Introduction
Acute myocardial infarction (AMI) usually leads to
myocardial injury and heart failure due to a sudden reduction in
oxygen and blood supply (1), and
is one of the leading cause of morbidity and mortality worldwide
(2). The number of studies on
stem cells as one of the potential therapeutic applications have
increased markedly, and researchers have used various experimental
models and human clinical trials to demonstrate that stem cells
have the capacity to improve acute and chronic myocardial injury
(3–6). Additionally, the majority of the
beneficial effects of stem cell-based therapies have been
attributed to paracrine effects (7,8).
As an important macromolecular substance secreted by stem cells,
exosomes contain numerous bioactive molecules and they can produce
similar effects to stem cells (9,10).
Our earlier studies demonstrated that human umbilical cord
mesenchymal stem cell (hucMSC)-exosomes may reduce cardiomyocyte
apoptosis, promote angiogenesis and improve cardiac function
following AMI (11,12); however, the underlying mechanism
remains to be investigated.
It is well established that mothers against
decapentaplegic homolog 7 (Smad7), a vital downstream regulator in
the transforming growth factor-β (TGF-β) signaling pathway, has
critical roles in cell proliferation, differentiation, apoptosis
and numerous other essential physiological activities (13,14). It can inhibit TGF-βI-induced
phosphorylation of Smad2/3 and interfere with the interaction
between other Smad proteins or receptors to inhibit downstream gene
transcription (13). Recent
studies suggested that Smad7 may also suppress the activation of
nuclear factor-κB and inhibit hypoxia/reoxygenation (H/R)-induced
cardiomyocyte apoptosis (15,16). Therefore, Smad7 is a major
negative regulator in the TGF-βI signaling pathway. Chen et
al (17) reported that Smad7
has a critical role in the development and function of the heart.
Wang et al (18)
demonstrated that decreased Smad7 expression contributes to cardiac
fibrosis in the infarcted rat heart. In a previous study (11), the results demonstrated that
hucMSC-exosomes improved myocardial repair; Smad7 expression was
increased in hypoxia cardiomyocytes in vivo and in
vitro. However, the molecular mechanism of
hucMSC-exosomes-induced upregulation of Smad7 expression is not yet
understood.
MicroRNAs (miRNAs/miRs) are small non-protein-coding
single-stranded RNAs that negatively control the expression of
target genes by binding to the 3′-untranslated region (3′UTR) of
target mRNAs (19). miRNAs have
been reported to exhibit critical roles in a variety of
cardiovascular diseases, including heart failure (20), myocardial hypertrophy (21), arrhythmia (22) and myocardial infarction (23). miR-125b-5p was previously reported
to be upregulated in patients with AMI and may serve as a novel
biomarker for the early diagnosis of AMI (24). Bie et al (25) demonstrated that miR-125b
contributes to the proliferation and migration of cardiac
fibroblasts. The present study reported that hucMSC-exosomes
inhibit miR-125b-5p expression following myocardial injury.
Furthermore, miR-125b-5p was predicted to a target Smad7 mRNA.
Therefore, the present study investigated whether hucMSC-exosomes
promoted Smad7 expression via the down-regulation of miR-125b-5p to
improve myocardial repair.
Materials and methods
Ethical statement
The present study was approved by the Laboratory
Animal Management Committee of Jiangsu University (Zhenjiang,
Jiangsu, China).
Cell culture and reagents
hucMSCs were isolated and cultured as previously
described (26). The collection
and use of hucMSCs was approved by the Ethics Committee of Jiangsu
University (Zhenjiang, China). All individuals provided informed
consent for the use of their umbilical cord in the present study.
The umbilical cords were collected between June 2016 and March 2017
at the Obstetrics Department of the Affiliated Hospital of Jiangsu
University (Zhenjiang, China). Maternal age distribution was from
25–32 years old. HucMSCs were cultured in low-glucose Dulbecco's
modified Eagle's medium (L-DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2. The rat embryonic cardiomyocyte cell line H9C2(2-1)
and 293T cells were purchased from the Cell Bank of Type Culture
Collection of Chinese Academy of Sciences (Shanghai, China) and
cultured in high-glucose DMEM (H-DMEM; Gibco; Thermo Fisher
Scientific, Inc.) containing 10% FBS at 37°C with 5%
CO2.
HucMSC-exosomes extraction, purif ication
and characterization
The hucMSC-exosomes were extracted and purified as
the previously described (11).
L-DMEM containing 10% FBS was replaced with L-DMEM containing 10%
exosomes-free FBS when cultured hucMSCs attained 80–90% confluence.
The conditioned medium of hucMSC (hucMSC-CM) was collected
following replacement with L-DMEM containing 10% exosomes-free FBS
for 48 h and centrifuged at 300 × g for 20 min, 2,000 × g for 20
min, 10,000 × g for 30 min at 4°C to remove dead cells and cellular
debris. The hucMSC-CM was then concentrated using a 100 kDa
molecular weight cutoff (MWCO) hollow fibre membrane (EMD
Millipore, Billerica, MA, USA) at 1,000 × g for 30 min at 4°C. The
concentrated hucMSC-CM was loaded onto 5 ml 30%
sucrose/D2O cushions and ultracentrifuged at 100,000 × g
for 2 h at 4°C (optimal-90k; Beckman Coulter, Inc., Brea, CA, USA).
The bottom of the cushion containing the hucMSC-exosomes was
collected and washed three times with PBS using a 100 kDa MWCO
centrifuge tube at 1,000 × g for 30 min at 4°C. The hucMSC-exosomes
were filtered through a 0.22 µm membrane filter (EMD
Millipore,) and stored at −70°C until use. The protein content of
hucMSC-exosomes was determined using a bicinchoninic acid (BCA)
protein assay kit (CWBiotech, Beijing, China). Nanoparticle
tracking analysis (NTA) was performed for the analysis of particle
size and particle concentration of hucMSC-exosomes using a digital
microscope LM10 system (NanoSight; Malvern Instruments, Ltd.,
Malvern, UK). The cluster of differentiation CD9, CD63 and CD81
molecules that are frequently located on the surface of
hucMSC-exosomes were analysed via western blotting.
Establishing the rat acute myocardial
infarction and evaluation of cardiac function
The animal protocol was approved by the Animal
Experimental Center of Jiangsu University (Zhenjiang, China).
Healthy male Sprague-Dawley rats (220–250 g; 8-week-old) were used
in the AMI model according to the previously reported method
(11). The rats were purchased
from the Animal Experimental Center of Jiangsu University and
housed in a specific pathogen-free animal facility under constant
temperature and humidity, and with a 12/12 h light/dark cycle with
sufficient qualified food and water. In brief, Sprague-Dawley rats
were anesthetized with 10% chloral hydrate (300 mg/kg; Sinopharm
Chemical Reagent Co., Ltd., Shanghai, China) by intraperitoneal
injection and were mechanically ventilated (Alcott Biotech Co.,
Ltd., Shanghai, China). The rat's thorax was opened, and the left
anterior descending (LAD) coronary artery was quickly and
accurately ligated with a 6-0 suture. The thorax was closed by
tightening the double purse suture. The animals were randomly
divided into three groups with 18 rats per group (6 rats for
western blot analysis, reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) at 2 days following surgery,
and echocardiography at 4 weeks following surgery, respectively).
AMI + hucMSC-exosomes group received hucMSC-exosomes (400 µg
proteins) diluted in 200 µl PBS and the AMI + PBS group
received 200 µl PBS. PBS or hucMSC-exosomes were injected
via the tail vein immediately after LAD ligation. The sham group
underwent neither LAD coronary artery ligation nor
PBS/hucMSC-exosomes injection. The borderline area (the juncture of
infract and non-infract site) of infraction myocardium was used for
RT-qPCR and western blot analyses at 2 days after surgery. The
tissue of the sham group was selected from near the LAD coronary
artery. Echocardiography of left ventricular performance was
assessed by a high-frequency colour ultrasound instrument
(Vevo2100; VisualSonics, Toronto, ON, Canada) at 4 weeks after
surgery.
Hypoxia experiments in vitro
To mimic ischemic injury and study whether
hucMSC-exosomes protected H9C2(2-1) cells against hypoxic injury,
three experimental groups were designed as follows: Hypoxia +
exosomes group, H9C2(2-1) cells were cultured in 6-well plates at
2×105 cells/plate in L-DMEM containing 0.2% FBS and
hucMSC-exosomes (200 µg/ml) at 37°C with 5% CO2,
94% N2 and 1% O2 for 48 h; hypoxia + PBS
group, H9C2(2-1) cells were cultured under the same conditions with
the hucMSC-exosomes replaced by isometric PBS; normoxia group,
H9C2(2-1) cells were cultured under normoxic conditions throughout
the experiments as the control.
Bioinformatics analysis
The potential targets of Smad7 were predicted using
miRbase (http://www.mirbase.org/) and TarBase
(http://www.microrna.gr/tarbase).
RNA isolation and detection
Total RNA of tissues and cell lines was extracted
with TRIzol Reagent (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocols. Bulge-loop™ miRNA qRT-PCR Primer Sets
(one RT primer and a pair of primers for each set) specific for
miR-21-5p, miR-92a-3p and miR-125b-5p (Table I) were designed by Guangzhou
RiboBio Co., Ltd. (Guangzhou, China). RT-qPCR was performed by
using the Bulge-Loop miRNA qRT-PCR Starter kit (R11067.1; Guangzhou
RiboBio Co., Ltd.) according to the manufacturer's protocols. The
reaction was performed at 95°C for 10 min, followed by 40 cycles of
95°C for 2 sec, 60°C for 20 sec and 70°C for 10 sec. The relative
expression levels of miRNA were analysed using the
2−∆∆Cq method (27).
The U6 small nuclear RNA served as the internal reference to
normalize the expression of miRNA.
 | Table IPrimer list. |
Table I
Primer list.
| miR | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| miR-21-5p |
GTGCAGGGTCCGAGGT |
GTGCGTGTCGTGGAGTCG |
| miR-92a-3p |
TATTGCACTTGTCCCGGCCTGT |
GTGCGTGTCGTGGAGTCG |
| miR-125b-5p |
GCTCCCTGAGACCCTAAC |
GTGCGTGTCGTGGAGTCG |
| U6 |
TGCGGGTGCTCGCTTCGGCAGC | CCA
GTGCAGGGTCCGAGGT |
Transient transfection
miR-125b-5p mimics (25 nM) (5′-UCC CUG AGA CCC UAA
CUU GUGA-3′) and mimic negative control (MNC; 25 nM; 5′-UUG UAC UAC
ACA AAA GUA CUG-3′) were synthesized and purified by Guangzhou
RiboBio. 293T cells and H9C2(2-1) cells were transfected with
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols. The
cells were undertaken to extract protein, isolate RNA or hypoxia
experiments following transfection for 24 h.
Trypan blue staining
H9C2(2-1) cells were cultured in hypoxia conditions
in vitro as described the above. Cell viability was analysed
by trypan blue (Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China). A total of 100 µl cell suspension
(104 cells) and 100 µl 0.4% trypan blue solution
were mixed well, then incubated for 1 min at room temperature; 10
µl of the mix was collected to carefully fill the
haemocytometer chamber. Cells were counted according to the
protocols of the haemocytometer. Viable cells remained unstained,
non-viable cells were stained blue.
Lactate dehydrogenase (LDH) release
detection
To evaluate the membrane integrity of H9C2(2-1)
cells with various treatments, the concentration of LDH in the
culture media was measured with a Lactate Dehydrogenase Assay kit
(Shanghai Rongsheng Biotech Co., Ltd., Shanghai, China) using the
continuous monitoring method according to the manufacturer's
protocols.
Dual-luciferase reporter gene assay
To verify the association between miR-125b-5p and
Smad7, the pmirGLO dual-luciferase reporter vector (Promega
Corporation, Madison, WI, USA) was employed to construct a 3′UTR
wild-type reporter vector of Smad7. Additionally, a 3′UTR mutant
reporter vector of Smad7 lacking miR-125b-5p binding site was also
constructed. 293T cells were transiently co-transfected with Smad7
3′UTR wild (or mutant) reporter vector (200 ng) and miR-125b-5p
mimics (25 nM) using Lipofectamine® 2000 reagent. The
cells were collected 24 h after co-transfection to measure the
luciferase activity using a Dual-Luciferase Reporter Assay system
(E1910; Promega Corporation) and normalized to that of
Renilla luciferase activity according to the manufacturer's
protocols.
Western blot analysis
Western blot analysis was conducted according to the
previously described procedure (11). In brief, protein was extracted
from hucMSC-exosomes, H9C2(2-1) cells and myocardial tissue using
radioimmunoprecipitation assay buffer and phenylmethylsulfonyl
fluoride (Invitrogen; Thermo Fisher Scientific, Inc.). The protein
concentration was determined with a BCA protein assay kit
(CWBiotech) according to the manufacturer's protocols. Equal
quantities of protein (30 µg) were separated by 12% SDS-PAGE
and subsequently transferred onto polyvinylidene fluoride membranes
(EMD Millipore). The membranes were blocked with 5% skimmed milk in
1X TBS-Tween for 1 h at room temperature and incubated with primary
antibodies against CD9 (1:1,000; cat. no. ab92726; Abcam,
Cambridge, UK), CD63 (1:500; cat. no. BS3474; Bioworld Technology,
Inc., St. Louis Park, MN, USA), CD81 (1:11,000; cat. no. ab109201;
Abcam), B-cell lymphoma 2 (Bcl-2; 1:2,000; cat. no. MAB8272;
R&D Systems, Inc., Minneapolis, MN, USA), Bcl-2-associated X
(Bax; 1:1,000; cat. no. 2772; Cell Signaling Technology, Inc.,
Danvers, MA, USA), Smad7 (1:1,000; cat. no. MAB2029; R&D
Systems, Inc.) and GAPDH (1:2,000; cat. no. CW0100M; CWBiotech) at
4°C overnight. Subsequently, membranes were washed three times with
1× TBS-Tween (10 min per wash) and then incubated with
HRP-conjugated secondary antibodies (1:5,000, cat. no. BS13278,
Bioworld Technology, Inc.; and 1:2,000, cat. no. CW0102S,
CWBiotech) at 37°C for 1 h. Then, membranes were washed three times
with 1X TBS-Tween (10 min per wash). The signals were visualized
using the Luminata Crescendo Western HRP substrate (EMD Millipore)
and the results were analyzed by Image-Pro Plus version 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Statistical analysis was performed by Graph Pad
Prism (v5.01; GraphPad Software, Inc., La Jolla, CA, USA). All
experimental data in vitro were obtained at least three
independent experiments and expressed as the mean ± standard
deviation. A Student's t-test was used to compare experimental and
relative control groups. Multiple comparisons were performed using
one-way analysis of variance followed by the Newman-Keuls post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Characterization of hucMSC-exosomes
The particle size distribution and particle
concentration of hucMSC-exosomes were recorded by NTA (Fig. 1A and B). The mean protein
concentration and mean particle concentration of hucMSC-exosomes
were 3.53 mg/ml and 4.17×1010 particles/ml, respectively
(Fig. 1C). Western blot analyses
indicated that purified hucMSC-exosomes expressed exosomal markers,
including CD9, CD63 and CD81 (Fig.
1D).
HucMSC-exosomes improve cardiac systolic
function, protected cardiomyocytes and upregulated the expression
of Smad7
The cardiac systolic function was analysed using
echocardiography at 4 weeks after AMI. Left ventricular ejection
fraction (LVEF) and left ventricular fractional shortening (LVFS)
reflected cardiac systolic function. Compared with sham group, LVEF
and LVFS were both significantly reduced in AMI + PBS group
(P<0.001), and were both significantly increased in AMI +
exosomes group compared with in the AMI + PBS group (Fig. 2A and B). The cardioprotective
effects of hucMSC-exosomes were analysed; Bax and Bcl-2 protein
expression levels in myocardial tissue were detected by western
blotting. Following LAD ligation for 48 h, Bcl-2 expression was
significantly decreased in AMI + PBS group compared with the sham
group; this phenomenon was reversed in the AMI + exosomes group.
Additionally, Bax expression exhibited the opposite trend to Bcl-2
expression (Fig. 2C–F).
Simultaneously, Smad7 expression levels were also significantly
decreased in the AMI + PBS group compared with in the sham group,
but increased in the AMI + exosomes group. To investigate the
cardioprotective effects of hucMSC-exosomes in vitro, trypan
blue staining of H9C2(2-1) cells was conducted following exposure
to hypoxic conditions for 48 h. The number of viable cells was
significantly increased in the hypoxia + exosomes group compared
with hypoxia + PBS group (Fig.
3A). In addition, the release of LDH in H9C2(2-1) cells'
culture media was detected. LDH release was significantly decreased
in hypoxia + exosomes group compared with in the hypoxia + PBS
group (Fig. 3B). Furthermore,
Bax, Bcl-2 and Smad7 protein expression levels in vitro
followed the same trends as observed in vivo (Fig. 3C–F).
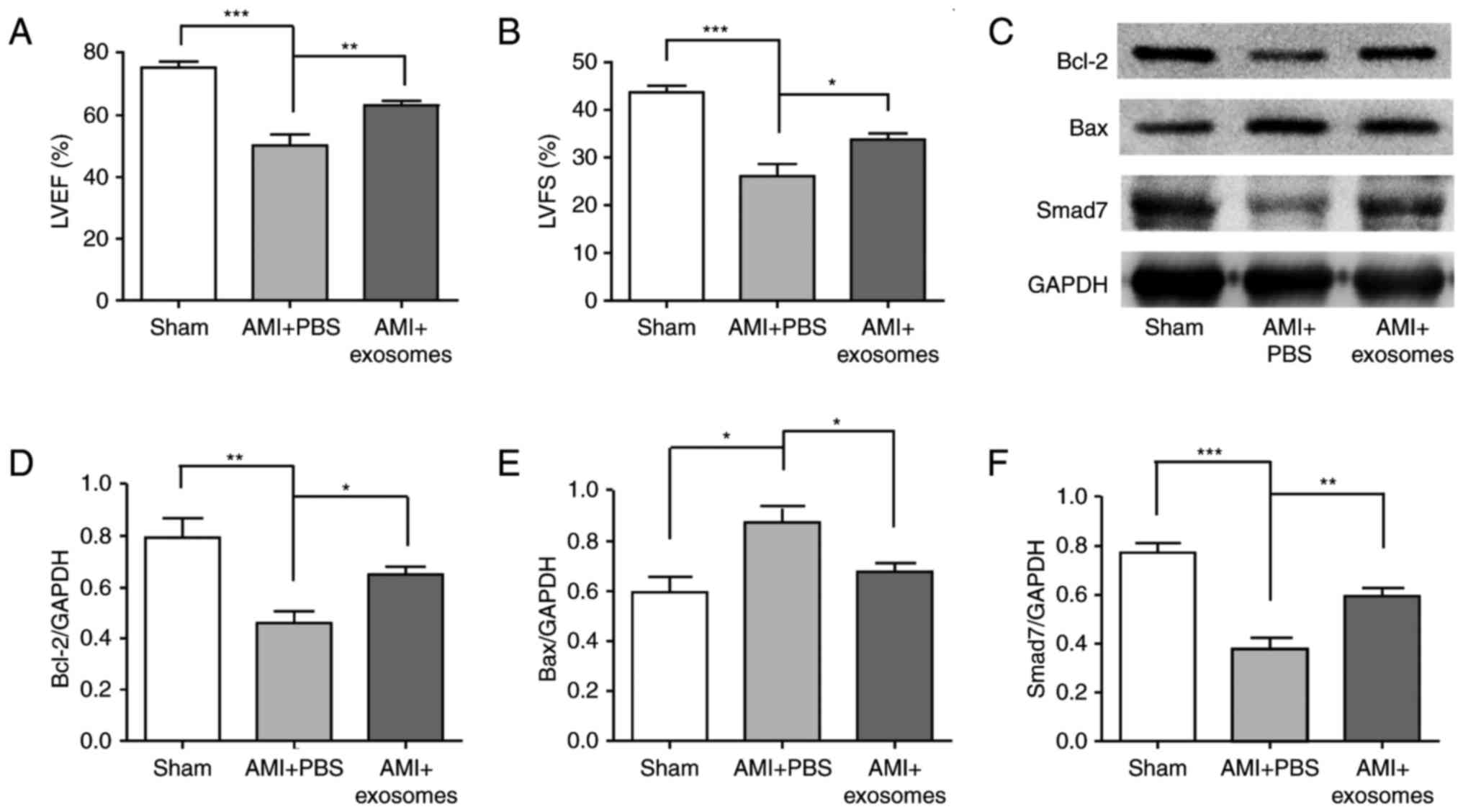 | Figure 2Cardiac systolic functions of AMI
rats following various treatments and the cardioprotective effect
of hucMSC-exosomes in vivo. Cardiac systolic
function-associated parameters: (A) LVEF, (B) LVFS, (C) Western
blot was used to detect markers and densitometry analysis was
performed to semi-quantify, (D) Bcl-2, (E) Bax and (F) Smad7
protein expression in different groups. HucMSC-exosomes exhibited a
cardioprotective effect in vivo and it may improve cardiac
systolic function of AMI rats. *P<0.05,
**P<0.01 and ***P<0.001.
hucMSC, human umbilical cord mesenchymal stem cell; LVEF, left
ventricular ejection fraction; LVFS, left ventricular fractional
shortening; AMI, acute myocardial infarction; Bcl-2, B-cell
lymphoma-2; Bax, Bcl-2-associated X; Smad7, mothers against
decapentaplegic homolog 7. |
HucMSC-exosomes inhibit miR-125b-5p
expression in injured cardiomyocytes in vivo and in vitro
The present study reported that Smad7 expression was
upregulated in hucMSC-exosomes to repair myocardial injury in
vivo and in vitro (Figs.
2 and 3). Notably, Smad7 is a
negative regulator of the TGF-β signaling pathway, which is
involved in cardiac remodeling; miRNAs that are relevant to damage
repair were investigated in the present study. To identify which
miRNAs may regulate Smad7 expression, bioinformatics analyses were
conducted using miRBase and TarBase. Three miRNAs (miR-21-5p,
miR-92a-3p and miR-125b-5p) (Table
II) were selected for further experiments.
 | Table IIList of predicted binding sites of
Smad7 and the targeting miRs. |
Table II
List of predicted binding sites of
Smad7 and the targeting miRs.
| Smad7 3′UTR bp
position | Smad7 3′UTR
sequence | miR | miR sequence |
|---|
| 1182–1189 |
5′-UGCUCACACUUUAAUAUAAGCUA-3′ | rno-miR-21-5p |
3′-AGUUGUAGUCAGACUAUUCGAU-5′ |
| 1393–1399 |
5′-CAUUAUUUAUGUAUUGUGCAAUG-3′ | rno-miR-92a-3p |
3′-GUCCGGCCCUGUUCACGUUAU-5′ |
| 3721–3727 |
5′-GACCAGCGAGGGGCAUCAGGGA-3′ |
rno-miR-125b-5p |
3′-AGUGUUCAAUCCCAGAGUCCCU-5′ |
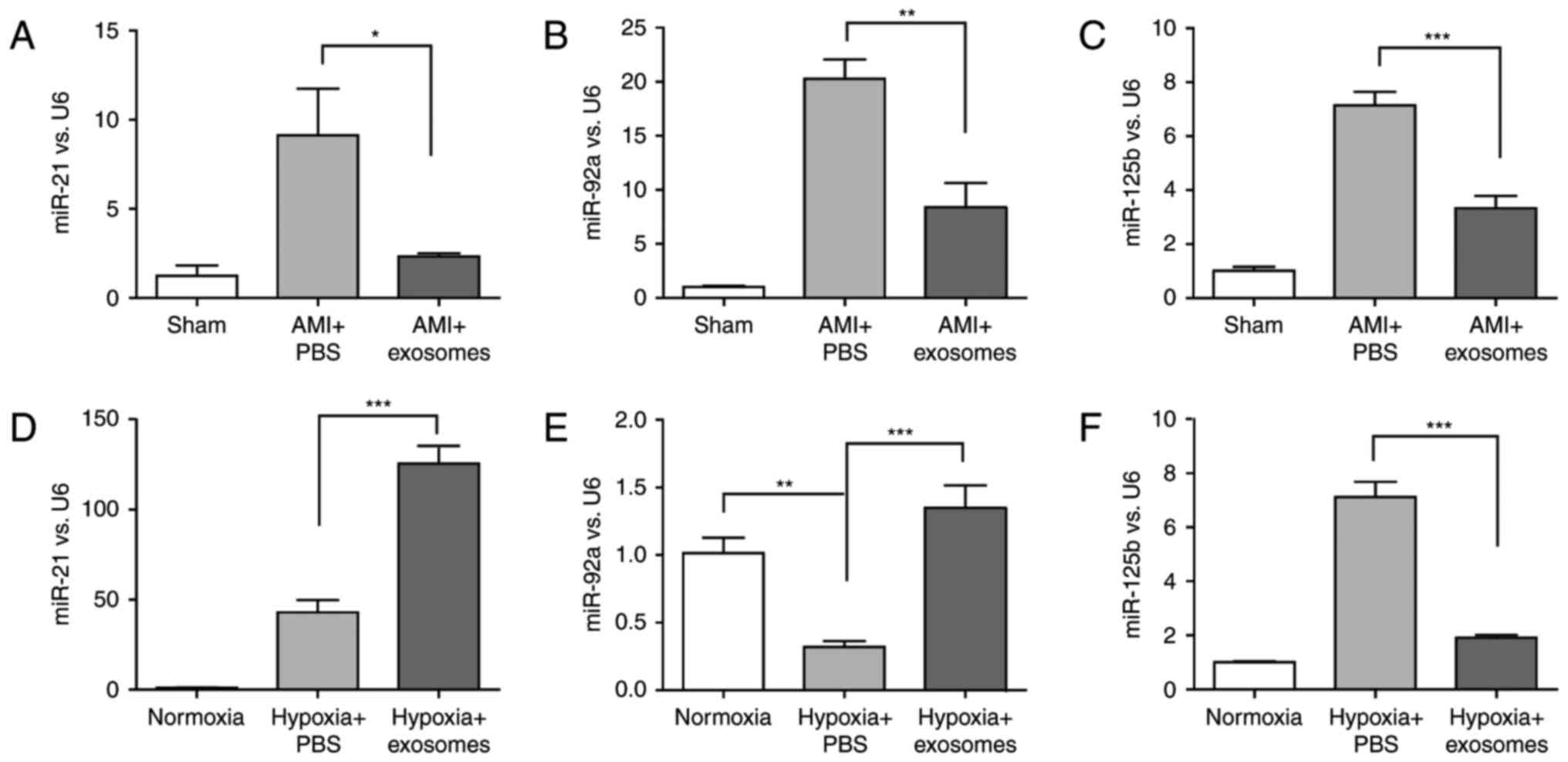
As presented in Fig.
4, miR-21-5p, miR-92a-3p and miR-125b-5p expression were
significantly altered the in vivo model after 2 days (sham
group, AMI + PBS group and AMI + exosomes group) and in H9C2(2-1)
cells exposed to normoxia or hypoxia for 48 h (normoxia, hypoxia +
PBS and hypoxia + exosomes groups). However, only the alterations
in miR-125b-5p expression were consistent in vivo and in
vitro. Simultaneously, the results revealed that there was a
negative association between Smad7 expression and miR-125b-5p
expression.
miR-125b-5p directly targets Smad7
To confirm whether miR-125b-5p directly targeted the
3′UTR of Smad7, 293T cells were used to perform a dual-luciferase
reporter gene assay. The 3′UTR of Smad7 was cloned downstream of a
luciferase gene and the predicted binding site of miR-125b-5p in
3′UTR of Smad7 was mutated to generate a mutant plasmid (Fig. 5A). As presented, the
overexpression of miR-125b-5p significantly decreased the
luciferase activity, whereas transfection miRNA negative control
did not (Fig. 5B). Additionally,
miR-125b-5p mimics were transfected into 293T cells to verify that
miR-125b-5p inhibited Smad7 expression. miR-125b-5p expression was
significantly increased in 293T cells following the transfection
miR-125b-5p mimics under normoxic conditions (Fig. 5C). Simultaneously, Smad7 protein
expression was significantly decreased (Fig. 5D and E).
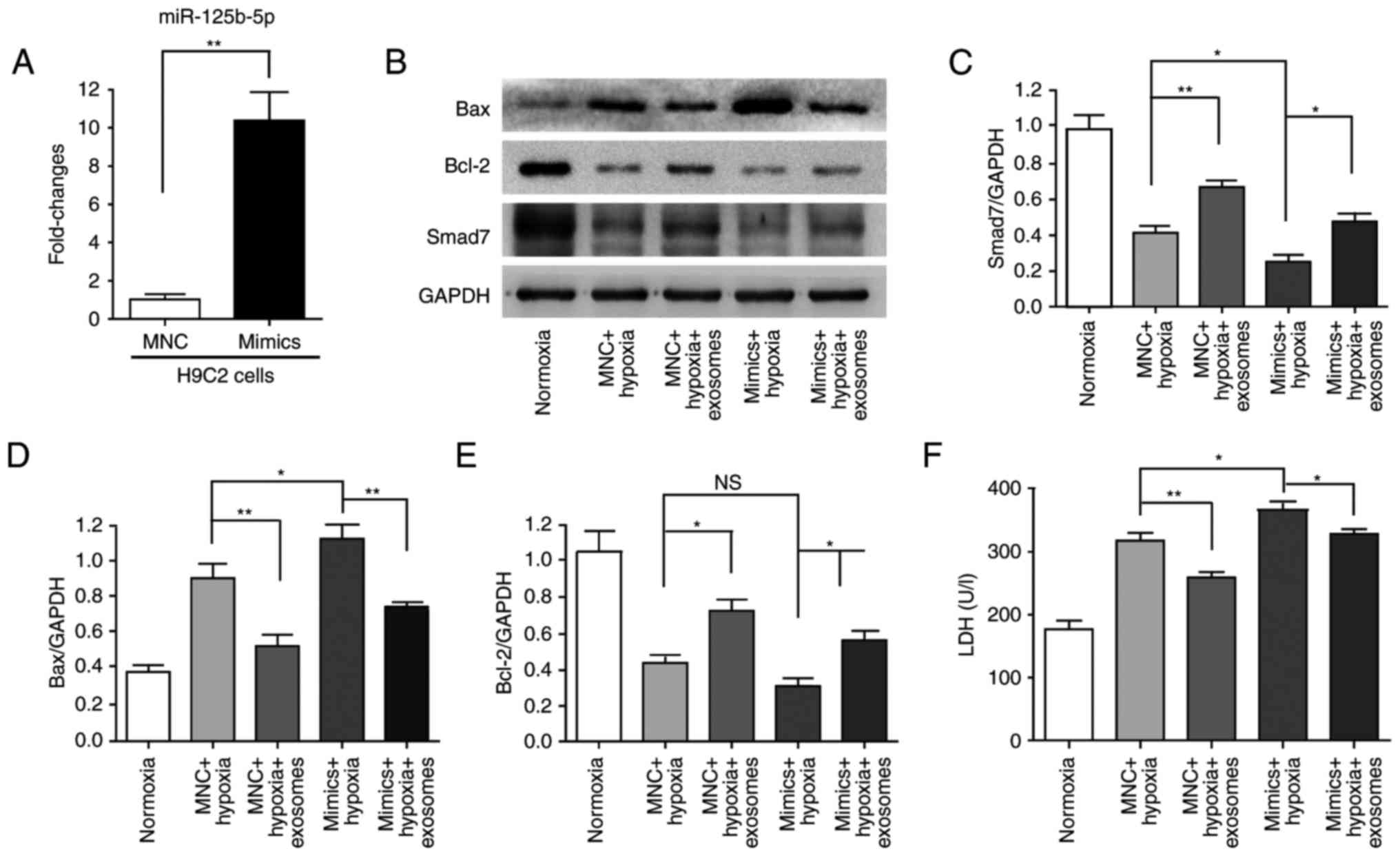
Subsequently, the cardioprotective effects of
hucMSC-exosomes were further investigated. miR-125b-5p expression
levels were also significantly increased following transfection
with miR-125b-5p mimics in H9C2(2-1) cells for 24 h (Fig. 6A). Subsequently, the transfected
H9C2(2-1) cells were maintained under hypoxia for 48 h. Western
blot analysis indicated hypoxia induced cell injury and
downregulation of Smad7 expression; hucMSC-exosomes partially
alleviated this phenomenon (Fig.
6B–E). The LDH assay revealed that LDH release was increased by
miR-125b-5p mimics compared with the MNC + hypoxia group;
hucMSC-exosomes also partially alleviated this phenomenon (Fig. 6F). According to these results,
H9C2(2-1) apoptosis and injury were increased by hypoxia and
miR-125b-5p, and hucMSC-exosomes protected H9C2(2-1) cells to
attenuate this effect. These results indicated that miR-125b-5p may
be a target gene of Smad7 and that hucMSC-exosomes may promote
Smad7 expression via downregulation of miR-125b-5p to improve
myocardial repair.
Discussion
An increasing number of studies have confirmed that
paracrine effects served a key role in stem cell mediated
myocardial repair (8,28–30). Exosomes are bioactive molecules
secreted by stem cells that can reduce myocardial injury (9). Our previous studies also reported
that hucMSC-exosomes may protect cardiomyocytes from injury,
promote angiogenesis and improve cardiac function following AMI
(11,12). However, the molecular reparation
mechanism of hucMSC-exosomes remains unclear. In the present study,
the effect of hucMSC-exosomes in repairing myocardial injury and
possible mechanisms were further investigated. The results
demonstrated that miR-125b-5p expression was increased in the
borderline area of the infraction myocardium and in H9C2(2-1) cells
following hypoxia injury, which indicated that hucMSC-exosomes may
promote Smad7 expression via the inhibition of miR-125b-5p to
improve myocardial repair.
Cell apoptosis and fibrosis have an important role
in myocardial injury. Notably, TGF-β/Smad is a classical key cell
signaling pathway involved in cardiac fibrosis and apoptosis during
myocardial injury (31,32). Smad7, a major negative regulator
that of TGF-β/Smad signaling, is be considered a protective protein
during myocardial injury. Smad7 expression was reported to be
decreased and associated with cardiac fibrosis in the infarcted rat
heart (18). Zhang et al
(15) reported that the
upregulation of Smad7 may prevent myocardial apoptosis induced by
H/R. Smad7 expression was significantly decreased in the borderline
area of the infraction myocardium or H9C2(2-1) cells following 48 h
of hypoxia in the present study. Following treatment with
hucMSC-exosomes, Smad7 expression was increased compared with the
AMI + PBS group or hypoxia + PBS group; simultaneously, myocardial
injury and apoptosis were attenuated.
Using bioinformatics analysis, miR-21-5p, miR-92a-3p
and miR-125b-5p were selected for verification using RT-qPCR.
Changes in miR-21-5p and miR-92a-3p following AMI and exosome
treatment demonstrated opposing results in vitro and in
vivo, only miR-125b-5p exhibited consistent results in
vitro and in vivo. These opposing results may be due to
two potential factors: i) Myocardial tissue is different to
H9C2(2-1) cells, it is a mixture of numerous cell types, in
addition to cardiomyocytes, it also includes stromal cells, immune
cells and other cells; ii) the environment is different between
H9C2(2-1) cells in vitro with myocardial tissue in
vivo, so the effects stimulus may vary to some extent; however,
further investigation is required. The experimental results of
miR-125b-5p were consistent in vivo and in vitro,
thus it was investigated further. Smad7 is a target gene of
miR-125b-5p, as confirmed by the dual luciferase activity assay;
miR-125b-5p may inhibit Smad7 expression by binding to the 3′UTR.
Studies on the effects of miR-125b have made great progress,
particularly in cancer. It may act as a tumor suppressor gene and
also as an oncogene; for example, miR-125b weakened the
epithelial-mesenchymal transitions phenotype in hepatocellular
carcinoma cells (33), whereas,
it may also promote tumor growth and favor malignant progression
(34). miR-125b-5p has gained
increasing attention associated with cardiovascular disease. Busk
and Cirera (35) reported that
miR-125b was increased in a rat model of early hypertrophic growth
of the left ventricle. Jia et al (24) reported that miR-125b-5p expression
levels were higher in patients with AMI than in healthy subjects
and higher expression levels may be of diagnostic value for early
diagnosis of AMI. Therefore, it may be an important regulator of
the development of cardiovascular disease.
In the present study, miR-125b-5p expression was
significantly increased in the borderline area of the infraction
myocardium and in H9C2(2-1) cells following hypoxia for 48 h,
whereas Smad7 expression was decreased. Following treatment with
hucMSC-exosomes in vivo or in vitro, this phenomenon
revealed a significant change: miR-125b-5p expression was decreased
while Smad7 expression was increased compared with in the AMI + PBS
or hypoxia + PBS groups, respectively. To further investigate the
effects of hucMSC-exosomes on miR-125b-5p/Smad7, H9C2(2-1) cells,
transfected with miR-125b-5p mimics, were exposed to hypoxia for 48
h. The present study reported that Bax expression was markedly
increased and Smad7 expression was further decreased compared with
in the MNC + hypoxia group. Additionally, hucMSC-exosomes
attenuated this phenomenon. However, some problems require further
investigation. miR-125b-5p has numerous target genes; it is unclear
whether Smad7 be the most important miR-125b-5p target gene in
cardiomyocytes. In addition, how hucMSC-exosomes induce
miR-125b-5p-mediated downregulation remains unknown.
hucMSC-exosomes may contain a competing endogenous RNA, for
example, long noncoding RNA, which may competitively bind
miR-125b-5p to facilitate Smad7 expression in cardiomyocytes.
However, this hypothesis requires further investigation in the
future.
In conclusion, the findings of the present study
demonstrated that hucMSC-exosomes may promote Smad7 expression by
inhibiting miR-125b-5p to improve myocardial repair. The results of
the present study provide novel insight into the mechanisms of
myocardial repair following AMI.
Acknowledgments
Not applicable.
Notes
[1]
Funding
The present study was supported by National Natural
Science Foundation of China (grant nos. 81270214 and 81472334).
[2] Availability
of data and materials
All data and materials are available from the
corresponding author on reasonable request.
[3] Authors'
contributions
All authors participated in the design,
interpretation of the studies and analysis of the data and review
of the manuscript. XLW and YYZ conducted the experiments and
performed the statistical analysis. LS, YS and ZQL performed the
statistical analysis. XDZ, CGX and HGJ designed the study. MW and
WRX designed the study and performed the statistical analysis. WZ
designed the study, provided financial support and wrote the
manuscript. All authors read and approved the final manuscript.
[4] Ethics
approval and consent to participate
The present study was approved by the Laboratory
Animal Management Committee of Jiangsu University (Zhenjiang,
China). The collection and use of hucMSCs was approved by the
Ethics Committee of Jiangsu University (Zhenjiang, China). All
individuals provided informed consent for the use of their tissue
in the present study.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Hinkel R, El-Aouni C, Olson T, Horstkotte
J, Mayer S, Müller S, Willhauck M, Spitzweg C, Gildehaus FJ,
Münzing W, et al: Thymosin beta4 is an essential paracrinf actor of
embryonic endothelial progenitor cell-mediated cardioprotection.
Circulation. 117:2232–2240. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leopez AD and Murray CC: The global burden
of disease, 1990–2020. Nat Med. 4:1242–1243. 1998.
|
|
3
|
Poynter JA, Herrmann JL, Manukyan MC, Wang
Y, Abarbanell AM, Weil BR, Brewster BD and Meldrum DR:
Intracoronary mesenchymal stem cells promote postischemic
myocardial functional recovery, decrease inflammation, and reduce
apoptosis via a signal transducer and activator of transcription 3
mechanism. J Am Coll Surg. 213:253–260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Uemura R, Xu M, Ahmad N and Ashraf M: Bone
marrow stem cells prevent left ventricular remodeling of ischemic
heart through paracrine signaling. Circ Res. 98:1414–1421. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quevedo HC, Hatzistergos KE, Oskouei BN,
Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu
Q, McNiece I, et al: Allogeneic mesenchymal stem cells restore
cardiac function in chronic ischemic cardiomyopathy via trilineage
differentiating capacity. Proc Natl Acad Sci USA. 106:14022–14027.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Angoulvant D, Ivanes F, Ferrera R,
Matthews PG, Nataf S and Ovize M: Mesenchymal stem cell conditioned
media attenuates in vitro and ex vivo myocardial reperfusion
injury. J Heart Lung Transplant. 30:95–102. 2011. View Article : Google Scholar
|
|
7
|
Gnecchi M, Zhang Z, Ni A and Dzau VJ:
Paracrine mechanisms in adult stem cell signaling and therapy. Circ
Res. 103:1204–1219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Imanishi Y, Saito A, Komoda H,
Kitagawa-Sakakida S, Miyagawa S, Kondoh H, Ichikawa H and Sawa Y:
Allogenic mesenchymal stem cell transplantation has a therapeutic
effect in acute myocardial infarction in rats. J Mol Cell Cardiol.
44:662–671. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khan M, Nickoloff E, Abramova T, Johnson
J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VN,
Benedict C, et al: Embryonic stem cell-derived exosomes promote
endogenous repair mechanisms and enhance cardiac function following
myocardial infarction. Circ Res. 117:52–64. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shao L, Zhang Y, Lan B, Wang J, Zhang Z,
Zhang L, Xiao P, Meng Q, Geng YJ, Yu XY and Li Y: MiRNA-sequence
indicates that mesenchymal stem cells and exosomes have similar
mechanism to enhance cardiac repair. Biomed Res Int.
2017:41507052017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao Y, Sun X, Cao W, Ma J, Sun L, Qian H,
Zhu W and Xu W: Exosomes derived from human umbilical cord
mesenchymal stem cells relieve acute myocardial ischemic injury.
Stem Cells Int. 2015:7616432015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma J, Zhao Y, Sun L, Sun X, Zhao X, Sun X,
Qian H, Xu W and Zhu W: Exosomes derived from AKt-modified human
umbilical cord mesenchymal stem cells improve cardiac regeneration
and promote angiogenesis via activating platelet-derived growth
factor D. Stem Cells Transl Med. 6:51–59. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miyazawa K and Miyazono K: Regulation of
TGF-β family signaling by inhibitory smads. Cold Spring Harb
Perspect Biol. 9:pii:a022095. 2017. View Article : Google Scholar
|
|
14
|
Duan Y, Zhu W, Liu M, Ashraf M and Xu M:
The expression of Smad signaling pathway in myocardium and
potential therapeutic effects. Histol Histopathol. 32:651–659.
2017.
|
|
15
|
Zhang B, Zhou M, Li C, Zhou J, Li H, Zhu
D, Wang Z, Chen A and Zhao Q: MicroRNA-92a inhibition attenuates
hypoxia/reoxygenation-induced myocardiocyte apoptosis by targeting
Smad7. PLoS One. 9:e1002982014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang C, Wan L and Liu J: Effect of
Xinfeng capsule on nuclear factor kappa B/tumor necrosis factor
alpha and transforming growth factor beta 1/Smads pathways in rats
with cardiac injuries induced by adjuvant arthritis. J Tradit Chin
Med. 36:92–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Q, Chen H, Zheng D, Kuang C, Fang H,
Zou B, Zhu W, Bu G, Jin T, Wang Z, et al: Smad7 is required for the
development and function of the heart. J Biol Chem. 284:292–300.
2009. View Article : Google Scholar :
|
|
18
|
Wang B, Omar A, Angelovska T, Drobic V,
Rattan SG, Jones SC and Dixon IM: Regulation of collagen synthesis
by inhibitory Smad7 in cardiac myofibroblasts. Am J Physiol Heart
Circ Physiol. 293:H1282–H1290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thum T, Galuppo P, Wolf C, Fiedler J,
Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J,
Haverich A, et al: MicroRNAs in the human heart: A clue to fetal
gene reprogramming in heart failure. Circulation. 116:258–267.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiao Y, Zhang X, Fan S, Cui G and Shen Z:
MicroRNA-497 inhibits cardiac hypertrophy by targeting Sirt4. PLoS
One. 11:e01680782016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liao C, Gui Y, Guo Y and Xu D: The
regulatory function of microRNA-1 in arrhythmias. Mol Biosyst.
12:328–333. 2016. View Article : Google Scholar
|
|
23
|
Lu C, Wang X, Ha T, Hu Y, Liu L, Zhang X,
Yu H, Miao J, Kao R, Kalbfleisch J, et al: Attenuation of cardiac
dysfunction and remodeling of myocardial infarction by
microRNA-130a are mediated by suppression of PTEN and activation of
PI3K dependent signaling. J Mol Cell Cardiol. 89:87–97. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jia K, Shi P, Han X, Chen T, Tang H and
Wang J: Diagnostic value of miR-30d-5p and miR-125b-5p in acute
myocardial infarction. Mol Med Rep. 14:184–194. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bie ZD, Sun LY, Geng CL, Meng QG, Lin XJ,
Wang YF, Wang XB and Yang J: MiR-125b regulates SFRP5 expression to
promote growth and activation of cardiac fibroblasts. Cell Biol
Int. 40:1224–1234. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qiao C, Xu W, Zhu W, Hu J, Qian H, Yin Q,
Jiang R, Yan Y, Mao F, Yang H, et al: Human mesenchymal stem cells
isolated from the umbilical cord. Cell Biol Int. 32:8–15. 2008.
View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
28
|
Kuraitis D, Ruel M and Suuronen EJ:
Mesenchymal stem cells for cardiovascular regeneration. Cardiovasc
Drugs Ther. 25:349–362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gnecchi M, Zhang Z, Ni A and Dzau VJ:
Paracrine mechanisms in adult stem cell signaling and therapy. Circ
Res. 103:1204–1219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tachibana A, Santoso MR, Mahmoudi M,
Shukla P, Wang L, Bennett M, Goldstone AB, Wang M, Fukushi M, Ebert
AD, et al: Paracrine effects of the pluripotent stem cell-derived
cardiac myocytes salvage the injured myocardium. Circ Res.
121:e22–e36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao M, Zheng S, Yang J, Wu Y, Ren Y, Kong
X, Li W and Xuan J: Suppression of TGF-β1/Smad signaling pathway by
sesamin contributes to the attenuation of myocardial fibrosis in
spontaneously hypertensive rats. PLoS One. 10:e01213122015.
View Article : Google Scholar
|
|
32
|
Schröder D, Heger J, Piper H and Euler G:
Angiotensin II stimulates apoptosis via TGF-beta1 signaling in
ventricular cardiomyocytes of rat. J Mol Med (Berl). 84:975–983.
2006. View Article : Google Scholar
|
|
33
|
Zhou JN, Zeng Q, Wang HY, Zhang B, Li ST,
Nan X, Cao N, Fu CJ, Yan XL, Jia YL, et al: MicroRNA-125b
attenuates epithelial-mesenchymal transitions and targets stem-like
liver cancer cells through small mothers against decapentaplegic 2
and 4. Hepatology. 62:801–815. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang L, Ge Y and Fuchs E: miR-125b can
enhance skin tumor initiation and promote malignant progression by
repressing differentiation and prolonging cell survival. Genes Dev.
28:2532–2546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Busk PK and Cirera S: MicroRNA profiling
in early hypertrophic growth of the left ventricle in rats. Biochem
Biophys Res Commun. 396:989–993. 2010. View Article : Google Scholar : PubMed/NCBI
|