Introduction
Breast cancer is the most common malignant tumor
affecting females (1). The
leading cause of tumor death is metastasis, and bone is one of the
most preferential metastatic sites for breast carcinoma (2). A number of studies have confirmed
the theory called 'soil and seed', that the microenvironment of
metastatic organs can be regarded as soil and the tumor cells are
seeds. Both the soil and seeds can promote the occurrence and
progress of tumor metastasis (3).
Particularly, as typical soil, bone tissue possesses biological
microenvironment which stores and releases various growth factors
for tumor growth (4). As seeds,
invasion ability of tumor cells play a decisive role (5). In fact, the main way that metastatic
breast cancer cells influence bone microenvironment and achieve
breast cancer bone metastasis is through regulating osteolytic
destruction, which is developed by increasing the number of
osteoclasts. It is abundantly clear that breast cancer cells could
in turn secrete a variety of tumor derived factors, such as
parathyroid hormone-related protein (PTHrP), tumor necrosis factor
(TNF), vascular endothelial growth factor (VEGF) and insulin-like
growth factor (IGF), to further promote osteolytic bone metastasis
(6). Of these, PTHrP plays an
important role. It controls the secretion of receptor activator of
nuclear factor-κB ligand (RANKL) and osteoprotegerin (OPG) to form
a self-sustaining vicious circle (7-9).
This vicious circle could break the balance of osteoclasts and
osteoblasts, which leads to osteolytic lesion in breast cancer bone
metastasis (10). Therefore,
inhibiting the expression of PTHrP and its downstream RANKL/OPG is
a promising approach to weaken breast cancer-induced bone
metastasis and osteolysis (11).
Currently, main medical treatments for breast
carcinoma bone metastasis are bisphosphonate and densumab which are
inhibitors against RANKL (12-14). However, metastasis to bone is
still uncontrollable and could greatly increase mortality and
morbidity in breast cancer patients. For further decreasing
skeletal morbidity and preventing disease progression, better
treatments are still needed.
Scutellarin barbata D. Don is a herbal plant
in Astragalus genus, known as Ban Zhi Lian in the field of
traditional Chinese medicine (TCM), and has been widely used for
treatment of various kinds of cancer, such as breast, colorectal,
liver and lung cancer in China (15). Although the chemical constituents
of Scutellarin barbata D. Don are complicated, some
alkaloids, flavones, steroids have been successfully extracted and
qualified. Moreover, the total flavonoids from Scutellarin
barbata D. Don (TF-SB) has been proven to be the effective
component and provides a favorable safety profile (16). In recent years, research in China
has shown that TF-SB could suppress the growth of various tumor
cells through several specific signaling pathways (17-22). In particular, it reportedly
inhibits the proliferation of several human breast cancer cell
lines, such as MCF-7, MCF-10A and MDA-MB-361 (23,24). As a well-known TCM, Scutel
larin barbata D. Don has attracted substantial research effort
in exploring the molecular mechanism of its antitumor activity, but
the details have not been clarified.
Based on these accurate mechanisms and existing
anti-tumor evidence of TF-SB, our study evaluated the therapeutic
effect of TF-SB on breast cancer and its bone metastasis.
Furthermore, by focusing on PTHrP and its downstream RANKL/OPG, we
explore the molecular mechanism of this inhibitory effect.
Materials and methods
Reagents and antibodies
Dulbecco's modified Eagle's medium (DMEM) (21063029;
Invitrogen Life Technologies, Carlsbad, CA, USA), 10% fetal bovine
serum (FBS, 0500; ScienCell Research Laboratories, Carlsbad, CA,
USA), trypsin-EDTA (15400054; Invitrogen Life Technologies), MTT
(M2128; Sigma-Aldrich, St. Louis, MO, USA), 4% paraformaldehyde
(sc-281692; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
DMSO (25-950-CQC; Cellgro, Manassas, VA, USA), methanol (K977-4L;
Amresco LLC, Solon, OH, USA), Transwell (3412; Corning Costar,
Inc., Corning, NY, USA), Matrigel (356234; BD Biosciences, Franklin
Lakes, NJ, USA), Transcriptor First Strand cDNA Synthesis kit
(04897030001) and FastStart Universal SYBR-Green Master
(04913914001) (both from Roche Diagnostics, Indianapolis, IN, USA),
DNase I, RNase-free kit (EN0521; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), TRIzol (Hualan Biological Engineering Inc.,
Henan, China), antibodies against PTHrP (5652-100; BioVision
Research Products, Mountain View, CA, USA), RANKL (GTX59855;
GeneTex, Inc., Irvine, CA, USA), OPG (5312-100; BioVision Research
Products) and GAPDH (622101; BioLegend, San Diego, CA, USA),
HRP-conjugated antibodies against mouse IgG (BW20330016; Bioworld
Technology Co., Ltd., Nanjing, China), enhanced chemiluminescence
western blotting kit (32109; Pierce, Rockford, IL, USA).
Preparation of TF-SB
The whole herbs of Scutellaria barbata D. Don
were purchased from a Chinese herbal medicine store called Tong Ren
Tang (Shanghai, China) and verified by TCM Department of Shanghai
Ninth People's Hospital (Shanghai, China). The original plant
materials were prepared through drying, coarse grinding, and 95%
alcohol extraction twice. Secondly, after the evaporation and
concentration, pH was adjusted to 2.0. The crude extracts were
filtered through an AB-8 macroporous adsorption resin column. Then
distilled water and 70% ethanol were used to elute TF-SB. Finally,
the experimental doses of TF-SB were determined according to
pharmacopoeia, references and results of pre-tests. In vitro
experiments, the concentrations of TF-SB were as follows: 40, 80,
120, 160 and 200 µg/ml, but in vivo, the concentrations were
50, 100 and 200 mg/ml.
High-performance liquid chromatography
(HPLC) analysis
TF-SB was analyzed through the HPLC system (Agilent
1100; Agilent Technologies, Inc., Santa Clara, CA, USA). According
to pharmacopoeia and relevant references, purified scutellarein,
apigenin, baicalein and luteolin were chosen as reference
substances. Then 100 mg TF-SB and these reference substances were
respectively dissolved in 50 ml methanol solution. Measuring
wavelength was set to 335 nm and the sample amount was 20 µl.
Apigenin, scutellarin, baicalein and luteolin were identified as
the main component of TF-SB (Fig.
1).
Cell line and cell culture
Breast cancer cell line MDA-MB-231 was the gift from
the Orthopaedic Laboratory of Shanghai Ninth People's Hospital and
was maintained in Tissue Engineering Laboratory of Shanghai Ninth
People's Hospital. These cells were cultured in DMEM medium,
supplemented with 10% FBS and penicillin-streptomycin-amphotericin
B (1×105 U/l). Cells were maintained at 37°C and 5%
CO2 under humidified atmosphere. Through changing the
culture media every two days and passaging every three days, the
cell lines were kept in optimal density and state. The cells in
logarithmic phase could be used in the in vitro
experiment.
Animals
Thirty female athymic BALB/c nude mice (4 weeks of
age) were provided by Experimental Animal Center of Shanghai Ninth
People's Hospital. These mice were housed five per cage and kept in
the same condition (specific pathogen-free, 26-27°C, humidity, 12-h
light/dark cycle, free access to water and food) strictly according
to international ethical guide lines. Animal procedures were
strictly in accordance with International Ethical Guidelines and
National Institutes of Health Guide concerning the Care and Use of
Laboratory Animals, and protocols were approved by the Ethics
Committee of Human Experimentation of Shanghai Ninth People's
Hospital. Observing the animals twice a day and starting
experiments after these mice had accustomed to the new
environment.
Cell viability test
Viability of TF-SB was determined by the MTT test.
Cells were manipulated to cell suspension, seeded into 96-well
plates at the density of 1×104 and cultured for 12 h. An
amount of 100 µl TF-SB solution (0, 40, 80, 120, 160 or 200 µg/ml)
was added into corresponding group and incubated at 37°C and 5%
CO2 under humidified atmosphere for 48 h. Then the media
in these plates were removed and 20 µl of MTT reagent was added
into each well. After being incubated as before for 4 h, 100 µl of
mixture which contains SDS, isobutanol and HCl was added. After
being shaked and incubated as before for 8 h, the absorbance of
each well was read at 540 nm by ELISA. Tumor inhibition rate =
(mean absorbance of control group - mean absorbance of TF-SB
group)/(mean absorbance of control group) ×100%.
Wound healing assay
The ability of cell migration was tested by wound
healing assay. MDA-MB-231 cells were seeded into 6-well plates to a
density of 5×105/well and cultured for 12 h. Then 200 µl
pipette tip was used to scratch cells in the centre points of the
plates. After washing twice with phosphate-buffered saline (PBS),
100 µl of TF-SB solution (0, 40, 80, 120, 160 or 200 µg/ml) was
added into corresponding group and incubated at 37°C and 5%
CO2 under humidified atmosphere for 48 h. The scratch
areas of these groups were photographed at 0, 6, 12 and 24 h.
ImageJ software was used to set boundaries and count the number of
migrated cells in these areas. The inhibition rate of cell
migration = (mean number of migrated cells in control group - mean
number of migrated cells in TF-SB group)/(mean number of migrated
cells in control group) ×100%.
Cell invasion test
Transwell chamber was used to perform the cell
invasion assay. Ten microliters of fibronectin (0.5 g/l) was used
to coat the outside of 8-µm pore Transwell chamber membrane and
Matrigel was used inside. Fifty microliters of FBS (10 g/l) was
used to block binding sites. Then 200 µl of MDA-MB-231 cell
suspensions (5×105/ml) with various concentrations (0,
40, 80, 120, 160 and 200 µg/ml) of TF-SB were added into Transwell
chambers. Then these Transwell chambers were set to keep suspension
in 24-well plates which contain 500 µl of DMEM. After being
incubated at 37°C and 5% CO2 under humidified atmosphere
for 24 h, residual cells in the Transwell chambers were wiped off
with cotton swabs. The invading cells were fixed with methanol,
stained with toluidine blue, washed with PBS, photographed under
microscope and counted. The inhibition rate of cell invasion =
(mean number of cells in control group - mean number of cells in
TF-SB group)/(mean number of cells in control group) ×100%.
Breast cancer bone metastasis nude mouse
model
One hundred microliters of MDA-MB-231 cell
suspension (8×107/ml) mixed with Matrigel (1:1) was
injected into the femur medullary cavity of 24 nude mice to
establish breast cancer bone metastasis nude mouse model. After two
weeks, micro-computed tomography (micro-CT) (IVIS Quantum FX) and
in vivo bioluminescence imaging (IVIS Lumina XR) (both from
Caliper Life Sciences, Hopkinton, MA, USA) were used to identify
neoplasm tissues and pick-out successful models. Then the nude mice
were randomized into 4 groups (n=10) and given a gavage of
distilled water (20 ml/kg) which contained corresponding
concentrations (0, 50, 100 or 200 mg/ml) of TF-SB for 4 weeks.
Survival rates of each group were recorded every week.
Micro-CT and in vivo bioluminescence
imaging
The mice were anesthetized and placed into a dark
room in a prone position. We used micro-CT to make full body scans
and 3D reconstruction of mice in the animal laboratory of Huashan
Hospital (Shanghai, China). Micro-CT was for observing tumor mass
and bone destruction. Then in vivo bioluminescence imaging
technique was performed in the same laboratory to identify the
property of lumps and observing luminous intensity by setting
MDA-MB-231 cell suspension as control every week. Tumor weights
were calculated by measuring, recording and comparing luminous
intensities between groups, which were evaluated according to the
total number of photons detected by in vivo bioluminescence
imaging technique. The tumor growth inhibition levels of TF-SB
groups were measured by comparing tumor weights with control
group.
Tumor and bone histology
Tumor tissues and corresponding bone tissues were
fixed in paraformaldehyde for 3 days and decalcified in EDTA for 3
weeks. Then these tissues were trimmed into homogeneous sections,
embedded in paraffin and serially cut for pathological examination.
Hematoxylin and eosin (H&E) staining was for contrasting
histological characteristics and tartrate-resistant acid
phosphatase (TRAP) staining was for observing osteoclast cells.
TRAP-positive cells were photographed and quantified in same
magnification, and data were expressed as number of osteoclasts per
field.
Quantitative polymerase chain reaction
(qPCR) of PTHrP, OPG and RANKL
The mRNA expression levels were tested by qPCR
(25). After extracting from
tumor tissue or corresponding bone tissue with TRIzol reagent, 1 µl
of total RNA was added into nucleic acid protein detector (ND-1000;
NanoDrop Technologies, Inc., Wilmington, DE, USA) to measure its
concentration and OD260/OD280 ratio.
According to the qPCR kit instruction, total RNA was reverse
transcribed into cDNA which worked as template. PCR proceeded in a
two-step method. PTHrP, OPG and RANKL were the objectives, and
GAPDH was used as internal control. PTHrP forward,
5′-ATCGCAGAAATCCACACAGC-3′ and reverse, 5′-TCATCATCAGACCCAAATCG-3′,
product length was 110 bp, annealing temperature was 58°C; OPG
forward, 5′-CCTTGCCCTGACCACTCTTA-3′ and reverse,
5′-CCCTTCCTCACACTCACACA-3′, product length was 141 bp, annealing
temperature was 60°C; RANKL forward, 5′-CTGATGAAAGGAGGGAGCAC-3′ and
reverse, 5′-AAGGGTTGGACACCTGAATG-3′, product length was 127 bp,
annealing temperature was 60°C; GAPDH forward,
5′-CAGGAGGCATTGCTGATGAT-3′ and reverse, 5′-GAAGGCTGGGGCTCATTT-3′,
product length was 138 bp, annealing temperature was 60°C. The
expression of PTHrP, OPG, RANKL and GAPDH were determined by
converting the fluorescence intensity into corresponding level of
mRNA and obtaining amplification curve. Finally, the
2−ΔΔCt method was used for calculating the relative
quantification of mRNA.
Western blot analysis of PTHrP, OPG and
RANKL
The protein expression levels of PTHrP, OPG and
RANKL were tested by western blot analysis kit. Firstly, the
protein concentrations of tumor tissue or corresponding tissue were
extracted by lypolysis-ultracentrifugation (4°C, 12,000 rpm for 2
min), quantified by manufacturing a standard curve and separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE). Secondly, these proteins were electrophoretically
transferred to polyvinylidene fluoride (PVDF) membranes. The PVDF
membranes were blocked with 5% skimmed milk for 2 h, and
respectively incubated with anti-PTHrP, anti-OPG or anti-RANKL
(1:500) at 4°C overnight. GAPDH (1:1,000) was used as internal
control to verify equivalent protein loading. Then all the PVDF
membranes were incubated with HRP conjugated goat anti-mouse
secondary IgG (1:2,000) for 1 h at room temperature. Each band was
revealed by enhanced chemiluminescence western blotting kit, then
photographed and quantified by luminescent image analyzer
(LAS-3000; Fujifilm, Tokyo, Japan).
Statistical analysis
Results are presented as mean ± SE. Statistical
analysis was performed with Student's t-test. Statistical
significance was determined at P≤0.05. Statistical evaluation was
done by SPSS 13.0 statistical software (SPSS, Inc., Chicago, IL,
USA).
Results
TF-SB inhibits MDA-MB-231 cell
proliferation
TF-SB has antitumor effects in the progression of
various tumors. To assess the role of TF-SB in breast cancer, we
initially performed MTT assay to determine its effects on breast
cancer cell viability. We chose the MDA-MB-231 cell line, which is
triple-negative and high invasive, for in vitro assays.
Cells were passaged into different groups and treated with
corresponding doses of TF-SB for 24, 48 or 72 h. As shown in
Fig. 2, cell viability was
suppressed after the TF-SB treatment with concentration from 40-200
µg/ml. Moreover, the anti-proliferative effects of TF-SB on
MDA-MB-231 cells occured in a dose- and time-dependent manner.
After 72 h TF-SB treatment the proliferation inhibition rates of
200 µg/ml group reached 51.3%.
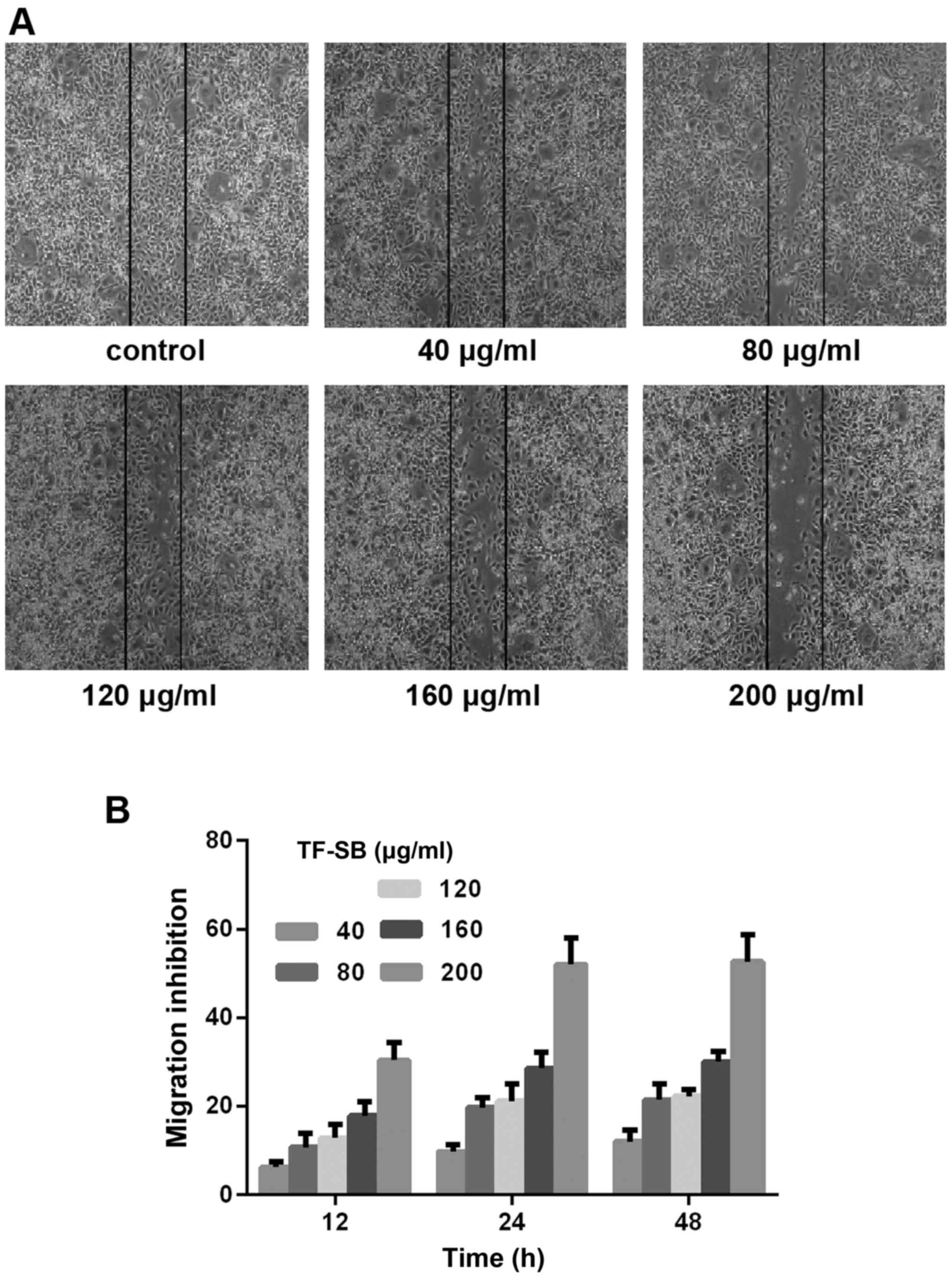
TF-SB inhibits MDA-MB-231 cell migration
and invasion
Tumor cell migration and invasion are necessary
parts of tumor metastasis. We performed wound healing assay and
Transwell chamber assay to determine the effect of TF-SB on
MDA-MB-231 cell migration and invasion. Cells were treated with
various doses of TF-SB (from 40-200 µg/ml) for 24 h. As shown in
Figs. 3 and 4, cell migration and invasion in TF-SB
groups were suppressed after 24 h treatment of TF-SB. The
inhibition degrees of TF-SB on MDA-MB-231 increased in a
dose-dependent manner and achieved maximum when received 200 µg/ml
TF-SB.
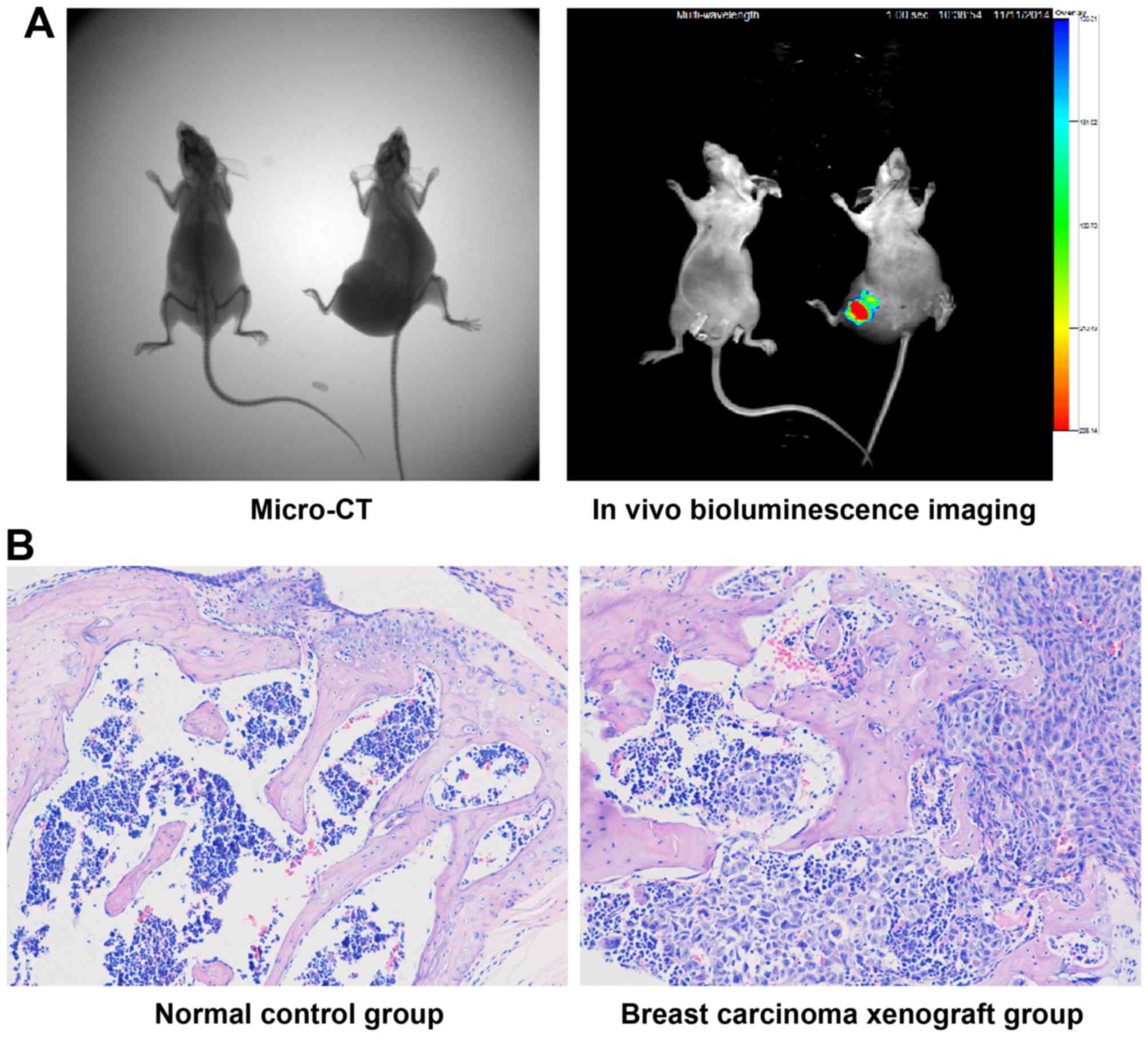
TF-SB inhibits bone destruction induced
by breast cancer bone metastasis while not altering tumor growth or
mouse survival
Having demonstrated the effect of TF-SB on
MDA-MB-231 cells, a well-established breast cancer bone metastasis
model was used to evaluate its effects in vivo. To observe
the degree of bone destruction, we used micro-CT and H&E
staining to compare the imaging and histological characteristics
between breast carcinoma xenograft and normal control groups
(Fig. 5). Osteoclast cells were
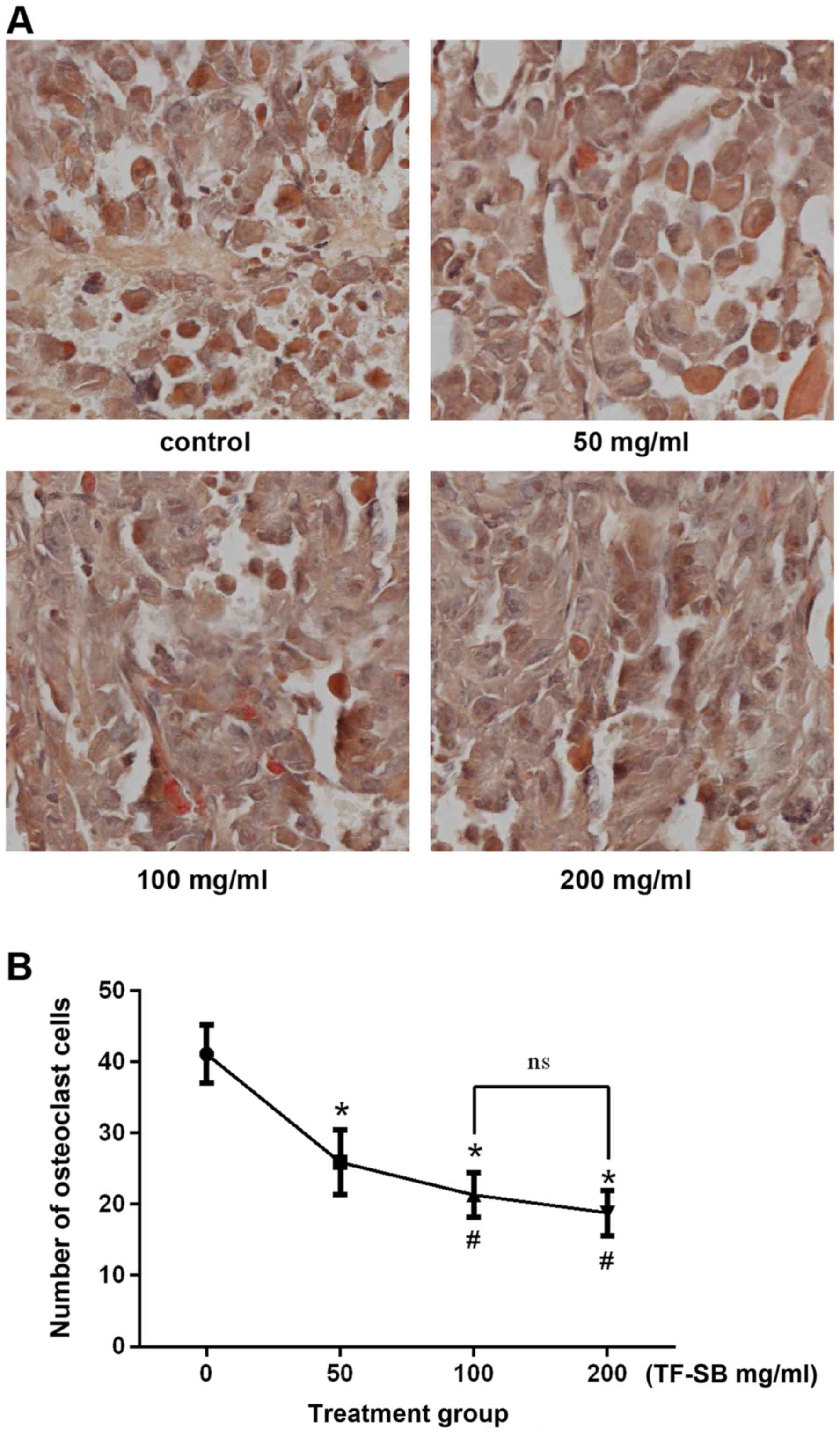
developed using TRAP staining, and bone destruction was measured by
counting osteoclast cells. As shown in Fig. 6, the number of osteoclast cells
per field in control and TF-SB treated groups (from low
concentration to high) were 42±1.3, 26±0.9, 20±0.7, 16±0.7,
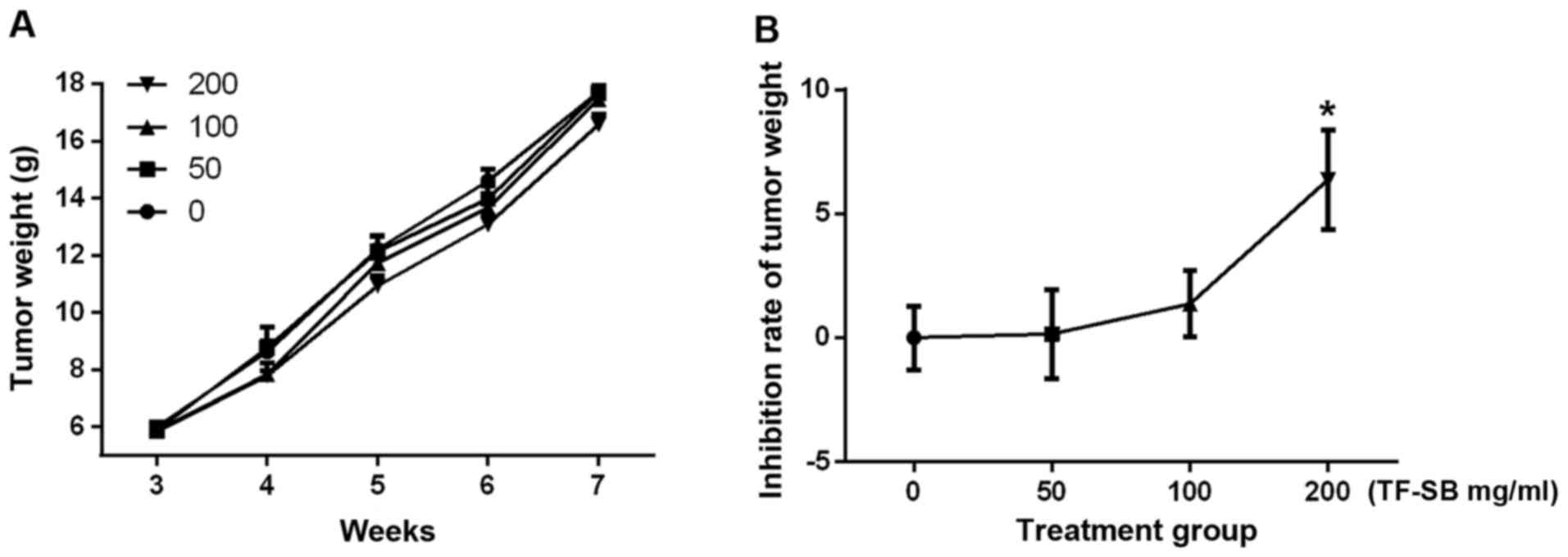
respectively. Bone destruction indu ced by metastatic tumor cells
were inhibited by TF-SB in a dose-dependent manner. But tumor
weights between groups were not statistically significant until the
concentration of TF-SB reached 200 mg/ml (Fig. 7). The survival in the nude mouse
model was 100% until sacrifice.
Effect of TF-SB on secretion of PTHrP and
its downstream RANKL/OPG in breast cancer bone metastasis
Having demonstrated the effect of TF-SB on
MDA-MB-231 cells and osteoclast cells, we continued to study the
underlying molecular mechanism. PTHrP and its downstream RANKL/OPG
play crucial roles in tumor progression and osteolytic lesion
induced by breast carcinoma bone metastasis. In order to confirm
that PTHrP pathway was responsible for explaining the inhibition
effect of TF-SB, mRNA expression levels of PTHrP, RANKL and OPG
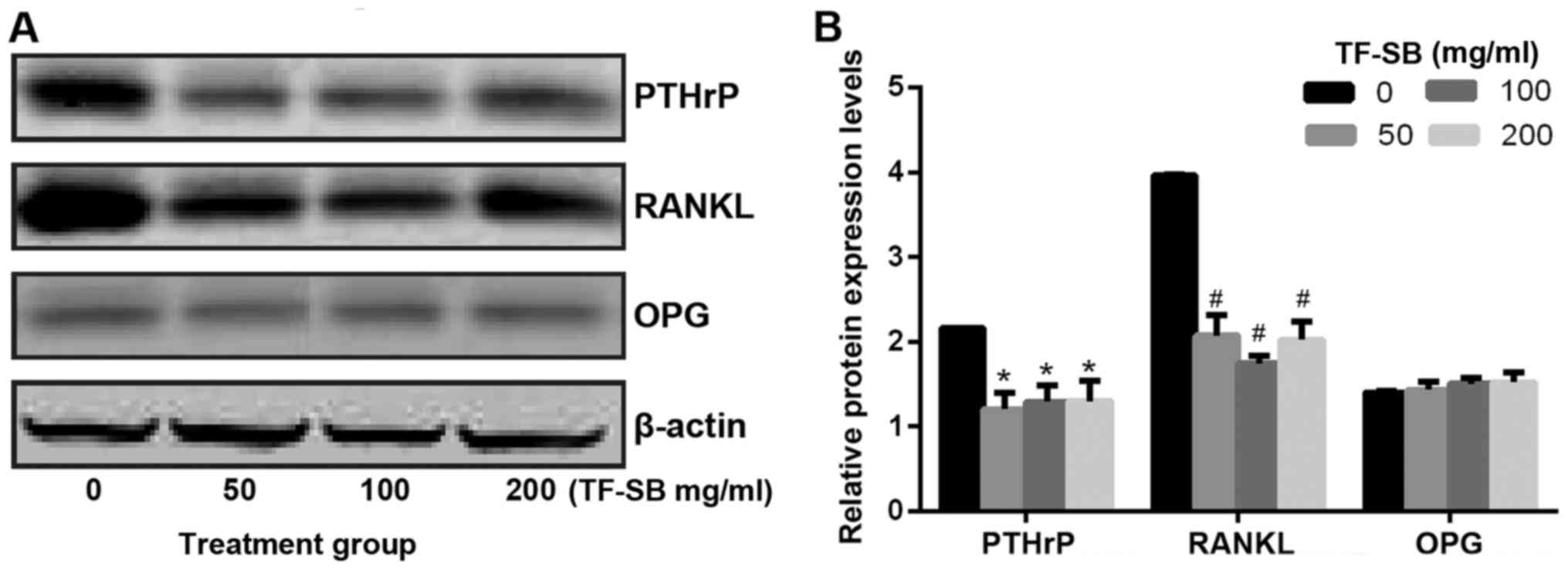
were measured by qPCR assays. As shown in Fig. 8, compared with control group,
PTHrP and RANKL expression levels were steeply decreased in the
presence of TF-SB, but OPG expression levels were slightly
increased. We concluded that the mRNA expression of PTHrP and its
downstream RANKL/ OPG could be affected by TF-SB in a
dose-dependent manner. Based on the positive results of qPCR, we
further detected the effects of TF-SB on protein expression levels.
In this study, protein expression of PTHrP, RANKL and OPG were
analyzed using western blot analysis. As shown in Fig. 9, the highest expression levels of
PTHrP and RANKL appeared in control group but decreased in TF-SB
treated groups. In contrast, OPG expression levels had the opposite
trend. The results indicated that TF-SB decreased the protein
expression of PTHrP and RANKL, but promoted OPG.
Disscussion
In the field of TCM, Scute llaria barbata D.
Don has been widely used in the treatment of inflammation and
tumors by clearing heat, activating blood and dissolving stasis
(15). Chromatographic analysis
has demonstrated that scutellarein, apigenin, baicalein and
luteolin are main components to the alcohol extract of
Scutellaria barbata D. Don (16). Moreover, TF-SB turned out to be
the main effective component in previous studies which had
favorable safety profile (14).
In our study, we had confirmed the existence of these main elements
in TF-SB. In recent years, Scutellarin barbata D. Don has
been involved in numerous antineoplastic studies (17-22). Specific to breast cancer, alcohol
extract of Scutellaria barbata D. Don has shown inhibition
effects on several cell lines (23,24).
It is generally known that breast carcinoma
frequently spreads to bone (26).
In fact, approximately 70% of patients with advanced breast cancer
metastasize to the skeleton and has risk of skeletal-related events
(3). Bone-target metastasis of
breast cancer contains multiple biological steps (27). The interaction between bone
microenvironment and metastasized breast cancer cells plays a major
role (4). It has been well
accepted that osteoclasts in the bone microenvironment are
responsible for metastatic osteolytic lesions in bone metabolism
(28). In fact, bone resorption
begins with the recruitment of large activated multinuclear
osteoclasts differentiated from non-functional multinuclear
pre-osteoclasts (29). Breast
cancer cells secrete various osteolytic factors, including PTHrP, a
protein, initially identified to be the factor in charge of
malignant hypercalcemia (30). In
primary breast cancer, PTHrP is expressed in 50% of patients
(31). But in breast cancer bone
metastasis samples, the percent rises to 90%. It is clear that
tumor-derived PTHrP works as a central contributor in promoting
osteolytic process of breast carcinoma bone metastasis (7,32).
Specifically, rather than stimulating osteoclast differentiation
directly, tumor-derived PTHrP could activate stromal cells and
osteoblasts to upregulate RANKL expression and cocurrently lower
OPG expression (8). This forms a
feed-forward pattern as mentioned above as a vicious circle with
multi-interactions between malignant cells, osteoblasts,
osteoclasts and bone microenvironment. RANKL is expressed on the
surface of osteoblast and could interact with the receptor RANK,
which is expressed on the surface of osteoclast precursor, to
further activate the osteoclast differentiation (9). RANKL binding to the RANK plays a
central role in the establishment of this vicious circle. This
circle could finally improve tumor cell proliferation, trigger
osteoclast activation and leads to osteolytic lesion of breast
cancer bone metastasis. Otherwise, OPG is a decoy receptor which
prevents RANKL binding to its receptor and inhibits osteoclast
activation (10). So the balance
between RANKL and OPG decides the degree of osteoclastic activity
and bone resorption. In our study, by particularly focusing on the
role of PTHrP and its downstream RANKL/ OPG, effects and relative
mechanisms of TF-SB on breast cancer bone metastasis were verified
at mRNA and protein levels.
As for human breast cancer cell line MDA-MB-231,
owing to its high potential of invasion and metastasis, it has been
identified as the ideal and most common cell line for modeling bone
metastasis of breast cancer. In vitro, our laboratory
firstly proved the inhibition capacity of TF-SB on cell
proliferation, migration and invasion which were detected,
respectively, by MTT test, wound healing assay and Transwell
chamber assay. In fact, at the experimental concentrations of TF-SB
ranging from 40 to 200 µg/ml, their inhibition tendencies were
dose-dependent. Evidence was shown that there was no benefit for
further improving the experimental concentration. These positive
results on MDA-MB-231 cell line were the basis for further study on
breast cancer bone metastasis in murine model.
In vivo, we injected MDA-MB-231 cell
suspension into the right femur medullary cavity of female nude
mice to build breast cancer bone metastasis animal model. According
to the result of random assignment, we further fed these animals
with corresponding concentrations of TF-SB for one month. Then
macroscopic and microcosmic angles, in vivo bioluminescence
and H&E staining were used to verify nude mouse models,
micro-CT and TRAP staining for observing osteolytic lesion caused
by tumor cells. As the results suggested, there was no effect of
TF-SB on tumor growth and mouse survival.
It had been well accepted that osteoclasts were the
major cause for metastatic osteolytic lesions in bone metabolism
(28). So focusing on the outcome
of TRAP staining, we drew the conclusion that TF-SB could diminish
osteoclast cells and ease bone destruction caused by metastatic
tumor cells in bone tissue. As tumor-derived PTHrP control the
secretion of RANKL and OPG, and the ratio of RANKL to OPG decides
the degree of bone degradation, so inhibiting the expression of
PTHrP and its downstream RANKL/OPG has been regarded as a hopeful
way to impair bone destruction (7). In order to confirm the relationship
between PTHrP pathway and inhibitory effects of TF-SB on breast
cancer bone metastasis, we offer evidence on mRNA and protein
levels. Our results suggested that TF-SB could decrease mRNA and
protein expression of PTHrP and RANKL, but on the contrary, promote
OPG expression. So a positive correlation had been established
between the phenomenon and mechanism. The data in vitro and
in vivo from our study suggest that TF-SB treatment has
beneficial effects on breast cancer bone metastasis by controlling
the expression of PTHrP and its downstream OPG/RANKL. Here we
reported that TF-SB targeting the PTHrP pathway offered a new
approach for therapeutic intervention in breast carcinoma bone
metastasis. However, for the pursuit of more effective and
selective treatments, further research could establish positive
control drugs, focus on purified components of TF-SB and extend
upstream of PTHrP pathway, such as TGF-β. More study should be done
to resolve the unconformity between bioactivity of tumor cells and
bioavailability of TF-SB.
Acknowledgments
We thank the Chemistry Experiment Center of Shanghai
Normal University, the Orthopedic Laboratory and Tissue Engineering
Laboratory of Shanghai Ninth People's Hospital for their
assistance.
Abbreviations:
|
TF-SB
|
total flavonoids from Scutellaria
barbata D. Don
|
|
HPLC
|
high-performance liquid
chromatography
|
|
PTHrP
|
parathyroid hormone-related
protein
|
|
RANKL
|
receptor or activator of nuclear
factor-κB ligand
|
|
OPG
|
osteoprotegerin
|
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
Notes
[1]
Funding
This study was supported by the Research Innovation
Program of Shanghai Municiple Education Commission (grant no.
09yz79).
[2] Availability
of data and material
The data and materials generated or analysed during
this study are available from the corresponding author on
reasonable request.
[3] Authors'
contributions
XZ performed the animal experiment. WK performed the
cell experiment. They made substantial contributions to design this
study and were major contributors in writing the manuscript. HL
analyzed and interpreted the data. As director of this study, SG
contributed to the conception of this study, gave guidance and
final approval of the version to be published. All authors read and
approved the final manuscript.
[4] Ethics
approval and consent to participate
The experimental protocol was established according
to the ethical guidelines and was approved by the Ethics Committee
of Shanghai Ninth People's Hospital.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
TA S: Genetic factors in the pathogenesis
of breast cancer: their role and relative importance. Nutr.
127(Suppl): 929–932. 1997. View Article : Google Scholar
|
|
2
|
Roodman GD: Mechanisms of bone metastasis.
N Engl J Med. 350:1655–1664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Paget S: The distribution of secondary
growths in cancer of the breast. Lancet. 1:571–573. 1889.
View Article : Google Scholar
|
|
4
|
Schulman KL and Kohles J: Economic burden
of metastatic bone disease in the U.S. Cancer. 109:2334–2342. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mundy GR: Metastasis to bone: causes,
consequences and therapeutic opportunities. Nat Rev Cancer.
2:584–593. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kominsky SL and Davidson NE: A 'bone' fide
predictor of metastasis? Predicting breast cancer metastasis to
bone. J Clin Oncol. 24:2227–2229. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dougall WC: Molecular pathways:
osteoclast-dependent and osteoclast-independent roles of the
RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin Cancer
Res. 18:326–335. 2012. View Article : Google Scholar
|
|
8
|
Schramek D, Sigl V and Penninger JM: RANKL
and RANK in sex hormone-induced breast cancer and breast cancer
metastasis. Trends Endocrinol Metab. 22:188–194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kennecke H, Yerushalmi R, Woods R, Cheang
MC, Voduc D, Speers CH, Nielsen TO and Gelmon K: Metastatic
behavior of breast cancer subtypes. J Clin Oncol. 28:3271–3277.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chiechi A, Waning DL, Stayrook KR, Buijs
JT, Guise TA and Mohammad KS: Role of TGF-β in breast cancer bone
metastases. Adv Biosci Biotechnol. 4:15–30. 2013. View Article : Google Scholar
|
|
11
|
Datta NS and Abou-Samra AB: PTH and PTHrP
signaling in osteoblasts. Cell Signal. 21:1245–1254. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roelofs AJ, Coxon FP, Ebetino FH, Lundy
MW, Henneman ZJ, Nancollas GH, Sun S, Blazewska KM, Bala JL,
Kashemirov BA, et al: Fluorescent risedronate analogues reveal
bisphosphonate uptake by bone marrow monocytes and localization
around osteocytes in vivo. J Bone Miner Res. 25:606–616. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Syddall SP, Ottewell PD and Holen I:
Combined therapies of bone disease with bisphosphonates. Curr Pharm
Des. 16:2988–2997. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lacey DL, Boyle WJ, Simonet WS, Kostenuik
PJ, Dougall WC, Sullivan JK, San Martin J and Dansey R: Bench to
bedside: elucidation of the OPG-RANK-RANKL pathway and the
development of denosumab. Nat Rev Drug Discov. 11:401–419. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chinese Pharmacopoeia Commission:
Pharmacopoeia of the People's Republic of China. China Medical
Science and Technology Press; Beijing: pp. 109–110. 2010
|
|
16
|
Ruan C, Xiao XH and Li GK:
Microwave-assisted extraction coupled with countercurrent
chromatography for the rapid preparation of flavonoids from
Scutellaria barbata D. Don. J Sep Sci. 37:1364–1369. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin J, Chen Y, Cai Q, Wei L, Zhan Y, Shen
A, Sferra TJ and Peng J: Scutellaria barbata D. Don inhibits
colorectal cancer growth via suppression of multiple signaling
pathways. Integr Cancer Ther. 13:240–248. 2014. View Article : Google Scholar
|
|
18
|
Wei L, Lin J, Wu G, Xu W, Li H, Hong Z and
Peng J: Scutellaria barbata D. Don induces G1/S arrest via
modulation of p53 and Akt pathways in human colon carcinoma cells.
Oncol Rep. 29:1623–1628. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei L, Lin J, Xu W, Cai Q, Shen A, Hong Z
and Peng J: Scutellaria barbata D. Don inhibits tumor angiogenesis
via suppression of Hedgehog pathway in a mouse model of colorectal
cancer. Int J Mol Sci. 13:9419–9430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao J, Lu WF, Dai ZJ, Lin S, Zhao Y, Li S,
Zhao NN, Wang XJ, Kang HF, Ma XB, et al: Induction of apoptosis by
total flavonoids from Scutellaria barbata D. Don in human
hepatocarcinoma MHCC97-H cells via the mitochondrial pathway.
Tumour Biol. 35:2549–2559. 2014. View Article : Google Scholar
|
|
21
|
Tang PM, Chan JY, Zhang DM, Au SW, Fong
WP, Kong SK, Tsui SK, Waye MM, Mak TC and Fung KP: Pheophorbide a,
an active component in Scutellaria barbata, reverses
P-glycoprotein-mediated multidrug resistance on a human hepatoma
cell line R-HepG2. Cancer Biol Ther. 6:504–509. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yin X, Zhou J, Jie C, Xing D and Zhang Y:
Anticancer activity and mechanism of Scutellaria barbata extract on
human lung cancer cell line A549. Life Sci. 75:2233–2244. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi Y, Zhou ZY, Cheng LJ and Lin MB: The
effects of scutellarin in barbated skullcup herb on galactophore
cancer MCF-7 cell proliferation and invasion potentia. Acta
Academiae Medicinae Jiangxi. 49:12–14. 2009.In Chinese.
|
|
24
|
Fong S, Shoemaker M, Cadaoas J, Lo A, Liao
W, Tagliaferri M, Cohen I and Shtivelman E: Molecular mechanisms
underlying selective cytotoxic activity of BZL101, an extract of
Scutellaria barbata, towards breast cancer cells. Cancer Biol Ther.
7:577–586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bustin SA and Mueller R: Real-time reverse
transcription PCR (qRT-PCR) and its potential use in clinical
diagnosis. Clin Sci (Lond). 109:365–379. 2005. View Article : Google Scholar
|
|
26
|
Weilbaecher KN, Guise TA and McCauley LK:
Cancer to bone: a fatal attraction. Nat Rev Cancer. 11:411–425.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kearns AE, Khosla S and Kostenuik PJ:
Receptor activator of nuclear factor kappaB ligand and
osteoprotegerin regulation of bone remodeling in health and
disease. Endocr Rev. 29:155–192. 2008. View Article : Google Scholar
|
|
28
|
Blanco MA and Kang Y: Signaling pathways
in breast cancer metastasis - novel insights from functional
genomics. Breast Cancer Res. 13:2062011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yin JJ, Selander K, Chirgwin JM, Dallas M,
Grubbs BG, Wieser R, Massagué J, Mundy GR and Guise TA: TGF-beta
signaling blockade inhibits PTHrP secretion by breast cancer cells
and bone metastases development. J Clin Invest. 103:197–206. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hastings RH, Burton DW, Nefzi A, Montgrain
PR, Quintana R and Deftos LJ: Combinatorial library discovery of
small molecule inhibitors of lung cancer proliferation and
parathyroid hormone-related protein expression. Cancer Biol Ther.
10:1067–1075. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Soki FN, Park SI and McCauley LK: The
multifaceted actions of PTHrP in skeletal metastasis. Future Oncol.
8:803–817. 2012. View Article : Google Scholar : PubMed/NCBI
|