Introduction
The consequences of blood deprivation have been
commonly acknowledged as important concerns in the clinical
manifestations of myocardial infarction, stroke and shock (1,2).
The restoration of blood flow is crucial to prevent irreversible
neuron death, with reperfusion leading to augmented inflammation,
apoptosis and reactive oxygen species (ROS), in excess of that
triggered by ischemia alone (1,2).
The pathogenesis and interventional options for cerebral
ischemia/reperfusion (I/R) injury have been expanded and derived
form a series of experimental observations, although these advances
have yet to be fully integrated into clinical practices (1-3).
According to the concept that cerebral I/R injury involves complex
pathophysiological processes, increasing investigations aimed at
elucidating the interferences of multiple etiologies may provide
promising approaches to minimize the detrimental effects of
cerebral I/R injury.
Transmembrane receptors, particularly for
radioprotective 105 kDa protein (RP105) and toll-like receptor 4
(TLR4), function as bridges by sensing extracellular stimuli and
thereby transferring signals to intercellular effectors (4-6).
In this manner, they may be implicated in neuronal injury in
response to I/R or oxygen-glucose deprivation/reoxygenation (OGD/R)
insult with respect to inflammation, apoptosis and ROS (7,8).
RP105 is a member of the TLR family and acts as an endogenous
inhibitor of TLR4, regulating acute/chronic myocardial ischemia and
pressure overload-induced cardiac remodeling (9-11).
Although the benefits have been well established for RP105 with
regard to dependence on TLR4-limited patterns, data since has shown
the effects of RP105 in TLR4-independent approaches (10). The phosphoinositide 3-kinase
(PI3K) and serine/threonine kinase, protein kinase B (AKT)
signaling pathways have been suggested as potent downstream
effectors of RP105 under I/R stimulation and the immune response
(10,12). In terms of their functional and
physical connections, the fundamental detection of RP105 and the
PI3K/AKT pathway in cerebral I/R injury have generated substantial
interest.
Substantial evidence has indicated that the activity
of the PI3K/AKT pathway exerts neuroprotective effects against I/R
injury, which is reinforced by the inhibition of PI3K/AKT driving
the progression of I/R (13). Due
to the potential of PI3K/AKT signaling as a substrate of RP105, it
is of interest to examine the existence of an RP105-PI3K-AKT axis
in cerebral I/R, whereby RP105 exerts pleiotropic protective
functions. In the present study, it was demonstrated that the
OGD/R-induced impairment of PC12 cells was markedly ameliorated by
the overexpression of RP105, as exhibited by weakened lactate
dehydrogenase (LDH) release, reductions in inflammation, ROS and
apoptosis, and increased cellular viability. The mechanistic
estimations revealed that the favorable RP105-induced effects on
OGD/R were due, in part, to the activation of the PI3K/AKT
pathways, and elimination of the PI3K/AKT axis inhibited the
inhibitory potency of RP105 on OGD/R. These results indicated that
RP105 may be a potent therapeutic candidate for the prevention of
cerebral I/R.
Materials and methods
Cell incubation and adenoviral vector
transfection
The PC12 cells (American Type Cell Culture
Collections, Manassas, VA, USA) were cultured in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin in a humidified atmosphere at 37°C with 5%
CO2 and 95% air (2).
The cells were seeded into microplates at a suitable density,
satisfying each experimental protocol, and the medium was replaced
every 24 h.
Adenoviruses encoding RP105 (Ad-RP105) or GFP
(Ad-GFP) as a control were provided by GenePharma Company
(Shanghai, China) with the viral titer at 1.5×1010
PFU/ml. For viral transduction, the PC12 cells were incubated at
37°C with Ad-RP105 or Ad-GFP at different multiplicities of
infections (MOI) for 4 h. An MOI of 50 was used in the subsequent
experiments due to its ~95% transduction efficiency and no
significant effect on cellular viability. At 48 h
post-transduction, the cells were subjected to OGD/R treatment
(14,15). Each experiment was performed in
triplicate.
OGD/R establishment and experimental
design
Following 48 h of adenoviral transduction, the PC12
cells underwent OGD/R treatment according to a prior demonstration
with minor modifications (16).
In detail, the cells were washed twice with glucose-free Earle's
balanced salt solution, and maintained in pre-warmed (37°C)
glucose-free DMEM without FBS. Subsequently, the cells were
transferred into an oxygen-deprived incubator (5% CO2
and 95% N2) at 37°C for 4 h. Reoxygenation was initiated
by exposing the cells to normal medium and maintaining the cells in
a normal incubator for another 24 h.
To examine the potential function of RP105 in PC12
cells during OGD/R injury, the cells were divided into four groups:
i) Control group, cells were cultured in normal medium in a normal
incubator; ii) OGD/R group, cells underwent 4 h of OGD followed by
24 h reoxygenation; iii) Ad-GFP+OGD/R, cells were transduced with
Ad-GFP and underwent OGD/R; iv) Ad-RP105+OGD/R, cells were
transduced with Ad-RP105 and then subjected to OGD/R. To estimate
the potential molecular mechanisms of RP105 in PC12 cells under the
OGD/R condition, the virus-transduced PC12 cells at a density of
1×106 cells/6-well plate were incubated at 37°C with or
without 10 μM LY294002 for 1 h, a specific PI3K inhibitor
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany), prior to the
OGD/R establishment.
Biochemical analysis
Following OGD/R treatment, the supernatants of the
cultured PC12 cells were collected for biological analysis of LDH,
a well-regarded marker of neuronal death (2). The supernatant was immediately
centrifuged at 1,370 × g for 5 min at 4°C, and was subjected to
commercial kits using standard protocols (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) according to the
manufacturer (2). The results
were determined in international units per liter.
Cell Counting Kit (CCK)-8 assays
A CCK-8 assay was performed to quantitatively detect
cell survival according to a previous method (2). The PC12 cells were seeded into
96-well plates at a density of 6×103 cells/well for 24 h
of incubation. Following the experimental procedures, 20 μl
of CCK-8 reagent (Dojindo Molecular Laboratories, Inc., Kumamoto,
Japan) was added into each well, followed by continuous incubation
at 37°C for 4 h. The optical density values were measured with a
microplate spectrophotometer (Bio-Rad Laboratories, Inc., Waltham,
MA, USA) at a 450 nm wavelength. Cell viabilities were determined
as the percentages relative to the control group.
Measurement of pro-inflammatory
mediators
For the estimations of inflammatory responses, the
levels of tumor necrosis factor (TNF)-α and interleukin (IL)-6 were
determined using commercial ELISA kits (Nanjing Jiancheng
Bioengineering Institute) according to the manufacturer's protocols
(2).
Apoptotic evaluation using flow
cytometry
For apoptotic evaluation, Annexin V-FITC/PI double
staining was performed as described previously (14,15). Following experimental treatment,
the PC12 cells were collected, washed with PBS and stained using
the commercial apoptosis detection kit (Sigma-Aldrich; Merck
Millipore) in accordance with the manufacturer's protocol. The PC12
cells (1×106 cells/ml) were then resuspended in PBS
(Gibco; Thermo Fisher Scientific, Inc.) and incubated with 5
μl of PI dye at room temperature for 15 min. Subsequently, 1
μl of Annexin V was added into the cell suspension and
maintained for another 15 min shielded from light. Finally, the
cells were analyzed by fluorescence-activated cell sorting via flow
cytometric analysis (CytoFLEX; Beckman Coulter, Inc., Brea, CA,
USA). Cells revealed to stain positively for Annexin V or PI were
considered apoptotic cells.
Detection of ROS generation
A DHE fluorescent probe (Beyotime Institute of
Biotechnology Co., Ltd., Haimen, China) and flow cytometric assays
were performed to determine the generation of ROS in PC12 cells, as
in a previously described method (16). In detail, the cells in each group
were adjusted to 1.5×105 per well, and seeded into a
24-well plate. Following OGD/R treatment, the medium was removed
and a DHE fluorescent probe (10 μmol/l) dissolved in
serum-free medium was added to the wells for 30 min at room
temperature. Flow cytometry (FACSCalibur; BD Biosciences, Franklin
Lakes, NJ, USA) was performed to calculate the DHE-positive
ratio.
To further evaluate the intercellular superoxide
generation and lipid peroxidation induced by OGD/R, the
concentration of malondialdehyde (MDA) and activity of superoxide
dismutase (SOD) of the PC12 cells in each group were measured using
commercially available kits (Nanjing Jiancheng Bioengineering
Institute) in accordance with the manufacturer's protocol (17).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA from the treated PC12 cells was extracted
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) as
previously described (1,2,16).
The transcription first strand cDNA synthesis kit (Roche
Diagnostics, Basel, Switzerland) was used to synthesize cDNA. The
mRNA expression of RP105 was determined by RT-qPCR analysis, which
was performed using SYBR-Green PCR Master mix (Roche Diagnostics).
The reverse transcriptase product (2 μl) was reacted with
the StepOnePlus system (Thermo Fisher Scientific, Inc.) in a
mixture (20 μl) containing 0.4 μl of TaKaRa
SYBR-Green (Roche Diagnostics), 0.4 mol/l each primer, and 10
μl of SYBR Premix Ex Taq (Roche Diagnostics). Thermocycling
conditions were as follows: 50°C for 2 min, 95°C for 10 min, and 40
cycles of 95°C for 30 sec and 60°C for 30 sec. The results were
normalized against the gene expression of β-actin. The
2−ΔΔCq method was used to calculate relative gene
expression (14). The primers
sequences for the amplification were as follows: RP105, forward
5′-TGAGGGCCTCTGTGAAATGT-3′ and reverse, 5′-GGAAGCACTGATTTGGCACA-3′;
β-actin, forward 5′-CACGATGGAGGGGCCGGACTCATC-3′ and reverse
5′-TAAAGACCTCTATGCCAACACAGT-3′.
Western blot analysis
The protocols used for western blot analysis have
been reported previously (1,2,16).
In detail, PC12 cells were washed twice with PBS (Gibco; Thermo
Fisher Scientific, Inc.), and were lysed using 200 μl of
lysis buffer containing phenylmethylsulfonyl fluoride (1 mM) on ice
for 30 min. The lysates were collected with cell scraper, and then
centrifuged at 20,000 × g at 4°C for 5 min. The BCA protein assay
kit (Beyotime Institute of Biotechnology) was utilized to determine
the concentrations. Extracts containing 40 μg of protein in
each group were subjected to 10% SDS-PAGE and transferred onto
polyvinylidene fluoride (PVDF) membranes (EMD Millipore, Billerica,
MA, USA). The membranes were then blocked with 5% non-fat dried
milk dissolved in Tris-buffered saline with 0.1% Tween-20 for 1 h
at room temperature. The PVDF membranes were then incubated with
primary antibodies targeting RP105 (1:800 dilution; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; cat. no. sc-27841), PI3K
(1:1,000 dilution; Cell Signaling Technology, Inc., Danvers, MA,
USA; cat. no. 4255), phosphorylated (p-)AKT (Ser473; Cell Signaling
Technology, Inc.; cat. no. 4060), AKT (1:1,000 dilution; Cell
Signaling Technology, Inc.; cat. no. 9272), p-glycogen synthase
kinase 3β (1:2,000 dilution; p-GSK-3β; Abcam, Cambridge, UK; cat.
no. ab75745), GSK-3β (1:2,000 dilution; Abcam; cat. no. ab93926),
cleaved caspase-9 (1:500 dilution; Abscitech, College Park, MD,
USA; cat. no. 40503-1), cleaved caspase-3 (1:2,000 dilution; Abcam;
cat. no. ab2302) and GAPDH (1:1,000 dilution; Abcam; cat. no.
ab37168) overnight at 4°C. Subsequently, the blots were incubated
with horseradish peroxidase-conjugated rabbit anti-rat IgG
secondary antibodies (1:1,000 dilution; cat. no. bs-0346R-HRP;
BIOSS, Beijing, China) for 2 h at room temperature. Finally, the
target bands were visualized using ECL reagent (Thermo Fisher
Scientific, Inc.). The signal intensities were quantified using
BandScan 5.0 software (Glyko, Inc., Novato, CA USA), and normalized
to GAPDH.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical comparisons were performed using one-way
analysis of variance followed by Tukey's test for post-hoc
analysis, and a Student's t-test for comparisons between two
groups. P<0.05 was considered to indicate a statistically
significant difference. Data and statistical analyses were
performed by SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA).
Results
OGD/R leads to the downregulation of
RP105 in PC12 cells
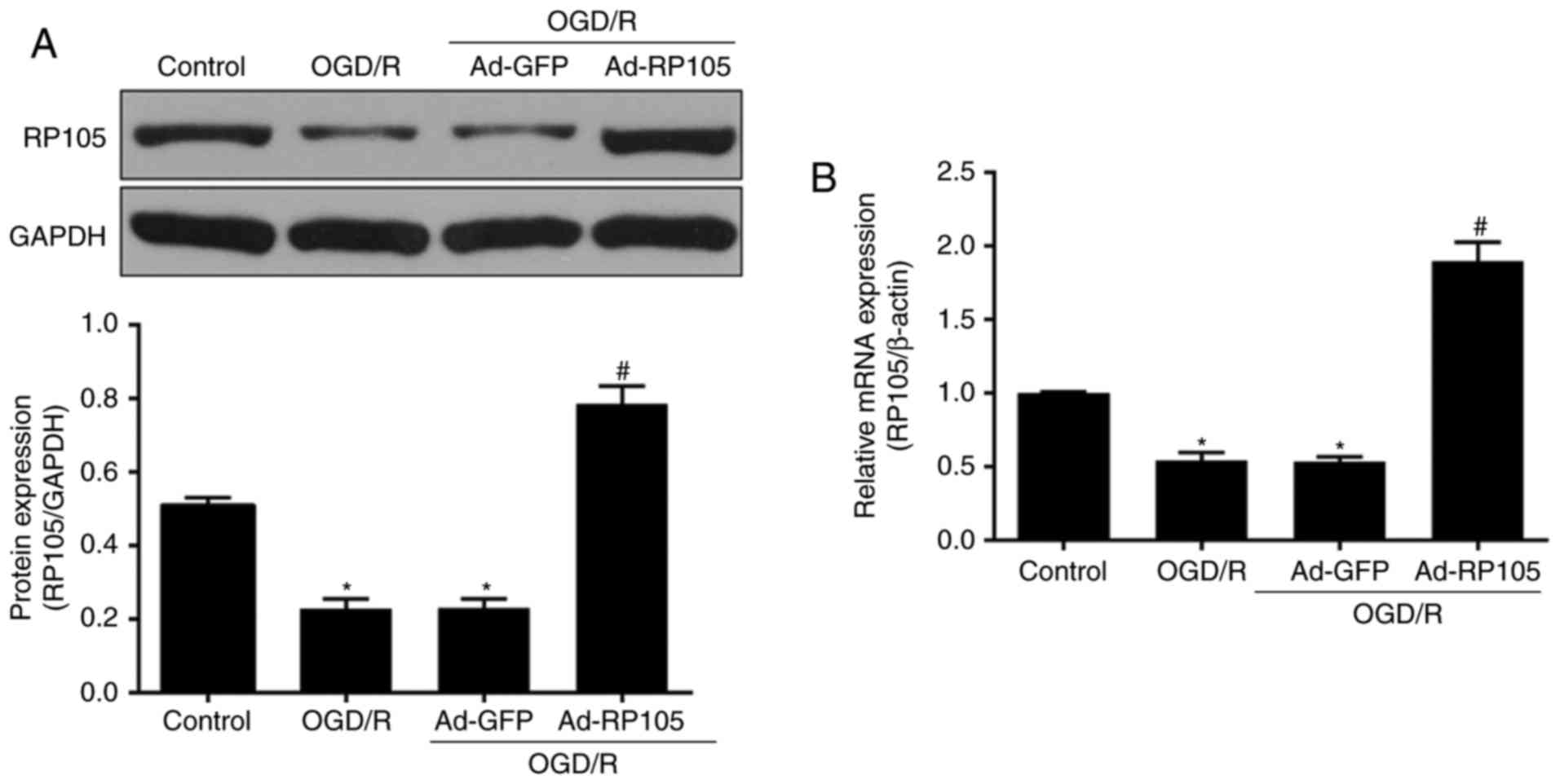
To examine the potential effects of RP105 in
neuronal OGD/R injury, levels of RP105 were measured following the
OGD/R procedure in PC12 cells. Compared with the control group, the
expression of RP105 at the protein and mRNA levels was
significantly reduced in the OGD/R-treated PC12 cells (P<0.05;
Fig. 1A and B). Ad-RP105
transduction prior to OGD/R significantly promoted the expression
of RP105 in PC12 cells (Ad-RP105+OGD/R, vs. OGD/R group;
P<0.05), which confirmed the successful transduction of the
adenoviral vector. No significant difference was found in the
expression of RP105 between the Ad-GFP+OGD/R group and the OGD/R
group (P>0.05). These findings indicated the possibility that
RP105 may be crucial in the neuronal OGD/R process.
Upregulation of RP105 increases cell
viability following OGD/R treatment
To estimate whether the overexpression of RP105
affects OGD/R injury, cellular viability and necrotic markers were
evaluated using a CCK-8 assay and LDH release detection,
respectively. As shown in Fig.
2A, OGD/R induced a significant decrease of cellular viability
in the PC12 cells, compared with those in the control group
(P<0.05). By contrast, Ad-RP105 transduction increased
viability, compared with that in the OGD/R group (P<0.05). A
similar trend was shown in the OGD/R-induced elevation of LDH
release (Fig. 2B). A lower
concentration of LDH was observed in the Ad-RP105+OGD/R group,
compared with that in the OGD/R group (P<0.05). Transduction
with Ad-GFP had no significant effect on the results of the CCK-8
or LDH assays (P>0.05).
Upregulation of RP105 represses
OGD/R-induced inflammation, apoptosis and ROS
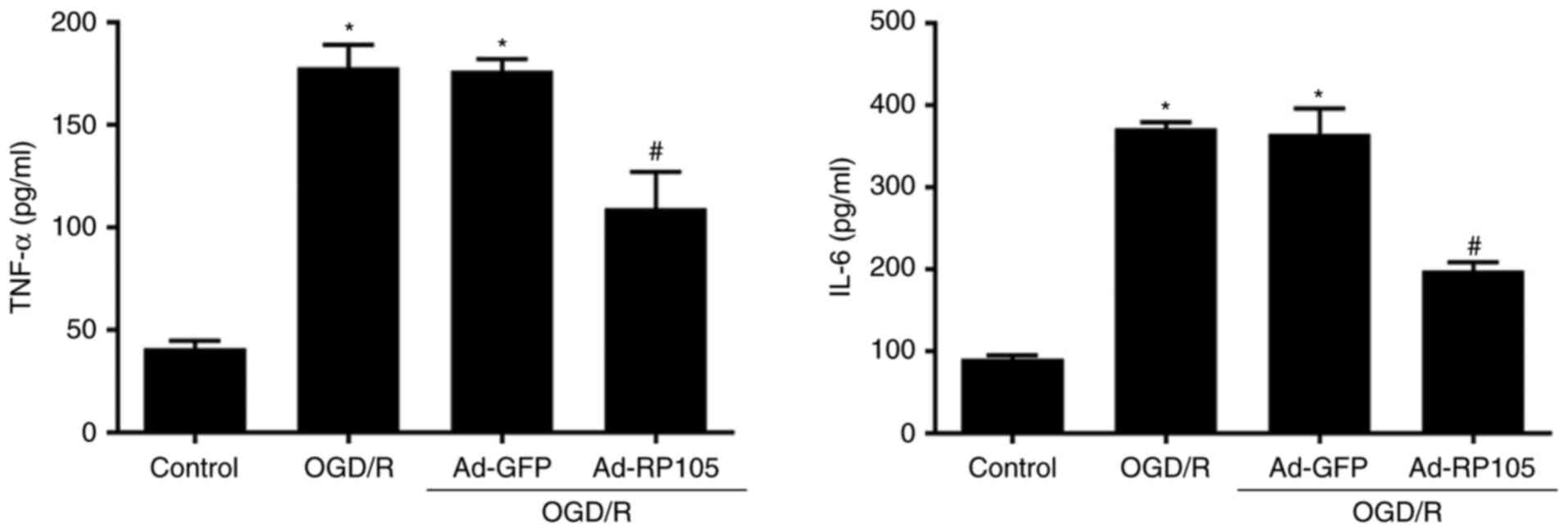
OGD/R injury is closely associated with the
stimulation of inflammation, apoptosis and ROS (1,2,16,17). In the subsequent experiments, the
present study investigated the involvement of RP105 in the changes
of pro-inflammatory mediators (IL-6 and TNF-α), apoptotic molecules
(cleaved caspase-9/-3) and ROS-associated markers (MDA and SOD)
during OGD/R of PC12 cells. In addition, a flow cytometer was
utilized to quantify apoptosis and ROS. As shown in Fig. 3, minimal concentrations of IL-6
and TNF-α were observed in the control group, whereas OGD/R
significantly accelerated inflammatory responses, compared with
those in the control group (P<0.05). As expected, the cells in
the Ad-RP105 transfection group exhibited lower IL-6/TNF-α release,
compared with those in the OGD/R group (P<0.05). Similarly, the
OGD/R-induced elevation of apoptosis, as exhibited by increased
apoptotic rate (Fig. 4A) and
protein expression levels of cle-caspase-9/-3 (Fig. 4B), was reversed by the
overexpression of RP105 (Ad-RP105+OGD/R, vs. OGD/R group,
P<0.05).
The oxidative status of OGD/R injury on PC12 cells
elicited similar patterns. As shown in Fig. 5A, the results from quantitative
evaluations of DHE-positive cells using flow cytometry revealed
that OGD/R promoted intercellular ROS generation in PC12 cells,
compared with those in the control group (P<0.05) and, in
contrast Ad-RP105 transduction effectively limited OGD/R-induced
ROS (Ad-RP105+OGD/R, vs. OGD/R group, P<0.05). Furthermore, the
accumulation of MDA, a by-product of lipid peroxidation, and
activity of SOD, an indicator of antioxidant defense, are widely
used to reflect oxidative responses to OGD/R injury (Fig. 5B). It was found that OGD/R
significantly alleviated the activity of SOD but aggravated the
production of MDA, compared with the control group (P<0.05).
Following transduction with Ad-RP105, the effects on these two
parameters of oxidative stress were reversed, compared with those
in the OGD/R group (P<0.05), indicating favorable effects of
RP105 against ROS in OGD/R. The cells transduced with Ad-GFP prior
to OGD/R exhibited no differences in inflammation, apoptosis or
ROS, compared with those in the OGD/R group (P>0.05).
Upregulation of RP105 activates the
PI3K/AKT pathways during OGD/R injury
The PI3K/AKT pathway acts as one of the major
pro-survival mediators, and its activation is fundamental in
decreasing inflammation, apoptosis and ROS in OGD/R-affected PC12
cells (18,19). To detect whether the PI3K/AKT
pathway was involved in the OGD/R-inhibitory effects of RP105, the
present study examined the expression levels of PI3K/AKT and
downstream effector GSK-3β using western blot analysis. As shown in
Fig. 6, OGD/R caused marked
reductions in the expression of PI3K, and phosphorylation of AKT
and GSK-3β, which were upregulated following Ad-RP105 transduction
(Ad-RP105+OGD/R, vs. OGD/R group; P<0.05). Ad-GFP treatment had
no significant effects on the expression levels of these proteins,
compared with levels in the OGD/R group (P>0.05).
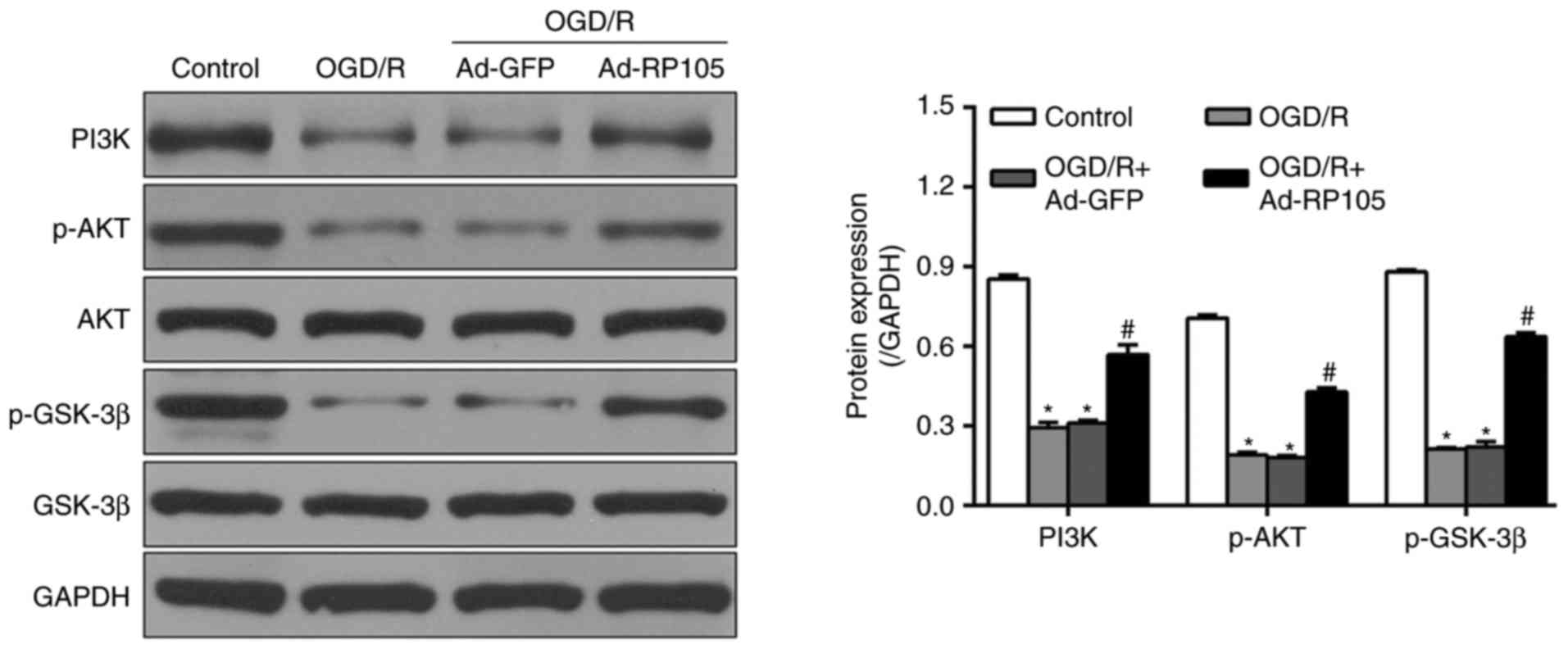 | Figure 6Upregulation of RP105 activates the
PI3K/AKT pathways in OGD/R injury. The original immunoblots of
PI3K, p-Akt, Akt, GSK-3β and p-GSK-3β, and their corresponding bar
graphs of densitometry were measured by western blot analysis.
GAPDH was used as internal control. Data are expressed as the mean
± standard deviation (n=3). *P<0.05, compared with
the control group; #P<0.05, compared with the OGD/R
group. OGD/R, oxygen-glucose deprivation/reoxygenation; PI3K,
phosphatidylinositol 3-kinase; AKT, protein kinase B; GSK-3β,
glycogen synthase kinase-3β; p-, phosphorylated; Ad, adenovirus;
RP105, radioprotective 105 kDa protein. |
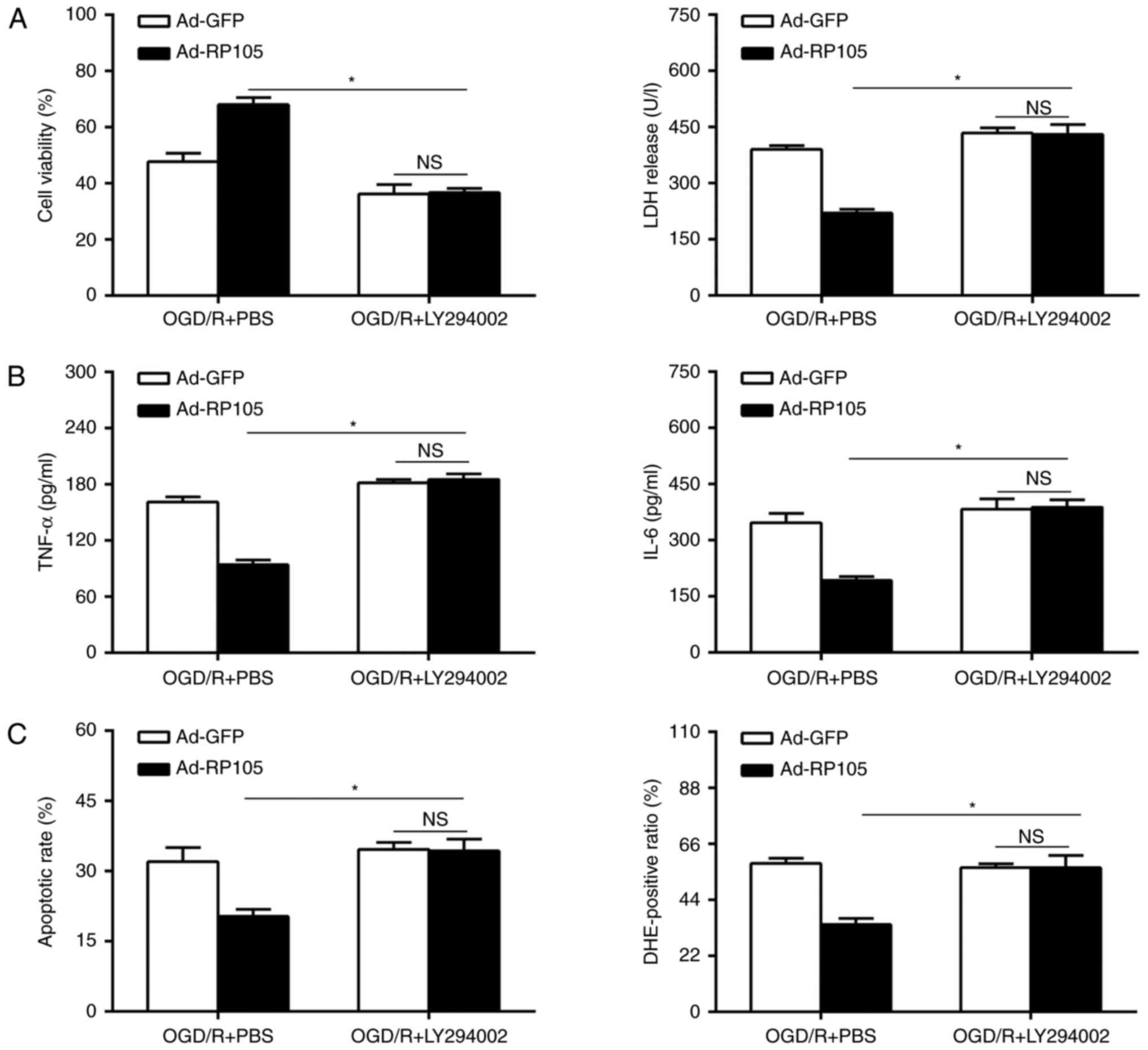
Inhibition of the PI3K/AKT pathway
eliminates the neuroprotective effects of RP105
In the subsequent investigations, whether the
neuroprotective effect of the overexpression of RP105 during OGD/R
injury is dependent on the PI3K/AKT-mediated pathways was
investigated. The Ad-RP105 and Ad-GFP-transduced PC12 cells were
subjected to a specific PI3K/AKT inhibitor (LY294002) or PBS
vehicle, followed by OGD/R insult. Cell viability was reduced in
the LY294002-treated PC12 cells, compared with the PBS-treated
cells following Ad-RP105 transduction during OGD/R insult (Fig. 7A). The administration of LY294002
notably reversed the anti-OGD/R effects of RP105, as indicated by
the reinforced LDH activity, and elevated levels of inflammation,
apoptosis and ROS (Fig. 7B and
C). Of note, the overexpression of RP105 had no effect on these
parameters following Ad-GFP transduction in the presence of
LY294002 in OGD/R (P>0.05). Together, these findings indicated
that there was a RP105-PI3K-AKT survival axis in the OGD/R
paradigm, and that RP105 ameliorated cerebral I/R injury, at least
in part, by activating the PI3K/AKT signaling pathway.
Discussion
The present study demonstrated that the
overexpression of RP105 serves as an approach to generate
anti-OGD/R effects in PC12 cells. The following evidence confirmed
this hypothesis: i) Adenoviral transduction followed by OGD/R
contributed to the overexpression of RP105 in PC12 cells; ii)
overexpression of RP105 prior to OGD/R markedly promoted cell
survival, and reduced inflammation, apoptosis and ROS; iii)
mechanistic evaluations suggested that RP105 triggered the PI3K/AKT
pro-survival signaling pathway in the OGD/R condition, which was
confirmed by the fact that PI3K/AKT inhibition eliminated the
neuroprotective effects yielded by RP105 reinforcement in PC12
cells. Taken together, these results indicated that RP105 may be a
potential target for attenuating neuronal OGD/R injury.
Due to a lack of direct extracellular ligands,
transmembrane receptor RP105, as one of the pivotal mediators in
intercellular cascade events including apoptosis and inflammation,
elicited multiple pathological functions via functional and
structural connections with its homology TLRs (9-11).
For example, Yang et al (6) reported that RP105 protected the
myocardium from I/R injury by inhibiting the TLR4/P38
mitogen-activated protein kinase (MAPK)/activator
protein-1-mediated mitochondrial apoptotic cascades. Its
anti-inflammatory property was also verified in their following
investigations; as expected, the molecular mechanisms were, at
least in part, due to the limitation of TLR4/nuclear factor
(NF)-κB-induced inflammation (20,21). Similarly, Xiong et al
(5) highlighted the involvement
of RP105 in pressure overload-induced cardiac remodeling, in which
RP105 markedly inhibited the expression of pro-inflammatory
mediators, including MAPK kinase-extracellular signal-regulated
kinase 1/2 and NF-κB, through a cooperative pattern with the
secreted protein myeloid differentiation-1. These results indicate
the potential of RP105 as a common therapeutic target against
cardiovascular diseases. However, intervention with RP105 through
TLR4-dependent pathways appeared to be unable to reduce I/R or
hypertrophy, which suggested that there may exist unrecognized
modulatory models independent of TLR4. In the present study, it was
confirmed that the activity of the RP105-PI3K-AKT axis acted as an
important pro-survival mechanism in neuronal OGD/R injury.
Therefore, the data obtained expands on the beneficial
characteristics of RP105 to include the treatment of cerebral I/R
disorder in addition to cardiovascular disease and, more
prospectively, confirmed the previously undefined mechanism beyond
TLR4-associated mechanisms.
Consistent with the present study, investigations
have focused increased attention on the TLR4-independent functions
of RP105, which appear to improve its regulatory networks (10,12). Among these, the facilitated vein
lesions in RP105-/- mice have been associated with an
increased level of chemoattractant chemokine (C-C motif) ligand-2
(22). The loss of RP105 has been
shown to alleviate atherosclerotic performance through reducing C-C
chemokine receptor-2 (23). In
addition, RP105 serves as a pro-inflammatory regulator in adipose
tissue independent of TLR4 (24).
In addition to direct or indirect pathways mediated by RP105,
results showing a physical connection between RP105 and PI3K have
attracted interest. Yu et al (12) revealed that p110δ, as the
catalytic subunit of PI3K, was a potent downstream effector reliant
on RP105. It was suggested that Bruton's tyrosine kinase acted as
one of the tyrosine residues of RP105 in the cytoplasmic tail,
which physically linked RP105 with PI3K (12). In this manner, it functioned as an
essential adaptor in inducing RP105-dependent stimulations of PI3K
and AKT. Based on the above, the present study hypothesized that
there may be functional associations between RP105 and the PI3K/AKT
axis. As expected, the association between the RP105-PI3K-AKT axis
and neuroprotective benefits was confirmed in OGD/R-exposed PC12
cells in the present study. This was also indicated by the adverse
effects observed from the inhibition of PI3K/AKT. Further
investigations may be required to verify whether other molecular
mechanisms are involved in RP105-induced pleiotropic protection
against cerebral I/R injury.
The PI3K/AKT axis is a well-known pro-survival
mediator, and is commonly regarded as an upstream negative mediator
of inflammation, apoptosis and ROS in cerebral I/R injury (17,18,25). The AKT-mediated phosphorylation of
GSK-3β at Ser9, and its subsequent inactivation, function as a
pivotal switch in regulating multiple protective genes that are
responsible for resistance to cerebral I/R injury (2). In accordance with this concept, the
present study confirmed that the activation of PI3K/AKT in an
RP105-dependent manner reversed the OGD/R-induced damage to PC12
cells, accompanied by weakened inflammation, apoptosis and ROS.
Notably, it was increasingly clear that PI3K/AKT acted as a direct
downstream effector of RP105, and that the increase of RP105
resulted in higher levels of PI3K, p-AKT and p-GSK-3β. In addition,
by applying PI3K/AKT inhibitor, the anti-OGD/R benefits rendered by
RP105 were markedly inhibited. In combination with the above data,
the results of the present study reinforced the neuroprotective
potency of RP105 against I/R injury, specifically regarding
modulation of the PI3K/AKT/GSK-3β pathway.
In conclusion, the present study demonstrated that
RP105 was an intrinsic positive mediator of cerebral I/R injury via
interference of inflammation, apoptosis and ROS. The RP105-PI3K-AKT
axis provides an appealing target for cerebral I/R treatment. Of
note, further investigations are urgently required to identify
possible molecules, particularly microRNAs and long non-coding
RNAs, which directly target the RP105-PI3K-AKT axis, and may offer
novel understanding and innovate therapeutic strategies for
cerebral I/R injury.
Glossary
Abbreviations
Abbreviations:
|
RP105
|
radioprotective 105 kDa protein
|
|
OGD/R
|
oxygen-glucose
deprivation/reoxygenation
|
|
I/R
|
ischemia/reperfusion
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
AKT
|
protein kinase B
|
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81671238).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang N, Zhang L, Lu Y, Zhang M, Zhang Z,
Wang K and Lv J: Down-regulation of microRNA-142-5p attenuates
oxygen-glucose deprivation and reoxygenation-induced neuron injury
through up-regulating Nrf2/ARE signaling pathway. Biomed
Pharmacother. 89:1187–1195. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang JF, Zhang L, Shi LL, Zhao ZH, Xu H,
Liang F, Li HB, Zhao Y, Xu X, Yang K and Tian YF: Parthenolide
attenuates cerebral ischemia/reperfusion injury via Akt/GSK-3β
pathway in PC12 cells. Biomed Pharmacother. 89:1159–1165. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jian Z, Ding S, Deng H, Wang J, Yi W, Wang
L, Zhu S, Gu L and Xiong X: Probenecid protects against
oxygen-glucose deprivation injury in primary astrocytes by
regulating inflammasome activity. Brain Res. 1643:123–129. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li X, Yang J, Yang J, Dong W, Li S, Wu H
and Li L: RP105 protects against myocardial ischemia-reperfusion
injury via suppressing TLR4 signaling pathways in rat model. Exp
Mol Pathol. 100:281–286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiong X, Liu Y, Mei Y, Peng J, Wang Z,
Kong B, Zhong P, Xiong L, Quan D, Li Q, et al: Novel protective
role of myeloid differentiation 1 in pathological cardiac
remodeling. Sci Rep. 7:418572017. View Article : Google Scholar
|
|
6
|
Yang J, Guo X, Yang J, Ding JW, Li S, Yang
R, Fan ZX and Yang CJ: RP105 protects against apoptosis in
ischemia/reperfusion-induced myocardial damage in rats by
suppressing TLR4-mediated signaling pathways. Cell Physiol Biochem.
36:2137–2148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Chen G, Yu X, Li Y, Zhang L, He Z,
Zhang N, Yang X, Zhao Y, Li N and Qiu H: Salvianolic acidB
ameliorates cerebral ischemia/reperfusion injury through inhibiting
TLR4/MyD88 signaling pathway. Inflammation. 39:1503–1513. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tao X, Sun X, Yin L, Han X, Xu L, Qi Y, Xu
Y, Li H, Lin Y, Liu K and Peng J: Dioscin ameliorates cerebral
ischemia/reperfusion injury through the downregulation of TLR4
signaling via HMGB-1 inhibition. Free Radic Biol Med. 84:103–115.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Louwe MC, Karper JC, de Vries MR, Nossent
AY, Bastiaansen AJ, van der Hoorn JW, Willems van Dijk K, Rensen
PC, Steendijk P, Smit JW and Quax PH: RP105 deficiency aggravates
cardiac dysfunction after myocardial infarction in mice. Int J
Cardiol. 176:788–793. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo X, Jiang H and Chen J: RP105-PI3K-Akt
axis: A potential therapeutic approach for ameliorating myocardial
ischemia/reperfusion injury. Int J Cardiol. 206:95–96. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
J Peng Y, Liu X, Xiong X, Huang C, Mei Y,
Wang Z, Tang Y, Ye J, Kong B, Liu W, et al: Loss of MD1 exacerbates
pressure overload-induced left ventricular structural and
electrical remodelling. Sci Rep. 7:51162016. View Article : Google Scholar
|
|
12
|
Yu CH, Micaroni M, Puyskens A, Schultz TE,
Yeo JC, Stanley AC, Lucas M, Kurihara J, Dobos KM, Stow JL and
Blumenthal A: RP105 engages phosphatidylinositol 3-kinase p110δ to
facilitate the trafficking and secretion of cytokines in
macrophages during mycobacterial infection. J Immunol.
195:3890–3900. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Zhang J, Han M, Liu B, Gao Y, Ma
P, Zhang S, Zheng Q and Song X: SMND-309 promotes neuron survival
through the activation of the PI3K/Akt/CREB-signalling pathway.
Pharm Biol. 54:1982–1990. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang J, Chen L, Ding J, Zhang J, Fan Z,
Yang C, Yu Q and Yang J: Cardioprotective effect of miRNA-22 on
hypoxia/reoxygenation induced cardiomyocyte injury in neonatal
rats. Gene. 579:17–22. 2016. View Article : Google Scholar
|
|
15
|
Yang J, Chen L, Ding J, Fan Z, Li S, Wu H,
Zhang J, Yang C, Wang H, Zeng P and Yang J: MicroRNA-24 inhibits
high glucose-induced vascular smooth muscle cell proliferation and
migration by targeting HMGB1. Gene. 586:268–273. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu J, Hua C, Pan X, Fu X and Wu W:
Eupatilin inhibits OGD/R-induced neuronal injury in PC12 cells. Int
J Clin Exp Med. 10:6728–6734. 2017.
|
|
17
|
Liu X, Zhu X, Chen M, Ge Q, Shen Y and Pan
S: Resveratrol protects PC12 cells against OGD/R-induced apoptosis
via the mitochondrial-mediated signaling pathway. Acta Biochim
Biophys Sin. 48:342–353. 2016. View Article : Google Scholar :
|
|
18
|
Luo T, Liu G, Ma H, Lu B, Xu H, Wang Y, Wu
J, Ge P and Liang J: Inhibition of autophagy via activation of
PI3K/Akt pathway contributes to the protection of ginsenoside Rb1
against neuronal death caused by ischemic insults. Int J Mol Sci.
15:15426–15442. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi HS, Kim MK, Choi YK, Shin YC, Cho SG
and Ko SG: Rhus verniciflua Stokes (RVS) and butein induce
apoptosis of paclitaxel-resistant SKOV-3/PAX ovarian cancer cells
through inhibition of AKT phosphorylation. BMC Complement Altern
Med. 16:1222016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo X, Jiang H, Yang J, Chen J, Yang J,
Ding JW, Li S, Wu H and Ding HS: Radioprotective 105 kDa protein
attenuates ischemia/reperfusion-induced myocardial apoptosis and
autophagy by inhibiting the activation of the TLR4/NF-κB signaling
pathway in rats. Int J Mol Med. 38:885–893. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu Q, Yang J, Dong W, Li X, Guo X and Yang
R: Increased expression of RP105 decreases cardiomyocytes
hypoxia/reoxygenation injury through the TLR4/NF-κB signaling
pathway. Int J Clin Exp Pathol. 9:960–968. 2016.
|
|
22
|
Wezel A, de Vries MR, Maassen JM, Kip P,
Peters EA, Karper JC, Kuiper J, Bot I and Quax PH: Deficiency of
the TLR4 analogue RP105 aggravates vein graft disease by inducing a
pro-inflammatory response. Sci Rep. 6:242482016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wezel A, van der Velden D, Maassen JM,
Lagraauw HM, de Vries MR, Karper JC, Kuiper J, Bot I and Quax PH:
RP105 deficiency attenuates early atherosclerosis via decreased
monocyte influx in a CCR2 dependent manner. Atherosclerosis.
238:132–139. 2015. View Article : Google Scholar
|
|
24
|
Nagai Y, Watanabe Y and Takatsu K: The TLR
family protein RP105/MD-1 complex: A new player in obesity and
adipose tissue inflammation. Adipocyte. 2:61–66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiao S, Zhu H, He P and Teng J: Betulinic
acid protects against cerebral ischemia/reperfusion injury by
activating the PI3K/Akt signaling pathway. Biomed Pharmacother.
84:1533–1537. 2016. View Article : Google Scholar : PubMed/NCBI
|