Introduction
Infantile hemangiomas are the most common tumors in
children, characterized by endothelial cell proliferation and
disorganized blood vessels (1).
Infantile hemangiomas are benign, but could result in severe
complications, such as life-altering disfigurement or ulceration
(2,3). Propranalol, a non-selective
β-blocker, is a promising treatment for infantile hemangiomas
(4). Hydrochloride propranalol
(also known as Hemangeol) has become the first and only US Food and
Drug Administration (FDA)-approved anti-infantile hemangioma drug
(4). Although Hemangeol is
effective and relatively safe, its frequency of oral administration
is high, and could induce complications, such as aggravated
respiratory tract infections (5).
Thus, it is necessary to develop other candidate reagents for
treating infantile hemangiomas.
Rapamycin is an inhibitor of the mammalian target of
rapamycin (mTOR), and inhibits neovascularization (6,7).
It is noteworthy that rapamycin inhibits the proliferation of
hemangioma endothelial cells and secretion of vascular endothelial
growth factor (VEGF), and rapamycin has been already used as a
medication in renal transplantation, making it a promising
candidate for treating infantile hemangiomas (8–10).
Nevertheless, rapamycin also has the disadvantages of high
frequency of administration and of several complications, including
anemia and acute renal toxicity (11). Local controlled systems directly
targeting diseased regions can reduce a body-wide distribution,
leading to undesirable side effects, and can also reduce the high
frequency of administration (12). Therefore, we previously fabricated
rapamycin lipid polymer nanoparticles (R-PLNPs) to attain
controlled rapamycin release locally, and to decrease the frequency
of administration and side effects of rapamycin (13). Poly(lactic-co-glycolic acid)
(PLGA) was selected as the core of R-PLNPs, since PLGA is a
hydrophobic FDA-approved material for use in humans (13).
Targeted nanoparticles coupled with antibodies can
promote the therapeutic effectiveness of conventional medications
in various diseases (14–16). In order to target infantile
hemangiomas with nanoparticles coupled with antibodies, the
identification of suitable targets in infantile hemangiomas is
pivotal. It has been reported that VEGF and its receptor (vascular
endothelial growth factor receptor; VEGFR) is critical in the
angiogenesis of infantile hemangiomas (17). VEGFR2, one of the three receptors
of VEGF, is a critical receptor for blood vasculature development,
and infantile hemangiomas are induced by elevated VEGF signaling
through VEGFR2 (8,18). Thus, it can be hypothesized that
VEGFR2 may be a suitable target for treating infantile hemangiomas.
In fact, VEGFR2 has been previously targeted to promote
urea-encapsulated liposomes to target hemangioma vascular
endothelial cells (19). In the
present study, it was hypothesized that an antibody targeting
VEGFR2 could be utilized to promote the therapeutic effectiveness
of the previously developed R-PLNPs towards infantile hemangiomas.
Rapamycin lipid polymer nanoparticles coupled with an anti-VEGFR2
antibody (R-PLNPs-V) were constructed as a controlled and targeted
release drug delivery system to treat infantile hemangiomas. It was
hypothesized that R-PLNPs-V could release rapamycin lastingly, and
promote the targeted delivery of rapamycin to infantile
hemangiomas.
Materials and methods
Chemical reagents, antibody, kits, and
cell culture
All analytical-grade organic reagents, PLGA (MW,
40–75 kDa; lactide: Glycolide, 50:50) and coumarin 6 were purchased
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Rapamycin and
its standards were purchased from Dalian Meilun Biotech Co., Ltd.
(Dalian, China). Soybean lecithin, DSPE-PEG2000
[1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-(methoxypolyethylene
glycol)-2000)], and DSPE-PEG2000-Mal [1,2-distearoyl-sn-glycero-3-
phosphoethanolamine-N-(maleimide (polyethylene glycol)-2000)] were
purchased from Avanti Polar Lipids, Inc. (Alabaster, AL, USA). The
Cell Counting Kit-8 (CCK-8 kit) was purchased from Dojindo
Molecular Technologies, Inc. (Kumamoto, Japan). Fetal bovine serum
(FBS) was purchased from Thermo Fisher Scientific, Inc. (Waltham,
MA, USA). The complex protein mixture, Matrigel, was purchased from
BD Biosciences (Franklin Lakes, NJ, USA). The mouse anti-human
VEGFR2 monoclonal antibody (MAB3571; 1:1,000), and the basic
fibroblast growth factor (bFGF) (DFB50) and VEGF-A (DVE00) ELISA
kits were purchased from R&D Systems, Inc. (Minneapolis, MN,
USA). The secondary antibody [goat anti-mouse immunoglobulin G
(IgG) coupled with horseradish peroxidase] (sc-2005; 1:2,000), and
β-actin antibody (sc-47778; 1:200) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA).
Human umbilical vein endothelial cells (HUVECs) were
purchased from ScienCell (Carlsbad, CA, USA). HUVECs were cultured
in a humidified 5% CO2 incubator at 37°C, in completed
endothelial cell medium (ECM; (ScienCell). The trypsinization of
cells was performed with 0.15% trypsin-EDTA, and cells of passages
3–10 were used in the present study.
Human hemangioma endothelial cells (HemECs) isolated
from the hemangiomas of a patient were obtained as described
previously (13). The Research
Ethics Committee of Wuhan Union Hospital (Wuhan, China) approved
the present study, and written informed consent was obtained from
the patient. The specimens were treated on the basis of the legal
and ethical standards. Specimens of infantile hemangiomas were
obtained from Wuhan Union Hospital (patient 2 years old, female,
date of sample collection April, 2016), and when the cells reached
confluence, they were subcultured at 1:3 ratio.
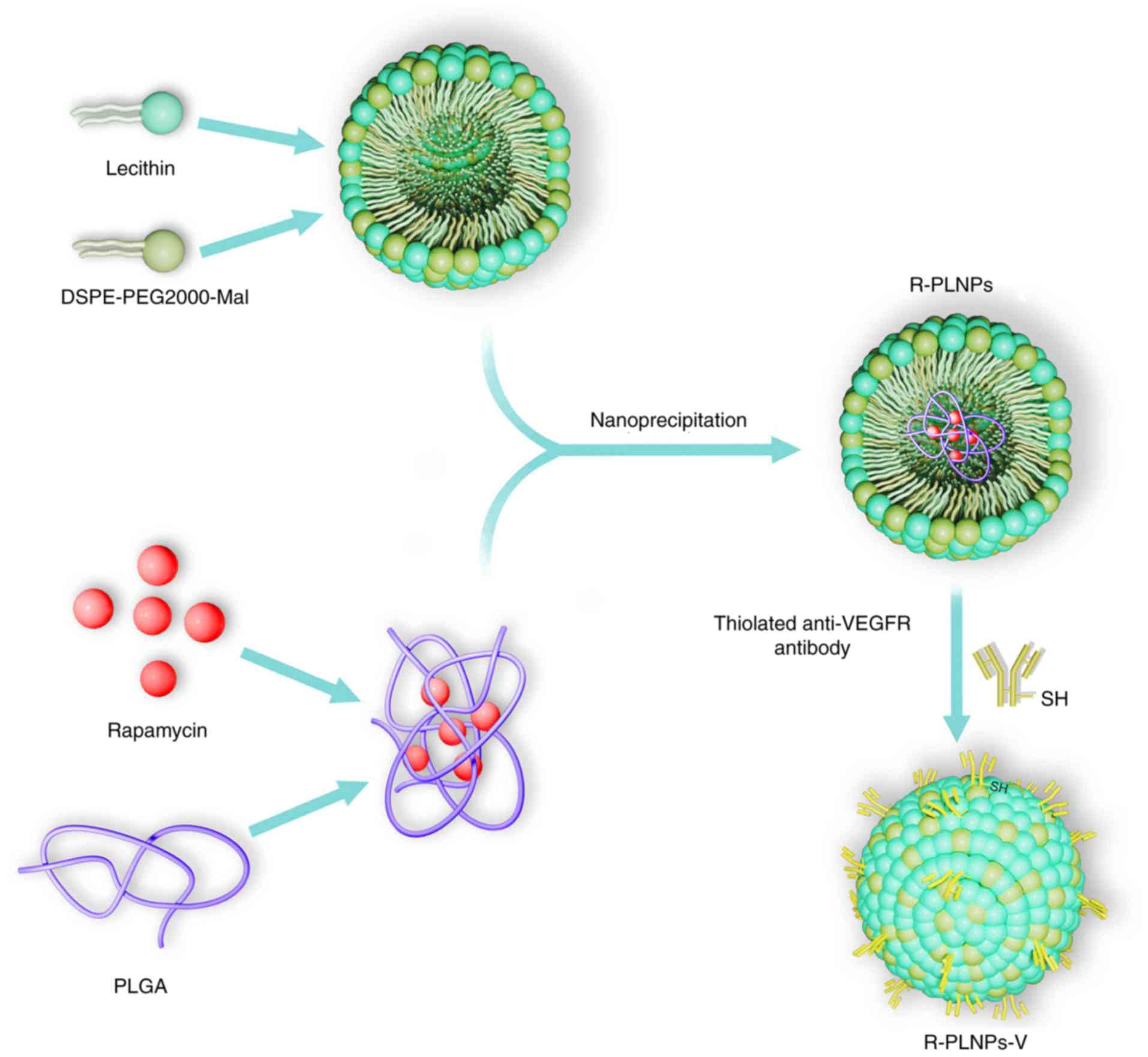
Fabrication of R-PLNPs
R-PLNPs were constructed using a one-step
nanoprecipitation approach, as previously described (13) (Fig.
1). In brief, the two solutions (A and B solutions) were
prepared as follows: Solution A was developed by dissolving
rapamycin (2 mg) in acetonitrile solution dissolved with 1 mg/ml
PLGA; Solution B was developed by dissolving DSPE-PEG2000 (0.2 mg)
and soybean lecithin (0.5 mg) in 4% ethanol aqueous solution at
65°C. Afterwards, A solution was mixed with B solution at a slow
speed of 1 ml/min. Following mixing, the solution was vortexed
vigorously for 5 min. The resulting solution was gently stirred for
6 h, and then dialysis to PBS (pH 7.4) with Spectra/pro 6 dialysis
membrane (MWCO 3500) was performed. Amicon Ultra-4 centrifugal
filter devices (Amicon Corporation; EMD Millipore, Billerica, MA,
USA) were used to concentrate the nanoparticles and to achieve the
desired concentration. Blank lipid polymer nanoparticles (PLNPs)
and coumarin 6-loaded nanoparticles (C-PLNPs) were constructed
similar to R-PLNPs.
Fabrication of rapamycin-encapsulated
lipid polymer nanoparticles coupled with anti-VEGFR2 antibody
(R-PLNPs-V)
R-PLNPs-V were fabricated by coupling thiolated
anti-VEGFR2 antibody to R-PLNPs as described before (8) (Fig.
1). In brief, 2-iminothiolane was adopted to thiolate the
anti-VEGFR2 antibody at a molar ratio of 2-iminothiolane: VEGFR2
antibodies. R-PLNPs were fabricated as described above, using
DSPE-PEG2000-Mal to replace DSPE-PEG2000. Then, 0.2 mg thiolated
anti-VEGFR2 antibody was incubated with 4 mg R-PLNPs containing
maleimide-terminated linker, and the mixed solution was incubated
for 6 h at ambient temperature under nitrogen. The obtained
R-PLNPs-V were washed and centrifuged (15,000 × g, 30 min) to
eliminate any uncoupled antibody. As controls, blank lipid polymer
nanoparticles coupled with anti-VEGFR antibody (PLNPs-V) and
coumarin 6-encapsulated lipid polymer nanoparticles coupled with
anti-VEGFR2 antibody (C-PLNPs-V) were also constructed.
Characteristics of nanoparticles
Following dispersion in deionized water, the
nanoparticles were examined for their size and zeta potential using
a Zetasizer Nano S (Malvern Instruments, Ltd., Malvern, UK). For
analysis of morphology, the nanoparticles were stained by 2%
phosphotungstic acid, and analyzed by Hitachi H-600 transmission
electron microscopy (TEM; Hitachi Ltd., Tokyo, Japan).
Drug loading and encapsulation efficiency
of rapamycin in nanoparticles
Reverse phase high performance liquid chromatography
(HPLC) was utilized to analyze the rapamycin encapsulation efficacy
and drug loading of nanoparticles, as previously described
(13). Briefly, the analysis was
performed by HPLC, using Inertsil C-18 column (Octadecylsilane-3V,
4.6×250 mm in dimension) at 278 nm wavelength. The mobile phase was
methanol-water (90:10 v/v). The flow rate was 1 ml/min. Drug
encapsulation efficacy (EE) was calculated by the formula: amount
of rapamycin loaded in nanoparticles/ total rapamycin added
initially. Drug loading was calculated by the formula: Rapamycin
loaded in nanoparticles/total amount of nanoparticles. The coumarin
6 loading of coumarin 6-loaded nanoparticles was calculated by a
coumarin-6 calibration curve.
Rapamycin release in vitro
A total of 2 mg nanoparticles were placed inside a
membrane dialysis bag (MWCO, 3500; Spectra/pro 6 membrane). The bag
was immersed in a vial containing PBS (200 ml; pH 7.4) with or
without the addition of 10% FBS while being stirred at 100 rpm in a
water bath at 37°C. One ml of dialysate was removed at different
time points for HPLC analysis, and 1 ml fresh solution was added
instead.
In vitro cellular uptake
HUVECs or HemECs (2×105/well) were
inoculated in 12-well cell culture plates at 37°C, and incubated
for 12 h. Free coumarin 6 or coumarin 6-loaded nanoparticles (an
equivalent concentration of 20 ng/ml coumarin 6) were added to the
cells, and incubated with the cells for 6 h. For competitive
assays, 5 mg/m anti-VEGFR2 antibody was pre-incubated with the
cells for 30 min. Following pre-incubation, the cells were rinsed
with PBS to eliminate any unbound drugs. Following rinsing, the
trypsinized cells were analyzed using a BD FACScan flow cytometer
(Becton Dickinson, San Jose, CA). The data were analyzed using
FlowJo software version 10 (FlowJo LLC, Ashland, OR, USA).
HUVECs or HemECs (2×105/well) were
inoculated in 12-well cell culture plates at 37°C, and incubated
for 12 h. Rapamycin or rapamycin-loaded nanoparticles (an
equivalent concentration of 200 ng/ml rapamycin) were added to the
cells, and the cells were incubated with the drugs for 6 h at 37°C.
For competitive assays, 5 mg/ml anti-VEGFR2 antibody was
pre-incubated with the cells for 30 min. Following pre-incubation,
unbound drugs were eliminated by rinsing the cells with PBS. Then,
the col lected cells were homogenized by the lysis buffer
consisting of 10% SDS. The rapamycin in the treated cells was
quantitated by HPLC as described above. The % of rapamycin uptake
was calculated by the following formula: Amount of internalized
rapamycin/amount of rapamycin added initially ×100%.
CCK-8 assay
HUVECs or HemECs were inoculated in 96-well culture
plates (10,000/well) at 37°C, and incubated for 12 h. The cells
were incubated with of the indicated concentrations of drugs for 72
h. The cell viability was determined by the CCK-8 assay, according
to the manufacturer's protocols.
ELISA
HUVECs or HemECs (2×105/well) were
inoculated in 12-well culture plates at 37°C overnight. The cells
were incubated with various concentrations of rapamycin and
nanoparticles. After 72 h of treatments, the supernatant of the
treated cells was isolated, and the cytokine levels were measured
by ELISA kits, according to the manufacturer's protocols.
Western blot analysis
Total protein was extracted from the cells using
ice-cold RIPA-Buffer (Beyotime Institute of Biotechnology, Jiangsu,
China). The protein concentration was determined by the BCA assay
with the BCA protein quantitative kit (Beyotime Institute of
Biotechnology) and separated by 10% SDS-PAGE (20 µg of
protein per lane). The protein was then transferred onto
polyvinylidene fluoride membranes. The membrane was blocked with
10% BSA at 25°C overnight. The primary antibody was the mouse
anti-human VEGFR2 antibody (1:1,000) overnight at 4°C. The
secondary antibody was horseradish peroxidase-coupled goat
anti-mouse IgG (1:2,000) 1 h at 25°C). An Enhanced
Chemiluminiscence kit (GE Healthcare) was used to detect the bands,
and the bands were visualized with the ChemiDoc XRS+
system with Image Lab™ image acquisition and analysis software
version 4.1 (Bio-Rad Laboratories Inc., Hercules, CA, USA).
In vivo anti-hemangioma activity of
nanoparticles
A total of 40 BALB/c nude mice (6–8 weeks, female; 8
mice per group/5 groups) were provided by the Experimental Animal
Center of Tongji Medical College, Huazhong University of Science
and Technology (Wuhan, China). The mice were housed under
conditions of controlled temperature (22±2°C), humidity (45–65%),
and artificial light (12-h light/dark cycle) with free access to
food and water. All the procedures in animal studies were performed
in accordance with the guidelines of the Committee on Animals of
Tongji Medical College, Huazhong University of Science and
Technology (Wuhan, China).
The mice were inoculated at the flank with
7.5×106 HemECs suspended in Matrigel. When the
hemangioma had reached about 25 mm3 on day 0, the mice
received single intratumoral (i.t.) injections of R-PLNPs (2 mg
rapamycin/kg), R-PLNPs-V (2 mg rapamycin/kg), rapamycin (2 mg
rapamycin/kg) or PLNPs-V (40 mg/kg). Free rapamycin was first
dissolved in dimethyl sulfoxide, and then dissolved in 5%
polyethylene glycol, 0.2% carboxymethylcellulose, and 0.25%
Tween-80 in H2O. The i.t. injections were carried out on
days 0, 5, 10, 15 and 20. The hemangioma volume of the mice,
calculated as V=(L x W2)/2 (L indicates length, and W
indicates width), was measured once every five days. On day 35,
after the mice were euthanized, the weight of the excised
hemangioma was measured. The excised hemangioma was stained by
H&E. The analysis of the microvessel density (MVD) of the
sections was done as previously described (8). Microvessels were quantified by
counting lumens containing red blood cells. A total of 4 fields per
slide were reviewed under a Zeiss Axiophot 2 microscope (Zeiss
GmbH, Jena, Germany). MVD was calculated as
vessels/mm2.
Statistical analysis
Statistical analysis was performed with Student's
non-paired t-tests or one-way analysis of variance followed by
Newman-Keuls or Dunnett's test, using SPSS 13.0 (SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference. Unless otherwise stated, all
data were expressed as the mean ± standard deviation.
Results
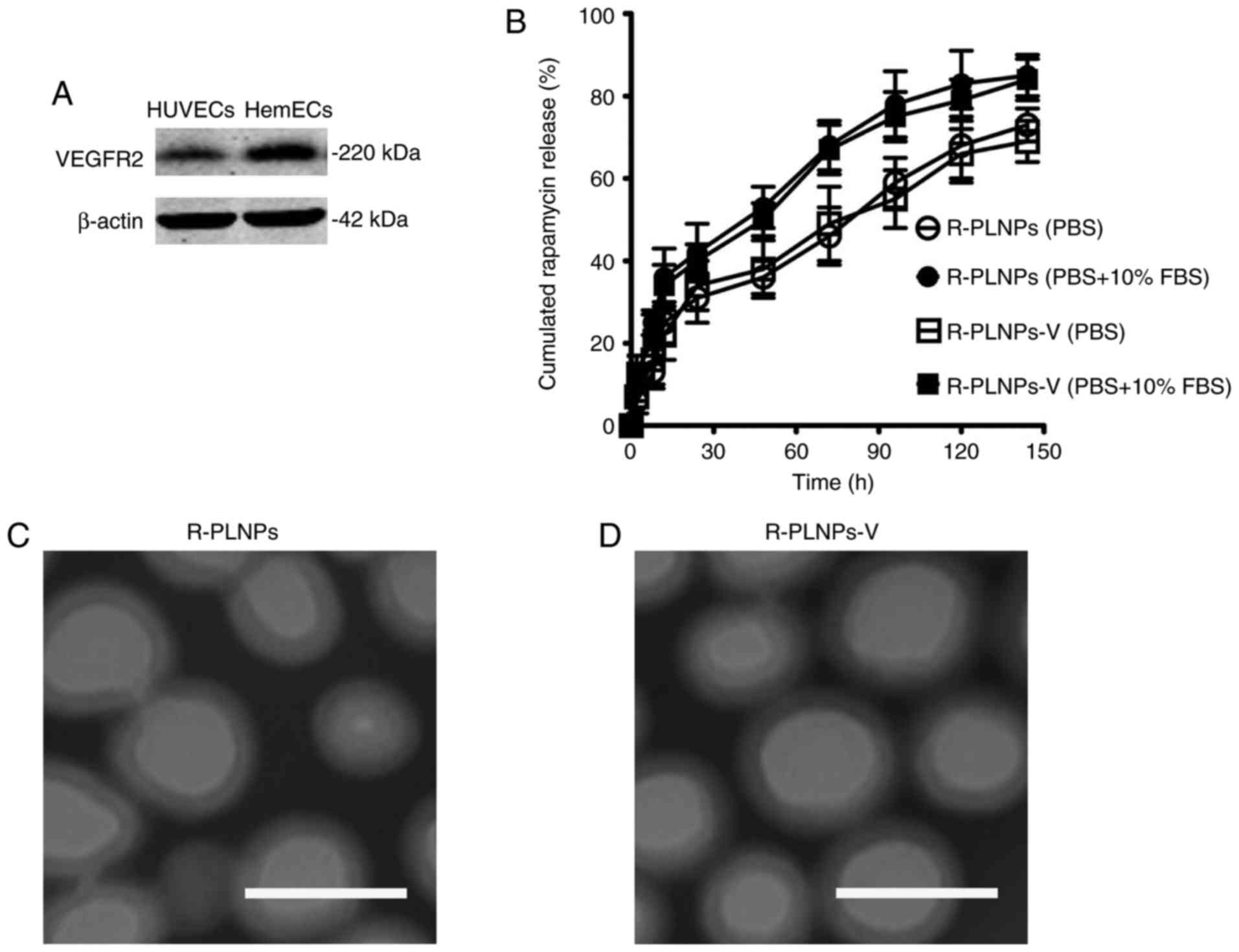
VEGFR2 expression of endothelial cells
and characteristics of nanoparticles
VEGFR2 was expressed in the endothelial cells
(HUVECs and HemECs; Fig. 2A),
suggesting that it could serve as a potential target in promoting
the targeting of nanoparticles to the endothelial cells. The
characteristics of nanoparticles are presented in Table I. The size of the nanoparticles
was slightly >100 nm, with a narrow polydispersity <0.2. The
drug encapsulation efficacy (EE) of R-PLNPs and R-PLNPs-V was 67.8
and 62.9%, respectively. The drug release profile of rapamycin from
the nanoparticles was examined (Fig.
2B). The rapamycin release of both nanoparticles was faster in
PBS with 10% FBS compared with PBS alone. In the early 48 h, both
nanoparticles exhibited a burst release (~40% for PBS, and ~50% for
PBS with 10% FBS). In the next 96 h, the cumulative release of both
nanoparticles achieved ~65% in PBS and 80% in PBS with 10% FBS.
Taken together, both nanoparticles exhibited a sustained release
during a period of 192 h. The surface morphology of the
nanoparticles was analyzed by TEM (Fig. 2C and D). TEM analysis demonstrated
that both nanoparticles displayed smooth and round shape. The dim
ring of the nanoparticles may reflect the structure of lipid layers
which surround the polymer core.
 | Table ICharacteristics of nanoparticles. |
Table I
Characteristics of nanoparticles.
| Size (nm) | Zeta potential
(mv) | PDI | EE (%) | Drug loading (%) |
|---|
| R-PLNPs | 110±21 | −20±7 | 0.15±0.05 | 67.8±9.3 | 6.3±3.3 |
| R-PLNPs-V | 116±26 | −24±9 | 0.16±0.06 | 62.9±8.1 | 5.8±3.7 |
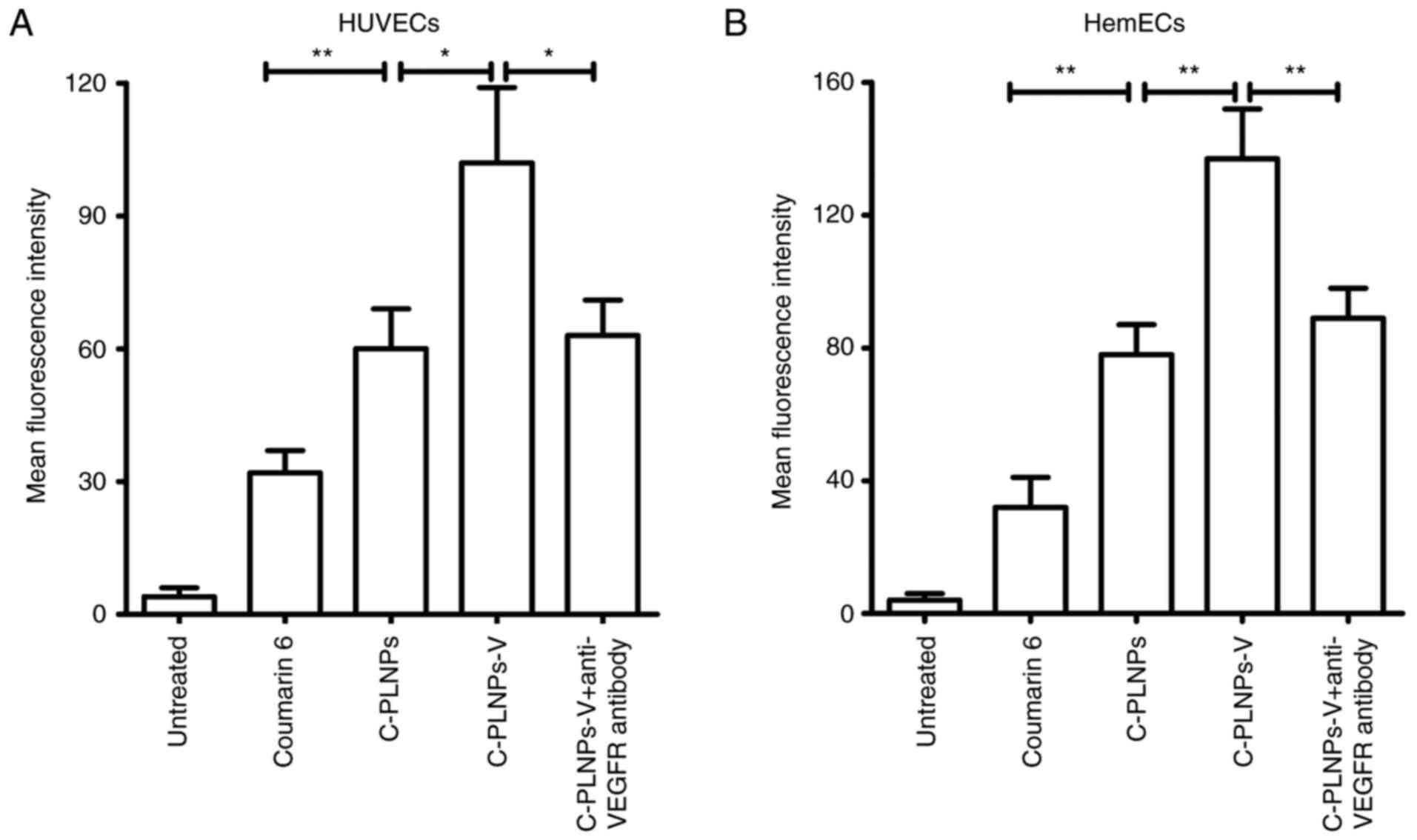
Nanoparticle uptake by endothelial
cells
First, coumarin 6 was used as the fluorescence probe
to evaluate the nanoparticle uptake in endothelial cells (Fig. 3A). In HUVECs, coumarin 6-loaded
nanoparticles (C-PLNPs) exhibited higher fluorescence intensity
than coumarin 6 alone (P<0.01; Fig. 3A), indicating that the formation
of coumarin 6 in the nanoparticles formulation facilitated its
uptake. After being coupled with the anti-VEGFR2 antibody, the
fluorescence intensity of nanoparticles was further increased, as
reflected by the higher fluorescence intensity of C-PLNPs-V
compared with C-PLNPs (P<0.05; Fig. 3A). By contrast, the fluorescence
intensity of C-PLNPs-V was significantly decreased after
pre-incubation with the anti-VEGFR2 antibody (P<0.05; Fig. 3A), indicating that the conjugated
anti-VEGFR2 antibody promoted the binding of C-PLNPs-V to HUVECs.
Similar results were obtained in HemECs (Fig. 3B).
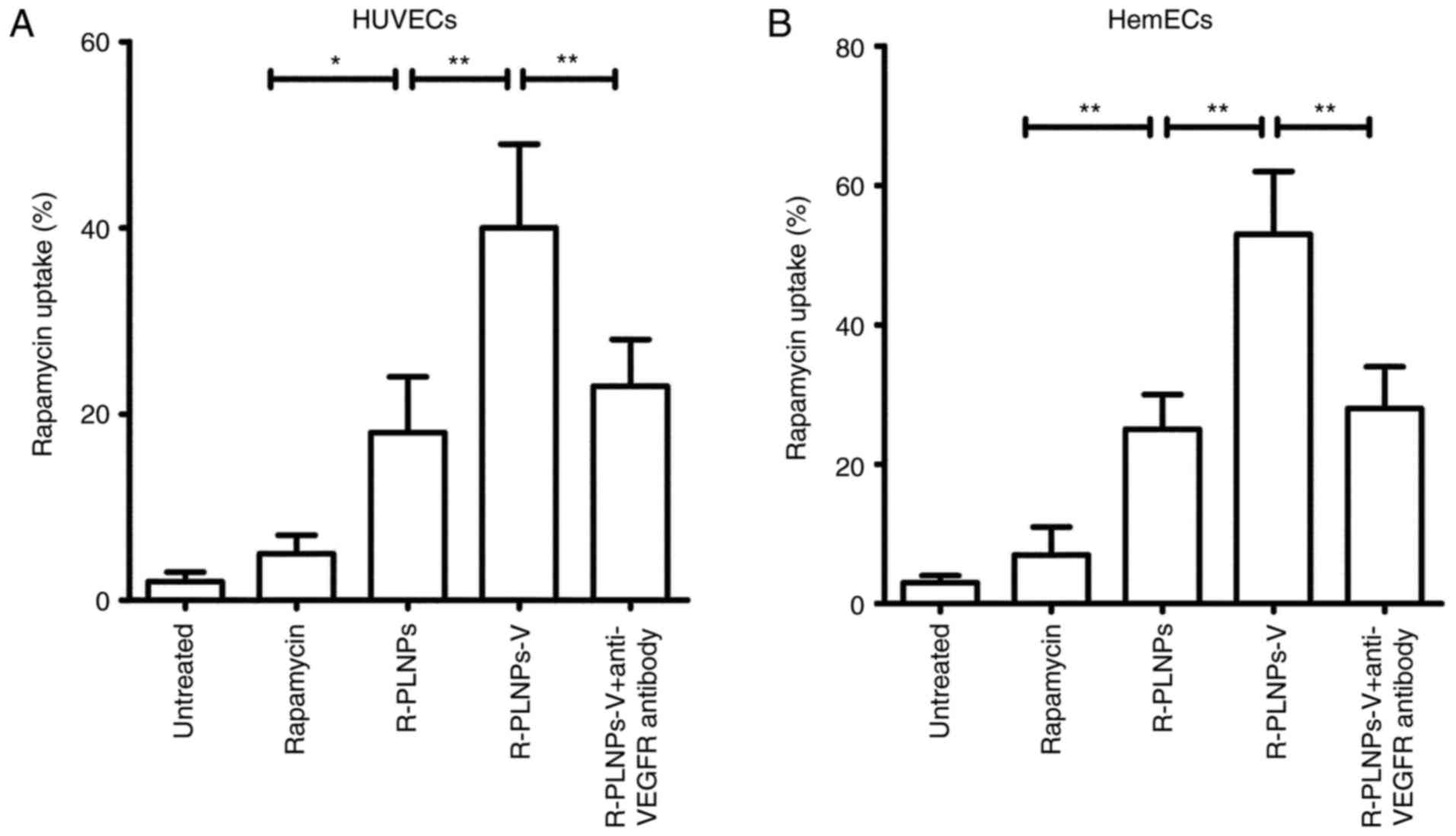
Subsequently, the rapamycin uptake of nanoparticles
in HUVECs and HemECs was explored (Fig. 4). Similar results, as the
fluorescence assay described above, were obtained. In HUVECs,
nanoparticles significantly increased the uptake of rapamycin, as
reflected by the fact that ~20% of rapamycin uptake was observed
for R-PLNPs, whereas only ~5% of rapamycin uptake was observed for
rapamycin alone (Fig. 4A). Once
again, R-PLNPs-V exhibited significant rapamycin uptake (~40%),
significantly higher compared with R-PLNPs (~20%; P<0.01;
Fig. 4A). However, the uptake of
R-PLNPs-V was decreased significantly to ~20% following anti-VEGFR2
antibody pretreatment (P<0.01; Fig. 4A), suggesting that the anti-VEGFR
antibody mediated the efficient rapamycin uptake of R-PLNPs-V.
Similar results were obtained in HemECs (Fig. 4B).
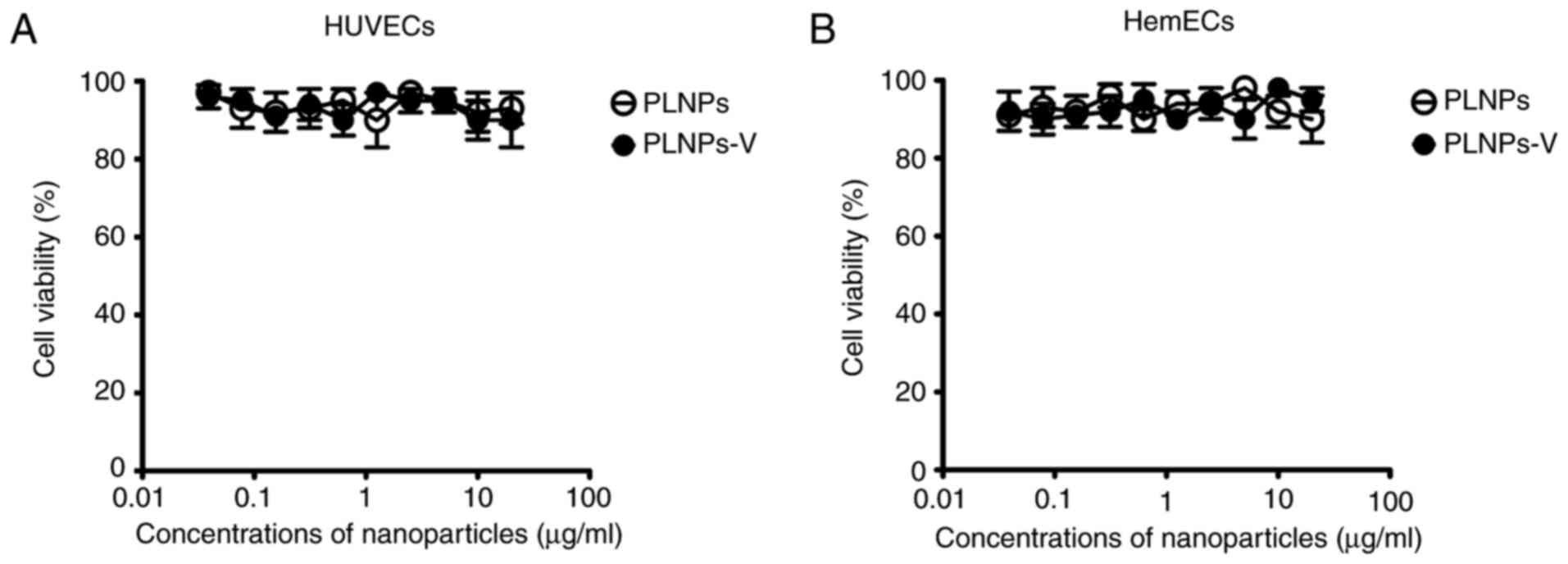
Effect of nanoparticles in cell viability
by CCK-8 assay
The biocompatibility of nanoparticles is an
important issue, since their toxicity may pose potential damage to
humans. As illustrated in Fig. 5,
the blank nanoparticles, PLNPs, and the PLNPs-V, did not result in
any significant toxicity to HUVECs and HemECs at 72 h at
nanoparticles concentrations of 0.04–20 µg/ml.
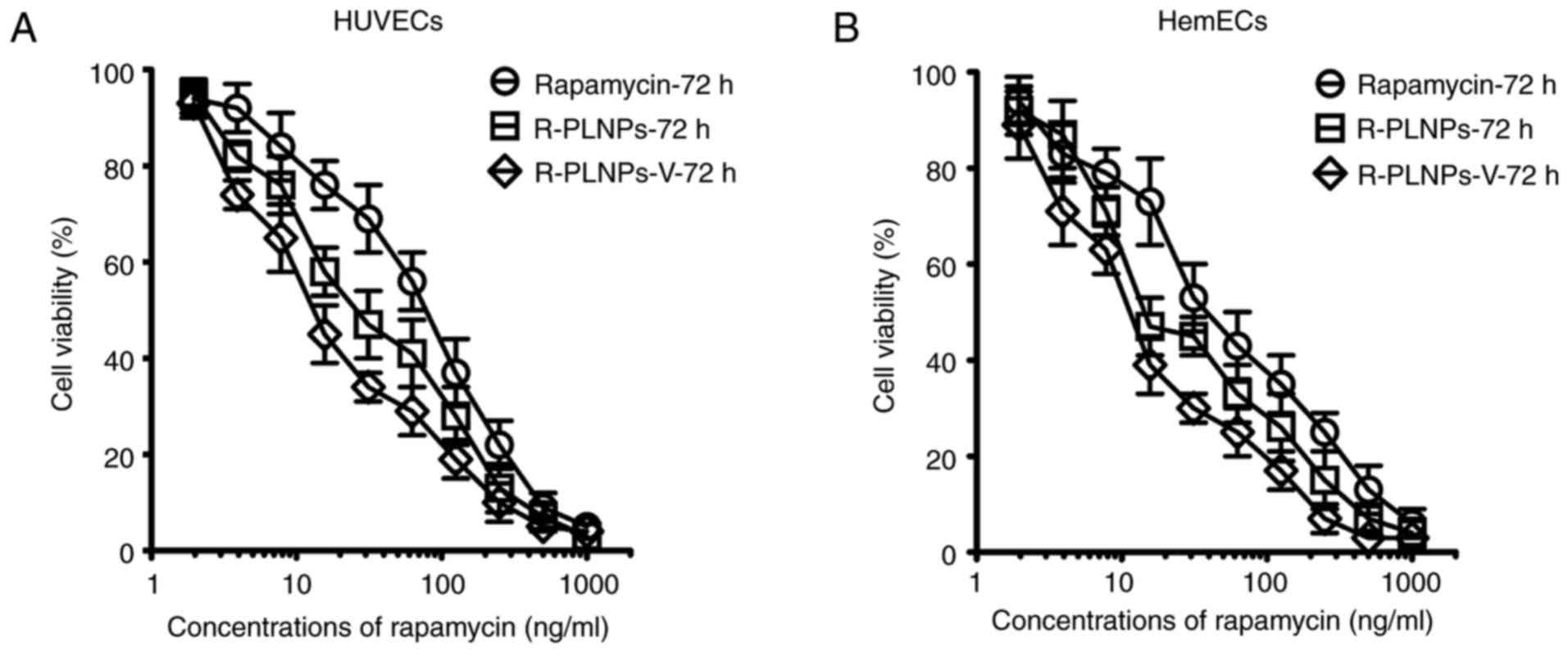
The cytotoxic effects of rapamycin, R-PLNPs, and
R-PLNPs-V were then evaluated in HUVECs and HemECs, and all of them
displayed a dose-dependent cytotoxicity in both cell lines
(Fig. 6). As presented in
Table II, the IC50 of
rapamycin, R-PLNPs, and R-PLNPs-V was 101.3, 39.1, and 21.3 ng/ml
in HUVECs, respectively. These results suggest that R-PLNPs-V were
2.6- and 4.8-fold more effective than R-PLNPs and rapamycin,
respectively. In HemECs, similar results were obtained (Table II). R-PLNPs-V were 2- and
3.8-fold more effective than R-PLNPs and rapamycin, respectively.
Thus, R-PLNPs-V displayed significantly increased cytotoxic effects
towards the endothelial cells compared with rapamycin and
R-PLNPs.
 | Table IIIC50 of various treatments
in HUVECs and HemECs. |
Table II
IC50 of various treatments
in HUVECs and HemECs.
| IC50,
ng/ml | 72 h |
|---|
| Rapamycin | R-PLNPs | R-PLNPs-V |
|---|
| HUVECs | 101.3±18.3 | 39.1±7.5 | 21.3±7.3 |
| HemECs | 75.6±7.2 | 38.5±7.1 | 19.8±8.7 |
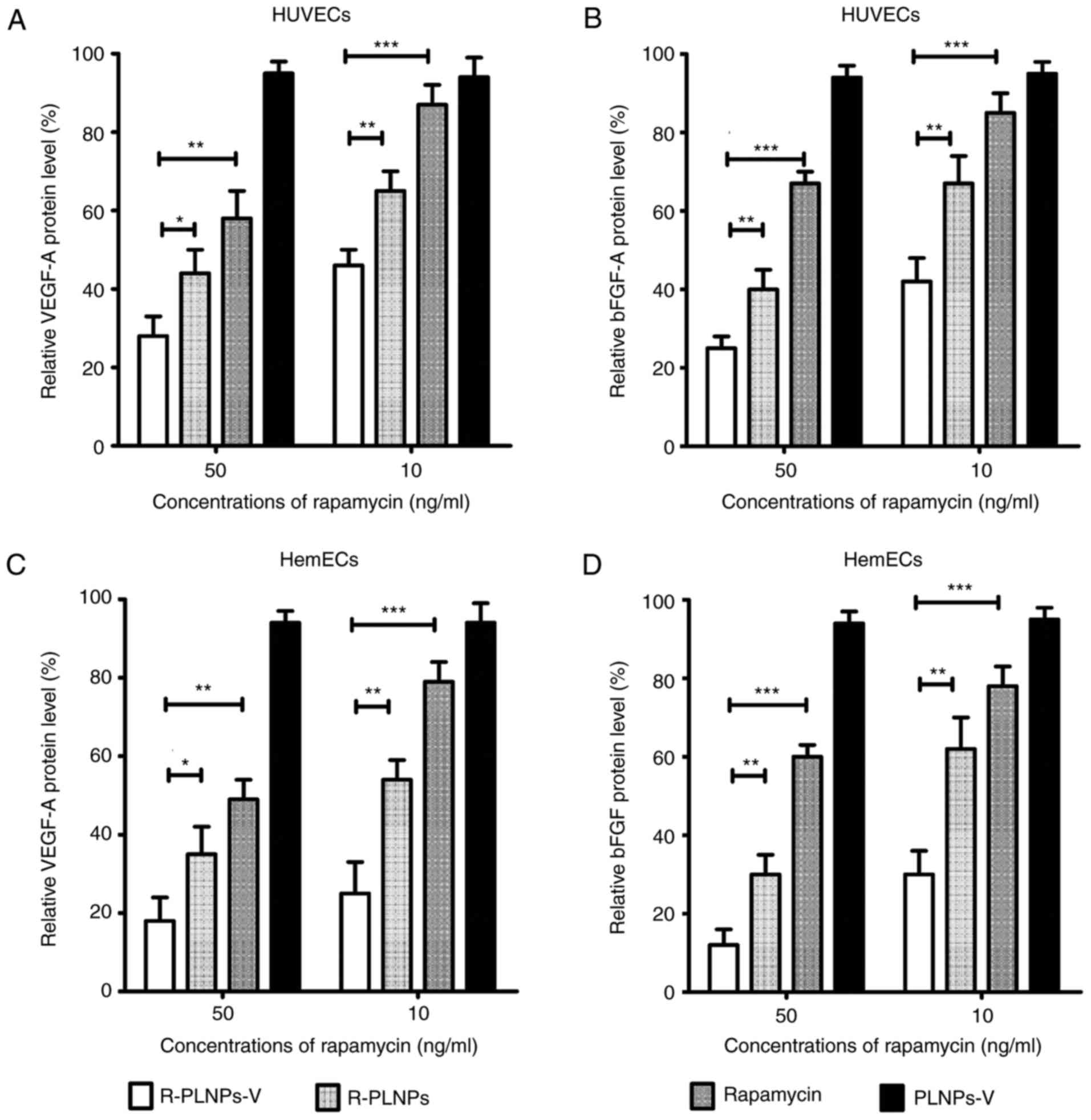
Effect of nanoparticles in cytokine
production by ELISA
The production of VEGF-A and bFGF is dependent on
mTOR activation, and rapamycin could decrease their production by
mTOR inhibition (20). As
illustrated in Fig. 7, levels of
secreted VEGF-A and bFGF were examined following various treatments
in the endothelial cells. The relative protein levels of the
treated groups are presented as the % of the levels of the
untreated group. Although the blank nanoparticles PLNPs-V barely
affected VEGF-A and bFGF production, R-PLNPs-V, R-PLNPs and
rapamycin treatments inhibited VEGF-A and bFGF production in HUVECs
and HemECs, in a dose-dependent manner (Fig. 7). At 50 ng/ml, R-PLNPs-V inhibited
the production of VEGF-A in HUVECs more effectively than rapamycin
(P<0.01) and R-PLNPs (P<0.05), and R-PLNPs-V at 10 ng/ml were
also more effective than rapamycin (P<0.01) and R-PLNPs
(P<0.05). In the case of bFGF in HUVECs, similar results were
obtained. R-PLNPs-V were more effective in inhibiting the
production of bFGF in HUVECs compared with rapamycin (P<0.001)
and R-PLNPs (P<0.01), and R-PLNPs-V was also more effective than
rapamycin (P<0.001) and R-PLNPs (P<0.01) at 10 ng/ml. In the
case of HemECs, similar results were obtained. Taken together,
these results demonstrated that R-PLNPs-V were more effective in
inhibiting VEGF-A and bFGF production in the endothelial cells,
compared with rapamycin and R-PLNPs.
Effect of nanoparticles in
anti-hemangioma activity in vivo
The therapeutic efficacy of the various treatments
was evaluated in hemangioma-bearing mice (Fig. 8). The blank nanoparticle, PLNPs-V,
did not exhibit any activity against hemangiomas, as reflected by
the progressive growth of tumors following treatment with PLNPs-V
(Fig. 8A). By contrast,
rapamycin, R-PLNPs and R-PLNPs-V inhibited the growth of
hemangiomas at different degrees. At the end time point, R-PLNPs-V
treatment resulted in a striking 86% decrease in the volume of
hemangiomas, whereas R-PLNPs and rapamycin alone had induced a 68
and 50% decrease in the volume of hemangiomas, respectively.
Compared with other groups, the R-PLNPs-V-treated group had a
significantly smaller hemangioma volume (P<0.001, compared with
PLNPs-V; P<0.01, compared with R-PLNPs; Fig. 8B). In addition, compared with
other groups, the R-PLNPs-V-treated group had a significantly lower
hemangioma weight (P<0.001, compared with PLNPs-V; P<0.01,
compared with R-PLNPs; Fig. 8C).
The overall body weight of the mice did not change significantly
with any of the treatments (Fig.
8D).
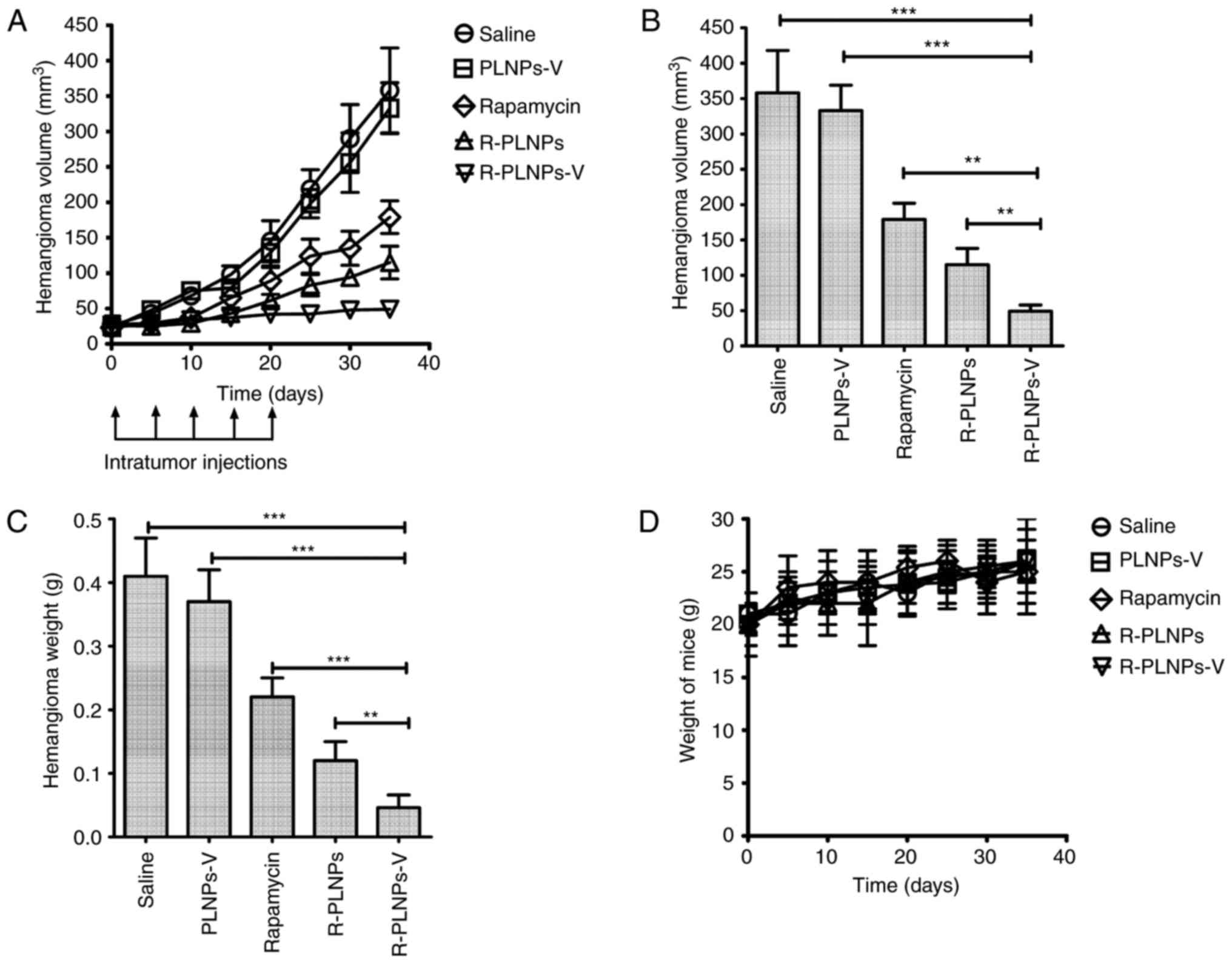 | Figure 8Anti-hemangioma activity of
nanoparticles in vivo. After hemangioma was ~25
mm3 in size (day 0), mice received single intratumoral
injections of saline, R-PLNPs (2 mg rapamycin/kg), R-PLNPs-V (2 mg
rapamycin/kg), rapamycin (2 mg rapamycin/kg) or PLNPs-V (40 mg/kg).
Additional intratumoral injections were performed on days 5, 10, 15
and 20 (indicated by black arrows in the schematic). (A) Growth
curve of hemangiomas during the course of treatments. (B) Tumor
volume of each experimental group analyzed at the end point. (C)
Weight of excised hemangiomas analyzed at the end point. (D)
Measurements of the total body weight of the mice during the
treatment. Data are expressed as mean ± standard deviation (n=8).
*P<0.05, **P<0.01 and
***P<0.001, with comparisons indicated by brackets.
PLNPs-V, lipid polymer nanoparticles coupled with anti-VEGR2
antibody; R-PLNPs, rapamycin-encapsulated lipid polymer
nanoparticles; R-PLNPs-V, R-PLNPs coupled with anti-VEGR2
antibody. |
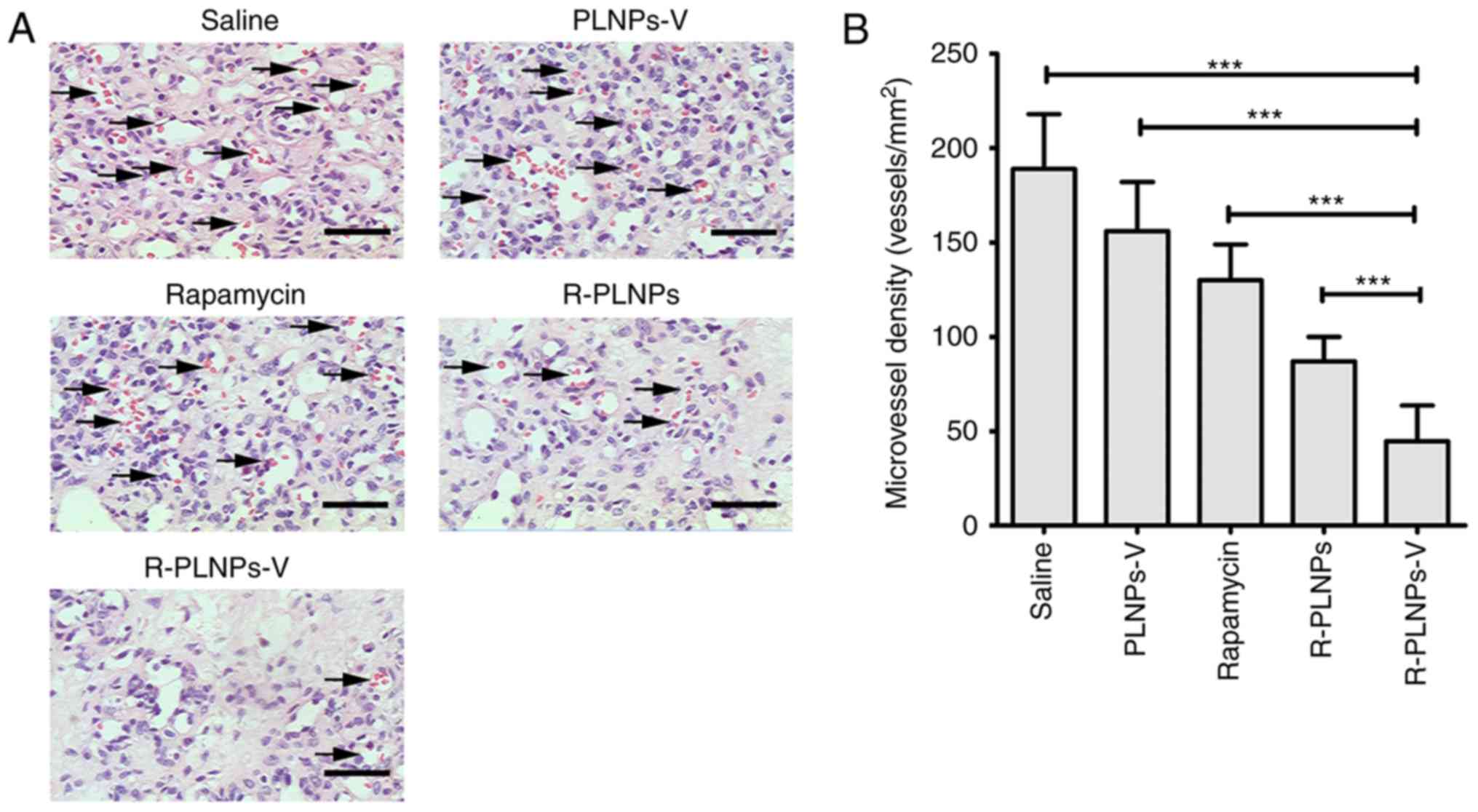
Furthermore, histology staining and microvessel
density (MVD) analysis were performed on the excised hemangiomas
(Fig. 9). Compared with other
groups, the R-PLNPs-V-treated group had the lowest MVD (P<0.001,
compared with saline, PLNPs-V, rapamycin or R-PLNPs; Fig. 9B). Thus, these results
demonstrated that R-PLNPs-V inhibited the microvessel density of
hemangiomas and displayed the best efficiency against hemangiomas
among the tested treatments.
Discussion
Hemangeol is the only FDA-approved drug for
infantile hemangiomas, but has several disadvantages and adverse
effects. Based on the previously developed R-PLNPs, anti-VEGFR2
antibody was coupled to the nanoparticles in the present study, in
order to enhance the targeting of R-PLNPs to infantile hemangiomas.
The results demonstrated that R-PLNPs-V displayed sustained
rapamycin release lastingly and superior efficiency in reducing
hemangioma activity in vitro and in vivo.
The superior safety profile of nanoparticles could
facilitate their clinic use due to their reduced damage to humans
(21). The components of the
R-PLNPs-V generated in the present study include rapamycin,
lecithin, PEGylated lipid, PLGA, and VEGFR2 antibodies (Fig. 1). Lecithin, PEGylated lipid, and
PLGA are biocompatible, and all of them are FDA-approved
pharmaceutical materials. The use of these FDA-approved
pharmaceutical materials may facilitate a potential transition of
the presented nanoparticles in the clinic. Rapamycin is an
FDA-approved drug for renal transplantation. Due to the numerous
antibodies approved by FDA to treat various diseases, the safety of
a large amount of monoclonal antibodies has been well-demonstrated
(22). Although the anti-VEGFR2
antibody has not been approved by FDA, its safety is expected to be
sufficient, although this will need to be demonstrated in future
studies. In the present study, the results from cyto-toxicity
assays demonstrated the good biocompatibility of the blank
nanoparticles coupled with anti-VEGFR2 antibody (PLNPs-V).
Furthermore, the present results from the mice study suggested that
the prepared R-PLNPs-V did not cause significant weight changes in
the mice. Therefore, R-PLNPs-V is anticipated to possess good
safety properties which could facilitate the clinical translation
of R-PLNPs-V.
A series of targeted nanoparticles have been
developed to target various cancers (14,15). The most prominent example is the
doxorubicin-loaded immunoliposomes that exhibited enhanced
cytotoxic effects towards human epidermal growth factor receptor 2
(HER2)-expressing breast cancers (23). However, there has been few
targeted nanoparticles that have been developed for infantile
hemangiomas. To the best of our knowledge, the only nanoparticle
developed for infantile hemangiomas to date is the
urea-immunoliposomes coupled with the anti-VEGFR antibody (19). The R-PLNPs-V developed in the
present study have three advantages over the urea-immunoliposomes.
First, rapamycin is a FDA-approved drug, whereas urea has not been
approved. Second, liposomes possess a structure of soft-membrane,
making its drug release rather quick. By contrast, the PLGA core of
R-PLNPs-V will be more rigid, making its drug release last for a
longer period. The drug release assay demonstrated that R-PLNPs-V
released rapamycin gradually in 6 days, thus having the possibility
to maintain a high level of the drug in infantile hemangiomas.
Third, the therapeutic efficacy of the urea-immunoliposomes was
only tested in hemangioma vascular endothelial cells in
vitro, and not in vivo (19). In the present study, R-PLNPs-V
have not only been demonstrated to inhibit HUVECs and HemECs in
vitro, but also significantly impede hemangioma growth in
vivo, using a patient-derived xenograft.
The present results have confirmed that the
anti-VEGFR2 antibody was pivotal for the special targeting of
R-PLNPs-V to HUVECs and HemECs. Confirmed by flow cytometry, the
anti-VEGFR2 antibody promoted the nanoparticle uptake in both the
endothelial cells. The quantitative HPLC assay demonstrated that
40–50% of rapamycin could be taken by R-PLNPs-V, in contrast to the
only ~5% of rapamycin uptake for the treatment with rapamycin
alone, suggesting that both the anti-VEGFR2 antibody conjugation
and the nanoparticle formulation significantly facilitated the
rapamycin uptake. The enhanced rapamycin uptake dramatically
increased the cytotoxic effects of R-PLNPs-V. R-PLNPs-V was 2.6-
and 4.8-fold more effective than R-PLNPs and rapamycin in HUVECs,
respectively, and 2- and 3.8-fold more effective than R-PLNPs and
rapamycin in HemECs, respectively. Notably, the in vivo
results demonstrated that the therapeutic efficacy of R-PLNPs-V was
superior to rapamycin alone or R-PLNPs, as reflected by the fact
that the R-PLNPs-V treatment resulted in the lowest hemangioma
volume, weight and MVD. These results suggested that the
therapeutic efficacy of rapamycin was promoted by R-PLNPs-V, and
the interaction of VEGFR/anti-VEGFR antibody could facilitate
effective rapamycin delivery to endothelial cells. Furthermore, the
local administration of rapamycin by R-PLNPs-V could target
endothelial cells directly and efficiently, resulting in minimal
unpredictable absorption and side effects of rapamycin. The lack of
anti-hemangioma activity for PLNPs-V, the blank nanoparticles with
anti-VEGFR2 antibody, may be attributed to the low amount of
anti-VEGFR2 antibody on PLNPs-V, which may not be sufficient to
induce obvious cytotoxic effects towards endothelial cells. In
summary, the superior activity of R-PLNPs-V is attributed to its
targeted delivery and sustained release of rapamycin.
The present data aid in clarifying the mechanism
underlying the anti-hemangioma activity of R-PLNPs-V. In brief,
following local administration, due the interaction of VEGFR with
the anti-VEGFR2 antibody, R-PLNPs-V bound to hemangioma endothelial
cells and inhibited the growth of hemangioma endothelial cells.
Additionally, R-PLNPs-V were also able to reduce VEGF-A and bFGF
production in hemangioma endothelial cells, while hemangioma growth
was inhibited in vivo to a great extent.
In conclusion, R-PLNPs-V released rapamycin
lastingly, and achieved superior therapeutic efficacy in inhibiting
hemangiomas in vitro and in vivo, compared with
rapamycin. Taken together, the present results suggest that
R-PLNPs-V may represent a promising and safe treatment for
infantile hemangiomas.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Kilcline C and Frieden IJ: Infantile
hemangiomas: How common are they? A systematic review of the
medical literature. Pediatr Dermatol. 25:168–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang LC, Haggstrom AN, Drolet BA, Baselga
E, Chamlin SL, Garzon MC, Horii KA, Lucky AW, Mancini AJ, Metry DW,
et al: Growth characteristics of infantile hemangiomas:
Implications for management. Pediatrics. 122:360–367. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen TS, Eichenfield LF and Friedlander
SF: Infantile hemangiomas: An update on pathogenesis and therapy.
Pediatrics. 131:99–108. 2013. View Article : Google Scholar
|
|
4
|
Sethuraman G, Yenamandra VK and Gupta V:
Management of infantile hemangiomas: Current trends. J Cutan
Aesthet Surg. 7:75–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Léaute-Labrèze C, Boccara O,
Degrugillier-Chopinet C, Mazereeuw-Hautier J, Prey S, Lebbé G,
Gautier S, Ortis V, Lafon M, Montagne A, et al: Safety of oral
rapamycin for the treatment of infantile hemangioma: A Systematic
Review. Pediatrics. 138:pii: e20160353. 2016. View Article : Google Scholar
|
|
6
|
Kaur A and Sharma S: Mammalian target of
rapamycin (mTOR) as a potential therapeutic target in various
diseases. Inflammopharmacology. 25:293–312. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Del Bufalo D, Ciuffreda L, Trisciuoglio D,
Desideri M, Cognetti F, Zupi G and Milella M: Antiangiogenic
potential of the Mammalian target of rapamycin inhibitor
temsirolimus. Cancer Res. 66:5549–5554. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Medici D and Olsen BR: Rapamycin inhibits
proliferation of hemangioma endothelial cells by reducing
HIF-1-dependent expression of VEGF. PLoS One. 7:e429132012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng N, Ding X and Jahan R: Low
concentration of rapamycin inhibits hemangioma endothelial cell
proliferation, migration, and vascular tumor formation in mice.
Curr Ther Res Clin Exp. 76:99–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johnson RW: Sirolimus (Rapamune) in renal
transplantation. Curr Opin Nephrol Hypertens. 11:603–607. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stallone G, Infante B, Grandaliano G and
Gesualdo L: Management of side effects of sirolimus therapy.
Transplantation. 87(8 Suppl): S23–S26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Almeida H, Amaral MH, Lobao P, Frigerio C
and Sousa Lobo JM: Nanoparticles in ocular drug delivery systems
for topical administration: Promises and challenges. Curr Pharm
Des. 21:5212–5224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li H, Teng Y, Sun J and Liu J: Inhibition
of hemangioma growth using lipid polymer nanoparticles for delivery
of rapamycin. Biomed Pharmacother. 95:875–884. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao J, Chen H, Song H, Su X, Niu F, Li W,
Li B, Dai J, Wang H and Guo Y: Antibody-targeted immunoliposomes
for cancer treatment. Mini Rev Med Chem. 13:2026–2035. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao J, Feng SS and Guo Y: Antibody
engineering promotes nanomedicine for cancer treatment.
Nanomedicine (Lond). 5:1141–1145. 2010. View Article : Google Scholar
|
|
16
|
Li W, Li J, Gao J, Li B, Xia Y, Meng Y, Yu
Y, Chen H, Dai J, Wang H and Guo Y: The fine-tuning of
thermosensitive and degradable polymer micelles for enhancing
intracellular uptake and drug release in tumors. Biomaterials.
32:3832–3844. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eskens FA and Verweij J: The clinical
toxicity profile of vascular endothelial growth factor (VEGF) and
vascular endothelial growth factor receptor (VEGFR) targeting
angiogenesis inhibitors; a review. Eur J Cancer. 42:3127–3139.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tugues S, Koch S, Gualandi L, Li X and
Claesson-Welsh L: Vascular endothelial growth factors and
receptors: Antiangiogenic therapy in the treatment of cancer. Mol
Aspects Med. 32:88–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Z, Li J, Xu X, Duan X and Cao G: Urea
immunoliposome inhibits human vascular endothelial cell
proliferation for hemangioma treatment. World J Surg Oncol.
11:3002013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Humar R, Kiefer FN, Berns H, Resink TJ and
Battegay EJ: Hypoxia enhances vascular cell proliferation and
angiogenesis in vitro via rapamycin (mTOR)-dependent signaling.
FASEB J. 16:771–780. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nyström AM and Fadeel B: Safety assessment
of nanomaterials: Implications for nanomedicine. J Control Release.
161:403–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nelson AL, Dhimolea E and Reichert JM:
Development trends for human monoclonal antibody therapeutics. Nat
Rev Drug Discov. 9:767–774. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park JW, Hong K, Kirpotin DB, Colbern G,
Shalaby R, Baselga J, Shao Y, Nielsen UB, Marks JD, Moore D, et al:
Anti-HER2 immunoliposomes: Enhanced efficacy attributable to
targeted delivery. Clin Cancer Res. 8:1172–1181. 2002.PubMed/NCBI
|