Introduction
A mammalian ovary consists of a large number of
follicles at the fetal stage. However, > 99% of follicles fail
to ovulate due to follicular atresia, which is the spontaneous
degeneration of a follicle (1).
Although follicular atresia may occur at any stage of growth and
development (2), it usually
occurs during the early antral stages of follicular development. As
a result, apoptosis is originally induced in the region of the
granulosa cells, as opposed to the cumulus cells, oocytes, or inner
or extra theca cells, and subsequently the majority of the
granulosa cells, theca cells and cumulus cells undergo apoptosis.
This firmly suggests that follicular development and atresia
predominantly depend on apoptosis of the granulosa cells. In
addition, previous studies have demonstrated that numerous factors,
including cell death ligands and receptors, growth factors, pro-
and anti-apoptotic factors, apoptotic genes and cytokines, may
modulate apoptosis of the granulosa cells (2).
Aurora B (also termed serine/threonine-protein
kinase 12) is a key regulator in mitosis. It forms the enzymatic
core of the chromosomal passenger complex (CPC) (3), which also contains three
non-enzymatic components: Survivin, inner centromere protein
(INCENP) and borealin (4). CPC is
a crucial upstream regulator of centromere-kinetochore function.
Aurora B exerts an important role in chromosomal cohesion and
arrangement, chromosomal separation, the spindle assembly
checkpoint, and cytokinesis (5).
During mitosis, the localization of Aurora B changes during cell
cycle division: For example, Aurora B is localized on the inner
centromeres during metaphase to regulate chromosomal cohesion and
spindle-kinetochore attachment (6); subsequently, it is localized on the
spindle midzone and equatorial cortex, and then it accumulates in
the midbody to accomplish cell division (6,7).
Aurora B may be activated by autophosphorylation of the T-loop
(activation loop) after it binds to the IN-box region of INCENP
(8,9). Post-translational modifications,
including ubiquitination and SUMOylation, are necessary for the
proper activity and control of the dynamic behavior of the CPC.
SUMOylation is a post-translational modification by
the covalent conjugation of small ubiquitin-related modifiers
(SUMOs), which are found in all eukaryotes (10-12). There are at least three forms of
SUMOs that have been identified in humans: SUMO1, 2 and 3. SUMO2
and 3 have a high degree of similarity (97%), whereas SUMO1 shares
43% of its identity with SUMO2/3 (13). Similarly to ubiquitination,
SUMOylation features a conserved sequence that covalently
conjugates to lysine residues within the target protein using the
sequential action of SUMO-specific E1 (activation), E2
(conjugation) and E3 (ligation) enzymes (14). This process may result in a
modulation of the subcellular localization of its targets (15), alter protein stability (16), and antagonize other types of
post-translational modifications (17,18) in at least two ways: An allosteric
effect that induces substrate conformational change, or an effect
that involves the creation/inhibition of a binding interface
(6). SUMOylation is also a
reversible process that is catalyzed by the SUMO-specific proteases
of the sentrin-specific protease (SENP) family (19).
The majority of studies have focused on the role of
Aurora B in cancer therapy; however, the role of Aurora B and its
SUMOylation in follicular development and atresia has yet to be
elucidated. A recent study has verified that, in humans, Aurora B
is modified by PIAS3 [protein inhibitor of activated signal
transducer and activator of transcription (STAT)]-mediated SUMO2
conjugation at Lys-202, and that SUMO2 conjugation facilitates the
autophosphorylation of Aurora B, but does not directly affect its
kinase activity during mitosis (6). Furthermore, Lys-207 of Aurora B may
be conjugated to SUMOs in 293 cells, and this modification requires
Aurora B to bind to its activator, INCENP. The defective
SUMOylation of Aurora B has resulted in abnormal chromosome
segregation, reduced cell viability, and altered cytokinesis and
chromosomal dynamics of the CPC (20).
However, upon sequencing cDNA obtained from the
granulosa cells of Kunming female white mice, the individual base
(134 bp) of Mus musculus Aurora B has been identified and
verified that is different, as reported by The National Center for
Biotechnology Information (NCBI; cDNA clone MGC:5803
IMAGE:3501444). In addition, recent proteomics studies have shown
that a considerable proportion of SUMOylated proteins do not
contain the consensus sites (21-23), and that not all consensus
sequences are SUMOylated, as this process often occurs outside of
the consensus sequence (24).
Therefore, the aim of the present study was to determine how Aurora
B and its SUMOylation may influence follicular development and
atresia in primary cultured granulosa cells in mice.
The findings of the present study have provided
evidence that, in mice, Aurora B serves a crucial role in
follicular development through mediating the viability of granulosa
cells, with possibly other molecular mechanisms being in operation.
Furthermore, this study has explored whether Aurora B may be
SUMO-modified at Lys-207 in vivo and in vitro. How
SUMOylation impacts on follicular development and atresia, as
mediated by granulosa cells, was also examined.
Materials and methods
Experimental animals and ethics
Female Kunming mice were purchased from the Center
of Laboratory Animals (Wuhan, China). All animal treatment
procedures were approved by the Ethics Committee of the Hubei
Research Center of Experimental Animals (ref. no. ETH-201509). The
mice were provided with food and water, and housed under controlled
conditions of temperature (20-24°C) and lighting (12-h light/12-h
darkness).
Immunohistochemistry (IHC)
The ovaries from mice aged 7, 14 or 21 days, or from
21-day mice that had been injected with 10 IU pregnant mare serum
gonadotropin (PMSG; Ningbo Sansheng Pharmaceutical Co., Ltd.,
Ningbo, China) for 48 h, or from 21-day mice that had been injected
with 10 IU human chorionic gonadotropin (hCG; Ningbo Sansheng
Pharmaceutical Co., Ltd.) for 7 h after treatment with PMSG for 48
h, were collected. The ovaries were fixed in 4% paraformaldehyde
(PFA; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
overnight at 4°C, passed through an ethanol gradient series for
dehydration, and embedded in paraffin wax. The ovaries were then
cut into 4-5 μm sections, which were placed on slides. The
slides were dewaxed in xylene and rehydrated in gradient ethanol.
Heat-mediated antigen retrieval of tissue sections was performed
before the sections were allowed to cool (25). Endogenous peroxidases were blocked
using 3% hydrogen peroxide for 15 to 20 min, and non-specific
antibody binding was blocked using 5% bovine serum albumin (BSA;
Sigma-Aldrich, St. Louis, MO, USA) for 1 h. The tissue sections
were incubated with polyclonal rabbit anti-Aurora B antibody (1:200
dilution; sc-25426; Santa Cruz Biotechnology, Inc.) overnight at
4°C, and then incubated with a secondary antibody and
streptavidin-biotin complex (SA1022; Wuhan Boster Biological
Technology, Ltd., Wuhan, China) for 20 min at 37°C. The signals
were visualized using 3,3′-diaminobenzidine (DAB) for 1 min and
hematoxylin for 2 min. Slides were subsequently dehydrated using
graded ethanol, cleared in xylene, mounted, and images were
captured.
Immunocytochemistry (ICC)
For immunofluorescence, granulosa cells from antral
follicles were obtained from the ovaries of 21-day-old female
Kunming mice injected with 10 IU PMSG for 48 h. To obtain purified
granulosa cells, it is necessary that the ovaries are not minced
too extensively; the tissues were then suspended in Dulbecco's
modified Eagle's medium/Nutrient F12 (DMEM/F12; Hyclone, Logan, UT,
USA) or phosphate-buffered saline (PBS) three times; the suspension
time was reduced from 1 min to 30 sec. The supernatant containing
the granulosa cells was centrifuged at 252 × g for 5 min at room
temperature, and cultured in DMEM/ F12 medium supplemented with 10%
fetal bovine serum (FBS; gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 37°C in a humidified atmosphere containing 5%
CO2. After 12 h, granulosa cells were washed with
DMEM/F12 to remove impurities and tissues, and cultured in fresh
medium continuously. Cells were fixed in 4% PFA and permeabilized
with 0.5% Triton X-100 (Santa Cruz Biotechnology, Inc.). The cells
were subsequently blocked with 5% BSA for 30 min at 37°C and
incubated with primary antibodies overnight at 4°C, a polyclonal
rabbit anti-Aurora B antibody (1:100 dilution; sc-25426; Santa Cruz
Biotechnology, Inc.), a monoclonal mouse anti-HA antibody (1:500
dilution; M180-3; Medical and Biological Laboratories, Co., Ltd.,
Nagoya, Japan), and a polyclonal rabbit anti-Flag antibody (1:200
dilution; 20543-1-AP; Proteintech, Chicago, IL, USA). The next day,
the cells were incubated with fluorescein isothiocyanate (FITC)/CY3
conjugated to a secondary antibody (1:100 dilution; Wuhan Boster
Biological Technology, Ltd.) for 2 h at room temperature in the
dark. The nuclei were stained with 10 μl/ml propidium iodide
(PI; Santa Cruz Biotechnology, Inc.) for 5 min in the dark. Images
were then captured using a microscope (TE2000-U; Nikon, Tokyo,
Japan).
Plasmid construction
The Aurora B- and SUMO2-coding sequences of Mus
musculus were obtained from the National Center for
Biotechnology Information (Bethesda, MD, USA) (Aurora B,
NM_011496.2; SUMO2, NM_133354), and were amplified using the
polymerase chain reaction (PCR) and primer pair sequences (Table I). To generate the Aurora B lysine
207-to-arginine (K207R) mutant, two primer pairs were used for PCR:
The Aurora B cDNA sequence was used as the template using primer
pair 1 (forward 1 and reverse 1) and primer pair 2 (forward 2 and
reverse 2), respectively, for generating two fragments including
the mutant base, and then the two fragments (1:1) were taken as the
template again, this time using the primer pair, forward 1 and
reverse 2, for the second reaction. The genes were then subcloned
into pCMV-N-HA or Flag vector to generate the plasmids, named as
HA-Aurora B, HA-Aurora BK207R and HA-SUMO2, and
Flag-Aurora B, Flag-Aurora BK207R and Flag-SUMO2. The
results were confirmed by sequencing.
 | Table ISequences of primer pairs for
PCR. |
Table I
Sequences of primer pairs for
PCR.
| Gene | | Primer sequences
(5′→3′) | Length (bp) |
|---|
| Aurora
B | Forward |
CAAGCTTTGTTTCCCTCTCTGTCCA | 1,099 |
| Reverse |
GGAATTCAACCAAGGAGCAGGCTA | |
| Aurora
BK207R | Forward 1 |
CAAGCTTTGTTTCCCTCTCTGTCCA | 678 |
| Reverse 1 |
AGGTTCTCCGGCTTTATGTCT | |
| Forward 2 |
TAATTCACAGAGACATAAAGCCGG | 451 |
| Reverse 2 |
GGAATTCAACCAAGGAGCAGGCTA | |
| SUMO2 | Forward |
GCTCCTGGTGCTGCTTGTG | 360 |
| Reverse
CCCTTTTTA |
GTAGACACCTCCAGT | |
Cell culture, inhibitor treatment and
transfection
Ovaries from 21-day mice were injected with PMSG for
48 h. The ovaries were subsequently minced using a scalpel, and
suspended in DMEM/F12 or PBS three times; the time of the
suspensions was reduced from 1 min to 30 sec. The supernatant
containing the granulosa cells was cultured in DMEM/F12
supplemented with 10% FBS at 37°C in a humidified atmosphere
containing 5% CO2. ZM447439 (Axon Medchem LLC, Reston,
VA, USA), an inhibitor of Aurora B, was dissolved in DMSO at
concentrations of 10, 50, and 100 mM, and stored at −20°C.
Appropriate concentrations were added to the culture medium for 48
h after the cells were starved for 6 h; the final concentration of
DMSO never exceeded 1 μl/ml. HA-Aurora B, HA-Aurora
BK207R, Flag-SUMO2 and HA or Flag (as control) were
transfected using Lipofectamine® LTX and Plus™ reagent
(Invitrogen Life Technologies; Thermo Fischer Scientific, Inc.)
according to the manufacturer's protocol. Lipofectamine®
LTX and plasmids were diluted into Opti-MEM (Gibco; Thermo Fischer
Scientific, Inc.) for 5 min, and then mixed in a ratio of 2:1 for
30 min. After 48 h, the granulosa cells were collected for protein
extraction or subsequent assay.
Protein extraction, immunoprecipitation
(IP) and western blotting
Granulosa cells with or without treatment were
washed with ice-cold PBS and harvested in 80 μl RIPA buffer
(Santa Cruz Biotechnology, Inc.) that was supplemented with 1% PMSF
(Dingguo Biotechnology Co., Beijing, China), 1% cocktail
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and 20 mM
N-ethylmaleimide (NEM; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) for 20 min. The lysates were centrifuged at 11,600 × g for
10 min, and the supernatant was collected and stored at −80°C.
In order to examine the interaction of the proteins
in vivo and in vitro, IP was performed. The granulosa
cells were washed with ice-cold PBS and lysed on ice in lysis
buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA and 1%
Triton X-100] that was supplemented with 1% PMSF, 1% cocktail and
20 mM NEM for 20 min; the protein samples were incubated with 1
μg of the respective primary antibodies overnight at 4°C, a
polyclonal rabbit anti-SUMO1 antibody (10329-1-AP) and a polyclonal
rabbit anti-SUMO2 antibody (11251-1-AP) (both from Proteintech, as
described above), a monoclonal mouse anti-HA antibody (M180-3;
Medical and Biological Laboratories, Co., Ltd.), and a polyclonal
rabbit anti-Flag antibody (20543-1-AP; Proteintech), and
subsequently 40 μl protein A+G Agarose (Beyotime
Biotechnology Co., Ltd., Shanghai, China) was added for 2 h at 4°C.
Finally, the samples were boiled with 40 μl 2X SDS loading
buffer for 5 min, and used immediately for the western
blotting.
The proteins were separated on SDS-PAGE and
transferred to polyvinylidene fluoride (PVDF) membranes (Millipore,
Bedford, MA, USA), blocked with 5% skimmed milk diluted with TBST
[10 mM Tris (pH 7.5), 150 mM NaCl and 0.1% Tween-20], and incubated
with the corresponding primary antibodies diluted in blocking
buffer overnight at 4°C. [The antibodies were rabbit anti-Aurora B
immunoglobulin g (IGG) (1:1,000 dilution; 3094; Cell Signaling
Technology, Inc., Danvers, MA, USA), mouse anti-β-actin IGG
(1:1,000 dilution; sc-517582; Santa Cruz Biotechnology, Inc.),
mouse anti-HA IGG (1:1,000 dilution; M180-3; Medical and Biological
Laboratories, Co., Ltd.), rabbit anti-Flag IGG (1:500 dilution;
20543-1-AP; Proteintech), rabbit anti-phospho-p38 mitogen-activated
protein kinase (MAPK) (Thr-180) IGG (1:1,000 dilution; AF4001;
Affinity Biosciences, Cincinnati, OH, USA), rabbit
anti-cyclin-dependent kinase 4 (CDK4) IGG (1:1,000 dilution; A0366;
ABclonal Biotech Co., Ltd., Wobrun, MA, USA), mouse
anti-proliferating cell nuclear antigen (PCNA) IGG (1:500 dilution;
BM0104; Boster Biological Technology, Ltd.), Fas (1:1,000 dilution;
A2639; ABclonal Biotech Co., Ltd.), mouse anti-caspase-8 IGG
(1:1,000 dilution; 66093- 1-IG; Proteintech), and caspase-3 (1:200
dilution; sc-136219) and B-cell lymphoma 2 (Bcl-2; 1:200 dilution;
sc-509) (both from Santa Cruz Biotechnology, Inc.)]. The following
day, the proteins were incubated with matching horseradish
peroxidase (HRP)-conjugated secondary anti bodies (1:3,000
dilution; Santa Cruz Biotechnology, Inc.), diluted in TBST for 1 h,
and washed three times in TBST. Chemiluminescent detection was
performed by enhanced chemiluminescence (ECL; Amersham Biosciences,
Piscataway, NJ, USA). The images were captured with a gel-Pro
analyzer 4.0 (Media Cybernetics, Silver Spring, MD, USA). The
scanning intensities of the western blots was analyzed using ImageJ
software to quantify the target bands in comparison with the
corresponding β-actin bands.
Preparation of ovarian follicles
Different follicular development stages, the
different granulosa cell layers and diameters were measured.
Primary follicles obtained from ovaries of 7-day-old female mice
were gently separated using the needle of a 1 ml syringe under a
stereo microscope (CKX41SF; Olympus Optical Technology Philippines
Inc., Lapu-Lapu City, Philippines), and observed again under an
inverted microscope (TE2000-U; Nikon) to collect the follicles with
single layer granulosa cells and a diameter <90 μm.
Secondary follicles obtained from the ovaries of 14-day-old female
mice were measured using same procedure as described above, and
follicles between 90 and 120 μm in diameter with two or
three layers of granulosa cells were collected. Antral follicles
were obtained from ovaries of 21-day-old female mice following
injection with 10 IU PMSG for 48 h, separating out the follicles
with a cavity measuring 400-500 μm in diameter, and
puncturing the cavity to remove follicular fluid. Preovlatory
follicles were obtained from the ovaries of 21-day-old female mice
following injection with 10 IU PMSG for 48 h, and then 10 IU hCG 7
h orderly, separating out the follicles with an obvious cavity and
measuring >500 μm in diameter, and then similarly
puncturing the cavity to remove the follicular fluid. Finally, the
four-stage follicles were washed in PBS three times, and
centrifuged at 112 × g for 5 min at room temperature prior to
immediate extraction of the proteins.
Quantitative real-time PCR (RT-qPCR)
analysis
Total RNA was extracted and reverse-transcribed
using a Total RNA extraction kit and RevertAid Strand cDNA
Synthesis kit, according to the manufacturer's protocol. Relative
mRNA levels of cdk4, PCNA, caspase-3 and bcl-2 were quantified
using special primer pairs (Table
II) and QuantiFast® SyBR®-Green PCR kit
(Qiagen, Hilden, Germany). All samples were performed in triplicate
to calculate the statistical significance. The gene expression
results were normalized to the basal level of β-actin. The Ct
(2−ΔΔCq) method (26)
was used to analyze the relative gene expression data.
 | Table IISequences of primer pairs for
RT-qPCR. |
Table II
Sequences of primer pairs for
RT-qPCR.
| Genes | Primer
sequences |
|---|
| PCNA | Forward
CAACTTGGAATCCCAGAAC |
| Reverse
AGACAGTGGAGTGGCTTTT |
| cdk4 | Forward
TCACGCCTGTGGTGGTTAC |
| Reverse
GGTCGGCTTCTGAGTTTCC |
| bcl-2 | Forward
GCTACCGTCGTGACTTCGC |
| Reverse
ACCCAGCCTCCGTTATCC |
|
caspase-3 | Forward
GGCGTGAAGACATTTTGAA |
| Reverse
CGGGAGTAGTCGCCTCTGAA |
| β-actin | Forward
CCCATCTACGAGGGCTAT |
| Reverse
TGTCACGCACGATTTCC |
Cell cycle, proliferation and apoptosis
assay
Granulosa cells were collected for cell cycle assay
using the Cell Cycle Detection kit (Keygen Biotech Co., Ltd.,
Nanjing, China) according to the manufacturer's protocol. Cells
were fixed with 70% ethanol overnight at 4°C, and incubated in 100
μl RNase A for 30 min at 37°C, and 400 μl PI solution
was added in the dark for 30 min at 4°C. Flow cytometric analysis
was conducted using a BD FACSCalibur [excitation wavelength (Ex),
488 nm; emission wavelength (Em), 530 nm; Becton-Dickinson,
Mountain view, CA, USA].
Apoptosis was performed using the Annexin V-FITC
Apopt osis Detection kit (Keygen Biotech Co., Ltd.) according to
the manufacturer's protocol. Granulosa cells were digested by the
pancreatin enzymes without EDTA; each sample was then added to 190
μl 1X binding buffer and incubated in 5 μl Annexin
V-FITC for 3 min in the dark, and then added to 10 μl PI
solution in the dark for 10 min. Flow cytometric analysis was then
performed.
Cell proliferation was assessed using a WST-1 Cell
Proliferation Assay kit (Beyotime Institute of Biotechnology)
according to the manufacturer's protocol. Briefly, granulosa cells
were seeded in 96-well plates (4×103 cells/well), and 10
μl freshly prepared WST-1 solution was added to each well,
along with the culture medium. The absorbance of the samples was
measured after 2 h using a microplate reader (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) at 450 nm.
Statistical analysis
All experiments were performed independently at
least three times, and the data are presented as the mean ±
standard deviation. The differences between the groups were
analyzed by one-way analysis of variance (ANOVA) followed by LSD
test using SPSS statistical software (version 17.0; SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Localization and expression of Aurora B
in the mouse follicle
To investigate whether Aurora B affects follicular
development and atresia, an IHC study was initially conducted to
detect the localization of Aurora B in the mouse follicles at
different stages, including primary, secondary, antral and
preovulatory follicles (Fig.
1Ab–e). A high concentration of Aurora B was identified in the
granulosa cells, whereas a lower concentration appeared in the
oocyte (Fig. 1A).
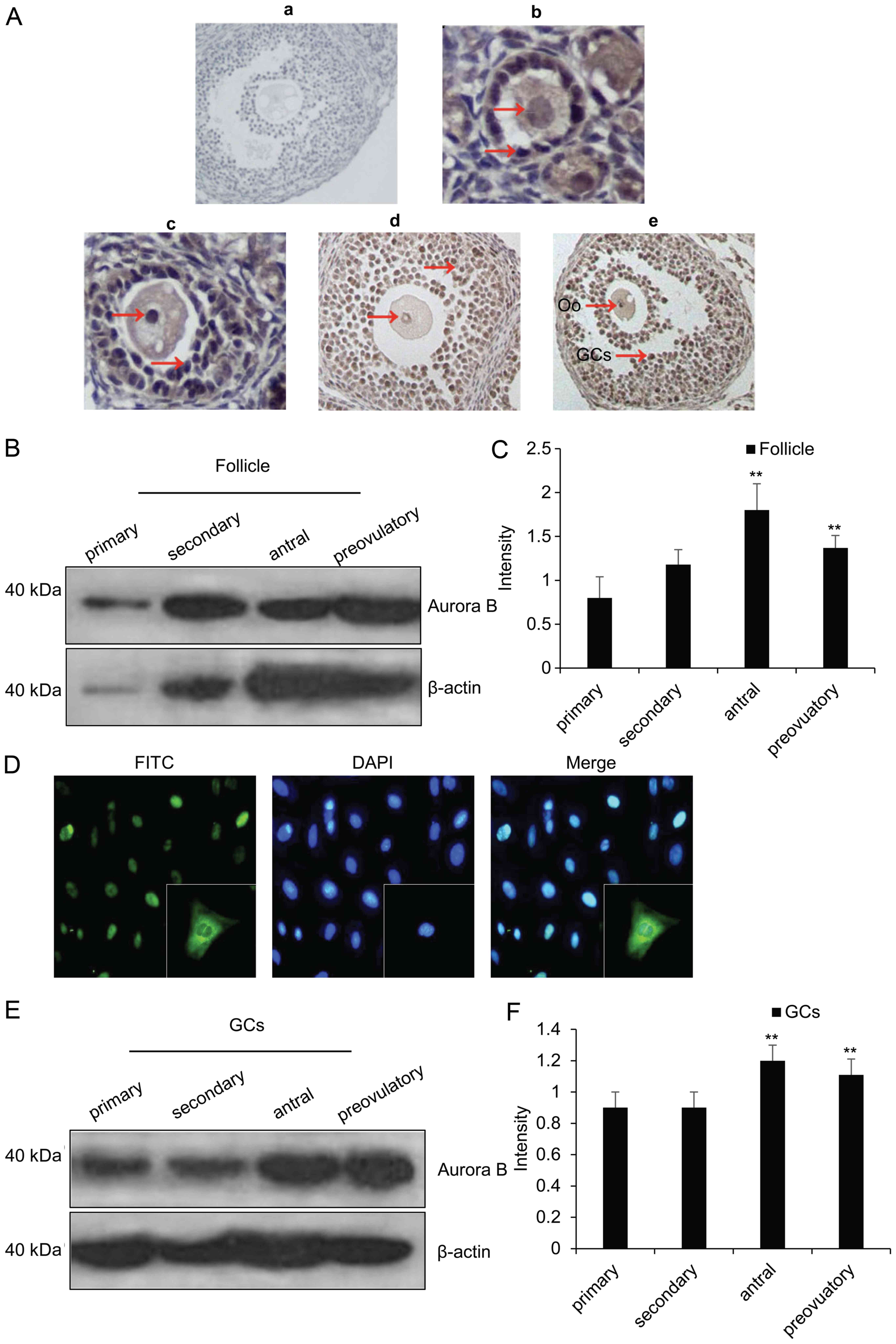 | Figure 1The protein levels, and localization,
of Aurora B in the follicles and granulosa cells. (A) Localization
of Aurora B in mouse ovarian follicles determined through
performing immunohistochemistry of the follicles. (a) Negative
control; (b) primary follicle (diameter, <90 μm); (c)
secondary follicle (diameter, 90-120 μm); (d) antral
follicle (diameter, 400-500 μm); and (e) preovulatory
follicle (diameter, >500 μm). The red arrows represent
the oocyte and the GCs. (B) Protein level of Aurora B in the
follicles at different development stages, as determined using
western blotting. (C) Scanning intensity of the western blotting
experiments. (D) Localization of Aurora B in the granulosa cells is
shown by the green fluorescence (FITC), whereas the nucleus is
shown by the blue fluorescence (DAPI). The position of green
fluorescence between two granulosa cells shows the midbody during
cytokinesis in the lower right-hand corner (images magnified ×40).
(E) Protein level of Aurora B in the granulosa cells at different
follicular development stages as determined using western blotting.
(F) The scanning intensity of western blotting. The values are
shown using bars. Each experiment was repeated three times, and the
values are presented as the mean ± standard deviation.
**P<0.01. GCs, granulosa cells; FITC, fluorescein
isothiocyanate.. |
To further explore the protein level of Aurora B in
the follicles, western blotting was performed, which determined
that Aurora B was expressed in each stage of follicular development
(Fig. 1B). The protein level of
Aurora B increased with the extent of follicular development,
particularly in the antral and preovulatory follicles, where the
levels were higher compared with the primary and secondary
follicles (P<0.01) (Fig. 1C).
It was surmised that the increased protein level of Aurora B was
caused by the proliferation of granulose cells in the follicles.
The localization of Aurora B in granulosa cells was determined
using ICC. As shown in Fig. 1D,
Aurora B was detected in the nucleus and, notably, on the midbody
and in the cytoplasm around the nucleus during cytokinesis. In
addition, the protein level of Aurora B in the granulosa cells was
also determined using western blotting. The results demonstrated
that Aurora B was expressed in all the stages (Fig. 1E). As expected, protein levels of
Aurora B in the antral and preovulatory granulosa cells were
significantly higher than in the primary and secondary granulosa
cells (P<0.01) (Fig. 1F).
These results suggested that Aurora B was localized
in all stages, and that the protein level increased with follicular
development and expansion of the granulosa cells, particularly at
the antral stage, where it attained the maximum level. Thus, it was
inferred that Aurora B affected the development of the follicles by
directly modulating the proliferation and apoptosis of the
granulosa cells. However, the role of Aurora B in granulosa cells
remains unclear, and continues to be a topic of intense research
investigation.
Aurora B impacts the viability of
granulosa cells
To determine the role of Aurora B in follicular
development, granulosa cells were co-cultured with ZM447439, which
inhibits the phosphorylation of Aurora B (data not shown). Serial
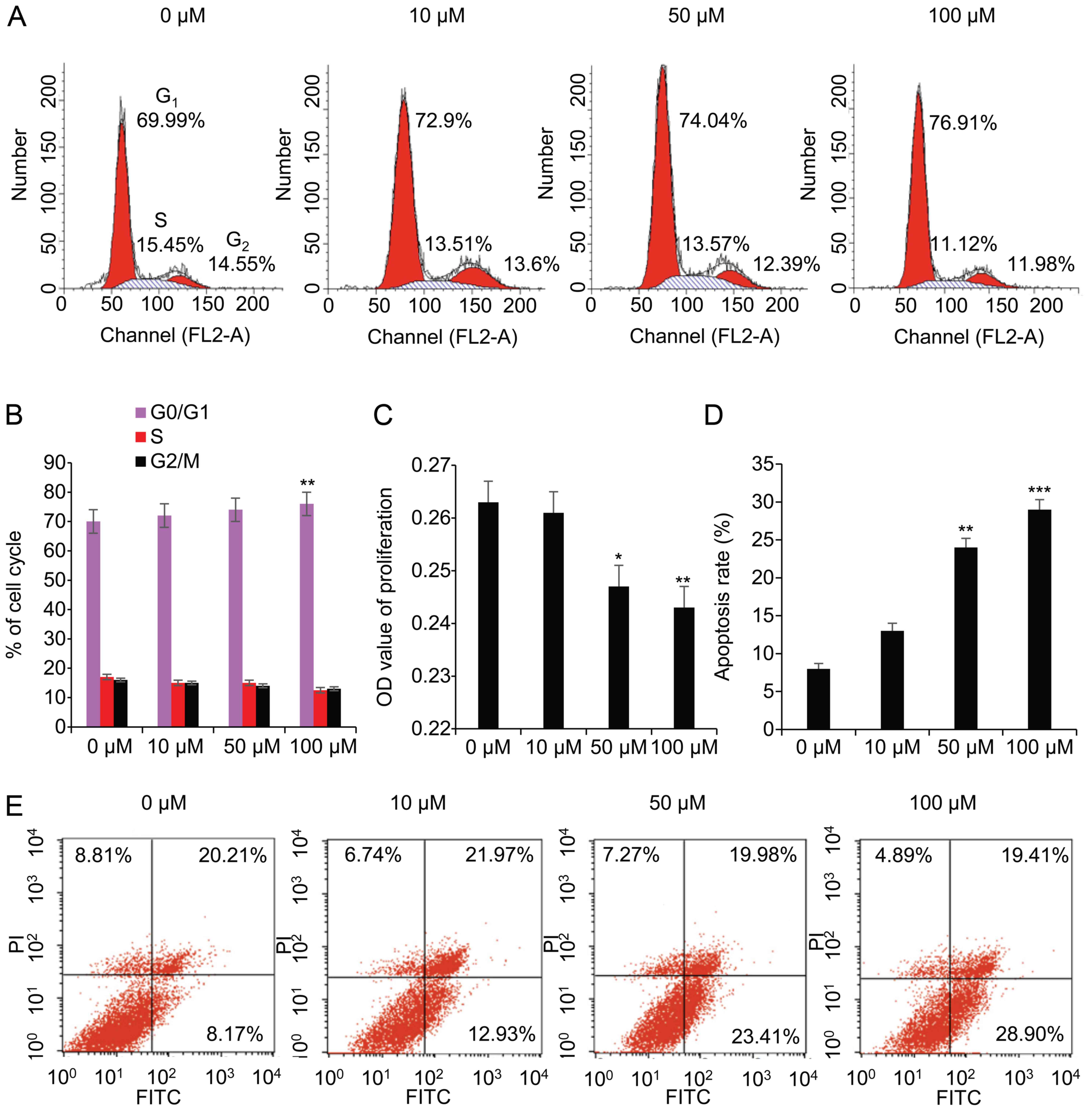
concentrations (0, 10, 50, and 100 μM) were used, and
following a period of 48 h, the ratio of the cell cycle, levels of
proliferation, and apoptosis rates were assessed.
As shown in Fig.
2A, the ratio of the cell cycle increased from 69.99 to 76.91%
in the G0/G1 phase, decreased from 15.45 to
11.12% in the S phase, and decreased from 14.55 to 11.98% in the
G2/M phase. These data suggested that, following
treatment with 100 μM ZM, the ratio was significantly
increased (P<0.01) in the G0/G1 phase,
significantly decreased (P<0.05) in the S phase (Fig. 2B), and cell cycle was arrested at
the G1/S phase. A proliferation assay using WST-1
absorbance at 490 nm revealed that the levels of proliferation were
moderately reduced in a dose-dependent manner, which was
particularly notable at 50 μM (P<0.05) and 100 μM
(P<0.01) compared with 0 μM ZM (Fig. 2C). The apoptotic rate was 8.17,
12.93, 23.41 and 28.90% at 0, 10, 50, and 100 μM ZM,
respectively (Fig. 2E), and
rapidly increased at 50 μM (P<0.01) and 100 μM
(P<0.001) (Fig. 2D).
These data indicated that blocking Aurora B exerted
an impact on the viability of the granulosa cells. The inhibition
of Aurora B led to an arrest of the cell cycle at the
G1/S phase, reduced the proliferation of the cells, and
induced apoptosis.
Aurora B mediates p38 MAPK and the
FasL/Fas pathway in the granulosa cells
To better understand the molecular mechanisms of
Aurora B, western blotting and RT-qPCR were performed using
viability-associated proteins and genes, i.e., p38 MAPK, CDK4
(cell-cycle-associated genes) and PCNA (proliferation-associated
gene), Fas (apoptotic membrane surface molecule), caspase-8 and
caspase-3 (apoptosis genes) and Bcl-2 (anti-apoptosis gene).
The western blotting results demonstrated that the
protein levels of phosphorylated p38 MAPK (p-p38 MAPK) (P<0.01),
CDK4 (P<0.05), PCNA (P<0.01) and Bcl-2 (P<0.01) were
significantly decreased at 50 μM ZM (Fig. 3A and B). However, the protein
level of Fas (P<0.01), caspase-8 (P<0.001) and caspase-3
(P<0.001) were moderately increased at 100 μM ZM
(Fig. 3A and B). Similarly, the
RT-qPCR results revealed that, at 100 μM ZM, the mRNA levels
of cdk4 (P<0.01), PCNA (P<0.05) and bcl-2 (P<0.01) were
reduced, whereas caspase-3 (P<0.01) was induced (Fig. 3C).
 | Figure 3Aurora B impacts the viability
related proteins and genes. (A) The protein levels of p-p38 MAPK,
CDK4, PCNA, Fas, caspase-8, caspase-3, Bcl-2 were performed by
western blotting. (B) Scanning intensities of western blotting are
shown. (C) The mRNA levels of cdk4, PCNA, caspase-3 and bcl-2 were
determined using RT-qPCR. Each experiment was repeated three times,
and the values are presented as the mean ± standard devidation;
*P<0.05, **P<0.01 and
***P<0.001 cf. the 0 μM values. p-p38 MAPK,
p-p38 mitogen-activated protein kinase; CDK4, cyclin-dependent
kinase 4; PCNA, proliferating cell nuclear antigen; Bcl-2, B-cell
lymphoma 2. |
The present study further demonstrated that
inactivated Aurora B exerted an effect on the p38 MAPK and FasL/Fas
pathways, causing the downregulation of p-p38 MAPK, CDK4, PCNA and
Bcl-2 and the upregulation of caspases-8 and -3, which led to an
arrest of the cell cycle at G1/S phase, decreased cell
proliferation and increased apoptosis of the granulosa cells.
Identification of SUMOylation of Aurora B
in vivo and in vitro
To investigate whether Aurora B is modified by SUMOs
in vivo, the lysates of granulosa cells were
immunoprecipitated with IGG, anti-SUMO1 and anti-SUMO2 antibodies,
and immunoblotted with Aurora B antibody (Fig. 4A). It was revealed that the band
of the Aurora B-SUMO2 complex (52 kDa) was more intense compared
with that of the Aurora B-SUMO1 complex, which was almost not
discernible; this suggested that Aurora B could be modified by
SUMO2 in vivo.
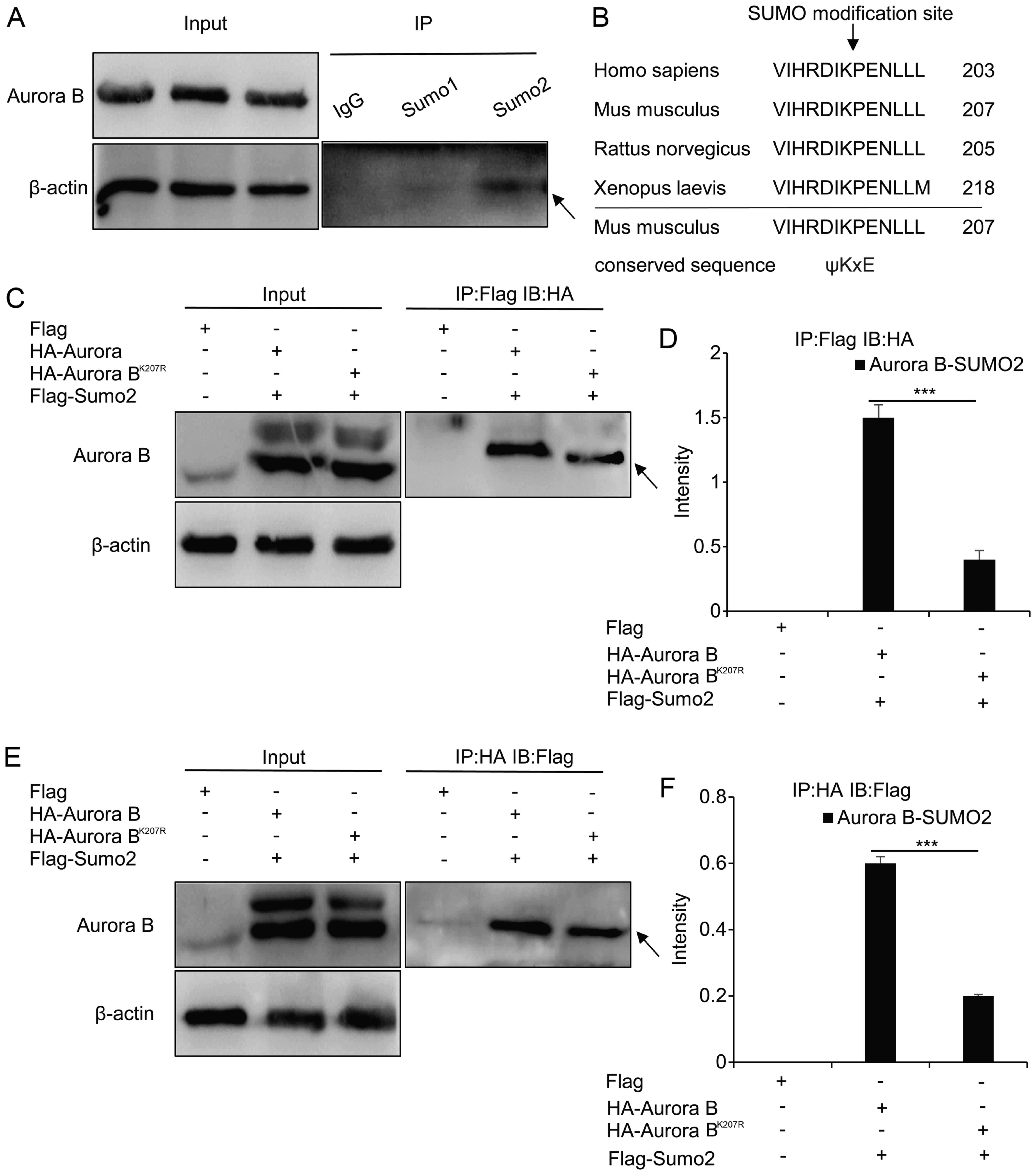 | Figure 4Identification of SUMOylation of
Aurora B in vivo and in vitro. (A) Lysates of
granulosa cells were immunoprecipitated with IGG, SUMO1 and
anti-SUMO2 antibodies, prior to immunoblotting with anti-Aurora B
antibody. The arrow represents the complex protein of Aurora
B-SUMO2. On the left, the input was immunoblotted with anti-Aurora
B antibody and anti-β-actin antibody, respectively. (B) The
SUMOylation site was predicted by SUMOsp2.0 to be at Lys-207 in
Mus musculus. (C and D) The co-transfection of HA-Aurora B
or HA-Aurora BK207R with Flag-SUMO2, followed by
immunoprecipitation with the anti-Flag antibody and immunoblotting
with the anti-HA antibody. The arrow represents the complex protein
of Aurora B-SUMO2, and the intensity of the bands was analyzed
using ImageJ software. (E and F) Co-transfection of HA-Aurora B or
HA-Aurora BK207R with Flag-SUMO2, followed by
immuno-precipitation with the anti-HA antibody and immunoblotting
with the anti-Flag antibody. The arrow represents the complex
protein of Aurora B-SUMO2, and the intensity of the bands was
analyzed using ImageJ software. Each experiment was repeated three
times, and the values are presented as the mean ± standard
deviation. ***P<0.001. SUMO1, anti-small
ubiquitin-related modifier 1; IP, immunoprecipitate; IB,
immunoblot. |
The SUMOylation site of Aurora B was also
investigated in vitro. Using SUMOsp2.0 software, data from
studies on Homo sapiens and Xenopus laevis were used
to predict that the SUMOylation site of Aurora B was located in
Lys-207 in Mus musculus (Fig.
4B). Wild HA-Aurora B or mutant HA-Aurora BK207R was
co-transfected with Flag-SUMO2 plasmids, and subsequently the cell
lysates were immunoprecipitated with the anti-Flag antibody, and
immunoblotted with anti-HA antibody. Notably, the complex protein
of Aurora B-SUMO2 was evident, whereas, although the mutant complex
protein was also visible (Fig.
4C), the band was less dense compared with the wild-type
(P<0.001) (Fig. 4D). To verify
these results, the cell lysates were immunoprecipitated with the
anti-HA antibody and immunoblotted with the anti-Flag antibody a
second time, which produced similar results (P<0.001) (Fig. 4E and F).
IP analysis confirmed that Aurora B and SUMO2
interacted in vivo and in vitro, and that Lys-207 was
the site of SUMOylation, although it may not be a unique one.
Impact of SUMOylation on the protein
stability and localization of Aurora B
The protein stability and localization of Aurora B
in the granulosa cells were examined to identify whether
SUMOylation could modulate the function of Aurora B. Granulosa
cells were co-transfected with Aurora B (2 μg) and
increasing concentrations of SUMO2 (2, 3, 4 and 5 μg) for 48
h. Western blotting revealed that the protein level of Aurora B was
increased upon increasing the concentration of SUMO2 (Fig. 5A), which reached the highest level
of significance with 4 μg SUMO2 (P<0.01) (Fig. 5B). Furthermore, it was revealed
that Aurora B was localized in the nuclei of granulosa cells
(Fig. 5C, top). However, certain
signals of Aurora BK207R were also observed in the
cytoplasm and in the nuclei, but not in the midbody, during
cytokinesis (Fig. 5C,
bottom).
These results demonstrated that SUMOylation was able
to stabilize the protein level of Aurora B, and that, in terms of
localization, Aurora B accumulated in the nuclei of granulosa
cells.
SUMOylation of Aurora B exerts an impact
on the viability of granulosa cells
It is known that SUMOylation is able to affect the
protein stability and localization of Aurora B. However, this
raises the question about whether the SUMOylation of mutant Aurora
B would have a significant impact on viability of granulosa cells.
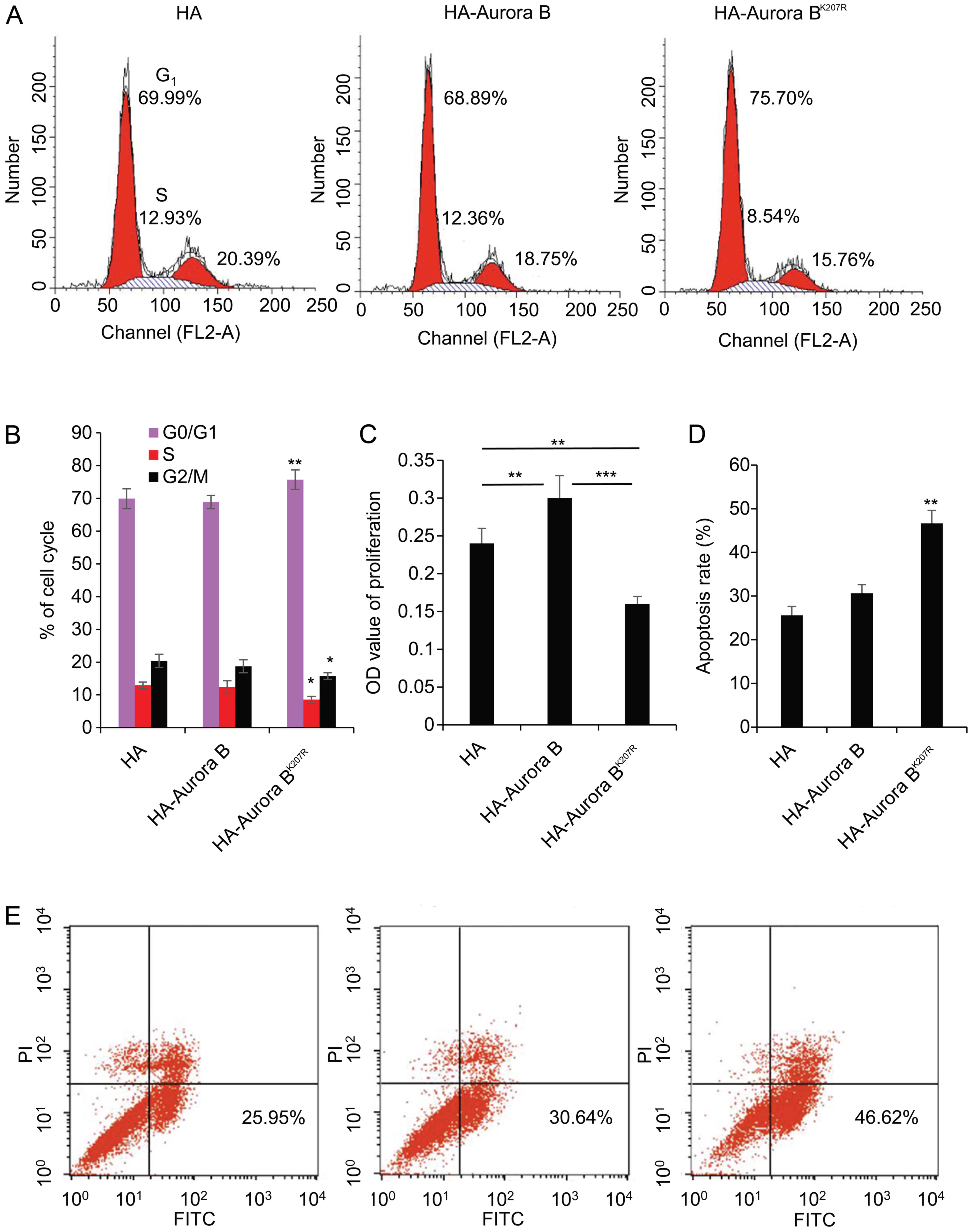
To investigate this, granulosa cells were transfected with HA,
HA-Aurora B, or HA-Aurora BK207R for 48 h, and
subsequently assayed using FACS and WST-1. As shown in Fig. 6A, the ratio of the cell cycle
increased from 66.69 to 75.70% in the G0/G1
phase, decreased from 12.93 to 8.54% in the S phase, and decreased
from 20.39 to 15.76% in the G2/M phase. These data
indicated that the ratio was significantly increased (P<0.01) in
the G0/G1 phase, and was decreased
(P<0.05) in the S and G2/M phases by the
overexpression of HA-Aurora BK207R (Fig. 6B). As expected, the proliferation
of granulosa cells was accelerated by the overexpression of
HA-Aurora B compared with HA (P<0.01), although it was
significantly suppressed by the overexpression of HA-Aurora
BK207R (P<0.001) (Fig.
6C). Similarly, apoptosis rates were significantly increased
from 25.95 to 46.62% by the overexpression of HA-Aurora
BK207R (P<0.01) (Fig.
6D and E).
These results strongly confirm that the SUMOylation
of mutant Aurora B affected the viability of granulosa cells by
arresting the cell cycle in the G1/S phase, inhibiting
proliferation, and inducing apoptosis. The results further proved
that the SUMOylation of Aurora B exerts a significant role in
follicular development through the granulosa cells.
Discussion
In the present study, following follicular
development, the protein level of Aurora B was revealed to increase
significantly until a maximum level was reached in the antral
follicle; a similar pattern of protein levels were observed in the
granulosa cells. It was inferred that Aurora B in the granulosa
cells may contribute to the development of follicles. The present
study also showed that follicular growth occurred slowly; the
atresia rate increased when follicles were supplemented with an
Aurora B inhibitor (data not shown), and the early follicular
atresia was mainly caused by the apoptosis of granulosa cells in
the follicular wall rather than of cumulus cells, oocytes or the
inner and outer theca cells (2).
These results further confirmed that Aurora B may modulate
follicular growth and atresia by mediating the proliferation and
apoptosis of granulosa cells.
Previous studies have shown that Aurora B exerts a
critical role in chromosome-microtubule interactions (27), as it loca lizes in the kinetochore
from prophase to metaphase, and relocalizes in the central spindle
and midbody during cytokinesis (28). These studies agree with our
results, which revealed that Aurora B was localized in the nucleus
and trans-localized on the midbody during cytokinesis. During the
shift from the G1 phase to the S phase, the cells must
obtain all the necessary materials to maintain a normal growth
level using the appropriate regulatory mechanism, particularly at
the restriction point, which includes members of the tumor
suppressor Rb gene family (pRb, p107 and p130), to prevent
excessive proliferation (29).
Furthermore, the CDK4/6 and cyclin D kinase activity of the complex
serves to release the transcription factor E2F, which is associated
with the S phase and maintains the cell at the checkpoint as it
shifts from the G1 phase to the S phase through the
phosphorylation of Rb (29).
Recent studies have shown that the inhibition of CDK4/6 reduced the
growth of neuroblastoma tumors in murine xenograft models (30,31). The p38 MAPK pathway is an
important signal transduction pathway, involved in the regulation
of cell inflammation, stress response, survival, differentiation,
and apoptosis (32). In the
present study, the phosphorylation level of p38 MAPK clearly
decreased, whereas the mRNA and protein levels of CDK4 and PCNA
significantly decreased, which caused the cell cycle to arrest at
the G1/S phase. Inactivated Aurora B in the granulosa
cells also reduced proliferation and induced apoptosis. These
results confirmed that Aurora B exerts an impact on the viability
of granulosa cells via the mediation of the p38 MAPK signal
pathway.
However, CDK4/6 is able to stop cell division, and
may even kill cancer cells by other hub proteins, including p53, a
stress-inducible transcription factor that induces cell cycle
arrest and apoptosis following DNA damage or cellular stress
(33-37). Studies have implied that CDK4 may
also be indirectly involved in the regulation of cell apoptosis
(34); it could also induce the
apoptosis of granulosa cells. On the other hand, the FasL/Fas
system induces apoptotic signaling in both normal and tumor cells,
whereas in normal mice, the injection of an agonistic anti-Fas
antibody induces the number of atretic follicles (38). In vitro studies revealed
that Fas mediated apoptosis in cultured granulosa cells, luteal
cells and ovarian surface epithelial cells (39-41).
To explore the impact of SUMOylation of Aurora B in
follicular development and atresia in mice, the present study,
revealed Aurora B conjugation of SUMO2, rather than SUMO1, in
vivo and in vitro, Lys-207 was discovered to be a major
SUMO2 modification site in the primary granulosa cells, although it
may not be a unique one. Recently, studies have indicated that a
considerable proportion of SUMOylated proteins do not contain the
consensus sites (21-23) and that not all the consensus
sequences are SUMOylated, as this process often occurs outside of
the consensus sequence (24);
furthermore, using software SUMOsp2.0, Lys-292/296 sites of Aurora
B were predicted with a high probability. This posed a limitation
in our study, and further studies to verify the results identified
in the primary granulosa cells are required in the future. It was
noted that SUMO2 modification modulated the function of Aurora B,
including maintenance of a higher protein level of Aurora B
following an increase in SUMO2, and an increase in the localization
of Aurora B in the nuclei of the granulosa cells.
The impact of SUMOylation of Aurora B was examined
on the granulosa cells, and the results revealed that null
SUMOylation of Aurora B (Aurora BK207R) arrested the
cell cycle at the G1/S phase, suppressed proliferation,
and promoted apoptosis. Notably, these results are consistent with
the results observed with the inhibition of Aurora B. It was
possible to deduce that D205, as a dead kinase domain of Aurora B,
would be inactivated by mutant K207R, which triggers structural
reorganization. Recently, a study revealed that the overexpression
of the SUMO-null form induced polyploidy to the same extent as the
Aurora BD205A mutant, and that it severely reduced the
number of colonies of U2OS cells (20); the two mutants, K207A and D205A,
of Aurora B promoted the level of ubiquitination (29). In addition, the facilitation of
Aurora B autophosphorylation was decreased by the inhibition of
SUMO2 conjugation by transfection with SENP2, which specifically
deconjugated SUMO from the target (6). From that study, it was clear that
Aurora BK207A decreased autophosphorylation, and
increased ubiquitination caused by the structural reorganization
that was triggered by the inactivation of the D205 kinase dead
domain, resulting in an abnormal localization, cell cycle,
proliferation and apoptosis.
Taken together, the results of the present study
suggested that Aurora B modulates follicular growth and atresia by
affecting the cell cycle of granulosa cells, cell proliferation and
apoptosis through regulation of the p-p38 MAPK and FasL/Fas
pathways, which causes the downregulation of CDK4, PCNA and Bcl-2,
and upregulation of Fas, caspase-8 and caspase-3. Aurora B may be
modified by SUMO2 rather than SUMO1 in the granulosa cells, and
SUMO2 stabilizes the protein level of Aurora B, leading to an
increase in its localization in the nucleus. Lys-207, a conserved
sequence, is a major SUMOylation site of Aurora B. The cell cycle
is arrested at the G1/S phase, proliferation is
inhibited, and apoptosis is increased through the null SUMOylation
of Aurora B.
In conclusion, the present study explored the
protein level pattern of Aurora B in follicular development, the
impact of Aurora B and its SUMOylation on follicular development
and atresia as mediated by granulosa cells, and the variety of
pathways that are involved in its regulation. Our study may provide
the theoretical basis for control measures for follicular
development and atresia, and further therapy for ovarian disease
and infertility, in humans in the future.
Acknowledgments
This work was supported by the National Key Research
and Development Program (2017yFD0501701). We are indebted to Dr Zia
Ur Rehman and Dr Rahim Dad Brohi for their important help in
revising the manuscript.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu XM, Yang FF, Yuan YF, Zhai R and Huo
LJ: SUMOylation of mouse p53b by SUMO-1 promotes its pro-apoptotic
function in ovarian granulosa cells. PLoS One. 8:e636802013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Inoue N, Matsuda F, Goto Y and Manabe N:
Role of cell-death ligand-receptor system of granulosa cells in
selective follicular atresia in porcine ovary. J Reprod Dev.
57:169–175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Porcelli L, Guida G, Quatrale AE, Cocco T,
Sidella L, Maida I, Iacobazzi RM, Ferretta A, Stolfa DA, Strippoli
S, et al: Aurora kinase B inhibition reduces the proliferation of
metastatic melanoma cells and enhances the response to
chemotherapy. J Transl Med. 13:262015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gohard FH, St-Cyr DJ, Tyers M and Earnshaw
WC: Targeting the INCENP IN-box-Aurora B interaction to inhibit CPC
activity in vivo. Open Biol. 4:1401632014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giet R and Glover DM: Drosophila Aurora B
kinase is required for histone H3 phosphorylation and condensin
recruitment during chromosome condensation and to organize the
central spindle during cytokinesis. J Cell Biol. 152:669–682. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ban R, Nishida T and Urano T: Mitotic
kinase Aurora-B is regulated by SUMO-2/3 conjugation/deconjugation
during mitosis. Genes Cells. 16:652–669. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Andrews PD, Knatko E, Moore WJ and Swedlow
JR: Mitotic mechanics: the auroras come into view. Curr Opin Cell
Biol. 15:672–683. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bishop JD and Schumacher JM:
Phosphorylation of the carboxyl terminus of inner centromere
protein (INCENP) by the Aurora B kinase stimulates Aurora B kinase
activity. J Biol Chem. 277:27577–27580. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Honda R, Körner R and Nigg EA: Exploring
the functional interactions between Aurora B, INCENP, and survivin
in mitosis. Mol Biol Cell. 14:3325–3341. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hay RT: SUMO: a history of modification.
Mol Cell. 18:1–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Geiss-Friedlander R and Melchior F:
Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol.
8:947–956. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ulrich HD: The fast-growing business of
SUMO chains. Mol Cell. 32:301–305. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klein UR, Haindl M, Nigg EA and Muller S:
RanBP2 and SENP3 function in a mitotic SUMO2/3
conjugation-deconjugation cycle on Borealin. Mol Biol Cell.
20:410–418. 2009. View Article : Google Scholar :
|
|
14
|
Makhnevych T, Sydorskyy Y, Xin X, Srikumar
T, Vizeacoumar FJ, Jeram SM, Li Z, Bahr S, Andrews BJ, Boone C, et
al: Global map of SUMO function revealed by protein-protein
interaction and genetic networks. Mol Cell. 33:124–135. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kamitani T, Nguyen HP and Yeh ET:
Preferential modification of nuclear proteins by a novel
ubiquitin-like molecule. J Biol Chem. 272:14001–14004. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sacher M, Pfander B, Hoege C and Jentsch
S: Control of Rad52 recombination activity by double-strand
break-induced SUMO modification. Nat Cell Biol. 8:1284–1290. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Desterro JM, Rodriguez MS and Hay RT:
SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation.
Mol Cell. 2:233–239. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hoege C, Pfander B, Moldovan GL,
Pyrowolakis G and Jentsch S: RAD6-dependent DNA repair is linked to
modification of PCNA by ubiquitin and SUMO. Nature. 419:135–141.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hay RT: SUMO-specific proteases: a twist
in the tail. Trends Cell Biol. 17:370–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fernández-Miranda G, Pérez de Castro I,
Carmena M, Aguirre-Portolés C, Ruchaud S, Fant X, Montoya G,
Earnshaw WC and Malumbres M: SUMOylation modulates the function of
Aurora-B kinase. J Cell Sci. 123:2823–2833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blomster HA, Hietakangas V, Wu J, Kouvonen
P, Hautaniemi S and Sistonen L: Novel proteomics strategy brings
insight into the prevalence of SUMO-2 target sites. Mol Cell
Proteomics. 8:1382–1390. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Golebiowski F, Matic I, Tatham MH, Cole C,
Yin Y, Nakamura A, Cox J, Barton GJ, Mann M and Hay RT: System-wide
changes to SUMO modifications in response to heat shock. Sci
Signal. 2:ra242009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hsiao HH, Meulmeester E, Frank BT,
Melchior F and Urlaub H: 'ChopNSpice', a mass spectrometric
approach that allows identification of endogenous small
ubiquitin-like modifier-conjugated peptides. Mol Cell Proteomics.
8:2664–2675. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Blomster HA, Imanishi SY, Siimes J, Kastu
J, Morrice NA, Eriksson JE and Sistonen L: In vivo identification
of sumoylation sites by a signature tag and cysteine-targeted
affinity purification. J Biol Chem. 285:19324–19329. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Davies EJ, Dong M, Gutekunst M, Närhi K,
Van Zoggel HJ, Blom S, Nagaraj A, Metsalu T, Oswald E,
Erkens-Schulze S, et al: Capturing complex tumour biology in vitro:
histological and molecular characterisation of precision cut
slices. Sci Rep. 5:171872015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
KJ and Schmittgen TD: Analysis of relative
gene expression data using real-time quantitative PCR and the
2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
27
|
Hauf S, Cole RW, LaTerra S, Zimmer C,
Schnapp G, Walter R, Heckel A, Van Meel J, Rieder CL and Peters JM:
The small molecule Hesperadin reveals a role for Aurora B in
correcting kinetochore-microtubule attachment and in maintaining
the spindle assembly checkpoint. J Cell Biol. 161:281–294. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carmena M and Earnshaw WC: The cellular
geography of aurora kinases. Nat Rev Mol Cell Biol. 4:842–854.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kouraklis G, Theocharis S, Vamvakas P,
Vagianos C, Glinavou A, Giaginis C and Sioka C: Cyclin D1 and Rb
protein expression and their correlation with prognosis in patients
with colon cancer. World J Surg Oncol. 4:52006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rader J, Russell MR, Hart LS, Nakazawa MS,
Belcastro LT, Martinez D, Li Y, Carpenter EL, Attiyeh EF, Diskin
SJ, et al: Dual CDK4/CDK6 inhibition induces cell-cycle arrest and
senescence in neuroblastoma. Clin Cancer Res. 19:6173–6182. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rihani A, Vandesompele J, Speleman F and
Van Maerken T: Inhibition of CDK4/6 as a novel therapeutic option
for neuroblastoma. Cancer Cell Int. 15:762015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang YN, Xiao KQ, Liu JL, Dallner G and
Guan ZZ: Effect of long term fluoride exposure on lipid composition
in rat liver. Toxicology. 146:161–169. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bukholm IK and Nesland JM: Protein
expression of p53, p21 (WAF1/CIP1), bcl-2, Bax, cyclin D1 and pRb
in human colon carcinomas. Virchows Arch. 436:224–228. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Elkady AI, Hussein RA and El-Assouli SM:
Mechanism of action of Nigella sativa on human colon cancer cells:
the suppression of AP-1 and NF-kappaB transcription factors and the
induction of cytoprotective genes. Asian Pac J Cancer Prev.
16:7943–7957. 2015. View Article : Google Scholar
|
|
35
|
Kollareddy M, Dimitrova E, Vallabhaneni
KC, Chan A, Le T, Chauhan KM, Carrero ZI, Ramakrishnan G, Watabe K,
Haupt Y, et al: Regulation of nucleotide metabolism by mutant p53
contributes to its gain-of-function activities. Nat Commun.
6:73892015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu J, Sammons MA, Donahue G, Dou Z,
Vedadi M, Getlik M, Barsyte-Lovejoy D, Al-awar R, Katona BW,
Shilatifard A, et al: Gain-of-function p53 mutants co-opt chromatin
pathways to drive cancer growth. Nature. 525:206–211. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsou SH, Hou MH, Hsu LC, Chen TM and Chen
YH: Gain-of-function p53 mutant with 21-bp deletion confers
susceptibility to multidrug resistance in MCF-7 cells. Int J Mol
Med. 37:233–242. 2016. View Article : Google Scholar
|
|
38
|
Quirk SM, Cowan RG, Joshi SG and Henrikson
KP: Fas anti- gen-mediated apoptosis in human granulosa/luteal
cells. Biol Reprod. 52:279–287. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Quirk SM, Cowan RG and Huber SH: Fas
antigen-mediated apoptosis of ovarian surface epithelial cells.
Endocrinology. 138:4558–4566. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Quirk SM, Porter DA, Huber SC and Cowan
RG: Potentiation of Fas-mediated apoptosis of murine granulosa
cells by interferon-gamma, tumor necrosis factor-alpha, and
cycloheximide. Endocrinology. 139:4860–4869. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vickers SL, Cowan RG, Harman RM, Porter DA
and Quirk SM: Expression and activity of the Fas antigen in bovine
ovarian follicle cells. Biol Reprod. 62:54–61. 2000. View Article : Google Scholar
|