Introduction
Osteoporosis is a type of bone disease characterized
by osteoclastic bone resorption, which overtakes bone synthesis
(1). Osteoclasts are
multinucleated cells that are differentiated from hematopoietic
precursors and are involved in bone remodeling (2). An increased number of osteoclasts
and enhanced bone resorption contribute to excessive bone
breakdown; therefore, osteoclasts are considered to serve a crucial
physiological and pathological role in osteoporosis, via
osteoclastic bone resorption. Glucocorticoid-induced osteoporosis
is the most common secondary form of osteoporosis, which leads to
decreased bone formation and increased bone resorption and increase
the expression of cytokines, including RANKL (3). Dexamethasone (Dex) is a synthetic
glucocorticoid, which causes loss of bone mass, apoptosis in both
osteoblastic and osteocytic cells and ROS generation (4). However, the underlying molecular
mechanism remains unclear.
Osteoclasts are formed and differentiated under the
control of numerous cytokines, including macrophage
colony-stimulating factor (M-CSF), receptor activator of nuclear
factor (NF)-κB (RANK) ligand (RANKL) and osteoprotegerin (OPG)
(5,6). M-CSF is associated with the
proliferation, survival and differentiation of early precursors,
and is constitutively produced by various types of cell; regulated
secretion of M-CSF has pathological consequences in the context of
osteoclasts (7). The interaction
between RANKL and its cognate RANK initiates a cascade of
intracellular signaling events, including NF-κB, protien kinase B,
mitogen-activated protien kinase and calcium-dependent kinase
(8,9). OPG, which is a soluble decoy
receptor of RANKL, negatively regulates osteoclast differentiation
and bone resorption (10).
Modulation of these differentiation-associated signaling pathways
may be considered a therapeutic strategy for the treatment of
certain skeletal diseases.
Recently, novel agents that promote osteoblastic
differentiation, thus increasing bone formation, have been
considered a novel therapeutic approach for the treatment of
osteoporosis. Bone morphogenetic protein 2 (BMP2) serves a role in
postnatal bone formation in animal models and in clinical studies
(11,12). BMP2 signals have been reported to
be mediated by activation of SMAD family member (Smad)-1, -5 and -8
upon ligand binding (13).
Furthermore, activation of the p38 pathway is important for
BMP2-induced signaling and influences osteoblastic differentiation
(14).
Mangiferin is a xanthone glucoside that is commonly
found in mangoes and papayas, and possesses antioxidant, antiviral,
antitumor and anti-inflammatory functions, and exerts gene
regulatory effects (15–17). Luo et al previously
demonstrated that mangiferin attenuates contusive spinal cord
injury in rats via oxidative stress and the B-cell lymphoma 2
(Bcl-2)/Bcl-2-associated X protein (Bax) pathway (18). RANKL-induced activation of NF-κB
and extracellular signal-regulated kinase pathways in
osteoclastogenesis has also been reported to be inhibited by
mangiferin treatment (1). Due to
its anti-NF-κB properties, mangiferin may be considered a potential
alternative medicine for the treatment of osteolytic bone
diseases.
The present study aimed to investigate the effects
of mangiferin on osteoblast function and oxidative modification
following exposure of MC3T3-E1 cells to 1 µM Dex. The
results indicated that mangiferin may protect MC3T3-E1 cells
against apoptosis and oxidative stress via activating the
BMP2/Smad-1 signaling pathway, thus suggesting that it may have
potential therapeutic value for the treatment of osteoporosis.
Materials and methods
Cell culture
The MC3T3-E1 murine preosteoblastic calvarial cell
line was obtained from Shanghai Institute of Biochemistry and Cell
Biology (Shanghai, China). MC3T3-E1 cells were cultured in
α-minimum essential medium (HYClone; GE healthcare Life Sciences,
Logan, UT, USA) supplemented with 10% fetal calf serum (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml
penicillin G and 100 µg/ml streptomycin (both from Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China) at
37°C in a humidified atmosphere containing 5% CO2.
Cell treatment
MC3T3-E1 cells were treated with different doses of
Dex (0.01, 0.1, 1 and 10 µM) or mangiferin (10, 20, 30, 40
and 60 µM) for screening optimum concentration. Then,
MC3T3-E1 cells were treated with 1 µM Dex and different
doses of mangiferin (30, 40 and 60 µM). Last, MC3T3-E1 cells
were treated with 1 µM Dex with or without BMP2
overexpression in the absence or presence of 60 µM
mangiferin treatment. MC3T3-E1 cells without treatment used as
control.
Vector construction
pLVX-AcGFP-C1, psPAX2 and pMD2G were purchased from
Addgene, Inc. (Cambridge, MA, USA). Plasmid containing full length
BMP2 was purchased from Sangon Biotech Co., Ltd. (Shanghai, China).
Full length BMP2 was amplified using polymerase chain reaction
(PCR). The primers used were as follows: BMP2, forward
5′-GCGAATTCATGGTGGCCGGGACCCGCTGT-3′ and reverse
5′-CGGGATCCACGACACCCGCAGCCCTCCACA-3′. Subsequently, the PCR
products were digested using EcoRI and BamHI, and
were cloned into pLVX-AcGFP-C1.
Lentiviral production and
transduction
BMP2 was delivered into MC3T3-E1 cells using a
lentiviral transfection system. Briefly, 239T cells were seeded in
60 mm dishes, and after 24 h were cotransfected with 2 µg
plasmid vector, 1 µg pLVX-AcGFP-C1-BMP2, 0.1 µg
psPAX2 and 0.9 µg pMD2G using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The recombinant lentivirus
pLVX-AcGFP-C1-BMP2 (pLVX-BMP2) was collected 48 h post-transfection
and was used to infect MC3T3-E1 cells at a multiplicity of
infection of 20 in the presence of 8 µg/ml polybrene
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Blank
pLVX-AcGFP-C1 was used as a negative control.
Cell viability assay
Cell viability was evaluated using Cell Counting
kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto,
Japan), as previously described (19). Briefly, MC3T3-E1 cells were seeded
in 96-well plates at a density of 5×104 cells/well, and
were cultured at 37°C in a humidified atmosphere containing 5%
CO2. Once the cells reached 80% confluence, they were
treated with conditioned medium prepared from α-minimum essential
medium containing either different doses of Dex (0.01, 0.1, 1 and
10 µM), mangiferin (10, 20, 30, 40 and 60 µM), 1 μM
Dex and different doses of mangiferin (30, 40 and 60 µM) or
1 µM Dex with or without 60 µM mangiferin treatment.
At the indicated timepoints, the culture supernatant was removed,
cells were washed with PBS, and 100 µl fresh medium mixed
with CCK-8 solution was added to each well, followed by a further 1
h incubation at 37°C. Absorbance of the supernatant was measured at
a wavelength of 450 nm using a spectrophotometric microplate reader
(BioTek Instruments, Inc., Winooski, VT, USA).
ALP activity assay
Induction of ALP is an unequivocal marker of bone
cell differentiation. Briefly, MC3T3-E1 cells were plated in
96-well plates. After the cells (5×104 cells/well) were
treated as indicated, ALP activity in the supernatant was measured
according to a previously described method (20). Ultraviolet absorbance of the
samples and standards was measured at 520 nm. Total protein was
assessed according to the Bradford method (21).
Cell apoptosis assay
Cell apoptosis was evaluated by flow cytometry (BD
Biosciences, Franklin Lakes, NJ, USA). MC3T3-E1 cells were plated
in 6-well plates. After the cells (3×105 cells/well)
were treated as indicated, 195 µl Annexin V-fluorescein
isothiocyanate (Beyotime Institute of Biotechnology, Haimen, China)
and 5 µl propidium iodide were added, according to the
manufacturer's protocol, and were then incubated for 10 min in the
dark at room temperature prior to flow cytometric analysis (BD
Accuri C6; software version 1.0.264.21; BD Biosciences).
Intracellular reactive oxygen species
(ROS) assay
Intracellular ROS content was determined using the
fluorescent probe dihydroethidium (DHE; Beyotime Institute of
Biotechnology), followed by flow cytometry. DHE is a poorly
fluorescent 2-electron reduction product of ethidium that, on
oxidation, produces DNA-sensitive fluorochromes that generate a red
nuclear fluorescence when excited at 510 nm. Briefly, MC3T3-E1
cells were plated in 6-well plates. After the cells
(3×105 cells/well) were treated as indicated, 50
µM DHE was added, according to the manufacturer's protocol,
and fluorescence intensity was measured using flow cytometry.
ELISA
Tumor necrosis factor (TNF)-α, interleukin (IL)-6
and M-CSF secretion was measured by ELISA. Briefly, MC3T3-E1 cells
were plated in 96-well plates. Following centrifugation at 400 × g
for 1 min at 4°C, the supernatant was obtained. After the cells
(5×104 cells/well) were treated as indicated, the
relative content of each secreted cytokine in the supernatant was
measured by ELISA assay, according to the manufacturer's protocols
[IL-6 mouse ELISA kit (KMC0061; Thermo Fisher Scientific, Inc.);
mouse GM-CSF ELISA kit (MBS494169; MyBioSource Inc., San Diego, CA,
USA); mouse TNF-α ELISA kit (RAB0477; Sigma-Aldrich; Merck
KGaA)].
Reverse transcription-quantitative PCR
(RT-qPCR) assay
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. cDNA was synthesized using a cDNA
synthesis kit (Thermo Fisher Scientific Inc.) according to
manufacturer's protocol. qPCR was performed using SYBR-Green
(Takara Biotechnology Co., Ltd., Dalian, China), and data
collection was conducted using an ABI 7500 (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The PCR cycling conditions were as
follows: 95°C for 10 min, followed by 40 cycles at 95°C for 15 sec
and 60°C for 45 sec, a final extension step of 95°C for 15 sec,
60°C for 1 min, 95°C for 15 sec and 60°C for 15 sec. Primer
sequences are as follows: BMP2, forward 5′-AGGATTAGCAGGTCTTTG-3′,
reverse 5′-TCACTGAAGTCCACATAC-3′; and GAPDH, forward
5′-ATCACTGCCACCCAGAAG-3′ and reverse 5′-TCCACGACGGACACATTG-3′.
GAPDH was used as an internal control for normalization. Gene
expression was calculated using the 2−ΔΔCq method
(22).
Western blot analysis
MC3T3-E1 cells were plated in 35 mm Petri dishes.
Once cells (3×105 cells/well) had reached 80%
confluence, they were treated as indicated. Subsequently, MC3T3-E1
cells were harvested and resuspended in ice-cold cell lysis
solution (Beijing Solarbio Science & Technology Co., Ltd.) and
the homogenate was centrifuged at 400 × g for 15 min at 4°C. The
protein concentration was determined using a Bicinchoninic Acid
Protein Assay kit (cat. no. PICPI23223; Thermo Fisher Scientific,
Inc.). The supernatant was transferred to a fresh tube and 100
µg protein from each sample was separated by 12% SDS-PAGE.
The proteins were then transferred onto polyvinylidene difluoride
membranes. The membranes were blocked with 5% fat-free milk
overnight at 4°C and were incubated with primary antibodies
specific to BMP2 (1:1,000; ab82511; Abcam, Cambridge, MA, USA),
osterix (OSX) (1:400; sc-22538; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), OCN (1:500; ab93876; Abcam), OPG (1:1,000;
ab183910; Abcam), RANK (1:200; ab200369; Abcam), RANKL (1:1,000;
ab45039; Abcam), Bcl-2 (1:300; sc-492; Santa Cruz Biotechnology,
Inc.), Bax (1:300; sc-493; Santa Cruz Biotechnology, Inc.),
phosphorylated (p)-Smad-1 (1:500; ab73211; Abcam), Smad-1 (1:1,000;
9743; Cell Signaling Technology, Inc., Danvers, MA, USA) and GAPDH
(1:1,500; 9743; Cell Signaling Technology, Inc.) overnight with
gentle agitation at 4°C. Subsequently, membranes were incubated
with horseradish peroxidase-conjugated secondary antibodies
(1:1,000; A0208, A0181 and A0216; Beyotime Institute of
Biotechnology) for 1 h at 37°C. The blots were visualized using
enhanced chemiluminescence (EMD Millipore, Billerica, MA, USA) and
signals were semi-quantified using ImageJ 1.41o software (National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All data are presented as the means ± standard
deviation and are representative of experiments conducted in
triplicate. Statistical analyses were performed using GraphPad
Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA).
One-way analysis of variance followed by Tukey's posthoc test was
used to compare data from the various groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Mangiferin attenuates Dex-induced
inhibition of MC3T3-E1 cell viability
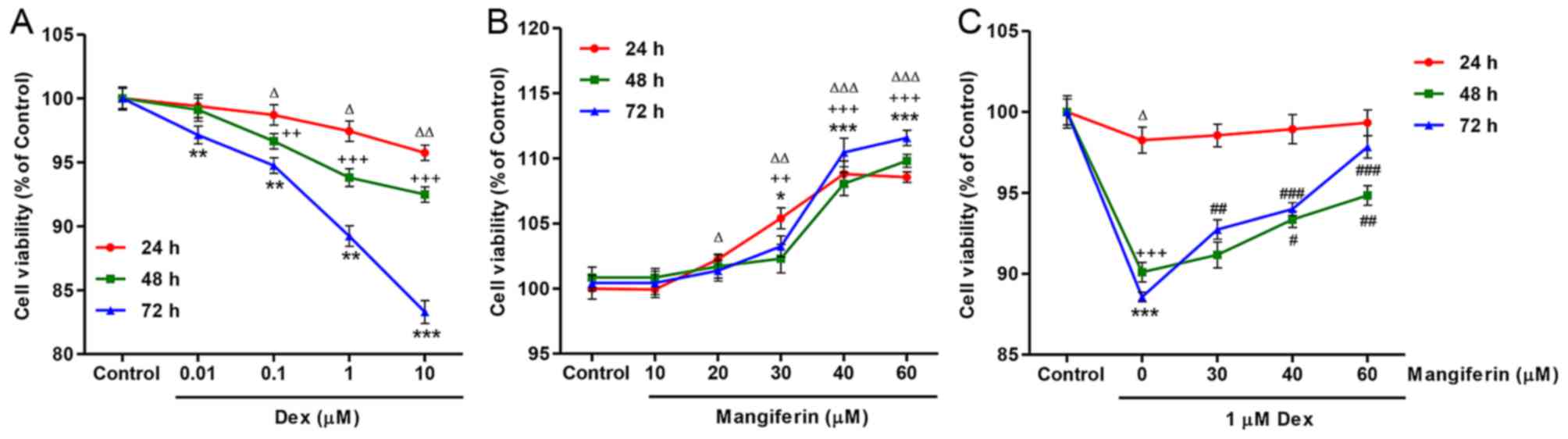
To investigate the effects of mangiferin on
Dex-induced cytotoxicity, cell viability was detected by CCK-8
assay. As presented in Fig. 1A,
exposure of MC3T3-E1 cells to Dex, at concentrations ranging
between 0.01 and 10 µM, for 24, 48 and 72 h led to a
decrease in cell viability in dose-and time-dependent manners.
Conversely, exposure of MC3T3-E1 cells to mangiferin, at
concentrations ranging between 10 and 60 µM, for 24, 48 and
72 h led to an increase in cell viability in dose- and
time-dependent manners (Fig. 1B).
In addition, the decreased cell viability induced by 1 µM
Dex treatment for 24, 48 and 72 h was significantly inhibited by
pretreatment with mangiferin at 30, 40 and 60 µM for 3 h
(Fig. 1C).
Mangiferin attenuates Dex-induced
inhibition of MC3T3-E1 cell differentiation
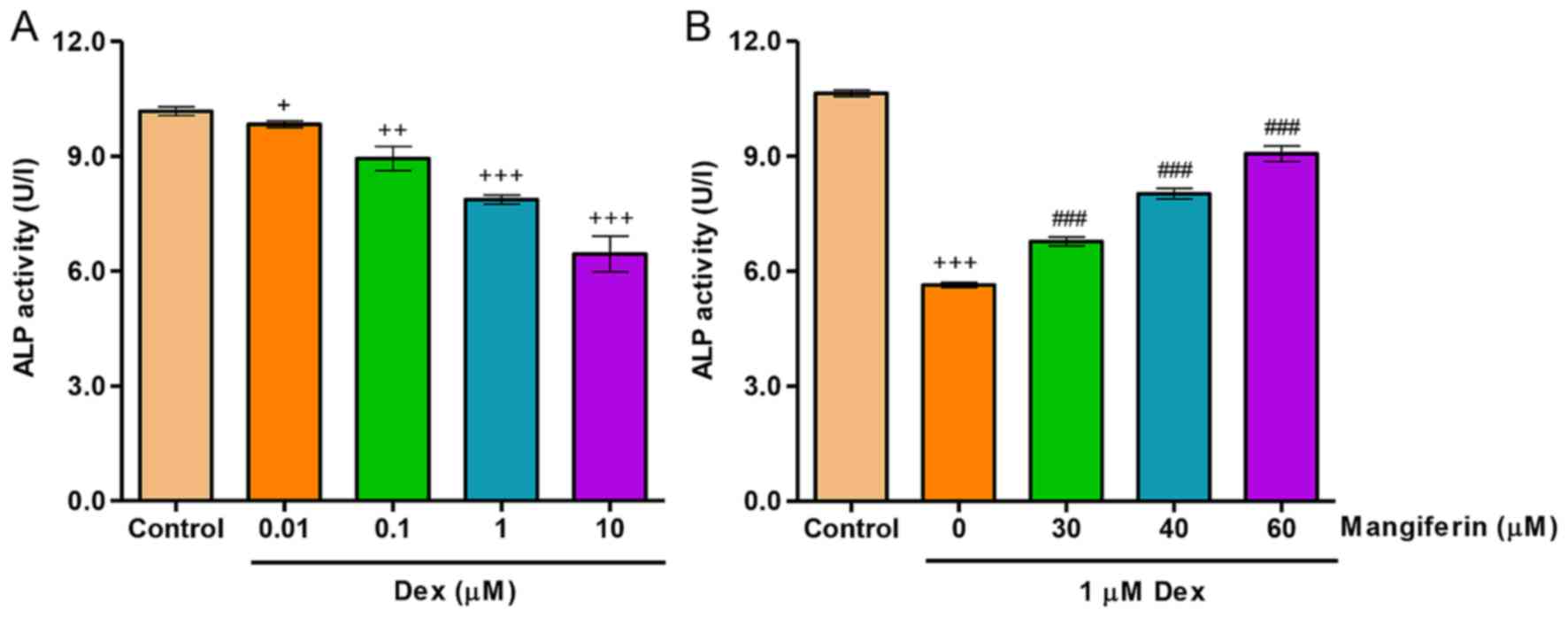
To investigate the effects of mangiferin on
Dex-induced differentiation, ALP activity was detected in MC3T3-E1
cells. As shown in Fig. 2A,
exposure of MC3T3-E1 cells to Dex, at concentrations ranging
between 0.01 and 10 µM, for 48 h led to a decrease in ALP
activity in a dose-dependent manner. Conversely, decreases in ALP
activity induced by 1 µM Dex treatment for 48 h were
significantly inhibited by pretreatment with mangiferin at 30, 40
and 60 µM for 3 h (Fig.
2B).
Mangiferin ameliorates Dex-induced
apoptosis of MC3T3-E1 cells
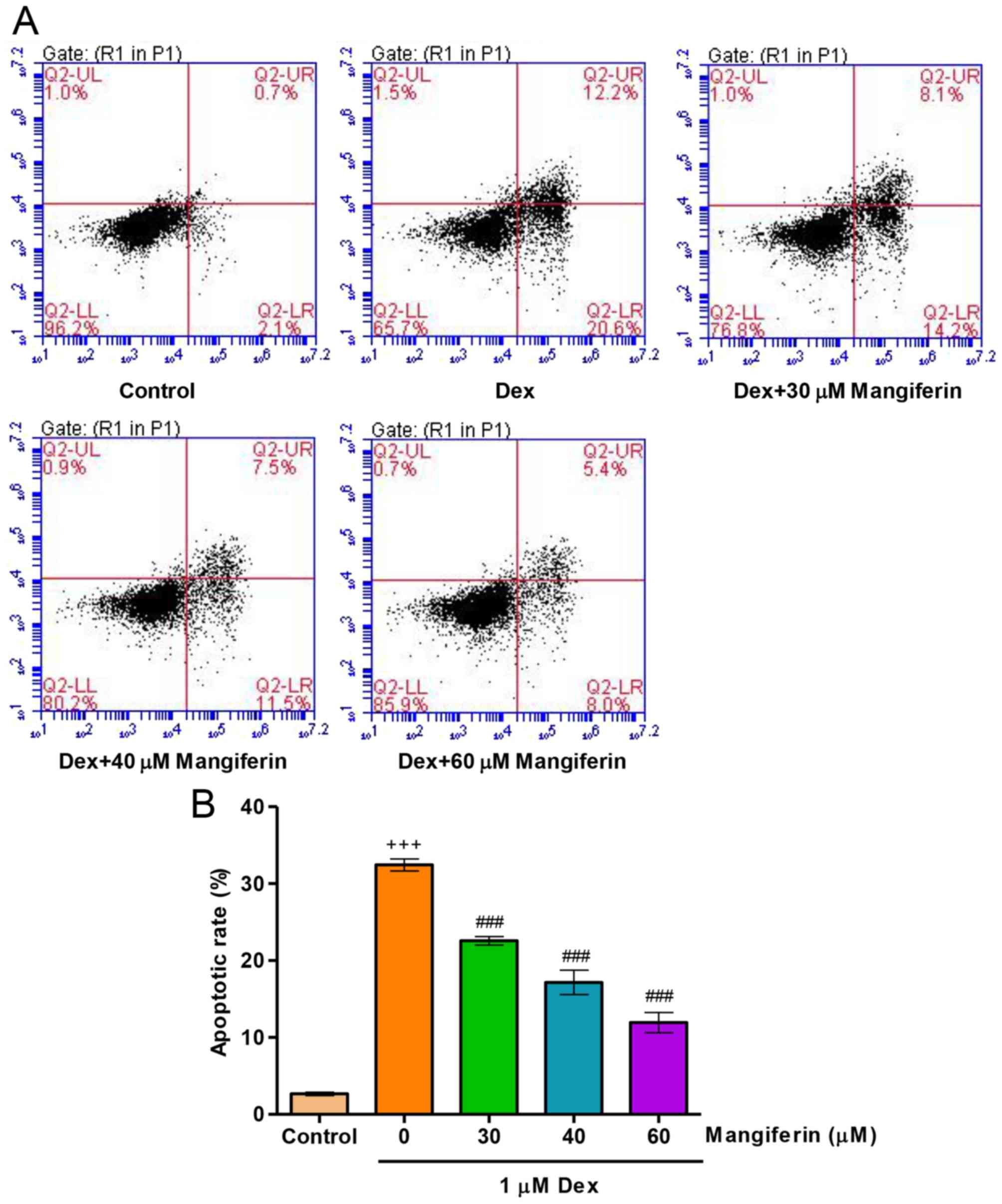
To elucidate whether the cytoprotective effects of
mangiferin were associated with the amelioration of Dex-induced
apoptosis of MC3T3-E1 cells, the apoptotic rate was measured by
flow cytometry. Exposure of MC3T3-E1 cells to 1 µM Dex for
48 h led to an increase in apoptotic rate (Fig. 3A and B). Prior to Dex exposure,
pretreatment with mangiferin at 30, 40 and 60 µM for 3 h
significantly decreased apoptotic rate in Dex-induced MC3T3-E1
cells. These findings indicated that Dex treatment impairs the
endogenous anti-apoptotic defense mechanism.
Mangiferin suppresses Dex-induced
oxidative stress in MC3T3-E1 cells
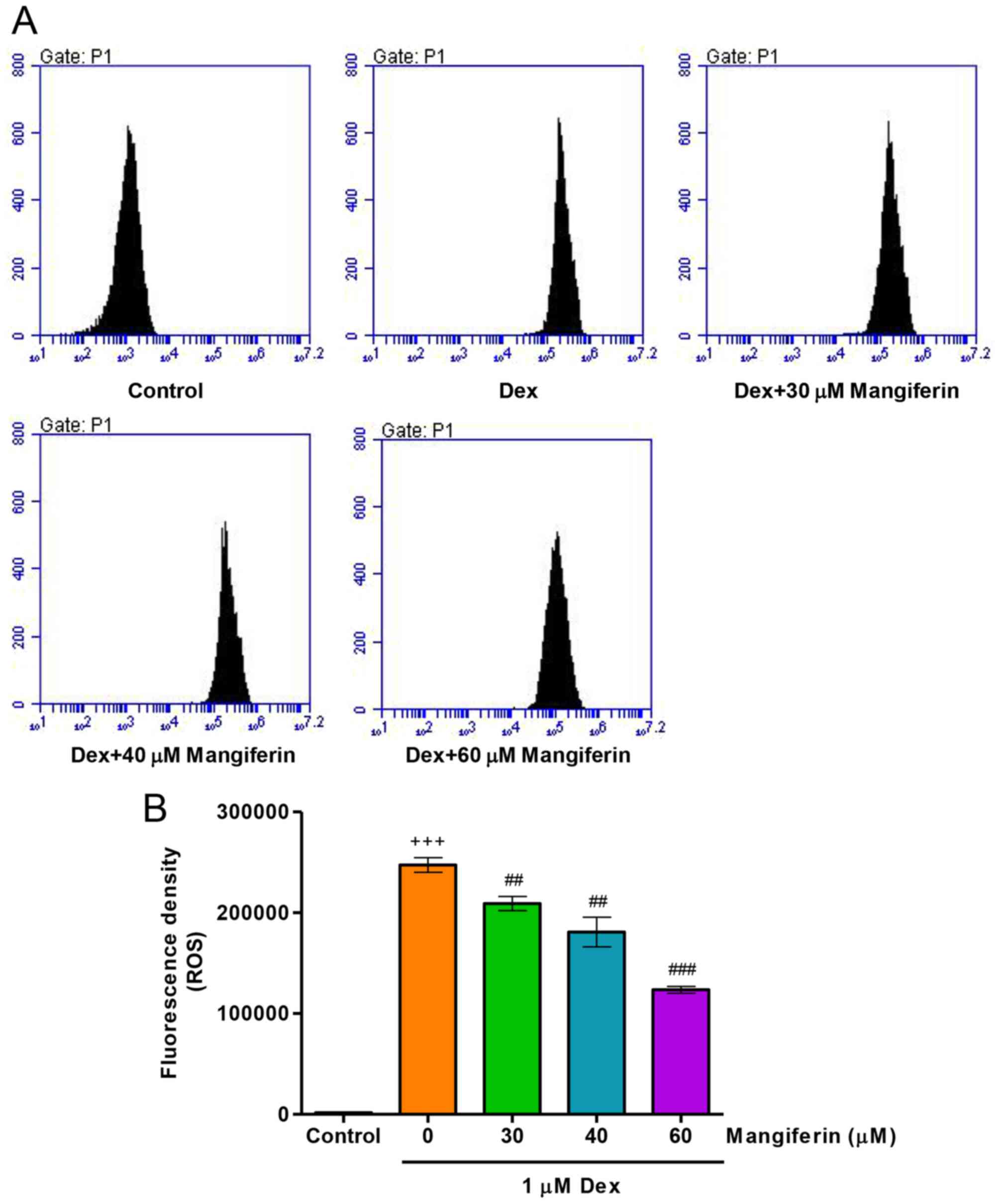
To elucidate whether the cytoprotective effects of
mangiferin were associated with antioxidation in Dex-induced
MC3T3-E1 cells, intracellular ROS levels were measured by flow
cytometry. Treatment of MC3T3-E1 cells with 1 µM Dex for 24
h significantly increased intracellular ROS levels (Fig. 4A and B). Notably, pretreatment
with mangiferin at 30, 40 and 60 µM for 3 h markedly
attenuated the augmented effects of Dex on intracellular ROS levels
in MC3T3-E1 cells. These findings suggested that mangiferin-induced
inhibition of cytotoxicity may be associated with its antioxidant
effects.
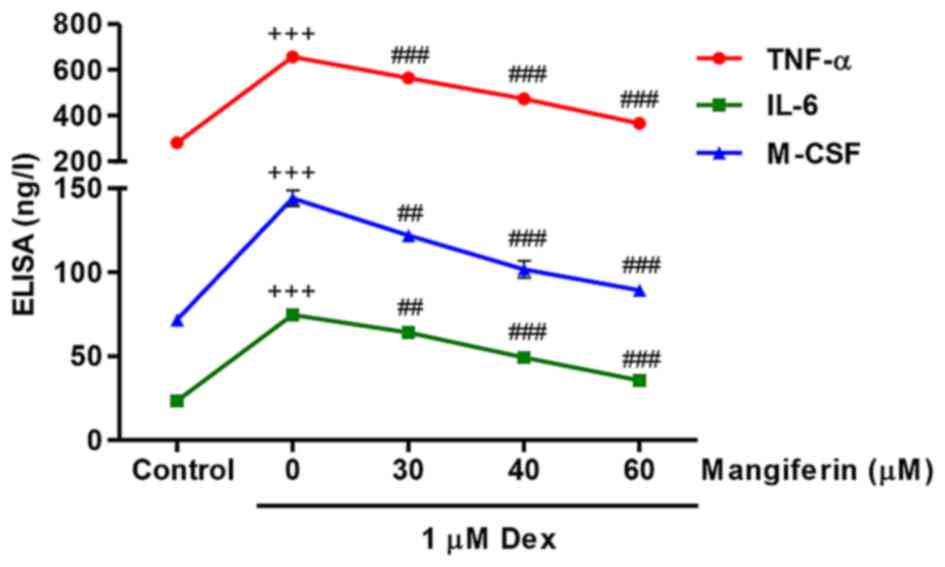
Mangiferin inhibits Dex-induced TNF-α,
IL-6 and M-CSF secretions from MC3T3-E1 cells
The present study measured TNF-α, IL-6 and M-CSF
secretions from MC3T3-E1 cells in response to Dex and mangiferin.
Following exposure of MC3T3-E1 cells to 1 µM Dex for 48 h,
TNF-α, IL-6 and M-CSF secretions were significantly increased
(Fig. 5). Conversely,
pretreatment with 30, 40 and 60 µM mangiferin for 3 h prior
to Dex exposure markedly inhibited TNF-α, IL-6 and M-CSF secretions
from MC3T3-E1 cells. These results indicated that mangiferin may
inhibit inflammation and M-CSF secretion in Dex-treated MC3T3-E1
cells.
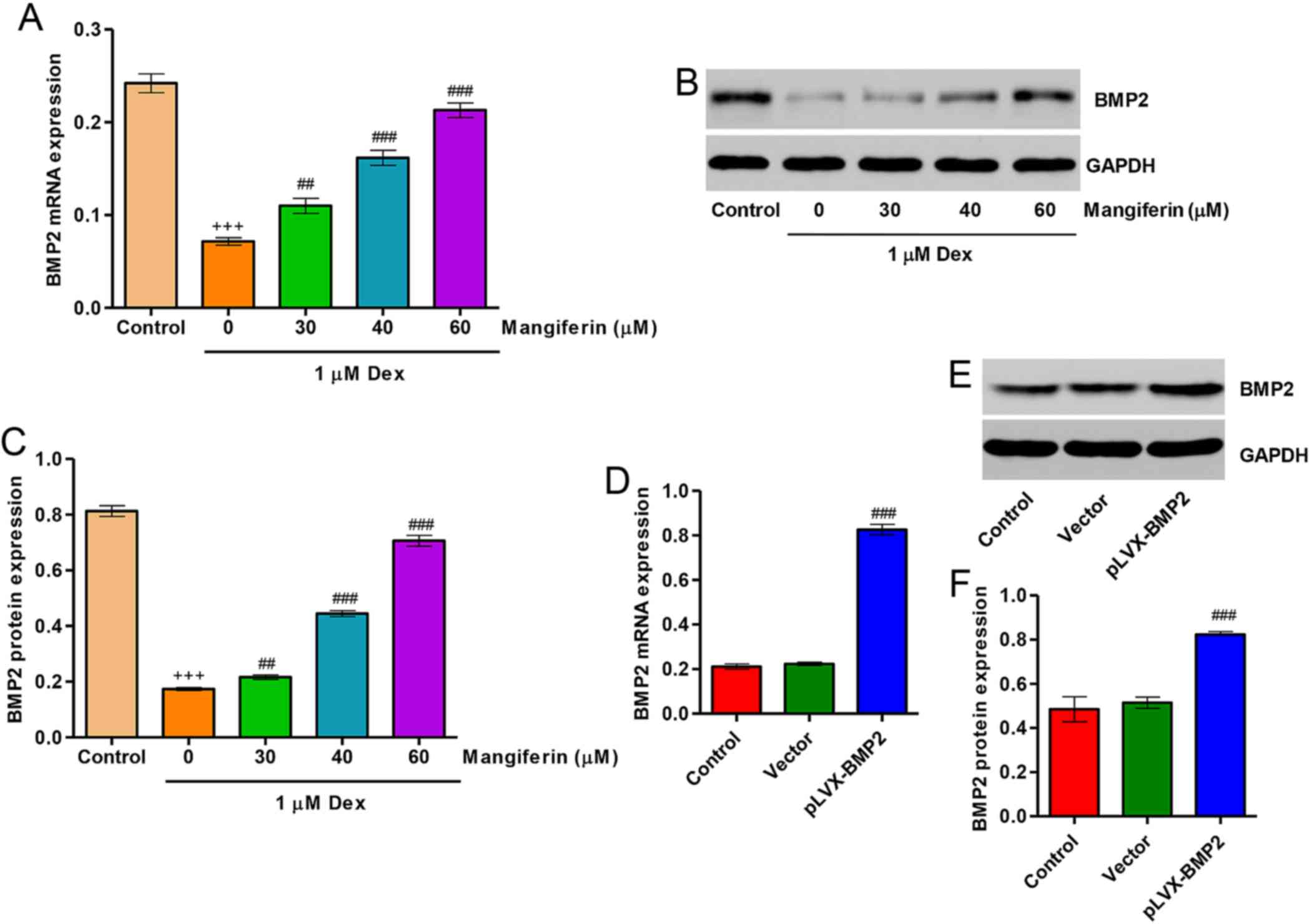
Upregulation of BMP2 contributes to the
cytoprotective effects of mangiferin on Dex-treated MC3T3-E1
cells
Following treatment of MC3T3-E1 cells with 1
µM Dex for 48 h, the expression levels of BMP2 were
significantly decreased. Pretreatment with 30, 40 and 60 µM
mangiferin for 3 h markedly attenuated the downregulation of BMP2
induced by Dex treatment (Fig.
6A–C). In addition, to investigate the role of BMP2 in
Dex-treated MC3T3-E1 cells, BMP2 overexpression was constructed in
MC3T3-E1 cells via infection with the pLVX-BMP2 lentivirus. As
shown in Fig. 6D–F, MC3T3-E1
cells infected with the pLVX-BMP2 lentivirus prior to Dex treatment
for 48 h exhibited significantly increased BMP2 expression at mRNA
and protein levels.
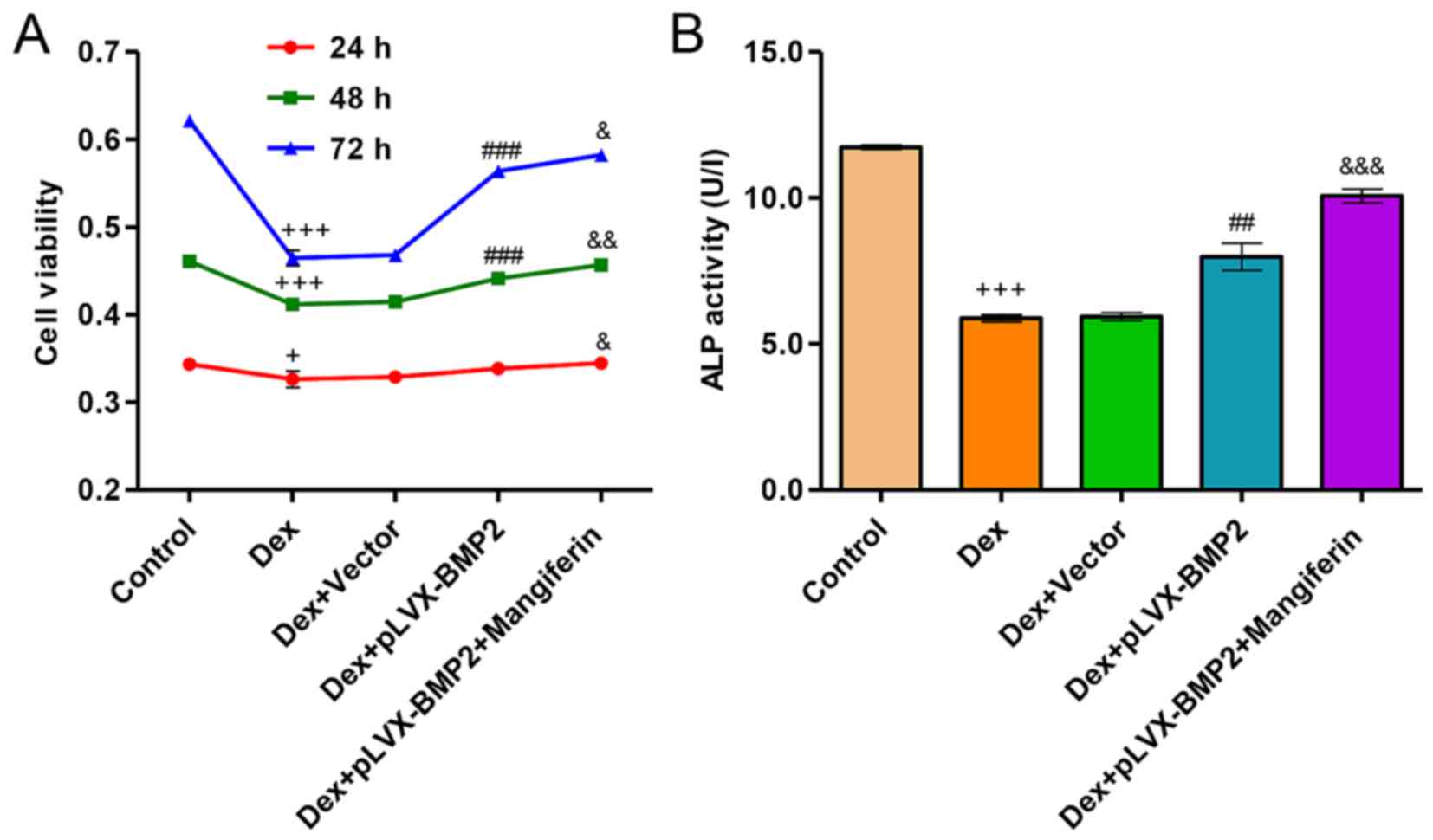
Overexpression of BMP2 attenuates
Dex-induced inhibition of viability and differentiation of MC3T3-E1
cells
Exposure of MC3T3-E1 cells to 1 µM Dex for
24, 48 and 72 h significantly decreased cell viability (Fig. 7A). However, overexpression of BMP2
in MC3T3-E1 cells significantly attenuated the inhibitory effects
of Dex on cell viability. Conversely, in Dex-treated MC3T3-E1 cells
infected with a blank vector no effect was detected on cell
viability compared with in the cell group treated with Dex alone.
Furthermore, exposure of MC3T3-E1 cells to 1 µM Dex for 48 h
significantly decreased ALP activity levels (Fig. 7B). However, overexpression of BMP2
in MC3T3-E1 cells significantly attenuated the Dex-induced decrease
in ALP activity. Conversely, in Dex-treated MC3T3-E1 cells infected
with a blank vector no effect was detected on ALP activity levels
compared with in the cell group treated with Dex alone. Notably,
treatment of Dex-induced MC3T3-E1 cells with the BMP2
overexpression vector and 60 µM mangiferin resulted in
augmented effects on cell viability and differentiation compared
with in the Dex-induced MC3T3-E1 cells treated with the BMP2
overexpression vector alone (Fig. 7A
and B).
Overexpression of BMP2 affects
Dex-induced protein expression in MC3T3-E1 cells
To clarify the mechanism underlying the effects of
Dex on the inhibition of MC3T3-E1 cell viability and
differentiation, and on the induction of apoptosis, the expression
levels of associated proteins were detected by western blot
analysis. As shown in Fig. 8A and
B, treatment with 1 µM Dex for 48 h decreased BMP2
expression and Smad-1 phosphorylation, whereas treatment of
MC3T3-E1 cells with 1 µM Dex for 48 h had no effect on the
protein expression levels of total Smad-1. Overexpression of BMP2
in MC3T3-E1 cells prior to Dex stimulation significantly reversed
the effects of Dex on BMP2 expression and Smad-1 phosphorylation.
Furthermore, treatment with Dex decreased the expression levels of
differentiation-associated markers, including OSX, OCN and OPG
(Fig. 8C and D), and increased
the expression levels of other differentiation-associated markers,
including RANK and RANKL. In addition, the ratio of Bax/Bcl-2 was
increased in Dex-induced MC3T3-E1 cells (Fig. 8C and E). Notably, Dex-induced
MC3T3-E1 cells treated with the BMP2 overexpression vector and 60
µM mangiferin exhibited augmented effects, with regards to
these protein levels, compared with in Dex-induced MC3T3-E1 cells
treated with the BMP2 overexpression vector alone (Fig. 8A–E).
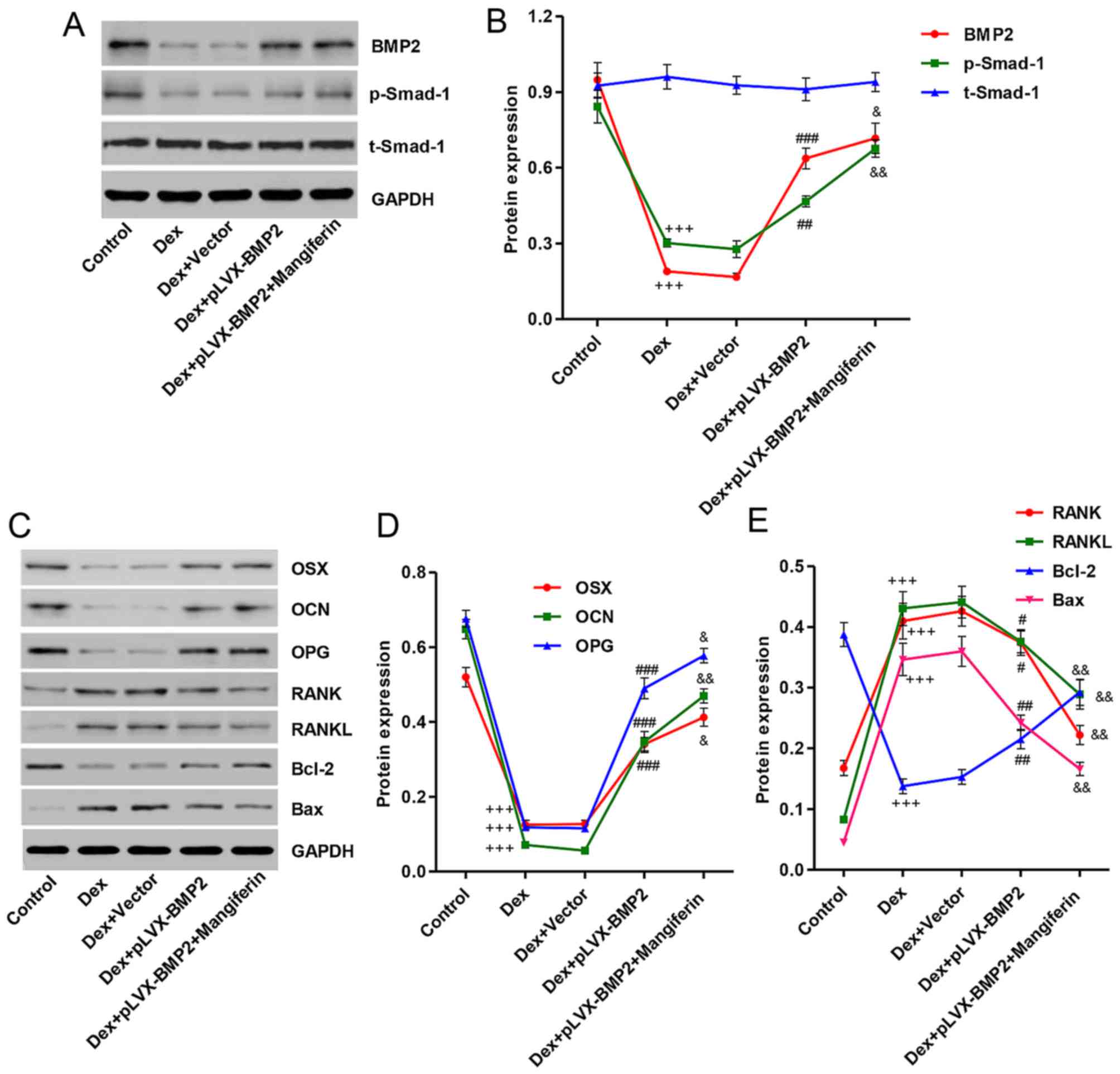 | Figure 8Effects of BMP2 overexpression on
Dex-induced protein alterations in MC3T3-E1 cells. MC3T3-E1 cells
were infected with pLVX-BMP2 prior to 1 µM Dex treatment for
48 h, in the absence or presence of 60 µM mangiferin
pretreatment for 3 h. (A) Cell lysates were subjected to western
blot analysis using BMP2, p-Smad-1 and Smad-1-specific antibodies.
(B) Intensity of the protein bands of a typical experiment was
semi-quantified using ImageJ 1.41o software. (C) Cell lysates were
subjected to western blot analysis using OSX, OCN, OPG, RANK,
RANKL, Bcl-2, and Bax-specific antibodies. (D and E) Intensity of
the protein bands of a typical experiment was semi-quantified using
ImageJ 1.41o software. +++P<0.001 compared with the
control group; #P<0.05, ##P<0.01 and
###P<0.001 compared with the Dex treatment group;
&P<0.01 and &&P<0.01
compared with the Dex + pLVX-BMP2 treatment group. Bax,
Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; BMP2, bone
morphogenetic protein 2; Dex, dexamethasone; OCN, osteocalcin; OPG,
osteoprotegerin; OSX, osterix; p-Smad-1, phosphorylated-Smad-1;
t-Smad-1, total Smad-1; RANK, receptor activator of nuclear
factor-κB; RANKL, RANK ligand; Smad-1, SMAD family member 1. |
Discussion
Glucocorticoid-induced injury occurs in numerous
diseases, including cardiovascular disease, Alzheimer's disease,
type II diabetes, obesity and cognitive deficits (23–26). Apoptosis and oxidative stress are
two key risk factors of these diseases. In addition,
glucocorticoids have been reported to modify the proliferative and
metabolic activity of bone cells, which results in the inhibition
of osteoblastogenesis and osteoclastogenesis, and reduces the
lifespan of osteoblasts (27).
Mangiferin is a naturally occurring polyphenol that is commonly
found in mangoes and papayas, which exhibits antitumor, antiviral,
anti-diabetic, antioxidant and anti-apoptotic properties (15–17,28). Therefore, the present study
hypothesized that mangiferin may exert protective effects against
glucocorticoid-induced osteoblast injury.
In the present study, glucocorticoid-induced injury
was initiated in the MC3T3-E1 murine preosteoblastic calvarial cell
line by exposure to Dex. This synthetic glucocorticoid promotes
differentiation of bone marrow stromal cells and increases the
number of mineralized bone nodules in primary fetal rat calvarial
osteoblast cultures (29,30). The results of the present study
demonstrated that exposure of MC3T3-E1 cells to Dex induced
cytotoxicity, as determined by decreased cell viability and ALP
activity levels. To investigate whether mangiferin may protect
MC3T3-E1 cells against Dex-induced cytoxicity, the cells were
pretreated with mangiferin at concentrations ranging between 10 and
60 µM for 3 h prior to Dex exposure. Notably, the results
indicated that pretreatment with mangiferin significantly
attenuated Dex-induced decreases in cell viability and ALP activity
levels in MC3T3-E1 cells. ALP activity is considered an early
marker of osteogenic differentiation, which is characterized by
increased OCN. A previous study reported that Dex significantly
suppresses ALP activity in mouse osteoblastic MC3T3-E1 and rat
osteoblastic UMR-106 cells (31).
However, another study reported that Dex increased ALP activity,
which is not in agreement with the present findings (32). However, in the previous study, the
concentrations of Dex used ranged between 10−4 and 1
µM, and the treatment period ranged between 2 and 6 days.
The differences between these previous results and the findings of
the present study may be due to differences in Dex treatment.
Another important finding of the present study was
that mangiferin inhibited apoptosis and oxidative stress induced by
Dex in MC3T3-E1 cells. In agreement with a previous study, which
indicated that Dex induces oxidative stress in cloned bone marrow
mesenchymal stem cells (33), the
present study demonstrated that exposure of Dex elicited marked
increases in apoptosis, as evidenced by an increased ratio of
Bax/Bcl-2, and ROS generation in MC3T3-E1 cells; these effects were
reversed following pretreatment with mangiferin. Similarly, it has
been reported that treatment with mangiferin suppresses
12-O-tetradecanoylphorbol-13-acetate-induced injury by inhibiting
ROS generation (34). Therefore,
mangiferin pretreatment may trigger cytoprotective effects, at
least in part, via its antioxidative function.
The inflammatory response is an important injury
factor in Dex-induced osteoporosis. Proinflammatory cytokines,
including IL-1α, TNF-α and IL-17, exhibit osteoclastogenic
properties (35), whereas others,
including IL-6, may produce stimulatory and suppressive actions on
osteoclasts (36). In the present
study, besides cytotoxicity and oxidative stress, Dex induced an
inflammatory response, as evidenced by increases in TNF-α and IL-6
secretions. Notably, pretreatment with mangiferin significantly
attenuated Dex-stimulated TNF-α and IL-6 secretions from MC3T3-E1
cells, thus suggesting that mangiferin may protect MC3T3-E1 cells
against gluco-corticoid-induced inflammatory responses.
A previous study, which used a combined linkage and
association analysis, indicated that low BMP2 expression is
associated with a combined phenotype of low bone mineral density
and high fracture risk (37). The
present study demonstrated that exposure of MC3T3-E1 cells to Dex
downregulated the expression of BMP2. Conversely, pretreatment with
mangiferin for 3 h suppressed Dex-stimulated BMP2 downregulation.
Similar to the protective effects of mangiferin, overexpression of
BMP2 attenuated not only Dex-induced cytotoxicity, but also ALP
activity levels. In agreement with the previous evidence that BMP2
acts on bone cells by binding to their cell surface receptors and
subsequently phosphorylates Smad-1 (13), the present study demonstrated that
overexpression of BMP2 significantly reversed the decrease in
Smad-1 phosphorylation induced by Dex in MC3T3-E1 cells. These
findings suggested that BMP-2 may exert an ameliorative effect on
osteoporosis via activation of Smad-1. Mangiferin inhibited
Dex-induced decrease of cell viability and ALP activity, and
corrected Dex-induced the expression of differentiation- and
apoptosis-associated markers in MC3T3-E1 cells with or without BMP2
overexpression.
Osteoblasts are not only involved in bone formation,
but also in the production of RANKL and OPG, in order to modulate
the formation and differentiation of osteoclasts. RANKL provides a
signal to osteoclast progenitors via RANK to activate osteoclast
differentiation and function (8).
OPG inhibits the interaction between RANKL and RANK (10). Therefore, bone remodeling can be
assessed by the relative ratio of OPG to RANKL. Chen et al
(38) reported that
ethanol-induced RANKL expression in osteoblasts was able to promote
osteoclastogenesis, and pretreatment of cells with 17β-estradiol or
the antioxidant N-acetylcysteine blocked these effects. The present
study examined the effects of BMP2 overexpression and mangiferin on
the protein expression levels of RANK, RANKL and OPG, and
demonstrated that BMP2 overexpression and mangiferin prevented the
increase in RANK and RANKL, and attenuated the decrease in OPG
levels in MC3T3-E1 cells treated with Dex, thus suggesting that
mangiferin may act on osteoblasts to alter RANKL/OPG and inhibit
osteoclastogenesis. Furthermore, the protein expression levels of
key osteogenic markers, OCN and OSX, were examined in MC3T3-E1
cells; the results indicated that Dex decreased the expression
levels of OCN and OSX, whereas BMP2 overexpression and mangiferin
prevented the decrease in OCN and OSX expression.
In conclusion, the present study is the first, to
the best of our knowledge, to demonstrate that mangiferin exerts a
cytoprotective effect against glucocorticoid-induced apoptosis and
oxidative stress via activation of the BMP2/Smad-1 signaling
pathway in MC3T3-E1 cells. The present study provides novel
insights into the roles of mangiferin in attenuating
glucocorticoid-induced osteoporosis. Administration of mangiferin
may therefore be considered a novel therapeutic strategy for the
treatment of glucocorticoid-induced osteoporosis.
Acknowledgments
Not applicable.
Notes
[1]
Funding
No funding was received.
[2] Availability
of data and materials
All data generated or analyzed during this study are
included in this published article.
[3] Authors'
contributions
LZD and XT conceived and designed the experiments.
ZBZ and CJZ performed the experiments and analyzed the data. SHC
contributed as regards the reagents/materials/analysis tools. LZD
wrote the paper. All authors read and approved the final
manuscript.
[4] Ethics
approval and consent to participate
Not applicable.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Ang E, Liu Q, Qi M, Liu HG, Yang X, Chen
H, Zheng MH and Xu J: Mangiferin attenuates osteoclastogenesis,
bone resorption, and RANKL-induced activation of NF-κB and ERK. J
Cell Biochem. 112:89–97. 2011. View Article : Google Scholar
|
|
2
|
Bar-Shavit Z: The osteoclast: a
multinucleated, hematopoietic-origin, bone-resorbing osteoimmune
cell. J Cell Biochem. 102:1130–1139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Whittier X and Saag KG:
Glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am.
42:177–189. 2016. View Article : Google Scholar
|
|
4
|
Wang Y, Liu J, Pang Q and Tao D:
Alpinumisoflavone protects against glucocorticoid-induced
osteoporosis through suppressing the apoptosis of osteoblastic and
osteocytic cells. Biomed Pharmacother. 96:993–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boyce BF and Xing L: Functions of
RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem
Biophys. 473:139–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fujita K and Janz S: Attenuation of WNT
signaling by DKK-1 and -2 regulates BMP2-induced osteoblast
differentiation and expression of OPG, RANKL and M-CSF. Mol Cancer.
6:712007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ross FP: M-CSF, c-Fms, and signaling in
osteoclasts and their precursors. Ann N Y Acad Sci. 1068:110–116.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamaguchi Y, Sakai E, Sakamoto H, Fumimoto
R, Fukuma Y, Nishishita K, Okamoto K and Tsukuba T: Inhibitory
effects of tert-butylhydroquinone on osteoclast differentiation via
up-regulation of heme oxygenase-1 and down-regulation of HMGB1
release and NFATc1 expression. J Appl Toxicol. 34:49–56. 2014.
View Article : Google Scholar
|
|
9
|
Boyce BF and Xing L: Biology of RANK,
RANKL, and osteoprotegerin. Arthritis Res Ther. 9(Suppl 1): S12007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xue L, Jiao L, Wang Y, Nie Y, Han T, Jiang
Y, Rahman K, Zhang Q and Qin L: Effects and interaction of icariin,
curculigoside, and berberine in er-xian decoction, a traditional
chinese medicinal formula, on osteoclastic bone resorption. Evid
Based Complement Alternat Med. 2012:4908432012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cancedda R, Giannoni P and Mastrogiacomo
M: A tissue engineering approach to bone repair in large animal
models and in clinical practice. Biomaterials. 28:4240–4250. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bishop GB and Einhorn TA: Current and
future clinical applications of bone morphogenetic proteins in
orthopaedic trauma surgery. Int Orthop. 31:721–727. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Langenfeld EM, Kong Y and Langenfeld J:
Bone morphogenetic protein 2 stimulation of tumor growth involves
the activation of Smad-1/5. Oncogene. 25:685–692. 2006. View Article : Google Scholar
|
|
14
|
Tang CH, Yang RS, Chien MY, Chen CC and Fu
WM: Enhancement of bone morphogenetic protein-2 expression and bone
formation by coumarin derivatives via p38 and ERK-dependent pathway
in osteoblasts. Eur J Pharmacol. 579:40–49. 2008. View Article : Google Scholar
|
|
15
|
Wilkinson AS, Monteith GR, Shaw PN, Lin
C-N, Gidley MJ and Roberts-Thomson SJ: Effects of the mango
components mangiferin and quercetin and the putative mangiferin
metabolite norathyriol on the transactivation of peroxisome
proliferator-activated receptor isoforms. J Agric Food Chem.
56:3037–3042. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Campos-Esparza MR, Sánchez-Gómez MV and
Matute C: Molecular mechanisms of neuroprotection by two natural
antioxidant polyphenols. Cell Calcium. 45:358–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rajendran P, Ekambaram G and Sakthisekaran
D: Protective role of mangiferin against benzo(a)pyrene induced
lung carcinogenesis in experimental animals. Biol Pharm Bull.
31:1053–1058. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo Y, Fu C, Wang Z, Zhang Z, Wang H and
Liu Y: Mangiferin attenuates contusive spinal cord injury in rats
through the regulation of oxidative stress, inflammation and the
Bcl-2 and Bax pathway. Mol Med Rep. 12:7132–7138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang YH, Wang Y, Yusufali AH, Ashby F,
Zhang D, Yin ZF, Aslanidi GV, Srivastava A, Ling CQ and Ling C:
Cytotoxic genes from traditional Chinese medicine inhibit tumor
growth both in vitro and in vivo. J Integr Med. 12:483–494. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Owen TA, Aronow M, Shalhoub V, Barone LM,
Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB and Stein
GS: Progressive development of the rat osteoblast phenotype in
vitro: reciprocal relationships in expression of genes associated
with osteoblast proliferation and differentiation during formation
of the bone extracellular matrix. J Cell Physiol. 143:420–430.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun M, Xia R, Jin F, Xu T, Liu Z, De W and
Liu X: Downregulated long noncoding RNA MEG3 is associated with
poor prognosis and promotes cell proliferation in gastric cancer.
Tumour Biol. 35:1065–1073. 2014. View Article : Google Scholar
|
|
23
|
Walker BR: Glucocorticoids and
cardiovascular disease. Eur J Endocrinol. 157:545–559. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Green KN, Billings LM, Roozendaal B,
McGaugh JL and LaFerla FM: Glucocorticoids increase amyloid-β and
tau pathology in a mouse model of Alzheimer's disease. J Neurosci.
26:9047–9056. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vegiopoulos A and Herzig S:
Glucocorticoids, metabolism and metabolic diseases. Mol Cell
Endocrinol. 275:43–61. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sato H, Takahashi T, Sumitani K, Takatsu H
and Urano S: Glucocorticoid generates ROS to induce oxidative
injury in the hippocampus, leading to impairment of cognitive
function of rats. J Clin Biochem Nutr. 47:224–232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hurson CJ, Butler JS, Keating DT, Murray
DW, Sadlier DM, O'Byrne JM and Doran PP: Gene expression analysis
in human osteoblasts exposed to dexamethasone identifies altered
developmental pathways as putative drivers of osteoporosis. BMC
Musculoskelet Disord. 8:122007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin H, Lan J, Guan M, Sheng F and Zhang H:
Spectroscopic investigation of interaction between mangiferin and
bovine serum albumin. Spectrochim Acta A Mol Biomol Spectrosc.
73:936–941. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Holtorf HL, Jansen JA and Mikos AG: Flow
perfusion culture induces the osteoblastic differentiation of
marrow stroma cell-scaffold constructs in the absence of
dexamethasone. J Biomed Mater Res A. 72:326–334. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vali B, Rao LG and El-Sohemy A:
Epigallocatechin-3-gallate increases the formation of mineralized
bone nodules by human osteoblast-like cells. J Nutr Biochem.
18:341–347. 2007. View Article : Google Scholar
|
|
31
|
Iu MF, Kaji H, Sowa H, Naito J, Sugimoto T
and Chihara K: Dexamethasone suppresses Smad3 pathway in
osteoblastic cells. J Endocrinol. 185:131–138. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mori K, Shioi A, Jono S, Nishizawa Y and
Morii H: Dexamethasone enhances in vitro vascular calcification by
promoting osteoblastic differentiation of vascular smooth muscle
cells. Arterioscler Thromb Vasc Biol. 19:2112–2118. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu H, Yang X, Zhang Y, Dighe A, Li X and
Cui Q: Fullerol antagonizes dexamethasone-induced oxidative stress
and adipogenesis while enhancing osteogenesis in a cloned bone
marrow mesenchymal stem cell. J Orthop Res. 30:1051–1057. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sánchez GM, Re L, Giuliani A, Núñez-Sellés
AJ, Davison GP and León-Fernández OS: Protective effects of
Mangifera indica L. extract, mangiferin and selected antioxidants
against TPA-induced biomolecules oxidation and peritoneal
macrophage activation in mice. Pharmacol Res. 42:565–573. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kudo O, Fujikawa Y, Itonaga I, Sabokbar A,
Torisu T and Athanasou NA: Proinflammatory cytokine
(TNFalpha/IL-1α) induction of human osteoclast formation. J Pathol.
198:220–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kudo O, Sabokbar A, Pocock A, Itonaga I,
Fujikawa Y and Athanasou NA: Interleukin-6 and interleukin-11
support human osteoclast formation by a RANKL-independent
mechanism. Bone. 32:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Styrkarsdottir U, Cazier JB, Kong A,
Rolfsson O, Larsen H, Bjarnadottir E, Johannsdottir VD,
Sigurdardottir MS, Bagger Y, Christiansen C, et al: Linkage of
osteoporosis to chromosome 20p12 and association to BMP2. PLoS
Biol. 1:e692003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen JR, Shankar K, Nagarajan S, Badger TM
and Ronis MJ: Protective effects of estradiol on ethanol-induced
bone loss involve inhibition of reactive oxygen species generation
in osteoblasts and downstream activation of the extracellular
signal-regulated kinase/signal transducer and activator of
transcription 3/receptor activator of nuclear factor-kappaB ligand
signaling cascade. J Pharmacol Exp Ther. 324:50–59. 2008.
View Article : Google Scholar
|