Introduction
Acute pancreatitis (AP) is an inflammatory disorder
of the pancreas, with an incidence of 13 to 45/100,000 people
(1). AP has been reported as one
of the most frequent principal gastrointestinal discharge diagnoses
in the USA (2). According to the
2012 Atlanta classification for acute pancreatitis, there are three
types of AP: mild acute pancreatitis (MAP), moderately acute
pancreatitis and severe acute pancreatitis (SAP), which are defined
based on the extent of organ failure and its duration (3). MAP is self-limiting, but SAP is a
life-threatening condition with a high mortality rate and rapid
progression that is associated with many complications (4). Several mechanisms of pancreatic
damage have been proposed in recent studies, such as pancreatic
duct obstruction, trypsinogen activation, pancreatic
microcirculation malfunction (5),
calcium (Ca2+) overload (6) and the activation of inflammatory
pathways (7).
Under normal physiological conditions,
Ca2+ signals are transient and localized in granules at
the apical pole; however, the sustained elevation of cytosolic
Ca2+ concentrations is fatal (8,9).
Ca2+ cell entry is mediated by voltage-dependent
Ca2+ channels (principally L-type Ca2+
channels) and is involved in a variety of Ca2+-dependent
processes, including muscle contraction, hormone or
neurotransmitter release and gene expression (10). In recent years, Ca2+
overload has received increasing attention, and its role is being
extensively investigated in the context of pancreatic acinar cells
injury (11). Digestive enzymes
have been reported to be produced by pancreatic acinar cells and
packaged in zymogen granules in the apical pole (12). When Ca2+ overload
occurs, it activates several signaling pathways, including
mitogen-activated protein kinases, phosphoinositide 3-kinase, and
nuclear factor-κB (NF-κB) cascades, which leads to the induction of
several proinflammatory mediators (13). However, Ca2+ overload
causes intracellular trypsin activation, vacuolization and necrosis
(14–16), which aggravates subsequent cell
injury and increases mortality in human acute pancreatitis
(17). Voltage-dependent
Ca2+ channels have recently been demonstrated to be
regulated by growth hormone secretagogue receptor type 1a (GHSR1a).
Ghrelin-dependent GHSR1a inhibition is reversible and involves the
altered function of Ca2+ channels via Gi/o or
Gq signaling pathways (18). Voltage-gated ion channels are
widely known to be involved in the control of growth hormone (GH)
synthesis and release (19). The
molecular basis of these regulatory actions has not been
determined.
Ghrelin is a novel GH-releasing peptide that was
initially isolated from gastric X/A-like cells and is a natural
ligand for GHSR (20). Acute
treatment with ghrelin increases intracellular calcium
[Ca2+]i (21). In somatotropes, this may
upregulate voltage-activated Ca2+ influx in a larger
time scale through activated L-type Ca2+ channels
(22). Several studies showed
that ghrelin has an anti-inflammatory role in acute pancreatitis
due to its involvement in NF-κB inhibition, an increase in
pancreatic blood flow and DNA synthesis, anti-oxidation, and the
stimulation of pancreatic cell proliferation (23–25). However, the molecular mechanism of
endogenous ghrelin calcium channel regulation in pancreatic acinar
cells in acute pancreatitis remains unclear. Therefore, this study
examined the level of serum ghrelin in acute edematous pancreatitis
(AEP) and acute necrotizing pancreatitis (ANP) rat models.
Additionally, Cav 1.2 (L-type of Ca2+ channel) and Cav
2.2 (N-type of Ca2+ channel) expression were examined in
rat pancreatic tissues and transfected AR42J cells with ghrelin
overexpression and knockdown.
Materials and methods
Antibodies and reagents
Antibody against ghrelin (cat. no. ab134978) was
purchased from Abcam PLC (Cambridge, MA, USA). Antibodies against
Cav 1.2 (cat. no. sc-16229-R) and Cav 2.2 (cat. no. sc-20129) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Antibody against glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
(cat. no. 2118) was purchased from Cell Signaling Technology
(Beverly, MA, USA). Pierce ECL Western Blotting Substrate (cat. no.
32209) was obtained from Thermo Fisher Scientific (Waltham, MA,
USA). Blasticidin (cat. no. 203351) was purchased from EMD
Millipore (Darmstadt, Germany). Fluo-4/AM (cat. no. F14217) was
purchased from Invitrogen (Grand Island, NY, USA). The kit for
enzyme-linked immunosorbent assay (ELISA) ghrelin detection (cat.
no. EIA-GHR) was purchased from RayBiotech, Inc. (Norcross, GA,
USA). Interleukin-1β (IL-1β) (cat. no. ERC007.96) and TNF-α (cat.
no. ERC102a.96) ELISA kits were purchased from Neobioscience
Biotechnology (Shenzhen, China). Caerulein and sodium taurocholate
were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
Animal groups
For the in vivo experiments, 40 healthy male
Sprague-Dawley rats weighing 200–250 g were purchased from the
Guangxi Medical University Animal Experimentation Center, China
(certificate no. SCXK GUI 2009-0002). All rats were maintained in
an environment with controlled temperature (20–24°C) and humidity
(55–58%), a 12-h light/dark cycle and fed with standard pellet rat
food (210 kcal/100 g/day). Before each experiment, the animals were
fasted overnight but allowed free access to water. The following
day, the rats were randomly divided into five groups: the normal
group, an acute edematous pancreatitis (AEP) group, an AEP-control
group, an acute necrotizing pancreatitis (ANP) group and an
ANP-control group.
Acute pancreatitis rat models and
pathological scores of pancreatic tissues
In this study, AEP was induced by the administration
of 50 μg/kg of caerulein with intraperitoneal injections
five times per day in 1-h intervals, and the same volume of normal
saline was injected intraperitoneally in the AEP control rats. ANP
was induced by the injection of 1 ml/kg of 5% sodium taurocholate
into a biliopancreatic duct for 5 min, following the closure of the
surgical incision with a double layer of stitching. After the
operation, the rats were subcutaneously injected with 30 ml/kg of
the same volume of normal saline. The pancreas and duodenum
treatments were switched as in the ANP control group. After
surgery, the rats were provided with water ad libitum. Next,
all rats were anesthetized and blood samples (2 ml) were collected
from the inferior vena cava 24 h following surgery or from the last
intraperitoneal injection. All animals were checked daily to
monitor their health. They were finally euthanized by cervical
dislocation. All animal care and studies were conducted in
accordance with the approval of the Medical Ethics Committee of the
First Affiliated Hospital of Guangxi Medical University for Ethical
Approval for Research Involving Animals (Nanning, China, permit no.
KY-113). All surgeries were performed under 10% chloral hydrate,
and all efforts were made to minimize suffering. Pancreatic tissue
was excised and fixed in 10% formalin and embedded in paraffin. For
pathological observation, tissue blocks were cut into sections and
stained with hematoxylin and eosin. A double-blind microscopic
analysis was performed by two senior pathologists. Pathological
scores for pancreatic tissues on a scale from 0 to 4 were
determined with regard to the degree of edema, inflammation,
hemorrhage and necrosis according to the method described by Kusske
et al (26).
Cell culture and transfection
For the in vitro experiments, the rat
pancreatic exocrine cell line AR42J was obtained from American Type
Culture Collection (Manassas, VA, USA) and used for stable ghrelin
overexpression or knockdown transfections. In brief, AR42J cells
were grown in high glucose Dulbecco's modified Eagle's medium
(Invitrogen) supplemented with 10% fetal bovine serum (FBS) and
penicillin/streptomycin (100 U/ml) in an atmosphere of 5%
CO2 at 37°C. In this study, cells were transfected with
ghrelin-overexpressing vector, knockdown ghrelin short hairpin RNA
(shRNA) vector or blank vector [negative control (NC)]. Lentiviral
vector encoding human ghrelin was generated by cloning ghrelin PCR
fragments (full sequence) into a pcDNA3.1-GFP vector (Invitrogen)
through EcoRI/XhoI digestion sites. The ghrelin shRNA
(3′-AGAAAGGAATCCAAGAAGCCACC-5′, 5′-TGCCAACATCGAAGGGAGC-3′) was
cloned into a pcDNA6.2-EGFP-ghrelin-miR vector (Invitrogen). For
the lentiviral infection of cells, cells were cultured in medium
and inoculated with lentivirus at a multiplicity of infection (MOI)
of 10 for 48 h, and the percentage of cells that became infected at
this MOI was ~95%. Blasticidin S (0.2 μg/ml) was added into
the medium for 2 weeks followed by another 2 weeks at 0.1
μg/ml. Stable ghrelin overexpression or knockdown cell lines
were then isolated via fluorescence-activated cell sorting and
verified using western blot analysis. Finally, stably transfected
AR42J cell clones with ghrelin overexpression or knockdown were
chosen for subsequent experiments.
Western blot analysis
In this study, the protein expression of ghrelin,
Cav 1.2, and Cav 2.2 in AR42J cells was examined using western blot
analysis. For western blot analysis, cells were lysed in Triton
X-100-based lysis buffer. The protein concentration in the
supernatant was determined using Bradford colorimetry. Next, 40
μg of protein from each sample was denatured and separated
using sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and electroblotted onto a PVDF membrane (Bio-Rad,
Hercules, CA, USA). Following blocking in 5% non-fat milk in TBST
for 1 h, the membranes were incubated overnight at 4°C with
appropriate antibodies as follows: ghrelin (diluted 1:250), Cav 1.2
(diluted 1:300), Cav 2.2 (diluted 1:300) and GAPDH (diluted
1:3,000). After washing with phosphate-buffered saline (PBS), PVDF
membranes were incubated with goat anti-rabbit horseradish
peroxidase (HRP)-conjugated secondary antibody (1:2,000) (Santa
Cruz Biotechnology, Inc.) for 2 h. Protein signals were visualized
using enhanced chemiluminescence reagents according to the
manufacturer's instructions. Optical density of the imaged bands
was normalized using a GAPDH signal obtained on the same blot. The
data were summarized as the means ± SD of three independent
experiments.
Immunohistochemistry
All pancreatic tissue samples were fixed with 4%
paraformaldehyde for 12 h, embedded in paraffin and cut into
4-μm sections. For the immunohistochemical analysis,
sections were deparaffinized, rehydrated, and endogenous
peroxidases were blocked in methanol with 3%
H2O2 for 10 min. After antigen retrieval
induced by heat in a microwave at 93°C for 30 min, sections were
blocked in 10% normal goat serum for 1 h and incubated with primary
antibody (Cav 1.2, diluted 1:100; Cav 2.2, diluted 1:100) for 4 h.
Next, biotinylated secondary antibody (Santa Cruz Biotechnology,
Inc.) was applied for 30 min. The immunohistochemical reaction was
visualized using 0.01% DAB chromogen (Santa Cruz Biotechnology,
Inc.) for 2 min. All slides were evaluated by two pathologists.
Evaluation of the staining reaction was performed in accordance
with the immunoreactive score (IRS) (27): IRS = SI (staining intensity) × PP
(percentage of positive cells). SI was defined as 0, negative; 1,
weak; 2, moderate; and 3, strong. PP was defined as 0, no positive
cells present; 1, 10% positive cells; 2, 11–50% positive cells; 3,
51–80% positive cells; and 4, >80% positive cells. Ten visual
fields from different areas of each tissue were used for IRS
evaluation. Pancreatic tissue slides with at least 3 IRS points in
this study were classified as immunoreactive.
[Ca2+]i imaging in
the AR42J cells
For [Ca2+]i imaging, AR42J
cells were seeded in a 24-well culture plate containing glass
coverslips for 24 h and fixed with 4% formaldehyde. Next, the cells
were washed three times with PBS and incubated in 5 μM
fluo-4/AM (Invitrogen) for 30 min at room temperature. The cells
were then washed five times with PBS, antifade mounting medium was
added and cells were examined under fluorescence microscope (IX83
system; Olympus, Tokyo, Japan). Fluo-4 was excited at 495 nm, and
fluorescence emissions were separately collected at 510 nm.
[Ca2+]i was quantified from fluo-4 levels
(red fluorescence). Each sample was analyzed three times.
Enzyme-linked immunosorbent assay
(ELISA)
Levels of ghrelin, IL-1β and TNF-α in rat serum were
measured using commercially available ELISA kits according to the
manufacturer's instructions. In brief, supernatants were collected
at the 24 h time point and centrifuged at 1,500 rpm for 20 min.
Next, a 100-μl aliquot of supernatant, standard sample, or
positive control sample was added into a 96-well plate and
incubated for 1 h at 37°C. Then, 100 μl of enzyme-linked
antibodies were added, and the plate was incubated for 30 min at
4°C. After washing nine times with washing buffer and incubation
for 30 min at 37°C, 2 M H2SO4 was added to
terminate the reaction. Absorbance at 450 nm was determined using a
microplate reader (Thermo Fisher Scientific). Each sample was
analyzed three times.
Statistical analysis
The data are presented as the means ± SD. The
statistical significance of differences between the means was
evaluated using the one-way analysis of variance test. Statistical
analysis was performed using SPSS 20.0 (IBM Corp., Armonk, NY,
USA). A value of p<0.05 was considered significant.
Results
Histopathological scores of pancreatic
tissues, serum ghrelin, IL-1β and TNF-α in AEP and ANP rats
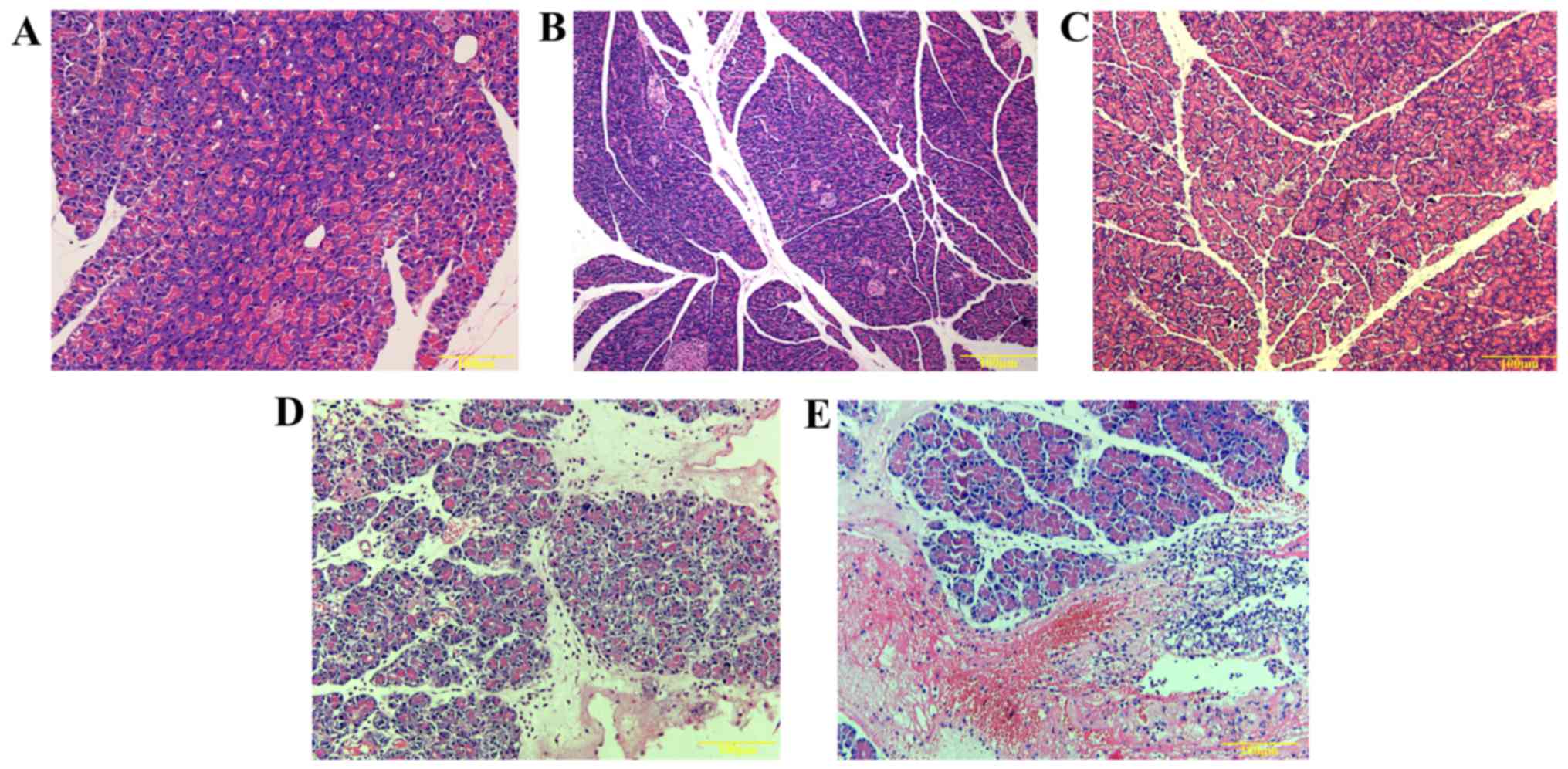
In this study, no obvious pathological changes were
observed in the normal group, the AEP-control group or ANP-control
group of animals (Fig. 1A–C).
When pancreatic tissues of AEP rats were examined, fewer foci were
observed. Additionally, hemorrhagic ascites in the pancreas and
saponifying spots in the mesentery or the greater omentum were not
observed in AEP rats. Under light microscope, edema and
inflammatory cells infiltrating the pancreatic stroma were
observed; nevertheless, diffuse bleeding and piecemeal necrosis did
not appear in the pancreas of AEP rats (Fig. 1D).
However, hemorrhagic ascites, necrosis foci in the
pancreas and several saponifying spots in the mesentery and greater
omentum were observed in rats with ANP. Infiltrating inflammatory
cells in the pancreatic stroma and glandular lobule, as well as
diffuse bleeding and necrosis were also observed under light
microscope in these rats (Fig.
1E). Pathohistological scores of pancreatic tissues in the ANP
group were significantly higher than in the other groups
(p<0.05). Additionally, these scores were also higher in the AEP
group compared with the AEP-control and normal rats (Table I).
 | Table IPathohistological scores of
pancreatic tissues, serum levels of ghrelin, IL-1β and TNF-α, and
the IRS of Cav 1.2 and Cav 2.2 in AEP and ANP rats. |
Table I
Pathohistological scores of
pancreatic tissues, serum levels of ghrelin, IL-1β and TNF-α, and
the IRS of Cav 1.2 and Cav 2.2 in AEP and ANP rats.
| Group | N | Pathohistological
score | Ghrelin
(pg/ml) | IL-1β (pg/ml) | TNF-α (pg/ml) | Cav 1.2 IRS | Cav 2.2 IRS |
|---|
| Normal | 6 | 0.33±0.52 | 71.15±6.28 | 40.45±7.05 | 7.98±1.29 | 1.76±0.57 | 1.74±0.47 |
| AEP-control | 6 | 1.67±1.03 | 74.94±11.95 | 46.07±27.81 | 9.34±3.15 | 1.76±0.36 | 1.74±0.33 |
| AEP | 6 | 4.50±1.64a,b | 98.96±9.06a,b | 67.52±25.38 | 28.02±11.60 | 3.69±0.52a,b | 2.89±0.51a,b |
| ANP-control | 6 | 2.83±1.72 | 87.11±7.90 | 60.80±21.58 | 14.70±5.47 | 3.06±0.29 | 3.56±0.58 |
| ANP | 6 | 10.83±2.04a–d |
291.37±57.35a–d |
182.82±65.28a–d | 54.59±16.60a,b,d | 5.74±1.04a–d | 5.74±1.04a–d |
Furthermore, ghrelin serum levels were significantly
increased in the ANP group compared with those in the other groups
(p<0.05). Additionally, ghrelin serum levels in the AEP group
were higher than the normal group and AEP-control group
(p<0.05). Finally, IL-1β and TNF-α serum levels were
significantly higher in the ANP group compared with the other
groups (p<0.05) (Table I).
Expression of calcium channels in the
pancreas of AEP and ANP rats
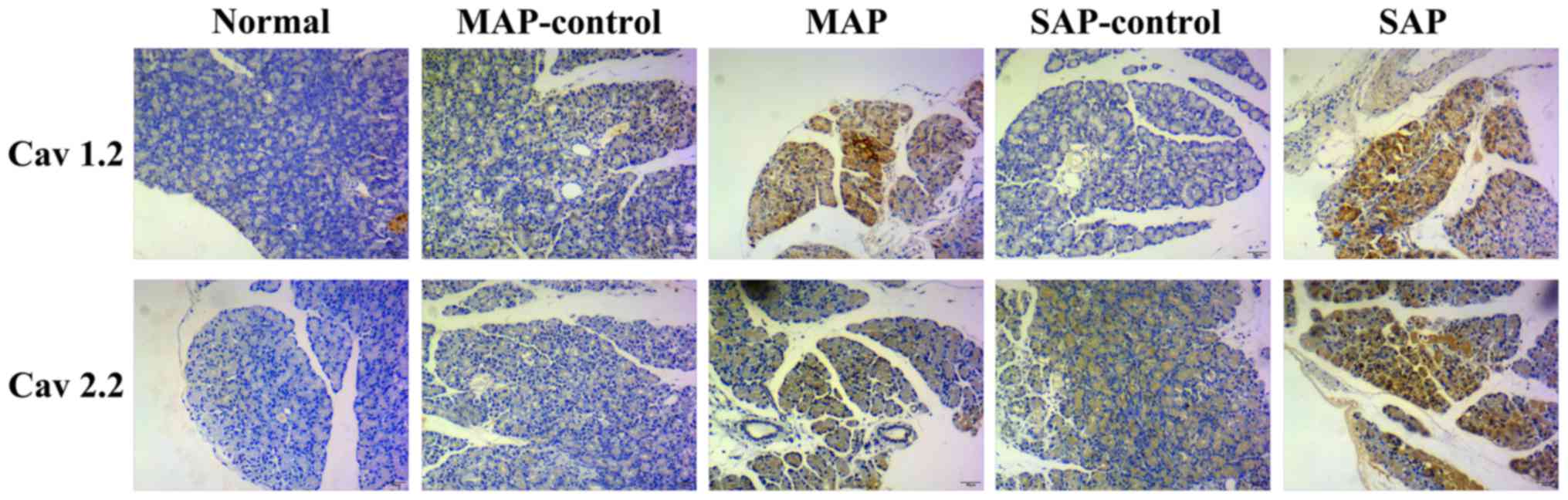
In this study, Cav 1.2 and Cav 2.2 expression in the
pancreas of AEP and ANP rats were examined using
immunohistochemistry. The IRS of Cav 1.2 and Cav 2.2 were higher in
the ANP group compared with the ANP-control group (p<0.05). IRS
scores in the AEP rats were higher than those obtained for the
AEP-control and normal rats (Fig.
2 and Table I).
The expression of calcium channels in
AR42J cells with endogenous ghrelin
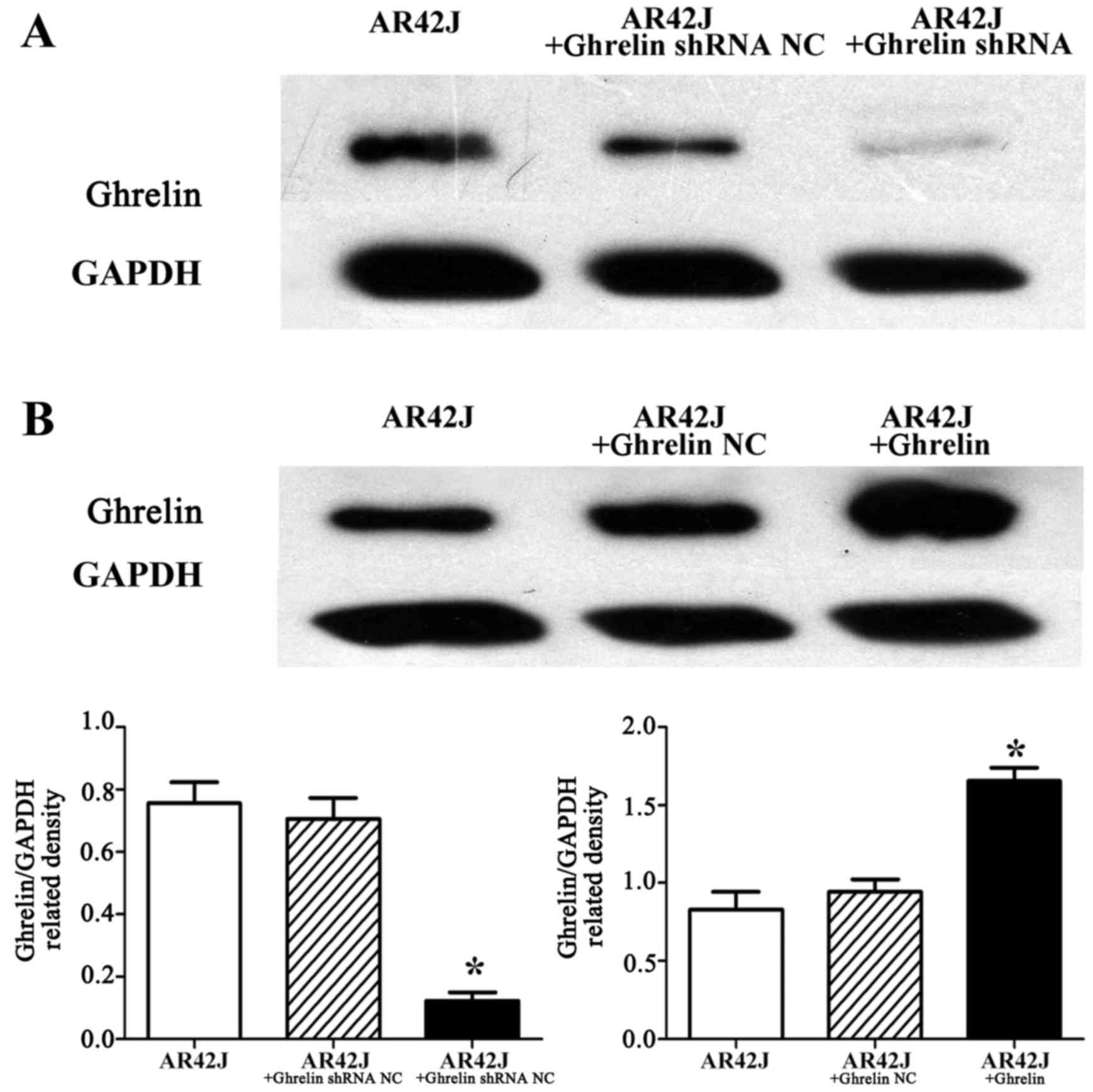
In this study, stable ghrelin knockdown in AR42J
cells resulted in low ghrelin protein expression, whereas cells
transfected with an empty vector (ghrelin shRNA NC) had similar
ghrelin expression as that detected for control untransfected AR42J
cells (p<0.05) (Fig. 3A).
Additionally, the stable ghrelin overexpression in AR42J cells
resulted in high ghrelin expression, whereas the cells transfected
with an empty vector (ghrelin NC) had a low ghrelin expression that
was similar to the control untransfected AR42J cells (p<0.05)
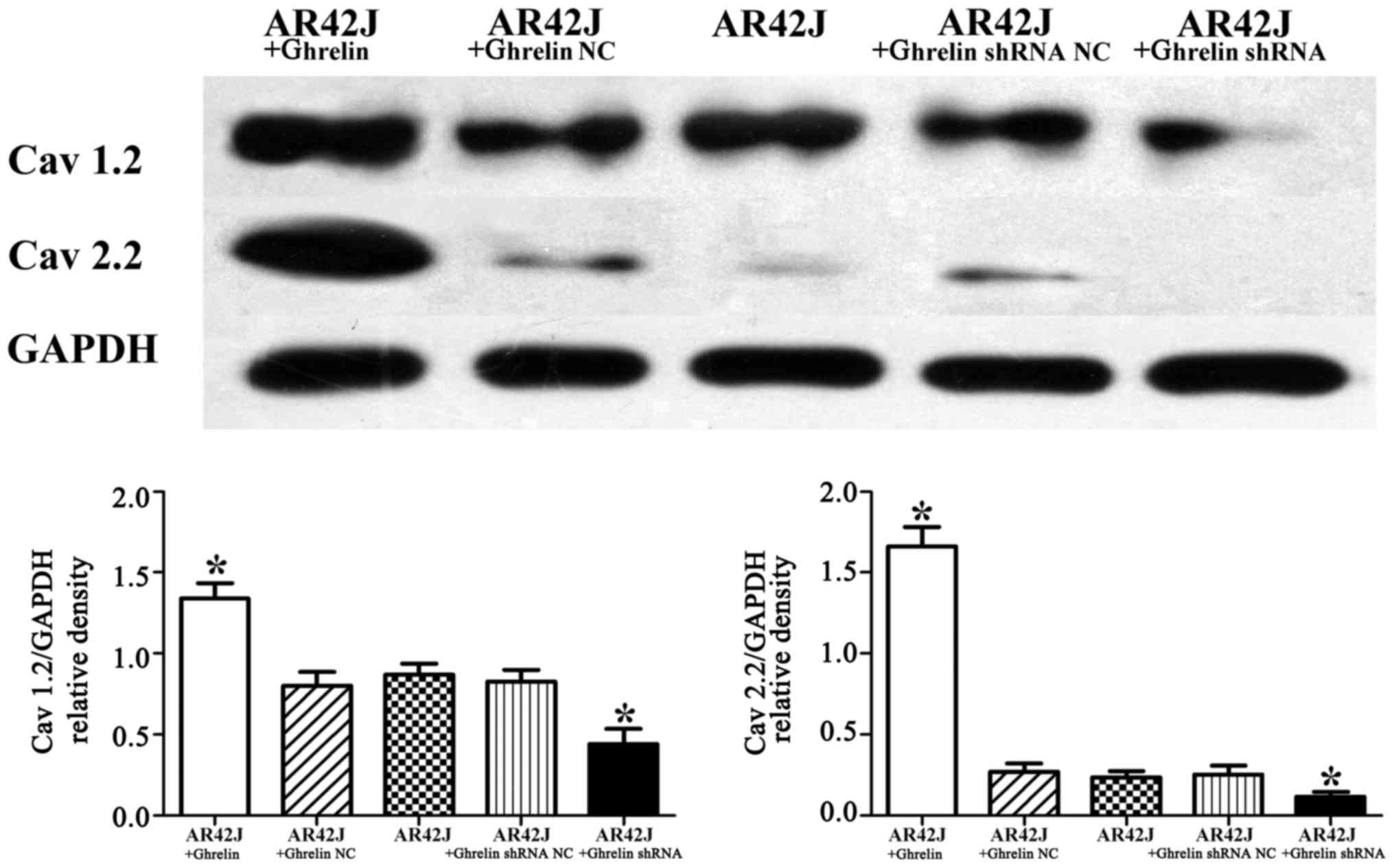
(Fig. 3B). Furthermore, in this
study, control untransfected AR42J cells had low Cav 2.2
expression. Compared with the control untransfected AR42J cells,
Cav 1.2 and Cav 2.2 expression decreased in AR42J cells with
ghrelin knockdown, whereas the expression of these calcium channels
was increased in AR42J cells with ghrelin overexpression
(p<0.05) (Fig. 4).
Collectively, these results indicate that endogenous ghrelin
changes the expression of calcium channels in AR42J cells.
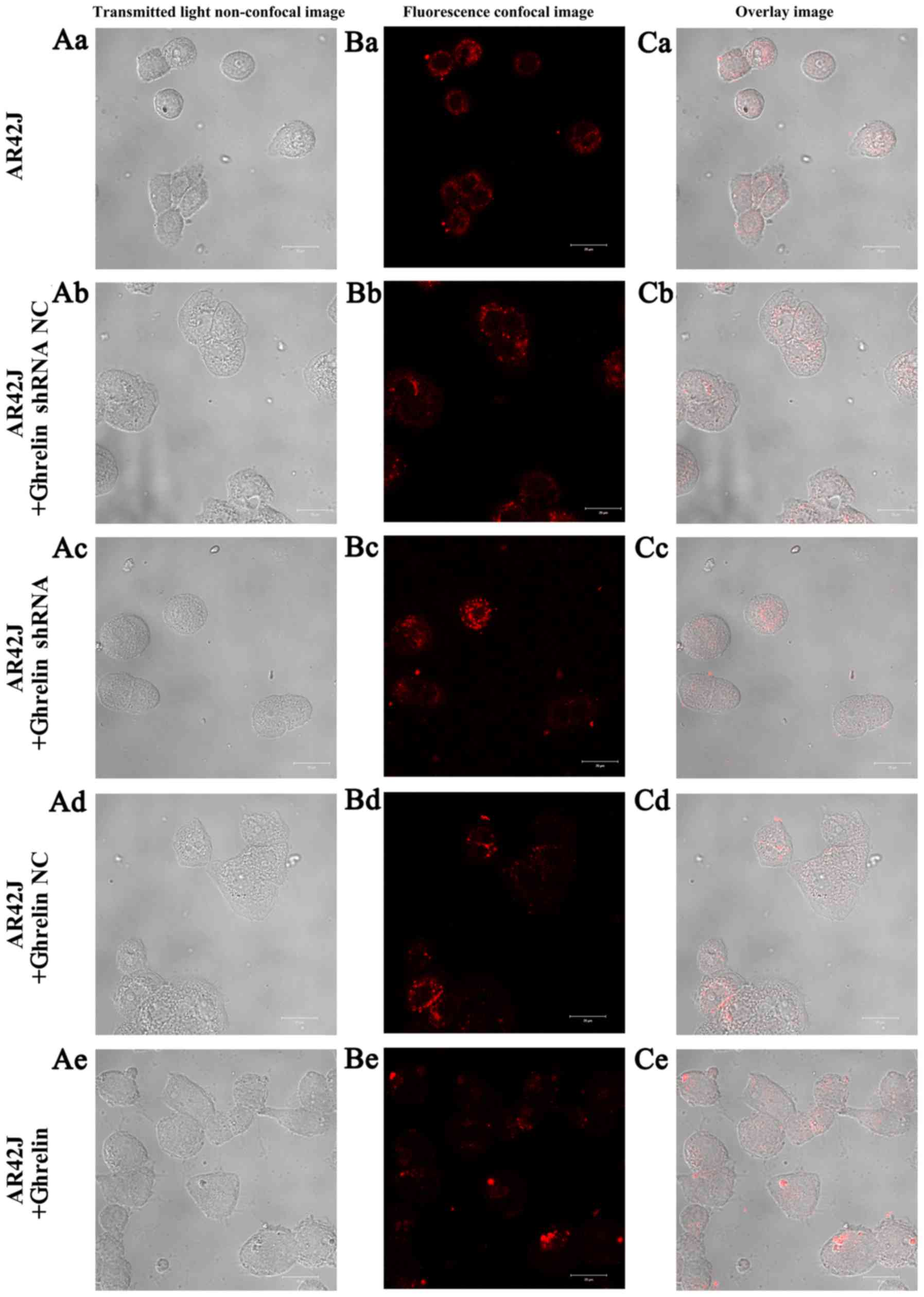
The [Ca2+]i imaging
in AR42J cells with ghrelin overexpression and knockdown
The [Ca2+]i imaging of AR42J
cells showed that red fluorescence was similar between the
untransfected AR42J cells and two groups of NC transfected cells
(Fig. 5). Red fluorescence was
weakened in the ghrelin knockdown AR42J cells but increased in
cells with ghrelin overexpression. These results collectively
suggest that a difference in ghrelin expression could affect the
[Ca2+]i in AR42J cells.
Discussion
AP is a relatively common inflammatory disorder of
the pancreas. Though most cases of AP are of the MAP type, which is
a self-limiting disease, SAP accounts for substantial additional
morbidity, with mortality rates as high as 10–20% (28).
Many molecular signaling pathways, such as
intra-acinar trypsinogen activation, local inflammation, systemic
inflammatory response, intra-acinar NF-κB activation, abnormal
intracellular calcium [Ca2+]i, mitochondrial
dysfunction, autophagy, ER stress and oxidative stress, have been
proposed to play a role in the etiology of pancreatic cellular
injury in acute pancreatitis (29). Among these possible pathways,
Ca2+ overload induced by abnormal
[Ca2+]i is receiving increasing attention as
an important molecular change in the pathogenesis of acute
pancreatitis (11).
Ca2+ entry pathway was previously described to be
provided by voltage-dependent Ca2+ channels, including
L-, N-, T-, P- and R-type Ca2+ channels (30). Of these channels, L-type calcium
channels may play a critical role in enhancing the selectivity and
regulating specific targets via complexes with G protein-coupled
receptors; N-type Ca2+ channels are thought to directly
interact with proteins of the synaptic vesicle docking and fusion
machinery (31). As proposed by
Gerasimenko et al (32), a
Ca2+ channel blocker has been proven useful in
preventing the premature digestive enzyme activation,
vacuolization, skeletal disruption and pancreatic acinar cell
necrosis induced by Ca2+ overload (33).
The aim of this study was to examine the role of
endogenous ghrelin in the expression of Cav 1.2 (L-type of
Ca2+ channel) and Cav 2.2 (N-type of Ca2+
channel) in acute pancreatitis. For this purpose, we established
AEP and ANP rat models, which were induced by caerulein and sodium
taurocholate, respectively. In this study, the expression of Cav
1.2 was higher in ANP rats compared with other groups; however, the
expression of Cav 2.2 showed no difference between the groups.
These results indicate that Cav 1.2 has a potential role in the
Ca2+ overload in acute pancreatitis. Additionally, in
this study, ghrelin serum levels in ANP rats were higher than those
in other groups, as were the IL-1β and TNF-α serum levels. Ghrelin
serum levels in AEP rats were also higher than control and normal
rats. Collectively, these results indicate that endogenous ghrelin
is involved in acute pancreatitis development and may influence the
severity of pancreatitis.
In clinical studies, the ghrelin serum level was not
found to be a predictor of the severity of disease; however, its
combination with the Gastroparesis Cardinal Symptom Index improved
its predictive accuracy (34,35). Other studies reported that ghrelin
could be implicated in the natural protection of the pancreatic
tissue through the activation of the innate immune system to
prevent the development of the inflammatory process in the
pancreas. This protective pancreatic effect appears to be indirect
and depends on the release of GH and insulin-like growth factor-1
by ghrelin (23,36,37). Our study used AR42J cells, which
have many characteristics of normal pancreatic acinar cells and
have been used as an in vitro model to study pancreatic
acinar cellular secretion, proliferation, and apoptosis (38,39). Previous study showed that ghrelin
increases [Ca2+]i through activated L-type
Ca2+ channel expression (22). This study performed the knockdown
and overexpression of ghrelin in AR42J cells, which retained many
characteristics of normal pancreatic acinar cells, such as the
synthesis and secretion of digestive enzymes. The expression of Cav
1.2 and Cav 2.2 decreased following ghrelin knockdown; however, the
expression of these two calcium channels increased in the
ghrelin-overexpressing AR42J cells. Additionally,
[Ca2+]i showed the same trend as ghrelin
expression in AR42J cells.
In conclusion, our results suggest that Cav 1.2 and
Cav 2.2 expression are increased in ANP rats and that serum ghrelin
levels may be involved in the severity of acute pancreatitis.
Additionally, the [Ca2+]i levels mediated by
Cav 1.2 and Cav 2.2 expression are regulated by ghrelin expression
in pancreatic acinar cells, at least in part. Nevertheless, the
molecular implications of ghrelin-mediated
[Ca2+]i regulation in the acute pancreatitis
remain to be elucidated.
Acknowledgments
Not applicable.
Notes
[1]
Funding
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81060043 and 81260087)
and Self-Raised Topic of the Guangxi Zhuang Autonomous Region
Health Department (no. Z2012105).
[2] Availability
of data and material
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
MQ and GT conceived and designed the study. HW, JH,
HF, HS performed the animal experiments. JZ, MQ and ZL performed
the cell experiments. JZ and MQ wrote the manuscript. All authors
read and approved the final manuscript.
[4] Ethics
approval and consent to participate
All animal care and studies were conducted in
accordance with the approval of the Medical Ethics Committee of the
First Affiliated Hospital of Guangxi Medical University for Ethical
Approval for Research Involving Animals (Nanning, China; permit no.
KY-113)
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Yadav D and Lowenfels AB: The epidemiology
of pancreatitis and pancreatic cancer. Gastroenterology.
144:1252–1261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peery AF, Dellon ES, Lund J, Crockett SD,
McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K,
Morgan DR, et al: Burden of gastrointestinal disease in the United
States: 2012 update. Gastroenterology. 143:1179–87.e1. –3. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Banks PA, Bollen TL, Dervenis C, Gooszen
HG, Johnson CD, Sarr MG, Tsiotos GG and Vege SS; Acute Pancreatitis
Classification Working Group: Classification of acute pancreatitis
- 2012: Revision of the Atlanta classification and definitions by
international consensus. Gut. 62:102–111. 2013. View Article : Google Scholar
|
|
4
|
Kingsnorth A and O'Reilly D: Acute
pancreatitis. BMJ. 332:1072–1076. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feng JY and Li YY: Alteration and role of
heat shock proteins in acute pancreatitis. J Dig Dis. 11:277–283.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Petersen OH and Sutton R: Ca2+
signalling and pancreatitis: Effects of alcohol, bile and coffee.
Trends Pharmacol Sci. 27:113–120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sah RP, Dawra RK and Saluja AK: New
insights into the pathogenesis of pancreatitis. Curr Opin
Gastroenterol. 29:523–530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Montell C: The latest waves in calcium
signaling. Cell. 122:157–163. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ashby MC and Tepikin AV: Polarized calcium
and calmodulin signaling in secretory epithelia. Physiol Rev.
82:701–734. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carabelli V, Marcantoni A, Comunanza V and
Carbone E: Fast exocytosis mediated by T- and L-type channels in
chromaffin cells: Distinct voltage-dependence but similar
Ca2+-dependence. Eur Biophys J. 36:753–762. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Frick TW: The role of calcium in acute
pancreatitis. Surgery. 152(Suppl 1): S157–S163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raraty M, Ward J, Erdemli G, Vaillant C,
Neoptolemos JP, Sutton R and Petersen OH: Calcium-dependent enzyme
activation and vacuole formation in the apical granular region of
pancreatic acinar cells. Proc Natl Acad Sci USA. 97:13126–13131.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Perides G, van Acker GJ, Laukkarinen JM
and Steer ML: Experimental acute biliary pancreatitis induced by
retrograde infusion of bile acids into the mouse pancreatic duct.
Nat Protoc. 5:335–341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Criddle DN, Raraty MG, Neoptolemos JP,
Tepikin AV, Petersen OH and Sutton R: Ethanol toxicity in
pancreatic acinar cells: Mediation by nonoxidative fatty acid
metabolites. Proc Natl Acad Sci USA. 101:10738–10743. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim JY, Kim KH, Lee JA, Namkung W, Sun AQ,
Ananthanarayanan M, Suchy FJ, Shin DM, Muallem S and Lee MG:
Transporter-mediated bile acid uptake causes
Ca2+-dependent cell death in rat pancreatic acinar
cells. Gastroenterology. 122:1941–1953. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Voronina S, Sherwood M, Barrow S, Dolman
N, Conant A and Tepikin A: Downstream from calcium signalling:
Mitochondria, vacuoles and pancreatic acinar cell damage. Acta
Physiol (Oxf). 195:161–169. 2009. View Article : Google Scholar
|
|
17
|
Reed AM, Husain SZ, Thrower E, Alexandre
M, Shah A, Gorelick FS and Nathanson MH: Low extracellular pH
induces damage in the pancreatic acinar cell by enhancing calcium
signaling. J Biol Chem. 286:1919–1926. 2011. View Article : Google Scholar :
|
|
18
|
López Soto EJ, Agosti F, Cabral A, Mustafa
ER, Damonte VM, Gandini MA, Rodríguez S, Castrogiovanni D, Felix R,
Perelló M, et al: Constitutive and ghrelin-dependent GHSR1a
activation impairs CaV2.1 and CaV2.2 currents in hypothalamic
neurons. J Gen Physiol. 146:205–219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stojilkovic SS, Tabak J and Bertram R: Ion
channels and signaling in the pituitary gland. Endocr Rev.
31:845–915. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Date Y, Kojima M, Hosoda H, Sawaguchi A,
Mondal MS, Suganuma T, Matsukura S, Kangawa K and Nakazato M:
Ghrelin, a novel growth hormone-releasing acylated peptide, is
synthesized in a distinct endocrine cell type in the
gastrointestinal tracts of rats and humans. Endocrinology.
141:4255–4261. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fang H, Hong Z, Zhang J, Shen DF, Gao FF,
Sugiyama K, Namba H and Asakawa T: Effects of ghrelin on the
intracellular calcium concentration in rat aorta vascular smooth
muscle cells. Cell Physiol Biochem. 30:1299–1309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Glavaski-Joksimovic A, Jeftinija K, Scanes
CG, Anderson LL and Jeftinija S: Stimulatory effect of ghrelin on
isolated porcine somatotropes. Neuroendocrinology. 77:367–379.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Warzecha Z, Ceranowicz P, Dembinski A,
Cieszkowski J, Kusnierz-Cabala B, Tomaszewska R, Kuwahara A and
Kato I: Therapeutic effect of ghrelin in the course of
cerulein-induced acute pancreatitis in rats. J Physiol Pharmacol.
61:419–427. 2010.PubMed/NCBI
|
|
24
|
Granata R, Settanni F, Trovato L,
Destefanis S, Gallo D, Martinetti M, Ghigo E and Muccioli G:
Unacylated as well as acylated ghrelin promotes cell survival and
inhibit apoptosis in HIT-T15 pancreatic beta-cells. J Endocrinol
Invest. 29:RC19–RC22. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kerem M, Salman B, Ozsoy S, Pasaoglu H,
Bedirli A, Haziroglu R and Yilmaz TU: Exogenous ghrelin enhances
endocrine and exocrine regeneration in pancreatectomized rats. J
Gastrointest Surg. 13:775–783. 2009. View Article : Google Scholar
|
|
26
|
Kusske AM, Rongione AJ, Ashley SW,
McFadden DW and Reber HA: Interleukin-10 prevents death in lethal
necrotizing pancreatitis in mice. Surgery. 120:284–289. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Friedrichs K, Gluba S, Eidtmann H and
Jonat W: Overexpression of p53 and prognosis in breast cancer.
Cancer. 72:3641–3647. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Haney JC and Pappas TN: Necrotizing
pancreatitis: diagnosis and management. Surg Clin North Am.
87:1431–1446. ix2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sah RP, Garg P and Saluja AK: Pathogenic
mechanisms of acute pancreatitis. Curr Opin Gastroenterol.
28:507–515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ertel EA, Campbell KP, Harpold MM, Hofmann
F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T,
Birnbaumer L, et al: Nomenclature of voltage-gated calcium
channels. Neuron. 25:533–535. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hofmann F, Flockerzi V, Kahl S and Wegener
JW: L-type CaV1.2 calcium channels: From in vitro findings to in
vivo function. Physiol Rev. 94:303–326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gerasimenko JV, Gerasimenko OV and
Petersen OH: The role of Ca2+ in the pathophysiology of
pancreatitis. J Physiol. 592:269–280. 2014. View Article : Google Scholar
|
|
33
|
Petersen O: Can specific calcium channel
blockade be the basis for a drug-based treatment of acute
pancreatitis? Expert Rev Gastroenterol Hepatol. 8:339–341. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Türkoğlu A, Böyük A, Tanrıverdi MH, Gündüz
E, Dusak A, Kaplan İ and Gümüş M: The potential role of BMI, plasma
leptin, nesfatin-1 and ghrelin levels in the early detection of
pancreatic necrosis and severe acute pancreatitis: A prospective
cohort study. Int J Surg. 12:1310–1313. 2014. View Article : Google Scholar
|
|
35
|
Wu LM, Premkumar R, Phillips AR, Windsor
JA and Petrov MS: Ghrelin and gastroparesis as early predictors of
clinical outcomes in acute pancreatitis. Pancreatology. 16:181–188.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jaworek J: Ghrelin and melatonin in the
regulation of pancreatic exocrine secretion and maintaining of
integrity. J Physiol Pharmacol. 57(Suppl 5): 83–96. 2006.
|
|
37
|
Dembiński A, Warzecha Z, Ceranowicz P,
Cieszkowski J, Pawlik WW, Tomaszewska R, Kuśnierz-Cabala B,
Naskalski JW, Kuwahara A and Kato I: Role of growth hormone and
insulin-like growth factor-1 in the protective effect of ghrelin in
ischemia/reperfusion-induced acute pancreatitis. Growth Horm IGF
Res. 16:348–356. 2006. View Article : Google Scholar
|
|
38
|
Christophe J: Pancreatic tumoral cell line
AR42J: An amphicrine model. Am J Physiol. 266:G963–G971.
1994.PubMed/NCBI
|
|
39
|
Masamune A, Sakai Y, Satoh A, Fujita M,
Yoshida M and Shimosegawa T: Lysophosphatidylcholine induces
apoptosis in AR42J cells. Pancreas. 22:75–83. 2001. View Article : Google Scholar : PubMed/NCBI
|