Introduction
Neurodegeneration consists of various cellular
processes whereby neuronal cells progressively deteriorate, lose
function and ultimately die. In 2014, Alzheimer's disease (AD) and
Parkinson's disease (PD), were the seventh and 14th leading causes
of mortality, respectively, in the US (1). Therefore, more research is required
to understand the intricate molecular components of these diseases
and their risk factors, including neuroinflammation. This may
enable the development of novel therapeutic strategies to treat
neurodegenerative diseases. Unlike associated genetic risk factors,
including polyglutamine (2) and
glucocerebrocidase (3),
neuroinflammation is a major risk factor of neurodegeneration as it
progressively develops throughout life (4). Coupled with increasing age, a
build-up of reactive oxygen species (ROS) levels (5) and protein misfolding (6), neuroinflammation may act as one of
the various triggers of neurodegeneration. Previous studies have
suggested that excessive neuroinflammation worsens the symptoms of
AD and PD by promoting the excessive generation of amyloid β
plaques and destruction of dopaminergic neurons (4). Although the nervous system is known
to be an immunologically privileged site, it still hosts astrocytes
and glial cells that express major histocompatibility complexes,
which propagate neuroinflammation (7). Despite the ability of immune cells
associated with the nervous system, such as microglia, to subdue
imminent infections and damages, this process typically causes
excessive neuroinflammation that harms the surrounding tissue
(7).
To produce a model of neuroinflammation, the present
study utilized lipopolysaccharide (LPS), which triggers the
activation of toll-like receptor 4 that is ubiquitously present on
the surface of microglia, subsequently triggering neuroinflammation
(8). LPS also elicits prolonged
responses in vivo by upregulating the expression of the
following pro-inflammatory components: Tumor necrosis factor α;
cyclooxygenase 2 (COX)-2, which is coded by the gene
prostaglandin-endoperoxide synthase 2 (PTGS)-2; inducible nitric
oxide synthase (iNOS), which is coded by the gene nitric oxide
synthase 2 (NOS2); and nuclear factor (NF)-κB, which is coded by
the NF-κB subunit 1 gene (9).
These pro-inflammatory components form a negative feedback system
with superoxide dismutase (SOD) and heme oxygenase 1 (HO-1)
(10) to produce
anti-neuroinflammatory components, including interleukin (IL)-10
(11). However, in the majority
of biological systems, pro-neuroinflammatory signaling pathways
typically outweigh anti-neuroinflammatory signaling pathways
(10).
Various compounds that trigger
anti-neuroinflammatory signaling pathways may therefore help to
treat neuroinflammation. Out of the several natural compounds that
have been screened, madecassoside, a triterpenoid saponin
extractable from Centella asiatica, known locally as Pegaga
(12), may be considered as a
potential method of treating excessive neuroinflammation. Various
studies have suggested that this compound may exhibit
anti-inflammatory, antioxidant and anti-cancer effects (12,13). Furthermore, madecassoside induces
the modulation of inflammatory cytokines, translocation of NF-κB
and regulation of COX-2 and iNOS (14,15). This compound, which is commonly
used in cosmetology, has also been assessed in neuronal settings
(10,16). Our preliminary study profiled the
production of pro- and anti-neuroinflammatory cytokines in
LPS-stimulated BV2 microglia (data not published), however, the
molecular activity of madecassoside has yet to be fully elucidated.
Subsequently, the objective of the present study was to analyze the
effects of madecassoside on ROS generation and to determine the
molecular effects of madecassoside at the genomic and proteomic
levels.
Materials and methods
Preparation of madecassoside and
indomethacin stock solution
Madecassoside powder (≥95% purity) was purchased
from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). A stock
solution of 50 μg/μl was prepared in molecular grade
dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA). To obtain the
maximum non-toxic dose (MNTD) of 9.50 μg/ml and ½ MNTD of
4.75 μg/ml, as determined by Mohan (17), the stock solution was diluted with
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The positive control
indomethacin (Sigma-Aldrich; Merck KGaA), a type of non-steroidal
anti-inflammatory drug known to elicit anti-inflammatory effects
(18), was dissolved in DMSO to a
concentration of 10 mM, which was further diluted to 25 μM
working solution with fresh DMEM.
Cell culture
BV2 microglia cells were provided by Dr Sharmili
Vidyadaran from Universiti Putra Malaysia (Selangor, Malaysia) and
subsequently cultured in DMEM supplemented with 10% fetal bovine
serum, 1% penicillin and streptomycin, 0.1% fungizone and 0.1%
gentamycin (all Gibco; Thermo Fisher Scientific, Inc.). Cells were
maintained in a humidified incubator containing 5% CO2
at 37°C. To determine the intracellular ROS levels, cells were
seeded at a desired concentration of 5×105 cells/well in
24-well tissue culture plates (Corning Incorporated, Corning, NY,
USA) and the trypan blue exclusion method (19) was then performed using 0.4% trypan
blue staining (Gibco; Thermo Fisher Scientific, Inc.) to calculate
the number of trypsinized cells, while for RNA and protein
extraction, 1×106 cells/well were seeded in 60-mm tissue
culture dishes (Corning Incorporated).
Treatment with madecassoside and LPS
stimulation
Culture medium was aspirated from the culture vessel
and BV2 cells were treated with madecassoside at the MNTD (9.50
μg/ml) and ½ MNTD (4.75 μg/ml), or they were treated
with indomethacin (25 μM). Untreated cells were used as the
control. Cells were subsequently incubated in a humidified
incubator containing 5% CO2 at 37°C for 3 h and were
thoroughly mixed at 30 min intervals. Following 3 h incubation, 0.1
μg/ml LPS (Sigma-Aldrich; Merck KGaA) (20,21) was introduced into treatment groups
as indicated (Table I). Cells
were then further incubated for 24 h within a humidified incubator
containing 5% CO2 at 37°C.
 | Table ITreatment groups used in the present
study. |
Table I
Treatment groups used in the present
study.
| Treatment
groups | Components |
|---|
| Control | Untreated BV2
cells |
| LPS | LPS (0.1
μg/ml) |
| MNTD + LPS | MNTD (9.5
μg/ml) + LPS (0.1 μg/ml) |
| ½ MNTD + LPS | ½ MNTD (4.75
μg/ml) + LPS (0.1 μg/ml) |
| Indo + LPS | Indomethacin (25
μM) + LPS (0.1 μg/ml) |
Determination of intracellular ROS
levels
DMEM was removed, cells were subjected to
trypsinization and the pellet was resuspended in phosphate-buffered
saline (PBS). Cell suspension (cell density, 1×105/well)
and non-sterile PBS were transferred into 96-well plates (Corning
Incorporated). Subsequently, 40 μM 2′,7′-dichlorofluorescin
diacetate (Sigma-Aldrich; Merck KGaA) was added. Following 30 min
incubation at 37°C, fluorescence was measured using SpectraMax M
Series Multi-Mode Microplate Reader (Molecular Devices, LLC,
Sunnyvale, CA, USA) at excitation and emissions wavelengths of 485
and 538 nm, respectively. The number of cells in each treatment
group was determined using the trypan blue exclusion method and
values were used to calculate the relative fluorescence unit of
each treatment group.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
RNA extraction and purification of BV2 cells was
performed using a PureLink RNA Mini kit (Ambion; Thermo Fisher
Scientific, Inc.) following the manufacturer's protocol. Lysis
buffer solution was prepared by adding 50 μl mercaptoethanol
to 5 ml lysis buffer from the kit. Subsequently, cell dislodgment
was performed using a cell scraper (Techno Plastic Products AG,
Trasadingen, Switzerland). Scraped off cells were transferred into
a sterile 1.5-ml microcentrifuge tube on ice. The contents of the
microcentrifuge tubes were passed through an 18-gauge syringe
needle 5-10 times. Subsequently, the lysate was subjected to the
PureLink RNA Mini kit protocol and extracted RNA was stored in
volumes of 35 μl at −30°C. To analyse RNA purity, a Tecan
Infinite 200 PRO microplate reader was utilized at 260/280 nm on a
NanoQuant plate (both Tecan Group Ltd., Mannedorf,
Switzerland).
RNA samples were normalized to 2 mg/ml and converted
to cDNA using the qPCRBIO cDNA Synthesis kit (PCR Biosystems Ltd.,
London, UK) following the manufacturer's protocol and an MJ
Research PTC-100 Thermal Cycler (GMI, Ramsey, MN, USA). qPCR was
performed in a 15-μl reaction mixture containing 2X qPCRBIO
SyGreen Mix (qPCRBIO SyGreen Mix with Fluorescein kit; PCR
Biosystems Ltd.) and primers (First Base Laboratories Sdn Bhd,
Selangor, Malaysia), the sequences of which are listed in Table II. qPCR results were analyzed
using iQ5 Optical System Software version 2.0 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) for 1 initial denaturation
cycle at 95°C for 2 min, followed by 40 cycles of denaturation 95°C
for 5 sec, annealing at 60°C for 30 sec and final elongation at
72°C for 30 sec. Relative gene expression values were normalized to
β-actin and fold changes of each gene were calculated using the
2−ΔΔCq method (22),
whereby the baseline of PCR efficiencies were set at 90%.
 | Table IIForward and reverse sequences of the
primers utilized for reverse transcription-quantitative polymerase
chain reaction. |
Table II
Forward and reverse sequences of the
primers utilized for reverse transcription-quantitative polymerase
chain reaction.
| Gene | Forward sequence
(5′-3′) | Reverse sequence
(5′-3′) | Amplicon size
(bps) |
|---|
| iNOS |
TTGCCACGGACGAGACGGATAGG |
GGGCACATGCAAGGAAGGGAACTC | 131 |
| COX-2 |
TGGGTGTGAAAGGAAATAAGGA |
GAAGTGCTGGGCAAAGAATG | 128 |
| HO-1 |
AGAGTTTCCGCCTCCAACCA |
CGGGACTGGGCTAGTTCAGG | 107 |
| STAT1 C |
TGAATATTTCCCTCCTGGG |
TCCCGTACAGATGTCCATGAT | 103 |
| NF-κB |
CTGGTGGACACATACAGGAAGAC |
ATAGGCACTGTCTTCTTTCACCTC | 198 |
| β-actin |
TCCTCCTGAGCGCAAGTACTCT |
GCTCAGTAACAGTCCGCCTA | 153 |
Western blotting
Proteins were extracted using
radioimmunoprecipiation lysis buffer consisting of 23.145 mg
dithioethreitol (Bio-Rad Laboratories, Inc.), 30 μl
proteinase inhibitor and 1.5 ml lysis buffer (Ambion; Thermo Fisher
Scientific, Inc.) with the aid of a cell scraper (Techno Plastic
Products AG, Switzerland). Lysates were centrifuged at 20,000 × g
for 20 min in 4°C. Protein concentration was determined using the
Bradford assay standard curve of bovine serum albumin (BSA; Nacalai
Tesque, Inc., Kyoto, Japan) analyzed using a 96-well plate (Corning
Incorporated) administered with Bradford Reagent (Bio-Rad
Laboratories, Inc.).
Protein samples at 100 mg/ml were loaded and
separated using 12% SDS-PAGE and a PowerPac Basic Machine (Bio-Rad
Laboratories, Inc.). Samples were transferred to 0.45-μm
poly-vinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA) and membranes were blocked with 5% BSA in Tris-buffered saline
with Tween-20 (TBS-T) at room temperature for 1 h. Membranes were
subsequently washed three times with TBS-T, incubated overnight
with primary antibodies against iNOS (cat. no., sc-8310), COX-2
(cat. no., sc-23984), HO-1 (cat no., sc-10789), signal transducer
and activator of transcription 1 (STAT1; cat. no., sc-271661),
NF-κB (cat. no., sc-372) and β-actin (cat., no. sc-130656; all
1:1,000 dilution; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
at 4°C. Following this, membranes were washed three times with
TBS-T and incubated with secondary antibodies (1:5,000 dilution;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) against rabbit
(cat. no., sc-2004), goat (cat. no., sc-2020) and mouse (cat. no.,
sc-358914) primary antibodies conjugated with horseradish
peroxidase at room temperature for 1 h. Proteins were visualized
using SuperSignal West Femto Chemiluminescent Substrate (Thermo
Fisher Scientific, Inc.) on a ChemiDoc XRS+ imaging system (Bio-Rad
Laboratories, Inc.).
Statistical analysis
Data were generated in triplicate from three
independent repeats and presented as the mean ± standard deviation.
SPSS 11.0 software (SPSS, Inc., Chicago, IL, USA) was used to
assess differences between groups using one way analysis of
variance followed by Tukey's multiple comparison test. P<0.05
was considered to indicate a statistically significant
difference.
Results and discussion
Determination of intracellular ROS
levels
ROS production is associated with inflammation,
including neuroinflammation (23). The production of various ROS,
including superoxide anions and its derivatives, such as hydroxyl
radicals, is required in normal physiological conditions, including
during the modulation of synaptic and non-synaptic communication,
and conditions whereby antimicrobial defence is required (23). Notably, exogenous ROS attained
from pollutants or xenobiotics (24), coupled with excess endogenous ROS
production, which occurs primarily via NADPH oxidase (25), may cause excessive
neuroinflammation.
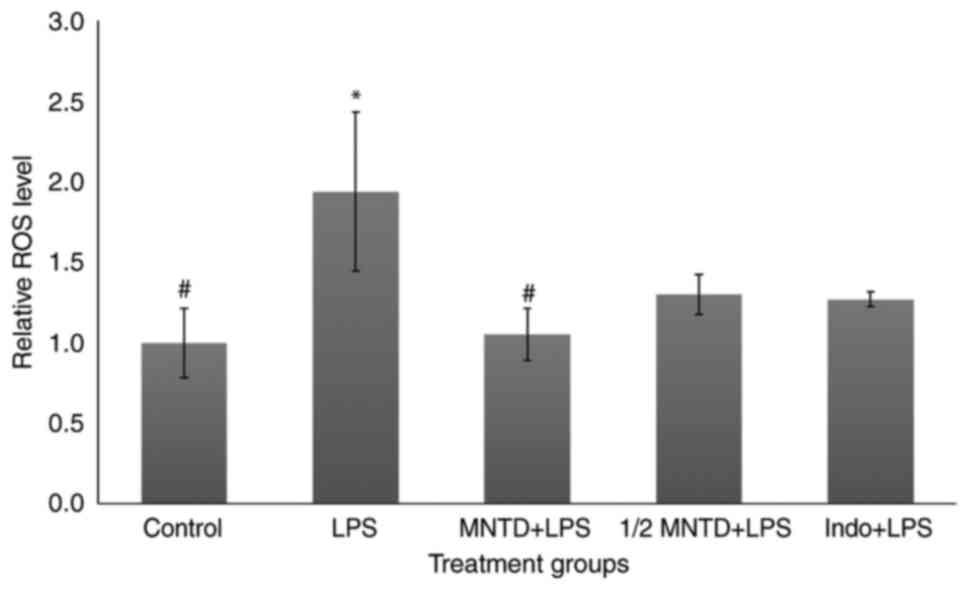
In the present study, madecassoside significantly
reduced ROS levels in BV2 cells by 56.84% in the MNTD + LPS group
compared with the group stimulated with LPS alone (P<0.05;
Fig. 1). These results were in
accordance with the results of a previous study (26). The MNTD + LPS treatment group also
exhibited superior ROS reduction compared with the Indo + LPS
group, which only caused a 34.62% reduction in ROS levels compared
with LPS-treated cells. It has been demonstrated that
trypsinization at high concentrations (U/ml) within cell culture
produces a certain level of ROS (27); therefore, for more accurate
measurement, the results from the treatment groups were all
normalized to those of the control group, which consisted of
untreated BV2 cells.
Nurlaily et al (14) indicated that madecassoside has
anti-oxidative effects. Notably, madecassoside has been studied
within the neuronal and other bodily systems, and similar trends
have been indicated regarding the reduction of ROS levels. Al Mamun
et al (26) utilized
madecassoside to observe its neuroprotective effects against
amyloid β plaques within in vivo and in vitro models
of AD. It has been demonstrated that the plaques propagate excess
oxidative stress in the form of ROS and reactive nitrogen species
(RNS) (2), which may react with
each other to form the toxic product peroxynitrite (10,28). Following exposure to madecassoside
there was a significant reduction in lipid peroxidation (LPO),
which is indicative of oxidative stress, and ROS levels, in
vivo and in vitro, amongst other pro-neuroinflammatory
components (26,28). This suggests that madecassoside
may mitigate the worsening symptoms of neurodegenerative diseases,
including AD. In addition, Li et al (15) indicated that madecassoside may
regulate ROS levels by generating SOD and increasing catalase
activity to inhibit the production of destructive free radicals and
superoxide anions.
In the present study, although this difference was
not significant, the reduction of ROS in the ½ MNTD + LPS group was
32.96% compared with the LPS-treated group; similar to what was
observed in the Indo + LPS group (Fig. 1). Despite the well-known
anti-inflammatory properties of NSAIDs, including ibuprofen and
indomethacin, the results of certain studies have suggested that
NSAIDs may cause more harm than good following chronic use and may
result in nephrotoxicity (29) or
gastric ulcers (30). This
suggests that the identification of alternative compounds capable
of effectively reducing ROS levels produced by neuroinflammation
that also induce minimal side effects may be beneficial.
Determination of pro-inflammatory iNOS
gene expression
The expression of the inducible form of NOS is
heavily upregulated following inflammation (10). As a widely-acknowledged downstream
process of transcription factors, including NF-κB and STAT1
(10,31), the expression of iNOS is commonly
upregulated in response to various stimuli, including infection,
ischemia or mechanical injury (32). During NO production, the iNOS
enzyme will behave as a RNS and may react with ROS to form harmful
intermediates (7,29). NO also causes endothelial
vasculature damage by upregulating interferon regulatory
transcription factor 1, which mediates cellular apoptosis and
pro-neuroinflammation (33).
Notably, Olivera et al (34) indicated that iNOS-deficient mice
had a higher degree of Trypanosoma infection; this suggests that,
despite having a negative effect with respect to inflammation, the
balanced presence of iNOS is required to maintain the integrity of
the blood brain barrier and blood vessels.
Within the present study, the expression of iNOS was
significantly downregulated at the MNTD and ½ MNTD of madecassoside
(79.81 and 74.21%, respectively) compared with the LPS group (both
P<0.05; Fig. 2A). These
results suggest that madecassoside may modulate
anti-neuroinflammatory signalling pathways and their subsequent
downstream activities, as iNOS expression was significantly reduced
compared the control group (P<0.05). The results of the present
study are in accordance with those of previous studies that
identified the anti-inflammatory effects of other triterpenoid
saponins, including tormentic acid (35) and madecassic acid (36). Madecassoside alone is also able to
downregulate iNOS amongst various other pro-inflammatory components
in a nephrotoxic in vivo setting (13). The results of the present study
demonstrated the potent effect of madecassoside in reducing NO
expression by downregulating iNOS expression in a neuronal
setting.
Determination of COX-2 expression
The PTGS2 gene codes for the COX-2 enzyme, which is
involved in the production of prostanoids and has been identified
at high quantities during inflammation, resulting in subsequent
tissue damage (37). The
conversion of prostaglandin H2 to the active PGE2 yields
pro-inflammatory ROS by-products (38). As indicated in Fig. 2B, significant downregulation of
COX-2 expression following treatment with madecassoside and in the
Indo + LPS group was observed compared with the LPS group
(P<0.05). The MNTD and ½ MNTD groups exhibited a reduction of
70.85 and 66.65% in COX-2 expression, respectively, which was
similar to the results obtained regarding iNOS expression. These
results indicated that the downregulation of COX-2 and iNOS
expression was not particularly dose-dependent. Although various
studies have not clarified whether changes in the expression of
pro-inflammatory factors including iNOS and COX-2 are
dose-dependent or -independent, a number of studies using RAW 264.7
macrophages yielded downregulatory trends, similar to the results
of the present study. Furthermore, the results of previous studies
indicated that, although iNOS is a signaling pathway activated by
NF-κB and STAT1, COX-2 expression is downstream of NF-κB but is
inhibited by the activation of the STAT1 pro-inflammatory component
(39,40). Notably, in the present study,
madecassoside treatment significantly downregulated NF-κB and STAT1
gene expression, compared with the LPS group (P<0.05; Fig. 2B and C).
Determination of pro-inflammatory STAT1
expression
STAT1 upregulation has been indicated to promote
interferon-γ expression during inflammation and STAT1 induces
inflammation-induced apoptosis by activating apoptosis-stimulating
of p53 protein 2 (ASPP2) (41).
Under normal physiological conditions, ASPP2 monitors and keeps
neuroinflammation in check; however, following the upregulation of
STAT1, ASPP2 mediates cell death (41). Other functions of STAT1 in
mediating tissue damage during inflammation include modulating the
expression of receptor-interacting serine/threonine-protein kinase
3, which stimulates macrophages during inflammation to increase
cell death (42). In the present
study, a significant reduction of STAT1 gene expression within
LPS-stimulated BV2 cells was observed in MNTD and ½ MNTD treated
groups, but only the latter group exhibited downregulated STAT1
expression to the same extent as in the Indo + LPS group
(P<0.05; Fig. 2C). These
results suggest that the utilization of madecassoside may
potentially mitigate the 'cytokine storm' by downregulating the
JAK/STAT signaling pathway. Triterpenes, including avicin D
(43) and betulinic acid
(44) are also able to
downregulate the JAK/STAT1 signaling pathway, which is in
accordance with the results of the present study.
Determination of pro-inflammatory NF-κB
expression
Considered to be the 'master transcription factor',
particularly with regards to inflammation, it has been demonstrated
that NF-κB is upregulated during inflammation and specifically
following stimulation with LPS (45). Following its localization to the
nucleus, NF-κB activates downstream pro-neuroinflammatory
components, including iNOS, COX-2 and NADPH oxidase, which elevates
ROS levels (10) and the mediator
of arachidonic acid, lipoxygenase (46). As well as its pro-inflammatory
properties, NF-κB activation also regulates the anti-inflammatory
components during inflammation, including HO-1 expression, which is
coded by HMOX1 and thioredoxin-1 (TRX1) (10). However, during neuroinflammation,
there is an imbalance between the pro- and anti-inflammatory
components, which may cause excessive neuroinflammation (10).
As indicated in Fig.
2D, NF-κB expression was significantly downregulated in the
MNTD and ½ MNTD-treated groups compared with the LPS group (84.78
and 89.73%, respectively; P<0.05). Notably, this effect was
greater in MNTD and ½ MNTD-treated groups compared with the Indo +
LPS group. NF-κB expression did not decrease significantly in the
Indo + LPS group compared with the LPS group. This may be due to
the fact that NSAIDs such as indomethacin commonly block the
downstream processes involved in inflammation, including COX-2 and
its subsequent prostanoid production, but may not affect NF-κB
expression (47). Previous
studies investigating indomethacin have unveiled that its mechanism
differs from aspirin, as it was demonstrated that
indomethacin-induced COX-2 inhibition did not downregulate upstream
NF-κB processes (48,49). The effects of madecassoside on
NF-κB expression were previously reported by Su et al
(13) and Patil et al
(50). These results suggest that
the downregulation of NF-κB may be caused by the inhibitory
properties of madecassoside on essential complexes within the
cascade, including the various subunits of inhibitory κB
kinase.
Determination of anti-neuroinflammatory
HO-1 gene expression
The gene HMOX1 codes for the antioxidative enzyme
HO-1, a key enzyme associated with various antioxidative signaling
pathways (51). NF-κB activates
many downstream transcription factors (10); a few of which being HO-1 and few
other antioxidative enzymes, including SOD and TRX1. In the context
of neuroinflammation during multiple sclerosis, the results of a
previous study provided an interesting outlook as to how the HMOX1
gene behaves and its effects on excessive neuroinflammation
(52). Activation of HO-1, which
is associated with upstream antioxidative signalling pathways,
including nuclear factor like 2 (Nrf2), produces components
including biliverdin, carbon monoxide, iron and IL-10, all of which
are antioxidants and function to prevent inflammation at varying
stages. These antioxidative elements are potent enough to protect
cells against apoptosis during oxidative stress (53).
As indicated in Fig.
2E, HO-1 gene expression in the MNTD + LPS group were
significantly upregulated (by 175.22%) compared with BV2 cells
treated with LPS alone (P<0.05). This response was significantly
greater in the MNTD + LPS group (P<0.05) compared with the
positive control (P<0.05). These results suggest that
madecassoside has the potential to upregulate antioxidative
signaling pathways. Unlike the downregulation of the
pro-neuroinflammatory components discussed previously, the
upregulation of HO-1 seems to be dose-dependent (Fig. 2E). Compared with the gene
expression profile of upstream NF-κB presented in Fig. 2D, the upregulation of HO-1
activity seems to be independent from the transcription factors
previously assessed in the current study, further strengthening the
hypothesis that HO-1 activity may be mediated by the Nrf2 signaling
pathway.
Nrf2 signaling pathways may mediate gain-of-function
mutations in squamous cell carcinomas, which provide cancer cells
with the necessary protection against treatments and thus may
confer a pro-tumorigenic property (54). Similar to results obtained
regarding STAT1, a limited number of studies have analyzed the
HMOX1 and HO-1 molecular profile of triterpenoid saponins,
including madecassoside, asiatic acid and boswellic acid. The
results of these studies indicate that HO-1 expression is increased
due to upregulation of the Nrf2 pathway and therefore protects
cells from apoptosis and oxidative damage (55,56).
Modulation of neuroinflammatory
pathway-associated protein expression
The expression of neuroinflammation-associated
proteins was determined using western blotting to confirm the gene
expression profiles. As indicated in Fig. 3, the immunoblots of these proteins
from the cell lysates of LPS-induced BV2 microglial cells following
pre-treatment with madecassoside were assessed. The results
indicated that the protein expression of the pro-neuroinflammatory
components iNOS, COX-2, STAT1 and NF-κB was markedly increased in
cells treated with LPS alone compared with the control group,
whereas in madecassoside-treated groups, the expression of
pro-inflammatory proteins was decreased in the MTND and ½ MNTD
groups. Furthermore, the expression pattern of NF-κB protein in the
Indo + LPS group was consistent with its gene expression.
Furthermore, the protein expression of the anti-neuroinflammatory
component HO-1 was notably increased in the MNTD + LPS group, which
was also in accordance with its gene expression profile. In
addition, indomethacin treatment also had similar effects to
madecassoside on the protein expression of iNOS, COX-2, STAT1 and
HO-1. The increase in NF-kB protein expression in the Indo + LPS
group increased, which is consistent with the changes in its gene
expression. Notably, the results of a previous study demonstrated
the effects of madecassoside on inflammatory-associated signaling
pathways, including iNOS and NF-κB, in murine kidneys, which
indicated similar results to those demonstrated in a previous study
(13). Furthermore, the
anti-inflammatory effects of other triterpenoids extracted from
Centella asiatica, including asiatic acid, have been
demonstrated in a study utilizing murine liver samples (57), which further support the
anti-neuroinflammatory abilities of madecassoside.
 | Figure 3Western blotting of pro- and
anti-neuroinflammatory proteins from cell lysates of LPS-induced
BV2 microglial cells following pre-treatment with madecassoside or
indomethacin. The expression of the pro-neuroinflammatory
components iNOS, COX-2, STAT1 and NF-κB and the
anti-neuroinflammatory protein HO-1 were assessed using western
blotting. β-actin was used as housekeeping protein. LPS,
lipopolysaccharide; MNTD, maximum non-toxic dose; Control,
untreated BV2 cells; iNOS, inducible nitric oxide synthase; COX-2,
cyclooxygenase 2; STAT1, signal transducer and activator of
transcription 1; NF-κB, nuclear factor-κB; HO-1, heme oxygenase
1. |
In conclusion, the present study indicated that
madecassoside, a triterpenoid saponin, is a potent
anti-neuroinflammatory agent, as it is able to reduce intracellular
ROS levels and influence genomic and proteomic components that have
been implicated in neuroinflammation. The results of the present
study suggest that madecassoside successfully and significantly
downregulated the gene and protein expression of iNOS, COX-2, STAT1
and NF-κB, all of which are pro-neuroinflammatory components.
Furthermore, madecassoside at the MNTD significantly increased the
gene expression of the anti-inflammatory component HO-1 by 175.22%
compared with the LPS group. The primary results and the likely
anti-neuroinflammatory mechanisms of madecassoside are presented in
Fig. 4, whereby protein
expression of pro-neuroinflammatory components were reduced while
the anti-neuroinflammatory HO-1 was increased following
madecassoside treatment. The antioxidative nature of madecassoside,
which has been suggested to activate the Nrf2/HO-1 signaling
pathways at the gene and protein levels, suggests that this
compound may be an ideal treatment for neuroinflammation. The
results of the present study warrant further in vivo studies
investigating the effects of madecassoside to treat
neuroinflammation, as it may be developed as a novel
anti-neuroinflammatory agent.
 | Figure 4Summary of the primary findings and
likely anti-neuroinflammatory mechanism of madecassoside. Shaded in
the grey rectangular section are the pathways that madecassoside
activated and thus subsequently down-regulated other
pro-neuroinflammatory signaling pathways, and upregulated
antioxidants and its associated signaling pathways.
ASPP2, apoptosis-stimulating of p53 protein 2; CO,
carbon monoxide; HO-1, heme oxygenase 1; iNOS, inducible nitric
oxide synthase; IFN, interferon; IL, interleukin; JAK/STAT1, janus
kinase/signal transducer and activator of transcription 1; LPS,
lipopolysaccharide; ROS, reactive oxygen species; NO, nitric oxide;
NF-κB, nuclear factor κB; Nrf2, nuclear factor-like 2;
ONOO-, peroxynitrite; PG, prostaglandin;
TXA2, thromboxane A2; TNF, tumor necrosis
factor. |
Acknowledgments
The authors would like to thank Dr Sharmili
Vidyadaran (Universiti Putra Malaysia Selangor, Malaysia) for
generously providing the BV2 microglial cells. The present study
was supported by the International Medical University [grant no.
MBT I-2016(02)]. The authors also wish to thank Henry Ling of
Columbia University (New York, USA) for his editorial input.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Kochanek KD, Murphy SL, Xu J and
Tejada-Vera B: Deaths: Final data for 2014. Natl Vital Stat Rep.
65:1–122. 2016.PubMed/NCBI
|
|
2
|
Zoghbi HY and Orr HT: Pathogenic
mechanisms of a polyglutamine-mediated neurodegenerative disease,
spinocerebellar ataxia type 1. J Biol Chem. 284:7425–7429. 2009.
View Article : Google Scholar :
|
|
3
|
Tsuji S: Genetics of neurodegenerative
diseases: Insights from high-throughput resequencing. Hum Mol
Genet. 19:R65–R70. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen WW, Zhang X and Huang WJ: Role of
neuroinflammation in neurodegenerative diseases (Review). Mol Med
Rep. 13:3391–3396. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uttara B, Singh AV, Zamboni P and Mahajan
RT: Oxidative stress and neurodegenerative diseases: A review of
upstream and downstream antioxidant therapeutic options. Curr
Neuropharmacol. 7:65–74. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soto C and Estrada LD: Protein misfolding
and neurodegeneration. Arch Neurol. 65:184–189. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cherry JD, Olschowka JA and O'Banion MK:
Neuroinflammation and M2 microglia: the good, the bad, and the
inflamed. J Neuroinflammation. 11:982014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yao L, Kan EM, Lu J, Hao A, Dheen ST, Kaur
C and Ling EA: Toll-like receptor 4 mediates microglial activation
and production of inflammatory mediators in neonatal rat brain
following hypoxia: Role of TLR4 in hypoxic microglia. J
Neuroinflammation. 10:232013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Banks WA, Gray AM, Erickson MA, Salameh
TS, Damodarasamy M, Sheibani N, Meabon JS, Wing EE, Morofuji Y,
Cook DG and Reed MJ: Lipopolysaccharide-induced blood-brain barrier
disruption: Roles of cyclooxygenase, oxidative stress,
neuroinflammation, and elements of the neurovascular unit. J
Neuroinflammation. 12:2232015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morgan MJ and Liu ZG: Crosstalk of
reactive oxygen species and NF-κB signaling. Cell Res. 21:103–115.
2011. View Article : Google Scholar
|
|
11
|
Hutchins AP, Diez D and Miranda-Saavedra
D: The IL-10/STAT3-mediated anti-inflammatory response: Recent
developments and future challenges. Brief Funct Genomics.
12:489–498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hashim P: Centella asiatica in food and
beverage applications and its potential antioxidant and
neuroprotective effect. Int Food Res J. 18:1215–1222. 2011.
|
|
13
|
Su Z, Ye J, Qin Z and Ding X: Protective
effects of madecassoside against doxorubicin induced nephrotoxicity
in vivo and in vitro. Sci Rep. 5:183142015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nurlaily A, Noor BA and Musalmah M:
Comparative antioxidant and anti-inflammatory activity of different
extracts of Centella asiatica (L.) Urban and its active compounds,
asiaticoside and madecassoside. Med Health. 7:62–72. 2012.
|
|
15
|
Li SQ, Xie YS, Meng QW, Zhang J and Zhang
T: Neuroprotective properties of Madecassoside from Centella
asiatica after hypoxic-ischemic injury. Pak J Pharm Sci.
29:2047–2051. 2016.
|
|
16
|
Kumar H, More SV, Han SD, Choi JY and Choi
DK: Promising therapeutics with natural bioactive compounds for
improving learning and memory-a review of randomized trials.
Molecules. 17:10503–10539. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mohan JRJ: Investigating
anti-neuroinflammatory responses in lipopolysaccharide-stimulated
BV2 microglia cells upon treatment with madecassoside
(Dissertation). Kuala Lumpur (MY): International Medical
University; 2016
|
|
18
|
Ajmone-Cat MA, Bernardo A, Greco A and
Minghetti L: Non-steroidal anti-inflammatory drugs and brain
inflammation: Effects on microglial functions. Pharmaceuticals.
3:1949–1964. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Strober W: Trypan blue exclusion test of
cell viability. Curr Protoc Immunol Appendix. 3:Appendix3B2001.
|
|
20
|
Park E, Kim DK and Chun HS: Resveratrol
inhibits lipopolysaccharide-induced phagocytotic activity in BV2
cells. J Korean Soc App Biolo Chemistry. 55:803–807. 2012.
View Article : Google Scholar
|
|
21
|
Zhang T, Gong X, Hu G and Wang X: EP2-PKA
signaling is suppressed by triptolide in lipopolysaccharide-induced
microglia activation. J Neuroinflammation. 12:502015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Popa-Wagner A, Mitran S, Sivanesan S,
Chang E and Buga AM: ROS and brain diseases: The good, the bad, and
the ugly. Oxid Med Cell Longev. 2013:9635202013. View Article : Google Scholar
|
|
24
|
Phanjedra A, Jestadi DB and Periyasamy L:
Free radicals: Properties, sources, targets, and their implication
in various diseases. Indian J Clin Biochem. 30:11–26. 2015.
View Article : Google Scholar
|
|
25
|
Ma MW, Wang J, Zhang Q, Wang R, Dhandapani
KM, Vadlamudi RK and Brann DW: NADPH oxidase in brain injury and
neurodegenerative disorders. Mol Neurodegener. 12:72017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Al Mamun A, Hashimoto M, Katakura M,
Matsuzaki K, Hossain S, Arai H and Shido O: Neuroprotective effect
of madecassoside evaluated using amyloid B1-42-mediated in vitro
and in vivo Alzheimer's Disease models. Int J Indigenous Med
Plants. 47:2051–4263. 2014.
|
|
27
|
Aoshiba K, Yasuda K, Yasui S, Tamaoki J
and Nagai A: Serine proteases increase oxidative stress in lung
cells. Am J Physiol Lung Cell Mol Physiol. 281:L556–L564. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo Y, Yang YP, Liu J, Li WH, Yang J, Sui
X, Yuan X, Nie ZY, Liu YQ, Chen D, et al: Neuroprotective effects
of madecassoside against focal cerebral ischemia reperfusion injury
in rats. Brain Res. 1565:37–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hörl WH: Nonsteroidal anti-inflammatory
drugs and the kidney. Pharmaceuticals. 3:2291–2321. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maity P, Bindu S, Dey S, Goyal M, Alam A,
Pal C, Mitra K and Bandyopadhyay U: Indomethacin, a non-steroidal
anti-inflammatory drug, develops gastropathy by inducing reactive
oxygen species-mediated mitochondrial pathology and associated
apoptosis in gastric mucosa. J Biol Chem. 284:3058–3068. 2009.
View Article : Google Scholar
|
|
31
|
Wang X, Zhao Q, Matta R, Meng X, Liu X,
Liu CG, Nelin LD and Liu Y: Inducible Nitric-oxide synthase
expression is regulated by mitogen-activated protein kinase
phosphatase-1. J Biol Chem. 284:27123–27134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Garry PS, Ezra M, Rowland MJ, Westbrook J
and Pattinson KT: The role of the nitric oxide pathway in brain
injury and its treatment-From bench to bedside. Exp Neurol.
263:235–243. 2015. View Article : Google Scholar
|
|
33
|
Nascimento FR, Gomes EA, Russo M and
Lepique AP: Interferon regulatory factor (IRF)-1 is a master
regulator of the cross talk between macrophages and L929
fibrosarcoma cells for nitric oxide dependent tumoricidal activity.
PLoS One. 10:e01177822015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Olivera GC, Ren X, Vodnala SK, Lu J, Coppo
L, Leepiyasakulchai C, Holmgren A, Kristensson K and Rottenberg ME:
Nitric oxide protects against infection-induced neuroinflammation
by preserving the stability of the blood brain barrier. PLoS
Pathog. 12:e10054422016. View Article : Google Scholar
|
|
35
|
An HJ, Kim IT, Park HJ, Kim HM, Choi JH
and Lee KT: Tormentic acid, a triterpenoid saponin, isolated from
Rosa rugosa, inhibited LPS-induced iNOS, COX-2, and TNF-α
expression through inactivation of the nuclear factor-κb pathway in
RAW 264.7 macrophages. Int Immunopharmacol. 11:504–510. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Won JH, Shin JS, Park HJ, Jung HJ, Koh DJ,
Jo BG, Lee JY, Yun K and Lee KT: Anti-inflammatory effects of
madecassic acid via the suppression of NF-kappaB pathway in
LPS-induced RAW 264.7 macrophage cells. Planta Med. 76:251–257.
2010. View Article : Google Scholar
|
|
37
|
Ricciotti E and FitzGerald GA:
Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol.
31:986–1000. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang G, Sarkar P, Peterson JR, Anrather J,
Pierce JP, Moore JM, Feng J, Zhou P, Milner TA, Pickel VM, et al:
COX-1-derived PGE2 and PGE2 type 1 receptors
are vital for angiotensin II-induced formation of reactive oxygen
species and Ca2+ influx in the subfornical organ. Am J
Physiol Heart Circ Physiol. 305:H1451–H1461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hanna N, Bonifacio L, Reddy P, Hanna I,
Weinberger B, Murphy S, Laskin D and Sharma S: IFN-gamma-mediated
inhibition of COX-2 expression in the placenta from term and
preterm labor pregnancies. Am J Reprod Immunol. 51:311–318. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Klampfer L, Huang J, Kaler P, Sasazuki T,
Shirasawa S and Augenlicht L: STAT1-independent inhibition of
cyclo-oxygenase-2 expression by IFNgamma; a common pathway of
IFNgamma-mediated gene repression but not gene activation.
Oncogene. 26:2071–2081. 2007. View Article : Google Scholar
|
|
41
|
Turnquist C, Wang Y, Severson DT, Zhong S,
Sun B, Ma J, Constaninescu SN, Ansorge O, Stolp HB, Molnár Z, et
al: STAT1-induced ASPP2 transcription identifies a link between
neuroinflammation, cell polarity, and tumor suppression. Proc Natl
Acad Sci USA. 111:9834–9839. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stockinger S and Decker T: Novel functions
of type I interferons revealed by infection studies with Listeria
monocytogenes. Immunobiology. 213:889–897. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang C, Li B, Gaikwad AS, Haridas V, Xu
Z, Gutterman JU and Duvic M: Avicin D selectively induces apoptosis
and downregulates p-STAT-3, bcl-2, and survivin in cutaneous T-cell
lymphoma cells. J Invest Dermatol. 128:2728–2735. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pandey MK, Sung B and Aggarwal BB:
Betulinic acid suppresses STAT3 activation pathway through
induction of protein tyrosine phosphatase SHP-1 in human multiple
myeloma cells. Int J Cancer. 127:282–292. 2010.
|
|
45
|
Lawrence T: The nuclear factor NF-κappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1:a0016512009. View Article : Google Scholar
|
|
46
|
Jatana M, Giri S, Ansari MA, Elango C,
Singh AK, Singh I and Khan M: Inhibition of NF-kappaB activation by
5-lipoxygenase inhibitors protects brain against injury in a rat
model of focal cerebral ischemia. J Neuroinflammation. 3:122006.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yamamoto Y and Gaynor RB: Therapeutic
potential of inhibition of the NF-kappaB pathway in the treatment
of inflammation and cancer. J Clin Invest. 107:135–142. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yin MJ, Yamamoto Y and Gaynor RB: The
anti-inflammatory agents aspirin and salicylate inhibit the
activity of I(kappa)B kinase-beta. Nature. 396:77–80. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pierce JW, Read MA, Ding H, Luscinskas FW
and Collins T: Salicylates inhibit I kappa B-alpha phosphorylation,
endothelial-leukocyte adhesion molecule expression, and neutrophil
transmigration. J Immunol. 156:3961–3969. 1996.PubMed/NCBI
|
|
50
|
Patil KR, Mohapatra P, Patel HM, Goyal SN,
Ojha S, Kundu CN and Patil CR: Pentacyclic Triterpenoids Inhibit
IKKβ mediated activation of NF-κB Pathway: In Silico and in vitro
evidences. PLoS One. 10:e01257092015. View Article : Google Scholar
|
|
51
|
Rotblat B, Grunewald TG, Leprivier G,
Melino G and Knight RA: Anti-oxidative stress response genes:
Bioinformatic analysis of their expression and relevance in
multiple cancers. Oncotarget. 4:2577–2590. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chora AA, Fontoura P, Cunha A, Pais TF,
Cardoso S, Ho PP, Lee LY, Sobel RA, Steinman L and Soares MP: Heme
oxygenase-1 and carbon monoxide suppress autoimmune
neuroinflammation. J Clin Invest. 117:438–447. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gozzelino R, Jeney V and Soares MP:
Mechanisms of cell protection by heme oxygenase-1. Annu Rev
Pharmacol Toxicol. 50:323–354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kim YR, Oh JE, Kim MS, Kang MR, Park SW,
Han JY, Eom HS, Yoo NJ and Lee SH: Oncogenic NRF2 mutations in
squamous cell carcinomas of oesophagus and skin. J Pathol.
220:446–451. 2010. View Article : Google Scholar
|
|
55
|
Qi Z, Ci X, Huang J, Liu Q, Yu Q, Zhou J
and Deng X: Asiatic acid enhances Nrf2 signaling to protect HepG2
cells from oxidative damage through Akt and ERK activation. Biomed
Pharmacother. 88:252–259. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ding Y, Chen M, Wang M, Wang M, Zhang T,
Park J, Zhu Y, Guo C, Jia Y, Li Y and Wen A: Neuroprotection by
acetyl-11-Keto-β-Boswellic acid, in ischemic brain injury involves
the Nrf2/HO-1 defense pathway. Sci Rep. 4:70022014. View Article : Google Scholar
|
|
57
|
Yan SL, Yang HT, Lee YJ, Lin CC, Chang MH
and Yin MC: Asiatic acid ameliorates hepatic lipid accumulation and
insulin resistance in mice consuming a high fat diet. J Agric Food
Chem. 62:4625–4631. 2014. View Article : Google Scholar : PubMed/NCBI
|