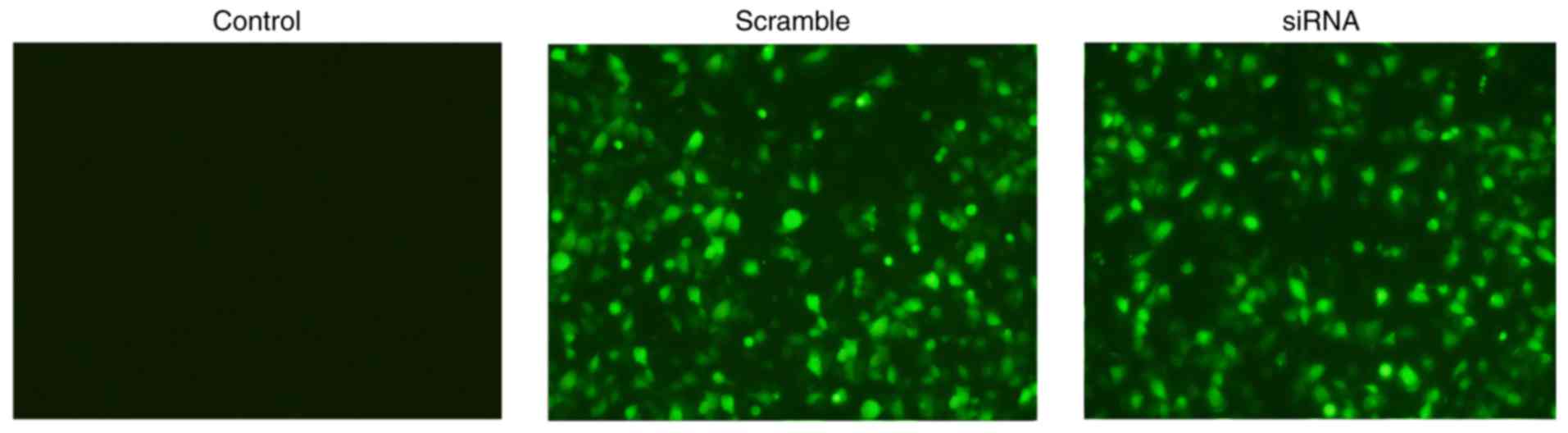
Titer determination of lentivirus and
results of 293T cell infection
The titer of the lentivirus concentrate was
5.62×108 TU/ml following serial dilution, and the virus
was successfully packaged. Under fluorescence microscopy, cells
that produced green fluorescence were infected successfully. There
were no fluorescent cells in the control group. The number of green
fluorescent cells in the scramble and siRNA groups accounted for
>90% of the total cells, indicating that the infection rate of
the lentivirus to the scramble and siRNA groups was >90%
(Fig. 2).
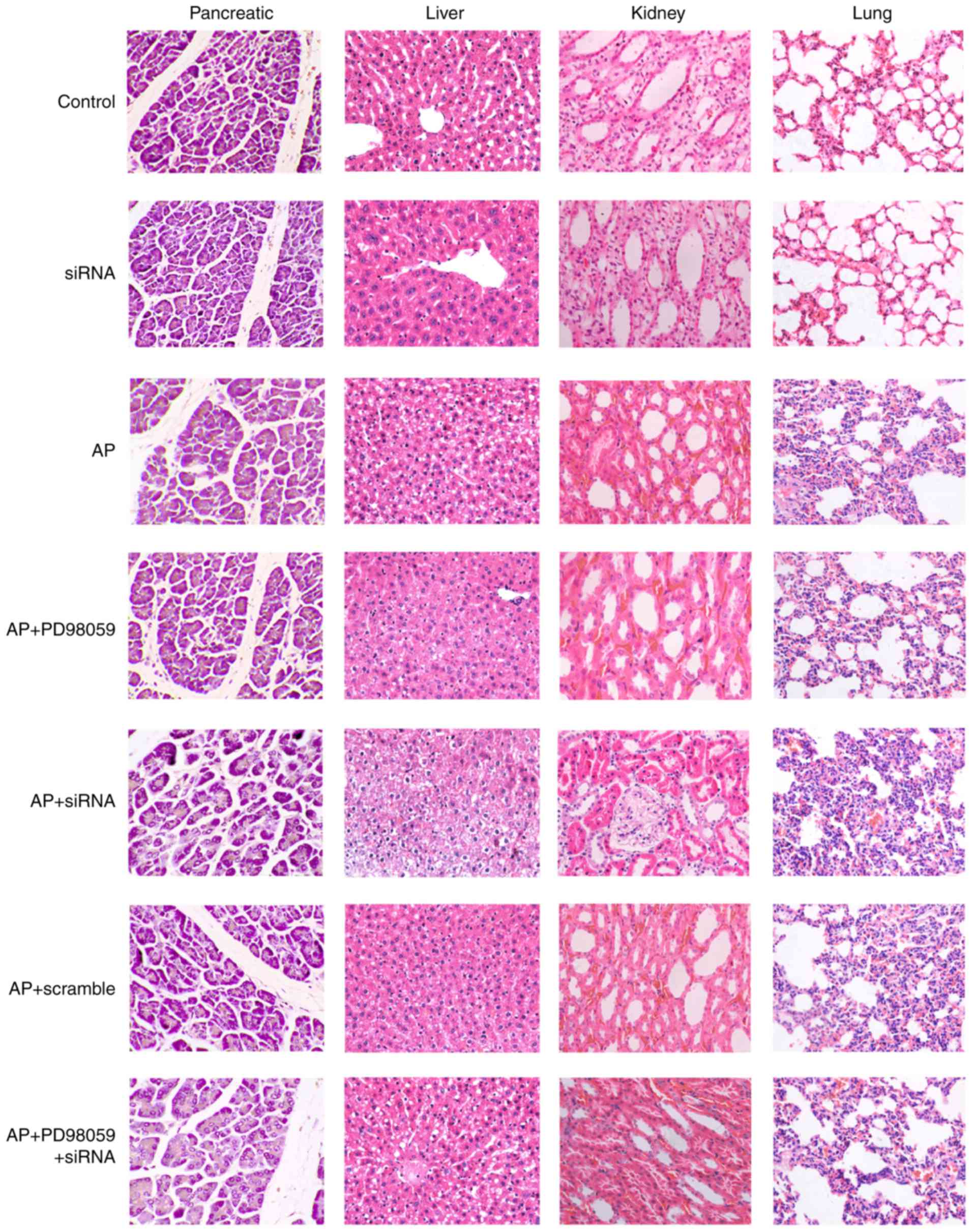
Pathological changes in pancreatic,
liver, kidney and lung tissues of mice in each group
The pancreatic tissues were stained using HE and
observed under a light microscope (Fig. 3). In the control and siRNA groups,
the acini and lobules of the pancreatic tissues were intact and the
stroma was normal, with no bleeding or necrosis. In the AP+PD98059
group, there was infiltration of neutrophils and monocytes with no
notable vascular lesion. In the AP, AP+scramble and
AP+PD98059+siRNA groups, the stroma of the pancreas exhibited
hyperemia, and edema was accompanied by obscure cell structure,
inflammatory cell infiltration, vascular dilatation and congestion.
The AP+siRNA group demonstrated regions of focal acinar necrosis,
inflammatory cell infiltration, occasional bleeding and marginal
fat necrosis.
The liver tissue was observed under light
microscopy. In the control and siRNA groups, the stem cells were
larger, with a polyhedral shape, large and round nuclei, and an
intact nuclear membrane. The AP+PD98059 group exhibited lymphocytic
infiltration in liver tissues and a loose hepatocyte cytoplasm. The
AP, AP+scramble and AP+PD98059+siRNA groups exhibited impaired lung
tissues and inflammatory cell infiltration. In the AP+siRNA group,
the hepatic lobules were not clear, and there were numerous fat
vacuoles of different sizes, spotty necrosis of hepatocytes, and
marked inflammatory cell infiltration in the cytoplasm.
The kidney tissue was observed under a light
microscope. In the control and siRNA groups, there were no marked
changes in the kidney tissue. In the AP+PD98059 group, the
glomerular endothelial cells were swollen with interstitial edema
and inflammatory cell infiltration. In the AP, AP+scramble and
AP+PD98059+siRNA groups, the cortical renal tubular epithelial
cells were vacuolar and drip shaped, the brush border disappeared
and there was a marked band of congestion. In the AP+siRNA group,
there was marked congestion, endotheliocytic swelling, interstitial
edema, and inflammatory cell infiltration in the glomerulus, and
degeneration, necrosis and a disappearing cell striated border were
observed in renal tubular epithelial cells.
The lung tissue was observed under light microscopy.
In the control and siRNA groups, the lung tissue was structurally
complete, the airway epithelial cells were arranged regularly with
complete alveolar spaces, and the interstitial cells were free of
edema without notable inflammatory cell infiltration, bleeding or
edema fluid. The AP+PD98059 group exhibited interstitial edema and
inflammatory cell infiltration. In the AP, AP+scramble and
AP+PD98059+siRNA groups, inflammatory cells had infiltrated and
diffused into the lung stroma. In the AP+siRNA group, there was
marked inflammatory cell infiltration, evidence of alveolar
structure damage, and evidence of epithelial cell denaturing and
detachment.
TNF-α, IL-1β, IL-6, HMGB1 and S100A12 are
expressed at high levels in AP mice
Compared with the control group, at 12, 24 and 48 h
post-model establishment, the expression levels of TNF-α, IL-1β,
IL-6, HMGB1 and S100A12 in serum were increased in the siRNA group,
but without statistical significance (all P>0.05); however,
these levels increased significantly in the other five groups
(P<0.05). Compared with the AP group, at 12, 24 and 48 h
post-model establishment, the AP+PD98059 group exhibited decreased
expression levels of TNF-α, IL-1β, IL-6, HMGB1 and S100A12 in serum
(P<0.05) and the AP+siRNA group had increased expression levels
of TNF-α, IL-1β, IL-6, HMGB1 and S100A12 in serum (P<0.05). No
significant differences were observed between the AP group and the
AP+scramble and AP+PD98059+siRNA groups (P>0.05; Table II).
 | Table IIExpression of proinflammatory
factors, HMGB1 and S100A12 in serum of mice in each group at 12, 24
and 48 h post model establishment (n=5). |
Table II
Expression of proinflammatory
factors, HMGB1 and S100A12 in serum of mice in each group at 12, 24
and 48 h post model establishment (n=5).
| Group | Control
(pg/ml) | siRNA (pg/ml) | AP (pg/ml) | AP+PD98059
(pg/ml) | AP+siRNA
(pg/ml) | AP+scramble
(pg/ml) | AP+PD98059+siRNA
(pg/ml) |
|---|
| TNF-α | | | | | | | |
| 12 h | 96.28±18.14 | 106.67±9.87 |
230.12±27.12a |
165.68±22.57a,b |
321.83±36.74a,b |
234.78±32.26a |
226.78±34.09a |
| 24 h | 92.02±15.14 | 98.67±7.57 |
199.58±25.65a |
145.65±14.14a,b |
310.78±18.26a,b |
204.52±31.19a |
201.58±32.93a |
| 48 h | 79.55±8.54 | 85.56±3.56 |
189.43±30.22a |
132.55±12.99a,b |
300.99±38.13a,b |
191.67±25.92a |
188.67±26.34a |
| IL-1β | | | | | | | |
| 12 h | 79.57±11.86 | 88.46±5.65 |
208.68±28.07a |
152.64±21.57a,b |
311.90±27.60a,b |
209.88±29.29a |
210.63±38.28a |
| 24 h | 82.31±8.60 | 91.34±8.55 |
229.68±12.37a | 178.28±10.0a,b |
343.85±37.78a,b |
234.18±27.43a |
239.68±29.39a |
| 48 h | 78.79±11.62 | 86.45±6.56 |
189.68±20.04a |
129.62±24.83a,b |
288.49±32.85a,b |
189.68±19.16a |
195.67±29.25a |
| IL-6 | | | | | | | |
| 12 h | 152.59±19.36 | 112.34±10.65 |
352.54±34.87a |
275.55±24.96a,b |
457.51±42.17a,b |
350.24±36.18a |
354.54±44.99a |
| 24 h | 156.32±24.43 | 187.67±12.34 |
384.64±31.31a |
289.43±39.15a,b |
472.45±46.05a,b |
381.66±35.51a |
386.16±41.22a |
| 48 h | 150.43±14.02 | 178.88±14.56 |
332.13±36.30a |
254.32±32.88a,b |
444.54±36.92a,b |
329.13±33.09a |
334.13±31.06a |
| HMGB1 | | | | | | | |
| 12 h | 44.16±5.09 | 48.56±2.45 |
137.55±18.64a | 82.41±8.13a,b |
214.55±29.24a,b |
139.52±21.54a |
133.43±15.97a |
| 24 h | 46.11±5.39 | 53.56±4.56 |
152.51±16.24a | 96.17±10.50a,b |
237.55±23.87a,b |
154.57±33.52a |
150.34±21.85a |
| 48 h | 42.12±5.68 | 47.77±3.79 |
133.11±22.91a | 86.32±9.36a,b |
208.51±26.45a,b |
135.86±24.21a |
131.99±20.87a |
| S100A12 | | | | | | | |
| 12 h | 40.56±3.62 | 46.67±5.35 |
567.99±38.36a |
267.45±35.14a,b |
783.34±23.61a,b |
562.13±61.29a |
572.42±46.17a |
| 24 h | 45.16±6.50 | 58.89±6.67 |
531.12±47.35a |
259.54±37.28a,b |
765.99±63.79a,b |
527.17±71.50a |
536.13±59.37a |
| 48 h | 41.89±5.59 | 52.45±6.76 |
497.91±48.69a |
243.53±32.08a,b |
747.34±69.61b |
492.12±53.93a |
492.41±53.98a |
Higher serum levels of amylase, lipase
and urinary trypsinogen-2 are identified in AP mice
The serum levels of amylase, lipase and urinary
trypsinogen-2 level of mice in the control and siRNA groups
demonstrated no significant difference with time extension (12, 24
and 48 h). The serum levels of amylase, lipase and urinary
trypsinogen-2 of mice in the other five groups initially increased
and then decreased as time was extended (12, 24 and 48 h), reaching
a peak at 24 h post-model establishment. Compared with the control
group, the serum levels of amylase, lipase and urinary
trypsinogen-2 of mice in the other five groups increased
significantly at 12, 24 and 48 h post-model establishment
(P<0.05). Compared with the AP group, the AP+PD98059 group
demonstrated significantly decreased serum levels of amylase,
lipase and urinary trypsinogen-2 at 12, 24 and 48 h post-model
establishment (P<0.05). The AP+siRNA group demonstrated
significantly increased serum levels of amylase, lipase and urinary
trypsinogen-2 of mice at 12, 24 and 48 h post-model establishment
(P<0.05), whereas no significant differences were observed in
the AP+scramble and AP+PD98059+siRNA groups (P>0.05; Table III).
 | Table IIISerum levels of amylase, lipase and
urinary trypsinogen-2 of mice in each group at 12, 24 and 48 h
post-model establishment. |
Table III
Serum levels of amylase, lipase and
urinary trypsinogen-2 of mice in each group at 12, 24 and 48 h
post-model establishment.
| Factor | Group
(h) | Control
(pg/ml) | siRNA
(pg/ml) | AP
(pg/ml) |
AP+PD98059
(pg/ml) | AP+siRNA
(pg/ml) |
AP+scramble
(pg/ml) |
AP+PD98059+siRNA
(pg/ml) |
|---|
| Serum amylase | 12 | 1,008.3±98.23 |
1,243.53±111.98 |
11,048.3±526.44a |
6,218.58±388.32a,b |
17,625.11±131.82a,b |
11,012.3±773.3a |
11,031.39±562.98a |
| 24 | 993.15±167.05 |
1,465.45±116.45 |
12,139.36±512.33a |
6,413.58±230.77a,b |
18,182.58±567.81a,b |
12,021.22±554.77a |
12,479.31±506.99a |
| 48 | 987.67±120.19 |
1,198.56±109.78 |
10,508.3±421.36a |
6,092.38±301.59a,b |
16,792.44±497.02a,b |
10,945.36±614.14a |
10,904.3±438.28a |
| Lipase | 12 | 103.34±12.02 | 134.63±12.96 |
739.25±24.02a |
435.32±18.02a,b |
906.25±25.96a,b |
709.23±22.03a |
713.45±25.02a |
| 24 | 131.59±11.36 | 155.29±12.02 |
795.32±26.32a |
505.63±18.24a,b |
1,090.12±29.25a,b |
805.11±22.12a |
811.32±24.05a |
| 48 | 120.50±12.96 | 139.63±13.65 |
703.25±19.68a |
485.20±18.96a,b |
948.56±22.65a,b |
730.90±20.13a |
731.25±20.69a |
| Urinary
trypsinogen-2 | 12 | 23.25±7.96 | 38.65±7.98 | 95.35±11.25a | 65.85±7.89a,b |
137.25±14.25a,b | 92.36±7.65a | 94.58±8.96a |
| 24 | 32.41±6.98 | 41.23±8.96 |
118.52±12.01a | 81.25±8.22a,b |
159.52±15.11a,b | 115.21±8.90a | 123.25±8.96a |
| 48 | 25.18±8.96 | 40.15±7.85 |
101.66±11.59a | 72.02±8.96a,b |
148.52±14.02a,b | 100.02±8.96a | 105.25±9.89a |
mRNA expression of TNF-α, IL-1β, IL-6,
DUSP1, HMGB1 and S100A12 in pancreatic, liver, kidney and lung
tissues of mice in each group
The result of the RT-qPCR analysis (Fig. 4A–H) demonstrated that, compared
with the control group, there were no significant differences in
the levels of inflammatory factors in the siRNA group (all
P>0.05), whereas the expression of DUSP1 was decreased. The
other five groups exhibited significantly increased mRNA expression
levels of TNF-α, IL-1β, IL-6, DUSP1, HMGB1 and S100A12 in
pancreatic, liver, kidney and lung tissues, among which pancreatic
tissues demonstrated the most marked increase (all P<0.05).
Compared with the AP group, there was no significant difference in
the AP+scramble group (P>0.05), whereas the AP+PD98059 group had
significantly decreased mRNA expression of TNF-α, IL-1β, IL-6,
HMGB1 and S100A12 in pancreatic, liver, kidney and lung tissues,
among which pancreatic tissues demonstrated the most marked
decrease (all P<0.05), however, no significant difference was
identified in the mRNA expression of DUSP1. The AP+siRNA group had
increased mRNA expression levels of TNF-α, IL-1β, IL-6, HMGB1 and
S100A12 in pancreatic, liver, kidney and lung tissues, among which
pancreatic tissues demonstrated the most marked increase (all
P<0.05), and the mRNA expression of DUSP1 was decreased. The
AP+PD98059+siRNA group demonstrated no significant difference in
the mRNA expression of TNF-α, IL-1β, IL-6, HMGB1 and S100A12 in
pancreatic, liver, kidney and lung tissues (P>0.05), however,
the mRNA expression of DUSP1 decreased significantly
(P<0.05).
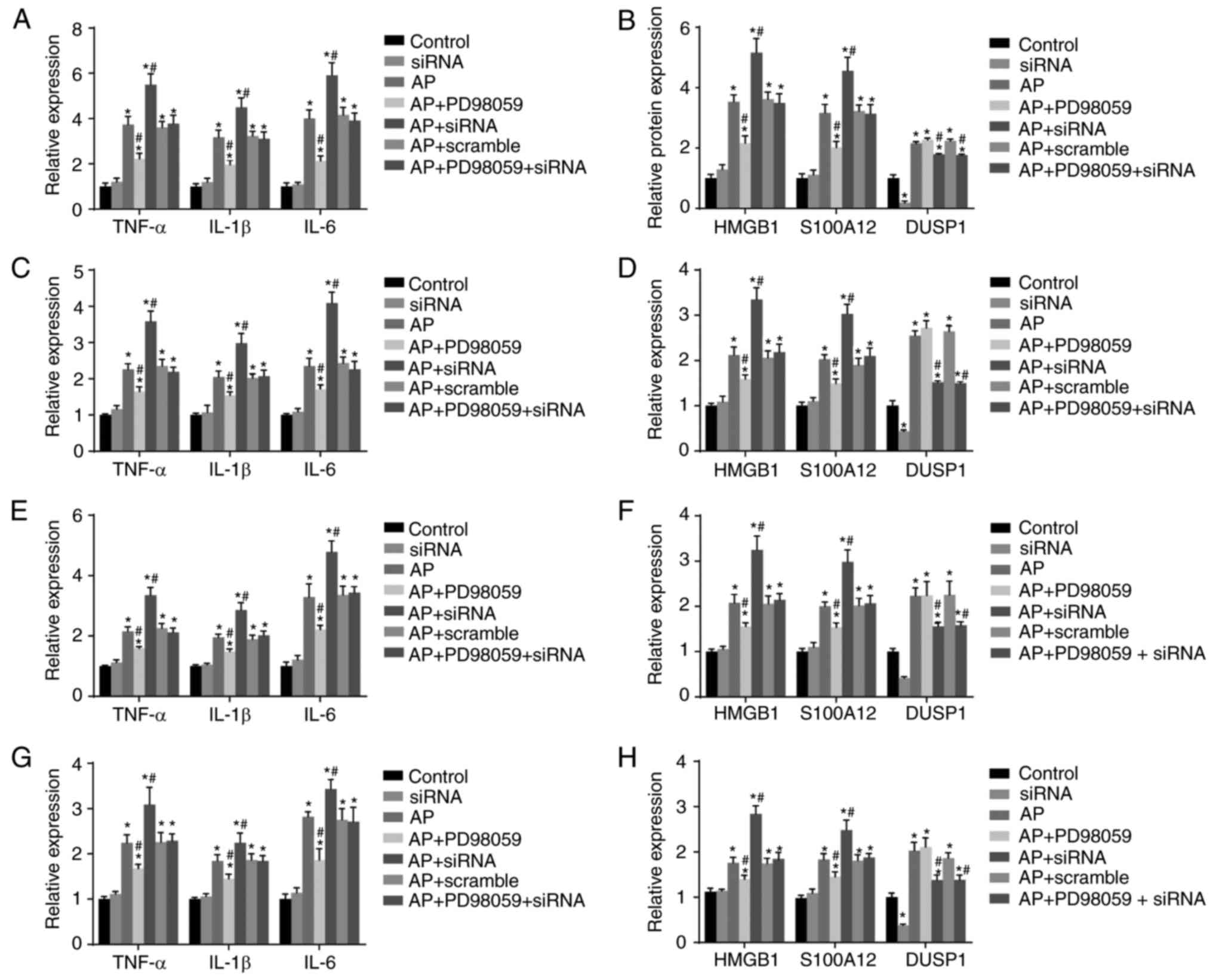 | Figure 4mRNA expression of proinflammatory
cytokines (TNF-α, IL-1β and IL-6), DUSP1, HMGB1 and S100A12 in the
pancreas, liver, kidney and lung tissues of mice in each group
detected using reverse transcription-quantitative polymerase chain
reaction analysis. (A) mRNA expression of TNF-α, IL-1β and IL-6 in
pancreatic tissues of mice in each group; (B) mRNA expression of
DUSP1, HMGB1 and S100A12 in pancreatic tissues of mice in each
group; (C) mRNA expression of TNF-α, IL-1β and IL-6 in liver
tissues of mice in each group; (D) mRNA expression of DUSP1, HMGB1
and S100A12 in liver tissues of mice in each group; (E) mRNA
expression of TNF-α, IL-1β and IL-6 in kidney tissues of mice in
each group; (F) mRNA expression of DUSP1, HMGB1 and S100A12 in
kidney tissues of mice in each group; (G) mRNA expression of TNF-α,
IL-1β and IL-6 in lung tissues of mice in each group; (H) mRNA
expression of DUSP1, HMGB1 and S100A12 in lung tissues of mice in
each group; *P<0.05, compared with the control group;
#P<0.05, compared with the AP group. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; DUSP1,
dual-specificity phosphatase-1; TNF-α, tumor necrosis factor-α;
IL-6, interleukin-6; IL-1β, interleukin-1β; HMGB1, high mobility
group box-1; AP, acute pancreatitis; siRNA, small interfering
RNA. |
Expression of DUSP1 and MAPK
pathway-related genes (p-ERK, p-JNK and p-p38) in pancreatic,
liver, kidney and lung tissues of mice in each group
The results of the western blot analysis (Fig. 5) demonstrated no significant
differences in protein expression levels of ERK, JNK or p38 in the
pancreatic, liver, kidney and lung tissues among the seven
treatment groups (P>0.05). Compared with the control group, the
expression levels of p-ERK, p-JNK and p-p38 in the pancreatic,
liver, kidney and lung tissues in the other six treatment groups
were significantly increased, among which the pancreatic tissue
exhibited the most marked changes (P<0.05). The protein
expression of DUSP1 was significantly decreased in the siRNA group,
but increased in the other five groups (all P<0.05). No
statistically significant difference was observed in the
AP+scramble group when compared with the AP group (P>0.05).
Compared with the AP group, the AP+PD98059 group exhibited
significantly decreased expression levels of p-ERK, p-JNK and p-p38
in the pancreatic, liver, kidney and lung tissues, among which the
pancreatic tissue exhibited the most marked reduction (P<0.05),
whereas no significant change in the expression of DUSP1 was
observed. The AP+siRNA group had significantly increased expression
levels of p-ERK, p-JNK and p-p38 in the pancreatic, liver, kidney
and lung tissues, among which the pancreatic tissue exhibited the
most marked increase, whereas the expression of DUSP1 was decreased
(P<0.05). In the AP+PD98059+siRNA group, no significant
differences in the expression levels of p-ERK, p-JNK or p-p38 were
observed in the pancreatic, liver, kidney and lung tissues
(P>0.05), however, the expression of DUSP1 was decreased
(P<0.05).
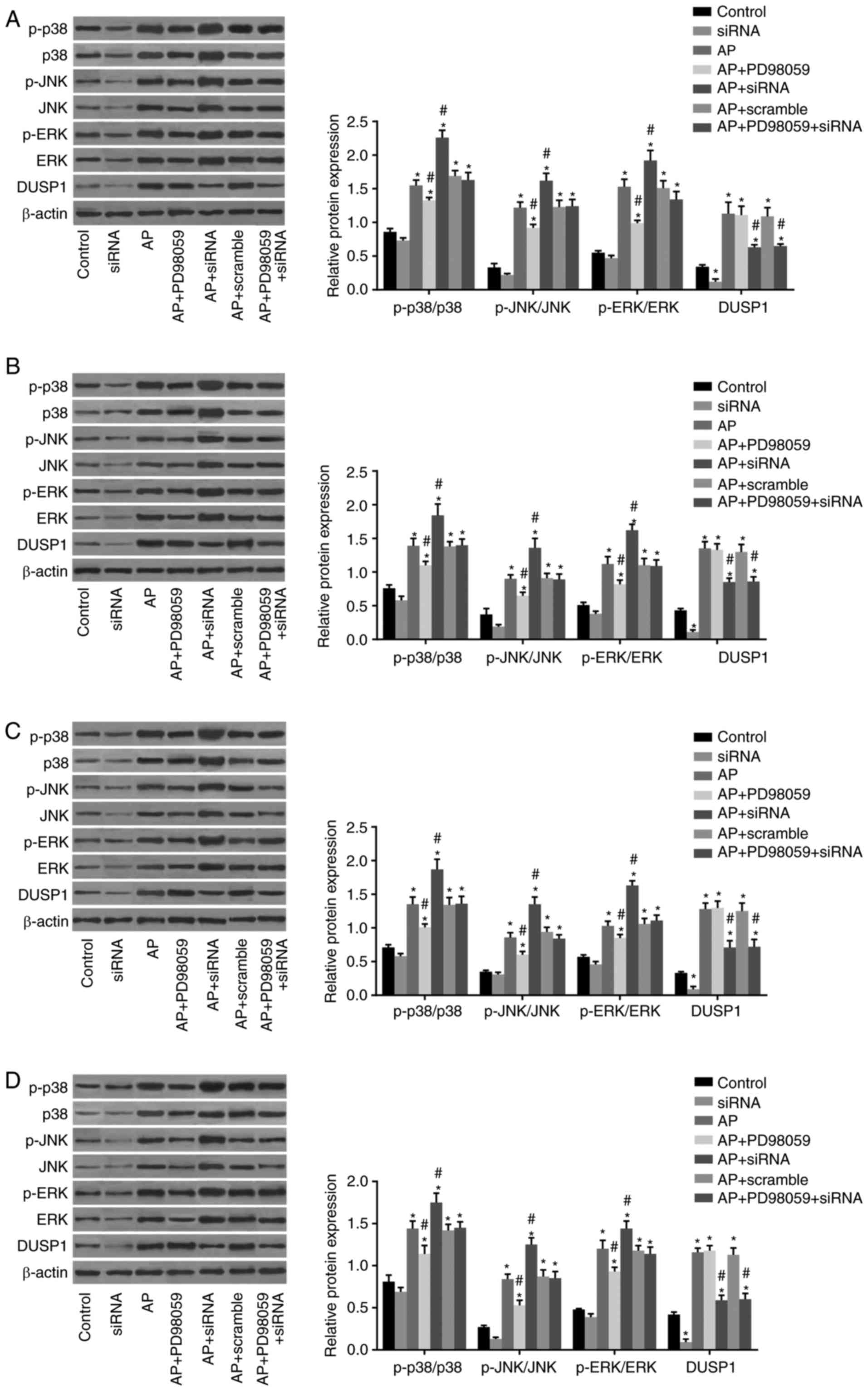 | Figure 5Expression of DUSP1 and MAPK
pathway-related genes (p-ERK, p-JNK and p-p38) in pancreatic,
liver, kidney and lung tissues of mice in each group detected using
western blot analysis. (A) Electrophoresis band chart and histogram
for expression of DUSP1 and MAPK pathway-related proteins in
pancreatic tissues of mice in each group. (B) Electrophoresis band
chart and histogram for expression of DUSP1 and MAPK
pathway-related proteins in liver tissues of mice in each group;
(C) electrophoresis band chart and histogram for expression of
DUSP1 and MAPK pathway-related proteins in kidney tissues of mice
in each group; (D) electrophoresis band chart and histogram for
expression of DUSP1 and MAPK pathway-related proteins in lung
tissues of mice in each group; *P<0.05, compared with
the control group; #P<0.05, compared with the AP
group. DUSP1, dual-specificity phosphatase-1; AP, acute
pancreatitis; siRNA, small interfering RNA; MAPK, mitogen-activated
protein kinase; ERK, extracellular signal-regulated kinase; JNK,
c-Jun N-terminal kinase; p-, phosphorylated. |
Discussion
AP is known as a common inflammatory disease and the
incidence of adult AP has increased in recent decades (4,22).
Previous studies have demonstrated that MAPKs are important in AP
and that the DUSP1 gene is involved in the acute inflammatory
response (23,24). In the present study, the role of
DUSP1 gene silencing on the release of proinflammatory cytokines
through regulation of the MAPK signaling pathway was investigated
in AP mice. The experimental results indicated that DUSP1 gene
silencing promoted the release of proinflam-matory cytokines
through activation of the MAPK signaling pathway in mice with
AP.
Initially, the AP mice exhibited increased serum
levels of TNF-α, IL-1β, IL-6, HMGB1, S100A12 and amylase, and mice
in the AP+siRNA group had higher expression levels of these
factors. TNF-α, IL-1 and IL-6, as proinflammatory cytokines, are
key regulators of acute inflammation and are important in the
pathogenesis of inflammatory diseases (25,26). HMGB1 and S100A12, as mediators of
inflammation, are considered to contribute to the proinflammatory
response in acute respiratory distress syndrome (27). The results of a study performed by
Xiang et al also demonstrated that there were increased
serum levels of HMGB1, TNF-α and IL-6 in traumatic pancreatitis
(28). Serum amylase is widely
used as a biochemical marker of pancreatic inflammation (29). A previous study indicated that
serum amylase is always high in AP (30). Matas-Cobos reported increased
serum amylase levels in AP, which was consistent with the present
study (31). Therefore, the
results indicated that DUSP1 gene silencing upregulated the
expression of proinflammatory cytokines, HMGB1, S100A12 and
amylase.
The AP mice also had significantly increased mRNA
expression levels of TNF-α, IL-1β, IL-6, HMGB1 and S100A12 in
pancreatic, liver, kidney and lung tissues, among which pancreatic
tissues exhibited the most marked changes. The expression levels of
these factors were increased in the AP+siRNA group, but decreased
in the AP+PD98059 group. PD98059, serving as an inhibitor of the
MAPK signaling pathway, can inhibit the activation of MAPK kinase
1, phosphorylation of ERK and expression of MAPK proteins (32). A previous study identified that
the activation of MAPK is associated with the upregulation of gene
expression involved in the proliferation and apoptosis of
pancreatic cancer cells (33).
The p38 MAPK signaling pathway can function as a regulator of the
inflammatory response, and inhibition of the p38 MAPK signaling
pathway can alleviate experimental AP in mice (34). A study by Sun et al also
demonstrated that inhibition of the MAPK signaling pathway
decreased the mRNA expression of proinflammatory cytokines
(35). A previous study by Wang
et al identified increased mRNA expression levels of TNF-α,
IL-1β and IL-6 in lung and pancreatic tissues (36,37). The results suggested that
inhibition of the MAPK signaling pathway downregulated the mRNA
expression of proinflammatory cytokines, HMGB1 and S100A12 in AP
mice, whereas silencing the DUSP1 gene upregulated their expression
levels.
Finally, the AP mice exhibited increased expression
levels of p-ERK, p-JNK and p-p38 in pancreatic, liver, kidney and
lung tissues, among which the pancreatic tissue exhibited the most
marked changes; the expression of these factors was increased in
the AP+siRNA group, but decreased in the AP+PD98059 group. It is
known that p38 MAPK, p-ERK, p-JNK, p-p38 and p54/p46 are members of
the MAPK family (38). ERK and
p38 are key in the overproduction of exocrine pancreatic cytokines
(39). DUSPs are involved in
regulating the phosphorylation of Erk1/2, p38 and JNK1/2 (40). It has been demonstrated that the
phosphorylation of ERK and p38 is regulated by DUSP1 (41). A previous study by Zhang et
al also reported the upregulation of downstream signaling
molecules, p-ERK, p-JNK and p-p38, mediated by the inhibition of
DUSP1 (42). These results
indicated that DUSP1 gene silencing increased the expression levels
of p-ERK, p-JNK and p-p38.
In conclusion, the data obtained in the present
study indicated that silencing the DUSP1 gene using lentiviral
vector-mediated siRNA promoted the release of proinflammatory
cytokines via activation of the MAPK signaling pathway in AP mice,
which aggravated AP. Several factors affect the DUSP1 and MAPK
signaling pathway; therefore, further investigations are required
to identify effective therapeutic regimens for patients with AP in
the future.
Acknowledgments
The present study was supported by the Shandong
Provincial Medical Science and Technology Development Project
(grant no. 2016WS0198).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Munsell MA and Buscaglia JM: Acute
pancreatitis. J Hosp Med. 5:241–250. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu B, Huang J and Zhang B: Nobiletin
protects against murine l-arginine-induced acute pancreatitis in
association with down-regulating p38MAPK and AKT. Biomed
Pharmacother. 81:104–110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wittau M, Mayer B, Scheele J, Henne-Bruns
D, Dellinger EP and Isenmann R: Systematic review and meta-analysis
of antibiotic prophylaxis in severe acute pancreatitis. Scand J
Gastroenterol. 46:261–270. 2011. View Article : Google Scholar
|
|
4
|
Kylänpää ML, Repo H and Puolakkainen PA:
Inflammation and immunosuppression in severe acute pancreatitis.
World J Gastroenterol. 16:2867–2872. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tonsi AF, Bacchion M, Crippa S, Malleo G
and Bassi C: Acute pancreatitis at the beginning of the 21st
century: The state of the art. World J Gastroenterol. 15:2945–2959.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zerem E: Treatment of severe acute
pancreatitis and its complications. World J Gastroenterol.
20:13879–13892. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bradley EL III and Dexter ND: Management
of severe acute pancreatitis: A surgical odyssey. Ann Surg.
251:6–17. 2010. View Article : Google Scholar
|
|
8
|
Moncho-Amor V, Ibañez de Cáceres I,
Bandres E, Martínez-Poveda B, Orgaz JL, Sánchez-Pérez I, Zazo S,
Rovira A, Albanell J, Jiménez B, et al: DUSP1/MKP1 promotes
angiogenesis, invasion and metastasis in non-small-cell lung
cancer. Oncogene. 30:668–678. 2011. View Article : Google Scholar
|
|
9
|
Horita H, Wada K, Rivas MV, Hara E and
Jarvis ED: The dusp1 immediate early gene is regulated by natural
stimuli predominantly in sensory input neurons. J Comp Neurol.
518:2873–2901. 2010.PubMed/NCBI
|
|
10
|
Liu F, Gore AJ, Wilson JL and Korc M:
DUSP1 is a novel target for enhancing pancreatic cancer cell
sensitivity to gemcitabine. PLoS One. 9:e849822014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang X, Hyer JM, Yu H, D'Silva NJ and
Kirkwood KL: DUSP1 phosphatase regulates the proinflammatory milieu
in head and neck squamous cell carcinoma. Cancer Res. 74:7191–7197.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dauletbaev N, Herscovitch K, Das M, Chen
H, Bernier J, Matouk E, Bérubé J, Rousseau S and Lands LC:
Down-regulation of IL-8 by high-dose vitamin D is specific to
hyperinflammatory macrophages and involves mechanisms beyond
up-regulation of DUSP1. Br J Pharmacol. 172:4757–4771. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun X, Liu C, Qian M, Zhao Z and Guo J:
Ceramide from sphingomyelin hydrolysis differentially mediates
mitogen-activated protein kinases (MAPKs) activation following
cerebral ischemia in rat hippocampal CA1 subregion. J Biomed Res.
24:132–137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qin Z, Dai L, Defee M, Findlay VJ, Watson
DK, Toole BP, Cameron J, Peruzzi F, Kirkwood K and Parsons C:
Kaposi's sarcoma-associated herpesvirus suppression of DUSP1
facilitates cellular pathogenesis following de novo infection. J
Virol. 87:621–635. 2013. View Article : Google Scholar :
|
|
16
|
Sandoval J, Escobar J, Pereda J, Sacilotto
N, Rodriguez JL, Sabater L, Aparisi L, Franco L, Lopez-Rodas G and
Sastre J: Pentoxifylline prevents loss of PP2A phosphatase activity
and recruitment of histone acetyltransferases to proinflammatory
genes in acute pancreatitis. J Pharmacol Exp Ther. 331:609–617.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singer O and Verma IM: Applications of
lentiviral vectors for shRNA delivery and transgenesis. Curr Gene
Ther. 8:483–488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Giry-Laterrière M, Verhoeyen E and Salmon
P: Lentiviral vectors. Methods Mol Biol. 737:183–209. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schaible HG, von Banchet GS, Boettger MK,
Bräuer R, Gajda M, Richter F, Hensellek S, Brenn D and Natura G:
The role of proinflammatory cytokines in the generation and
maintenance of joint pain. Ann N Y Acad Sci. 1193:60–69. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang QL, Wang JM, Jiang S, Wen LM and
Zhou H: Large-scale real-time titration of
green-fluorescence-protein-marked recombinant retrovirus:
Comparison with standard titration method. Di Yi Jun Yi Da Xue Xue
Bao. 23:1101–1103. 2003.In Chinese. PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
22
|
Morinville VD, Barmada MM and Lowe ME:
Increasing incidence of acute pancreatitis at an American pediatric
tertiary care center: Is greater awareness among physicians
responsible? Pancreas. 39:5–8. 2010. View Article : Google Scholar
|
|
23
|
Alaoui-Jamali M: Comment on 'p38 MAPK
inhibition alleviates experimental acute pancreatitis in mice'.
Hepatobiliary Pancreat Dis Int. 14:3302015. View Article : Google Scholar
|
|
24
|
Korhonen R, Turpeinen T, Taimi V, Nieminen
R, Goulas A and Moilanen E: Attenuation of the acute inflammatory
response by dual specificity phosphatase 1 by inhibition of p38 MAP
kinase. Mol Immunol. 48:2059–2068. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dimitrakova E and Kostov I: Studies on the
level of proin-flammatory cytokines IIL-1a, IL-1b, IL-6, TNF-a in
pregnant women with acute pyelonephritis. Akush Ginekol. 50:3–6.
2011.In Bulgarian.
|
|
26
|
Jang CH, Choi JH, Byun MS and Jue DM:
Chloroquine inhibits production of TNF-alpha, IL-1beta and IL-6
from lipopolysac-charide-stimulated human monocytes/macrophages by
different modes. Rheumatology. 45:703–710. 2006. View Article : Google Scholar
|
|
27
|
Müller MC, Tuinman PR, Vlaar AP, Tuip AM,
Maijoor K, Achouiti A, Van t Veer C, Vroom MB and Juffermans NP:
Contribution of damage-associated molecular patterns to
transfusion-related acute lung injury in cardiac surgery. Blood
Transfus. 12:368–375. 2014.PubMed/NCBI
|
|
28
|
Xiang K, Cheng L, Luo Z, Ren J, Tian F,
Tang L, Chen T and Dai R: Glycyrrhizin suppresses the expressions
of HMGB1 and relieves the severity of traumatic pancreatitis in
rats. PLoS One. 9:e1159822014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hofmeyr S, Meyer C and Warren BL: Serum
lipase should be the laboratory test of choice for suspected acute
pancreatitis. S Afr J Surg. 52:72–75. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ghimire R, Thapa AS, Karki D and Shrestha
DK: Routine measurement of serum amylase in acute abdomen. JNMA J
Nepal Med Assoc. 52:982–985. 2014.PubMed/NCBI
|
|
31
|
Matas-Cobos AM, Redondo-Cerezo E,
Alegría-Motte C, Martínez-Chamorro A, Saenz-López P, Jiménez P,
Jiménez MR, González-Calvín JL, de Teresa J and Osuna FR: The role
of Toll-like receptor polymorphisms in acute pancreatitis
occurrence and severity. Pancreas. 44:429–433. 2015.
|
|
32
|
Di Paola R, Galuppo M, Mazzon E, Paterniti
I, Bramanti P and Cuzzocrea S: PD98059, a specific MAP kinase
inhibitor, attenuates multiple organ dysfunction syndrome/failure
(MODS) induced by zymosan in mice. Pharmacol Res. 61:175–187. 2010.
View Article : Google Scholar
|
|
33
|
Furukawa T, Tanji E, Kuboki Y, Hatori T,
Yamamoto M, Shimizu K, Shibata N and Shiratori K: Targeting of
MAPK-associated molecules identifies SON as a prime target to
attenuate the proliferation and tumorigenicity of pancreatic cancer
cells. Mol Cancer. 11:882012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cao MH, Xu J, Cai HD, Lv ZW, Feng YJ, Li
K, Chen CQ and Li YY: p38 MAPK inhibition alleviates experimental
acute pancreatitis in mice. Hepatobiliary Pancreat Dis Int.
14:101–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun HY, Hu KZ and Yin ZS: Inhibition of
the p38-MAPK signaling pathway suppresses the apoptosis and
expression of proinflammatory cytokines in human osteoarthritis
chondrocytes. Cytokine. 90:135–143. 2017. View Article : Google Scholar
|
|
36
|
Wang HT, Fang YQ, Bao XC, Yuan HR, Ma J,
Wang FF, Zhang S and Li KC: Expression changes of TNF-α, IL-1β and
IL-6 in the rat lung of decompression sickness induced by fast
buoyancy ascent escape. Undersea Hyperb Med. 42:23–31.
2015.PubMed/NCBI
|
|
37
|
Wang XY, Tang QQ, Zhang JL, Fang MY and Li
YX: Effect of SB203580 on pathologic change of pancreatic tissue
and expression of TNF-α and IL-1β in rats with severe acute
pancreatitis. Eur Rev Med Pharmacol Sci. 18:338–343. 2014.
|
|
38
|
Costa AP, Lopes MW, Rieger DK, Barbosa SG,
Gonçalves FM, Xikota JC, Walz R and Leal RB: Differential
activation of mitogen-activated protein kinases, ERK 1/2,
p38MAPK and JNK p54/p46 during postnatal development of
rat hippocampus. Neurochem Res. 41:1160–1169. 2016. View Article : Google Scholar
|
|
39
|
Samuel I, Zaheer A and Fisher RA: In vitro
evidence for role of ERK, p38, and JNK in exocrine pancreatic
cytokine production. J Gastrointest Surg. 10:1376–1383. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Z, Reinach PS, Zhang F, Vellonen KS,
Urtti A, Turner H and Wolosin JM: DUSP5 and DUSP6 modulate corneal
epithelial cell proliferation. Mol Vis. 16:1696–1704.
2010.PubMed/NCBI
|
|
41
|
Leyva-Ilades D, Cherla RP, Lee MS and Tesh
VL: Regulation of cytokine and chemokine expression by the
ribotoxic stress response elicited by Shiga toxin type 1 in human
macrophage-like THP-1 cells. Infect Immun. 80:2109–2120. 2012.
View Article : Google Scholar
|
|
42
|
Zhang Q, Yu J, Wang J, Ding CP, Han SP,
Zeng XY and Wang JY: The red nucleus TNF-α participates in the
initiation and maintenance of neuropathic pain through different
signaling pathways. Neurochem Res. 40:1360–1371. 2015. View Article : Google Scholar : PubMed/NCBI
|