Introduction
Socheongryong-Tang (SCRT), also known as
Xiao-Qing-Long-Tang or Sho-Seiryu-To, has been commonly used for
the treatment of inflammatory diseases, including allergic rhinitis
and bronchial asthma, for several centuries in Asian countries
(1-3). SCRT is composed of 8 herbal
components and is classically administered depending on the
specific diseases and symptoms of the patients (4). The major active ingredients of SCRT
include liquiritigenin, isoliquiritigenin, glycyrrhizin and
homogentisic acid, as well as paeoniflorin, kaempferol, gomisin B
and C, O-methoxycinnamaldehyde, higenamine, L-ephedrine and
6-gingerol (2-4).
The inflammatory response is regulated by
inflammatory mediators; among these, nitric oxide (NO),
prostaglandin E2 (PGE2) and cytokines,
including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and
IL-6, are considered to have central roles (5,6). A
significant feature of inducible NO synthase (iNOS) and
cyclooxygenase-2 (COX-2), which have a major role in the production
of NO and PGE2, is that they are inducible enzymes. NO
is a radical produced from L-arginine through the iNOS, which
produces high amounts of NO during inflammation (7-9).
Another enzyme involved in the generation of PGs, including
PGH2 and PGE2, is COX. Specifically, COX-2 is
responsible for mediating inflammation by producing PGE2
induced by factors including lipopolysaccharide (LPS) (10,11). IL-1β, which is immunologically
associated with TNF-α and IL-6, activates natural killer cells, B
cells and T cells and induces fever by acting on the hypothalamus.
IL-6 also has a central role in the acute immune response and
increases antibody production by activating lymphocytes. It has
also been reported that the levels of this cytokine are always
increased in inflammatory lesions (12,13). Furthermore, TNF-α is well known to
have key roles in numerous autoimmune diseases and as a primary
mediator in inflammatory reactions that occur during immune
responses via stimulation of the secretion of other inflammatory
cytokines (12,14).
Nuclear factor κB (NF-κB) is a transcription factor
associated with the transactivation of diverse genes involved in
the regulation of tumorigenesis, cellular proliferation and the
inflammatory response (15). In
addition, mitogen-activated protein kinases (MAPKs) are kinases
that are specific to serine and threonine. They include p38 MAPK
(p38), extracellular signal-regulated kinases (ERKs) and c-Jun
NH2-terminal kinases (JNKs), which have key roles in the
modulation of the inflammatory response (16). The signaling pathways of MAPKs may
activate NF-κB and cause the expression of pro-inflammatory genes
(12-14).
Several studies have reported on the anti-allergic
activity of SCRT in guinea pig and mouse models of airway
inflammation (1,17,18). In addition, SCRT was demonstrated
to exhibit an immunomodulaory effect in allergen-sensitized mice
(19). However, to the best of
our knowledge, the underlying molecular mechanisms of the
anti-inflammatory effects of SCRT have remained elusive. Therefore,
the present study evaluated the effects of SCRT on the NF-κB and
MAPKs signaling pathways and on NF-κB-dependent induction of
inflammatory cytokines, iNOS and COX-2 in RAW 264.7 cells induced
with LPS. Furthermore, the effects of SCRT on carrageenan
(CA)-induced acute edematous inflammation were examined by
histomorphometry and histopathology in vivo. The present
study provided a molecular basis for the inhibitory effects of SCRT
on inflammatory responses and expanded the current knowledge on the
mechanism of action to provide a scientific rationale for the use
of SCRT as complementary and alternative medicine.
Materials and methods
Chemicals and reagents
Five reference standards, namely ephedrine,
paeoniflorin, cinnamic acid, glycyrrhizin and gomisin-A, were
obtained from Wako Inc. (Wako, Japan). The purity of the 5
standards was >98%. Acetonitrile, methanol and other solvents
for ultra performance liquid chromatograpy (UPLC) analysis were
from J.T. Baker (Avantor, Center Valley, PA, USA). Anti-NF-κB (cat.
no. sc-8008), anti-lamin A/C (cat. no. sc-376248),
anti-phosphorylated (p) inhibitor of NF-κB (p-I-κBa; cat. no.
sc-8404) and anti-COX-2 (cat. no. sc-19999) antibodies were
purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA) and
JNK (cat. no. 9252), p-JNK (cat. no. 9255), ERK1/2 (cat. no. 9102),
p-ERK1/2 (cat. no. 9101), p38 (cat. no. 9212) and p-p38 (cat. no.
9211) antibodies were obtained from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Anti-β-actin (cat. no. sc-58673) and
peroxidase-conjugated secondary (cat. no. sc-51625) antibodies were
acquired from Santa Cruz Biotechnology Inc. Anti-iNOS antibody
(cat. no. MABN527) was acquired from Calbiochem (San Diego, CA,
USA). The immunoassay kit (cat. no. KGE004B) for PGE2
was obtained from R&D Systems (Minneapolis, MN, USA) and ELISA
kits for IL-1β (cat. no. EMIL1B), IL-6 (cat. no. EM2IL6) and TNF-α
(cat. no. EMTNFA) were purchased from Pierce (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). A peroxidase substrate kit
(cat. no. SK-4100) and vectastain elite ABC kit (cat. no. PK-6200)
were acquired from Vector Lab. Inc. (Burlingame, CA, USA). LPS,
sulfanilamide, sodium nitrite, MTT, dexamethasone (DEXA), CA and
other chemicals were obtained from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany).
Preparation of SCRT
SCRT (2X, 80 g) consisted of the following medicinal
herbs: 12 g of Ephedrae Herba (Ephedra sinica Stadf.), 12 g
of Paeoniae Radix (Paeonia lactiflora Pall.), 12 g of Turcz.
Ball. Schizandrae Fructus (Schizandra chinensis), 12 g of
Tenore et Breit. Pinelliae Rhizoma (Pinellia ternata), 8 g
of Asiasari Radix (Asarum sieboldii F. Maekawa), 8 g of
Zingiberis Rhizoma (Zingiber officinale Rosc.), 8 g of
Cinnamomi Ramulus (Cinnamomum cassia Blume.) and 8 g of
Glycyrrhizae Radix (Glycyrrhiza glabra L.). All medicinal
herbs were purchased from Daewon Pharmacy (Daegu, Korea) and
voucher specimens (no. PNU10-28) were deposited in the Herbarium at
the School of Korean Medicine, Pusan National University (Busan,
Korea). The mixture (80 g) was extracted with 1.2 l of boiling
distilled water for at least 3 h, then filtered through filter
paper (Hyundai Micro no. 20; Hyundai Pharmaceutical Co., Ltd.,
Seoul, South Korea) and the filtrate was then lyophilized. The
percentage yield of lyophilized SCRT extract was 14.8%. The
lyophilized powder of SCRT was dissolved in distilled water
immediately prior to use, and then filter-sterilized (Nalgene,
Rochester, NY, USA) using a 0.2-μm syringe filter to avoid
contamination.
Chemical profiling of SCRT by UPLC
The UPLC operating system was equipped with a Waters
ACQUITY™ (Waters Corp., Milford, MA, USA) pump and photodiode array
detector. Signal of the detector was indicated using the Empower
Data System. The separation was performed using a Waters ACQUITY™
BEH C18 column (2.1×100 cm; 1.7 μm particle size;
Waters Corp.). The mobile phase was comprised of 0.1% formic acid
in water and 0.1% formic acid in aceto-nitrile with application of
gradient elution (0.4 ml/min). The volume for injection was always
2 μl, the ultraviolet wavelength for detection was 254 nm
and the column temperature was kept at 25°C. To obtain the chemical
profile of SCRT, the lyophilized powder of SCRT aqueous extracts
was dissolved in methanol (10 mg/ml). Prior to UPLC analysis, the
sample solution was filtered using a 0.22-μm filter. In
addition, standard solutions of 5 components, ephedrine,
paeoniflorin, cinnamic acid, glycyrrhizin and gomisin-A, were
prepared (1,000 μg/ml in methanol). All solutions were
stored at 4°C.
Cell culture
RAW 264.7 cells (American Type Culture Collection,
Manassas, VA, USA) were cultured in Dulbecco's modified Eagle's
medium (HyClone; Thermo Fisher Scientific, Inc.) containing 10%
heat-inactivated fetal bovine serum (Sigma-Aldrich; Merck KGaA),
100 μg/ml streptomycin and 100 U/ml penicillin (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in a 5% CO2
incubator.
MTT assay
To examine the cytotoxicity of SCRT, RAW 264.7 cells
(5×104/well) were seeded in a 96-well plate. The cells
were serum-starved for 16-17 h, after which they were pre-treated
with diverse concentrations of SCRT for 1 h, followed by induction
with LPS (1 μg/ml) at 37°C with 5% CO2 for an
additional 20 h. After incubation, the cells were stained with MTT
(4 h, 0.5 mg/ml). The media were then removed and formazan crystals
were dissolved by adding dimethyl sulfoxide at 200 μl/well.
Next, the absorbance was read at 570 nm with an automated
microplate reader (Infinite 200 Pro; Tecan, Männedorf,
Switzerland). The viability of cells relative to that of the
untreated cells was calculated as follows: Viability (% of control)
= (optical density of treated sample)/(optical density of untreated
control) × 100%.
Measurement of NO
According to previously established procedures
(20-23), RAW 264.7 cells (5×105
cells/ml) were cultured for ~16 h and then treated with diverse
concentrations of SCRT for 1 h, followed by induction with LPS (1
μg/ml). Cells were then incubated in a 5% CO2
incubator (37°C) for 20 h, after which the culture supernatants
were collected. NO was measured by reaction with 100 μl
Griess reagent [0.1% N-(1-naphthy)-ethylenediamine dihydrochloride
+ 1% sulfa-nilamide in the 5% phosphoric acid; Hoffmann-La Roche
AG, Basel, Switzerland] with 100 μl supernatant of cells at
25°C for 15 min. The absorbance was read at 540 nm with a
microplate reader (Tecan). The standard curve was drawn using
NaNO2.
IL-1β, IL-6, TNF-α and
PGE2 assays
RAW 264.7 cells at a concentration of
5×105 cells/ml were cultured for 16 h, after which they
were pre-treated with diverse concentrations of SCRT for 1 h, and
then stimulated with 1 μg/ml LPS. The culture supernatants
were collected at 20 h after LPS stimulation and levels of IL-1β,
IL-6, TNF-α and PGE2 were quantified using a microplate
reader (Tecan).
Preparation of whole-cell lysates and
nuclear extracts
To prepare whole-cell lysates, control and
SCRT-treated RAW 264.7 cells were harvested by centrifugation and
rinsed with PBS. Washed pellets of cells were resuspended in lysis
buffer [250 mM NaCl, 50 mM
4-(2-hydroxyethyl)-1-piper-azineethanesulfonic acid (HEPES; pH
7.0), 0.1% Nonidet P (NP)-40, 5 mM EDTA, 1 mM phenylmethylsulfonyl
fluoride, 0.5 mM dithiothreitol (DTT), 0.5 mM sodium orthovanadate
and 5 mM NaF] containing 5 μg/ml each of aprotinin and
leupeptin, and then incubated at 4°C for 20 min.
Microcentrifugation was performed to remove cell debris,
accomplished by rapid freezing of supernatants. To prepare nuclear
extracts, cells were then swollen by adding lysis buffer [10 mM
KCl, protease inhibitor cocktail, 1 mM DTT, 10 mM HEPES (pH 7.9),
1.5 mM MgCl2 and 0.2% NP-40 (Roche Diagnostics,
Indianapolis, IN, USA)]. The samples were then incubated on ice for
10 min and centrifuged at 4°C for 5 min. Pellets containing the
nuclei were suspended in 50 μl buffer containing 1.5 mM
MgCl2, 20 mM HEPES (pH 7.9), protease inhibitor
cocktail, 420 mM NaCl, 1 mM DTT and 20% glycerol, followed by
incubation on a shaker at 4°C for 30 min. Finally, samples were
centrifuged (16,000 × g) for 10 min. A Bradford assay (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) was applied for the
determination of protein concentrations.
Western blot analysis
Protein from untreated or treated cell extracts (30
μg) was subjected to 8% SDS-PAGE, after which they were
electroblotted onto nitrocellulose membranes (Thermo Fisher
Scientific, Inc.). Samples were then blocked in 5% skim milk in
0.1% Tween-20/Tris-buffered saline (TTBS) at room temperature for 1
h, incubated overnight in the primary antibody solutions (1:1,000
dilution) at 4°C. The blots were subsequently rinsed with TTBS,
stirred with a dilute solution of horseradish peroxidase-conjugated
antibody (1:1,000) at 25°C for 1 h, and then washed 3 times with
TTBS. Western blot detection reagents (enhanced chemiluminescence;
GE Healthcare, Little Chalfont, UK) were utilized to develop the
blots.
CA-induced paw edema
Paw edema experiments were performed according to
previously established procedures (20-23). All animal procedures were
performed in accordance with the national regulations regarding the
welfare and usage of laboratory animals and protocols were approved
by the Institutional Animal Care and Use Committee of Daegu Haany
University (Gyeongsan, South Korea; approval no. DHU2011-020). Male
Sprague Dawley rats (age, 4 weeks; weight, 80-100 g) were obtained
from Samtako Co. (Osan, South Korea) and acclimatized for one week.
The animals were reared in a pathogen-free environment at 20-23°C
under a 12-h light/dark cycle and a relative humidity of 50% with
commercial chow (Nestle Purina PetCare Ltd., Seoul, South Korea)
and water provided ad libitum. Rats (n=25) were randomly
divided into 5 groups that consisted of 5 animals each. SCRT was
administered to rats by oral gavage at different doses (0.3 and 1.0
g/kg/day) for 3 consecutive days. An anti-inflammatory drug, DEXA,
was applied as a positive control. To cause acute-phase
inflammation, rats received a subcutaneous injection of a CA
solution (1% in saline; 0.1 ml/rat) into the right hind paw at 1 h
after SCRT or vehicle treatment. The paw volumes were measured for
4 h after injection with 1-h intervals. The paw volume was recorded
using a plethysmometer (Ugo Basile, Varese, Italy).
Histological examination
The paw skins (ventrum and dorsum) were separated
and fixed in neutral buffered formalin (10%), subsequently embedded
in paraffin, sliced (3-4 μm) and stained using hematoxylin
and eosin for histopathological profiles and toluidine blue for
mast cells. To observe the changes induced by CA treatment in
greater detail, the thicknesses of dorsal and ventral skins (from
the epidermis to the dermis, excluding keratin layers;
μm/paw) were measured using an automated image analyzer
(DMI-300; DMI, Daegu, South Korea) and a light microscope (Nikon,
Tokyo, Japan) under 40× magnification, and the mast and infiltrated
inflammatory cells were counted by an automated image analyzer and
denoted as cells/mm2 of dermis under 200×
magnification.
Statistical analysis
All values are expressed as the mean ± standard
deviation. Multiple-comparisons tests were performed for different
dose groups. Levene's test was used to test for homogeneity in
variance. If the Levene test indicated an insignificant deviation
from variance homogeneity, the results were analyzed by one way
analysis of variance, followed by the least-significant differences
multi-comparison test assess significant differences between pairs
of groups. For non-parametric analysis, the Kruskal-Wallis H test
was used. When a significant difference was indicated by a
Kruskal-Wallis H test, the Mann-Whitney U test was performed to
identify specific pairs that differed significantly. SPSS (version
14.0K; SPSS, Inc., Chicago, IL, USA) was applied for statistical
analyses. Differences were considered significant at P<0.05.
Results
Analysis of SCRT
Determination of 5 markers, ephedrine, paeoniflorin,
cinnamic acid, glycyrrhizin, and gomisin-A, in SCRT was established
using an UPLC system. The contents of the 5 components in SCRT were
calculated from the standard curve (Table I and Fig. 1).
 | Table IContents of 5 marker compounds in
Socheongryong-Tang as determined by ultra performance liquid
chromatography (n=3). |
Table I
Contents of 5 marker compounds in
Socheongryong-Tang as determined by ultra performance liquid
chromatography (n=3).
| Compound | Content
(μg/ml) |
|---|
| Ephedrine | 21.7±0.41 |
| Paeoniflorin | 10.7±1.09 |
| Cinnamic acid | 0.19±0.02 |
| Glycyrrhizin | 2.50±0.03 |
| Gomisin-A | 1.97±0.08 |
SCRT inhibits LPS-stimulated NO and
PGE2 production and reduction in cell viability
To measure the inhibitory effects of SCRT on
LPS-stimulated NO production in RAW 264.7 cells, 0.25, 0.5 and 1
mg/ml SCRT were analyzed. Compared with the control, treatment with
LPS (1 μg/ml for 20 h) significantly increased NO
production. However, treatment with SCRT significantly inhibited
LPS-stimulated NO production (Fig.
2A, left). SCRT (0.25-1 mg/ml) in the absence of LPS did not
change the basal levels of NO production in RAW 264.7 cells
(Fig. 2A, right). In addition,
the effects of SCRT on LPS-stimulated PGE2 production
were also measured. When compared with the control, LPS treatment
significantly increased PGE2 production. However,
treatment with SCRT significantly suppressed LPS-stimulated
PGE2 production (Fig.
2B). Furthermore, the possible cytotoxic effects of SCRT in RAW
264.7 cells were examined using an MTT assay. The viability of
cells was not affected by SCRT treatment, at least up to the SCRT
concentration of 1 mg/ml (Fig.
2C).
SCRT inhibits LPS-stimulated iNOS and
COX-2 expression
To examine whether the inhibitory effects of SCRT
were associated with the expression of iNOS and COX-2, western blot
analysis was performed. The protein levels of iNOS and COX-2 were
highly upregulated in response to LPS, while treatment with SCRT
significantly inhibited the upregulation of iNOS and COX-2 in a
dose-dependent manner (Fig. 3A and
B).
SCRT reduces LPS-inducible IL-1β, IL-6
and TNF-α production
The present study investigated the effects of SCRT
on LPS-inducible IL-1β, IL-6 and TNF-α production by ELISA. When
compared with the control, treatment with LPS (1 μg/ml for
20 h) significantly increased the production of IL-1β, IL-6 and
TNF-α (P<0.01). However, treatment with SCRT significantly
inhibited LPS-inducible IL-1β, IL-6 and TNF-α production in a
dose-dependent manner (Fig.
4).
SCRT inhibits LPS-stimulated activation
of NF-κB
To examine whether the reduction of nuclear
translocation of NF-κB (p65) by SCRT was due to the inhibition of
p-I-κBα, western blot analysis was performed to evaluate levels of
p-I-κBα and nuclear NF-κB (p65). Treatment with LPS for 15 min
increased the levels of p-I-κBα and SCRT significantly blocked this
LPS-stimulated increase (Fig.
5A). In addition, NF-κB (p65) was accumulated in the nucleus
after treatment with LPS for 15 min, which was significantly
inhibited by pretreatment with SCRT (Fig. 5B).
Inhibitory effects of SCRT on
LPS-stimulated phosphorylation of MAPKs
To examine the molecular targets of SCRT and
associated signaling pathways, the effects of SCRT on
LPS-stimulated phosphorylation of JNK, ERK1/2 and p38 in RAW 264.7
cells were evaluated. As presented in Fig. 6, the phosphorylation of JNK,
ERK1/2 and p38 was significantly increased after LPS treatment (1
μg/ml) for 15 min. However, treatment with SCRT (1 mg/ml)
significantly decreased the phosphorylation of JNK and ERK1/2,
whereas the phosphorylation of p38 was unaffected.
 | Figure 6Inhibitory effects of SCRT on the
phosphorylation of mitogen-activated protein kinases (JNK, ERK and
p38) in LPS-stimulated RAW 264.7 cells. The cells were treated with
the indicated concentrations of SCRT for 1 h, followed by induction
with 1 μg/ml LPS for 15 min. Western blot analysis was
performed using anti-phosphokinase antibodies for cell extract
analysis. Phosphorylated JNK, ERK1/2 and p38 vs. total JNK, ERK1/2
and p38 were measured via densitometry. Values are expressed as the
mean ± standard deviation of 3 replicates for each condition.
**P<0.01, ***P<0.001 vs.
vehicle-treated control; ##P<0.01,
###P<0.001 vs. LPS only group. SCRT,
Socheongryong-Tang; LPS, lipopolysaccharide; JNK, c-Jun N-terminal
kinase; p-ERK, phosphorylated extracellular signal-regulated
kinase. |
SCRT reduces CA-induced paw edema, as
well as iNOS and COX-2 protein expression in paw tissues
In accordance with the results of previous studies
(20-23), the present results indicated that
CA injection significantly increased the paw swelling relative to
the control group within 4 h. However, treatment with DEXA
(positive control; 1 mg/kg/day, per os) resulted in a
significant decrease in edema formation relative to that in the CA
group (P<0.01). Treatment with SCRT (0.3 and 1 g/kg/day, per os,
3 days) also significantly decreased paw edema volumes within 4 h
(Fig. 7A). CA significantly
increased the expression of iNOS and COX-2 protein relative to the
control in the paw tissues. However, DEXA significantly decreased
the expression of iNOS and COX-2 protein. In addition, SCRT (1
g/kg) also significantly decreased the expression of iNOS and COX-2
protein (P<0.001; Fig.
7B).
 | Figure 7Effects of SCRT on CA-induced paw
edema volume and expression of iNOS and COX-2 by CA in the paw
tissues. Rats were orally pretreated with DEXA (1 mg/kg, p.o., 3
days) or SCRT (0.3 or 1 g/kg, p.o., 3 days) and subcutaneously
injected with 1% CA (100 μl/rat, dissolved in sterilized
saline). (A) The volume of paw swelling was recorded at 0-4 h after
CA injection. (B) The protein from paw tissue samples of rats at 4
h after CA injection was isolated and the expression of iNOS and
COX-2 was determined using western blot analysis. β-actin was used
as a loading control. Values are expressed as the mean ± standard
deviation of 3 replicates for each condition.
**P<0.01, ***P<0.001 vs.
vehicle-treated control; #P<0.05,
##P<0.01, ###P<0.001 vs. CA only group.
SCRT, Socheongryong-Tang; LPS, lipopolysaccharide; DEXA,
dexamethasone; CA, carrageenan; iNOS, inducible nitric oxide
synthase; COX, cyclooxygenase; p.o., per os. |
Histological examination
Representative histological profiles of the dorsal
and ventral pedis skins observed after CA and DEXA or SCRT
treatment are presented in Figs.
8 and 9, respectively. In
addition, the histomorphometrical measurements of the dorsal and
ventral pedis skins are listed in Table II. The thicknesses of the dorsal
and ventral pedis skins at 4 h after CA injection were increased by
152.50 and 132.14%, respectively, as compared with those in the
control group. In addition, the thickness of the dorsal pedis skin
in rats treated with DEXA and SCRT at 0.3 g/kg (low dose) and 1
g/kg (high dose) was decreased by 44.80, 18.67 and 31.05%,
respectively, and that of the ventral pedis skin was decreased by
33.97, 5.27 and 19.73%, respectively, compared with that in the CA
group. Furthermore, the number of infiltrated inflammatory cells in
the dorsal and ventral pedis skins at 4 h after CA injection was
increased by 909.09 and 2,244.55% relative to that in the control
group, respectively. The number of infiltrated inflammatory cells
in the dorsal pedis skin of rats treated with DEXA and SCRT at 0.3
g/kg (low dose) and 1 g/kg (high dose) was decreased by 60.78,
10.33 and 39.92%, and that in the ventral pedis skin was decreased
by 58.32, 12.84 and 29.31% compared with that in the CA group,
respectively. Finally, the number of mast cells in the dorsal and
ventral pedis skin in the CA group was decreased by 66.67 and
65.71% relative to that in the control group, respectively. In
addition, the number of mast cells in the dorsal pedis skins of
rats treated with DEXA and SCRT at 0.3 g/kg (low dose) and 1 g/kg
(high dose) was changed by 106.19, 15.46 and 110.31%, and that in
the ventral pedis skin was changed by 131.67, −8.33 and 71.67%
compared with that in the CA group, respectively (Table II).
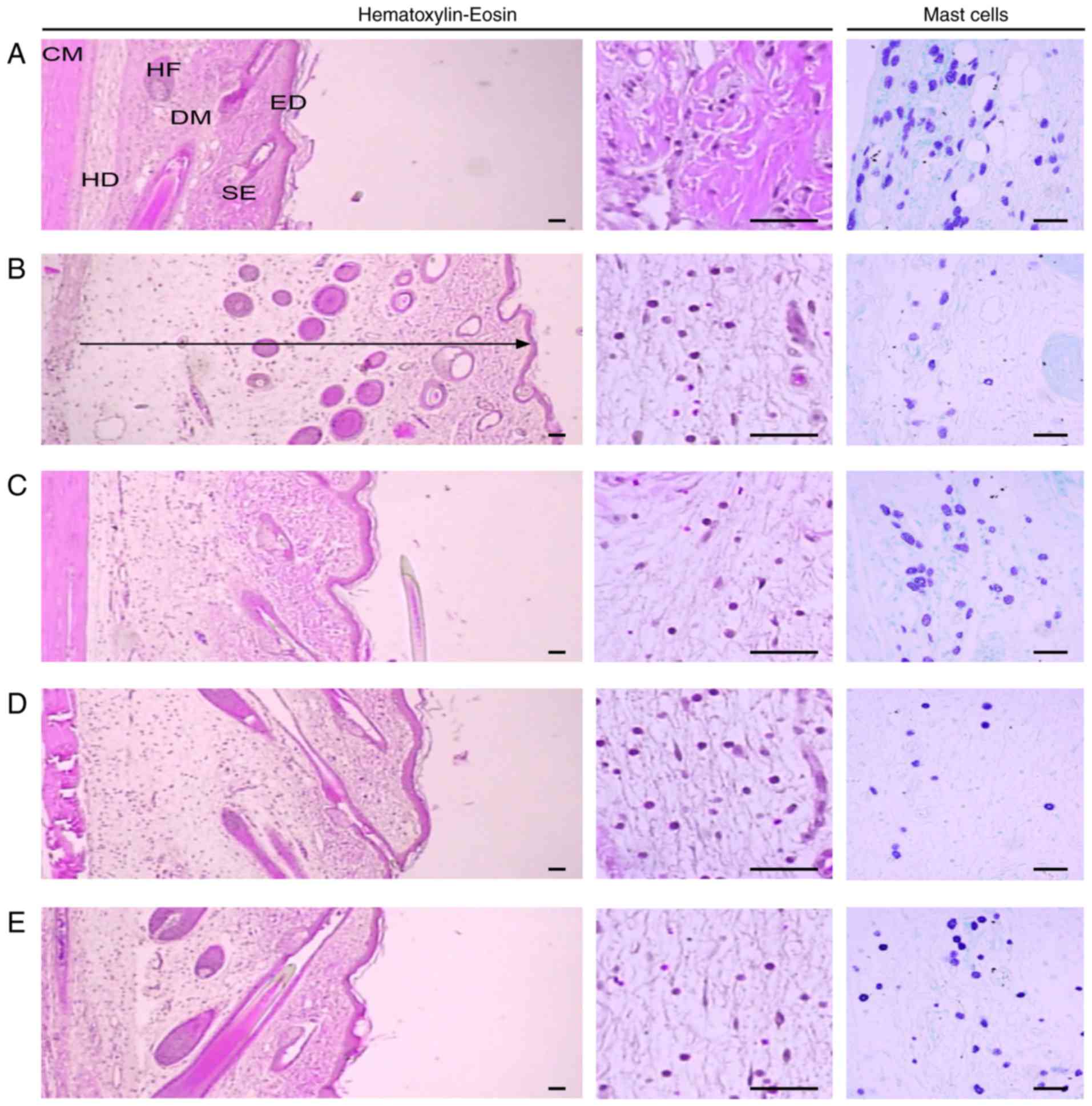 | Figure 8Histological images of the dorsal
pedis skin. Tissue sections from the dorsal pedis of the (A)
Control, (B) CA, (C) CA and dexamethasone-treated, (D) CA and SCRT
(0.3 g/kg)-treated and the (E) CA and SCRT (1.0 g/kg)-treated rats
were stained with hematoxylin and eosin or toluidine blue for
histo-pathological examination. The arrow indicates the total
thickness and scale bars indicate 40 μm. CM, cutaneous
muscle; DM, dermis; ED, epidermis; HD, hypodermis; HF, hair
follicle; SE, sebaceous gland; SCRT, Socheongryong-Tang; LPS,
lipopolysaccharide. |
 | Table IIChanges in the histomorphometrical
parameters of paw skins. |
Table II
Changes in the histomorphometrical
parameters of paw skins.
| Group | Dorsal pedis skin
| Ventral pedis skin
|
|---|
| Total thickness
(μm) | IF cell number
(cells/mm2) | Mast cell number
(cells/mm2) | Total thickness
(μm) | IF cell number
(cells/mm2) | Mast cell number
(cells/mm2) |
|---|
| Control | 937.49±221.08 | 28.60±11.80 | 58.20±9.98 | 595.58±53.73 | 20.20±5.89 | 35.00±5.10 |
| CA |
2,367.14±369.77a |
288.60±17.64a | 19.40±2.30a |
1,382.58±165.53a |
473.60±45.64b | 12.00±2.35a |
| Dexamethasone |
1,306.65±98.70c,d |
113.20±24.79a,c | 40.00±10.98a,c |
912.94±175.95a,c |
197.40±17.04b,e | 27.80±4.71c,d |
| SCRT (0.3
g/kg) |
1,925.25±217.35a,c |
258.80±38.32a | 22.40±6.91a |
1,309.70±162.60a |
412.80±55.88b | 11.00±2.55a |
| SCRT (1 g/kg) |
1,632.05±86.02a,c |
173.40±16.70a,c | 40.80±7.69a,c |
1,109.76±108.75a,c |
334.80±38.34b,e | 20.60±6.31a,c |
Discussion
Although the exact molecular mechanisms associated
with herbal medicines have remained to be fully elucidated, herbal
medications have been widely used for centuries and have become
attractive therapeutics due to fewer side-effects than certain
pharmaceutical drugs. In traditional Korean medicine, SCRT has been
commonly used to treat a variety of inflammatory allergic diseases,
including allergic rhinitis and bronchial asthma (1-3).
However, only few studies have provided a scientific assessment of
the benefits of SCRT. Accordingly, the present study investigated
the influences of SCRT on inflammatory responses of RAW 264.7 cells
and rats.
Inflammation has a key role in health as well as in
disease. Specifically, it is a response of the body to injury
linked to harmful chemical or physical stimuli and microbiological
toxins that is involved in multiple pathologies, including
arthritis, asthma, inflammatory bowel diseases and atherosclerosis.
The inflammatory response is intended to demolish or inactivate
invading organisms and set the stage for tissue repair (6). NO produced by NOS is associated with
the development of inflammation after CA administration, and NO
generated by iNOS is associated with the maintenance of the
inflammatory responses (24).
Increases of iNOS activity that influences inflammatory agents,
including endotoxin, interferon-γ, IL-1β and TNF-α, may cause shock
and inflammatory responses in humans (25,26). Furthermore, it has been reported
that COX-2 is activated during the inflammatory response to produce
PGE2, which contributes to the formation of tumors by
inhibiting apoptosis and inducing cell division, cancer metastasis
and angiogenesis (27). In the
present study, SCRT significantly inhibited the LPS-stimulated NO
production and expression of iNOS protein in RAW 264.7 cells.
Furthermore, the PGE2 assay and immunoblot analysis
revealed that SCRT significantly blocked the induction of
PGE2 and COX-2 protein by LPS. In paw tissues, treatment
with CA resulted in significantly increased expression of iNOS and
COX-2 protein in comparison with the control group. However, DEXA
significantly decreased the expression of iNOS and COX-2 protein.
SCRT (1 g/kg) also significantly inhibited the expression of iNOS
and COX-2 protein. These results may indirectly suggest that the
therapeutic effects of SCRT on various inflammatory symptoms of
allergic rhinitis and bronchial asthma are partly due to inhibition
of NO and PGE2 production as well as expression of iNOS
and COX-2 protein. TNF-α, IL-1β and IL-6 are frequently encountered
pro-inflammatory cytokines that are involved in the interaction
with various target cells as well as diverse immunological
functions (12-14). These cytokines also mediate
immunity and inflammation. Large amounts of TNF-α, IL-1β and IL-6
are released by LPS in macrophages. NF-κB is also known have a key
role in transmitting proinflammatory cytokine signals to the
nucleus (13). Treatment with
SCRT significantly inhibited the production of these cytokines,
suggesting that SCRT inhibits the expression of these specific
genes associated with the inflammatory process. The present results
demonstrated that SCRT is a strong inhibitor of the production of
IL-1β, IL-6 and TNF-α. Furthermore, the inhibitory mechanism of
SCRT on pro-inflammatory cytokines may indicate an important
strategy to limit pathological inflammation.
NF-κB is a typical transcription factor that
controls the expression of genes associated with apoptosis, immune
responses and the cell cycle. Unsuitable NF-κB activation may
mediate tumorigenesis and inflammation. However, suppression of
NF-κB activity should be useful in the treatment and prevention of
cancer (28). The present study
demonstrated that SCRT significantly prevented LPS-stimulated NF-κB
activation. It has been suggested that NF-κB is associated with the
regulation of COX-2 and iNOS protein expression, and that several
chemopreventive phytochemicals inhibited COX-2 and iNOS protein
expression by suppressing NF-κB activation (20,29). In addition, the NF-κB pathway is a
major regulator of LPS-induced pro-inflammatory cytokine release
(30). The present results
revealed that LPS increased the levels of p-I-κBα and NF-κB (p65),
whereas treatment with SCRT resulted in reduced p-I-κBα levels and
inhibited the nuclear translocation of NF-κB. Taken together, these
results indicate that suppression of NF-κB activation by SCRT was
correlated with inhibition of induction of iNOS, COX-2 and
pro-inflammatory cytokines by SCRT. Furthermore, MAPKs, a family of
protein threonine/serine kinases, represent a major component of
intracellular signaling (31,32). LPS has been reported to induce
macrophages, leading to increased activation and phosphorylation of
JNK, ERK1/2 and p38 (33). In the
present study, it was demonstrated that SCRT (1 mg/ml)
significantly suppressed the phosphorylation of JNK and ERK1/2,
whereas the phosphorylation of p38 was unaffected. Furthermore, the
results of the present demonstrated an association between
inhibited phosphorylation of JNK and ERK1/2 and inactivation of
NF-κB. Therefore, the aforementioned data suggests that the
anti-inflammatory activities of SCRT may be due to inhibition of
LPS-stimulated activation of NF-kB and phosphorylation of JNK and
ERK1/2 in vitro.
Local treatment with CA induces severe acute
edematous inflammation, and a variety of inflammatory mediators
produced from resident macrophages, damaged tissues,
polymorphonuclear cells (e.g., neutrophils) and mast cells are
involved in the associated pathogenesis (34-37). Histopathologically, infiltration
of inflammatory cells and loosening of connective tissues were
observed around CA-treated sites (38-40). Thus, the present study used
CA-induced paw edema as a representative model to assess the
anti-inflammatory activities of therapeutic candidates (20-23,37,41,42), while the in vivo and in
vitro models may exhibit differences of major cell types. In
the present study, prominent increases of inflammatory infiltrated
cells with increases in skin thicknesses on the dorsal and ventral
pedis were observed following treatment of rat paw skins with CA.
However, the CA-induced acute edematous inflammation was
significantly inhibited by treatment with the higher dosage of SCRT
and DEXA (P<0.01). In rats treated with the lower dose of SCRT,
a significant decrease in the dorsal pedis skin thickness relative
to that in the CA group was demonstrated, but no significant
changes in the ventral pedis skin thicknesses or number of
infiltrated inflammatory cells on the dorsal and ventral pedis skin
tissues were demonstrated. These results are considered as direct
evidence that the higher dosage of SCRT has beneficial
anti-inflammatory effects sufficient to reduce inflammatory edema
and cell infiltration, but that the lower dosage was only effective
against edematous changes restricted to dorsal pedis skin tissues
but that it was not sufficient to reduce inflammatory cell
infiltration and edema in ventral pedis skin tissues. Mast cells
are distributed in the body and have an important role in a variety
of inflammatory and allergic diseases. These cells also have cell
surface immunoglobulin (Ig)E receptors and are provoked by
interaction between bound IgE and the pertinent antigen (43). Following activation, mast cells
release a variety of bioactive substances, including various lipid
mediators and histamine that cause an immediate-type allergic
reaction. Mast cell degranulation has been also known to increase
in the acute (44,45), as well as the chronic stage
(46-48) of diverse inflammatory and allergic
diseases. Noticeable increases of mast cell degranulation in dermis
tissue have been observed in CA-induced acute inflammation, and the
inhibition of these mast cell activities (degranulation) has been
used as a beneficial index to predict the efficacy of
anti-inflammatory drugs (34,49,50). In the present study, treatment
with the higher dosage of SCRT and DEXA markedly and significantly
inhibited mast cell degranulation and maintained the mast cell
numbers in the dermis of the CA-induced rats (P<0.01), but no
significant changes in mast cell numbers were observed in rats
treated with the lower dosage of SCRT compared with those in the CA
group on the dorsal and ventral pedis skins. These results provide
direct evidence that the higher dosage of SCRT exerted
anti-inflammatory effects through the control of mast cell
activation, as indicated by at least partial degranulation, but
that the lower dosage of SCRT did not. Furthermore, as the results
of the toluidine blue staining of edematous tissues indicated that
SCRT inhibited the numbers of degranulated mast cells, SCRT may
have therapeutic potential to reduce type I hypersensitivity. In
the present in vivo study, rats were injected with CA once,
without any previous injection of any sensitizing agent, so that
hypersensitivity could not be assessed. As previously established,
CA injection causes a maximum increase in paw volumes within 4 h,
and in the present study, paw volumes were monitored at 1-h time
intervals for 4 h. Thus, the effect of SCRT on type IV
hypersensitivity reactions could also not be determined.
SCRT is an extract from a blend of 8 medicinal
herbs. Several active components of SCRT have been reported to
exert anti-inflammatory activities. Ephedrine and pseudoephedrine
are stereoisomers isolated from the plant Ephedra sinica
(Ephedraceae family) or the traditional Chinese medicinal herb Ma
Huang (51). These compounds have
also been reported to exert powerful anti-inflammatory effects
against D-galactosamine/LPS-stimulated acute liver failure in rats
(52). Furthermore, ephedrine and
pseudoephed-rine were reported to suppress CA-induced hind paw
edema in mice. Hind paw edema stimulated by prostaglandin E,
histamine and bradykinin was reported to be inhibited by these
compounds, demonstrating that they exert anti-inflammatory effects
(53). Paeoniflorin has
pharmacological properties, including anti-inflammatory and
immunomodulatory effects, as well as the ability to suppress
rheumatic diseases (18). In
addition, Liu et al (54)
reported that paeoniflorin reduced
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced toxicity by
suppression of neuroinflammation via activation of adenosine A1
receptor, and that paeoniflorin may be a useful neuroprotective
compound for the treatment of Parkinson's disease. Cinnamic acid
was reported to have significant anti-inflammatory activities in
vitro and in vivo (55). Furthermore, this compound acts as
a lipoxygenase inhibitor with antioxidant, anti-inflammatory and
anticancer activity (56,57). Glycyrrhizin, a triterpene
glycoside from the roots of licorice, resulted in widely inhibited
induction of inflammatory mediators stimulated by CpG-DNA in RAW
264.7 cells, as well as attenuated inflammatory responses
stimulated by toll-like receptor (TLR)3 and TLR4 (58). In addition, inhibition of NF-κB
activity and IL-8 production in lung cells and attenuation of
CA-induced lung damage in mice by glycyrrhizin have been
demonstrated (59,60). Gomisin A inhibits the activation
of NF-κB and oxidative stress, leading to the suppression of
pro-inflammatory mediators by attenuating
CCl4-stimulated acute liver damage (61). Furthermore, gomisin A was reported
to have neuroprotective effects by relieving the microglia-mediated
neuroinflammatory response through inhibition of TLR4-mediated
MAPKs and NF-κB signaling pathways (62). UPLC analysis revealed that the
major active components of SCRT are ephedrine, paeoniflorin,
cinnamic acid, glycyrrhizin and gomisin-A. Accordingly, it is
suggested that the anti-inflammatory properties of SCRT on
LPS-stimulated RAW 264.7 cells and CA-induced rat paw edema may be
due to the action of these 5 compounds.
In conclusion, the present results clearly
demonstrated that SCRT has anti-inflammatory activities through
decreasing the production of inflammatory mediators, including
PGE2, NO and pro-inflammatory cytokines via inhibition
of the signaling pathways of JNK, ERK1/2 and NF-κB in the
LPS-stimulated RAW 264.7 cells. In addition, the results from the
CA-induced paw edema model demonstrated an anti-edema effect of
SCRT. Furthermore, SCRT (1 g/kg) inhibited acute edematous
inflammation through inhibition of mast cell degranulation and
infiltration of inflammatory cells. Therefore, the present study
provided scientific evidence for the anti-inflammatory activities
of SCRT.
Glossary
Abbreviations
Abbreviations:
|
CA
|
carrageenan
|
|
COX-2
|
cyclooxygenase
|
|
DEXA
|
dexamethasone
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
IL
|
interleukin
|
|
iNOS
|
inducible nitric oxide synthase
|
|
I-κB
|
inhibitory-κB
|
|
JNK
|
c-Jun N-terminal kinase
|
|
LPS
|
lipopolysaccharide
|
|
MAPK
|
mitogen-activated protein kinase
|
|
NF-κB
|
nuclear factor-κB
|
|
NO
|
nitric oxide
|
|
PGE2
|
prostaglandin E2
|
|
SCRT
|
Socheongryong-Tang
|
|
TNF-α
|
tumor necrosis factor-α
|
|
TLR
|
toll-like receptor
|
|
UPLC
|
ultra performance liquid
chromatography
|
Acknowledgments
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (grant no. NRF-2015
R1D1A1A01059994) and by the NRF grant funded by the Korean
Government (Ministry of Science, ICT and Future Planning; grant no.
2012 R1A5A2A42671316).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Kao ST, Lin CS, Hsieh CC, Hsieh WT and Lin
JG: Effects of Xiao-Qing-Long-Tang (XQLT) on bronchoconstriction
and airway eosinophil infiltration in ovalbumin-sensitized guinea
pigs: In vivo and in vitro studies. Allergy. 56:1164–1171. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ko E, Rho S, Cho C, Choi H, Ko S, Lee Y,
Hong MC, Shin MK, Jung SG and Bae H: So-Cheong-Ryong-Tang,
traditional Korean medicine, suppresses Th2 lineage development.
Biol Pharm Bull. 27:739–743. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ko E, Rho S, Lee E, Seo Y, Cho C, Lee Y,
Min BI, Shin MK, Hong MC and Bae H: Traditional Korean medicine
(SCRT) modulate Th1/Th2 specific cytokine production in mice
CD4+ T cell. J Ethnopharmacol. 92:121–128. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakao M, Muramoto Y, Hisadome M, Yamano N,
Shoji M, Fukushima Y, Saruwatari J and Nakagawa K: The effect of
Shoseiryuto, a traditional Japanese medicine, on cytochrome P450s,
N-acetyltransferase 2 and xanthine oxidase, in extensive or
intermediate metabolizers of CYP2D6. Eur J Clin Pharmacol.
63:345–353. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lawrence T, Willoughby DA and Gilroy DW:
Anti-inflammatory lipid mediators and insights into the resolution
of inflammation. Nat Rev Immunol. 2:787–795. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guzik TJ, Korbut R and Adamek-Guzik T:
Nitric oxide and superoxide in inflammation and immune regulation.
J Physiol Pharmacol. 54:469–487. 2003.
|
|
7
|
Nathan C: Nitric oxide as a secretory
product of mammalian cells. FASEB J. 6:3051–3064. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
MacMicking J, Xie QW and Nathan C: Nitric
oxide and macrophage function. Annu Rev Immunol. 15:323–350. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagy G, Clark JM, Buzás EI, Gorman CL and
Cope AP: Nitric oxide, chronic inflammation and autoimmunity.
Immunol Lett. 111:1–5. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Botting RM: Cyclooxygenase: Past, present
and future. A tribute to John R. Vane (1927–2004). J Therm Biol.
31:208–219. 2006. View Article : Google Scholar
|
|
11
|
Blobaum AL and Marnett LJ: Structural and
functional basis of cyclooxygenase inhibition. J Med Chem.
50:1425–1441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Delgado AV, McManus AT and Chambers JP:
Production of tumor necrosis factor-alpha, interleukin 1-beta,
interleukin 2, and interleukin 6 by rat leukocyte subpopulations
after exposure to substance P. Neuropeptides. 37:355–361. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshimura A: Signal transduction of
inflammatory cytokines and tumor development. Cancer Sci.
97:439–447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beutler B and Cerami A: The biology of
cachectin/TNF-α primary mediator of the host response. Annu Rev
Immunol. 7:625–655. 1989. View Article : Google Scholar
|
|
15
|
Ghosh S and Ksarin M: Missing pieces in
the NF-kappaB puzzle. Cell. 109(Suppl): S81–S96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chan ED and Riches DW: IFN-gamma + LPS
induction of iNOS is modulated by ERK, JNK/SAPK, and p38 (mapk) in
a mouse macrophage cell line. Am J Physiol Cell Physiol.
280:C441–C450. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagai T, Arai Y, Emori M, Nunome SY, Yabe
T, Takeda T and Yamada H: Anti-allergic activity of a Kampo
(Japanese herbal) medicine 'Sho-seiryu-to (Xiao-Qing-Long-Tang)' on
airway inflammation in a mouse model. Int Immunopharmacol.
4:1353–1365. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang SD, Lin LJ, Chen CL, Lee SC, Lin CC,
Wang JY and Kao ST: Xiao-Qing-Long-Tang attenuates allergic airway
inflammation and remodeling in repetitive Dermatogoides
pteronyssinus challenged chronic asthmatic mice model. J
Ethnopharmacol. 142:531–538. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kao ST, Wang SD, Wang JY, Yu CK and Lei
HY: The Effect of herbal medicine, xiao-qing-long-tang (XQLT), on
allergen-induced bronchial inflammation in mite-sensitized mice.
Allergy. 55:1127–1130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim YW, Zhao RJ, Park SJ, Lee JR, Cho IJ,
Yang CH, Kim SG and Kim SC: Anti-inflammatory effects of
liquiritigenin as a consequence of the inhibition of
NF-kappaB-dependent iNOS and pro-inflammatory cytokines production.
Br J Pharmacol. 154:165–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee CW, Park SM, Kim YS, Jegal KH, Lee JR,
Cho IJ, Ku SK, Lee JY, Ahn YT, Son Y, et al: Biomolecular evidence
of anti-inflammatory effects by Clematis mandshurica Ruprecht root
extract in rodent cells. J Ethnopharmacol. 155:1141–1155. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee CW, Park SM, Zhao R, Lee C, Chun W,
Son Y, Kim SH, Jung JY, Jegal KH, Cho IJ, et al: Hederagenin, a
major component of Clematis mandshurica Ruprecht root, attenuates
inflammatory responses in RAW 264.7 cells and in mice. Int
Immunopharmacol. 29:528–537. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SY, Park SM, Hwangbo M, Lee JR, Byun
SH, Ku SK, Cho IJ, Kim SC, Jee SY and Park SJ:
Cheongsangbangpung-tang ameliorated the acute inflammatory response
via the inhibition of NF-κB activation and MAPK phosphorylation.
BMC Complement Altern Med. 17:462017. View Article : Google Scholar
|
|
24
|
Borthakur A, Bhattacharyya S, Dudeja PK
and Tobacman JK: Carrageenan induces interleukin-8 production
through distinct Bcl10 pathway in normal human colonic epithelial
cells. Am J Physiol Gastrointest Liver Physiol. 292:G829–G838.
2007. View Article : Google Scholar
|
|
25
|
Szabó C: Alterations in nitric oxide
production in various forms of circulatory shock. New Horiz.
3:2–32. 1995.PubMed/NCBI
|
|
26
|
Southan GJ and Szabó C: Selective
pharmacological inhibition of distinct nitric oxide synthase
isoforms. Biochem Pharmacol. 51:383–394. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gröesch S, Maier TJ, Schiffmann S and
Geisslinger G: Cyclooxygenase-2 (COX-2)-independent
anticarcinogenic effects of selective COX-2 inhibitors. J Natl
Cancer Inst. 98:736–747. 2006. View Article : Google Scholar
|
|
28
|
Aggarwal BB: Nuclear factor-kappaB: The
enemy within. Cancer Cell. 6:203–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Surh YJ, Chun KS, Cha HH, Han SS, Keum YS,
Park KK and Lee SS: Molecular mechanisms underlying chemopreventive
activities of anti-inflammatory phytochemicals: Down-regulation of
COX-2 and iNOS through suppression of NF-kappa B activation. Mutat
Res. 480–481:243–268. 2001. View Article : Google Scholar
|
|
30
|
Lappas M, Permezel M, Georgiou HM and Rice
GE: Nuclear factor kappa B regulation of pro-inflammatory cytokines
in human gestational tissues in vitro. Biol Reprod. 67:668–673.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guha M and Mackman N: LPS induction of
gene expression in human monocytes. Cell Signal. 13:85–94. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xiao ZY, Zhou WX, Zhang YX, Cheng JP, He
JF, Yang RF and Yun LH: Inhibitory effect of linomide on
lipopolysaccharide-induced proinflammatory cytokine tumor necrosis
factor-alpha production in RAW264.7 macrophages through suppression
of NF-kappaB, p38, and JNK activation. Immunol Lett. 114:81–85.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Choi MS, Lee SH, Cho HS, Kim Y, Yun YP,
Jung HY, Jung JK, Lee BC, Pyo HB and Hong JT: Inhibitory effect of
obovatol on nitric oxide production and activation of NF-kappaB/MAP
kinases in lipopolysaccharide-treated RAW 264.7 cells. Eur J
Pharmacol. 556:181–189. 2007. View Article : Google Scholar
|
|
34
|
Mazzari S, Canella R, Petrelli L,
Marcolongo G and Leon A: N-(2-hydroxyethyl)hexadecanamide is orally
active in reducing edema formation and inflammatory hyperalgesia by
down-modulating mast cell activation. Eur J Pharmacol. 300:227–236.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Antonio MA and Souza Brito AR: Oral
anti-inflammatory and anti-ulcerogenic activities of a
hydroalcoholic extract and partitioned fractions of Turnera
ulmifolia (Turneraceae). J Ethnopharmacol. 61:215–228. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Handy RL and Moore PK: A comparison of the
effects of L-NAME, 7-NI and L-NIL on carrageenan-induced hind paw
oedema and NOS activity. Br J Pharmacol. 123:1119–1126. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gupta M, Mazumder UK, Gomathi P and Selvan
VT: Antiinflammatory evaluation of leaves of Plumeria acuminate.
BMC Complement Altern Med. 6:362006. View Article : Google Scholar
|
|
38
|
Holt S, Comelli F, Costa B and Fowler CJ:
Inhibitors of fatty acid amide hydrolase reduce carrageenan-induced
hind paw inflammation in pentobarbital-treated mice: Comparison
with indomethacin and possible involvement of cannabinoid
receptors. Br J Pharmacol. 146:467–476. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu J, Zhang W, Zhou L, Wang X and Lian Q:
Anti-inflammatory effect and mechanism of osthole in rats. Zhong
Yao Cai. 28:1002–1006. 2005.
|
|
40
|
Beloeil H, Ababneh Z, Chung R, Zurakowski
D, Mulkern RV and Berde CB: Effects of bupivacaine and tetrodotoxin
on carrageenan-induced hind paw inflammation in rats (Part 1):
Hyperalgesia, edema, and systemic cytokines. Anesthesiol.
105:128–138. 2006. View Article : Google Scholar
|
|
41
|
Lee JH, Choi YH and Choi BT: The
anti-inflammatory effects of 2 Hz electroacupuncture with different
intensities on acute carrageenan-induced inflammation in the rat
paw. Int J Mol Med. 16:99–102. 2005.PubMed/NCBI
|
|
42
|
Rao CV, Verma AR, Gupta PK and Vijayakumar
M: Anti-inflammatory and anti-nociceptive activities of Fumaria
indica whole plant extract in experimental animals. Acta Pharm.
57:491–498. 2007. View Article : Google Scholar
|
|
43
|
Holgate ST: The role of mast cells and
basophils in inflammation. Clin Exp Allergy. 30(Suppl 1): S28–S32.
2000. View Article : Google Scholar
|
|
44
|
Crimi E, Chiaramondia M, Milanese M, Rossi
GA and Brusasco V: Increased numbers of mast cells in bronchial
mucosa after the late-phase asthmatic response to allergen. Am Rev
Respir Dis. 144:1282–1286. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gauvreau GM, Lee JM, Watson RM, Irani AM,
Schwartz LB and O'Byrne PM: Increased numbers of both airway
basophils and mast cells in sputum after allergen inhalation
challenge of atopic asthmatics. Am J Respir Crit Care Med.
161:1473–1478. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mitchell EB, Crow J, Williams G and
Platts-Mills TA: Increase in skin mast cells following chronic
house dust mite exposure. Br J Dermatol. 114:65–73. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chanez P, Lacoste JY, Guillot B, Giron J,
Barnéon G, Enander I, Godard P, Michel FB and Bousquet J: Mast
cells' contribution to the fibrosing alveolitis of the scleroderma
lung. Am Rev Respir Dis. 147:1497–1502. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Armbrust T, Batusic D, Ringe B and
Ramadori G: Mast cells distribution in human liver disease and
experimental rat liver fibrosis. Indications for mast cell
participation in development of liver fibrosis. J Hepatol.
26:1042–1054. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Weischer CH: Experimental studies on the
possibility of influencing mast cells in experimental
carrageenin-induced rat paw edema: Histological study on the
effects of some non-steroidal anti-inflammatory agents (author's
transl). Arzneimittelforschung. 26:1867–1870. 1976.In German.
|
|
50
|
Sin YM, Sedgwick AD, Chea EP and
Willoughby DA: Mast cells in newly formed lining tissue during
acute inflammation: A six day air pouch model in the mouse. Ann
Rheum Dis. 45:873–877. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mehendale SR, Bauer BA and Yuan CS:
Ephedra-containing dietary supplements in the US versus ephedra as
a Chinese medicine. Am J Chin Med. 32:1–10. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wu Z, Kong X, Zhang T, Ye J, Fang Z and
Yang X: Pseudo-ephedrine/ephedrine shows potent anti-inflammatory
activity against TNF-α-mediated acute liver failure induced by
lipopolysaccharide/D-galactosamine. Eur J Pharmacol. 724:112–121.
2014. View Article : Google Scholar
|
|
53
|
Kasahara Y, Hikino H, Tsurufuji S,
Watanabe M and Ohuchi K: Anti-inflammatory actions of ephedrines in
acute inflammations. Planta Med. 325–331. 1985. View Article : Google Scholar
|
|
54
|
Liu HQ, Zhang WY, Luo XT, Ye Y and Zhu XZ:
Paeoniflorin attenuates neuroinflammation and dopaminergic
neurodegeneration in the MPTP model of Parkinson's disease by
activation of adenosine A1 receptor. Br J Pharmacol. 148:314–325.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liao JC, Deng JS, Chiu CS, Hou WC, Huang
SS, Shie PH and Huang GJ: Anti-inflammatory activities of
Cinnamomum cassia constituents in vitro and in vivo. Evid Based
Complement Alternat Med. 2012:4293202012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu L, Hudgins WR, Shack S, Yin MQ and
Samid D: Cinnamic acid: A natural product with potential use in
cancer intervention. Int J Cancer. 62:345–350. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hadjipavlou-Litina D and Pontiki E:
Aryl-acetic and cinnamic acids as lipoxygenase inhibitors with
antioxidant, anti-inflammatory, and anticancer activity. Methods
Mol Biol. 1208:361–377. 2015. View Article : Google Scholar
|
|
58
|
Schröfelbauer B, Raffetseder J, Hauner M,
Wolkerstorfer A, Ernst W and Szolar OH: Glycyrrhizin, the main
active compound in liquorice, attenuates pro-inflammatory responses
by interfering with membrane-dependent receptor signaling. Biochem
J. 421:473–482. 2009. View Article : Google Scholar
|
|
59
|
Takei H, Baba Y, Hisatsune A, Katsuki H,
Miyata T, Yokomizo K and Isohama Y: Glycyrrhizin inhibits
interleukin-8 production and nuclear factor-kappa B activity in
lung epithelial cells, but not through glucocorticoid receptors. J
Pharmacol Sci. 106:460–468. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Menegazzi M, Di Paola R, Mazzon E,
Genovese T, Crisafulli C, Dal Bosco M, Zou Z and Suzuki H:
Glycyrrhizin attenuates the development of carrageenan-induced lung
injury in mice. Pharmacol Res. 58:22–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Teraoka R, Shimada T and Aburada M: The
molecular mechanisms of the hepatoprotective effect of gomisin A
against oxidative stress and inflammatory response in rats with
carbon tetrachloride-induced acute liver injury. Biol Pharm Bull.
35:171–177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang X, Hu D, Zhang L, Lian G, Zhao S,
Wang C, Yin J, Wu C and Yang J: Gomisin A inhibits
lipopolysaccharide-induced inflammatory responses in N9 microglia
via blocking the NF-κB/MAPKs pathway. Food Chem Toxicol.
63:119–127. 2014. View Article : Google Scholar
|























