Introduction
Mesenchymal stem cells (MSCs) have exhibited
potential for the treatment of ischemic heart diseases. Previous
studies demonstrated that MSCs derived from the bone marrow can
differentiate into cardiac myocytes in vitro and in
vivo (1–3). Furthermore, it has been reported
that MSCs transplanted into the acute ischemic heart and chronic
congestive heart may modify cardiac function by promoting
angiogenesis and reducing myocardial fibrosis (4–6).
However, the therapeutic potential of MSCs is limited by their low
survival rate following transplantation into damaged myocardium
(7,8). A previous study revealed that <1%
of MSCs were detected 24 h following transplantation into a rat
heart with experimental myocardial infarction (MI) (9). Cell apoptosis, which is caused by
the harsh hypoxic microenvironment, contributes to the low survival
rate of transplanted MSCs (10,11). Therefore, the present study aimed
to protect MSCs against apoptosis, in order to improve the
therapeutic efficacy of MSCs transplantation.
Adrenomedullin (ADM) is a ubiquitous peptide
synthesized by numerous cell types, including neurons, macrophages,
monocytes, lymphocytes, and epithelial and endothelial cells
(12–14). Although ADM was initially
described as a potent vasodilator and hypotensive factor, numerous
studies have reported that it may induce various biological
activities in a paracrine or autocrine manner. It has been reported
that ADM is not only able to enhance cell proliferation and
angiogenesis (15–17), but can inhibit cell apoptosis
(18). Furthermore, it has been
demonstrated that ADM protects numerous cell types, including
cardiomyocytes (19), rat Leydig
cells (20), endothelial
progenitor cells (21) and
vascular endothelial cells (22),
against apoptosis via the protein kinase B (Akt)/glycogen synthase
kinase (GSK)3β signaling pathway. Akt is a powerful survival
signal, which suppresses apoptosis and increases cell survival.
Activation of Akt can trigger GSK3β phosphorylation (23), which subsequently results in an
antiapoptotic effect via inactivation of caspase-3 (24,25). Furthermore, Akt has been reported
to serve an important role in regulating B-cell lymphoma 2 (Bcl-2)
family members (26). The Bcl-2
family members are important regulators of mitochondria-mediated
apoptosis, and can be divided into anti-apoptotic proteins, such as
Bcl-2, and proapoptotic proteins, including Bcl-2-associated X
protein (Bax). The Bcl-2/Bax ratio is often used to determine the
extent of apoptosis (27). Since
the Akt signaling pathway has also been reported to serve an
important role in mediating survival signaling in MSCs (28), the present study infected MSCs
with ADM, and investigated whether ADM overexpression could protect
MSCs from hypoxia and serum deprivation (H/SD)-induced apoptosis
via the Akt/GSK3β and Bcl-2 signaling pathways.
Materials and methods
Culture and identification of MSCs
MSCs were isolated from the bone marrow of
Sprague-Dawley rats (age, 4 weeks; weight, 60–80 g) according to a
previously published method (29,30). Rats were obtained from the
Laboratory Animal Science Department, The Second Affiliated
Hospital of Harbin Medical University (Harbin, China). The rats
were housed at a temperature of 22°C with a relative humidity of
40–70% and a 12-h light/dark cycle with food/water ad
libitum. The present study was approved by the Local Ethics
Committee for the Care and Use of Laboratory Animals of Harbin
Medical University. Briefly, the femurs and tibias were removed
from the rats, and the bone marrow was flushed out using 10 ml
Dulbecco's modified Eagle's medium/F12 (DMEM/F12; HyClone; GE
Healthcare, Logan, UT, USA) supplemented with 1%
penicillin/streptomycin (Beyotime Institute of Biotechnology,
Nantong, China). Following centrifugation at 1,000 × g at room
temperature for 5 min, the resulting cell pellets were resuspended
in 6 ml DMEM/F12 supplemented with 10% fetal bovine serum (FBS;
ScienCell Research Laboratories, Inc., San Diego, CA, USA) and 1%
penicillin/streptomycin, and were plated in a 25 cm2
plastic flask at 37°C in a humidified atmosphere containing 5%
CO2 and 95% air. After a 48 h culture, the medium was
replaced and non-adherent hematopoietic cells were removed. The
remaining spindle-shaped, adherent cells were MSCs. These adherent
MSCs were subsequently cultured; the culture medium was regularly
changed every 3–4 days. A total of 7–10 days after seeding, the
cells became 80–90% confluent. The adherent cells were released
from the flask using 0.25% trypsin (Beyotime Institute of
Biotechnology) and were expanded at a 1:2 or 1:3 dilution. A total
of 9 Sprague-Dawley rats were used in the present study. For each
primary cell culture, the bone marrow of 3 rats was mixed together.
Passage 2 MSCs were cryopreserved and passage 3–4 MSCs were used in
the present study. Primary cell culture was conducted three times,
and cells in each primary culture were repeatedly used for
subsequent experiments.
To investigate the expression of typical
MSC-associated cell surface antigens, MSCs were analyzed by
fluorescence-activated cell sorting (FACS). Briefly, MSCs were
trypsinized and 1×106 MSCs were incubated with 10
µg antibodies in 1 ml PBS at room temperature in the dark
for 15 min. The antibodies used in the present study were as
follows: Hematopoietic progenitor marker phycoerythrin
(PE)-conjugated mouse anti-rat cluster of differentiation (CD)34
(1:200; sc-74499; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
the pan-leukocyte marker fluorescein isothiocyanate
(FITC)-conjugated mouse anti-rat CD45 (1:100; MA5-17385; Caltag
Laboratories, Inc., Burlingame, CA, USA), and the MSC marker
PE-conjugated anti-rat CD29 (1:100; 102207; BioLegend, Inc., San
Diego, CA, USA). To investigate differentiative ability, according
to our previous study, MSCs were incubated with osteogenic
induction medium (RAWMD-90021; Cyagen Biosciences, Santa Clara, CA,
USA) or adipogenic induction medium (RAWMD-90021; Cyagen
Biosciences) for 3 or 4 weeks. Subsequently, the cells were stained
with von Kossa or Oil Red O (31). The staining results were observed
by optical microscope.
Adenoviral transduction of MSCs
The MSCs were transduced with a recombinant
adenoviral vector encoding the gene green fluorescent protein
(Ad-CMV-GFP). For infection, MSCs were seeded in 6-well plates at
1×105 cells/well and were allowed to grow overnight to
50–60% confluence. Subsequently, the MSCs were infected with
enhanced (E) GFP-recombinant adenovirus capsids (EGFP-Adv) or
EGFP-recombinant adenovirus capsids containing ADM cDNA (EGFP-ADM)
(BC061775; Shanghai GeneChem Co. Ltd., Shanghai, China) at a
multiplicity of infection (MOI) of 80 at 37°C. After 12 h, the
medium was replaced with normal medium containing 10% FBS and
antibiotics. To confirm infection was successful, the mRNA
expression levels of ADM were detected by reverse
transcription-polymerase chain reaction (RT-PCR). Briefly, 24, 48
and 72 h post-infection, total RNA was isolated from the MSCs using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The β-actin gene was used as a normalizing
control. The designed paired primers were as follows: ADM, forward
5′-GGACTTTGCGGGTTTTGC-3′, reverse 5′-TCTGGCGGTAGCGTTTGA-3′; and
β-actin, forward 5′-ATATCGCTGCGCTCGTCGTC-3′ and reverse
5′-GCATCGGAACCGCTCATTGC-3′. PCR conditions were as follows: 25
cycles of denaturation at 94°C for 30 sec, annealing at 55°C for 30
sec and extension at 72°C for 1 min (initial denaturation at 94°C
for 5 min; final extension is 72°C for 5 min). The PCR products
were subjected to 1.5% agarose gel electrophoresis, and were
scanned and semi-quantified using ImageQuant software (TL 7.0; GE
Healthcare, Chicago, IL, USA). Based on these results, 48 h
post-infection, the MSCs were used for subsequent experiments.
Furthermore, 48 h post-infection, the infection rate was also
assessed by fluorescence microscopy (DMI4000B; Leica Microsystems
GmbH, Wetzlar, Germany) and FACS analysis with FACSDiva software
(version 4.1; BD Biosciences, Franklin Lakes, NJ, USA). For FACS
analysis, the MSCs at 48 h post-infection were digested and
centrifuged at 1,000 × g for 5 min. After removing the supernatant,
500 µl PBS buffer was added and the MSCs were analyzed by
flow cytometry.
H/SD model and cell groups
MSCs were randomly separated into the following six
groups: Control, EGFP-Adv, EGFP-ADM, H/SD, EGFP-Adv + H/SD, and
EGFP-ADM + H/SD.
To mimic the in vivo ischemic
microenvironment, cells were cultured under H/SD conditions,
according to a previous study (10). Briefly, 48 h post-infection, the
cells in the EGFP-Adv + H/SD, EGFP-ADM + H/SD and H/SD groups were
washed with PBS, cultured in serum-free medium and incubated in a
glove box (855-AC; Plas-Labs, Inc., Lansing, MI, USA) to scavenge
free oxygen at 37°C for an additional 12 h. The cells in the
control, EGFP-Adv and EGFP-ADM groups were cultured in complete
medium in a general cell incubator for 12 h. Subsequently, the
following experiments were conducted.
Cell viability assay
The viability of MSCs was assessed using the Cell
Counting kit-8 assay kit (CCK-8; Dojindo Molecular Technologies,
Inc., Kumamoto, Japan) according to the manufacturer's protocol.
Cells were seeded into a 96-well plate (5,000 cells/well) after
being subjected to the aforementioned experimental treatments, and
cell viability was measured following the addition of 10 µl
CCK-8 into the culture medium for 1.5 h at 37°C. The absorbance of
each well was quantified at 450 nm. The mean optical density (OD)
of 5 wells in each group was used to calculate the percentage of
cell viability. The experiments were conducted in triplicate.
Terminal deoxynucleotidyl
transferase-mediated-dUTP nick-end labeling (TUNEL) staining
Apoptosis of MSCs was determined using a TUNEL assay
(Roche Applied Science, Penzberg, Germany) according to the
manufacturer's protocol with some modifications. Briefly, the MSCs
were grown in a 24-well plate. Following the aforementioned
experimental treatments, the cells were fixed in 4%
paraformaldehyde for 1 h at room temperature. Subsequently, the
cells (1×105 cells/well) were incubated with 3%
H2O2 in methanol for 10 min at room
temperature to block endogenous peroxidase activity, and were then
incubated with 0.1% Triton X-100 in 0.1% sodium citrate for 2 min
at 4°C. After washing in PBS, the cells were finally incubated with
the TUNEL reaction mixture containing 5 µl enzyme solution
and 45 µl fluorochrome-labeled solution at 37°C for 60 min
in the dark. For counterstaining, the cells were incubated with
DAPI (Beyotime Institute of Biotechnology) for 15 min. The cells
were observed using a fluorescence microscope, in which 10 fields
were randomly selected. The TUNEL+ and DAPI+
nuclei in the cells were counted manually. The percentage of
positive cells was calculated as the apoptotic index (AI) using the
following equation: AI = (number of positive cells/total number of
cells) × 100%, as previously described (32,33). The experiments were conducted in
triplicate.
Flow cytometric analysis of cell
apoptosis
Apoptosis of MSCs was also determined by detecting
phosphatidylserine exposure on cell plasma membranes using the
fluorescent dye Annexin V-PE/7-aminoactinomycin D (7-AAD) apoptosis
detection kit (BD Biosciences, San Diego, CA, USA) according to the
manufacturer's protocol. This assay is used to discriminate between
intact (Annexin V−/7-AAD−), early apoptotic
(Annexin V+/7-AAD−), late apoptotic (Annexin
V+/7-AAD+) and necrotic cells (Annexin
V−/7-AAD+). Briefly, at the end of the
treatment period, the cells were harvested with 0.25% trypsin and
washed twice with cold PBS. After being resuspended in 100
µl 1X binding buffer, 5 µl Annexin V-PE and 5
µl 7-AAD were added, and the cells (4–5×105
cells) were incubated for 15 min at room temperature in the dark.
Finally, 400 µl 1X binding buffer was added to the cells and
flow cytometric analysis was conducted. The experiments were
conducted in triplicate.
Protein extraction and western blot
analysis
After washing with PBS twice, the treated cells were
harvested and lysed in ice-cold radioimmunoprecipitation assay
lysis buffer (Beyotime Institute of Biotechnology) mixed with
protease and phosphatase inhibitors (Roche Applied Science). Total
protein in the supernatant was quantified using a bicinchoninic
acid protein assay kit (Beyotime Institute of Biotechnology). Equal
amounts of protein (50 µg/lane) were separated by 12%
SDS-PAGE and were transferred to polyvinylidene fluoride membranes.
The membranes were then incubated at room temperature in a blocking
solution composed of 5% skim milk powder dissolved in 1X TBS for 1
h at room temperature. Subsequently, the membranes were incubated
with the following primary antibodies at 4°C overnight: Rabbit
polyclonal antibodies against Bax (WL01637), Bcl-2 (WL01556), Akt
(WL0003b) and GSK3β (WL01456) (all from Wanleibio Co., Ltd.,
Shanghai, China), rabbit polyclonal antibody against phosphorylated
(p)-Akt (Ser-473) (sc-7985-R; Santa Cruz Biotechnology, Inc.),
rabbit monoclonal antibody against p-GSK3β (Ser-9, 9322s), rabbit
polyclonal antibody against caspase-3 (9662s) (both from Cell
Signaling Technology, Inc., Danvers, MA, USA) and mouse polyclonal
antibody against GAPDH (KC-5G4; Kangchen Bio-Tech Co., Ltd.,
Shanghai, China). The membrane was then washed three times with
TBS-0.1% Tween (TBST) for 5 min followed by incubation with
horseradish peroxidase-conjugated anti-mouse (ZB-2305; OriGene
Technologies, Inc., Beijing, China) or anti-rabbit secondary
antibodies (WLA023a; Wanleibio Co., Ltd.) for 60 min at 37°C. After
further washes with TBST, immunoreactive bands were visualized
using an enhanced chemiluminescence detection system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Image Pro Plus 5 software
(Bio-Rad Laboratories, Inc.) was used to semi-quantify the protein
levels in each lane.
Statistical analysis
Experimental values are presented as the means ±
standard deviation, and the difference between groups was analyzed
by one-way analysis of variance with Tukey's and Newman Keuls post
hoc tests. The experiments were conducted in triplicate.
Statistical analysis was performed using SPSS 19.0 software (IBM
Corp., Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Characterization and differentiation of
cultured MSCs
Surface molecular markers of MSCs were examined
after 3–4 passages using flow cytometry. As shown in Fig. 1A, 96.84% of isolated cells
expressed CD29. Conversely, few cells expressed the hematopoietic
markers CD34 (1.33%) and CD45 (18.08%) (Fig. 1A and B). In addition, as shown in
Fig. 1C and D, the cultured MSCs
possessed the ability to differentiate along the osteogenic and
adipogenic lineages, as determined by von Kossa and Oil Red O
staining, respectively.
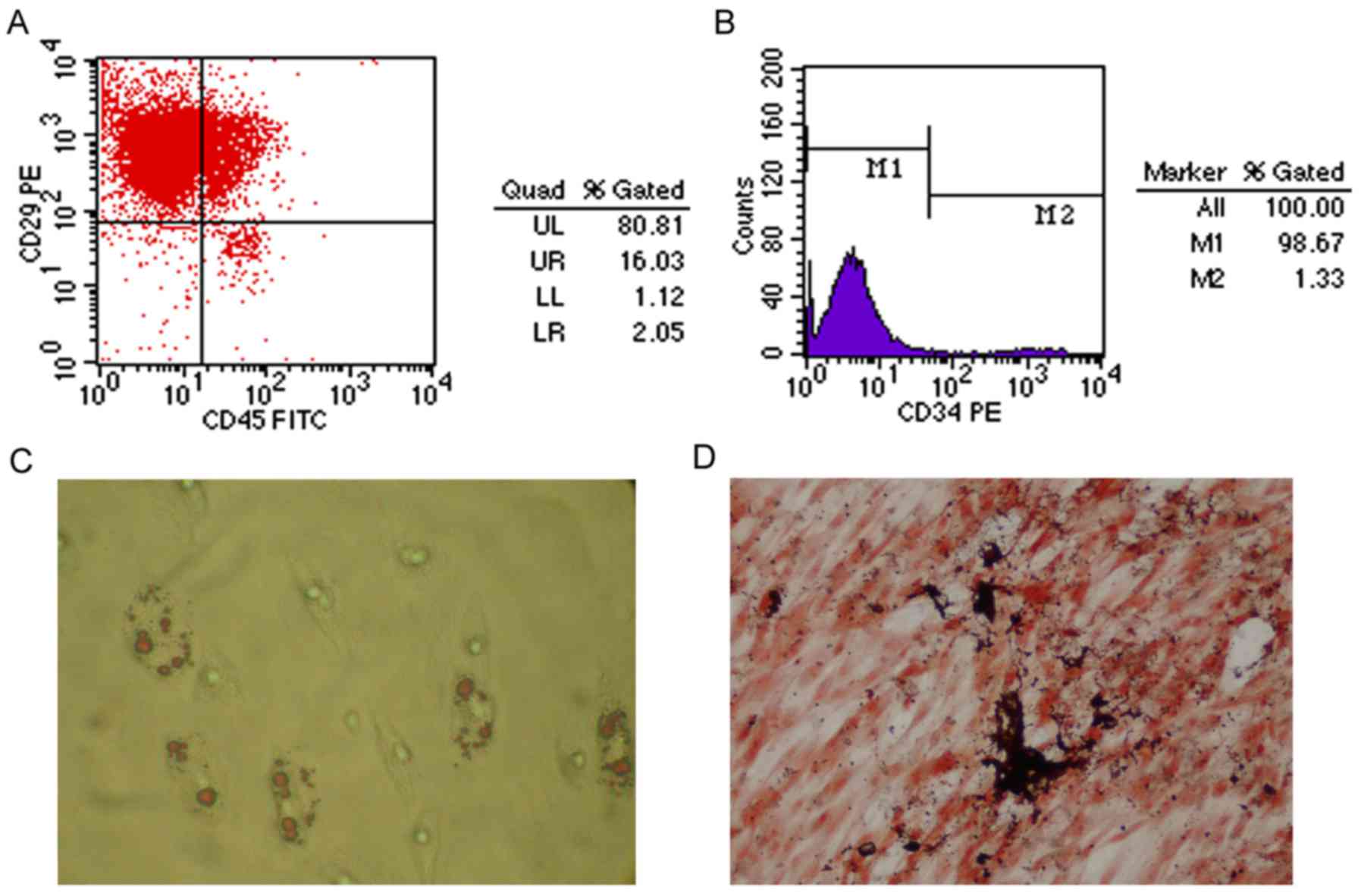 | Figure 1Characterization and differentiation
of cultured MSCs. (A and B) Flow cytometric analysis of adherent,
spindle-shaped MSCs (passage 3 or 4). Most cultured MSCs expressed
CD29. However, the majority of MSCs were CD34- and CD45-negative.
(C) Adiopogenic differentiation, as determined by Oil Red O
staining. MSCs developed some lipid droplets (magnification, ×400).
(D) Osteogenic differentiation, as determined using von Kossa
staining (magnification, ×400). Mineralized matrix was formed in
MSCs. CD, cluster of differentiation; FITC, fluorescein
isothiocyanate; MSCs, mesenchymal stem cells; PE,
phycoerythrin. |
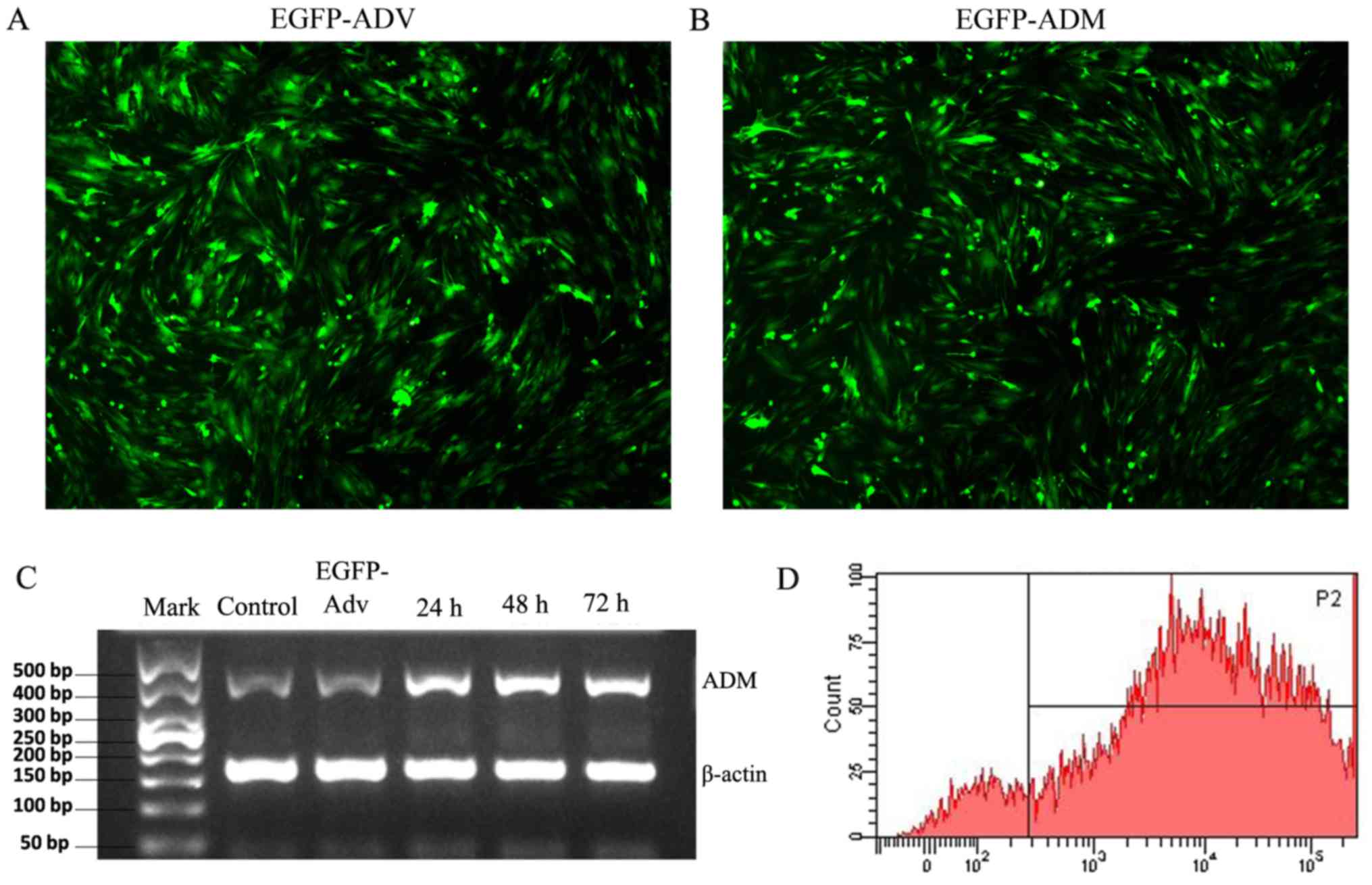
EGFP-ADM infection increases the
expression of ADM in MSCs
In the present study, infection efficiency was
determined by detecting the positive rates of GFP fluorescence.
Following infection with EGFP-Adv or EGFP-ADM for 48 h, ~100% of
MSCs exhibited green fluorescence, which suggested that MSCs were
successfully infected with the adenoviruses (Fig. 2A and B). Furthermore, to evaluate
the mRNA expression levels of ADM in the genetically modified MSCs
in vitro, RT-PCR analysis was performed on cell samples at
24, 48 and 72 h post-infection. The results demonstrated that the
expression levels of ADM were markedly increased in the EGFP-ADM
group as early as 24 h; the expression levels peaked at 48 h and
were maintained until 72 h (Fig.
2C). Therefore, 48 h post-infection, the MSCs were used for
subsequent experiments. Successful transduction at 48 h was also
assessed by FACS analysis. As shown in Fig. 2D, >91% of MSCs were
successfully transduced.
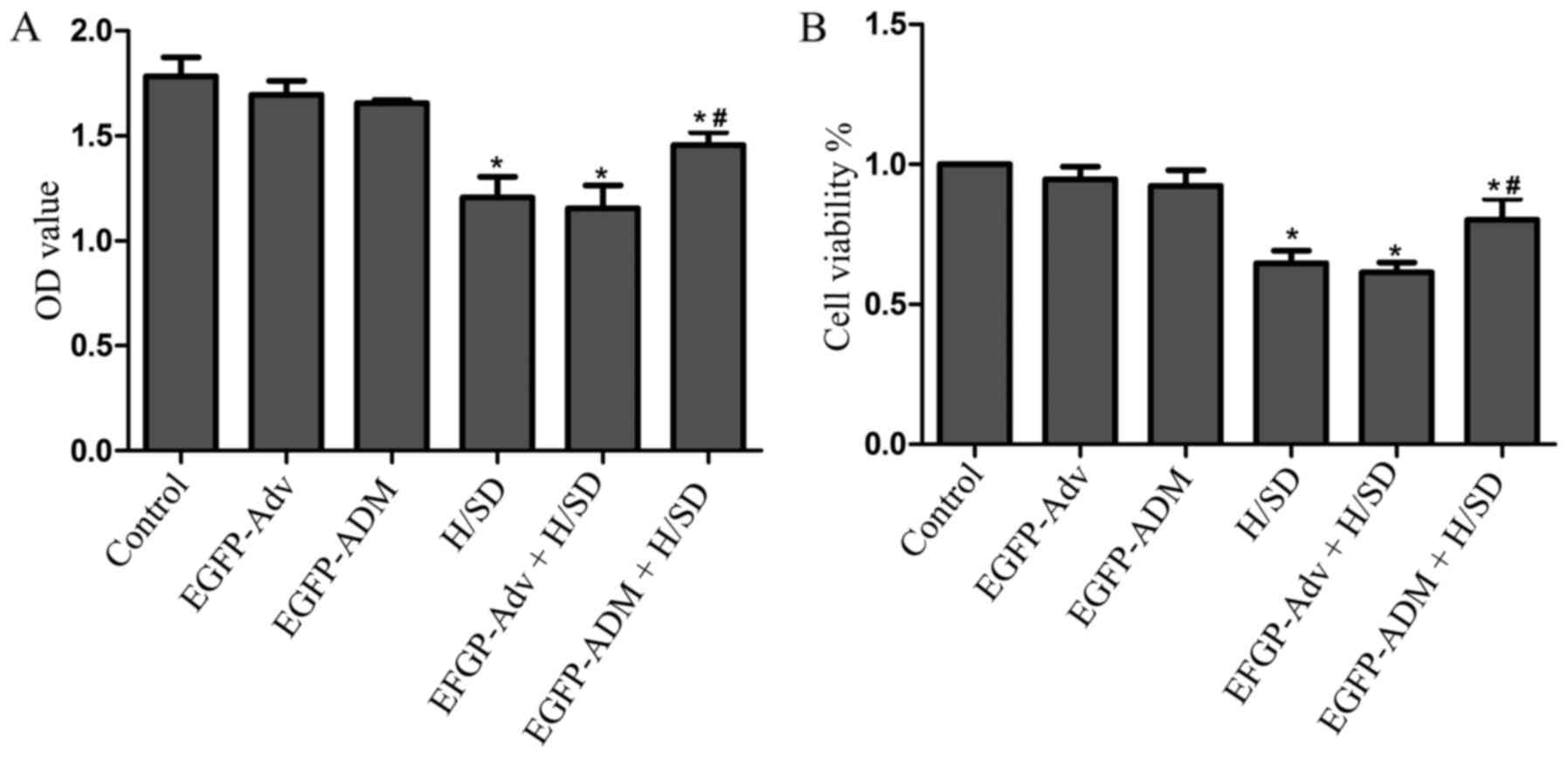
ADM increases MSC viability and
proliferation
To investigate whether ADM protects MSCs against
H/SD-induced injury, cell proliferation rate and viability rate
were assessed using the CCK-8 assay. The cell viability rate was
calculated using the following equation. Cell viability (%)=[OD
(treated group) − A (blank)] / [ OD (control) − OD (blank)] ×100
The results detected no significant difference among the control
(OD, 1.784±0.089; viability, 1±0), EGFP-Adv (OD, 1.696±0.066;
viability, 0.947±0.036) and the EGFP-ADM-treated groups (OD,
1.656±0.015; viability, 0.923±0.046), thus indicating that
adenovirus infection did not damage MSCs (Fig. 3). However, the proliferation and
viability of MSCs was significantly decreased in the H/SD group
(OD, 1.206±0.101; viability, 0.647±0.037) compared with in the
control group (P<0.05). Conversely, the H/SD-induced impairments
were markedly attenuated in the EGFP-ADM + H/SD group (OD,
1.457±0.062; viability, 0.802±0.061) compared with in the H/SD
group (P<0.05), but not in the EGFP-Adv + H/SD group (OD,
1.154±0.109; viability, 0.615±0.028; P>0.05). These results
suggested that ADM may protect MSCs against H/SD-induced injury
(Fig. 3).
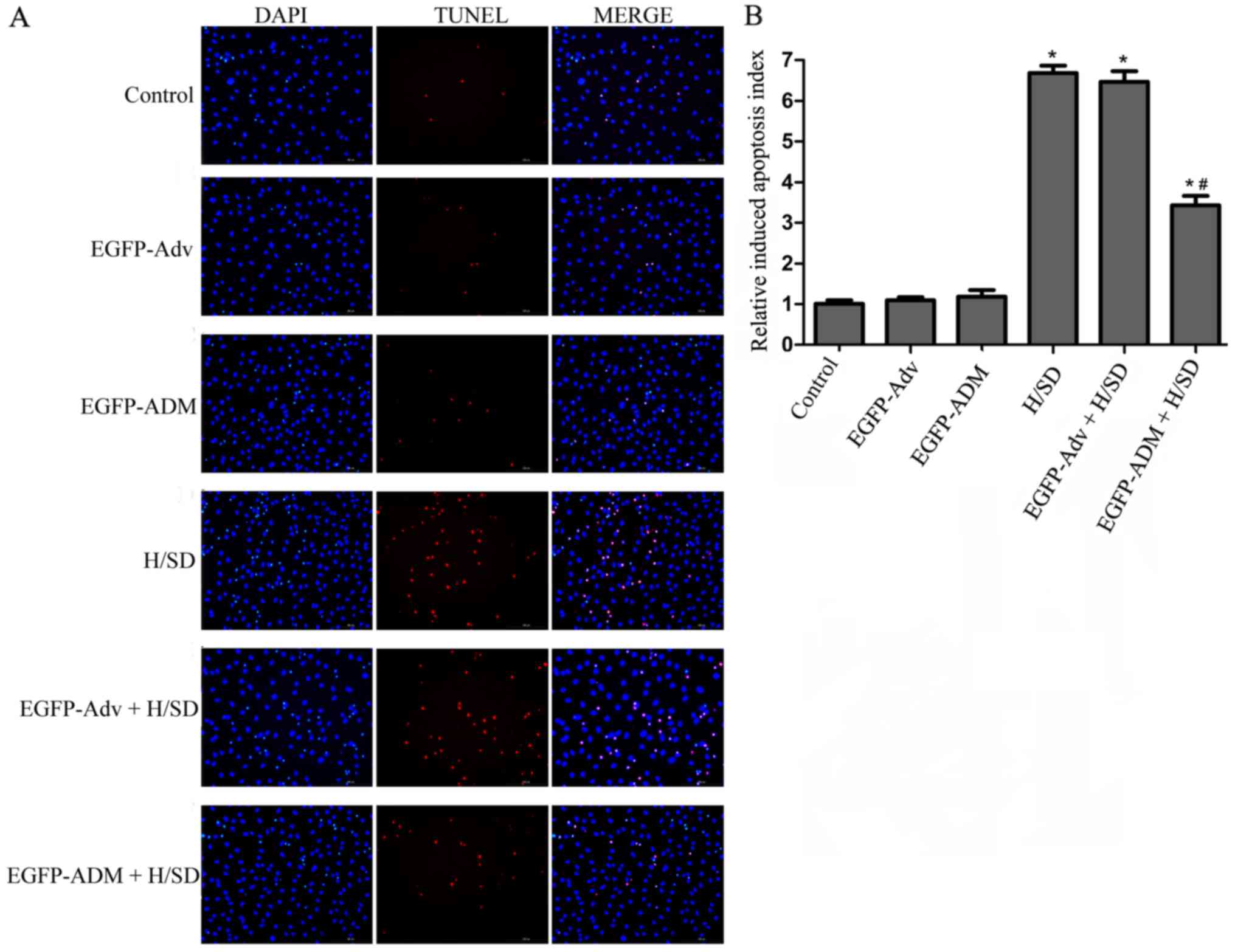
ADM protects MSCs from H/SD-induced
apoptosis
DAPI and TUNEL staining were used to evaluate the
protective effects of ADM on MSCs against H/SD-induced apoptosis.
Briefly, modified or not modified MSCs were treated under H/SD
conditions or normal conditions for 12 h; almost no TUNEL-positive
cells were observed in the control (1.005±0.087), EGFP-ADM
(1.179±0.165) and EGFP-Adv groups (1.091±0.079); however, cell
shrinkage and nuclear condensation were markedly increased in the
H/SD (6.683±0.178) and EGFP-Adv + H/SD groups (6.465±0.264)
compared with in the control group (P<0.05), and no significant
difference was detected between these two groups (P>0.05;
Fig. 4). Notably, ADM
transduction clearly exerted a protective effect on MSCs; the
EGFP-ADM + H/SD group (3.428±0.229) possessed a significantly lower
proportion of apoptotic cells compared with in the H/SD and
EGFP-Adv + H/SD groups (P<0.05; Fig. 4).
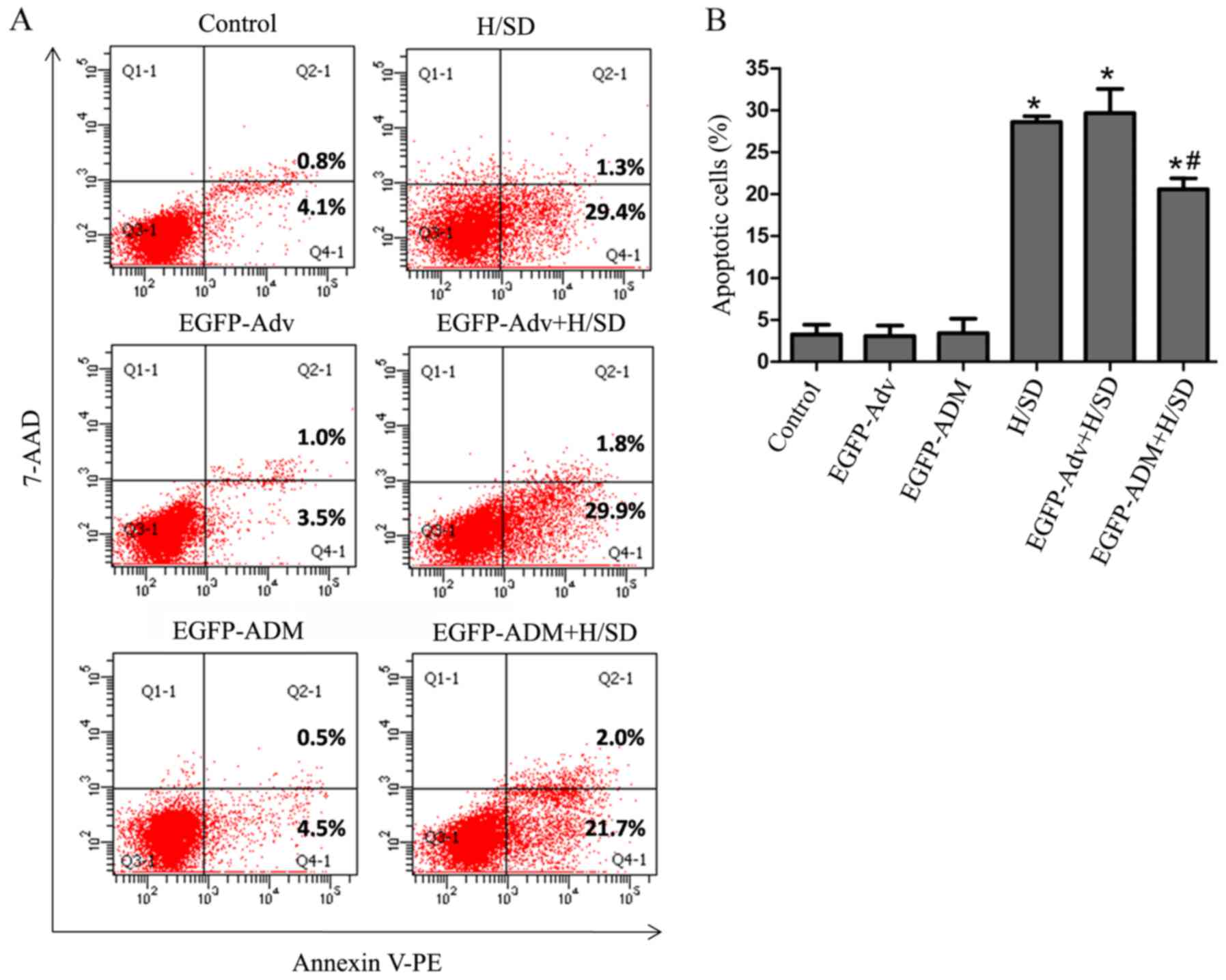
In further studies, cell apoptosis was determined by
flow cytometry. As shown in Fig.
5, the early apoptotic rate [Annexin
V+/AAD−] was markedly increased in the H/SD
(28.6±0.693) and EGFP-Adv + H/SD groups (29.667±1.857) compared
with in the control (3.267±1.150; P<0.05), EGFP-ADM
(3.433±1.709; P<0.05) and EGFP-Adv groups (3.067±1.266;
P<0.05). In addition, there was no significant difference in the
number of early apoptotic cells among the control, EGFP-ADM and
EGFP-Adv groups (P>0.05). Notably, the EGFP-ADM + H/SD group
(20.567±1.332) had a significantly lower proportion of cells in the
early apoptotic phase compared with in the H/SD (P<0.05) and
EGFP-Adv + H/SD groups (P<0.05); however, there were no
differences in the number of cells in the late apoptotic phase
(Annexin V+/AAD+) or necrosis (Annexin
V−/AAD+) among the groups (data not shown).
These findings indicated that overexpression of ADM in MSCs may
protect against apoptosis under H/SD conditions.
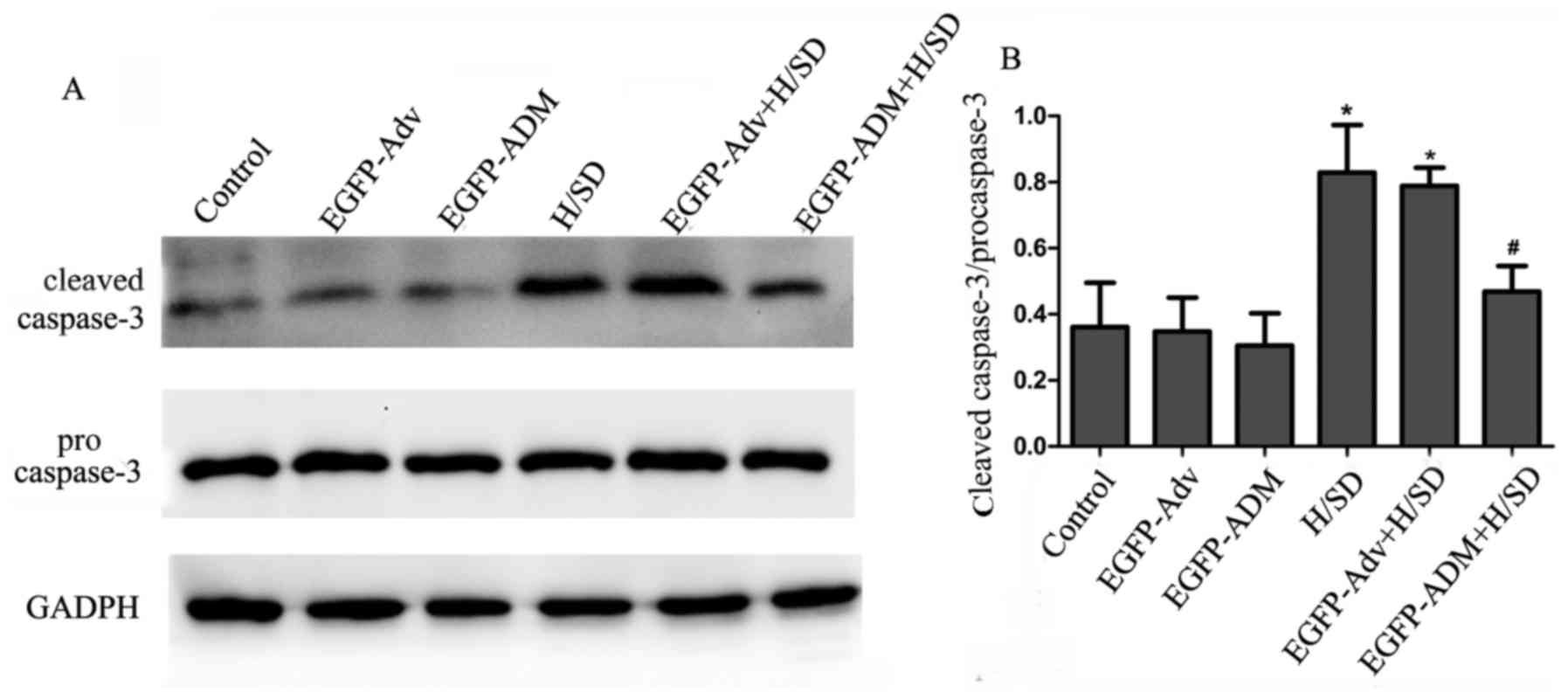
Caspase-3 is a key effector protease associated with
the execution of apoptosis. Under normal conditions, caspase-3
exists as inactivated procaspase-3. During apoptosis, procaspase-3
is activated and converted to cleaved caspase-3; therefore, the
present study investigated the effects of ADM transduction on the
expression of procaspase-3 and cleaved caspase-3. As shown in
Fig. 6, the results demonstrated
that the expression levels of cleaved caspase-3 were low in the
control, EGFP-ADM and EGFP-Adv groups, according to the ratio of
cleaved caspase-3/procas-pase-3 (control, 0.361±0.134; EGFP-ADM
group, 0.305±0.098; EGFP-Adv group, 0.348±0.103). Conversely, the
ratio of cleaved caspase-3/procaspase-3 was markedly increased in
the H/SD (0.829±0.144) and EGFP-Adv + H/SD groups (0.789±0.055)
compared with in the control group (P<0.05). Furthermore, the
present results demonstrated that this increase was attenuated in
the EGFP-ADM + H/SD group (0.469±0.077) compared with in the vs.
H/SD group (P<0.05). There was no significant difference between
the EGFP-ADM + H/SD and control groups (P>0.05).
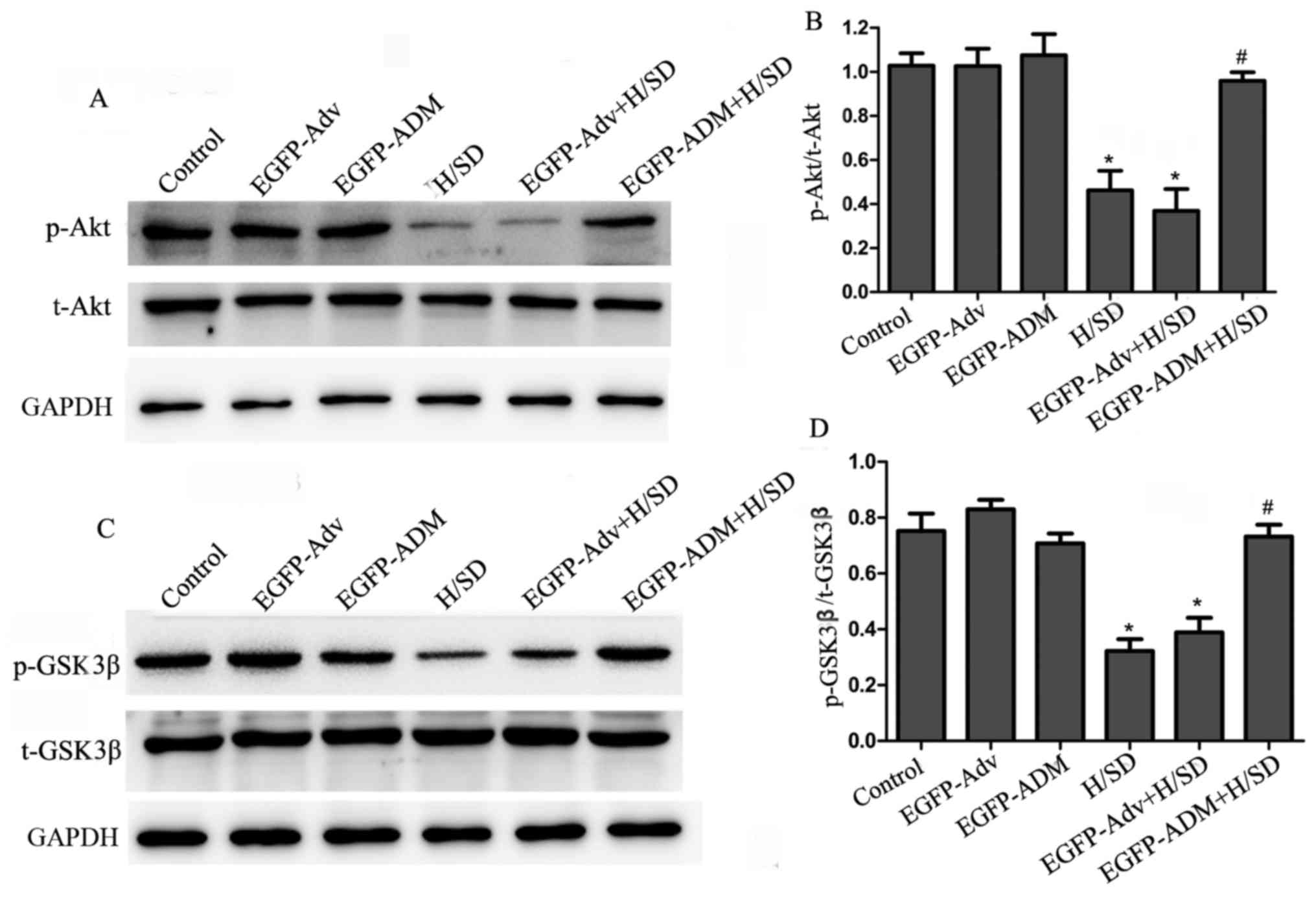
ADM prevents MSCs from H/SD-induced
apoptosis via the Akt/GSK3β pathway
The present study investigated the mechanisms
underlying the antiapoptotic effects of ADM. As aforementioned, the
Akt/GSK3β pathway has been reported to be important in promoting
survival in various cell systems. Therefore, the present study
investigated whether this pathway may mediate the antiapoptotic
effects of ADM in MSCs by western blot analysis. As shown in
Fig. 7A and B, compared with in
the control (1.027±0.056), EGFP-Adv (1.025±0.079) and EGFP-ADM
(1.074±0.097) groups, the ratio of p-Akt/Akt was significantly
decreased in the H/SD (0.462±0.089; P<0.05) and EGFP-Adv + H/SD
groups (0.369±0.098; P<0.05). However, the ratio of p-Akt/Akt
was markedly increased in the EGFP-ADM + H/SD group (0.958±0.039)
compared with in the H/SD (P<0.05) and EGFP-Adv + H/SD groups
(P<0.05). In addition, no obvious difference was detected among
the control, EGFP-Adv and EGFP-ADM groups (P>0.05). GSK3β is an
important downstream target of the Akt signaling pathway.
Phosphorylation of GSK3β at the inactivating residue serine-9 by
Akt results in GSK3β inactivation. Therefore, the present study
investigated the effects of ADM on p-GSK3β expression. As shown in
Fig. 7A and B, H/SD treatment
(H/SD, 0.322±0.043; EGFP-Adv + H/SD, 0.389±0.053) decreased p-GSK3β
expression compared with in the control (0.752±0.062; P<0.05),
EGFP-Adv (0.829±0.034; P<0.05) and EGFP-ADM groups (0.708±0.035;
P<0.05), whereas the expression levels of inactivated p-GSK3β
were significantly enhanced in the EGFP-ADM + H/SD group
(0.732±0.042) compared with in the H/SD group (P<0.05). There
was no significant difference between the EGFP-ADM + H/SD and
control groups (P>0.05).
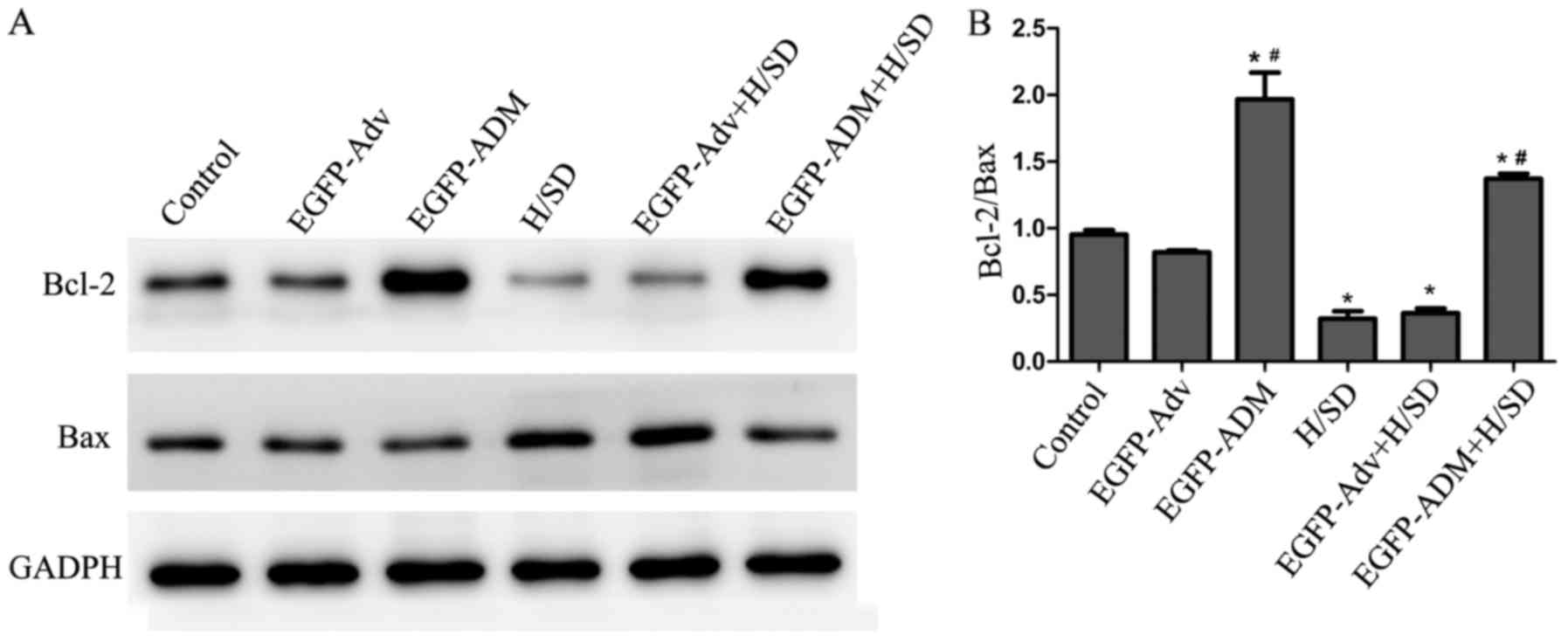
Antiapoptotic effects of ADM are involved
in inhibition of the Bcl-2 pathway
Bcl-2 family members are important regulators of
mitochondria-mediated apoptosis, and are classified as
antiapoptotic proteins, such as Bcl-2, and proapoptotic proteins,
such as Bax. The Bcl-2/Bax ratio is often used to determine the
extent of apoptosis. The present results indicated that exposure of
MSCs to H/SD conditions induced a significant decrease in the
Bcl-2/Bax ratio (0.322±0.055) compared with in the control
(0.954±0.033; P<0.05) and EGFP-Adv + H/SD groups (0.364±0.035;
P<0.05). Conversely, the Bcl-2/Bax ratio was significantly
increased in the EGFP-ADM (1.967±0.201) and EGFP-ADM + H/SD groups
(1.371±0.039) compared with in the control (P<0.05) and H/SD
(P<0.05) groups (Fig. 8).
Discussion
The present study demonstrated that ADM
overexpression exerted antiapoptotic effects on MSCs under H/SD
conditions, and indicated that the protective effects were mediated
via the Akt/GSK3β and Bcl-2 signaling pathways.
Due to the self-renewal ability, multi-directional
differentiation potential and low immunogenicity of MSCs, and the
ease with which they can be transfected/transduced in order to
express heterologous genes, MSCs are considered an ideal choice to
treat MI (29,34). However, previous studies reported
that only 1–5% transplanted cells survive 48 h post-transplantation
(7,8). In addition, three mechanisms have
been reported to mediate cell death following transplantation,
including nutrient and oxygen deprivation, inflammation, and a lack
of matrix support and adhesion to extracellular matrix (35–38). The present study focused on one of
these mechanisms, with the aim of increasing cell survival under
conditions of nutrient and oxygen deprivation. Therefore, in the
present study, a model of H/SD was used to induce apoptosis of MSCs
in vitro. Similar to the findings of previous studies
(10,28–30), the present results demonstrated
that the apoptosis of MSCs was significantly increased under H/SD
conditions, thus indicating that the H/SD model may successfully
mimic the in vivo microenvironment of nutrient and oxygen
deprivation in MI.
There are various strategies that may be used to
overcome the low survival rates of transplanted cells. These
approaches include pretreatment with growth factors or cytokines,
preconditioning with hypoxia, and genetic modification to
overexpress antideath or adhesion signals (35,36). The majority of preclinical and
clinical gene therapy applications have used virus-based transfer
of genetic material, due to high transduction efficiency, cell
tropism and levels of transgene expression; however, detrimental
immune reactions against the gene therapy vehicle, and transduced
cells or transgene products, has raised important concerns
(39). Therefore, it is desirable
to develop an efficient and safe carrier for gene transfection. Jo
et al reported that a non-viral carrier of cationized
polysaccharide may be used for genetic engineering of MSCs, and
indicated that it is a promising strategy to improve the
therapeutic effects of MSCs (40). In addition, compared with direct
adenovirus-injection as unbound adenovirus, recombinant adenovirus
modification, which has been reported to have no major influence on
the immunological properties of MSCs in vitro and in
vivo, has been considered a suitable gene vector for
therapeutic applications of MSCs and has been extensively used for
the genetic modification of MSCs (41). Therefore, recombinant adenovirus
modification was used in the present study. As expected, the
present results demonstrated that the adenovirus itself did not
harm MSCs with an MOI of 80, since there was no difference in MSC
proliferation and viability among the control, EGFP-Adv and
EGFP-ADM groups. Therefore, it was concluded that EGFP-ADM
transduction is a safe and effective method to protect MSCs against
H/SD-induced apoptosis. In the future, we aim to investigate more
carriers for genetic engineering of MSCs.
ADM has been confirmed as a hypoxia-induced peptide,
which is found in various human cancers, as well as in cell lines,
where it serves an antiapoptotic role (18,42). Therefore, overexpression of ADM in
MSCs was generated, in order to study its effect on the apoptosis
of MSCs in the present study. The results demonstrated that gene
modification with ADM could protect MSCs from H/SD-induced
apoptosis, since apoptosis of MSCs was significantly decreased in
the EGFP-ADM + H/SD group compared with in the H/SD and EGFP-Adv +
H/SD groups.
The underlying mechanisms were subsequently
investigated. Akt is a serine-threonine kinase, which has been
reported to act as a powerful survival signal that exerts
antiapoptotic effects and promotes cell survival. Akt can
phosphorylate various transcription factors and other regulatory
proteins. GSK3β is an important downstream signaling protein kinase
of Akt. Phosphorylation of GSK3β at serine 9 leads to inhibition of
its activity and reduces apoptosis (23–25,43,44). Furthermore, Yin et al
demonstrated that ADM could protect cardiomyocytes against
ischemia/reperfusion injury-induced apoptosis via the
Akt-GSK-caspase signaling pathway in a rat model (19). Based on these findings, it may be
hypothesized that ADM exerts protective effects on MSCs under H/SD
conditions via the Akt/GSK3β pathway. Therefore, in the present
study, the phosphorylation of Akt and GSK3β were detected by
western blotting. The results clearly indicated that H/SD
successfully induced MSC apoptosis and decreased the expression of
p-Akt and p-GSK3β, whereas pretreatment with EGFP-ADM attenuated
the apoptosis of MSCs and increased the expression levels of p-Akt
and p-GSK3β. Therefore, it may be concluded that ADM gene
transduction protects MSCs against H/SD-induced apoptosis through
the Akt/GSK3β pathway. Caspase-3 acts as a key effector of
apoptosis. Under normal conditions, it is initially synthesized as
inactive precursors. During the final step of the proapoptotic
signaling pathway in numerous cell lines, caspase-3 is activated to
form cleaved caspase-3. Cleaved caspase-3 is a crucial downstream
factor within the apoptotic cascade, which functions as an
important executor of apoptosis (45). The present results revealed that
cleaved caspase-3 was markedly increased in the H/SD group compared
with in the control group; however, this increase was attenuated in
the EGFP-ADM + H/SD group compared with in the EGFP-Adv + H/SD
group, thus indicating that ADM may protect MSCs through reducing
the activation of caspase-3.
Proteins in the Bcl-2 family serve an instrumental
role in regulating apoptosis by controlling mitochondrial
permeability and the release of cytochrome c. This protein
family includes antiapoptotic proteins, including Bcl-2 and
Bcl-extra large (xL), and proapoptotic proteins, such as
Bcl-2-associated death promoter (Bad), BH3 interacting-domain death
agonist, Bax and Bcl-2-like protein 11. Bcl-2 resides in the outer
mitochondrial wall, maintains membrane integrity and inhibits
cytochrome c release. Conversely, Bax resides in the cytosol
but translocates to the mitochondria following death signaling,
where it damages membrane integrity and promotes release of
cytochrome c. The Bcl-2/Bax ratio is often adopted to
represent the extent of apoptosis (12). Furthermore, Bcl-2 family proteins
have been reported to be associated with Akt/GSK3β. Bad is a well
known substrate of Akt. In addition, p-Akt and p-GSK3β have been
reported to upregulate the expression of Bcl-2 and Bcl-xL, and
increase the Bcl-2/Bax ratio (46,47). Zhou et al confirmed that
early administration of ADM significantly reduced apoptosis of
vascular endothelial cells, increased Bcl-2 protein levels and
decreased Bax gene expression (48). Therefore, the present study
further examined whether the Bcl-2 pathway was also involved in the
antiapoptotic activity of ADM. In the present study, the protein
expression levels of Bcl-2 and Bax were detected. Notably, the
present results demonstrated that ADM increased Bcl-2 protein
expression and improved the Bcl-2/Bax ratio, regardless of hypoxic
or normoxic conditions. Furthermore, although ADM could increase
Bcl-2 protein expression under basal conditions, no effect was
observed on the apoptotic rate between the control and EGFP-ADM
groups, due to the low basal levels of apoptosis in MSCs. However,
further investigation is required to elucidate the mechanisms
underlying the effects of ADM on the increased expression of
Bcl-2.
In conclusion, the results of the present study
provided evidence to suggest that ADM promotes MSC survival under
conditions mimicking myocardial ischemia. The prosurvival effects
of ADM against H/SD-induced apoptosis may be mediated via the
Akt/GSK3β and Bcl-2 signaling pathways.
Acknowledgments
The authors would like to thank Dr Wei Liu for her
expert assistance with cell culture and western blot analysis. Dr
Wei Liu is a member of the Key Laboratory of Myocardial Ischemia
Mechanism and Treatment (Harbin Medical University), Ministry of
Education (Harbin, China).
Notes
[1]
Funding
The present study was supported by grants from the
Natural Science Foundation of Heilongjiang Province (grant no.
QC2013C108), the Key Laboratory of Myocardial Ischemia, Harbin
Medical University, Ministry of Education (grant no. KF201313) and
the National Nature Science Foundations of China (grant no.
81700234).
[2] Availability
of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
LL conceived and designed the experiments. HS and YZ
performed the experiments. YS analyzed the data. LL and HS wrote
the paper.
[4] Ethics
approval and consent to participate
The present study was approved by the Local Ethics
Committee for the Care and Use of Laboratory Animals of Harbin
Medical University.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Rouhi L, Kajbafzadeh AM, Modaresi M,
Shariati M and Hamrahi D: Autologous serum enhances cardiomyocyte
differentiation of rat bone marrow mesenchymal stem cells in the
presence of transforming growth factor-β1 (TGF-β1). In Vitro Cell
Dev Biol Anim. 49:287–294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ishimine H, Yamakawa N, Sasao M, Tadokoro
M, Kami D, Komazaki S, Tokuhara M, Takada H, Ito Y, Kuno S, et al:
N-Cadherin is a prospective cell surface marker of human
mesenchymal stem cells that have high ability for cardiomyocyte
differentiation. Biochem Biophys Res Commun. 438:753–759. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fukuda K and Fujita J: Mesenchymal, but
not hematopoietic, stem cells can be mobilized and differentiate
into cardiomyocytes after myocardial infarction in mice. Kidney
Int. 68:1940–1943. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Montanari S, Dayan V, Yannarelli G, Billia
F, Viswanathan S, Connelly KA and Keating A: Mesenchymal stromal
cells improve cardiac function and left ventricular remodeling in a
heart transplantation model. J Heart Lung Transplant. 34:1481–1488.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mathiasen AB, Qayyum AA, Jørgensen E,
Helqvist S, Fischer-Nielsen A, Kofoed KF, Haack-Sørensen M, Ekblond
A and Kastrup J: Bone marrow-derived mesenchymal stromal cell
treatment in patients with severe ischaemic heart failure: A
randomized placebo-controlled trial (MSC-HF trial). Eur Heart J.
36:1744–1753. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mias C, Lairez O, Trouche E, Roncalli J,
Calise D, Seguelas MH, Ordener C, Piercecchi-Marti MD, Auge N,
Salvayre AN, et al: Mesenchymal stem cells promote matrix
metalloproteinase secretion by cardiac fibroblasts and reduce
cardiac ventricular fibrosis after myocardial infarction. Stem
Cells. 27:2734–2743. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Müller-Ehmsen J, Krausgrill B, Burst V,
Schenk K, Neisen UC, Fries JW, Fleischmann BK, Hescheler J and
Schwinger RH: Effective engraftment but poor mid-term persistence
of mononuclear and mesenchymal bone marrow cells in acute and
chronic rat myocardial infarction. J Mol Cell Cardiol. 41:876–884.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kolossov E, Bostani T, Roell W, Breitbach
M, Pillekamp F, Nygren JM, Sasse P, Rubenchik O, Fries JW, Wenzel
D, et al: Engraftment of engineered ES cell-derived cardiomyocytes
but not BM cells restores contractile function to the infarcted
myocardium. J Exp Med. 203:2315–2327. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Toma C, Pittenger MF, Cahill KS, Byrne BJ
and Kessler PD: Human mesenchymal stem cells differentiate to a
cardiomyocyte phenotype in the adult murine heart. Circulation.
105:93–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu W, Chen J, Cong X, Hu S and Chen X:
Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem
cells. Stem Cells. 24:416–425. 2006. View Article : Google Scholar
|
|
11
|
Suresh SC, Selvaraju V, Thirunavukkarasu
M, Goldman JW, Husain A, Alexander Palesty J, Sanchez JA, McFadden
DW and Maulik N: Thioredoxin-1 (Trx1) engineered mesenchymal stem
cell therapy increased pro-angiogenic factors, reduced fibrosis and
improved heart function in the infarcted rat myocardium. Int J
Cardiol. 201:517–528. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu XY, Hao CP, Ling M, Guo CH and Ma W:
Hypoxia-induced apoptosis is blocked by adrenomedullin via
upregulation of Bcl-2 in human osteosarcoma cells. Oncol Rep.
34:787–794. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Di Liddo R, Bridi D, Gottardi M, De Angeli
S, Grandi C, Tasso A, Bertalot T, Martinelli G, Gherlinzoni F and
Conconi MT: Adrenomedullin in the growth modulation and
differentiation of acute myeloid leukemia cells. Int J Oncol.
48:1659–1669. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ah Kioon MD, Asensio C, Ea HK, Velard F,
Uzan B, Rullé S, Bazille C, Marty C, Falgarone G, Nguyen C, et al:
Adrenomedullin(22-52) combats inflammation and prevents systemic
bone loss in murine collagen-induced arthritis. Arthritis Rheum.
64:1069–1081. 2012. View Article : Google Scholar
|
|
15
|
Kaafarani I, Fernandez-Sauze S, Berenguer
C, Chinot O, Delfino C, Dussert C, Metellus P, Boudouresque F,
Mabrouk K, Grisoli F, et al: Targeting adrenomedullin receptors
with systemic delivery of neutralizing antibodies inhibits tumor
angiogenesis and suppresses growth of human tumor xenografts in
mice. FASEB J. 23:3424–3435. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen P, Huang Y, Bong R, Ding Y, Song N,
Wang X, Song X and Luo Y: Tumor-associated macrophages promote
angiogenesis and melanoma growth via adrenomedullin in a paracrine
and autocrine manner. Clin Cancer Res. 17:7230–7239. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sakimoto S, Kidoya H, Kamei M, Naito H,
Yamakawa D, Sakaguchi H, Wakabayashi T, Nishida K and Takakura N:
An angiogenic role for adrenomedullin in choroidal
neovascularization. PLoS One. 8:e580962013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim JY, Park WD, Lee S and Park JH:
Adrenomedullin is involved in the progression of colonic
adenocarcinoma. Mol Med Rep. 6:1030–1034. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yin H, Chao L and Chao J: Adrenomedullin
protects against myocardial apoptosis after ischemia/reperfusion
through activation of Akt-GSK signaling. Hypertension. 43:109–116.
2004. View Article : Google Scholar
|
|
20
|
Zhou PH, Hu W, Zhang XB, Wang W2 and Zhang
LJ: Protective effect of adrenomedullin on rat leydig cells from
lipopolysaccharide-induced inflammation and apoptosis via the
PI3K/Akt signaling pathway ADM on rat leydig cells from
inflammation and apoptosis. Mediators Inflamm. 2016:72015492016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kong XQ, Wang LX, Yang CS, Chen SF, Xue YZ
and Liu YH: Effects of adrenomedullin on the cell numbers and
apoptosis of endothelial progenitor cells. Clin Invest Med.
31:E117–E122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim W, Moon SO, Sung MJ, Kim SH, Lee S,
Kim HJ, Koh GY and Park SK: Protective effect of adrenomedullin in
mannitol-induced apoptosis. Apoptosis. 7:527–536. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen L, Zhang Y, Sun X, Li H, LeSage G,
Javer A, Zhang X, Wei X, Jiang Y and Yin D: Synthetic resveratrol
aliphatic acid inhibits TLR2-mediated apoptosis and an involvement
of Akt/GSK3β pathway. Bioorg Med Chem. 17:4378–4382. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ying Y, Zhu H, Liang Z, Ma X and Li S:
GLP1 protects cardiomyocytes from palmitate-induced apoptosis via
Akt/GSK3b/b-catenin pathway. J Mol Endocrinol. 55:245–262. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yin H, Chao L and Chao J: Kallikrein/kinin
protects against myocardial apoptosis after ischemia/reperfusion
via Akt-glycogen synthase kinase-3 and Akt-Bad.14-3-3 signaling
pathways. J Biol Chem. 280:8022–8030. 2005. View Article : Google Scholar
|
|
26
|
Kim CH, Hao J, Ahn HY and Kim SW:
Activation of Akt/protein kinase B mediates the protective effects
of mechanical stretching against myocardial ischemia-reperfusion
injury. J Vet Sci. 13:235–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song JQ, Teng X, Cai Y, Tang CS and Qi YF:
Activation of Akt/GSK-3β signaling pathway is involved in
intermedin(1-53) protection against myocardial apoptosis induced by
ischemia/reperfusion. Apoptosis. 14:1061–1069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen J, Baydoun AR, Xu R, Deng L, Liu X,
Zhu W, Shi L, Cong X, Hu S and Chen X: Lysophosphatidic acid
protects mesenchymal stem cells against hypoxia and serum
deprivation-induced apoptosis. Stem Cells. 26:135–145. 2008.
View Article : Google Scholar
|
|
29
|
He J, Wang C, Sun Y, Lu B, Cui J, Dong N,
Zhang M, Liu Y and Yu B: Exendin-4 protects bone marrow-derived
mesenchymal stem cells against oxygen/glucose and serum
deprivation-induced apoptosis through the activation of the
cAMP/PKA signaling pathway and the attenuation of ER stress. Int J
Mol Med. 37:889–900. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang F, Cui J, Lv B and Yu B: Nicorandil
protects mesenchymal stem cells against hypoxia and serum
deprivation-induced apoptosis. Int J Mol Med. 36:415–423. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li L, Zhang S, Zhang Y, Yu B, Xu Y and
Guan Z: Paracrine action mediate the antifibrotic effect of
transplanted mesenchymal stem cells in a rat model of global heart
failure. Mol Biol Rep. 36:725–731. 2009. View Article : Google Scholar
|
|
32
|
Zhu X, Jiang Y, Shan PF, Shen J, Liang QH,
Cui RR, Liu Y, Liu GY, Wu SS, Lu Q, et al: Vaspin attenuates the
apoptosis of human osteoblasts through ERK signaling pathway. Amino
Acids. 44:961–968. 2013. View Article : Google Scholar
|
|
33
|
Yu D, Mu S, Zhao D, Wang G, Chen Z, Ren H
and Fu Q: Puerarin attenuates glucocorticoid-induced apoptosis of
hFOB1.19 cells through the JNK- and Akt-mediated mitochondrial
apoptotic pathways. Int J Mol Med. 36:345–354. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Parekkadan B and Milwid JM: Mesenchymal
stem cells as therapeutics. Annu Rev Biomed Eng. 12:87–117. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shi RZ and Li QP: Improving outcome of
transplanted mesenchymal stem cells for ischemic heart disease.
Biochem Biophys Res Commun. 376:247–250. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Robey TE, Saiget MK, Reinecke H and Murry
CE: Systems approaches to preventing transplanted cell death in
cardiac repair. J Mol Cell Cardiol. 45:567–581. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Amiri F, Jahanian-Najafabadi A and
Roudkenar MH: In vitro augmentation of mesenchymal stem cells
viability in stressful microenvironments: In vitro augmentation of
mesenchymal stem cells viability. Cell Stress Chaperones.
20:237–251. 2015. View Article : Google Scholar :
|
|
38
|
Potier E, Ferreira E, Meunier A, Sedel L,
Logeart-Avramoglou D and Petite H: Prolonged hypoxia concomitant
with serum deprivation induces massive human mesenchymal stem cell
death. Tissue Eng. 13:1325–1331. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Edelstein ML, Abedi MR and Wixon J: Gene
therapy clinical trials worldwide to 2007 - an update. J Gene Med.
9:833–842. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jo J, Nagaya N, Miyahara Y, Kataoka M,
Harada-Shiba M, Kangawa K and Tabata Y: Transplantation of
genetically engineered mesenchymal stem cells improves cardiac
function in rats with myocardial infarction: Benefit of a novel
nonviral vector, cationized dextran. Tissue Eng. 13:313–322. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Treacy O, Ryan AE, Heinzl T, O'Flynn L,
Cregg M, Wilk M, Odoardi F, Lohan P, O'Brien T, Nosov M, et al:
Adenoviral transduction of mesenchymal stem cells: In vitro
responses and in vivo immune responses after cell transplantation.
PLoS One. 7:e426622012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Park SC, Yoon JH, Lee JH, Yu SJ, Myung SJ,
Kim W, Gwak GY, Lee SH, Lee SM, Jang JJ, et al: Hypoxia-inducible
adrenomedullin accelerates hepatocellular carcinoma cell growth.
Cancer Lett. 271:314–322. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chung H, Seo S, Moon M and Park S:
Phosphati-dylinositol-3-kinase/Akt/glycogen synthase kinase-3 beta
and ERK1/2 pathways mediate protective effects of acylated and
unacylated ghrelin against oxygen-glucose deprivation-induced
apoptosis in primary rat cortical neuronal cells. J Endocrinol.
198:511–521. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yan X, Lyu T, Jia N, Yu Y, Hua K and Feng
W: Huaier aqueous extract inhibits ovarian cancer cell motility via
the AKT/GSK3β/β-catenin pathway. PLoS One. 8:e637312013. View Article : Google Scholar
|
|
45
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Linseman DA, Butts BD, Precht TA, Phelps
RA, Le SS, Laessig TA, Bouchard RJ, Florez-McClure ML and
Heidenreich KA: Glycogen synthase kinase-3beta phosphorylates Bax
and promotes its mitochondrial localization during neuronal
apoptosis. J Neurosci. 24:9993–10002. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kaga S, Zhan L, Altaf E and Maulik N:
Glycogen synthase kinase-3beta/beta-catenin promotes angiogenic and
anti-apoptotic signaling through the induction of VEGF, Bcl-2 and
survivin expression in rat ischemic preconditioned myocardium. J
Mol Cell Cardiol. 40:138–147. 2006. View Article : Google Scholar
|
|
48
|
Zhou M, Simms HH and Wang P:
Adrenomedullin and adrenomedullin binding protein-1 attenuate
vascular endothelial cell apoptosis in sepsis. Ann Surg.
240:321–330. 2004. View Article : Google Scholar : PubMed/NCBI
|