Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune
disease that is associated with high morbidity and high disability
rate. Pathogenesis of RA has not been illuminated yet (1), thus, exploring the
pathophysiological process of RA synovial proliferation is an
urgent problem to be solved (2).
The pathological manifestations of RA include recurrent attacks of
inflammation, excessive proliferation and dysfunction of synovial
tissues, invasion and destruction of articular cartilage and bone
by synoviocytes and vascular cells (3). At the initial stage of RA,
inflammatory cells aggregate to the articular cavity under the
action of inflammatory mediators and chemokines, and generate
inflammatory response targeting the synovial membrane (4). Macrophage-like synoviocytes and
fibroblast-like synoviocytes will produce a large amount of
inflammatory cytokines and matrix degrading enzymes under incentive
conditions, maintain and promote the persistence of inflammatory
response, promote angiogenesis, and induce destruction of joint
tissue (5).
It is known that autoimmune disorder can induce RA,
but the cause of such disturbance remains unclear (6). microRNA, which is most promising in
research in this direction, is an important component in the
cellular and genetic regulatory network (7). It is a kind of endogenous and tiny
non-coding single-strand RNA molecule with the average length of 22
nucleotides, which has become the hot research direction in
immunology at present as a result of its diversified species and
important roles (8). It plays an
important role in processes such as hematopoiesis, proliferation,
apoptosis, tissue differentiation and organ differentiation
(9). In addition, it is suggested
in a recent study to be involved in regulating body immune system
(9). Currently, increasing
literature illustrates the huge potential of microRNA at
fundamental and clinical levels, especially as the therapeutic
targets, inserted molecules and biomarkers (9). It is been known that the functions
of microRNA in animals are not restricted to the regulation of cell
proliferation and differentiation, lipid conversion, and hormone
secretion regulation. More importantly, it can prevent disturbance
of normal immune function (8).
Jiang and Wang (10) indicated
that histone deacetylase 3 was involved in ankylosing spondylitis
via miR-130a and enhancement of tumor necrosis factor-1α (TNF-1α)
expression. Li et al (11)
showed that miR-130a played an important role in regulating the
expression of TNF-α in osteoarthritis.
Akt, which is overexpressed in RA synoviocytes, can
not be detected in normal synoviocytes (12). Addition of Akt in RA synoviocytes
cultured in vitro endows cells with the ability to act
against apoptosis, suggesting that the PI3K/Akt signal pathway,
which is highly active in RA, may be involved in the course of RA
(12). PTEN is an important
negative regulatory factor in PI3K/Akt signal pathway, which can
downregulate the activities of pathway as well as its downstream
factors at multiple levels (13).
This characteristic has been verified in numerous studies on tumor
(13). Research on RA suggests
that PTEN may also be involved in activating the pathway (13). Expression quantity of PTEN is
lower than normal level in lining layer of affected joints, but it
is normal in the sub-lining layer and can hardly be detected in
invasive RA synoviocytes, indicating certain association between
PTEN afunction and the highly activated PI3K/Akt in RA (14). Therefore, expressing PTEN protein
in the pathway, targeting Akt mRNA sequence, upregulating PTEN or
downregulating Akt can inhibit the pathway activity, which thus
inhibits FLS proliferation, promotes its apoptosis and delays
injury in affected joints (15).
In this study, we sought to determine how microRNA-130a regulates
osteoarthritis.
Materials and methods
Patients
The serum levels of patients with osteoarthritis
(n=6, male, 55.45±5.23) and healthy volunteers (n=6, male,
50.56±7.62) were obtained from the department of Orthopaedics,
Shanghai Tongji Hospital at the time of total knee replacement
surgery. Ten milliliters of peripheral blood was collected and
centrifuged at 2,000 × g for 10 min at 4°C. Serum samples were
collected and saved at −80°C. This study was carried out in
accordance with the approved guidelines of Shanghai Tongji Hospital
and was approved by the Ethics Committee of Shanghai Tongji
Hospital.
RT-PCR
Total RNA was isolated from serum samples and cell
samples using TRIzol regent, and samples were treated with DNase I
(both from Invitrogen, USA). cDNA was performed using
oligo(dT)20 and Superscript II reverse transcriptase
(Invitrogen). Real-time PCR was performed using a StepOnePlus
Real-time PCR system (Applied Biosystems, Foster City, CA, USA)
with an ABI 7500 quantitative PCR instrument. The primer sequences
were as follows: U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-ACGCTTCACGAATTTGCGT-3′; miR-130a, 5′-CAGUGCAAUGUUAAAAG-3′. PCR
cycling conditions were as follows: 95°C for 10 min, 40 cycles of
94°C for 30 sec, 55°C for 30 sec, 60°C for 10 sec, and a final
extension at 72°C for 30 sec. The 2−ΔΔCt method was used
to represent microRNA-130a expression.
Cell isolation, culture conditions, and
cell transfection
The use of animals was approved by the Ethics
Committee of Shanghai Tongji Hospital. Male SD rats (220-250 g, 8-9
weeks) were obtained from Shanghai Slick Experimental Animal Co.,
Ltd. (Shanghai, China) and housed at 22-23°C, 55-60% humidity,
7:00-19:00. The cartilage tissues were acquired, washed with
phosphate-buffered saline (PBS), sterilized with 75%-ethyl alcohol
and cut into pieces using micro-scissors. The tissues were digested
with 0.25% Trypsin-EDTA for 30 min on ice, then digested with
collagenase II (both from Invitrogen) for 4 h on ice and then
filtered using 200-mesh sieve. Chondrocytes were cultured with
Dulbecco's modified Eagle's medium (DMEM) with high-dose (4.5 g/l)
glucose, 10% fetal bovine serum (FBS), and 1%
penicillin/streptomycin at 37°C with 5% CO2. The
microRNA-130a mimics, microRNA-130a inhibitors and their negative
controls (NC) were purchased from Sangon Biotech Co., Ltd.
(Shanghai, China). MicroRNA-130a inhibitors and VO-Ohpic trihydrate
(10 nM, PTEN inhibitor) or wortmannin (2 nM, PI3K inhibitor) were
added into cells for 48 h.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
After transfection or inhibitor treatment, 10
μl MTT (5 mg/ml; Invitrogen) was added into cultured medium
for 4 h at 37°C. After the removal of culture medium, dimethyl
sulphoxide (150 μl; Invitrogen) was added to each well,
leaving the cells at room temperature in the dark for 20 min, and
absorbance at 570 nm.
Apoptosis assay
After inhibitor treatment, cells were washed three
times with PBS and resuspend using buffer (BD Biosciences, San
Jose, CA, USA). Cells was stained using the 5 μl of Annexin
V-FITC and 5 μl of PI double (BD Biosciences) for 15 min in
the dark at room temperature. Flow cytometry (FACSCanto™) was used
to measure apoptosis rate, and analyzed using CellQuest Pro
software (both from BD Biosciences). Then enzyme-linked
immunosorbent assay (ELISA) assay was performed. After inhibitor
treatment, supernatant of all the cells was collected and used to
measure interleukin-1β (IL-1β), IL-6 and IL-18 levels using ELISA
kits (Nanjing Jiancheng Biology Engineering Institute, Nanjing,
China). Plates were read using a Multiskan Go Microplate
Spectrophotometer (Thermo Fisher Scientific) at 450 nm.
Western blot analysis
After transfection, cell was lysed in lysis buffer
on ice for 30 min, followed by a 12,000 rpm centrifugation at 4°C
for 10 min. The amount of BCA detection reagent needed was
calculated according to protein content. Proteins were resolved on
an 8-10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred to PVDF membranes (Millipore, Billerica,
MA, USA). Membranes were blocked with 5% nonfat milk in
Tris-buffered saline and were incubated with primary antibodies
against: Bax (sc-6236,1:500), caspase-3 (sc-98785, 1:500),
caspase-9 (sc-8355, 1:500), PTEN (sc-6817-R, 1:500), PI3K (sc-7174,
1:500), p-Akt (sc-33437, 1:500) and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) (sc-367714, 1:2,000) at 4°C overnight. After
washing with TBST, membranes were detected using HRP-conjugated
secondary anti-rabbit antibodies (7074, 1:5,000; Cell Signaling
Technology, Inc.) and The band was visualized with an enhanced
chemiluminescence kit and analyzed using AlphaEaseFC 4.0
software.
Statistical analysis
Results are expressed as the mean ± SD. Data were
analyzed between two groups using the Student's t-test, while among
more than two groups by the One-way ANOVA method. P<0.05
provided evidence of significant differences.
Results
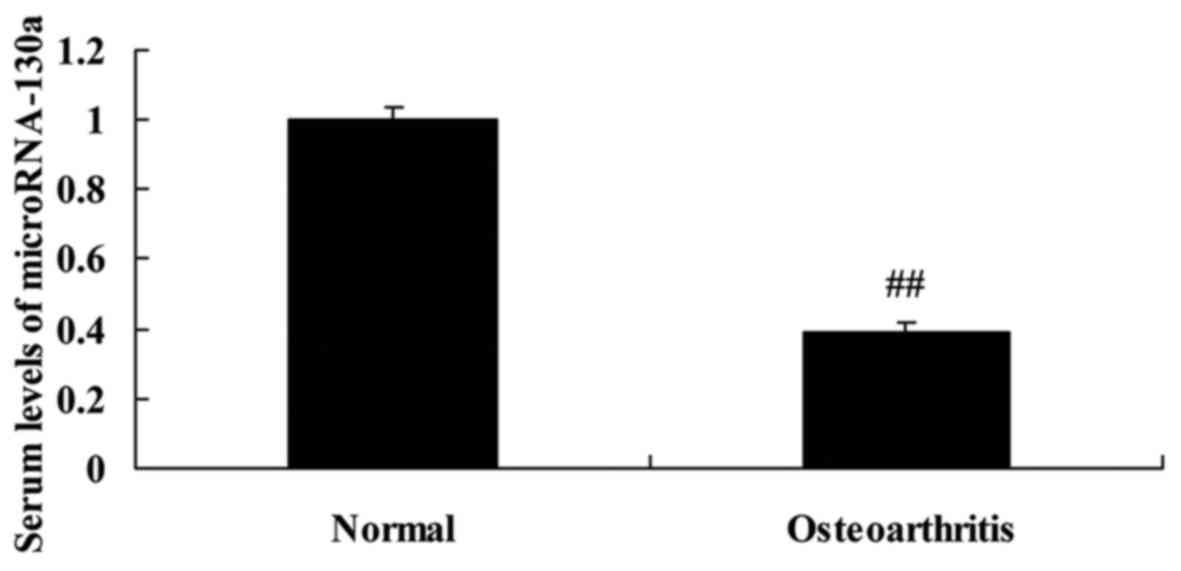
The serum levels of microRNA-130a in
osteoarthritis patients
To identify the function of microRNA-130a in
development and progression of osteoarthritis, microRNA-130a
expression was surveyed by RT-PCR. As shown in Fig. 1, the serum levels of microRNA-130a
were decreased, compared with normal group.
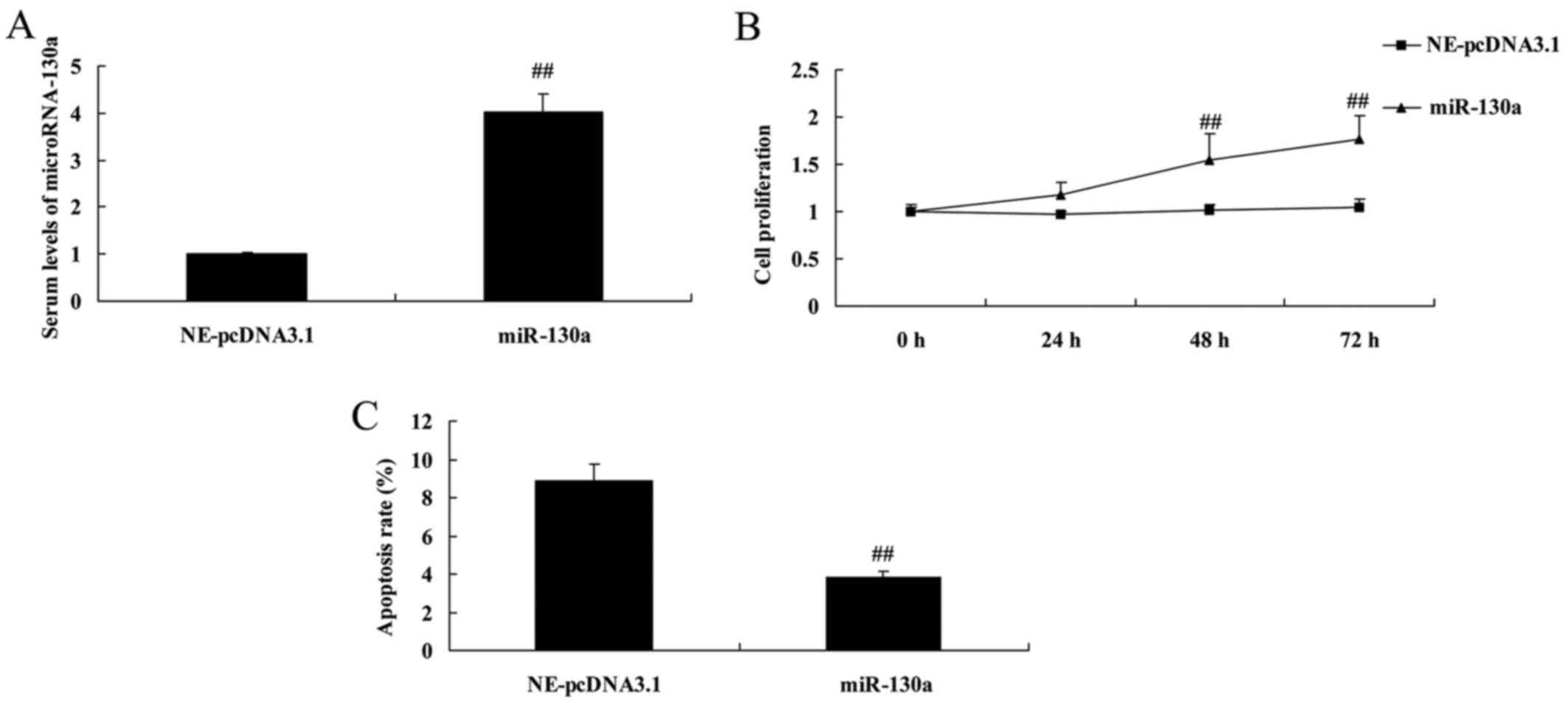
Overexpression of microRNA-130a increased
cell proliferation and decreased apoptosis of chondrocytes
Interestingly, we used microRNA-130a mimics to
increase microRNA-130a expression for the function of microRNA-130a
on osteoarthritis. Fig. 2 showed
that microRNA-130a mimics effectively increased microRNA-130a
expression. Overexpression of microRNA-130a increased cell
proliferation and decreased apoptosis of chondrocytes (Fig. 2).
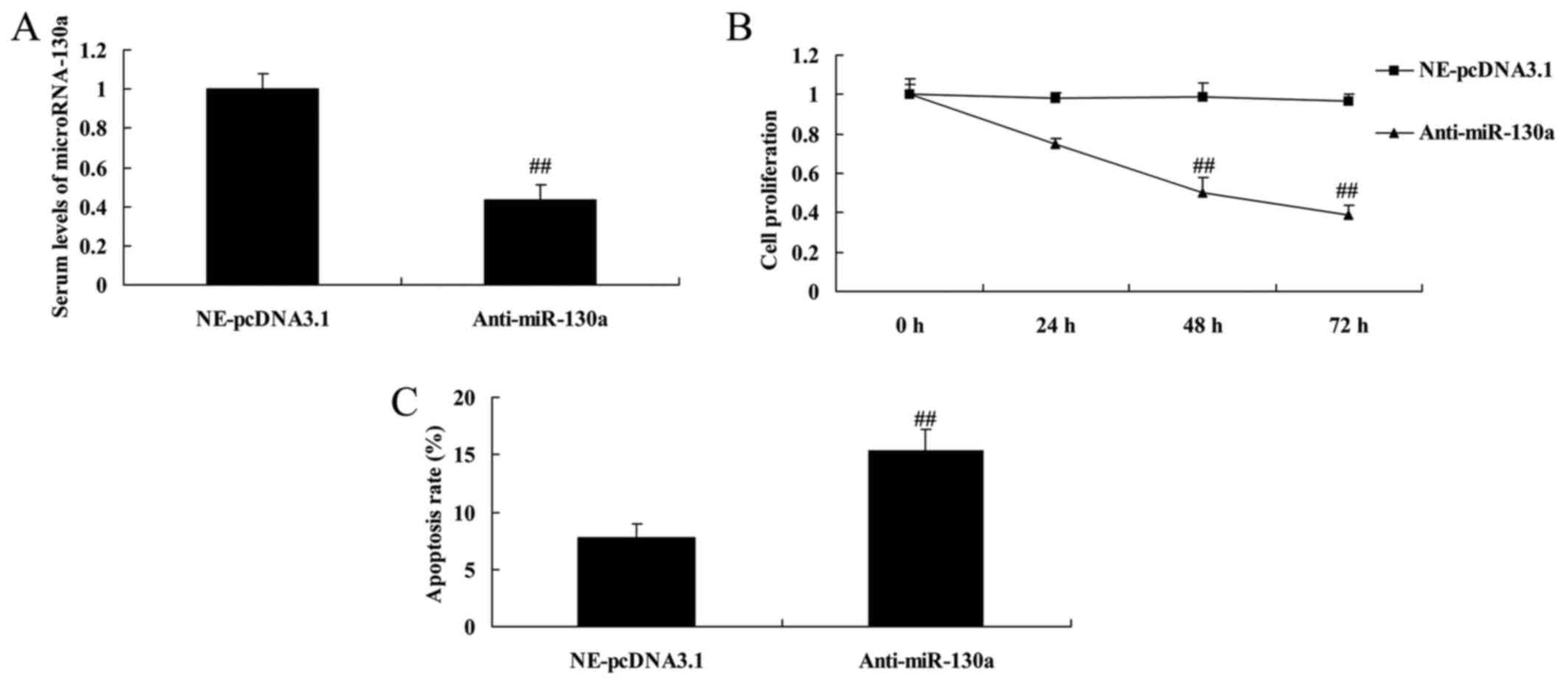
Downregulation of microRNA-130a also
decreased cell proliferation and induced apoptosis in
chondrocytes
Next, we also used anti-microRNA-130a mimics to
decrease microRNA-130a expression for the function of microRNA-130a
on osteoarthritis. Similar to the results of Fig. 3, anti-microRNA-130a mimics
effectively decreased microRNA-130a expression, however,
downregulation of microRNA-130a also decreased cell proliferation
and induced apoptosis in chondrocytes (Fig. 3).
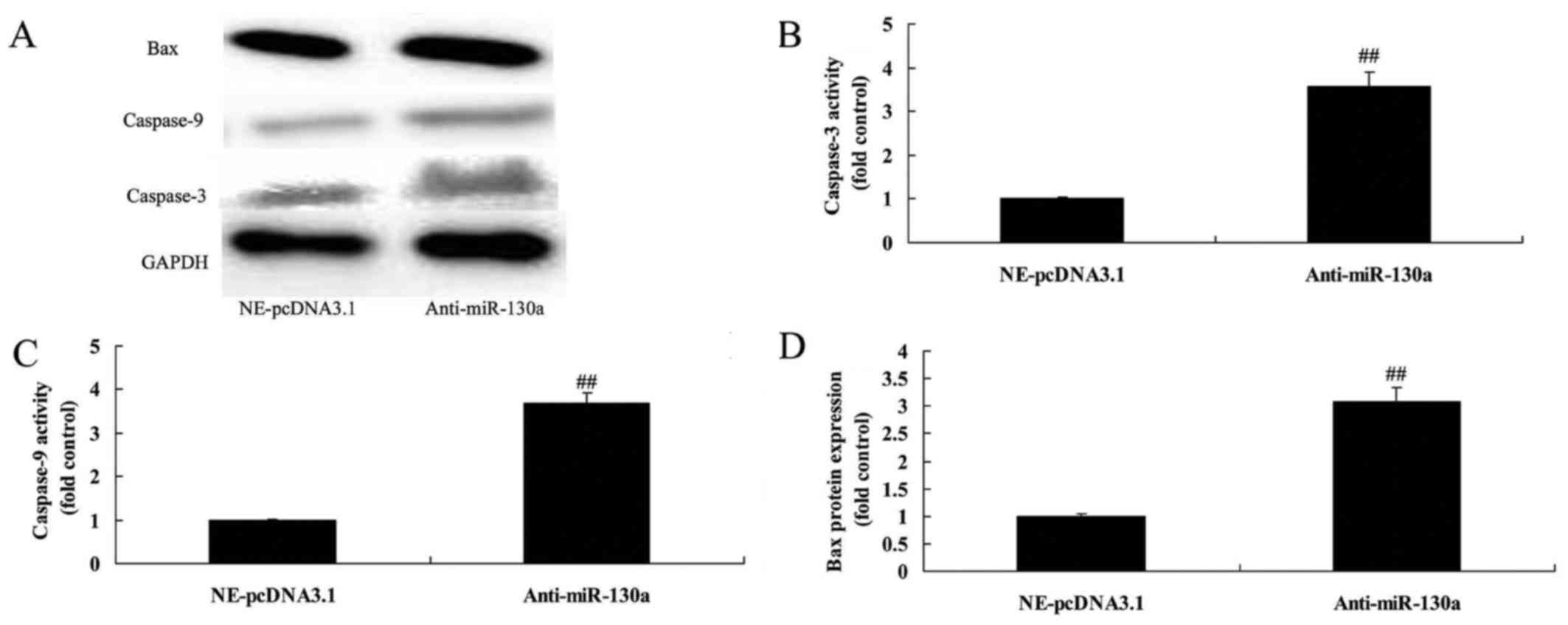
Downregulation of microRNA-130a promoted
Bax and caspase-3/9 protein expression in chondrocytes
We tested the hypothesis that the downregulation of
microRNA-130a on anticancer effects in osteoarthritis. Notably,
downregulation of microRNA-130a significantly promoted Bax and
caspase-3/9 protein expression in chondrocytes (Fig. 4).
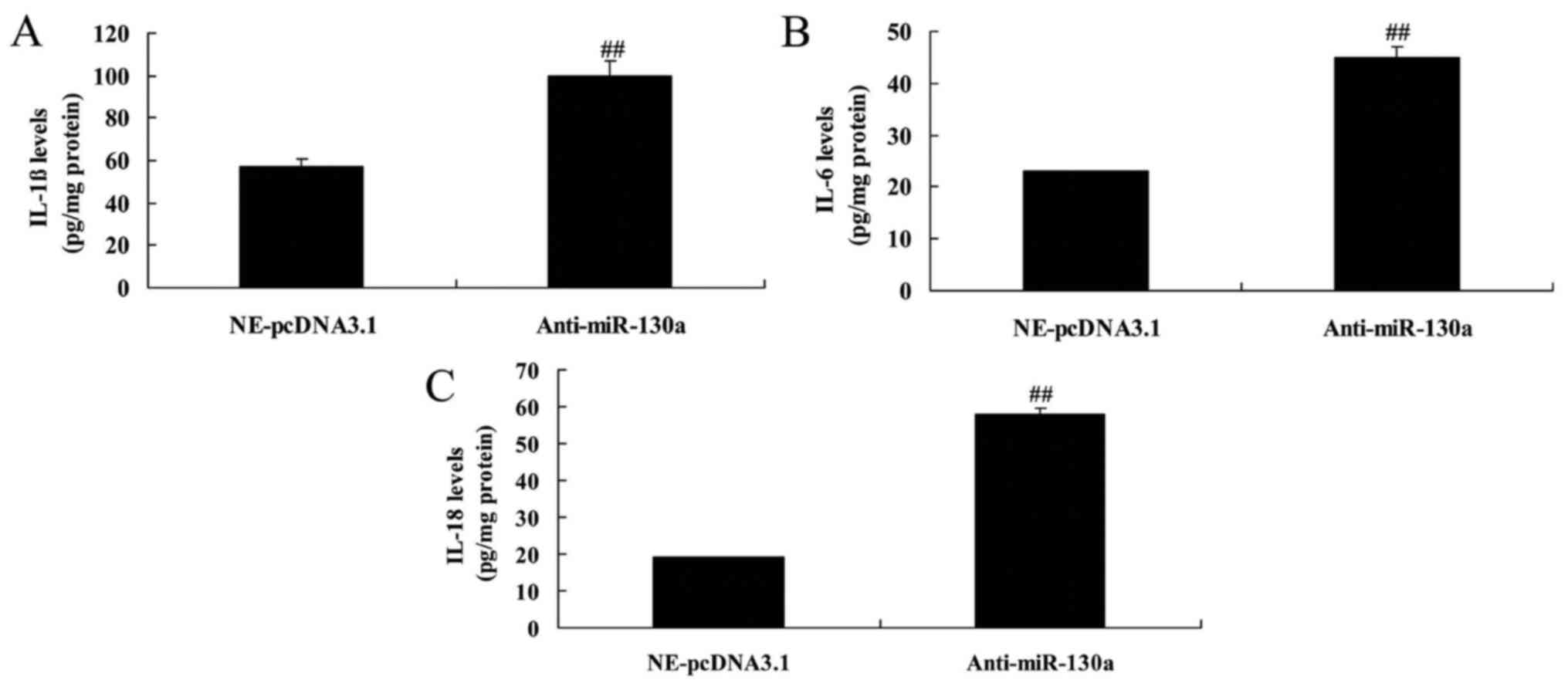
Downregulation of microRNA-130a increases
inflammation divisors in chondrocytes
To directly test the hypothesis that
osteoarthritis-induced inflammation divisors is a downstream target
of microRNA-130a, IL-1β, IL-6 and IL-18 levels were measured in
this study. There was a significant increases of IL-1β, IL-6 and
IL-18 levels in chondrocytes by microRNA-130a downregulation,
compared with negative control group (Fig. 5).
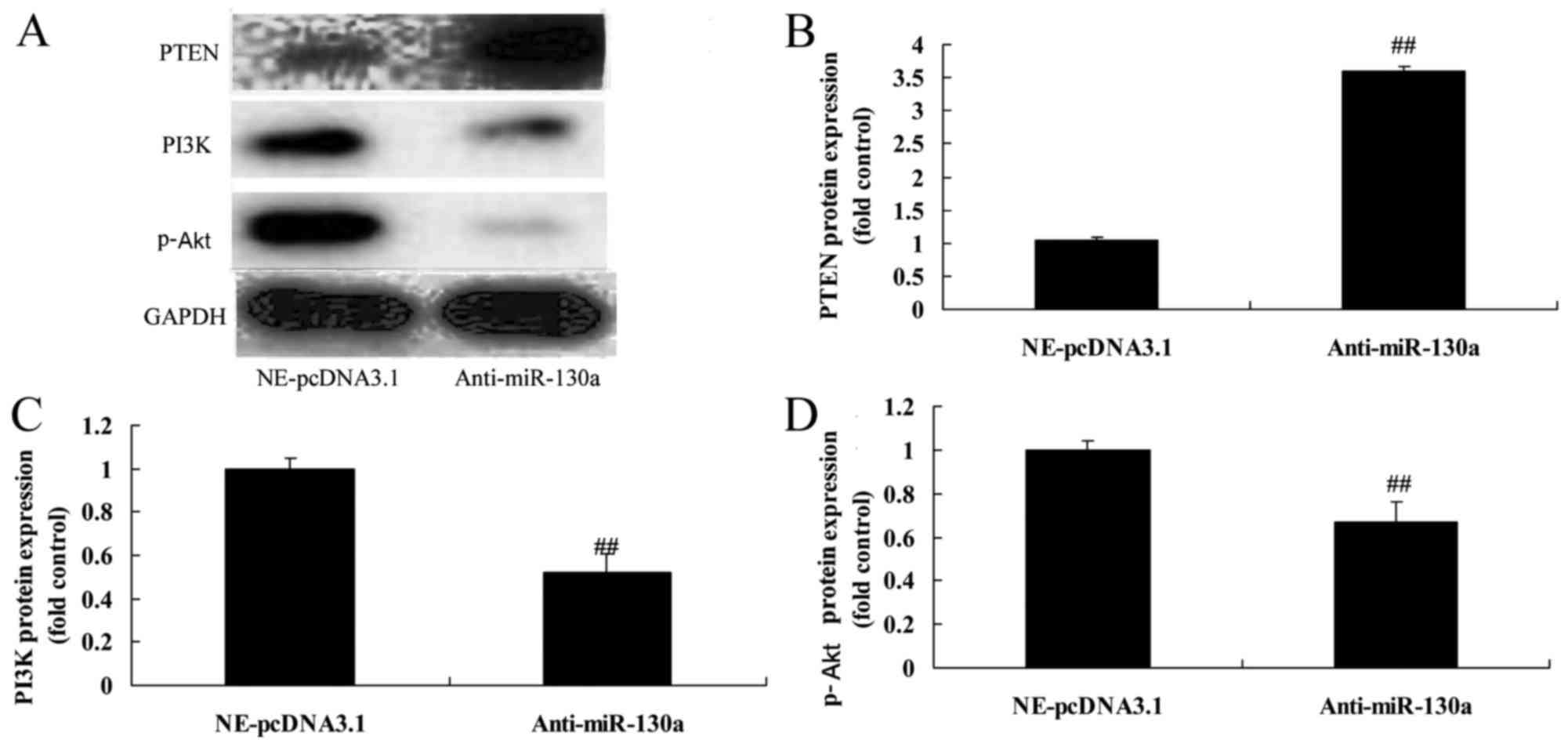
Downregulation of microRNA-130a increased
PTEN/PI3K/Akt signaling pathway in chondrocytes
We investigated which of PTEN/PI3K/Akt signaling
pathways regulates the function of microRNA-130a on osteoarthritis.
Compared with negative control group, the downregulation of
microRNA-130a significantly increased PTEN protein expression and
suppressed PI3K and p-Akt protein expression in chondrocytes
(Fig. 6). Summarily,
osteoarthritis patients have alterations in microRNA-130a
expression, and microRNA-130a may regulate osteoarthritis.
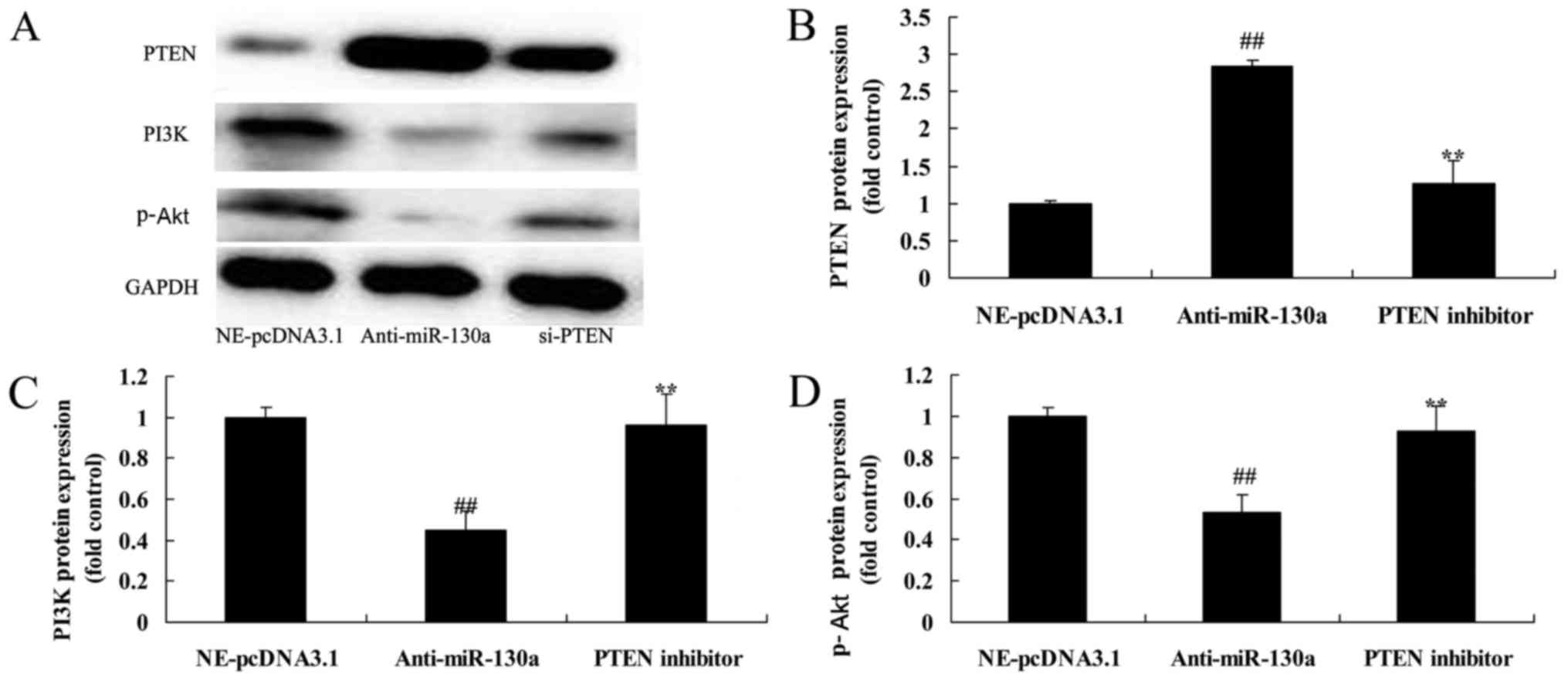
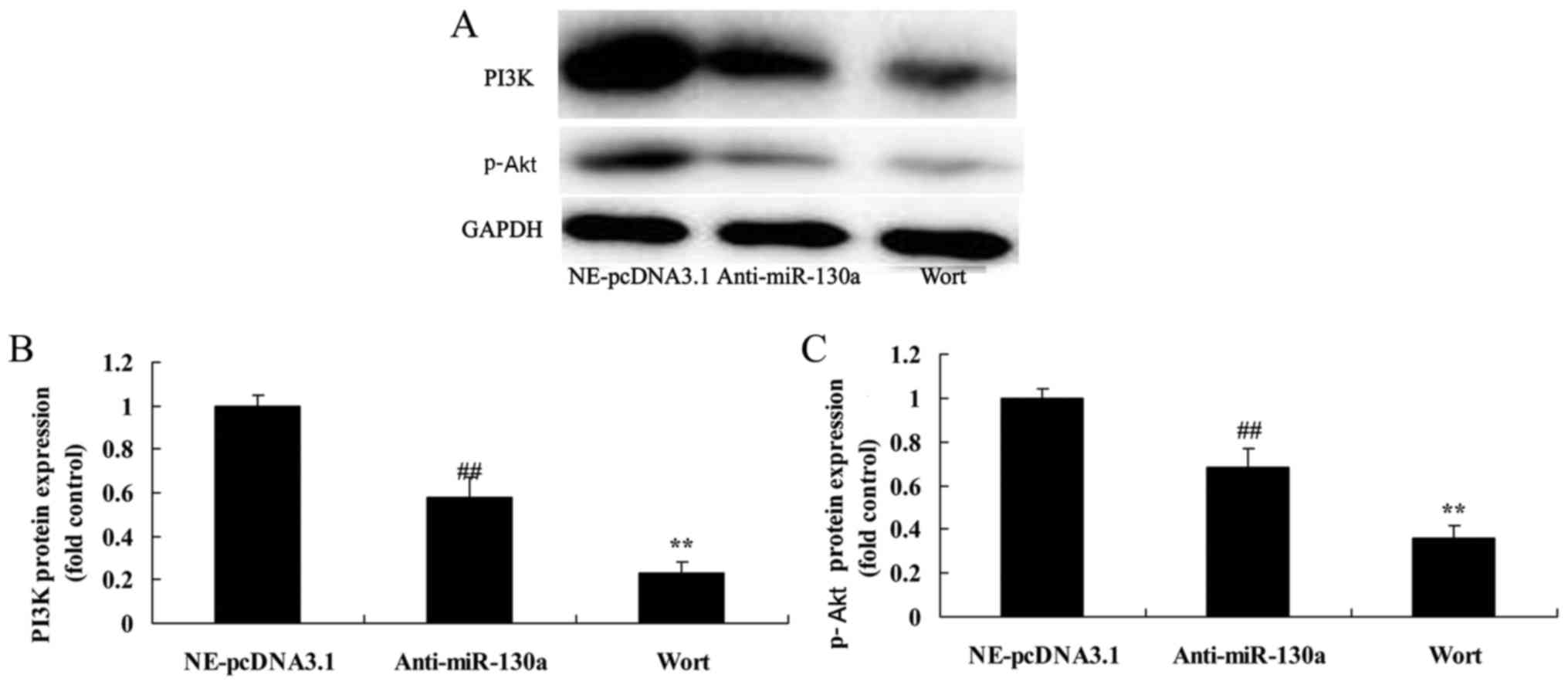
PTEN inhibitor decreased the destructive
effect of microRNA-130a on PTEN/PI3K/Akt signaling pathway of
chondrocytes
In contrast, we used VO-Ohpic trihydrate, PTEN
inhibitor, was used to inhibit PTEN expression in chondrocytes
after microRNA-130a downregulation. PTEN inhibitor significantly
suppressed PTEN protein expression, and induced PI3K and p-Akt
protein expression chondrocytes after microRNA-130a downregulation
(Fig. 7).
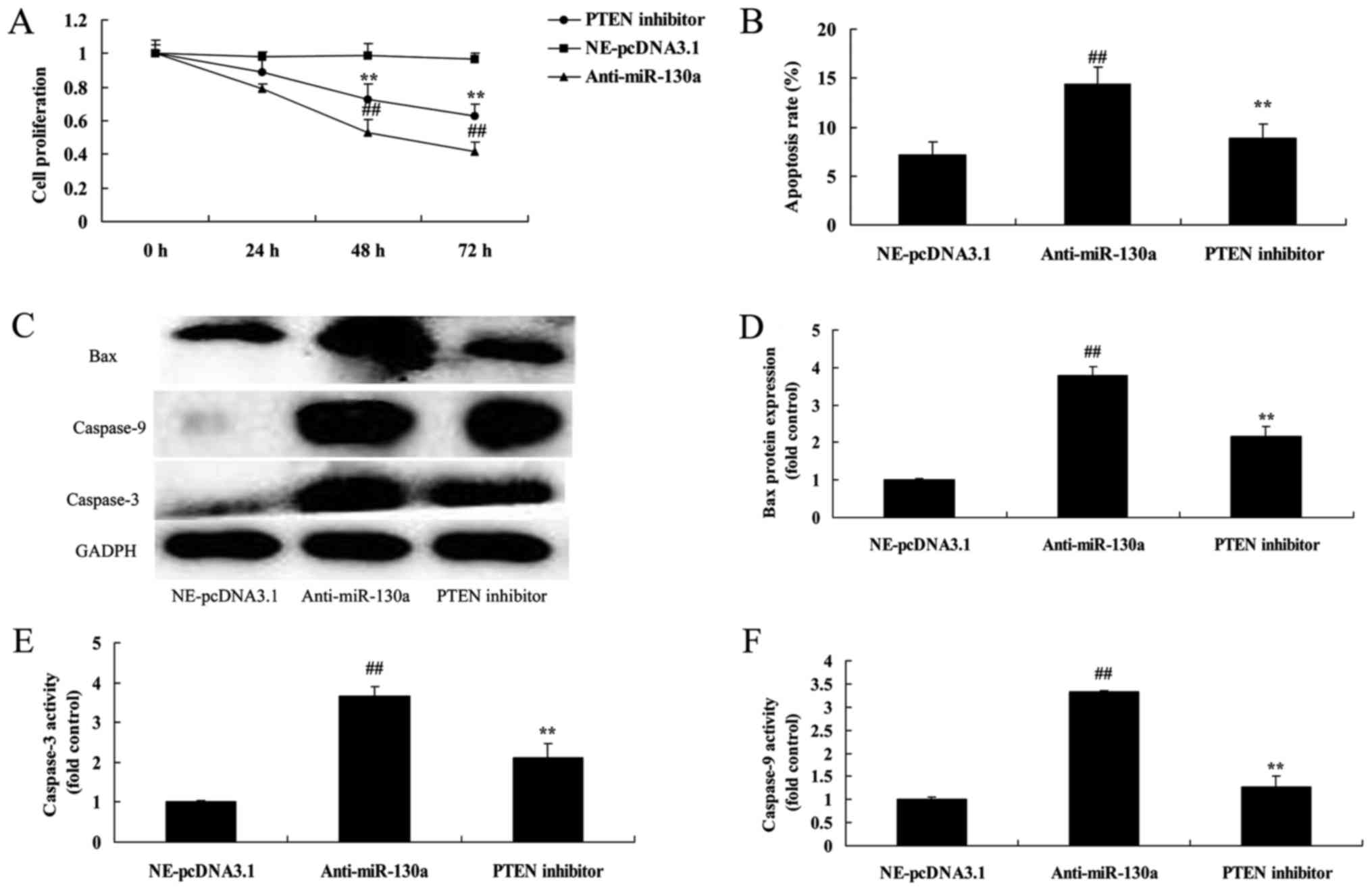
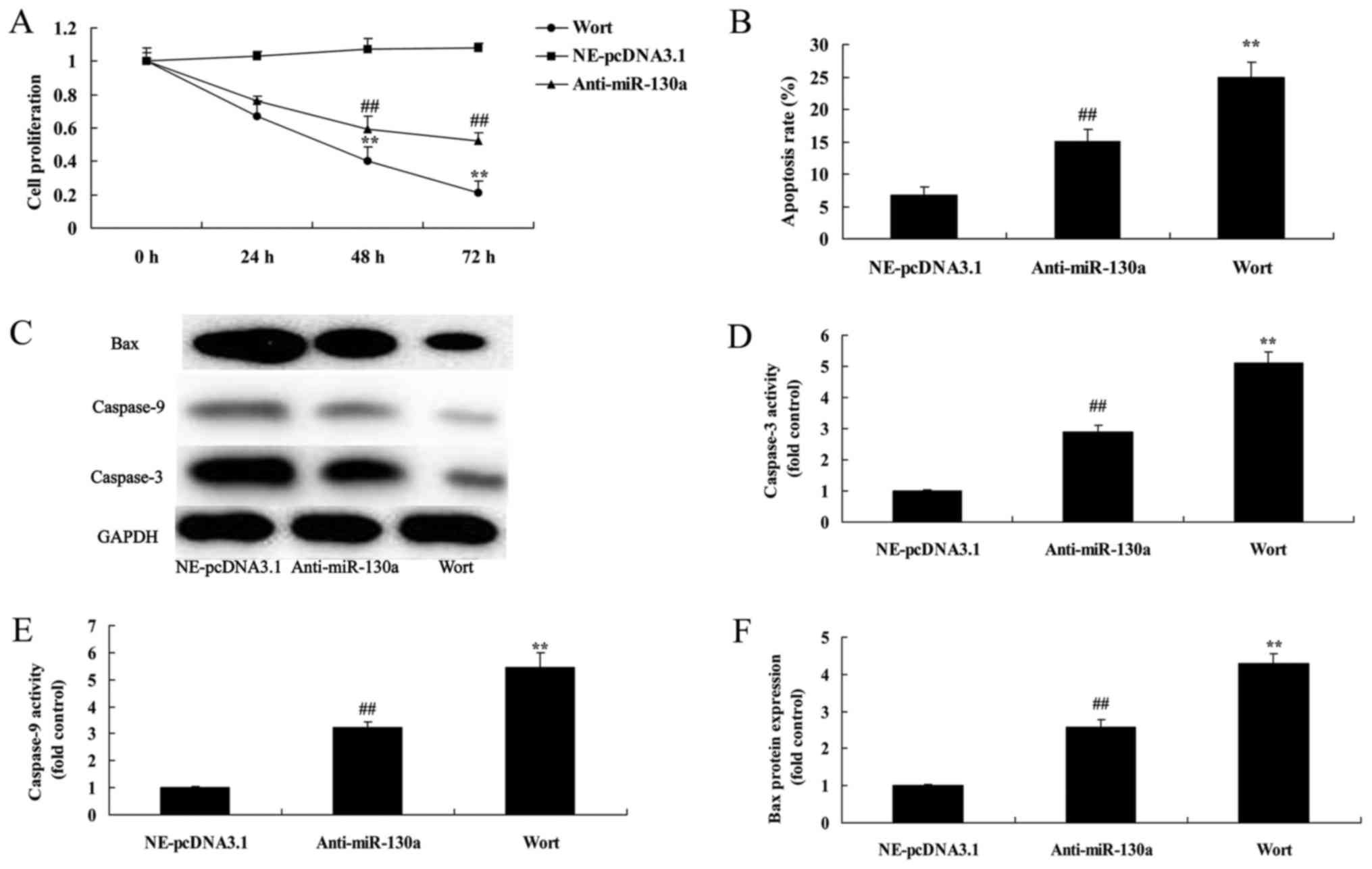
PTEN inhibitor decreases the destructive
effect of microRNA-130a on cell proliferation of chondrocytes
We assessed whether PTEN participates in the
function of microRNA-130a on osteoarthritis. PTEN inhibitor
significantly promoted cell proliferation, decreased apoptosis, and
suppressed Bax and caspase-3/9 protein expression in chondrocytes
after microRNA-130a downregulation (Fig. 8).
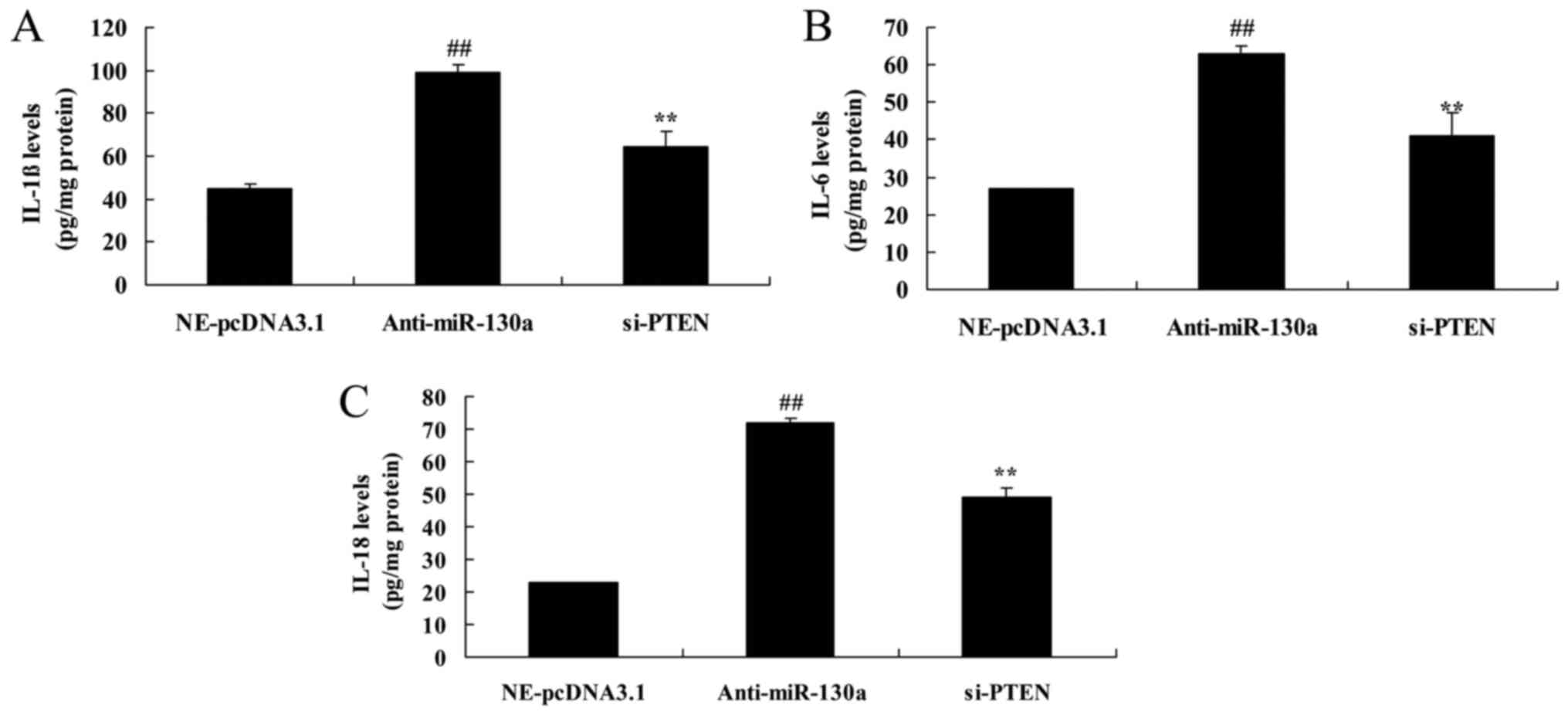
PTEN inhibitor decreases the destructive
effect of microRNA-130a on inflammation divisors of
chondrocytes
We analyzed whether PTEN participates in the
function of microRNA-130a on inflammation divisors of
osteoarthritis. As showed in Fig.
9, PTEN inhibitor significantly reduced IL-1β, IL-6 and IL-18
levels in chondrocytes after microRNA-130a downregulation.
Furthermore, PTEN regulates microRNA-130a effect on
osteoarthritis.
Wortmannin increases the destructive
effect of microRNA-130a on PI3K/Akt signaling pathway of
chondrocytes
We further clarified the molecular mechanism
underlying the suppressive effect of microRNA-130a on
osteoarthritis. PI3K inhibitor, wortmannin, significantly
suppressed PI3K and p-Akt protein expression in chondrocytes after
microRNA-130a downregulation (Fig.
10).
Wortmannin increases the destructive
effect of microRNA-130a on cell proliferation of chondrocytes
In order to determine the function of PI3K on
microRNA-130a in osteoarthritis, we revealed cell proliferation of
chondrocytes. As showed in Fig.
11, the inhibition of PI3K significantly decreased cell
proliferation, increased apoptosis, and induced Bax and caspase-3/9
protein expression in chondrocytes after microRNA-130a
downregulation.
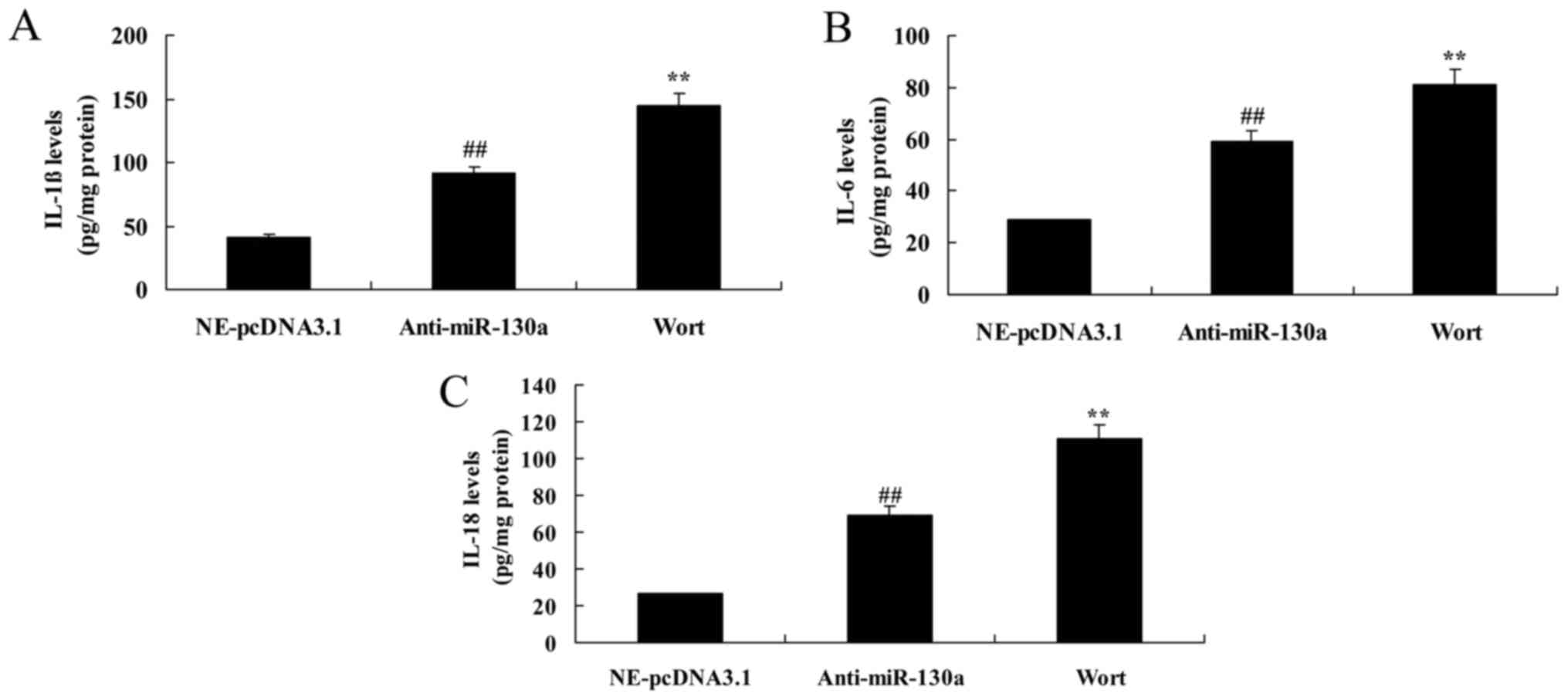
Wortmannin increases the destructive
effect of microRNA-130a on inflammation divisors of
chondrocytes
We then examined the inhibition of PI3K on the
function of microRNA-130a on IL-1β, IL-6 and IL-18 levels.
Interestingly, the inhibition of PI3K significantly reduced IL-1β,
IL-6 and IL-18 levels in chondrocytes after microRNA-130a
downregulation (Fig. 12). Taken
together, PI3K/Akt signaling pathway participates in the function
of microRNA-130a on inflammation in osteoarthritis.
Discussion
Present study generally focuses on the molecular
mechanisms by which RA initiates and develops; for instance,
expression, activity and function of disease-related factors under
specific tissue, cell and physiopathological conditions, as well as
function, regulation and signal communication of relevant signal
pathways (16). Illuminating the
mechanism that drives abnormal activities or functions of
cytokines, signal pathways and cells is an important component in
research on RA (17). As is
commonly suggested at present, RA is induced by imbalance of
cellular and molecular network, which gives rise to abnormal
expression and activities of cytokines [such as TNF-α, IL-1, IL-6
and nuclear factor-κB (NF-κB)], proteins and enzyme systems (like
FAK, shC and MMPS) (18). In this
study, we found that the serum levels of microRNA-130a were
decreased. Overexpression of microRNA-130a increased cell
proliferation and decreased apoptosis of chondrocytes, and
downregulation of microRNA-130a also decreased cell proliferation
and induced apoptosis in chondrocytes. Zumbrennen-Bullough et
al suggested that microRNA-130a is upregulated suppresses
hepcidin synthesis, and thereby promotes iron availability through
BMP and ALK2 (19).
Activation of PI3K signal transduction pathway has
been recently recognized to be one of the important mechanisms for
anti-apoptosis of cells and induction of abnormal proliferation.
PI3K/Akt signal pathway is closely associated with
inflammation-associated cytokines, such as TNF-α, IL-1, IL-6 and
NF-κB, and plays an important role in pathological process
(20). PI3K can be activated by
multiple extracellular stimuli, such as cytokines, growth factors,
G-protein, T-cell antigen, small G-protein, thrombin, cytoplasmic
protein tyrosine kinase, as well as other physical and chemical
factors (21). In addition, it
can induce extensive biological effects through PI3K/Akt pathway,
and regulate multiple cell functions, such as apoptosis,
proliferation, metabolism, growth and transformation, membrane
transport, secretion and chemotaxis. Moreover, it plays an
important role in the pathogeneses of inflammation, tumor,
metabolic and cardiovascular diseases (17). Our data suggest that
downregulation of microRNA-130a increased inflammation divisors in
chondrocytes.
Akt is an effector of PI3K/Akt signal pathway
locating in the important convergence point of multiple upstream
signal activities (15). Akt is a
serine/threonine protein kinase, the activation of which will
induce the interaction between downstream phosphorylation cascade
reaction and target proteins (22). On the contrary, it participates in
the regulation of multiple biological effects, such as cell growth
and survival, proliferation and apoptosis, carbohydrate metabolism,
gene transcription, neovascularization, cell migration and
movement, as well as cell cycle regulation (22). This study extends these
observations suggesting that the downregulation of microRNA-130a
significantly suppressed the PTEN/PI3K/Akt signaling pathway in
chondrocytes.
Akt can also indirectly act on proteins or cytokines
such as Bax, p53 and NF-κB, thus affecting cell survival, growth
and proliferation (12). Akt
plays a vital role of blocking PI3K/Akt pathway activity through
targeting Akt, thereby offering possibility to treat PI3K/Akt
signal activation-related diseases (23). Our data suggest that wortmannin
also increased the destructive effect of microRNA-130a on
inhibition of cell proliferation and apoptosis of chondrocytes
through PI3K/Akt signaling. Lu et al (24) reported that microRNA-130a
attenuated cardiac dysfunction and remodeling after myocardial
infarction via activation of PI3K/Akt signaling via suppression of
PTEN expression. This study shows that microRNA-130a regulates
PI3K/Akt signaling in osteoarthritis model in vitro.
PTEN is a tumor suppressor gene possessing
dual-phosphorylase activities, which is a natural inhibitor of
PI3K/Akt signal pathway (14).
Downregulated PTEN expression and afunction can frequently be seen
in multiple tumors (14). PTEN
downregulates PI3K/Akt pathway, and inhibits a series of downstream
anti-apoptosis, proliferation and invasion related signals through
the dephosphorylation of PI3K (15). Furthermore, PTEN can reduce the
phosphorylation levels of multiple key survival kinases, thus
promoting apoptosis and inhibiting proliferation as well as
infiltration (15). PTEN reduces
key protein activity through dephosphorylation, and blocks multiple
signal pathways that promote tumor growth, invasion, metastasis and
anti-apoptosis (25). It is
pointed out in literature that PTEN gene mutation or methylation
cannot be detected in RA synoviocytes, but PTEN has significantly
lowered activity than normal level (25). In our assays, we found that PTEN
inhibitor, decreased the destructive effect of microRNA-130a on
cell proliferation and apoptosis of chondro-cytes. Song et
al indicated that miR-130a alleviates coronary artery
endothelial cell injury through downregulating PTEN and activating
PI3K/Akt/eNOS signaling pathway (26). This study extends these
observations suggesting that miR-130a/PTEN/PI3K/Akt signaling
pathway participated in osteoarthritis apoptosis.
In conclusion, this study found that microRNA-130a
targets PTEN and PI3K/Akt signal to regulate osteoarthritis-induced
apoptosis and inflammation, which mediates the establishment and
development of osteoarthritis. These results provide insights into
the mechanism underlying directed differentiation of
osteoarthritis.
Acknowledgments
Not applicable.
Notes
[1]
Funding
The study was supported by the Natural Science
Foundation of China (no. 81472144).
[2] Availability
of data and material
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
YY designed the study. YZ, SX, EH, HZ, BL and CS
performed the experiments. YY and YZ analyzed the data. YY wrote
the manuscript. All authors read and approved the final
manuscript.
[4] Ethics
approval and consent to participate
This study was carried out in accordance with the
approved guidelines of Shanghai Tongji Hospital.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Wallenstein GV, Kanik KS, Wilkinson B,
Cohen S, Cutolo M, Fleishmann R, Genovese MC, Gomez Reino J, Gruben
D, Kremer J, et al: Effects of the oral Janus kinase inhibitor
tofacitinib on patient-reported outcomes in patients with active
rheumatoid arthritis: Results of two phase 2 randomised controlled
trials. Clin Exp Rheumatol. 34:430–442. 2016.PubMed/NCBI
|
|
2
|
Izumi K, Kaneko Y, Hashizume M, Yoshimoto
K and Takeuchi T: Baseline serum osteopontin levels predict the
clinical effectiveness of tocilizumab but not infliximab in
biologic-naïve patients with rheumatoid arthritis: A single-center
prospective study at 1 year (the Keio First-Bio Cohort study). PLoS
One. 10:e01454682015. View Article : Google Scholar
|
|
3
|
Xu Y, Zhu Q, Song J, Liu H, Miao Y, Yang
F, Wang F, Cheng W, Xi Y, Niu X, et al: Regulatory effect of
iguratimod on the balance of Th subsets and inhibition of
inflammatory cytokines in patients with rheumatoid arthritis.
Mediators Inflamm. 2015:3560402015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Durez P, Vandepapeliere P, Miranda P,
Toncheva A, Berman A, Kehler T, Mociran E, Fautrel B, Mariette X,
Dhellin O, et al: Therapeutic vaccination with TNF-Kinoid in TNF
antagonist- resistant rheumatoid arthritis: A phase II randomized,
controlled clinical trial. PLoS One. 9:e1134652014. View Article : Google Scholar
|
|
5
|
Genovese MC, Kremer J, Zamani O, Ludivico
C, Krogulec M, Xie L, Beattie SD, Koch AE, Cardillo TE, Rooney TP,
et al: Baricitinib in patients with refractory rheumatoid
arthritis. N Engl J Med. 374:1243–1252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maeda Y, Farina NH, Matzelle MM, Fanning
PJ, Lian JB and Gravallese EM: Synovium-derived microRNAs regulate
bone pathways in rheumatoid arthritis. J Bone Miner Res.
32:461–472. 2017. View Article : Google Scholar :
|
|
7
|
Krintel SB, Dehlendorff C, Hetland ML,
Hørslev-Petersen K, Andersen KK, Junker P, Pødenphant J, Ellingsen
T, Ahlquist P, Lindegaard HM, et al: Prediction of treatment
response to adalimumab: A double-blind placebo-controlled study of
circulating microRNA in patients with early rheumatoid arthritis.
Pharmacogenomics J. 16:141–146. 2016. View Article : Google Scholar
|
|
8
|
Abou-Zeid A, Saad M and Soliman E:
MicroRNA 146a expression in rheumatoid arthritis: Association with
tumor necrosis factor-alpha and disease activity. Genet Test Mol
Biomarkers. 15:807–812. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang G, Wu D, Zeng G, Jiang O, Yuan P,
Huang S, Zhu J, Tian J, Weng Y and Rao Z: Correlation between
miR-126 expression and DNA hypomethylation of CD4+ T
cells in rheumatoid arthritis patients. Int J Clin Exp Pathol.
8:8929–8936. 2015.
|
|
10
|
Jiang Y and Wang L: Role of histone
deacetylase 3 in ankylosing spondylitis via negative feedback loop
with microRNA-130a and enhancement of tumor necrosis factor-1α
expression in peripheral blood mononuclear cells. Mol Med Rep.
13:35–40. 2016. View Article : Google Scholar
|
|
11
|
Li ZC, Han N, Li X, Li G, Liu YZ, Sun GX,
Wang Y, Chen GT and Li GF: Decreased expression of microRNA-130a
correlates with TNF-α in the development of osteoarthritis. Int J
Clin Exp Pathol. 8:2555–2564. 2015.
|
|
12
|
Kuuliala K, Kuuliala A, Hämäläinen M,
Koivuniemi R, Kautiainen H, Moilanen E, Repo H and Leirisalo-Repo
M: Impaired Akt phosphorylation in monocytes of patients with
rheumatoid arthritis. Scand J Immunol. 85:155–161. 2017. View Article : Google Scholar
|
|
13
|
Zhang W, Du Z, Zhu J, Yu J and Xu Y:
Sprouty2 suppresses the inflammatory responses in rheumatoid
arthritis fibroblast-like synoviocytes through regulating the
Raf/ERK and PTEN/AKT signals. Mol Immunol. 67:532–539. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang CR, Shiau AL, Chen SY, Lin LL, Tai
MH, Shieh GS, Lin PR, Yo YT, Lee CH, Kuo SM, et al: Amelioration of
collagen-induced arthritis in rats by adenovirus-mediated PTEN gene
transfer. Arthritis Rheum. 58:1650–1656. 2008. View Article : Google Scholar
|
|
15
|
Malemud CJ: The PI3K/Akt/PTEN/mTOR
pathway: A fruitful target for inducing cell death in rheumatoid
arthritis? Future Med Chem. 7:1137–1147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Østergaard M, Jacobsson LT, Schaufelberger
C, Hansen MS, Bijlsma JW, Dudek A, Rell-Bakalarska M, Staelens F,
Haake R, Sundman-Engberg B, et al: MRI assessment of early response
to certolizumab pegol in rheumatoid arthritis: A randomised,
double-blind, placebo-controlled phase IIIb study applying MRI at
weeks 0, 1, 2, 4, 8 and 16. Ann Rheum Dis. 74:1156–1163. 2015.
View Article : Google Scholar :
|
|
17
|
Zhu Q, Huang J, Wang SZ, Qin ZH and Lin F:
Cobrotoxin extracted from Naja atra venom relieves arthritis
symptoms through anti-inflammation and immunosuppression effects in
rat arthritis model. J Ethnopharmacol. 194:1087–1095. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McWilliams DF, Ferguson E, Young A, Kiely
PD and Walsh DA: Discordant inflammation and pain in early and
established rheumatoid arthritis: Latent Class Analysis of Early
Rheumatoid Arthritis Network and British Society for Rheumatology
Biologics Register data. Arthritis Res Ther. 18:2952016. View Article : Google Scholar :
|
|
19
|
Zumbrennen-Bullough KB, Wu Q, Core AB,
Canali S, Chen W, Theurl I, Meynard D and Babitt JL: MicroRNA-130a
is up-regulated in mouse liver by iron deficiency and targets the
bone morphogenetic protein (BMP) receptor ALK2 to attenuate BMP
signaling and hepcidin transcription. J Biol Chem. 289:23796–23808.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jia Q, Cheng W, Yue Y, Hu Y, Zhang J, Pan
X, Xu Z and Zhang P: Cucurbitacin E inhibits TNF-α-induced
inflammatory cytokine production in human synoviocyte MH7A cells
via suppression of PI3K/Akt/NF-κB pathways. Int Immunopharmacol.
29:884–890. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang TT, Song Y, Ding YJ, Liao YH, Yu X,
Du R, Xiao H, Yuan J, Zhou ZH, Liao MY, et al: Atorvastatin
upregulates regulatory T cells and reduces clinical disease
activity in patients with rheumatoid arthritis. J Lipid Res.
52:1023–1032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao J, Zhou XL, Kong RN, Ji LM, He LL and
Zhao DB: microRNA-126 targeting PIK3R2 promotes rheumatoid
arthritis synovial fibro-blasts proliferation and resistance to
apoptosis by regulating PI3K/AKT pathway. Exp Mol Pathol.
100:192–198. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu X, Song Y, Huo R, Zhang J, Sun S, He
Y, Gao H, Zhang M, Sun X, Zhai T, et al: Cyr61 participates in the
pathogenesis of rheumatoid arthritis by promoting proIL-1β
production by fibroblast-like synoviocytes through an AKT-dependent
NF-κB signaling pathway. Clin Immunol. 157:187–197. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu C, Wang X, Ha T, Hu Y, Liu L, Zhang X,
Yu H, Miao J, Kao R, Kalbfleisch J, et al: Attenuation of cardiac
dysfunction and remodeling of myocardial infarction by
microRNA-130a are mediated by suppression of PTEN and activation of
PI3K dependent signaling. J Mol Cell Cardiol. 89:87–97. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee SH, Park JS, Byun JK, Jhun J, Jung K,
Seo HB, Moon YM, Kim HY, Park SH and Cho ML: PTEN ameliorates
autoimmune arthritis through down-regulating STAT3 activation with
reciprocal balance of Th17 and Tregs. Sci Rep. 6:346172016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song CL, Liu B, Shi YF, Liu N, Yan YY,
Zhang JC, Xue X, Wang JP, Zhao Z, Liu JG, et al: MicroRNA-130a
alleviates human coronary artery endothelial cell injury and
inflammatory responses by targeting PTEN via activating
PI3K/Akt/eNOS signaling pathway. Oncotarget. 7:71922–71936. 2016.
View Article : Google Scholar : PubMed/NCBI
|