Introduction
Globally and in high socio-demographic index (SDI)
countries, stomach cancer ranked fifth highest for cancer incidence
and third highest for cancer-associated mortality in 2015. In
high-middle, middle, low-middle, and low SDI countries, stomach
cancer incidence ranked third. For cancer-associated mortality in
high-middle, middle, and low SDI countries, stomach cancer ranked
as the third highest cause. For low-middle SDI countries, stomach
cancer was the second highest cause of cancer-associated mortality
(1). According to the National
Cancer Institute's Surveillance, Epidemiology, and End Results
program, ~26,370 new cases of gastric cancer were expected to be
diagnosed in 2016, with an estimated mortality rate of 10,730 and a
low survival rate of 30.4% at 5 years (2), indicating the need for novel
therapeutic approaches and an understanding of the biological
mechanisms of stomach cancer. In previous years, the treatment of
stomach cancer has focused on its surgical resection. Surgical
removal remains the optimal treatment in patients with resectable
tumors, and the overall survival (OS) range is between 5 and 90%
depending on disease staging at diagnosis (3). However, the recurrence rate of
gastric cancer is ~20-50% in all gastric resections (4). Improved prognosis has been achieved
through the use of adjuvant chemoradiotherapy and perioperative
chemotherapy. However, the majority of cases of gastric cancer are
diagnosed in an advanced and unresectable stage; therefore, the
optimal treatment for these patients includes chemotherapy, novel
target therapies and supportive care (5). Although there are a large number of
potential therapeutic targets, only a limited number are currently
known. Therefore, the development of more specifically targeted and
less toxic therapeutic methods, particularly those that target
common molecular pathways associated with disease progression and
maintenance, and those shared across a wide range of gastric cancer
types, is crucial for patients with gastric cancer.
Aurora kinases (AURKs) are a family of conserved
serine/threonine protein kinases, which are involved in several
stages of mitosis (6); three of
family members are AURKA, AURKB and AURKC. These members are
expressed in mitotic and meiotic cells (7), and AURKA localizes to the duplicate
centrosomes at the beginning of the S phase, shifts to the bipolar
spindle microtubules during mitosis, and moves to perinuclear
components of the daughter cell at the end of mitosis (8). AURKA is also important in
tumorigenesis and tumor progression. The overexpression of AURKA
has been found in several malignancies, including breast cancer,
esophageal squamous cell carcinoma and bladder cancer (9-11),
According to a previous study, the overexpression of AURKA was
significantly increased in differentiated gastric carcinoma,
suggesting that the high expression of AURKA is involved in the
development and progression of differentiated gastric carcinoma
(12).
Phosphatase and tensin homolog deleted on chromosome
10 (PTEN) is a tumor suppressor gene, which functions primarily as
a cellular lipid phosphatase (13). According to previous studies, the
overexpression of PTEN is protective against cancer (14). PTEN can dephosphorylate
phosphatidylinositol 3,4,5-trisphosphate, transforming it into
phosphatidylinositol-4,5-bisphosphate (15), and directly antagonizes the
phosphoinositide 3-kinase (PI3K)/Akt signaling pathway, which is
involved in cell survival, cell proliferation, angiogenesis and
anabolic metabolism (16).
However, PTEN has been found to be frequently mutated in various
types of human cancer (17-19). Studies have shown that alterations
of PTEN by inactivating mutations and/or chromosomal deletions
contribute to the progression of gastric cancer (20).
The present study showed for the first time, to the
best of our knowledge, that PTEN downregulated the expression of
phosphorylated (p)-AURKA and further affected the activation of
AURKA. In addition, the knockdown of the expression AURKA led to
decreased expression of p-AURKA, which further increased the
expression of PTEN, similar to the results using the AURKA
inhibitor alisertib (MLN8237). Additionally, the aberrant
expression of PTEN and AURKA induced malignant changes in the
phenotype of gastric cancer cells. The downregulation of PTEN and
use of AURKA inhibitor MLN8237 decreased the expression of p-AURKA
in MGC-803 and SGC-7901 cells. Knockdown of the expression of PTEN
led to significant changes in the expression levels of several
other important genes of the PI3K/AKT/glycogen synthase kinase 3β
(GSK3β)/β-catenin signaling pathway, which are associated with the
development of gastric cancer, including p-AKT, p-GSK3β and
β-catenin. The present study showed that p-AURKA is crucial in
gastric cancer and is the first to discuss how p-AURKA acts as a
key molecule in the activation of PTEN-associated regulation of
AURKA.
Materials and methods
Cell lines and culture
The MGC803 and SGC7901 human gastric cancer cell
lines were purchased from China Academia Sinica Cell Repository
(Shanghai, China) and cultured in RPMI-1640 medium supplemented
with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The GES-I cells were purchased from China
Academia Sinica Cell Repository (Shanghai, China) and cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.). The
cells were cultivated under in 5% CO2 at 37°C.
Antibodies and other reagents
Primary antibodies mouse anti-AURKA (cat. no.
ab13824; 1:1,000), rabbit anti-AKT1 (cat.no. ab32505; 1:5,000),
rabbit anti-p-AKT1 (s473; cat. no. ab81283; 1:5,000), rabbit
anti-GSK3β (cat. no. ab93926; 1:2,000), rabbit anti-p-GSK3β (s9;
cat. no. ab75814; 1:10,000) and rabbit anti-β-catenin (cat. no.
ab32572; 1:5,000) were purchased from Abcam (Cambridge, UK).
Primary antibodies rabbit anti-p-AURKA (Thr288; cat. no. 3079;
1:1,000), rabbit anti-PTEN (cat. no. 9188; 1:1,000) and rabbit
anti-p-β-catenin (Ser675; cat. no. 4176; 1:1,000) were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). Mouse
anti-GAPDH (cat. no. TA309157; 1:1,000) antibody was purchased from
Zhongshan Golden Bridge Biotechnology (Beijing, China). Secondary
antibodies included anti-rabbit (cat. no. ZB-2301; 1:5,000) and
anti-mouse (cat. no. ZB-2305; 1:5,000) purchased from Zhongshan
Golden Bridge Biotechnology (Beijing, China). The AURK inhibitor
alisertib was purchased from Selleck Chemicals (Houston, TX,
USA).
Immunocytochemistry
All tissue samples were gathered from the isolated
specimens of surgical patients of the Tianjin Medical University
General Hospital (Tianjin, China) between December 2016 and May
2017, and the tissue type includes the normal gastric mucosa and
the gastric carcinoma tissues. These patients are diagnosed with
gastric adenocarcinoma. The tumor tissue is from the gastric
antrum, cardia and gastric corpus. Formalin-fixed tissue samples
were prepared as paraffin-embedded sections, and immunostaining was
performed on the sections using the avidinbiotin-complex method.
The tissue sections were incubated with the indicated primary
antibodies (anti-AURKA antibody, anti-PTEN antibody, anti-AKT
antibody, anti-p-AKT antibody, anti-GSK3β antibody, anti-p-GSK3β
antibody, and anti-β-catenin antibody) overnight at 4°C. The
tissues were incubated with secondary antibodies at a dilution of
1:100 for 1 h at 37°C, washed with PBS, incubated with the
avidin-biotin complex (ABC)-peroxidase for 1 h, and washed with PBS
again. The tissues were counterstained with diaminobenzidine
buffer, hematoxylin and 0.1% TRIS and were then dehydrated in
alcohol, and visualized under an Olympus light microscope
(magnification, ×200) (Olympus Corporation, Tokyo, Japan).
Immunofluorescence analysis
Cells (3×104/500 μl/well)
transfected with si-AURKA and si-PTEN were seeded in 24-well plates
chamber for 24 h at 37°C, washed three times with PBS and fixed
with 4% paraformaldehyde in PBS. The cells were then washed three
times with PBS, followed by blocking with 5% bovine serum albumin
(BSA; Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) for 30 min. The cells were incubated with specific primary
antibodies against p-AURKA (1:1,600) overnight at 4°C, following
which the cells were washed three times with PBS and hybridized
with fluorescently conjugated secondary antibody (TRITC; 1:200) for
2 h at room temperature. The nuclei were stained with DAPI for 15
min at room temperature and visualized under an Olympus
fluorescence microscope (magnification, ×400). (Olympus
Corporation).
Transwell invasion assay
The Matrigel matrix was diluted with serum-free
(DMEM (Gibco; Thermo Fisher Scientific, Inc.) and layered into the
upper well of a Transwell chamber. Following incubation for 30 min
at 37°C, the Matrigel solidified and served as an extracellular
matrix for tumor cell invasion analysis. Cells
(5×104/500 μl/well) were seeded in the upper
chamber of the 24-well Transwell plate and incubated for 48 h at
37°C in 5% CO2. The cells were fixed with
paraformaldehyde for 15 min and stained with crystal violet for 10
sec. The chambers were detected under an Olympus microscope at 200×
magnification, using three randomly selected fields to count the
number of cells.
Cell proliferation measurement using a
CCK-8 assay
The tumor cells were transfected with si-AURKA and
si-PTEN, and seeded into 96-well plates (2,000 cells/well), with
three wells included for each group. The cells were incubated for 4
days at 37°C in an environment with 5% CO2.
Subsequently, 10 μl of CCK-8 solution was added to each
well, followed by additional incubation for 1 h. The absorbance
values were measured on a spectrophotometer at a wavelength of 450
nm.
Transient transfection
The sequences of si-AURKA and si-PTEN were designed
by Shanghai GenePharma Co., Ltd. (Shanghai, China). The targeted
sequences were as follows: Negative control, forward
5′-UUCUCCGAACGUGUCAGUTT-3′ and reverse 5′-ACGUGACACGUUCGGAGAATT-3′;
si-AURKA, forward 5′-GAAGAGAGUUAUUCAUAGAdtdt-3′ and reverse
5′-TdTCUUCUCUCAAUAAGUAUCU-3′; and si-PTEN, forward
5′-GCGUAUACAGGAACAAUATT-3′ and reverse 5′-UAUUGUUCCUGUAUACGCCTT-3′.
Prior to transfection, cells (25×104/2 ml/well) were
cultured for 24 h at 37°C in an environment containing 5%
CO2. X-tremeGENE siRNA transfection reagent (5
μl) was added with a combination of 50 μl serum-free
DMEM and 5 μl siRNA. Transfection of the MGC803 and SGC7901
cells was performed using the above prepared reagent in 6-well
plates. Following incubation for 48 h, the cells were used in
subsequent experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the prepared cells
using TRIzol reagent. The RNA (1 μg) was then reverse
transcribed into cDNA using a Promega Reverse Transcription kit
(Promega Corporation, Madison, WI, USA). The RT-PCR sample
contained 1 ng cDNA,10 μl SYBR-Green PCR Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and 2 μM
primers, and was analyzed using the ABI StepOne Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
PCR conditions were as follows: 40 cycles of 95°C for 10 min, 95°C
for 15 sec, 55°C for 40 sec. The primer sequences used were as
follows: GAPDH, forward, 5′-GGAGCCAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′; PTEN-001, forward,
5′-AAAGACTTGAAGGCGTATACAGGAA-3′ and reverse,
5′-ATGTCTTTCAGCACAAAGATTGTA-3′; PTEN-002, forward,
5′-ACATTATTGCTATGGGATTTCCTGC-3′ and reverse,
5′-ATGTCTTTCAGCCAAAGATTGTA-3′; PTEN-003, forward,
5′-TGAAGGCGTATACAGGAACAATA-3′ and reverse,
5′-ATGTCTTTCAGCACAAAGATTGTA T-3′; AURKA-001, forward,
5′-TCCCACCTTCGGCATCCTA-3′ and reverse, 5′-CGAATGACAGTAAGACAGGGC-3′,
AURKA-002, forward, 5′-TACAGTCCCACCTTCGGCAT-3′ and reverse,
5′-CAGGGCATTTGCCAATTCTG-3′; and AURKA-003, forward,
5′-TACAGTCCCACCTTCGGCA-3′ and reverse,
5′-GACAGGGCATTTGCCAATTCTG-3′. The results of PCR were analyzed
using the 2−ΔΔCT method (21).
Western blot analysis
Following incubation for 48 h post-transfection, the
MGC803 and SGC7901 cells were trypsinized and washed three times
with cold PBS, and were then lysed on ice for 30 min with RIPA
buffer. The lysate was collected and centrifuged at 12,000 × g at
4°C for 15 min. The protein concentrations were measured using a
BCA protein assay kit. A total of 20 mg proteins were separated
using 10% SDS-PAGE, followed by transfer onto polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). The
membranes were blocked for 1 h in BSA, and then incubated with the
appropriate antibodies at 4°C overnight. The primary antibodies
against PTEN, AURKA, p-AURKA, AKT1, p-AKT1, GSK3β, p-GSK3β and
β-catenin were diluted with BSA to a suitable concentration
(1:1,000). Secondary antibodies were added for incubation for 1 h
at room temperature (1:4,000 dilution).
Acquisition and analysis of the
database
The clinical features and survival data of patients
with pancreatic cancer were obtained from The Cancer Genome Atlas
(TCGA) database (http://cancergenome.nih.gov/). The cBioPortal for
Cancer Genomics provided visualization, analysis, and the ability
to download large-scale cancer genomics data sets (http://cbio-portal.org). The data necessary to analyze
PTEN and AURKA gene alterations, gene co-expression, and gene
enrichment in the cBioPortal for Cancer Genomics was obtained from
TCGA. To evaluate the association between the presence of different
genes and patient clinical outcome, the KM Plotter online tool
(http://www.kmplot.com) was used for different
gastric cancer subtypes.
Statistical analysis
GraphPad Prism 7.0 software (GraphPad Software,
Inc., La Jolla, CA, USA) was used for statistical analysis. Data in
each experimental group are presented as the mean ± standard
deviation and the difference between groups was analyzed by one-way
analysis of variance or a two-tailed Student's t-test. Kaplan-Meier
analysis was applied to analyze the effect of PTEN and AURKA on the
survival rates of patients with gastric cancer. P<0.05 was
considered to indicate a statistically significant difference.
Results
PTEN and AURKA gene alterations in 478
studies using the cBioPortal online resource
The data of 478 patients were collected from TCGA,
and the PTEN and AURKA gene alterations were analyzed in the
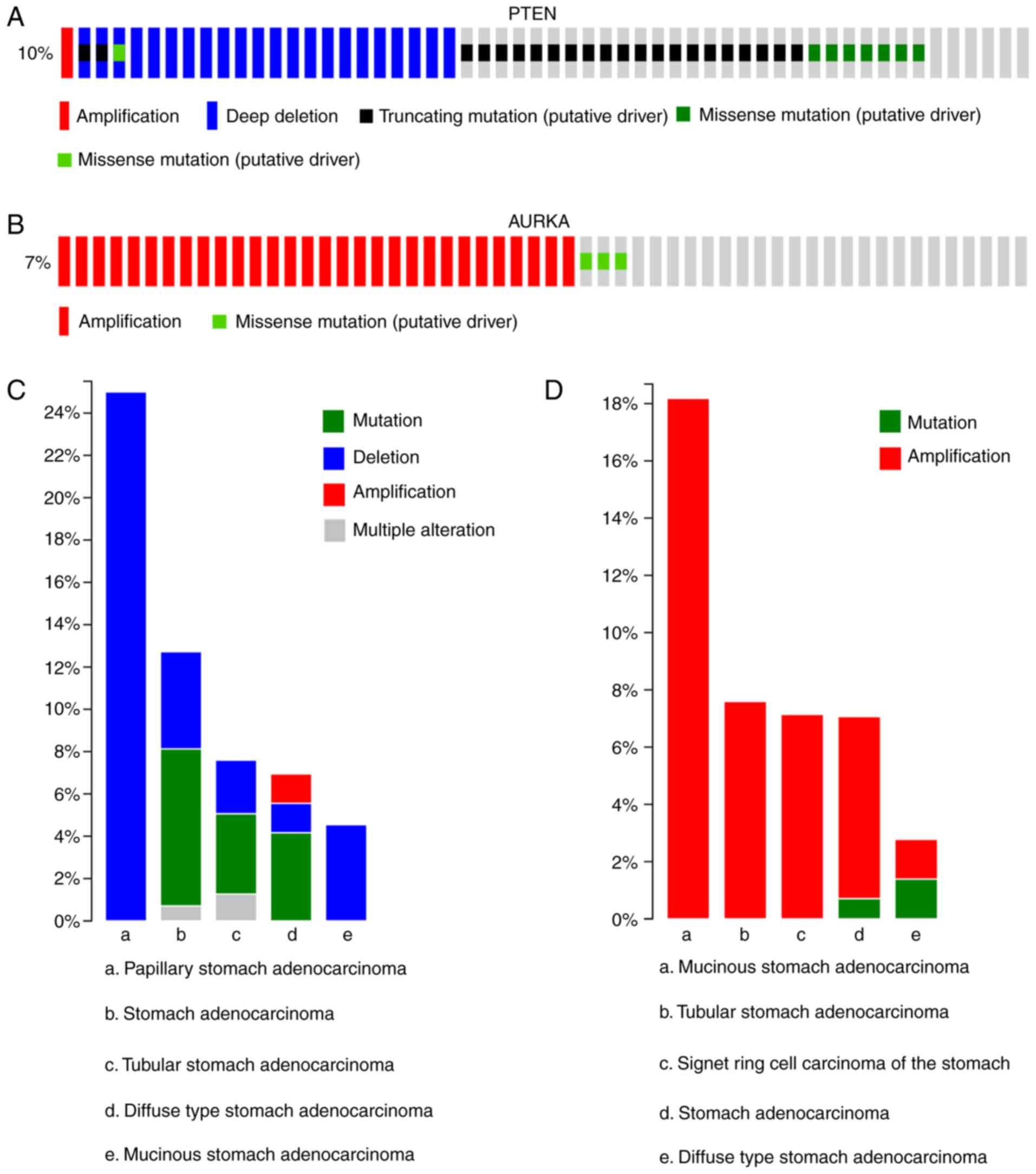
cBioPortal for Cancer Genomics. As shown in Fig. 1, PTEN alterations, including
amplifications, deep deletions, truncating mutations and missense
mutations, were observed in 50 patients and accounted for 10% of
the total mutations in all patients. In addition, two alterations
(amplification and missense mutations) of AURKA were detected and
visualized in 33 patients, accounting for 7% of the total mutations
in all patients (Fig. 1A and B).
AURKA and PTEN gene alterations have been found in mucinous stomach
adenocarcinoma, tubular adenocarcinoma of the stomach, signet ring
cell carcinoma of the stomach, stomach adenocarcinoma and
diffuse-type stomach adenocarcinoma. PTEN deletions accounted for
the majority of alterations, with the highest percentage of mutated
cases within one cancer type being 25% and the percentage of
mutated cases for all pathologies being >38%. However, there
were few PTEN amplifications in the 478 samples on examination
using cBioPortal, accounting for 1.4% of the mutated cases
(Fig. 1C). AURKA amplifications
accounted for the majority of alterations in mucinous stomach
adenocarcinoma, with 18.2% of tumors carrying this alteration
(TCGA, provisional data). Furthermore, AURKA amplification was
common in the three specific pathological types of stomach
adenocarcinoma (Fig. 1D).
PTEN and AURKA are aberrantly expressed
in gastric cancer cells
AURKA has been reported to be involved in the
formation of several types of gastrointestinal cancer (22-25). However, the mechanism of
interaction between AURKA and PTEN in gastric cancer has not been
addressed. To investigate this, the present study first examined
expression levels of PTEN and AURKA through RT-qPCR, western blot
and immunocytochemical analyses in gastric carcinoma and normal
mucosa samples. The immunocytochemistry showed that the expression
of AURKA in gastric carcinoma was higher, compared with that in
normal gastric mucosa, whereas the expression of PTEN in the normal
gastric mucosa was higher, compared with that in gastric carcinoma
(Fig. 2A). Furthermore, The
immunocytochemistry reveals that the expression levels of p-AKT,
p-GSK3β, AKT and β-catenin in normal gastric mucosa and gastric
carcinoma also differed (Fig.
2A). In addition, it was found that the expression of AURKA was
elevated in the MGC803 and SGC7901 cells at the mRNA and protein
levels, compared with the normal GES-I cells (P<0.05; Fig. 2B-D), indicating that AURKA may be
a key molecule in the pathogenesis of gastric cancer, as in other
malignant tumors.
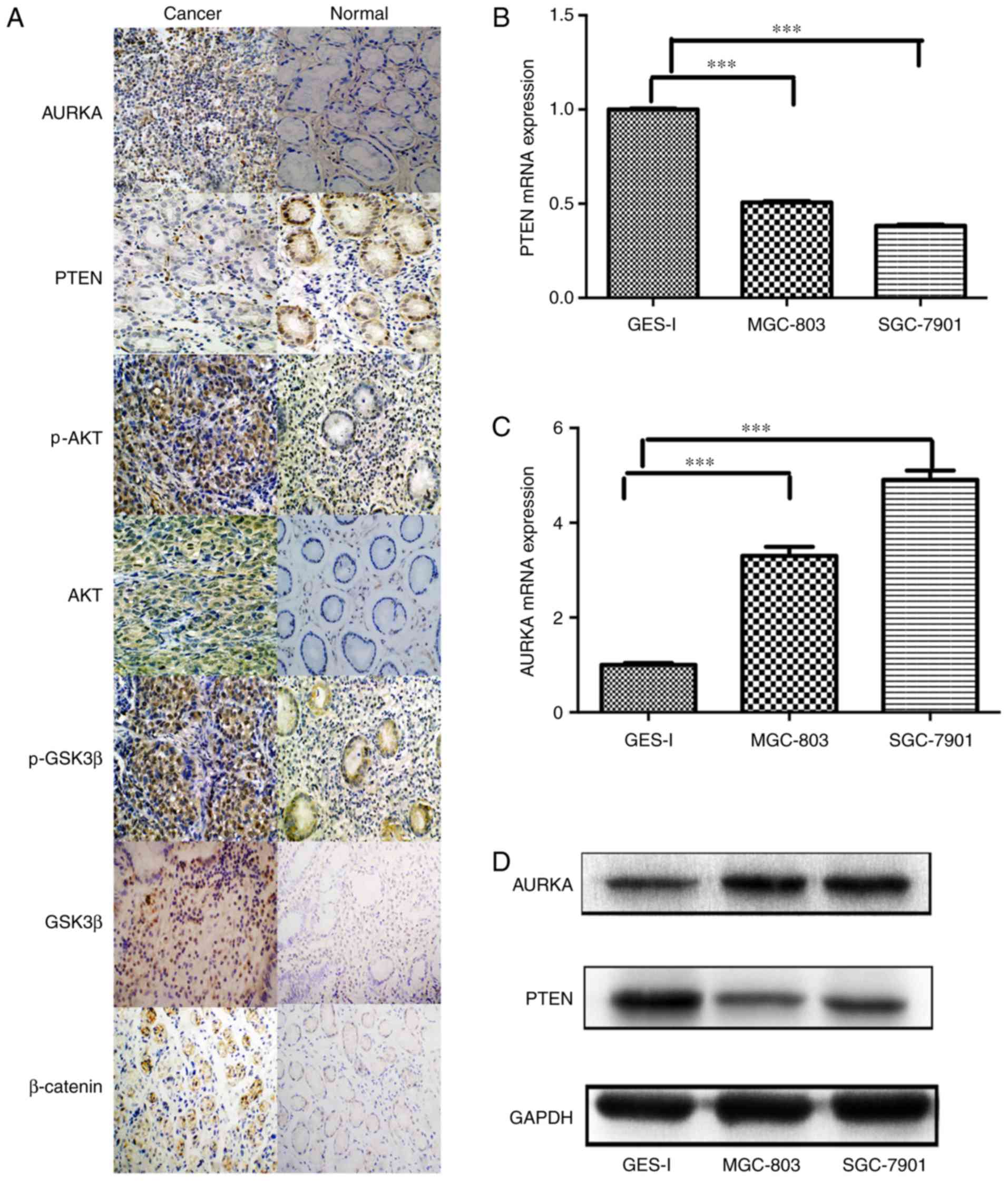 | Figure 2PTEN and AURKA are overexpressed in
gastric cancer cell lines. (A) Immunocytochemical analysis of the
expression of PTEN, AURKA, p-AKT, AKT, p-GSK3β, GSK3β and β-catenin
in patients with gastric cancer (magnification, ×200). Analysis of
expression levels of (B) PTEN and (C) AURKA in MGC803 and SGC7901
cells, determined using reverse transcription-quantitative
polymerase chain reaction analysis (***P<0.001). (D)
Western blot analysis of protein expression of PTEN and AURKA in
MGC803 and SGC7901 cells. PTEN, phosphatase and tensin homolog
deleted on chromosome 10; AURKA, aurora kinase A; GSK3β, glycogen
synthase kinase 3β; p-, phosphorylated. |
Studies have shown that PTEN and AURKA share certain
common signaling pathways in malignant tumors (26-31). Therefore, the present study
analyzed the expression of PTEN in MGC803 and SGC7901 cells. A
decreased mRNA level of PTEN was observed, and the opposite mRNA
expression pattern was observed for AURKA, as determined by RT-qPCR
analysis (P<0.05; Fig. 2B and
C). The protein levels of PTEN were also decreased in the
MGC803 and SGC7901 cells (Fig.
2D), suggesting a potential functional association between PTEN
and AURKA. Therefore, MGC803 and SGC7901 cells were used in
subsequent loss- and gain-of-function experiments to further
investigate the correlation between PTEN and AURKA.
PTEN and AURKA maintain malignant
phenotypes and predict survival rate in gastric cancer
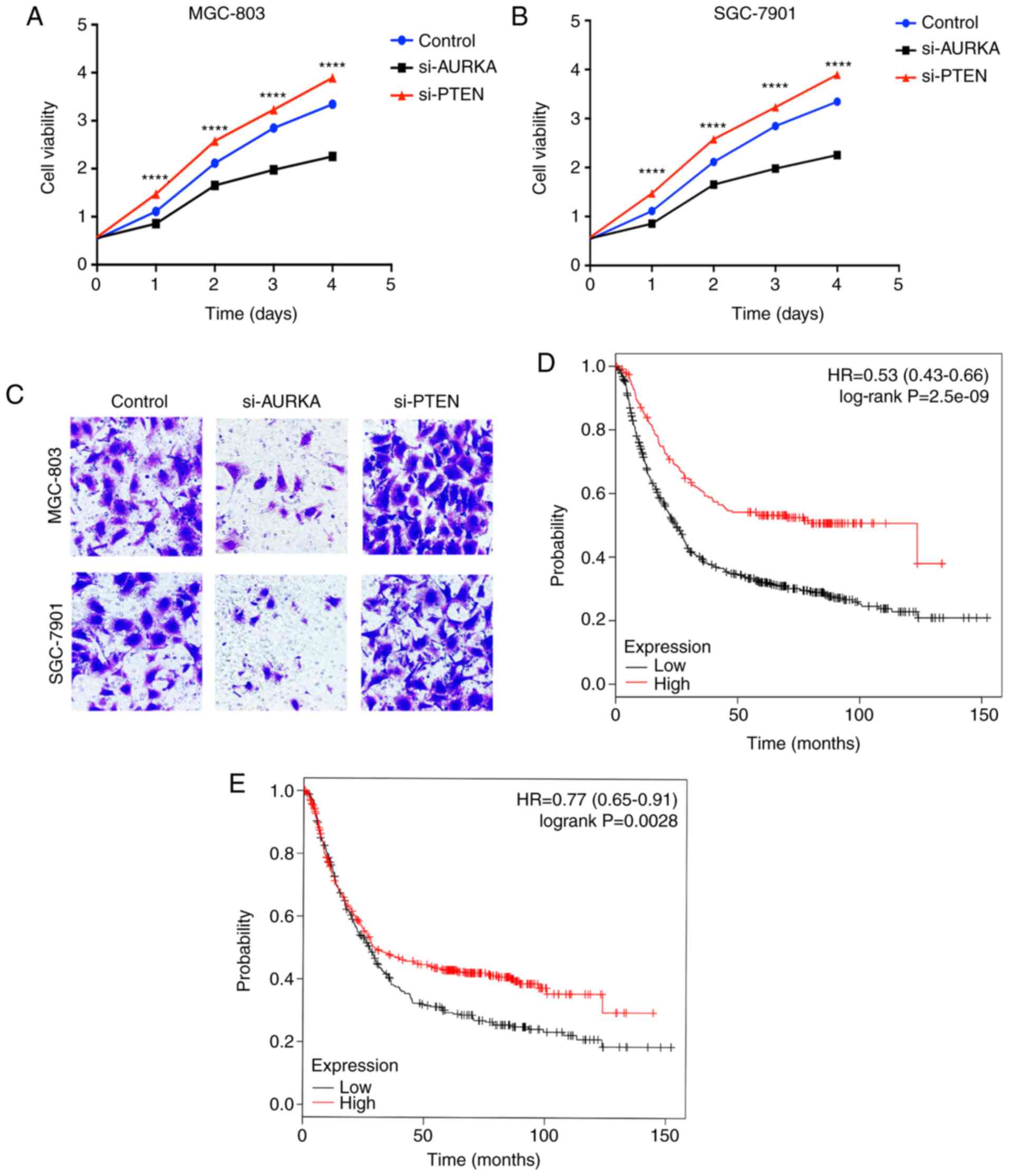
To examine the effect of PTEN and AURKA on the
malignant phenotype of gastric cancer cells, MGC803 and SGC7901
cells were transfected with PTEN siRNA or AURKA siRNA, and the
cells were monitored for malignant phenotype changes. As shown in
Fig. 3A and B, compared with the
control vector-transfected cells, transient transfection with
si-PTEN led to a marked increase in proliferation in the two
gastric cancer cell lines, whereas the knockdown of AURKA resulted
in decreased proliferation, compared with that in the untreated
group. In addition, the Transwell invasion assays showed that the
reduction in the expression of PTEN following transfection with
si-PTEN led to an increase in the invasion capacity of MGC803 and
SGC7901 cells, whereas the knockdown of AURKA led to significant
changes in the invasion capacity of the two cell types (Fig. 3C).
Although the overall incidence of gastric cancer has
been decreasing, it remains one of the most common types of
malignancy and is the leading cause of cancer-associated mortality
worldwide. To investigate whether the gene expression of PTEN and
AURKA can predict survival rates of patients with gastric cancer,
the present study analyzed the gene expression of PTEN and AURKA in
patients with gastric cancer using the KM Plotter. The KM Plotter
is a public database containing information from 1,065 patients
with gastric cancer to permit the investigation of gene
associations with OS. The samples were segregated into high and low
expression groups. It was observed that patients with a high
expression of PTEN were predicted to have improved OS (log-rank
P=2.5e-09). The median survival time of the PTEN high-expressing
group was 123.6 months, whereas that of the low expression group
was 24.9 months (Fig. 3D).
However, patients with gastric cancer with a high expression of
AURKA had poor OS, compared with patients with a low expression of
AURKA (log-rank P=0.0028). The median survival time of the AURKA
high-expressing group was 29.5 months, and that of the
low-expressing group was 27.4 months (Fig. 3E). These results revealed that
PTEN and AURKA are crucial in the maintenance of malignant
phenotypes in gastric cancer cells. The expression levels of PTEN
and AURKA can determine the clinical progression and outcome of
patients with gastric cancer.
Functional link between AURKA and PTEN in
gastric cancer
In the present study, data were collected from TCGA
to confirm the connection between PTEN and AURKA by using
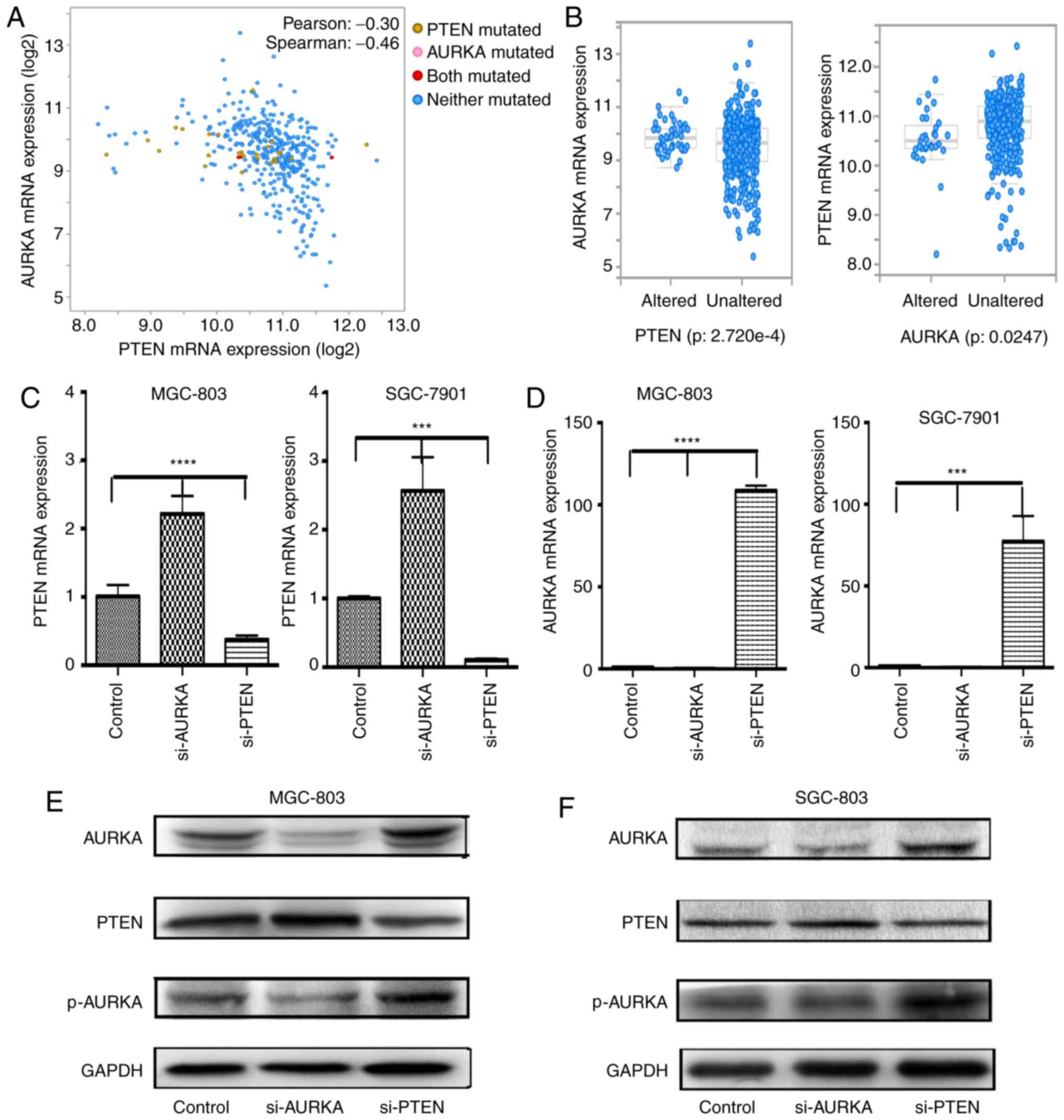
cBioPortal. Pearson's correlation analysis was performed to
investigate the correlations between the expression of PTEN and
AURKA. The results revealed that the expression of PTEN was
significantly negatively correlated with the expression of AURKA
(Fig. 4A). It was also observed
that the mRNA expression of PTEN was enriched in the
AURKA-unaltered group, and that the mRNA expression of AURKA was
enriched in the PTEN-altered group (Fig. 4B).
The outcomes of the above experiments on the
expression of PTEN and AURKA in gastric cancer cell lines also
suggested a close association between the overexpression of PTEN
and AURKA in gastric cancer cells. To further examine the
interaction mechanism of PTEN and AURKA, the present study analyzed
the gene and protein expression levels of PTEN and AURKA in MGC803
and SGC7901 cells following transfection by si-PTEN and si-AURKA.
As shown in Fig. 4C and D, the
results showed that the expression of AURKA was markedly higher in
the si-PTEN-treated cells than that in control cells at the mRNA
level in the gastric cancer cell lines (P<0.0001). By contrast,
compared with the control vector-transfected gastric cancer cells,
the expression of PTEN in the si-AURKA-transfected cells was
increased (P<0.0001). In addition, transient transfection with
si-AURKA led to an increase in the expression of PTEN at the
protein level. It was also found that a reduction in PTEN resulted
in the overexpression of AURKA in MGC803 and SGC7901 cells
(Fig. 4E and F). These results
supported the hypothesis that PTEN may regulate the expression or
function of AURKA, and that changes in the expression or function
of AURKA, in turn, may affect the activity of PTEN.
PTEN mediates the AURKA-associated
maintenance of a malignant state by activating p-AURKA
Previous studies have shown that the
autophosphorylation of AURKA is a key regulatory mechanism in
centrosomes in the early stages of mitosis, and that the
autophosphorylation of p-AURKA creates an activation loop, which
increases the catalytic activity of AURKA (32). The present study hypothesized that
PTEN can inhibit the activation of AURKA by suppressing the
formation of p-AURKA. Therefore, a potential regulatory association
among these three proteins was investigated. First, the protein
expression of p-AURKA was investigated in conditions of
siRNA-induced knockdown of PTEN and knockdown of AURKA in MGC803
and SGC7901 cells. The knockdown of PTEN caused a marked elevation
in expression levels of p-AURKA and AURKA in the gastric cancer
cell lines. When AURKA was inhibited by transient siRNA
transfection in the MGC803 and SGC7901 cells, the protein level of
p-AURKA decreased (Fig. 4E and
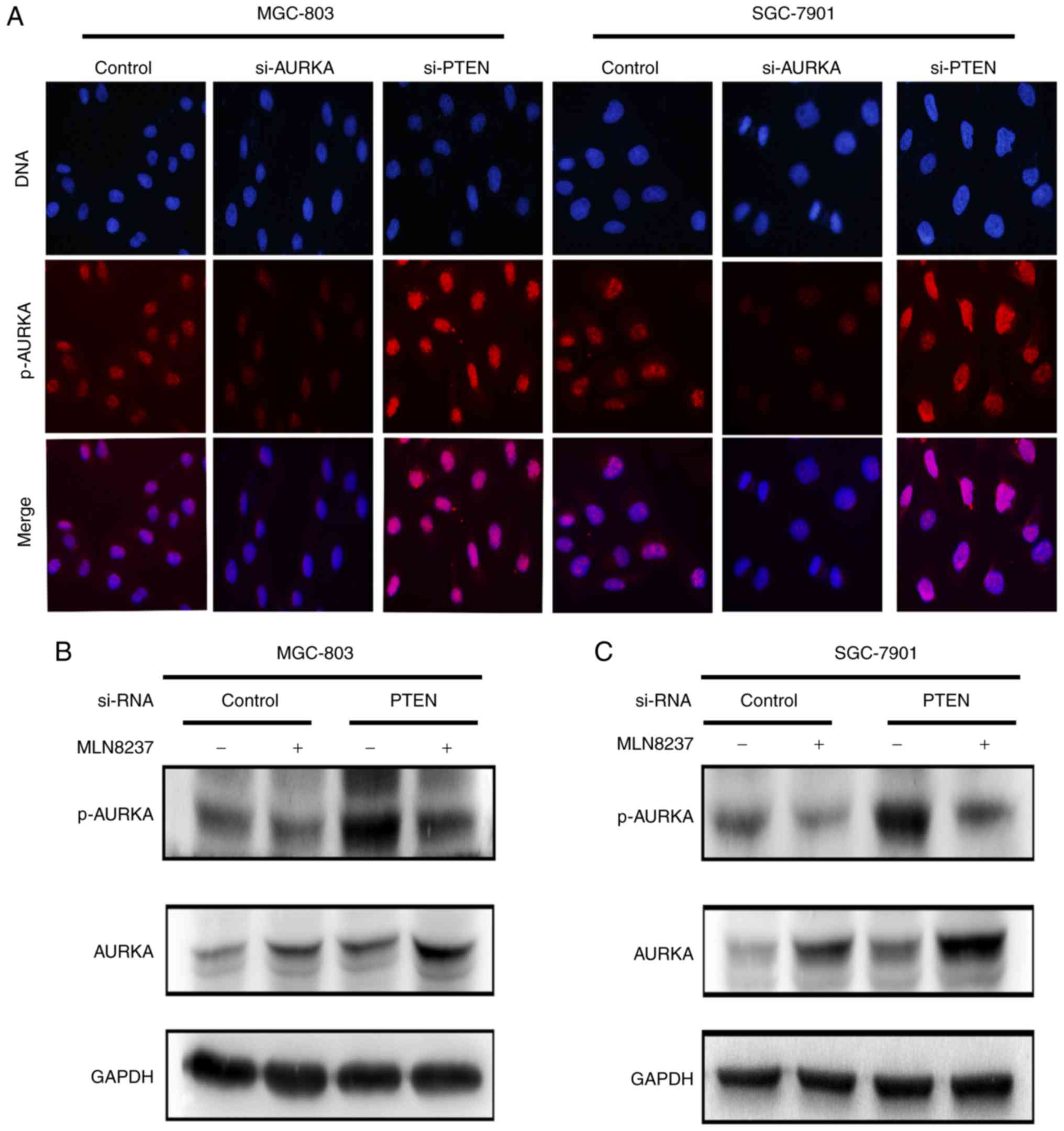
F). The immunofluorescence analysis suggested that si-PTEN
treatment in the MGC803 and SGC7901 cell lines resulted in the
increased expression of p-AURKA in the nucleus, whereas the
expression level of p-AURKA in the nucleus decreased when the
gastric cancer cells were treated with si-AURKA (Fig. 5A). Based on these observations, it
was hypothesized that p-AURKA is a downstream target of PTEN. Of
note, these outcomes also demonstrated a novel role of p-AURKA in
mediating the PTEN-induced activation of AURKA.
To confirm the above hypothesis, a double variable
experiment was performed to analyze the effect of PTEN on the
expression of p-AURKA and thus the activity of AURKA. Alisertib
(MLN8237) is known to selectively inhibit AURKA, markedly inhibit
the phosphorylation of AURKA, and lead to a reduction in the level
of p-AURKA; however, in the present study, there was a significant
increase in the level of AURKA when treated with ALS (33). As shown in Fig. 5B and C, the MGC803 and SGC7901
cells were treated with either MLN8237 or si-PTEN, or with si-PTEN
and MLN8237 (10 μM), and the protein expression levels of
p-AURKA and AURKA were examined in these different conditions. It
was observed that the expression of AURKA increased in cells
treated with MLN8237 and with si-PTEN transfection plus MLN8237 (10
μM) treatment. The expression of AURKA increased more
markedly in cells transfected with si-PTEN plus MLN8237 (10
μM) treatment, compared with the other groups. However,
under conditions of si-PTEN transfection plus MLN8237 treatment,
the opposite effect was observed on the protein levels of p-AURKA
(Fig. 5B and C) in the MGC803 and
SGC7901 cells. In addition, the expression of p-AURKA was increased
in gastric cancer cells transfected with si-PTEN only, compared
with the cells transfected with si-PTEN and treated with MLN8237.
These results confirmed that the upregulation of p-AURKA by the
downregulation of PTEN resulted in the overexpression of AURKA.
Suppression of PTEN affects several
signal pathways involved in the development of gastric cancer
According to the present study, PTEN inhibited
tumorigenesis by interacting with genes or modulating multiple
signal transduction pathways (34-36). To further examine the effects of
the reduced expression of PTEN on other intracellular signaling
pathways in gastric cancer cells, the expression of key molecules
in the signaling pathways of MGC803 and SGC7901 cells were
investigated. The MGC803 and SGC7901 cells were transfected with
si-PTEN or a control DNA vector, and then analyzed for protein
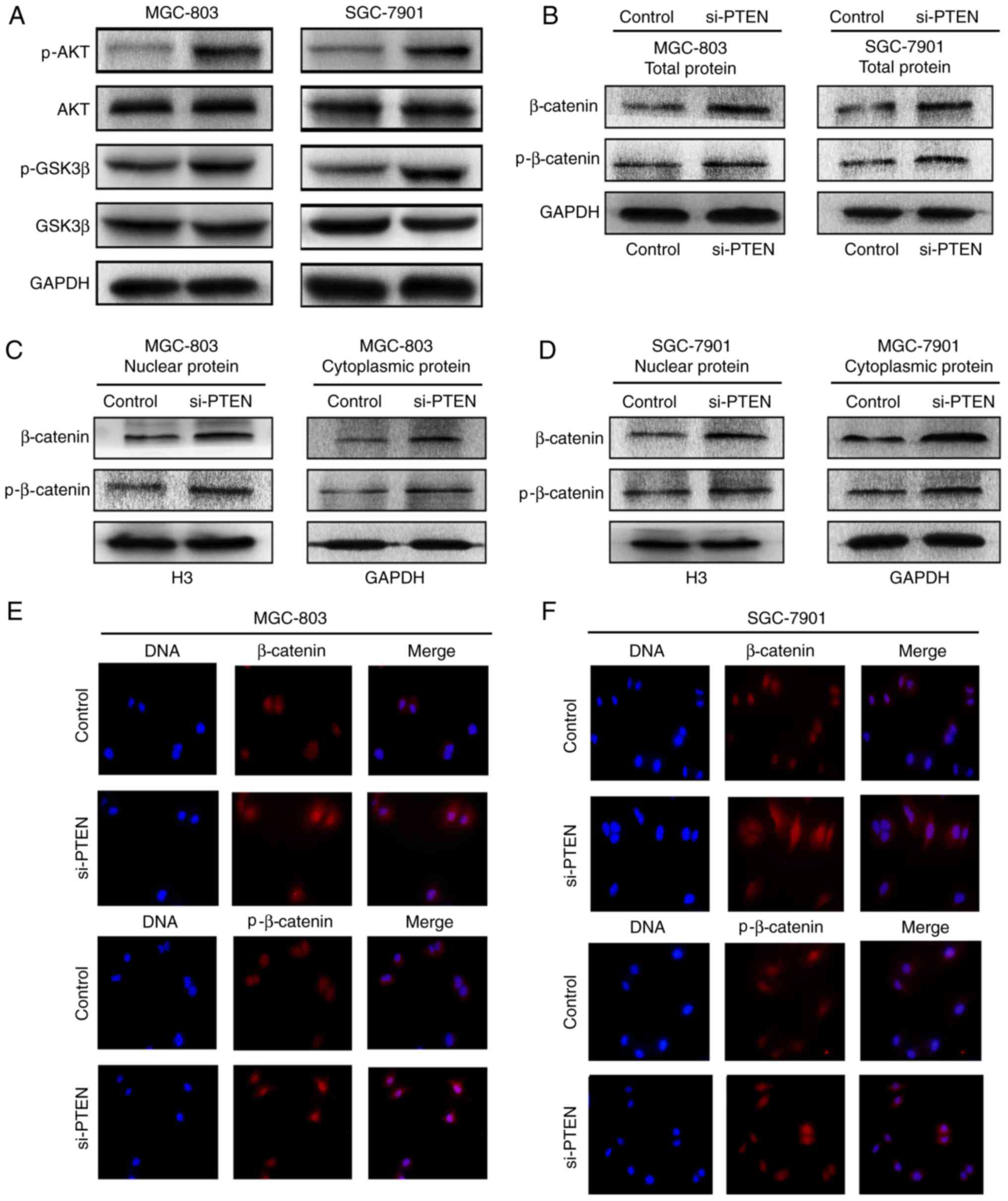
levels of p-AKT, p-GSK3β, AKT, GSK3β and β-catenin. The knockdown
of PTEN by si-PTEN transfection resulted in increases in the
expression of p-AKT and p-GSK3β, compared with that in the control
cells, whereas the levels of AKT and GSKβ did not alter markedly
(Fig. 6A). Furthermore, gastric
cancer cells showed increased total protein expression levels of
β-catenin and p-β-catenin (Ser675) following transfection with
si-PTEN (Fig. 6B). The cellular
distributions of β-catenin and p-β-catenin (Ser675) were also
detected in the gastric cancer cells with downregulated PTEN. The
downregulation of PTEN led to increases in the protein levels of
β-catenin and p-β-catenin (Ser675) in the nucleus and cytoplasm of
gastric cancer cells (Fig. 6C and
D). The immunofluorescence results also revealed that the
expression levels of β-catenin and p-β-catenin (Ser675) in the
nucleus and cytoplasm were increased following PTEN knockdown
(Fig. 6E and F). These results
showed that the knockdown of PTEN resulted in increased expression
of certain target, intracellular signaling pathway-associated
molecules to promote the development of gastric cancer.
Discussion
Due to the lack of typical symptoms, patients are
often diagnosed at an advanced stage of gastric cancer. Although
there have been increases in the efficiency of early diagnosis and
in the appearance of novel effective chemotherapeutic regimens, the
5-year-survival rate of advanced gastric cancer remains low at
<20%), and the majority of patients with advanced-stage or
metastatic disease only survive <1 year (37). The poor prognosis of patients with
gastric cancer indicates that current comprehension of the
molecular mechanisms involved in gastric carcinogenesis is lacking.
The present study revealed a novel molecular mechanism by which
aberrant expression of p-AURKA in gastric cancer cells was crucial
in the progression of gastric cancer through affecting the
PTEN-regulated activity of AURKA. Therefore, p-AURKA represents a
drug target warranting further investigation for gastric cancer. In
addition, the present study found that knockdown of the expression
of PTEN resulted in the upregulation of protein expression levels
of p-AKT and p-GSK3β, and an increase in protein levels of
β-catenin, which may enhance the carcinogenic properties of gastric
cancer cells.
p-AURKA is the product of autophosphorylation of
AURKA on the T288 residue in the activation or T-loop, and a number
of proteins, including TPX2, NEDD9 and Ajuba, directly associate
with AURKA to regulate this process (38). p-AURKA is important in mitosis,
phosphorylating BRCA1 protein to reduce G2/M checkpoint controls
(39) and phosphorylating the RAS
family protein RALA to regulate mitochondrial fusion, which is
critical for the equal post-mitotic segregation of mitochondria
between daughter cells (40).
AURKA can be autophosphorylated into p-AURKA to promote the
progression of gastric cancer. A previous study showed that AURKA
directly interacts with important oncogenes and tumor suppressor
genes; it phosphorylates Src, stabilizes N-myc, and phosphorylates
and downregulates the major tumor suppressor p53 (38). However, the mechanism by which
PTEN regulates AURKA remains to be fully elucidated. The present
study aimed to examine the regulatory mechanism of PTEN on AURKA.
Data were collected from TCGA, and the common PTEN and AURKA gene
alterations were analyzed in the cBioPortal for Cancer Genomics,
which revealed that PTEN and AURKA had various types of alterations
in gastric cancer (Fig. 1). In
addition, the expression of PTEN and AURKA were determined in
normal gastric mucosa and gastric carcinoma tissues, and their
expression in MGC-803, SGC-7901 and GES-I cells were determined at
the mRNA and protein levels (Fig.
2). Subsequently, gastric cancer cells were transfected by
control vector, si-AURKA and si-PTEN, and it was found that PTEN
knockdown using a PTEN siRNA expression vector in MGC-803 and
SGC-7901 cells resulted in a significant increase in cell
proliferation (Fig. 3A and B).
Transwell assays also showed an increase in invasion of
si-PTEN-transfected cells and a decrease in invasion of
si-AURKA-transfected cells (Fig.
3C). In addition, a high expression of PTEN predicted improved
outcomes in patients with gastric cancer, whereas patients with
gastric cancer with a high expression of AURKA had poor OS
(Fig. 3D and E). Therefore, these
results indicated that there is a close association between PTEN
and AURKA in carcinogenesis and in the maintenance of malignant
phenotypes in gastric cancer cells. In addition, KaplanMeyer
analysis indicated that the mRNA expression of PTEN and AURKA may
affect the prognosis of stomach adenocarcinoma.
Relational investigations have shown that
overexpression of Aurora-A promotes the protein expression of
nuclear inhibitor of nuclear factor (NF)-κB and enhances the
activity of NF-κB, thus promoting the transcription of microRNA-21,
which negatively regulates PTEN (41). According to a previous study in
mouse embryonic fibroblasts and mouse keratinocyte cell lines,
Fbxw7 and PTEN tumor suppressor pathways, which control the levels
of the oncoprotein AURKA, and the loss of PTEN attenuates the
degradation of Aurora-A by Fbxw7 through the AKT/GSK3β pathway
(42). In the present study, the
data of 478 patients from TCGA indicated that the expression of
PTEN was significantly negatively correlated with the expression of
AURKA (Fig. 4A). These results
showed that AURKA and PTEN interacted with each other. However,
there are no reports on the way in which PTEN affects the
expression of AURKA by affecting p-AURKA, which is important for
the development of gastric cancer. In the present study, it was
observed that AURKA was overexpressed in gastric carcinoma and
gastric cancer cell lines at the mRNA and protein levels (Fig. 2). Experiments showed that a low
expression of PTEN resulted in a significant decrease in the mRNA
and protein expression of AURKA in MGC-803 and SGC-7901 cells
(Fig 4C-F). In addition, PTNE
knockdown led to elevation in the expression levels of p-AURKA and
AURKA in gastric cancer cell lines, whereas AURKA knockdown lead to
a reduction in the expression of p-AURKA (Fig. 4E and F). si-PTEN transfection also
led to an increase in p-AURKA in the nucleus of MGC803 and SGC7901
cell lines (Fig. 5A). These
results suggested that p-AURKA may be one of the downstream targets
of PTEN, and the role of p-AURKA in mediating the regulation of
AURKA by PTEN requires further clarification. PTEN may downregulate
the formation of p-AURKA to affect the function of AURKA. As shown
in Fig. 5B and C, PTEN knockdown
with MLN8237 treatment led to an increase in the protein levels of
AURKA in MGC-803 and SGC-7901 cells, whereas p-AURKA increased more
markedly in cells transfected with si-PTEN than in other groups.
These results not only further confirmed that p-AURKA is a
downstream regulator of PTEN, but also confirmed the close
association between PTEN and AURKA.
Based on the above findings, it was confirmed that
p-AURKA is important in mediating the PTEN-associated regulation of
AURKA, thus indicating its involvement in carcinogenesis and in
maintaining the malignant phenotypes of gastric cancer cells. When
p-AURKA was inhibited in MGC-803 and SGC-7901 cells by transient
transfection with si-AURKA, the protein level of PTEN increased and
malignant phenotypes, including increased cell proliferation and
invasion, were observed in the gastric cancer cells. Transfection
of the gastric cancer cells with si-PTEN led to the increased
expression levels of AURKA and p-AURKA, and the overexpression of
AURKA has been shown result in the chemoresistance of several
malignant tumor cells (43-45) with poorer patient outcomes. These
results suggested that p-AURKA induced the malignant phenotype of
tumor cells, which revealed that p-AURKA may be a novel target of
anti-tumor drugs. In addition, it was found that the knockdown of
PTEN affected several signaling pathways to promote the development
of gastric cancer.
Although the incidence and mortality rates of
gastric cancer have been in gradual decline globally over the last
30 years, gastric cancer remains a major threat to the health of
patients across the world. In previous years, several methods have
emerged for the treatment of gastric cancer, including radical
surgery, radiotherapy and chemoradiotherapy, and novel technologies
have emerged, including laparoscopic gastrectomy (46) and robotic gastrectomy (47). Unlike the favorable prognosis of
early gastric cancer, a substantial number patients with advanced
gastric cancer experience tumor recurrence during their lifetime,
even following radical surgery. Therefore, additional strategies
are required to improve the survival rate of patients with advanced
gastric cancer. However, numerous trials have failed to show an
added benefit of chemotherapy to surgery (48). Combination chemotherapeutic
regimens produce higher response rates and longer survival rates,
compared with single agents, however, therapeutic options remain
limited, and the overall prognosis remains poor (5). Based on these findings, as patients
with gastric cancer have a high mortality rate, novel therapeutic
strategies, useful biomarkers and personalized treatments are
required to improve the outcomes of patients with gastric cancer.
Therefore, patients with gastric cancer require DNA testing to
screen out those with a high expression of AURKA. According to the
present study, PTEN downregulated the expression of p-AURKA and
further affected the activation of AURKA, and p-AURKA induced the
malignant phenotype of tumor cells. A combination of PTEN- and
AURKA-targeted therapies may be used for patients who benefit from
specific targeted treatments and improve the survival rate of
patients. Although several candidate biomarkers and therapeutic
targets have been reported, few are used in clinical practice.
Therefore, in the future, improvements in next-generation
sequencing technology are required to improve the therapy and
prognosis of patients with gastric cancer.
In conclusion, the present study presented the novel
finding that PTEN inhibited the expression of AURKA by suppressing
the activity of p-AURKA and thus regulated the malignant phenotype
of gastric cancer cells. These results provide a novel approach, in
which the combined targeting of PTEN and AURKA may potentially
serve as a therapeutic treatment for gastric cancer, which may
enable more accurate gastric cancer cell death that spares normal
cells.
Acknowledgments
The authors would like to express their gratitude to
everyone in the Laboratory of Neuro-Oncology, Tianjin Neurological
Institute (Tianjin, China) for their technical assistance.
Notes
[1]
Funding
This study was supported by grants from the National
High Technology Research and Development Program 863 (grant nos.
2014AA021102 and 2016YFC0902502) and from the China National
Natural Scientific Fund (grant nos. 81372703 and 81172356).
[2] Availability
of data and materials
The datasets generated and analyzed during the
current study are available in the cBioPortal for Cancer Genomics
and the KM Plotter online repositories, (http://cbioportal.org, and http://www.kmplot.com).
[3] Authors'
contributions
QL, XL, RW contributed to the conception of the
study. YS contributed significantly to analysis and manuscript
preparation. LL performed the data analyses and wrote the
manuscript. CK and QZ helped perform the analysis with constructive
discussions.
[4] Ethics
approval and consent to participate
The present study was approved by the Ethics
Committee of Tianjin Medical University General Hospital (Tianjin,
China). All subjects provided written informed consent prior to
enrollment.
[5] Consent for
publication
All patients whose tissue samples were used gave
written informed consent for publication of this study.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Global Burden of Disease Cancer
Collaboration; Fitzmaurice C, Allen C, Barber RM, Barregard L,
Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R,
Dandona L, et al: Global, regional, and national cancer incidence,
mortality, years of life lost, years lived with disability, and
disability-adjusted life-years for 32 cancer groups, 1990 to 2015:
A systematic analysis for the global burden of disease study. JAMA
Oncol. 3:524–548. 2017. View Article : Google Scholar
|
|
2
|
SEER Cancer Statistics Factsheets: Stomach
Cancer. National Cancer Institute; 2016
|
|
3
|
Arrington AK, Nelson R, Patel SS, Luu C,
Ko M, Garcia-Aguilar J and Kim J: Timing of chemotherapy and
survival in patients with resectable gastric adenocarcinoma. World
J Gastrointest Surg. 5:321–328. 2013. View Article : Google Scholar
|
|
4
|
Sakar B, Karagol H, Gumus M, Basaran M,
Kaytan E, Argon A, Ustuner Z, Bavbek SE, Bugra D and Aykan FN:
Timing of death from tumor recurrence after curative gastrectomy
for gastric cancer. Am J Clin Oncol. 27:205–209. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wagner AD, Unverzagt S, Grothe W, Kleber
G, Grothey A, Haerting J and Fleig WE: Chemotherapy for advanced
gastric cancer. Cochrane Database Syst Rev. Mar 17–2010.Epub ahead
of print. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Glover DM, Leibowitz MH, McLean DA and
Parry H: Mutations in aurora prevent centrosome separation leading
to the formation of monopolar spindles. Cell. 81:95–105. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nguyen AL and Schindler K: Specialize and
divide (twice): Functions of three aurora kinase homologs in
mammalian oocyte meiotic maturation. Trends Genet. 33:349–363.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sugimoto K, Urano T, Zushi H, Inoue K,
Tasaka H, Tachibana M and Dotsu M: Molecular dynamics of Aurora-A
kinase in living mitotic cells simultaneously visualized with
histone H3 and nuclear membrane protein importinalpha. Cell Struct
Funct. 27:457–467. 2002. View Article : Google Scholar
|
|
9
|
Sen S, Zhou H, Zhang RD, Yoon DS,
Vakar-Lopez F, Ito S, Jiang F, Johnston D, Grossman HB, Ruifrok AC,
et al: Amplification/overexpression of a mitotic kinase gene in
human bladder cancer. J Natl Cancer Inst. 94:1320–1329. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Staff S, Isola J, Jumppanen M and Tanner
M: Aurora-A gene is frequently amplified in basal-like breast
cancer. Oncol Rep. 23:307–312. 2010.PubMed/NCBI
|
|
11
|
Yang SB, Zhou XB, Zhu HX, Quan LP, Bai JF,
He J, Gao YN, Cheng SJ and Xu NZ: Amplification and overexpression
of Aurora-A in esophageal squamous cell carcinoma. Oncol Rep.
17:1083–1088. 2007.PubMed/NCBI
|
|
12
|
Kamada K, Yamada Y, Hirao T, Fujimoto H,
Takahama Y, Ueno M, Takayama T, Naito A, Hirao S and Nakajima Y:
Amplification/overexpression of Aurora-A in human gastric
carcinoma: Potential role in differentiated type gastric
carcinogenesis. Oncol Rep. 12:593–599. 2004.
|
|
13
|
Maehama T and Dixon JE: PTEN: A tumour
suppressor that functions as a phospholipid phosphatase. Trends
Cell Biol. 9:125–128. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ortega-Molina A and Serrano M: PTEN in
cancer, metabolism, and aging. Trends Endocrinol Metab. 24:184–189.
2013. View Article : Google Scholar
|
|
15
|
Maehama T and Dixon JE: The tumor
suppressor, PTEN/MMAC1, dephosphorylates the lipid second
messenger, phosphatidylino-sitol 3,4,5-trisphosphate. J Biol Chem.
273:13375–13378. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
VanderLaan PA, Rangachari D, Mockus SM,
Spotlow V, Reddi HV, Malcolm J, Huberman MS, Joseph LJ, Kobayashi
SS and Costa DB: Mutations in TP53, PIK3CA, PTEN and other genes in
EGFR mutated lung cancers: Correlation with clinical outcomes. Lung
Cancer. 106:17–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smith IN and Briggs JM: Structural
mutation analysis of PTEN and its genotype-phenotype correlations
in endometriosis and cancer. Proteins. 84:1625–1643. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu JC, Wang DY, Egan SE and Zacksenhaus
E: Common and distinct features of mammary tumors driven by
Pten-deletion or activating Pik3ca mutation. Oncotarget.
7:9060–9068. 2016.PubMed/NCBI
|
|
20
|
Mina S, Bohn BA, Simon R, Krohn A, Reeh M,
Arnold D, Bokemeyer C, Sauter G, Izbicki JR, Marx A and Stahl PR:
PTEN deletion is rare but often homogeneous in gastric cancer. J
Clin Pathol. 65:693–698. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta) C(T) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Wang J, Nikhil K, Viccaro K, Chang L,
White J and Shah K: Phosphorylation-dependent regulation of ALDH1A1
by aurora kinase a: Insights on their synergistic relationship in
pancreatic cancer. BMC Biol. 15:102017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pitts TM, Bradshaw-Pierce EL, Bagby SM,
Hyatt SL, Selby HM, Spreafico A, Tentler JJ, McPhillips K, Klauck
PJ, Capasso A, et al: Antitumor activity of the aurora a selective
kinase inhibitor, alisertib, against preclinical models of
colorectal cancer. Oncotarget. 7:50290–50301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen C, Song G, Xiang J, Zhang H, Zhao S
and Zhan Y: AURKA promotes cancer metastasis by regulating
epithelial-mesenchymal transition and cancer stem cell properties
in hepatocellular carcinoma. Biochem Bioph Res Commun. 486:514–520.
2017. View Article : Google Scholar
|
|
25
|
Katsha A, Arras J, Soutto M, Belkhiri A
and El-Rifai W: AURKA regulates JAK2-STAT3 activity in human
gastric and esophageal cancers. Mol Oncol. 8:1419–1428. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
You D, Xin J, Volk A, Wei W, Schmidt R,
Scurti G, Nand S, Breuer EK, Kuo PC, Breslin P, et al: FAK mediates
a compensatory survival signal parallel to I3K-AKT in PTEN-null
T-ALL Cells. Cell Rep. 10:2055–2068. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang LY, He CY, Chen XH, Su LP, Liu BY and
Zhang H: Aurora kinase a revives dormant laryngeal squamous cell
carcinoma cells via FAK/PI3K/Akt pathway activation. Oncotarget.
7:48346–4859. 2016.PubMed/NCBI
|
|
28
|
Wang Y, Chen B, Wang Z, Zhang W, Hao K,
Chen Y, Li K, Wang T, Xie Y, Huang Z and Tong X: Marsdenia
tenacissimae extraction (MTE) inhibits the proliferation and
induces the apoptosis of human acute T cell leukemia cells through
inactivating PI3K/AKT/mTOR signaling pathway via PTEN enhancement.
Oncotarget. 7:82851–82863. 2016.PubMed/NCBI
|
|
29
|
Li JP, Yang YX, Liu QL, Pan ST, He ZX,
Zhang X, Yang T, Chen XW, Wang D, Qiu JX and Zhou SF: The
investigational aurora kinase a inhibitor alisertib (MLN8237)
induces cell cycle G2/M arrest, apoptosis, and autophagy via p38
MAPK and Akt/mTOR signaling pathways in human breast cancer cells.
Drug Des Devel Ther. 9:1627–1652. 2015.PubMed/NCBI
|
|
30
|
Jiang X and Li H: Overexpression of LRIG1
regulates PTEN via MAPK/MEK signaling pathway in esophageal
squamous cell carcinoma. Exp Ther Med. 12:2045–2052. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Puig-Butille JA, Vinyals A, Ferreres JR,
Aguilera P, Cabré E, Tell-Martí G, Marcoval J, Mateo F, Palomero L,
Badenas C, et al: AURKA overexpression is driven by FOXM1 and
MAPK/ERK activation in melanoma cells harboring BRAF or NRAS
mutations: Impact on melanoma prognosis and therapy. J Invest
Dermatol. 137:1297–1310. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zorba A, Buosi V, Kutter S, Kern N,
Pontiggia F, Cho YJ and Kern D: Molecular mechanism of aurora a
kinase autophosphorylation and its allosteric activation by TPX2.
Elife. 3:e026672014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shu LP, Zhou ZW, Zi D, He ZX and Zhou SF:
A SILAC-based proteomics elicits the molecular interactome of
alisertib (MLN8237) in human erythroleukemia K562 cells. Am J
Transl Res. 7:2442–2461. 2015.
|
|
34
|
Narbonne P, Maddox PS and Labbe JC:
DAF-18/PTEN signals through AAK-1/AMPK to inhibit MPK-1/MAPK in
feedback control of germline stem cell proliferation. PLoS Genet.
13:e10067382017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Benitez JA, Ma J, D'Antonio M, Boyer A,
Camargo MF, Zanca C, Kelly S, Khodadadi-Jamayran A, Jameson NM,
Andersen M, et al: PTEN regulates glioblastoma oncogenesis through
chromatin-associated complexes of DAXX and histone H3.3. Nat
Commun. 8:152232017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
de la Rosa J, Weber J, Friedrich MJ, Li Y,
Rad L, Ponstingl H, Liang Q, de Quirós SB, Noorani I, Metzakopian
E, et al: A single-copy sleeping beauty transposon mutagenesis
screen identifies new PTEN-cooperating tumor suppressor genes. Nat
Genet. 49:730–741. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shah MA: Gastrointestinal cancer targeted
therapies in gastric cancer-the dawn of a new era. Nat Rev Clin
Oncol. 11:10–11. 2014. View Article : Google Scholar
|
|
38
|
Shagisultanova E, Dunbrack RL Jr and
Golemis EA: Issues in interpreting the in vivo activity of
Aurora-A. Expert Opin Ther Targets. 19:187–200. 2015. View Article : Google Scholar
|
|
39
|
Ouchi M, Fujiuchi N, Sasai K, Katayama H,
Minamishima YA, Ongusaha PP, Deng C, Sen S, Lee SW and Ouchi T:
BRCA1 phosphorylation by Aurora-A in the regulation of G2 to M
transition. J Biol Chem. 279:19643–19648. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kashatus DF, Lim KH, Brady DC, Pershing
NL, Cox AD and Counter CM: RALA and RALBP1 regulate mitochondrial
fission at mitosis. Nat Cell Biol. 13:1108–1115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang K, Chen J, Chen D, Huang J, Feng B,
Han S, Chen Y, Song H, De W, Zhu Z, et al: Aurora-A promotes
chemoresistance in hepatocelluar carcinoma by targeting
NF-kappaB/microRNA-21/PTEN signaling pathway. Oncotarget.
5:12916–12935. 2014.PubMed/NCBI
|
|
42
|
Kwon YW, Kim IJ, Wu D, Lu J, Stock WA Jr,
Liu Y, Huang Y, Kang HC, DelRosario R, Jen KY, et al: Pten
regulates Aurora-A and cooperates with Fbxw7 in modulating
radiation-induced tumor development. Mol Cancer Res. 10:834–844.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cirak Y, Furuncuoglu Y, Yapicier O, Aksu A
and Cubukcu E: Aurora A overexpression in breast cancer patients
induces taxane resistance and results in worse prognosis. J BUON.
20:1414–1419. 2015.
|
|
44
|
Mignogna C, Staropoli N, Botta C, De Marco
C, Rizzuto A, Morelli M, Di Cello A, Franco R, Camastra C, Presta
I, et al: Aurora Kinase A expression predicts platinum-resistance
and adverse outcome in high-grade serous ovarian carcinoma
patients. J Ovarian Res. 9:312016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu J, Yue CF, Zhou WH, Qian YM, Zhang Y,
Wang SW, Liu AW and Liu Q: Aurora-A contributes to cisplatin
resistance and lymphatic metastasis in non-small cell lung cancer
and predicts poor prognosis. J Transl Med. 12:2002014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kitano S, Shiraishi N, Uyama I, Sugihara K
and Tanigawa N; Japanese Laparoscopic Surgery Study G: A
multicenter study on oncologic outcome of laparoscopic gastrectomy
for early cancer in Japan. Ann Surg. 245:68–72. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang SY, Roh KH, Kim YN, Cho M, Lim SH,
Son T, Hyung WJ and Kim HI: Surgical outcomes after open,
laparoscopic, and robotic gastrectomy for gastric cancer. Ann Surg
Oncol. 24:1770–1777. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Choi YY, Noh SH and Cheong JH: Evolution
of gastric cancer treatment: From the golden age of surgery to an
era of precision medicine. Yonsei Med J. 56:1177–1185. 2015.
View Article : Google Scholar : PubMed/NCBI
|