Introduction
Low back pain (LBP) is a muscular disorder leading
to severe social and economic burden. It has been shown that LBP is
mainly associated with intervertebral disc (IVD) degeneration (IDD)
(1,2). IDD is characterized by a series of
pathogenic processes, including cellular, biochemical, and
structural impairment, which result in a metabolic imbalance of the
extracellular matrix (ECM) (3,4).
Previous studies have demonstrated that catabolic proteinases, such
as matrix metalloproteinases (MMPs) and a disintegrin and
metalloproteinase with thrombospondin motifs (ADAMTSs), are
upregulated in IDD; this is the main cause of ECM degradation
during IDD progression (5,6).
While the proteolytic activity of MMPs and aggrecanases plays an
important role in breaking down the disc matrix, another constant
feature of disc degeneration is angiogenesis.
Angiopoietin (Ang) plays crucial roles in successful
angiogenesis (7). Upregulation of
Ang-2 expression constitutes a critical step in the initiation of
the inflammatory process (8). On
its own, Ang-2 can recruit myeloid cells and induce inflammation
even in the absence of preceding pro-inflammatory stimuli (9). Mice lacking Ang-2 cannot elicit an
inflammatory response but administration of recombinant Ang-2
restores the inflammatory process (10). Ang-2 facilitates the activities of
inflammatory molecules such as tumor necrosis factor and
interleukin (IL)-1 exaggerates inflammatory responses (11,12). These key inflammatory cytokines
induce the expression of genes encoding matrix-degrading enzymes in
IDD (13). Previous studies have
shown that Ang-1 is crucial for the survival of nucleus pulposus
(NP) cells (14). However,
limited studies have explored the function of Ang-2 in degenerative
NP cells.
Here, we investigated the role of Ang-2 in human
degenerative NP cells and IDD pathology. We verified the expression
of Ang-2 in human degenerative NP cells and determined that its
level increase with progressing degeneration. We demonstrated that
Ang-2 participates in ECM degradation in degenerative NP cells, as
well as in the occurrence and development of IDD. This effect is
mediated by the nuclear factor-κB (NF-κB) signaling pathway. Ang-2
stimulated expression of IL-1β in human degenerative NP cells.
Materials and methods
The collection and staging of human NP
tissue samples
This study was approved by the Institutional Ethics
Review Board of Tongji Medical College of Huazhong University of
Science and Technology. The investigation was carried out following
the rules of the Declaration of Helsinki. The patients or their
parents (on behalf of children) provided informed consent prior to
the study. NP tissues were collected from patients (n=20)
undergoing spinal surgery. Patients undergoing surgery for burst
fracture (n=3), lumbar disc herniation (n=8), lumbar spinal
stenosis (n=5) or spondylolysis (n=4) were enrolled in this study.
The patients comprised 23 males and 17 females aged from 13 to 59
years. The T2 weighted magnetic resonance images were obtained at
1.5 Tesla resolution and the tissues were evaluated using the
Thompson grading system and Pfirrmann classification system
(15,16). Among the NP specimens, 3 specimens
were classified as grade II and 7 specimens as grade III; 5 disc
specimens from patients with lumbar disc herniation or
spondylolysis were classified as grade IV and the others were grade
V.
Immunohistochemistry
Samples from human NP tissues were fixed in 4%
paraformaldehyde in phosphate buffer (pH 7.4) for 24 h. Paraffin
sections were cut into 4-µm thick sections. The sections
were dewaxed in xylene, then rehydrated in graded alcohol series.
Antigen retrieval was performed by microwave in Tris-EDTA pH 9.0
for 15 min. Followed by washing with phosphate-buffered saline
(PBS), the sections were blocked with endogenous peroxidase
activities in 3% H2O2. Subsequently the
samples were incubated with anti-Ang-2 (sc-7015, 1:200; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), anti-collagen II
(ab34712, 1:200) and anti-MMP13 (ab39012, 1:200) (both from Abcam,
Cambridge, MA, USA). After labeling with a secondary antibody
(1:2,000) conjugated to horseradish peroxidase, the sections were
stained with diaminobenzidine (DAB) and counterstained with
hematoxylin. Images were captured using an IX71 phase microscope
(Olympus, Tokyo, Japan).
Isolation of NP cells
NP cells were isolated as previously described
(17,18). After isolation, the NP cells were
plated and expanded for 3 weeks in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 20% fetal bovine serum (FBS), and
1% penicillin/streptomycin at 37°C in a 5% CO2
incubator. The culture medium was replaced twice a week. All NP
cells were isolated from degenerated samples. The cells passaged
two to three times were used for subsequent experiments.
Exogenous Ang-2 treatment of NP
cells
When cultured NP cells reached 80% confluence,
2×104 cells were seeded into 96-well plates and
subjected to serum starvation overnight to synchronize the cell
cycles. The cells were then treated with various concentrations of
exogenous Ang-2 (R&D Systems, Minneapolis, MN, USA) for 24 h.
They were then harvested for mRNA and protein analysis. To evaluate
the signaling pathways in NP cells, cells were treated with various
concentrations of Ang-2 for 24 h, and indicated concentrations of
the NF-κB inhibitor BAY11-7082 (Beyotime, Nantong, China) were
added for 1 h. Each treatment was performed in 3 different
wells.
Quantitative western blotting
The culture supernatants were collected and cells
were lysed for 20 min in cold radioimmunoprecipitation (RIPA) lysis
buffer (Beyotime, Beijing, China). Protein lysates were resolved by
SDS-PAGE, and transferred onto polyvinylidene fluoride membranes
(Amersham Biosciences, Piscataway, NJ, USA). The membranes were
incubated with primary antibodies. In another experiment, nuclear
protein was extracted using the CelLytic NuClear extraction kit
(Sigma-Aldrich, St. Louis, MO, USA) following the manufacturer's
instructions. Nuclear proteins were incubated with anti-p65
antibodies for 2 h. Immunoreactive bands were quantified using
ImageQuant LAS 400 software (GE Healthcare Life Sciences, Logan,
UT, USA) and calculated by normalization to the reference bands of
β-actin or lamin A. The primary antibodies used were as follows:
anti-Ang-2 (sc-7015, 1:2,000) was purchased from Santa Cruz
Biotechnology, Inc. Anti-collagen II (ab34712, 1:2,000),
anti-aggrecan (ab3778, 1:1,500), anti-MMP13 (ab39012, 1:3,000),
anti-ADAMTS4 (ab185722, 1:3,000), IL-1β (ab2105, 1:3,000) and
anti-p65 antibodies (ab16502, 1:2,000) were all obtained from
Abcam.
RNA extraction and qRT-PCR
Total RNA was isolated from harvested cells and
tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer's instructions. qRT-PCR was carried
out using the Power SYBR-Green PCR Master Mix and a 7900 HT
thermocycler (Applied Biosystems, Foster City, CA, USA). Briefly,
RNA was denatured for 5 min at 70°C and placed on ice for 5 min.
Denatured RNA was added to a mixture of MMLV-RT, MMLV-RT buffer,
HRP (RRI)/RNase inhibitor, and dNTPs, and incubated for 60 min at
42°C. The reagent was then inactivated by heating at 95°C for 5
min. After amplification, relative gene expression levels were
determined using the 2−ΔΔCt method. β-actin was used as
an endogenous control to normalize mRNA levels. The expression of
the following genes was investigated: Ang-2 forward,
5′-ACTGTGTCCTCTTCCACCAC-3′ and reverse,
5′-GGATGTTTAGGGTCTTGCTTT-3′; MMP13 forward,
5′-CCCAACCCTAAACATCCAA-3′ and reverse, 5′-AAACAGCTCCGCATCAACC-3′;
ADAMTS4 forward, 5′-ACCCAAGCATCCGCAATC-3′ and reverse,
5′-TGCCCACATCAGCCATAC-3′; collagen II forward,
5′-TCCAGATGACCTTCCTACGC-3′ and reverse, 5′-GGTATGTTTCGTGCAGCCAT-3′;
aggrecan forward, 5′-TGAGCGGCAGCACTTTGAC-3′ and reverse,
5′-TGAGTACAGGAGGCTTGAG-3′; IL-1β forward, 5′-ATGGCTTATTACAGTGGCA-3′
and reverse, 5′-TGTAGTGGTGGTCGGAGA-3′, Homo β-actin forward,
5′-AGCGAGCATCCCCCAAAGTT-3′ and reverse,
5′-GGGCACGAAGGCTCATCATT-3′.
Statistical analysis
All results are representative of three independent
experiments, with three replicates per experiment, and expressed as
the mean ± SD. Two-tailed unpaired Student's t-test was used to
compare two groups, and one-way analysis of variance (ANOVA) was
used to compare three or more groups to test significant
differences. P<0.05 was considered to be statistically
significant.
Results
Expression of Ang-2 in human NP
tissues
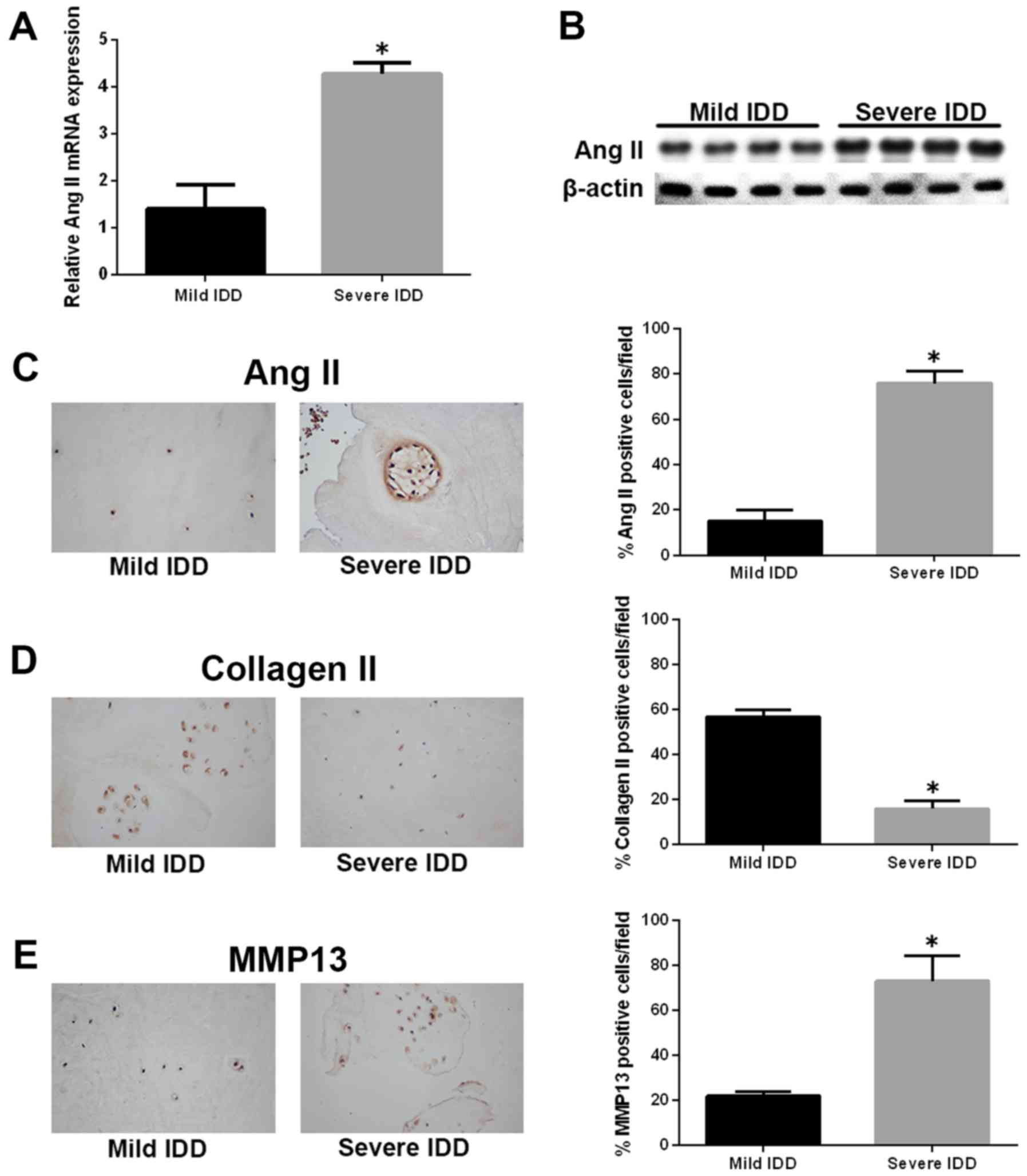
To explore the role of Ang-2 in the pathogenesis of
IDD, we first assessed the expression of the ANG2 gene in
human IVD samples by quantitative real-time polymerase chain
reaction (qRT-PCR). The expression of ANG2 gene was markedly
upregulated in NP tissues from severe IDD samples compared with
those from mild IDD samples (Fig.
1A). Similar results were observed by western blotting, and
Ang-2 protein levels were significantly increased in the severe IDD
group compared with the mild IDD group (Fig. 1B). Immunohistochemistry revealed
that Ang-2-positive cells were significantly more numerous in the
severe IDD group compared with the mild IDD group (Fig. 1C). Moreover, the amounts of type
II collagen-positive cells and MMP13-positive cells were decreased
and increased, respectively, in severe IDD compared with mild IDD
group (Fig. 1D and E).
Ang-2 treatment promotes ECM degradation
in human degenerative NP cells
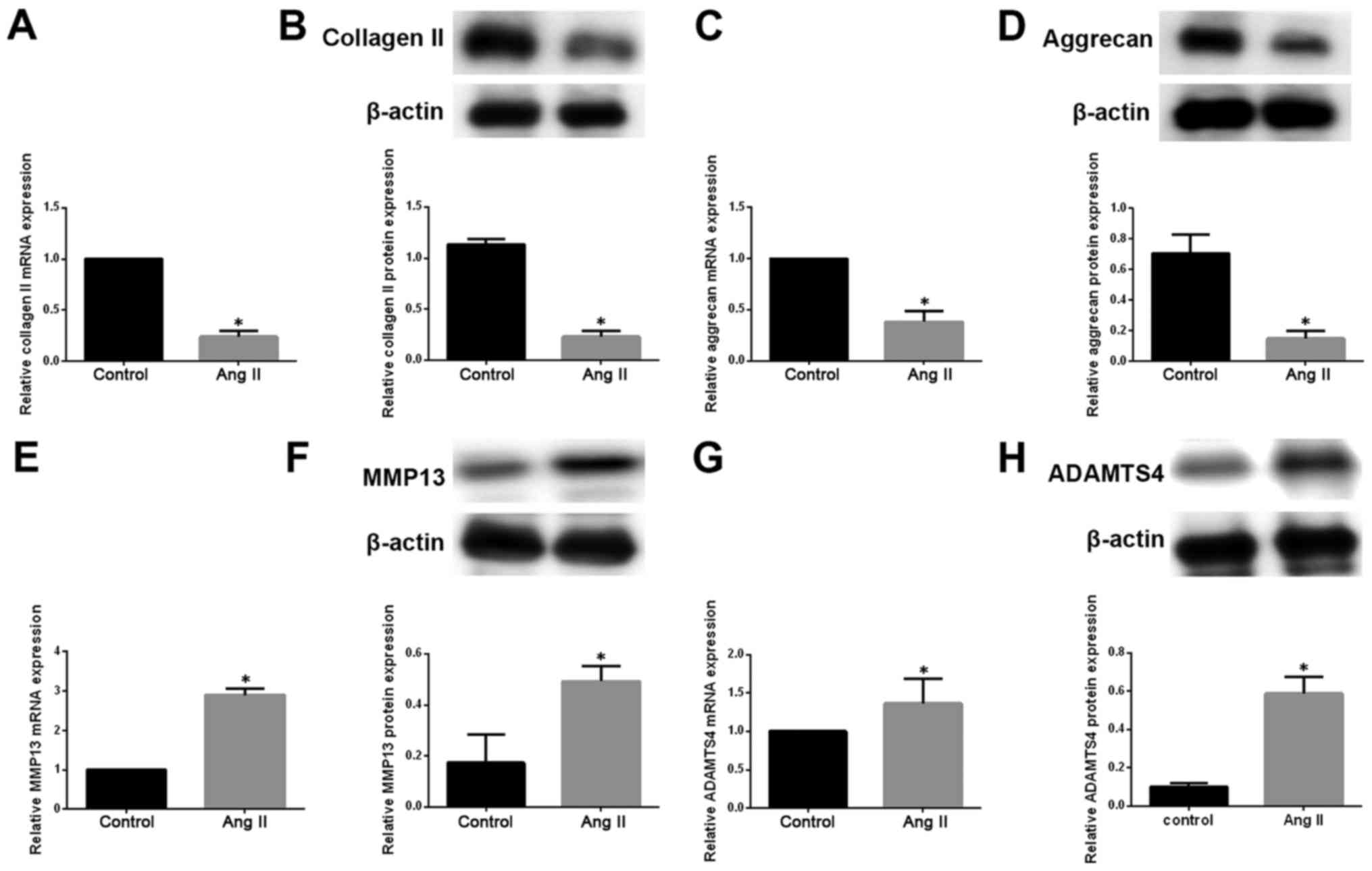
We then investigated the impact of Ang-2 on ECM
metabolism in degenerative NP cells. We measured the mRNA and
protein levels of type II collagen and aggrecan by qRT-PCR and
western blotting, respectively, in degenerative NP cells exposed to
exogenous Ang-2. Ang-2 treatment resulted in a pronounced
downregulation of type II collagen and aggrecan at both mRNA and
protein levels (Fig. 2A–D). ECM
catabolic proteinases, such as MMPs and ADAMTSs, are highly
expressed in degenerative IVD tissue and cells, and are involved in
ECM degradation during the development of IDD (19). We therefore measured the
expression of MMP13 and ADAMTS4 in degenerative NP cells. We
observed that the expression of MMP13 and ADAMTS4 on mRNA and
protein levels was significantly increased in degenerative NP cells
treated with exogenous Ang-2 compared with untreated controls
(Fig. 2E–H).
Ang-2 induces activation of the NF-κB
pathway
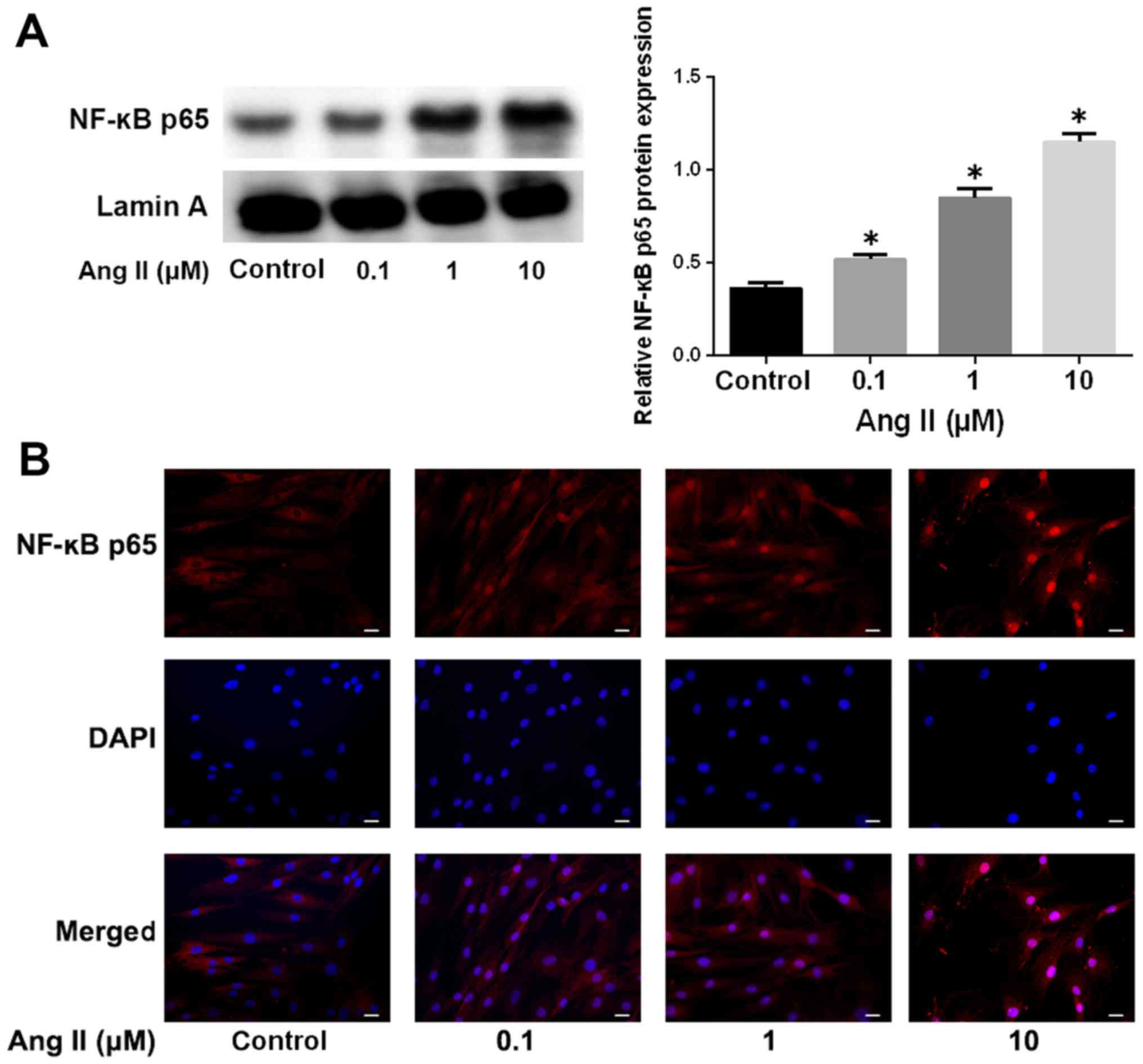
It has been reported that the NF-κB pathway is
aberrantly activated in IDD (20,21). We therefore asked whether the
NF-κB signaling pathway is involved in Ang-2-induced ECM
degradation. We analyzed NF-κB nuclear translocation by western
blotting and immunofluorescence. As shown in Fig. 3A, Ang-2 treatment markedly
increased the protein levels of NF-κB p65 in a nuclear extract, in
a dose-dependent manner, which was indicative of the activation of
the NF-κB pathway. The induction of NF-κB p65 nuclear translocation
by Ang-2 was also verified by immunofluorescence staining (Fig. 3B).
The effect of Ang-2 on ECM degradation is
mediated by NF-κB in human degenerative NP cells
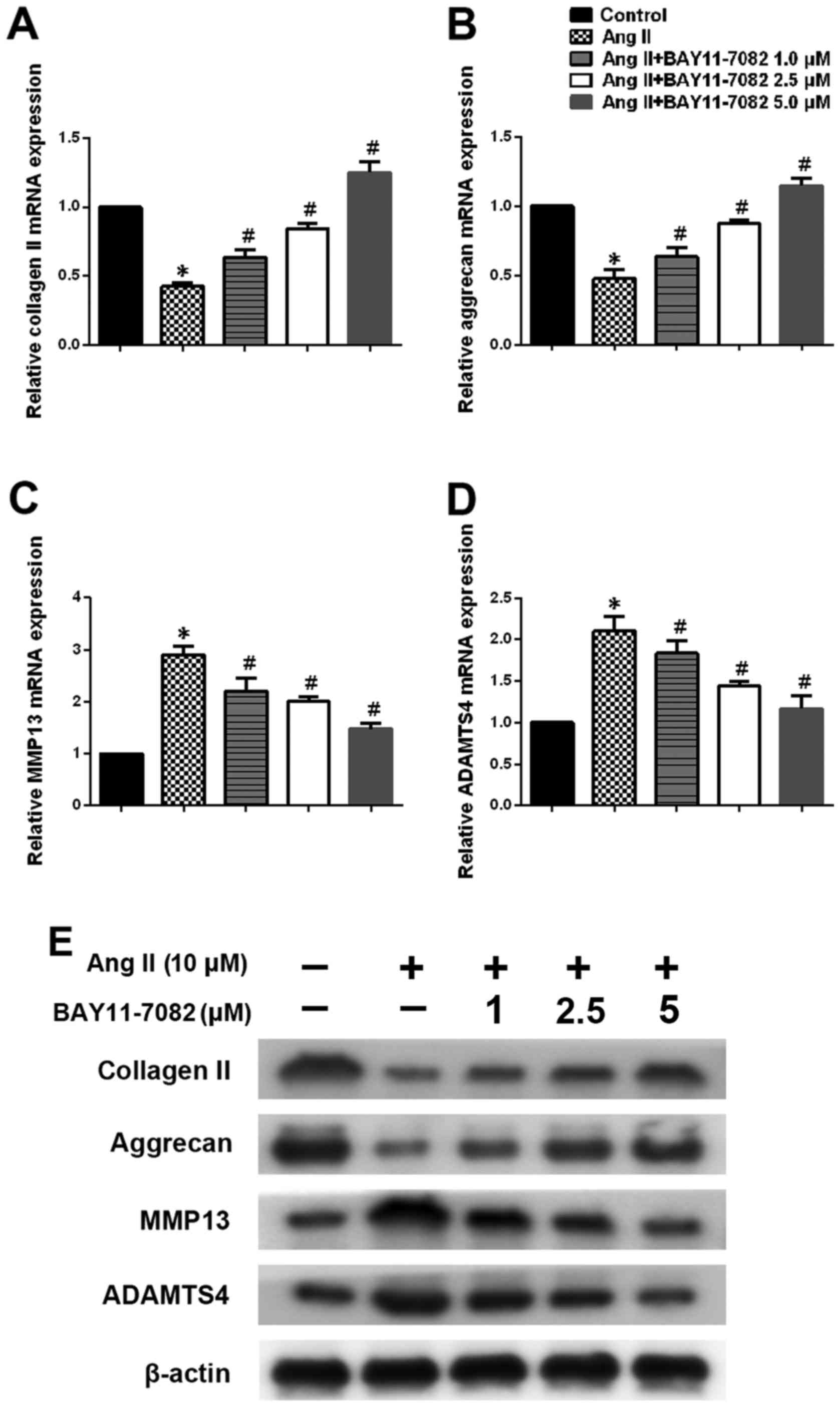
We next determined the expression of type II
collagen, aggrecan, MMP13 and ADAMTS4 in NP cells stimulated by
Ang-2 in the presence or absence of NF-κB inhibitor BAY11-7082.
Ang-2 treatment led to a marked downregulation of type II collagen
and aggrecan expression in NP cells, whereas NF-κB inhibition
reversed this effect in a dose-dependent manner (Fig. 4A, B and E). We also assessed the
expression of MMP13 and ADAMTS4 on mRNA and protein levels.
Upregulation of MMP13 and ADAMTS4 on stimulation with Ang-2 was
significantly attenuated in the presence of the NF-κB inhibitor
(Fig. 4C–E).
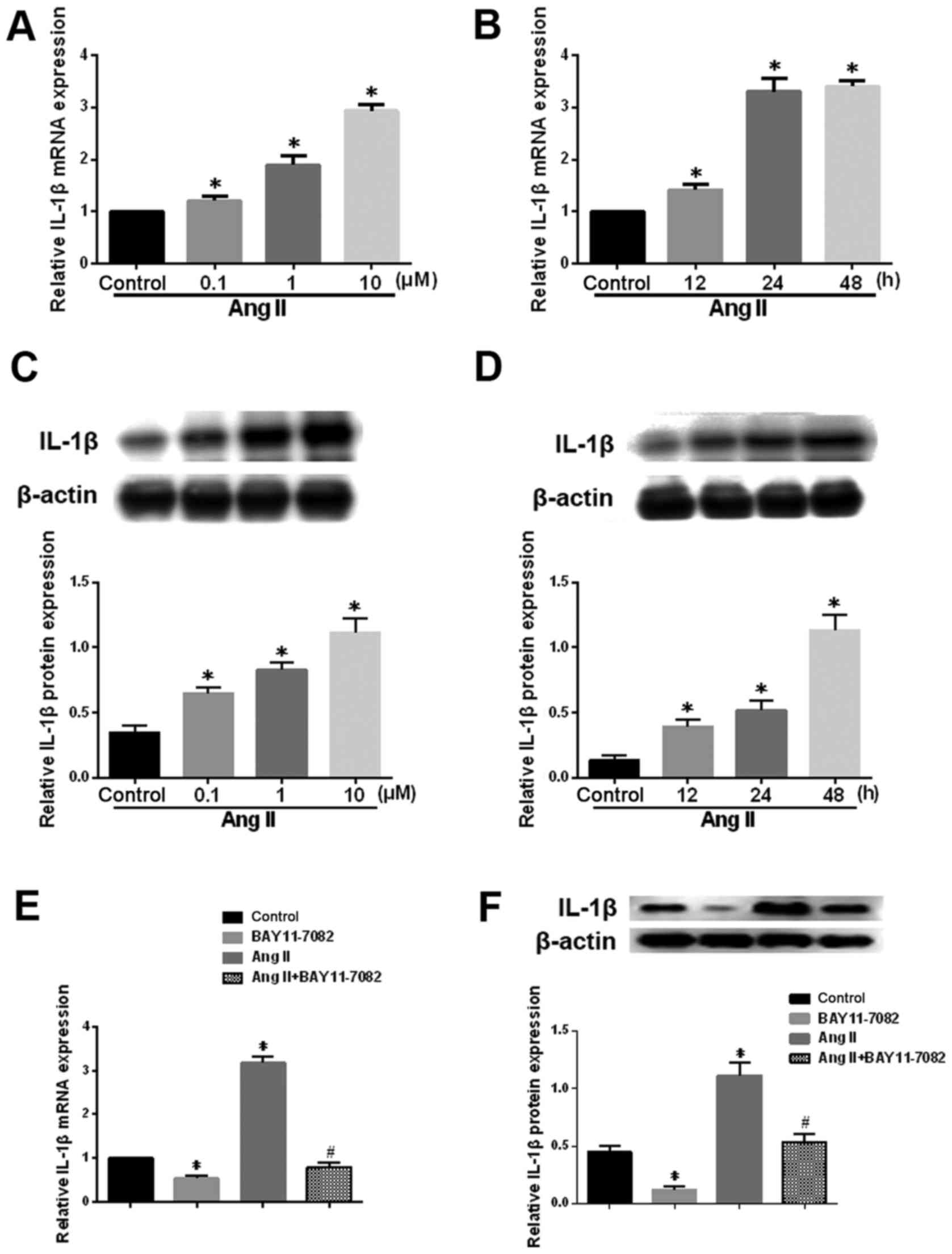
Ang-2 affects the expression of IL-1β in
human degenerative NP cells
The potent pro-inflammatory cytokine IL-1β plays an
important role in the pathogenesis of IDD by hampering matrix
synthesis and promoting the production of degradative enzymes
(22). Ang-2 treatment
significantly upregulated IL-1β mRNA and protein levels in NP cells
a dose- and time-dependent manner (Fig. 5A-D). Furthermore, this effect was
significantly attenuated in the presence of the NF-κB inhibitor
(Fig. 5E and F). These results
suggest that the NF-κB pathway mediates the Ang-2-induced
expression of IL-1β in human NP cells.
Discussion
Accumulating evidence implicates altered cell
function and decrease in the overall number of matrix-producing
cells as potential contributors to IDD (23). Therefore, the control of matrix
homeostasis, i.e., synthesis and degradation, is important.
Numerous studies have focused on the Ang-2 epigenetic regulation of
pathogenesis and potential Ang-2 targets in the tumor, specifically
Ang/Tie signaling; however, the role of Ang-2 in IDD remains
largely unknown. In the present study, we show that increased
secretion of Ang-2 by NP cells is involved in IDD. By employing
loss-of-function and gain-of-function approaches, we determined
that Ang-2 plays a role in the degradation of ECM. Ang-2 promoted
the activation of MMPs and ADAMTSs through the NF-κB signaling
pathway. Inhibition of NF-κB significantly suppressed this effect.
Our data additionally indicated that Ang2 stimulates the expression
of the pro-inflammatory cytokine IL-1β in human degenerative NP
cells.
While gene expression profiling studies have
identified endothelial cells as the primary source of Ang-2
(24), other studies have
revealed that Ang-1 and -2 are also located at sites of
endochondral bone formation in the growing skeleton (25). Mesenchymal stem cells, which
express Ang-1 and high levels of Ang-2, possess a pronounced
ability to elicit remodeling of preexisting vasculature at a site
of injury and support new vessel formation (26). In the present study, examination
of Ang-2 transcript and protein levels in NP cells derived from
mild IDD and severe IDD confirmed that the expression of Ang-2
considerably increases during IDD progression. In addition,
immunohistochemistry experiments revealed higher numbers of Ang-2
and MMP13-positive cells, and lower numbers of collage II-positive
cells, in severe IDD than in mild IDD.
Accumulating evidence suggests that
neovascularization of IVD is a pathological phenomenon as normal
(healthy) discs are primarily avascular structures. A recent study
evaluated the effect of extracellular endothelial microparticles
(EMPs) and soluble protein factors (SUP fraction) produced by ECs
on matrix catabolismin annulus fibrosus (AF) cells. This study
revealed enhanced matrix catabolism as a molecular consequence of
AF exposure to ECEMPs (27).
Another study showed that AF cells from degenerated discs secrete
factors that stimulate ECs to produce factors known to induce
matrix degradation, angiogenesis and innervation (28). In the present study, we
investigated the effect of Ang-2 on the expression of MMP13,
ADAMTS4, aggrecan and collagen II. Gene expression analysis
revealed that MMP13 and ADAMTS4 mRNA levels were
significantly higher in the Ang-2 treatment group than in the
control group. The effect of Ang-2 on aggrecan and type II collagen
showed the opposite trend. Similarly, it has been shown that the
treatment of cultured retinal microvascular endothelial cells with
increasing amounts of purified Ang-2 led to a significant increase
in MMP9 levels during retinal neovascularization (29).
In addition to the Ang/Tie system, numerous studies
have focused on novel signaling pathways in non-Tie-expressing
cells. Ang-2 has been shown to induce pericyte apoptosis via the
p53 pathway under high glucose in diabetic retinopathy (30). Ang-2 addtionally exerts
synergistic effects under high glucose conditions on astrocyte
apoptosis via the GSK-3β/β-catenin pathway (31). In another study, Ang-2 acts as a
vessel-destabilizing molecule in TIE2-expressing ECs, and
additionally as a direct proangiogenic molecule in TIE2-negative
angiogenic ECs via integrin signaling (32). Moreover, Ang-2 interacts directly
with integrins and activates the downstream focal adhesion kinase
(FAK), ILK, Akt and ERK signaling pathways in Tie2-deficent glioma
and breast cancer cells (33).
As a rapidly inducible transcription factor, NF-κB
regulates the expression of numerous genes to mediate various
cellular processes, including cell proliferation, survival and
differentiation (34).
Furthermore, NF-κB is an important pathway that plays a crucial
role in both anabolism and catabolism in IVD. The decreased gene
expression of aggrecan and type II collagen induced by IL-1β was
reversed by inhibiting NF-κB signaling (35). NF-κB pathway promotes catabolic
gene expression such as MMP-1, MMP-3, MMP-13, ADAMTS-4 and ADAMTS-5
expression in IL-1β stimulated animal NP cells (36). Here, we mainly studied the NF-κB
signaling pathway in Ang-2 treated degenerative NP cells. The
results showed that the effect of Ang-2 on ECM degradation is
mediated by NF-κB signaling pathway, at least in part. When we used
an inhibitor of NF-κB, the effect of Ang-2 on ECM degradation was
attenuated. Catabolism relative ADAMTS4 and MMP13 expression
induced by Ang-2 decreased dramatically. However, increased
synthesis collagen II and aggrecan were detected after BAY11-7082
treatment. Future studies should investigate the specific mechanism
whereby Ang-2 activates NF-κB signaling in degenerative NP cells.
In addition, Ang-2 induces the expression of IL-1β in a time- and
concentration-dependent manner in human degenerative NP cells. This
process was additionally weaken by the inhibitor of NF-κB.
David et al observed that expression of
neovascularization growth factors in the disc is associated with
post-surgical pain, implicating this process in a clinically
relevant pathology (37). A phase
III AVAGAST trial is currently under way to evaluate Ang-2 as a
biomarker in gastric cancer (38). To the best of our knowledge, this
study provides evidence for the first time that Ang-2 levels in
degenerative NP cells increase with increasing severity of IDD.
Upon Ang-2 exposure, MMPs and ADAMTSs become dysregulated in
degenerative NP cells and degrade aggrecan and type II collagen.
This contributes to the pathogenesis of IDD. Ang-2 is also involved
in the pathogenesis of IDD, at least partially, by inducing NF-κB
activation and IL-1β expression.
In conclusion, our study suggests that the
inhibition of Ang-2 may represent a novel therapeutic approach for
the treatment of IDD.
Acknowledgments
Not applicable.
Notes
[1]
Funding
This study was supported by the National Natural
Science Foundation of China (nos. 81272025 and 81541056).
[2] Availability
of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
CY designed the study. CY and KW wrote the
manuscript. KW, LK, and WL conducted the experiments. YS, XW, YZ,
WH, KZ, SL, LT and RL collected the data. YS, XW, and YZ analyzed
the data. CY reviewed and revised the manuscript.
[4] Ethics
approval and consent to participate
This study was approved by the Institutional Ethics
Review Board of Tongji Medical College of Huazhong University of
Science and Technology.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Deyo RA and Weinstein JN: Low back pain. N
Engl J Med. 344:363–370. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sakai D and Andersson GB: Stem cell
therapy for intervertebral disc regeneration: obstacles and
solutions. Nat Rev Rheumatol. 11:243–256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kepler CK, Ponnappan RK, Tannoury CA,
Risbud MV and Anderson DG: The molecular basis of intervertebral
disc degeneration. Spine J. 13:318–330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Phillips KL, Jordan-Mahy N, Nicklin MJ and
Le Maitre CL: Interleukin-1 receptor antagonist deficient mice
provide insights into pathogenesis of human intervertebral disc
degeneration. Ann Rheum Dis. 72:1860–1867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Le Maitre CL, Freemont AJ and Hoyland JA:
Localization of degradative enzymes and their inhibitors in the
degenerate human intervertebral disc. J Pathol. 204:47–54. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bachmeier BE, Nerlich A, Mittermaier N,
Weiler C, Lumenta C, Wuertz K and Boos N: Matrix metalloproteinase
expression levels suggest distinct enzyme roles during lumbar disc
herniation and degeneration. Eur Spine J. 18:1573–1586. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Makinde TO and Agrawal DK: Increased
expression of angiopoietins and Tie2 in the lungs of chronic
asthmatic mice. Am J Respir Cell Mol Biol. 44:384–393. 2011.
View Article : Google Scholar :
|
|
8
|
Kim H and Koh GY: Ang2, the instigator of
inflammation. Blood. 118:4767–4768. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scholz A, Lang V, Henschler R, Czabanka M,
Vajkoczy P, Chavakis E, Drynski J, Harter PN, Mittelbronn M, Dumont
DJ, et al: Angiopoietin-2 promotes myeloid cell infiltration in a
β2-integrin-dependent manner. Blood. 118:5050–5059.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fiedler U, Reiss Y, Scharpfenecker M,
Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S,
Suttorp N, et al: Angiopoietin-2 sensitizes endothelial cells to
TNF-alpha and has a crucial role in the induction of inflammation.
Nat Med. 12:235–239. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fiedler U and Augustin HG: Angiopoietins:
a link between angiogenesis and inflammation. Trends Immunol.
27:552–558. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tabruyn SP, Colton K, Morisada T, Fuxe J,
Wiegand SJ, Thurston G, Coyle AJ, Connor J and McDonald DM:
Angio-poietin-2-driven vascular remodeling in airway inflammation.
Am J Pathol. 177:3233–3243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Setton LA and Chen J: Mechanobiology of
the intervertebral disc and relevance to disc degeneration. J Bone
Joint Surg Am. 88(Suppl 2): 52–57. 2006.PubMed/NCBI
|
|
14
|
Sakai D, Nakamura Y, Nakai T, Mishima T,
Kato S, Grad S, Alini M, Risbud MV, Chan D, Cheah KS, et al:
Exhaustion of nucleus pulposus progenitor cells with ageing and
degeneration of the intervertebral disc. Nat Commun. 3:12642012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thompson JP, Pearce RH, Schechter MT,
Adams ME, Tsang IK and Bishop PB: Preliminary evaluation of a
scheme for grading the gross morphology of the human intervertebral
disc. Spine. 15:411–415. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pfirrmann CW, Metzdorf A, Zanetti M,
Hodler J and Boos N: Magnetic resonance classification of lumbar
intervertebral disc degeneration. Spine. 26:1873–1878. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu W, Zhang Y, Feng X, Li S, Gao Y, Wang
K, Song Y, Yang S, Tu J, Shao Z, et al: Inhibition of microRNA-34a
prevents IL-1β-induced extracellular matrix degradation in nucleus
pulposus by increasing GDF5 expression. Exp Biol Med (Maywood).
241:1924–1932. 2016. View Article : Google Scholar
|
|
18
|
Wu X, Liu W, Duan Z, Gao Y, Li S, Wang K,
Song Y, Shao Z, Yang S and Yang C: The involvement of protease
nexin-1 (PN1) in the pathogenesis of intervertebral disc (IVD)
degeneration. Sci Rep. 6:305632016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pockert AJ, Richardson SM, Le Maitre CL,
Lyon M, Deakin JA, Buttle DJ, Freemont AJ and Hoyland JA: Modified
expression of the ADAMTS enzymes and tissue inhibitor of
metalloproteinases 3 during human intervertebral disc degeneration.
Arthritis Rheum. 60:482–491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian Y, Yuan W, Fujita N, Wang J, Wang H,
Shapiro IM and Risbud MV: Inflammatory cytokines associated with
degenerative disc disease control aggrecanase-1 (ADAMTS-4)
expression in nucleus pulposus cells through MAPK and NF-κB. Am J
Pathol. 182:2310–2321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Wang H, Yang H, Li J, Cai Q,
Shapiro IM and Risbud MV: Tumor necrosis factor-α- and
interleukin-1β-dependent matrix metalloproteinase-3 expression in
nucleus pulposus cells requires cooperative signaling via syndecan
4 and mitogen-activated protein kinase-NF-κB axis: implications in
inflammatory disc disease. Am J Pathol. 184:2560–2572. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Phillips KL, Cullen K, Chiverton N,
Michael AL, Cole AA, Breakwell LM, Haddock G, Bunning RA, Cross AK
and Le Maitre CL: Potential roles of cytokines and chemokines in
human intervertebral disc degeneration: interleukin-1 is a master
regulator of catabolic processes. Osteoarthritis Cartilage.
23:1165–1177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roughley PJ: Biology of intervertebral
disc aging and degeneration: involvement of the extracellular
matrix. Spine. 29:2691–2699. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fiedler U, Scharpfenecker M, Koidl S,
Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G and Augustin HG:
The Tie-2 ligand angiopoietin-2 is stored in and rapidly released
upon stimulation from endothelial cell Weibel-Palade bodies. Blood.
103:4150–4156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Horner A, Bord S, Kelsall AW, Coleman N
and Compston JE: Tie2 ligands angiopoietin-1 and angiopoietin-2 are
coexpressed with vascular endothelial cell growth factor in growing
human bone. Bone. 28:65–71. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nagano M, Kimura K, Yamashita T, Ohneda K,
Nozawa D, Hamada H, Yoshikawa H, Ochiai N and Ohneda O: Hypoxia
responsive mesenchymal stem cells derived from human umbilical cord
blood are effective for bone repair. Stem Cells Dev. 19:1195–1210.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pohl PH, Lozito TP, Cuperman T, Yurube T,
Moon HJ, Ngo K, Tuan RS, St Croix C , Sowa GA, Rodrigues LM, et al:
Catabolic effects of endothelial cell-derived microparticles on
disc cells: implications in intervertebral disc neovascularization
and degeneration. J Orthop Res. 34:1466–1474. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moon HJ, Yurube T, Lozito TP, Pohl P,
Hartman RA, Sowa GA, Kang JD and Vo NV: Effects of secreted factors
in culture medium of annulus fibrosus cells on microvascular
endothelial cells: elucidating the possible pathomechanisms of
matrix degradation and nerve in-growth in disc degeneration.
Osteoarthritis Cartilage. 22:344–354. 2014. View Article : Google Scholar
|
|
29
|
Das A, Fanslow W, Cerretti D, Warren E,
Talarico N and McGuire P: Angiopoietin/Tek interactions regulate
mmp-9 expression and retinal neovascularization. Lab Invest.
83:1637–1645. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park SW, Yun JH and Kim JH, Kim KW, Cho CH
and Kim JH: Angiopoietin 2 induces pericyte apoptosis via α3β1
integrin signaling in diabetic retinopathy. Diabetes. 63:3057–3068.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yun JH, Park SW and Kim JH, Park YJ, Cho
CH and Kim JH: Angiopoietin 2 induces astrocyte apoptosis via
αvβ5-integrin signaling in diabetic retinopathy. Cell Death Dis.
7:e21012016. View Article : Google Scholar
|
|
32
|
Felcht M, Luck R, Schering A, Seidel P,
Srivastava K, Hu J, Bartol A, Kienast Y, Vettel C, Loos EK, et al:
Angiopoietin-2 differentially regulates angiogenesis through TIE2
and integrin signaling. J Clin Invest. 122:1991–2005. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee HS, Oh SJ, Lee KH, Lee YS, Ko E, Kim
KE, Kim HC, Kim S, Song PH, Kim YI, et al: Gln-362 of
angiopoietin-2 mediates migration of tumor and endothelial cells
through association with α5β1 integrin. J Biol Chem.
289:31330–31340. 2014. View Article : Google Scholar :
|
|
34
|
Hayden MS and Ghosh S: NF-κB, the first
quarter-century: remarkable progress and outstanding questions.
Genes Dev. 26:203–234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhongyi S, Sai Z, Chao L and Jiwei T:
Effects of nuclear factor kappa B signaling pathway in human
intervertebral disc degeneration. Spine. 40:224–232. 2015.
View Article : Google Scholar
|
|
36
|
Ellman MB, Kim JS, An HS, Kroin JS, Li X,
Chen D, Yan D, Buechter DD, Nakayama K, Liu B, et al: The
pathophysiologic role of the protein kinase Cδ pathway in the
intervertebral discs of rabbits and mice: in vitro, ex vivo, and in
vivo studies. Arthritis Rheum. 64:1950–1959. 2012. View Article : Google Scholar
|
|
37
|
David G, Ciurea AV, Iencean SM and Mohan
A: Angiogenesis in the degeneration of the lumbar intervertebral
disc. J Med Life. 3:154–161. 2010.PubMed/NCBI
|
|
38
|
Hacker UT, Escalona-Espinosa L, Consalvo
N, Goede V, Schiffmann L, Scherer SJ, Hedge P, Van Cutsem E,
Coutelle O and Büning H: Evaluation of angiopoietin-2 as a
biomarker in gastric cancer: results from the randomised phase III
AVAGAST trial. Br J Cancer. 114:855–862. 2016. View Article : Google Scholar : PubMed/NCBI
|