Introduction
Gastric cancer (GC) is a malignant tumor arising
from the lining of the stomach. GC is the fifth most common type of
cancer in the world, and remains the second most common cause of
cancer-associated mortality worldwide (1,2).
The development of GC may result from complex interactions between
multiple factors, including genetic and epigenetic alterations of
oncogenes and tumor-suppressor genes, cell-cycle regulators, cell
adhesion molecules and DNA repair genes (3,4).
Phosphatase and tensin homology deleted on
chromosome 10 (PTEN) is a tumor suppressor gene belonging to
the phosphatase family, and is one of the most frequently mutated
tumor suppressors in several types of human cancer (5). PTEN is important in cell
proliferation, migration and apoptosis (6). In GC, a significant reduction in the
expression of PTEN has been reported. In addition, the expression
of PTEN negatively correlates with tumor size, Borrmann
classification, lymph node metastasis and tumor staging (7,8).
Loss of a PTEN allele has been identified in 70% of patients
with prostate cancer at the time of diagnosis (9). The inactivation of PTEN can be
attributed to gene mutation, hypermethylation, microRNA-mediated
regulation and post-translational phosphorylation (10). Previous studies have shown that
the functional inactivation of PTEN has been detected in multiple
cases of GC and other tumors (11–13). These reports suggest that the
inactivation of PTEN is closely associated with the incidence,
progression and prognosis of GC.
Livin, a member of the inhibitor-of-apoptosis
protein (IAP) family is expressed in a variety of tumors, including
GC, melanoma, neuroblastoma, mesothelioma and osteosarcoma
(14–16). The expression of Livin is
increased in human GC, and correlates with tumor differentiation
and lymph node metastases. The underlying mechanism of its effect
indicates that Livin protein binds directly to caspases, thereby
inhibiting apoptosis and promoting tumor growth. By contrast, the
knockdown of Livin inhibits cell growth and invasion (17,18). Therefore, the results reported to
date suggest that the downregulation of Livin may be a potential
therapeutic strategy for GC.
PTEN and Livin are involved in the regulation of
tumor cell proliferation, migration and apoptosis, in which PTEN
suppresses and Livin promotes cancer development. Although the
modulation of PTEN or Livin has been investigated extensively in
various cancer models (7,8,19),
no studies have been performed to evaluate the combined effect on
the development of GC of concurrently modulating these two genes.
Therefore, the present study focused on evaluating the biological
effect of this dual gene modulation on the development of GC by
employing recombinant vectors with PTEN and/or Livin
gene modulation capacity, previously constructed in the laboratory
(20).
Materials and methods
Mice
All animal experiments were performed in accordance
with protocols approved by the Animal Ethics Committee of Zhengzhou
University (Zhengzhou, China). The 6–8-week-old BALB/c male mice
were purchased from the Laboratory Animal Center of Zhengzhou
University. All animal experimental procedures were approved by the
Institutional Animal Care and Use Committee of Zhengzhou
University. The mice were bred in the animal room with continuous
air circulation at 25–27°C, 40–60% humidity and a regular 12 h
light/dark cycle. Sterile food and water containing multi-vitamins
was provided to the mice.
BGC823 cell transfection
The following vectors were used in the present
study: pCL-neo-PTEN vector for the overexpression of PTEN;
pRNAT-U6.1-siLivin vector for silencing of the Livin gene;
pCL-neo-PTEN-siLivin vector for the simultaneous overexpression of
PTEN and silencing of the Livin gene; empty vector as
a transfection control. The vectors were constructed as described
previously (20). These vectors
were transfected into BGC823 cells (Culture Collection of the
Chinese Academy of Sciences, Shanghai, China) using a
Lipofectamine™2000 transfection kit (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The BGC823 cells were cultured
in a 6-well plate in RPMI-1640 culture medium at a concentration of
1×106 cells/ml at 37°C and in the presence of 5%
CO2. The stable transfection clones from the above
transfections were selected following multiple rounds of culture
and selection.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Tumor tissue (20–30 mg) from each mouse was ground
in liquid nitrogen. Total RNA was extracted and purified using an
RNA extraction kit (cat no. 15596026; Invitrogen; Thermo Fisher
Scientific, Inc.). The RNA sample was then reverse transcribed into
cDNA using a high capacity cDNA reverse transcription kit (cat no.
RR047A; Takara Biotechnology Co., Ltd., Dalian, China). RT-qPCR
analysis was performed using a real-time PCR kit (Baocheng Biotech
Co., Ltd., Dalian, China). Subsequently, qPCR was performed using
the SYBR Green Master mix (cat no. 4472908; Thermo Fisher
Scientific, Inc.) on a 7500 Fast Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Thermocycling
conditions were as follows; 50°C for 2 min; pre-denaturation at
95°C for 2 min; followed by 15 sec at 95°C then 1 min at 60°C for
40 cycles; melt curve was at 95°C for 15 sec, 1 min at 60°C, 15 sec
at 95°C and 15 sec at 60°C.
RNA was normalized to the expression levels of
β2-microglobulin (B2M) and relative expression was
calculated. The sequences of the PCR primers used were as follows:
B2M, forward 5′-TCCATCCGACATTGAAGTTG-3′ and reverse
5′-ACACGGCAGGCATACTCAT-3′; PTEN, forward 5′-TGGCGGAACTYGCAATCC-3′
and reverse 5′-GCTGAGGAACTCAAAGTAC-3′; Livin, forward
5′-TCCTGCTCCGGTCAAAAGG-3′ and reverse 5′-GCTGCGTCTTCCGGTTCTT-3′.
RNA levels were normalized to β-actin expression and relative
expression was calculated using the 2−ΔΔCq method
(21).
Western blot analysis
The cells or tumor tissues were harvested and
extracted using radioimmunoprecipitation assay lysis buffer (50 mM
Tris-HCl, pH 7.5, 150 mM NaCl, 1% deoxycholate, 1% Triton X-100, 2
mM EDTA, 0.1% SDS and 50 mM NaF) containing a protease inhibitor
cocktail (Roche Diagnostics GmbH, Mannheim, Germany). Protein
concentrations were determined using the bicinchoninic acid method
(Pierce; Thermo Fisher Scientific, Inc.). The extracts were heated
for 5 min in loading buffer, and equal quantities (40 µg) of cell
extracts were separated on 12% SDS-PAGE gels. The separated protein
bands were transferred onto polyvinylidene fluoride membranes and
then blocked in 10% skim milk for 1 h at room temperature. Primary
antibodies against PTEN (cat no. sc-133242; Santa Cruz
Biotechnology, Inc., California, CA, USA), Livin (cat no.
ab-127979; Abcam, Cambridge, MA, USA) and β-actin (cat no.
sc-130301, Santa Cruz Biotechnology, Inc.) were diluted 1:1,000
according to the manufacturer's protocol and incubated overnight at
4°C. The membranes were then incubated with the corresponding
horseradish peroxidase-conjugated secondary antibodies (1:5,000;
cat no. sc-2005; Santa Cruz Biotechnology, Inc.) at room
temperature for 1 h. Chemiluminescent solution (NEN Life Science,
Boston, MA, USA) was used to visualize the blot following exposure
onto hyper film (GE Healthcare Life Sciences, Pittsburgh, PA, USA)
for 5 min. Blots were analyzed and quantified by Quantity One
software (4.6.2 version; Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Cell proliferation assay
Cells in the exponential phase were collected and
cultured in a 96-well culture plate at a concentration of
1×104 cells in a volume of 200 µl per well at 37°C with
5% CO2 for 1–5 days. Each day, 20 µl of
3-(4,5-dimethylthi-azol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
solution (5 mg/ml) was added to the culture and incubated for
another 4 h at 37°C. The culture medium was discarded and 150 µl of
DMSO was added for 10 min to dissolve the crystals. The absorption
at 570 nm was detected in an automatic plate reader (Bio-Rad
Laboratories, Inc.).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) apoptosis assay
A TUNEL assay was used to detect apoptosis following
transfection with different vectors. The cells were deposited onto
glass slides, and cell climbing slides were produced and dried at
room temperature. Following washing three times with
phosphate-buffered saline (PBS), the cells on slides were fixed by
adding 4% paraformaldehyde and then washed twice with PBS. The
apoptotic cells were detected using a TUNEL kit (R&D Systems,
Inc., Minneapolis, MN, USA) according to the manufacturer's
protocol. PBS was used as a negative control. Cells presenting with
brown granules in the nuclei were classified as apoptotic with an
inverted microscope (X-71; Olympus Corporation, Tokyo, Japan). The
percentage of apoptotic cells and the apoptotic indices were
calculated by randomly counting 500 cells selected from five spots
in the slides.
Detection of caspase-3 and -9
activity
Following transfection, 2×106 BGC823
cells were collected, and active caspase-3 and -9 were examined
using a caspase activity detection kit (GenMed Scientifics,
Wilmington, DE, USA) according to the manufacturer's protocol. The
fluorescence of each sample was detected using a spectrophotometer
with absorption/emission maxima of ~511/533 nm (Bio-Rad
Laboratories, Inc.).
Cell migration assay
In vitro cell invasion was evaluated using
the Boyden Chamber Assay (Cell Biolabs, Inc., San Diego, CA, USA)
as previously reported (22).
Briefly, serum-free RPMI-1640 containing 3.9 µg/µl of Matrigel™ (BD
Biosciences, San Jose, CA, USA) was added to the upper chamber
above the filter membrane, and incubated at 37°C for 2 h.
Subsequently, 200 µl of serum-free cell culture supernatant from
NIH3T3 cells (purchased from the Shanghai Institute of
Pharmaceutical Industry, Shanghai, China) was added into the lower
chamber as the chemoattractant factor. The cells (400 µl)
transfected with different vectors were added to the upper chamber
at a concentration of 1×106 cells/ml and cultured for 24
h at 37°C with 5% CO2. The cells that had migrated into
the Matrigel were detected by hematoxylin and eosin (HE) staining
and those from five randomly selected spots were counted under an
inverted microscope (X-71; Olympus Corporation). Five chambers were
assessed for each cell line transfected by different vectors. At
least three independent experiments were performed.
In vivo mouse models of GC
In vivo tumorigenesis was investigated in
6–8-week-old BALB/c nude mice (Shanghai SLAC Laboratory Animal Co.
Ltd.). The 25 nude mice were divided into five groups (n=5/group)
for xenograft transplantation of non-transfected and transfected
BGC823 cells. The vectors used for transfection were pCL-neo-PTEN,
pRNAT-U6.1-siLivin, pCL-neo-PTEN-siLivin, and control. Briefly,
1×107 (1.0 ml) BGC823 cells were inoculated
subcutaneously. Tumor size was carefully measured from day 7
post-inoculation. The tumors were removed and weighed on day
28.
Statistical analysis
Data were processed and analyzed using SPSS 13.0
(SPSS, Inc., Chicago, IL, USA). Results are shown as the mean ±
standard error of the mean. One-way analysis of variance was used
to detect differences between groups. If a significant difference
was found by analysis of variance, the Fisher LSD test was used to
detect specific differences between the study groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
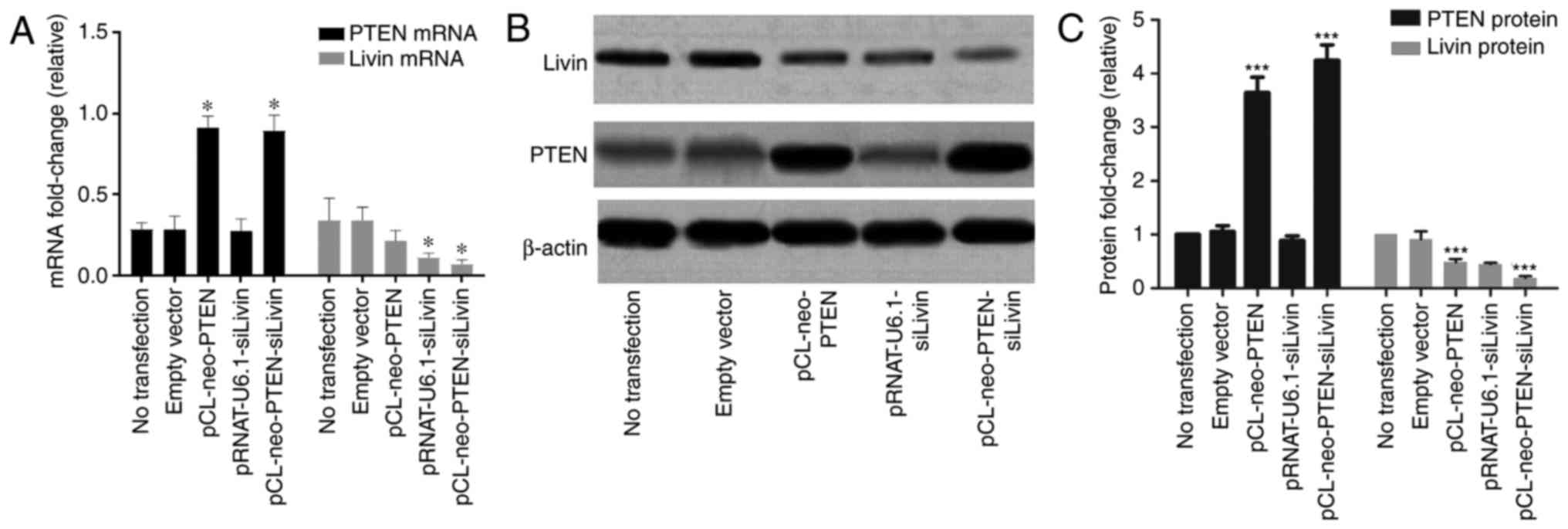
Characterization of the expression of
PTEN and Livin in transfected BGC823 cells
The constructs, which were previously constructed in
the laboratory (pCL-neo-PTEN, pRNAT-U6.1-siLivin and
pCL-neo-PTEN-siLivin) and the empty vector control were transfected
into BGC823 cells. To evaluate the quality of transfection, the
mRNA and protein expression levels were characterized. The results
showed that the cells transfected with either pCL-neo-PTEN or
pCL-neo-PTEN-siLivin vectors exhibited a significant increase in
mRNA and protein expression levels of PTEN, compared with
cells transfected with empty vector alone (Fig. 1A–C). In addition, a significant
decrease in the mRNA and protein expression levels of Livin
were observed in the cells transfected with either the
pRNAT-U6.1-siLivin or pCL-neo-PTEN-siLivin vector (Fig. 1A and C). pCL-neo-PTEN transfection
also resulted in a partial decrease in the mRNA and protein
expression levels of Livin, although the inhibitory effect
was not as pronounced as that observed in the Livin
silencing groups. By contrast, trans-fection with the
pRNAT-U6.1-siLivin vector had minimal effect on the expression of
PTEN. These data suggested that PTEN may be involved in
regulating the gene expression of Livin.
Effect of PTEN and Livin transfection on
tumor cell proliferation and migration
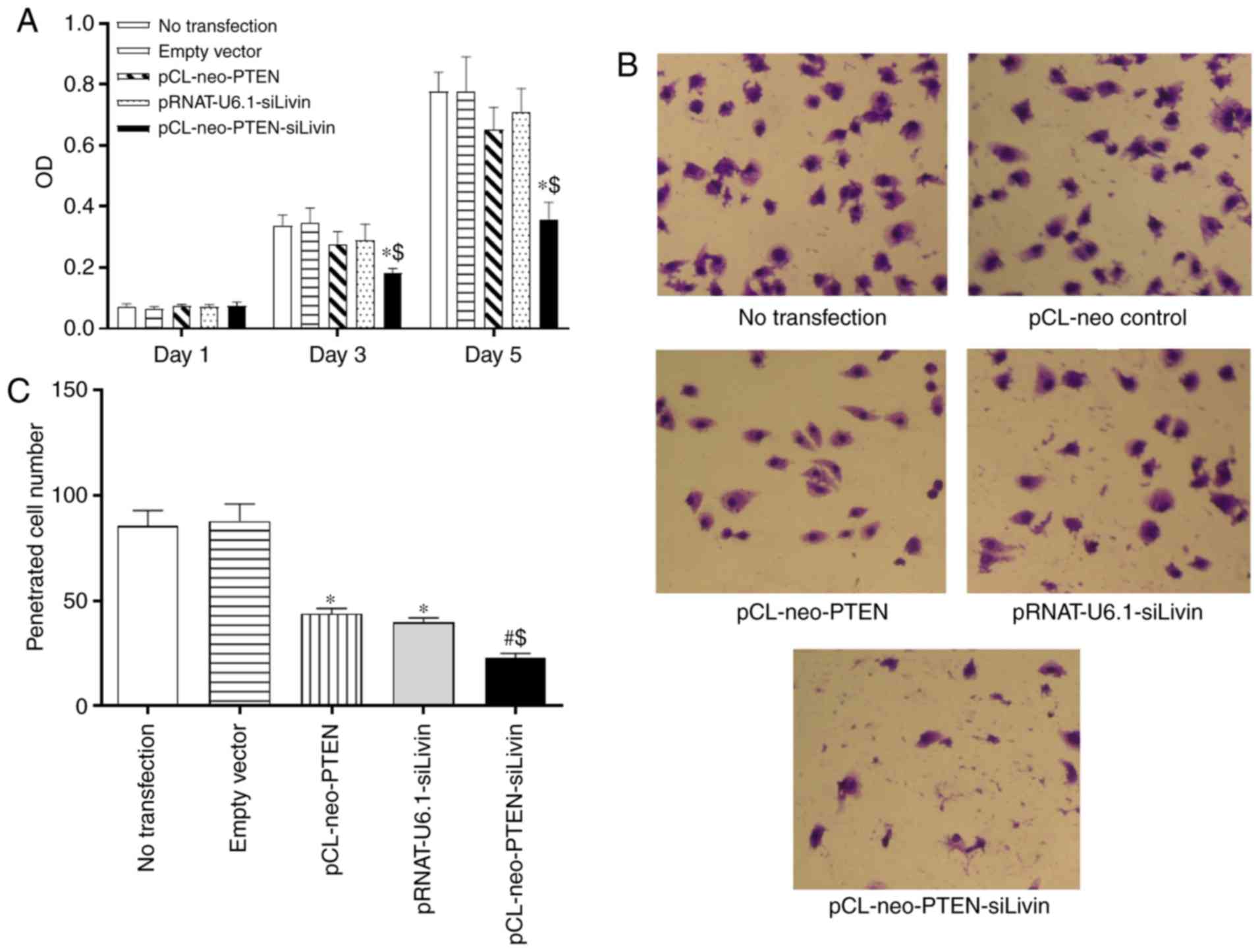
Subsequently, the present study characterized
whether the modulation of PTEN and Livin had any effect on BGC823
cell proliferation. Following transfection of the BGC823 cells with
the various constructs, cell proliferation was monitored on days 1,
3, and 5 using the MTT assay. The data demonstrated that neither
pCL-neo-PTEN nor pRNAT-U6.1-siLivin vector transfection alone
significantly affected cell proliferation at the monitored
time-points. By contrast, pCL-neo-PTEN-siLivin vector transfection,
in which PTEN and Livin were modulated concurrently,
significantly inhibited cell proliferation on days 3 and 5,
compared with either empty vector transfection or PTEN or Livin
monovector transfection (P<0.05, Fig. 2A).
To evaluate whether the modulation of PTEN
and Livin exerted any effect on the metastasis of
transfected cells, an in vitro Matrigel assay was performed.
The migrated cells were detected in the Matrigel by HE staining and
the numbers of cells in five randomly selected spots were counted
under the microscope. Compared with the cells transfected with the
empty vector, the single (pCL-neo-PTEN or pRNAT-U6.1-siLivin
transfection) and dual (pCL-neo-PTEN-siLivin transfection) gene
modulation resulted in a significant inhibition of cell migration
(P<0.05 and P<0.01 for single and dual modulation,
respectively; Fig. 2B and C).
Furthermore, compared with either pCL-neo-PTEN or
pRNAT-U6.1-siLivin single gene transfection, dual gene modulation
resulted in the maximal level of migration inhibition (P<0.05;
Fig. 2B and C). The data
suggested that PTEN and Livin are critical in cell migration, and
concurrent PTEN and Livin gene modulation may result in the most
marked inhibition of tumor cell migration.
Modulation of PTEN and Livin induces
apoptosis by activating the caspase-signaling pathway
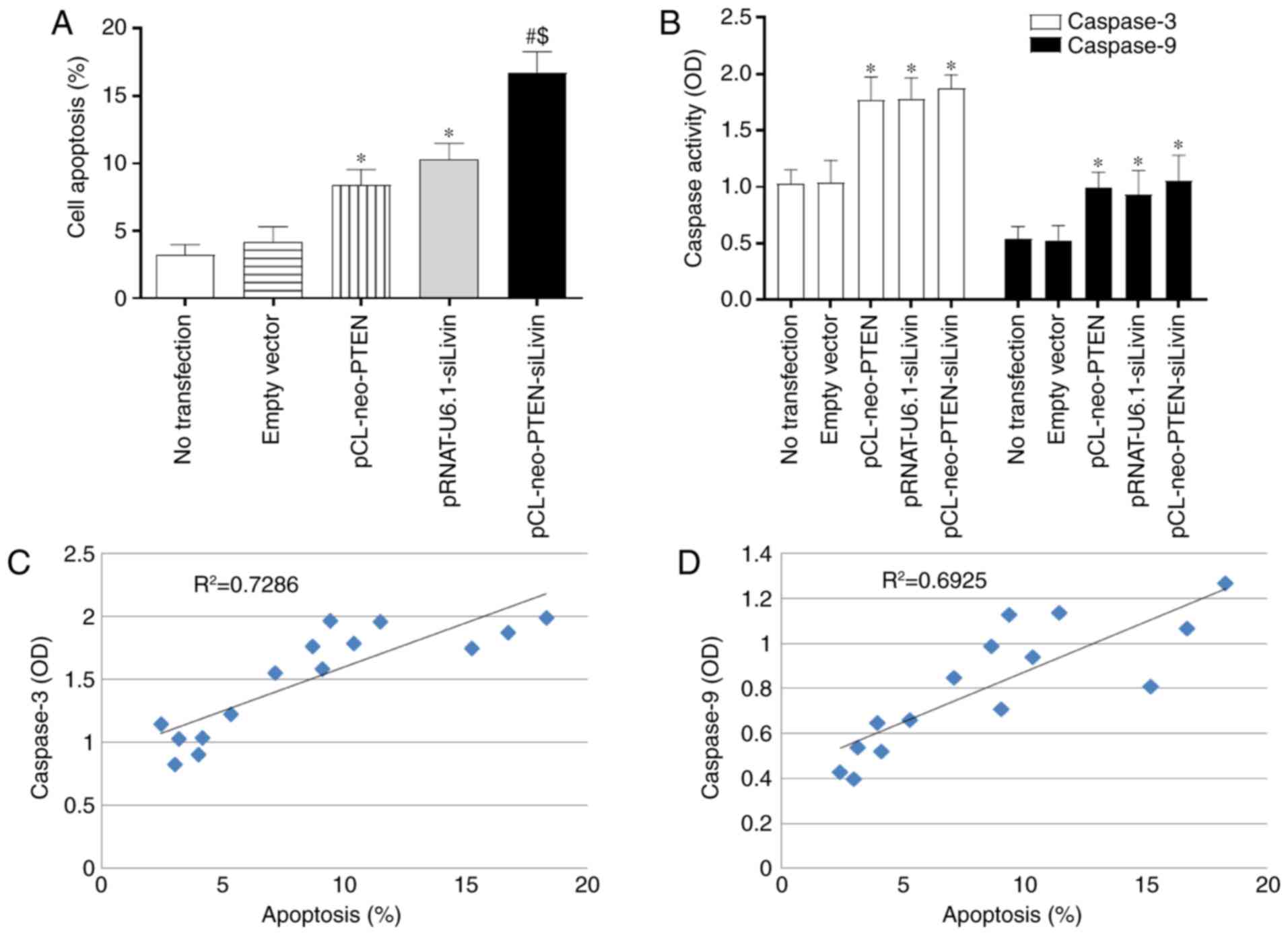
The present study used TUNEL staining to evaluate
whether modulation of the PTEN and Livin genes
affected apoptosis. Single (pCL-neo-PTEN or pRNAT-U6.1-siLivin
transfection) and dual (pCL-neo-PTEN-siLivin transfection) gene
modulation resulted in a significant increase in the percentage of
apoptotic cells, compared with that in the control (Fig. 3A). The percentages of apoptotic
cells observed following pCL-neo-PTEN, pRNAT-U6.1-siLivin, and
pCL-neo-PTEN-siLivin transfections were 8.67±1.27, 10.39±1.31 and
16.72±1.84, respectively. Furthermore, compared with either
pCL-neo-PTEN or pRNAT-U6.1-siLivin single gene transfection, dual
gene modulation resulted in the highest level of cell apoptosis
(P<0.05; Fig. 3A).
Caspase activity is a marker of apoptosis.
Therefore, the present study examined the activities of caspase-3
and caspase-9 in the transfected BGC823 cells. Single (pCL-neo-PTEN
or pRNAT-U6.1-siLivin transfection) and dual (pCL-neo-PTEN-siLivin
transfection) gene modulation induced significant increases in
caspase-3 and caspase-9 activities (P<0.05; Fig. 3B). However, there were no
significant differences between the three transfection groups.
Further examination revealed that the observed apoptosis was
positively correlated with caspase 3 and caspase 9 activity,
(R2=0.7286 and R2=0.6925, respectively;
Fig. 3C and D). These data
suggested that the modulation of PTEN and Livin
induced apoptosis via activation of the caspase signaling
pathway.
Modulation of PTEN and Livin inhibits
tumor growth in vivo
To determine the effect of PTEN and
Livin gene modulation on tumor growth in vivo,
various vector-transfected BGC823 cells were injected into nude
mice. Tumor growth was monitored weekly between days 0 and 28, when
the mice were sacrificed. The tumors grew continuously from day 0
to day 28 in the non-transfected and empty vector transfection
groups (Fig. 4A). By contrast,
tumor growth was suppressed in the single gene (pCL-neo-PTEN or
pRNAT-U6.1-siLivin) and dual gene (pCL-neo-PTEN-siLivin)
transfected groups, with the dual gene modulation group
demonstrating the most marked suppression of tumor growth (Fig. 4A). The percentage tumor growth
inhibition (% TGI) in the pCL-neo-PTEN, pRNAT-U6.1-siLivin and
pCL-neo-PTEN-siLivin transfection groups was 45%, 51 and 72%,
respectively (Fig. 4B).
Characterization of the gene expression
of PTEN and Livin in tumors
To verify whether the observed inhibitory effects on
tumor growth were specific to PTEN or Livin gene
modulation, the expression levels of PTEN and Livin
in the tumor tissues were examined. The mice were sacrificed 28
days following establishment of the xenograft model and the tumors
were collected. Tumor tissues from the mice with cells expressing
pCL-neo-PTEN or pCL-neo-PTEN-siLivin expressed significantly higher
mRNA and protein levels of PTEN, compared with those with
control or empty vector cells (P<0.05; Fig. 5A–C). Tumor tissues from mice with
cells expressing pRNAT-U6.1-siLivin or pCL-neo-PTEN-siLivin
expressed significantly lower mRNA and protein levels of
Livin, compared with those with control or empty vector
cells (P<0.05; Fig. 5A–C).
These data indicated that the transfected cells maintained stable
expression of the target genes in the tumors in vivo. These
results showed that the in vivo experiments on tumor tissues
and in vitro experiments using BGC823 cells produced
comparable results.
Discussion
Several studies have demonstrated an association
between the expression of PTEN and Livin and the malignancies of
renal cell carcinoma (23,24),
breast cancer (25) and
retinocytoma (26). Specifically,
a low expression of PTEN and high expression of Livin were
significantly associated with the clinical stage and lymph node
metastases of patient malignancies. However, no studies have
investigated a direct correlation between PTEN and
Livin gene expression and the malignancy of GC.
In the present study, it was shown that the
overexpression of PTEN or gene silencing of Livin
promoted apoptosis in BGC823 cells and inhibited cell
proliferation. The data demonstrated that the combined effect of
PTEN/Livin dual gene modulation on apoptosis and
proliferation was more marked, compared with that of either single
gene alone. The functional effects of PTEN and Livin
gene regulation on GC apoptosis and proliferation were further
supported by the xenograft experiments in nude mice. The results
suggested that the overexpression of PTEN concomitant with
the gene silencing of Livin may represent a potential
therapeutic strategy for gene therapy in the treatment of GC.
Caspase-3 is a proteinase with a crucial role in the
apoptotic pathway. Caspase-3 can be detected in almost all cell
types, emphasizing its importance in modulating cell survival and
death (23). The increased
expression of PTEN in cultured neonatal rat primary cardiomyocytes
leading to increased caspase-3 activity and apoptosis has been
reported (24), which suggests
that caspase-3 is the major effector of PTEN. PTEN has been shown
to inhibit cell proliferation and apoptotic function by
downregulating the AKT/PKB pathway (25). By contrast, Livin demonstrates
anti-apoptotic activity. Livin contains a unique Baculovirus IAP
repeat (BIR) domain and a Really Interesting New Gene (RING) finger
motif domain (26). The BIR
domain forms a novel zinc-fold, which is the critical motif for the
anti-apoptotic activity of the parent protein, its interaction with
caspase 3, −7 and −9, and its E3 ubiquitin ligase (27). The RING domain is critical in
tumor necrosis factor-α-mediated nuclear factor (NF)-κβ activation,
thereby providing an additional mechanism for the anti-apoptotic
activity of Livin (28). Livin
also promotes the degradation of the inhibitor of apoptosis
antagonist SMAC/DIABLO (29,30). These data suggest that PTEN and
Livin exert opposite effects on the caspase signaling pathway.
Consistent with this, the present study observed significantly
increased caspase-3 and caspase-9 activity in BGC823 cells
overexpressing PTEN, with silenced Livin gene
expression, or with dual modulation. Therefore, the results are
consistent with those previously published and showed that the
PTEN and Livin genes are important in apoptosis by
regulating caspase-3/9 activity. Although dual gene modulation led
to the most marked effect on apoptosis, no significant difference
in caspase activity was identified between the three transfection
groups (Fig. 3B). This suggests
that additional mechanisms, including Fas/Fasl signaling and
cytochrome c release, may trigger cell death. Further
investigations are warranted to address which signaling cascades
are involved and the underlying mechanisms through which they are
contributing to apoptosis in BGC823 cells.
PTEN is important in the regulation of tumor cell
metastasis. Hwang et al showed that PTEN enhanced tumor
metastasis through vascular endothelial factor and matrix
metalloproteinases (31). In
other studies, the overexpression of PTEN inhibited glioblastoma
cell migration (32), and
PTEN-knockdown enhanced cell migration in fibroblasts by regulating
FAK, a cytoplasmic phosphoprotein activated by integrin (33). Livin was shown to regulate tumor
cell invasion, the first step of metastasis, through the NF-κβ
signaling pathway (34,35). Furthermore, knockdown of the
Livin gene inhibited tumor invasion by inhibiting the
mitogen-activated protein kinase (MAPK) signaling (17,36). In the present study, the migration
of BGC823 cells was significantly inhibited following transfection
with a vector overexpressing PTEN, silencing Livin,
or modulating the two genes, with dual transfection having the
highest inhibitory effect. Further experiments are required to
characterize whether molecules, including FAK, NF-κβ and MAPK, are
involved in metastasis in GC; however, they are presently beyond
the scope of the present study.
The results of the present study are consistent with
those of previous reports showing that either the overexpression of
PTEN or silencing of the Livin gene significantly
inhibited cell proliferation and invasion, and induced apoptosis in
GC. The present study successfully established in vitro and
in vivo models with the simultaneous overexpression of
PTEN and silencing of Livin for the first time, to
the best of our knowledge. The results demonstrated that dual gene
modulation produced more marked antitumor and antimetastatic
effects, compared with either single gene modulation alone, in
vitro and in vivo. Therefore, the simultaneous
overexpression of PTEN and silencing of Livin may
represent a novel therapeutic approach for the treatment of GC.
Acknowledgments
This study was supported by the Major Public
Interest Foundation of Henan Province, China (grant no.
HNZB2010N91).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Guggenheim DE and Shah MA: Gastric cancer
epidemiology and risk factors. J Surg Oncol. 107:230–236. 2013.
View Article : Google Scholar
|
|
2
|
Yeoh KG: How do we improve outcomes for
gastric cancer? J Gastroenterol Hepatol. 22:970–972. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tan IB, Ng I, Tai WM and Tan P:
Understanding the genetic basis of gastric cancer: Recent advances.
Expert Rev Gastroenterol Hepatol. 6:335–341. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagini S: Carcinoma of the stomach: A
review of epidemiology, pathogenesis, molecular genetics and
chemoprevention. World J Gastrointest Oncol. 4:156–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stambolic V, Suzuki A, de la Pompa JL,
Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM,
Siderovski DP and Mak TW: Negative regulation of PKB/Akt-dependent
cell survival by the tumor suppressor PTEN. Cell. 95:29–39. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
She QB, Solit DB, Ye Q, O'Reilly KE, Lobo
J and Rosen N: The BAD protein integrates survival signaling by
EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor
cells. Cancer Cell. 8:287–297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang LL, Liu J, Lei S, Zhang J, Zhou W
and Yu HG: PTEN inhibits the invasion and metastasis of gastric
cancer via downregulation of FAK expression. Cell Signal.
26:1011–1020. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Katoh M: WNT/PCP signaling pathway and
human cancer (review). Oncol Rep. 14:1583–1588. 2005.PubMed/NCBI
|
|
9
|
Chen Z, Trotman LC, Shaffer D, Lin HK,
Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, et al:
Crucial role of p53-dependent cellular senescence in suppression of
Pten-deficient tumorigenesis. Nature. 436:725–730. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu WT, Yang Z and Lu NH: Roles of PTEN
(Phosphatase and Tensin Homolog) in gastric cancer development and
progression. Asian Pac J Cancer Prev. 15:17–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koike H, Nozawa M, De Velasco MA, Kura Y,
Ando N, Fukushima E, Yamamoto Y, Hatanaka Y, Yoshikawa K, Nishio K
and Uemura H: Conditional PTEN-deficient mice as a prostate cancer
chemoprevention model. Asian Pac J Cancer Prev. 16:1827–1831. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kechagioglou P, Papi RM, Provatopoulou X,
Kalogera E, Papadimitriou E, Grigoropoulos P, Nonni A, Zografos G,
Kyriakidis DA and Gounaris A: Tumor suppressor PTEN in breast
cancer: Heterozygosity, mutations and protein expression.
Anticancer Res. 34:1387–1400. 2014.PubMed/NCBI
|
|
13
|
Nakanishi A, Kitagishi Y, Ogura Y and
Matsuda S: The tumor suppressor PTEN interacts with p53 in
hereditary cancer (Review). Int J Oncol. 44:1813–1819. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim DK, Alvarado CS, Abramowsky CR, Gu L,
Zhou M, Soe MM, Sullivan K, George B, Schemankewitz E and Findley
HW: Expression of inhibitor-of-apoptosis protein (IAP) livin by
neuroblastoma cells: Correlation with prognostic factors and
outcome. Pediatr Dev Pathol. 8:621–629. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kleinberg L, Lie AK, Florenes VA, Nesland
JM and Davidson B: Expression of inhibitor-of-apoptosis protein
family members in malignant mesothelioma. Hum Pathol. 38:986–994.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li CJ, Cong Y, Liu XZ, Zhou X, Shi X, Wu
SJ, Zhou GX and Lu M: Research progress on the livin gene and
osteosarcomas. Asian Pac J Cancer Prev. 15:8577–8579. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ou JM, Ye B, Qiu MK, Dai YX, Dong Q, Shen
J, Dong P, Wang XF, Liu YB, Quan ZW and Fei ZW: Knockdown of Livin
inhibits growth and invasion of gastric cancer cells through
blokkade of the MAPK pathway in vitro and in vivo. Int J Oncol.
44:276–284. 2014. View Article : Google Scholar
|
|
18
|
Chung CY, Park YL, Kim N, Park HC, Park
HB, Myung DS, Kim JS, Cho SB, Lee WS and Joo YE: Expression and
prognostic significance of Livin in gastric cancer. Oncol Rep.
30:2520–2528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Crnković-Mertens I, Wagener N, Semzow J,
Gröne EF, Haferkamp A, Hohenfellner M, Butz K and Hoppe-Seyler F:
Targeted inhibition of Livin resensitizes renal cancer cells
towards apoptosis. Cell Mol Life Sci. 64:1137–1144. 2007.
View Article : Google Scholar
|
|
20
|
Zhao CL, Wang JX, Zhang XF, Zhao GQ and
Wang ZJ: Construction and identification of gene vector expressing
PTEN while simultaneously silencing Livin. Zhonghua Yi Xue Za Zhi.
90:2428–2432. 2010.In Chinese. PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Hwang TL, Changchien TT, Wang CC and Wu
CM: Claudin-4 expression in gastric cancer cells enhances the
invasion and is associated with the increased level of matrix
metalloproteinase-2 and -9 expression. Oncol Lett. 8:1367–1371.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krajewska M, Wang HG, Krajewski S, Zapata
JM, Shabaik A, Gascoyne R and Reed JC: Immunohistochemical analysis
of in vivo patterns of expression of CPP32 (Caspase-3), a cell
death protease. Cancer Res. 57:1605–1613. 1997.PubMed/NCBI
|
|
24
|
Schwartzbauer G and Robbins J: The tumor
suppressor gene PTEN can regulate cardiac hypertrophy and survival.
J Biol Chem. 276:35786–35793. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun CH, Chang YH and Pan CC: Activation of
the PI3K/Akt/mTOR pathway correlates with tumour progression and
reduced survival in patients with urothelial carcinoma of the
urinary bladder. Histopathology. 58:1054–1063. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kasof GM and Gomes BC: Livin, a novel
inhibitor of apoptosis protein family member. J Biol Chem.
276:3238–3246. 2001. View Article : Google Scholar
|
|
27
|
Hinds MG, Norton RS, Vaux DL and Day CL:
Solution structure of a baculoviral inhibitor of apoptosis (IAP)
repeat. Nat Struct Biol. 6:648–651. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chu ZL, McKinsey TA, Liu L, Gentry JJ,
Malim MH and Ballard DW: Suppression of tumor necrosis
factor-induced cell death by inhibitor of apoptosis c-IAP2 is under
NF-kappaB control. Proc Natl Acad Sci USA. 94:10057–10062. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang H and Schimmer AD: Livin/melanoma
inhibitor of apoptosis protein as a potential therapeutic target
for the treatment of malignancy. Mol Cancer Ther. 6:24–30. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma L, Huang Y, Song Z, Feng S, Tian X, Du
W, Qiu X, Heese K and Wu M: Livin promotes Smac/DIABLO degradation
by ubiquitin-proteasome pathway. Cell Death Differ. 13:2079–2088.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hwang PH, Yi HK, Kim DS, Nam SY, Kim JS
and Lee DY: Suppression of tumorigenicity and metastasis in B16F10
cells by PTEN/MMAC1/TEP1 gene. Cancer Lett. 172:83–91. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Leslie NR: PTEN: An intercellular
peacekeeper? Sci Signal. 5:pe502012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maehama T, Taylor GS and Dixon JE: PTEN
and myotubularin: novel phosphoinositide phosphatases. Annu Rev
Biochem. 70:247–279. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen F, Yang D, Che X, Wang J, Li X, Zhang
Z, Chen X and Song X: Livin mediates tumor cell invasion in the
DU-145 cell line via NF-κB. Oncol Rep. 27:2010–2016.
2012.PubMed/NCBI
|
|
35
|
Chen F, Yang D, Wang S, Che X, Wang J, Li
X, Zhang Z, Chen X and Song X: Livin regulates prostate cancer cell
invasion by impacting the NF-κB signaling pathway and the
expression of FN and CXCR4. IUBMB Life. 64:274–283. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yoon TM, Kim SA, Lee DH, Lee JK, Park YL,
Lee KH, Chung IJ, Joo YE and Lim SC: Expression of Livin and the
inhibition of tumor progression by Livin silencing in
laryngohypopharyngeal cancer. In Vivo. 28:751–759. 2014.PubMed/NCBI
|