Introduction
Corneal endothelial cells (CECs) form a single
monolayer on the posterior surface of the cornea and serve a
pivotal function in the regulation of stromal hydration and the
maintenance of corneal transparency (1). Adult human CECs are
G1-arrested, leading to a decline of endothelial cell
density and subsequent endothelial dysfunction and loss of vision,
particularly following injury, aging and surgery (2–4).
The conventional approach involves transplantation of healthy donor
CECs, but a current global shortage of donor corneas necessitates
other options and demands the development of novel therapeutic
agents and strategies to induce cell proliferation in the corneal
endothelium.
Bradykinin (BK), a nonapeptide, is a major effector
of the kallikrein-kinin system that demonstrates a wide range of
biological activities, being involved in inflammation, pain,
angiogenesis and cell proliferation (5–12).
In ocular tissues, BK receptors (B1 and B2 receptors) are
abundantly distributed and may trigger ocular allergies and
inflammatory responses on the ocular surface (13). BK has been demonstrated to promote
cell proliferation through the B2 receptor and epidermal growth
factor receptor (EFGR) in ex vivo corneas, including bovine
corneal endothelial cells (8),
canine/human corneal epithelial cells (9,10)
and corneal keratocytes or fibroblasts in the Statens Seruminstitut
Rabbit (11,12). However, this phenomenon has not
been reported in rabbit CECs, and the exact cellular mechanisms
underlying BK-induced proliferation in CECs remain unknown.
Tight junctions (TJs), which are major components of
the cell junctional complex, are essential for the barrier function
of epithelium, epithelial proliferation and differentiation
(14,15). Zonula occludens-1 (ZO-1) is a key
TJ-associated protein that links junctional membrane proteins to
the cytoskeleton (14).
ZO-1-associated nucleic-acid-binding protein (ZONAB) is a Y-box
transcription factor that is recruited to TJs by binding to the Src
homology 3(SH3) domain of ZO-1 (14–16). ZONAB interacts with ZO-1 and
regulates the transcriptional activity of cell cycle genes,
including cyclin D1 and proliferating cell nuclear antigen (PCNA),
that modulate cell cycle progression and cell proliferation
(16–18). The ZO-1- and ZONAB-associated
pathway (ZO-1/ZONAB pathway) has been demonstrated to regulate
proliferation in epithelial cells derived from the renal proximal
tubule and retinal pigment epithelium (RPE) (16–20). However, little is known about the
effect of ZO-1 and ZONAB on CECs; the involvement of the ZO-1/ZONAB
pathway in BK-stimulated cell proliferation remains to be
examined.
Therefore, the purpose of the present study was to
explore the effect of BK on cell proliferation in cultured rabbit
corneal endothelial cells (RCECs), and to determine the
contribution of the ZO-1/ZONAB pathway to BK-induced RCEC
proliferation. To the best of our knowledge, the present study is
the first to demonstrate BK-stimulated cell proliferation and cell
cycle progress in RCECs, and that the underlying mechanisms
involved the activation of the ZO-1/ZONAB signaling pathway.
Materials and methods
Animals
A total of 34 New Zealand white rabbits
(Experimental Animal Center, University of South China, Hengyang,
China; weight, 1.5–2.0 kg; age, 50 days) were employed in the
present study. Rabbits were housed in individual cages under
standard conditions (room temperature at 25–27°C, humidity at
45–55% with 12 h light/dark cycle) with free access to standard
laboratory chow and sterile acidified water. All experimental
protocols were conducted in accordance with the Experimental Animal
Regulations established by The Ministry of Science and Technology
of the People's Republic of China, and the Guidelines for the Care
and Use of Laboratory Animals published by the National Institutes
of Health (Bethesda, MD, USA) (21). The study received ethical approval
from the ethics committee of the University of South China.
Cell culture
Isolation and establishment of RCECs was performed
as previously described, with modifications (22,23). Briefly, the rabbit corneal buttons
were obtained following enucleation. Corneal endothelia with
Descemet's membrane were dissected and peeled off under a
stereoscopic dissecting light microscope (SMZ800; Nikon
Corporation, Tokyo, Japan). Cells were then incubated in
disaggregating solution (300 U type I collagenase and 1%
antibiotic/antimycotic) in Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for
3 h at 37°C in 5% CO2. The medium was changed every
other day. When cells reached confluence (within 10–14 days), they
were enzymatically detached with 0.25% trypsin (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) and subcultured. RCECs
that had been passaged 2–4 times were used for the following
experiments.
Small interfering (si)RNA preparation,
screening and transfection
Three siRNA duplexes targeting ZONAB (GenBank
accession ID: AF171061.1) were designed using the siRNA Target
Finder and Design Tool (http://www.ambion.com; Ambion; Thermo Fisher
Scientific, Inc.) and National Center for Biotechnology Information
Basic Local Alignment Search Tool. Another scrambled sequence
siRNA, with no homology to the rabbit ZONAB gene, was used as a
siRNA negative control (NC-siRNA). All siRNAs were commercially
synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The
sequences of each siRNA targeting ZONAB, as well as the scramble
control were presented in Table
I.
 | Table IsiRNA and RT-PCR primer
sequences. |
Table I
siRNA and RT-PCR primer
sequences.
| A, siRNA sequences
used for ZONAB silencing |
|---|
| ZONAB siRNA1 |
5′-GAAUCAACAAGCAGCCAAUTTdTdT-3′
(sense) |
|
5′-AUUGGCUGCUUGUUGCUUCTTdTdT-3′
(antisense) |
| ZONAB siRNA2 |
5′-GAUCGGAGAGAUGAAGGAUTTdTdT-3′
(sense) |
|
5′-AUCCUUCAUCUCUCCGAUCTT dTdT-3′
(antisense) |
| ZONAB siRNA3 |
5′-GGAAUUUGAUGUGGUGGAATTdTdT-3′
(sense) |
|
5′-UUCCACCACAUCAAAUUCCTTdTdT-3′
(antisense) |
| Scramble
control |
5′-CGAGGAGACUUCCGAAUCUAUdTdT-3′
(sense) |
|
5′-ACGUGACACGUUCGGAGAATTdTdT-3′
(antisense) |
|
B, Primer sequences
used for RT-PCR analysis of ZONAB
|
| ZONAB |
5′-GCCATCAAGAAGAATAACCCACG-3′
(forward) |
|
5′-GCGTAACGACTCCCTTCCACA-3′ (reverse) |
| β-actin |
5′-GTTCGAGACCTTCAACACCCC-3′ (forward) |
|
5′-CCGGCCAGCCAGGTCCAGA-3′ (reverse) |
Transient siRNA transfection was performed using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocols, due to the high
transfection efficiency and low cytotoxicity of lipofectin
transfection (24). In short,
three siRNA sequences or the scrambled control siRNA complex (5
µl of each at the concentration of 20 µM, diluted
with diethyl pyrocabonate-treated water) with transfection reagents
were added to cultured cells at 50% confluence. After 48 h, mRNA or
protein was extracted to detect the transfection efficiency. The
ZONAB-siRNA that had the maximum inhibition rate was selected and
used for further in vitro experiments.
BK administration and experimental
groups
In the present study, cells in the logarithmic
growth phase were incubated with various concentrations (0.01, 0.1,
1.0 and 10.0 µM) of BK (Abcam, Cambridge, MA, USA) for the
indicated time intervals (24, 48, 72 and 96 h) at 37°C. The time
intervals and concentrations of BK were selected based on the
results of previous studies and were confirmed to effectively
induce cell proliferation (9–12).
When the cultures reached confluence, cells were plated onto
12-well culture plates (1 ml/well) for the measurement of ZO-1 and
ZONAB protein expression. RCECs were plated onto 24-well plates
(0.5 ml/well) for the examination of cell growth and morphology
under a phase-contrast microscope (CH2; Olympus Corporation, Tokyo,
Japan).
In order to investigate the causal function of
ZONAB-associated signaling in BK-induced cell proliferation, RCECs
were treated with BK alone, or BK treatment was combined with
ZONAB-siRNA transfection. The cells were randomly divided into 6
groups (n=8 each) as follows: Control group (neither BK treatment
nor ZONAB-siRNA transfection), BK group (treated with 1.0 µM
BK), NC-siRNA group (transfected with NC-siRNA), ZONAB-siRNA group
(transfected with the ZONAB-siRNA sequence), NC-siRNA+BK group (1.0
µM BK was administrated to cells transfected with NC-siRNA)
and ZONAB-siRNA+BK group (1.0 µM BK was administrated to
cells transfected with the ZONAB-siRNA sequence). Cell samples were
collected at 72 h. MTT assays were performed to detect cell
proliferation, and cell cycle distribution was analyzed using flow
cytometry, as subsequently described. ZO-1, ZONAB, PCNA and cyclin
D1 protein expression was detected by western blotting, together
with immunofluores-cence assay for ZONAB.
Cell proliferation assay
Cells (5×103/plate) were loaded in
96-well plates, maintained in DMEM with 10% FBS, and then treated
with 0.3% dimethyl sulfoxide (DMSO) or BK (0.01, 0.1, 1.0 and 10.0
µM), or BK (1.0 µM) with ZONAB-siRNA or NC-siRNA
transfection as aforementioned. At each exact time point (24, 48,
72 and 96 h), cells were treated with MTT reagent (10 µl)
for 4 h at 37°C and then with 100 µl DMSO overnight at 37°C.
Absorbance at 490 nm was measured usinga Bio-Rad microplate reader
(Model-680; Bio-Rad Laboratories, Inc., Hercules, CA, USA). Wells
containing culture medium but no cells served as controls. All
experiments were repeated five times to ensure consistent
results.
Flow cytometry
Confluent cells, treated for 72 h, were collected
using 0.25% trypsin, fixed with 70% absolute ethyl alcohol at 4°C
overnight, washed twice in 3 ml PBS, and stained in darkness with
PBS containing propidium iodide (50 µg/ml; Roche Diagnostics
Co., Ltd., Shanghai, China) at 4°C for 1 h and RNAse (100
µg/ml; Thermo Fisher Scientific, Inc.) at 37°C for 30 min.
The samples were then analyzed using a FACSCanto II flow cytometer
(BD Biosciences, Franklin Lakes, NJ, USA). Based on DNA content,
the percentage of cells in each stage of the cell cycle
(G0/G1, S and G2/M phases) was
calculated using ModFit LT 3.0(Verity Software House, Inc.,
Topsham, ME, USA).
Semi-quantitative reverse transcription
polymerase chain reaction (RT-PCR)
Total RNA was extracted using an RNeasy Mini kit
(Invitrogen; Thermo Fisher Scientific, Inc.), and RT was performed
using a High Capacity Reverse Transcription kit (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
PCR primers targeting ZONAB were designed and synthesized by Sangon
Biotech Co., Ltd and are presented in Table I. Thermocycler conditions were as
follows: 10 min of initial activation at 95°C, followed by 40
cycles of 15 sec denaturation at 95°C, and 1 min annealing and
extension at 60°C. The identity of each PCR product was confirmed
by size determination using 2% agarose gels followed by ethidium
bromide staining and the PCR marker, using an EC3 Imaging System
(BioImaging Systems; UVP, Inc., Upland, CA, USA). The intensities
of the bands were densitometrically quantified using Quantity One
1D software (version 4.6.9, Bio-Rad Laboratories, Inc., Hercules,
CA, USA), and β-actin was used as an internal reference in each
reaction.
Western blotting
Confluent cells were washed, scraped, collected, and
centrifuged at 226,000 × g for 1 h at 4°C to yield whole cell
extract. Nuclear ZONAB protein and cytoplasmic ZO-1 protein were
extracted using a Bicinchoninic acid protein assay (Nanjing KeyGen
Biotech Co., Ltd.) according to the manufacturer's protocol.
Samples (50 µg protein) were denatured, subjected to 10%
SDS-PAGE, and transferred to nitrocellulose membranes.
Subsequently, the membrane was blocked with 5% non-fat milk in TBST
(containing 0.2% Tween-20, 20 mmol/l Tris-HCl, and 150 mmol/l NaCl,
pH 7.14; non-fat milk, Bio-Rad Laboratories, Inc., Hercules, CA,
USA; Tris, Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 1 h
at 37°C, then incubated withanti-ZO-1 (1:500; cat. no. ab-61357,
Abcam), anti-ZONAB (1:1,000; cat. no. 40–2800; Invitrogen; Thermo
Fisher Scientific, Inc.), anti-PCNA (1:1,000; cat. no. 60097-1-Ig;
ProteinTech Group, Inc., Chicago, IL, USA), anti-cyclin D1
(1:1,000; cat. no BS-0623R; BIOSS, Beijing, China) and anti-β-actin
(1:1,000; cat. no. 60008-1-Ig; ProteinTech Group, Inc.) at 4°C
overnight. The membrane was washed three times, blocked with 5%
non-fat milk in TBST and then incubated with the appropriate
horseradish peroxidase-conjugated secondary antibody at a final
dilution of 1:6,000 (cat. no. SA00001-2; ProteinTech Group, Inc.)
for 1 h at 37°C. An enhanced chemiluminescence system (Pierce;
Thermo Fisher Scientific, Inc.) was used for measuring the
protein-antibody complexes. The blots were quantified by Quantity
One 1-D software (version 4.6.9; Bio-Rad Laboratories, Inc.) and
β-actin was used as the control.
Immunofluorescence assay
Confluent cells were fixed in 4% paraformaldehyde in
PBS, pH 7.2, for 10 min at 37°C, then permeabilized in 0.1% Triton
X-100 (Sigma-Aldrich; Merck KGaA) in PBS for 5 min at room
temperature; rinsed three times in PBS, and blocked for 30 min in
PBS with 10% goat serum (Sigma-Aldrich; Merck KGaA) at 37°C.
Primary antibody staining was performed at 4°C overnight with the
following antibodies at a 1:50 dilution: Anti-ZO-1 and anti-ZONAB.
The sections were then incubated with an Alexa Fluor®
594-conjugated goat anti-mouse secondary antibody at a 1:100
dilution (cat. no. SA00006-3; ProteinTech Group, Inc.) for 2 h at
room temperature in the dark. Following DAPI staining at 37°C for
10 min, cells were imaged using an inverted fluorescence microscope
(TE2000U Eclipse; Nikon Corporation).
Statistical analysis
Statistical analysis was performed using SPSS 17.0
statistical software (SPSS, Inc., Chicago, IL, USA) for Windows.
All data are expressed as the mean ± standard deviation), and
analyzed via one-way analysis of variance followed by the post hoc
Bonferroni's t-test where appropriate. P<0.05 was considered to
indicate a statistically significant difference.
Results
BK treatment induces RCECs proliferation
in a time- and concentration-dependent manner
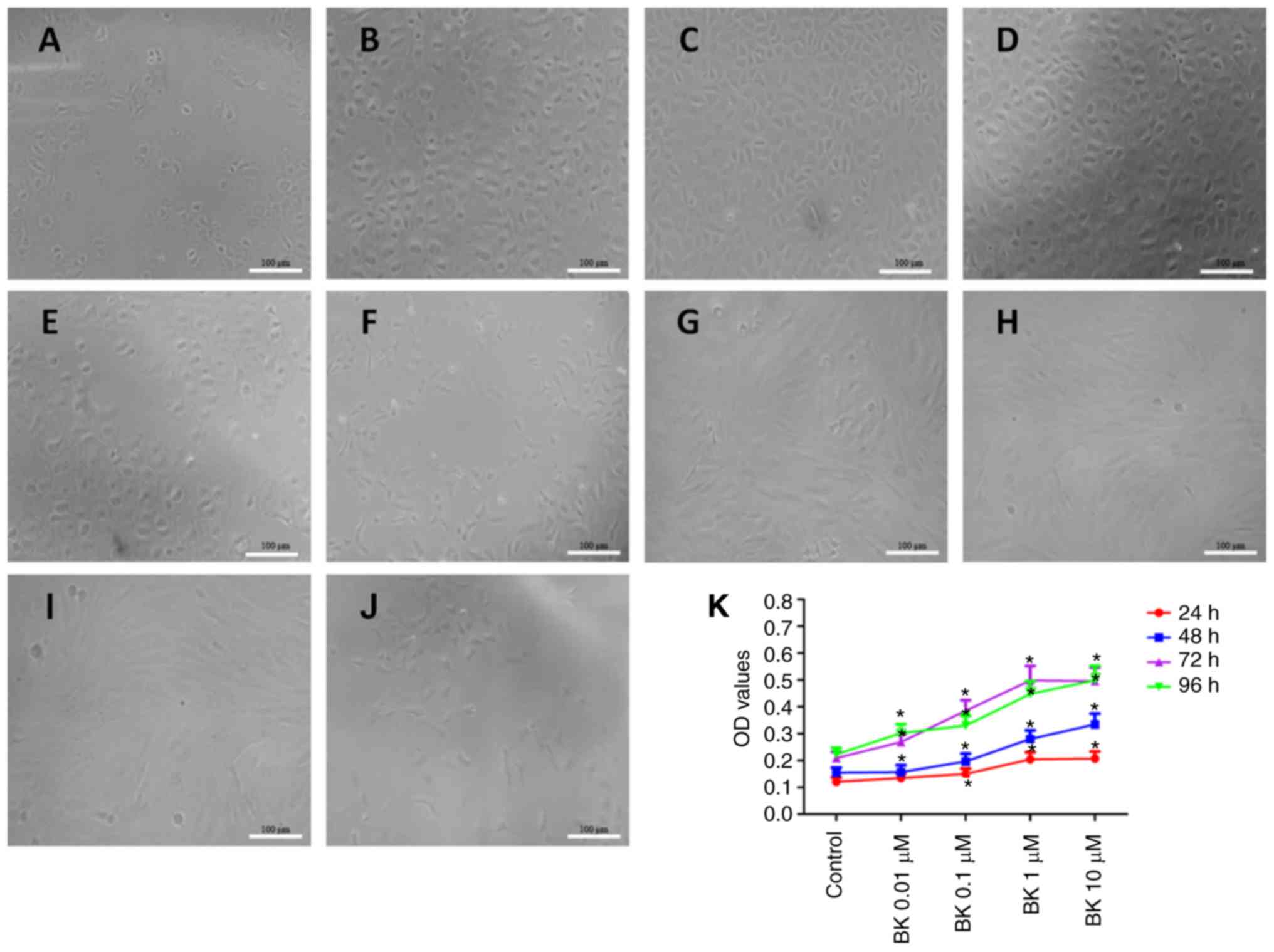
Under an inverted microscope, cells treated with
0.1–1.0 µM BK were revealed to have formed a monolayer with
a mosaic arrangement, cellular morphology was normal at 72 h
(Fig. 1A–D). However, at 96 h,
cells were irregular in shape with thin, long, neurite-like
processes, and cell extensions and increased detachment were
observed (Fig. 1F–J). In
addition, BK increased cell density in a concentration-dependent
manner when treated with 0.1–1.0 µM BK, while cell growth
was significantly inhibited following 10.0 µM BK treatment
(Fig. 1A–E). Thus, BK-induced
proliferation of RCECs was demonstrated to be time- and
concentration-dependent.
Next, BK-induced cell proliferation was analyzed
using an MTT assay. As presented in Fig. 1K, exposure of RCECs to BK at
0.1–1.0 µM resulted in a concentration-dependent increase in
optical density (OD) values, while 10.0 µM BK treatment
resulted in relatively limited proliferation, reflected by the
decrease of OD values. These results indicated that BK treatment at
1.0 µM significantly increased cell viability and induced
RCEC proliferation.
BK treatment increases the expression of
the tight junction ZO-1 and nuclear ZONAB during RCECs
proliferation
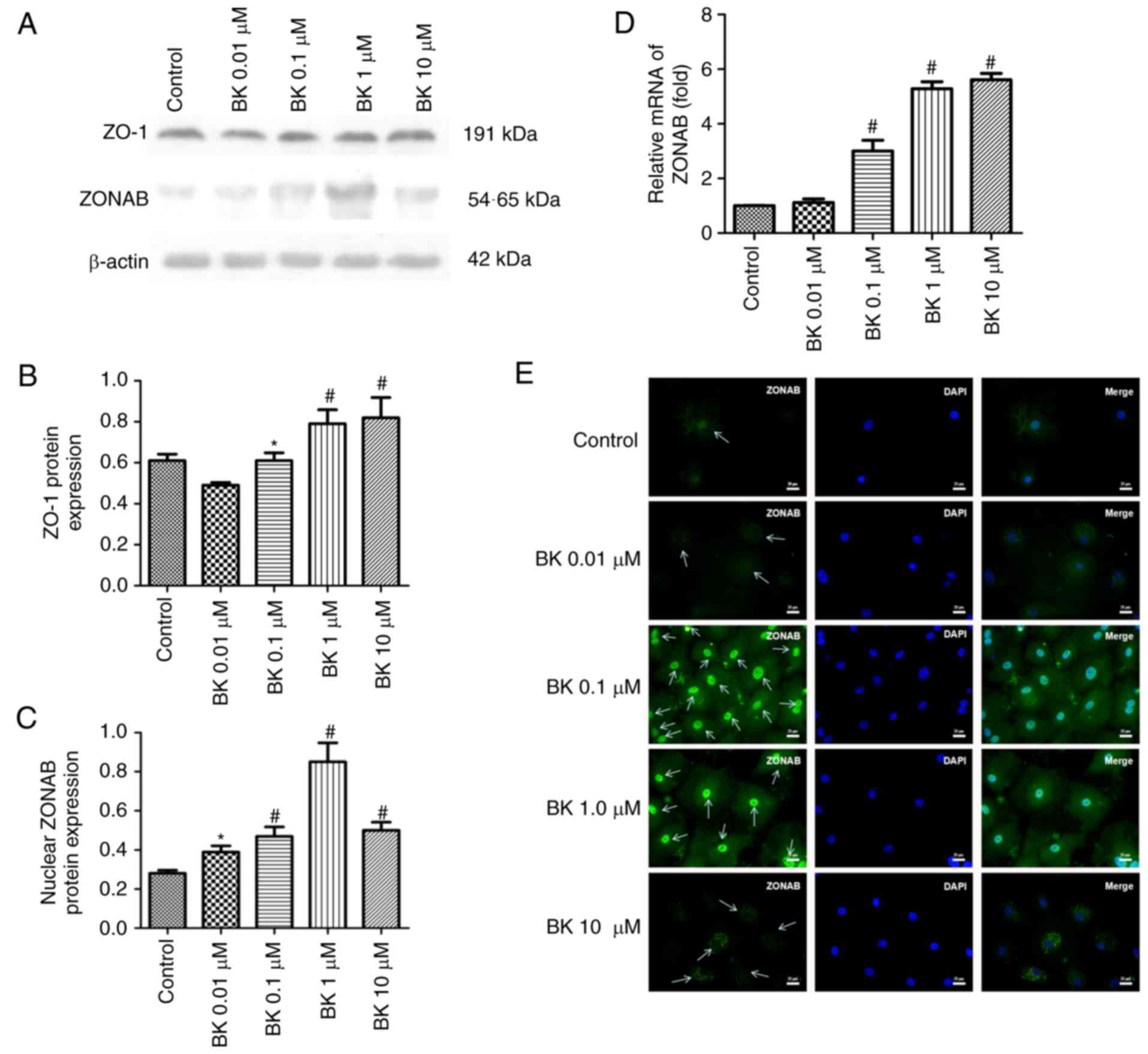
To determine the involvement of ZO-1 and ZONAB in
the regulation of RCEC proliferation, the localization and
transcription levels of ZONAB andZO-1 were assessed. Based on
immunofluorescence analysis of RCECs, ZONAB was revealed to be
primarily located with the nucleus or nuclear membrane, and there
was high luminescence for ZONAB in these areas of cells following
treatment with 0.1–1.0 µM BK and of cells in the control
group, but not cells treated with 0.01 µM BK. In cells
exposed to 10 µM BK, ZONAB was primarily localized within
the cytoplasm and was excluded from the nuclear region (Fig. 2E). These observations are
consistent with the data from western blotting and RT-PCR. Compared
with the control group, BK increased nuclear ZONAB mRNA and protein
expression in a concentration-dependent manner (0.01–1.0 µM
BK; P<0.05 or P<0.01; Fig.
2C and D). Similarly, this BK-induced concentration-dependent
effect was also was observed for ZO-1 protein (0.1–10.0 µM
BK; P<0.05 or P<0.01). These data suggested that ZO-1 and
ZONAB are crucial components in the regulation of cell
proliferation in RCECs, and are potentially associated with
BK-induced proliferation.
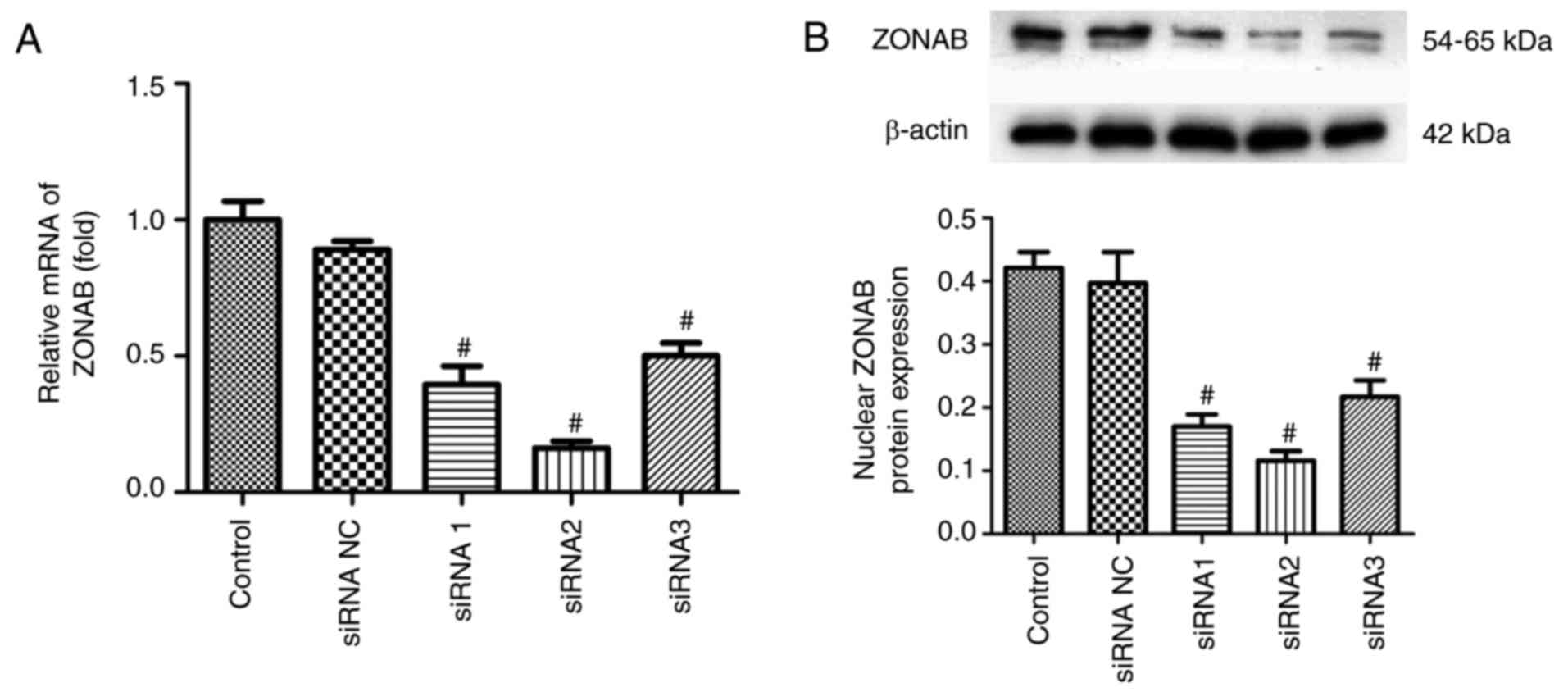
Knockdown with ZONAB siRNAs induces
significant downregulation of ZONAB mRNA and protein
Next, RCECs were transfected with small
ZONAB-directed RNA duplexes to induce RNA interference. RT-PCR data
(Fig. 3A) and western blotting
analysis (Fig. 3B) suggested that
transient transfection of three different regions of ZONAB resulted
in efficient reduction of ZONAB mRNA and protein expression, while
the negative control RNA duplex had no effect. In particular,
transfection of the second sequence, ZONAB-siRNA2, efficiently
decreased ZONAB mRNA expression with a reduction of ~85% in RCECs
(P<0.01; Fig. 3A). This
sequence was therefore used for the remaining experiments.
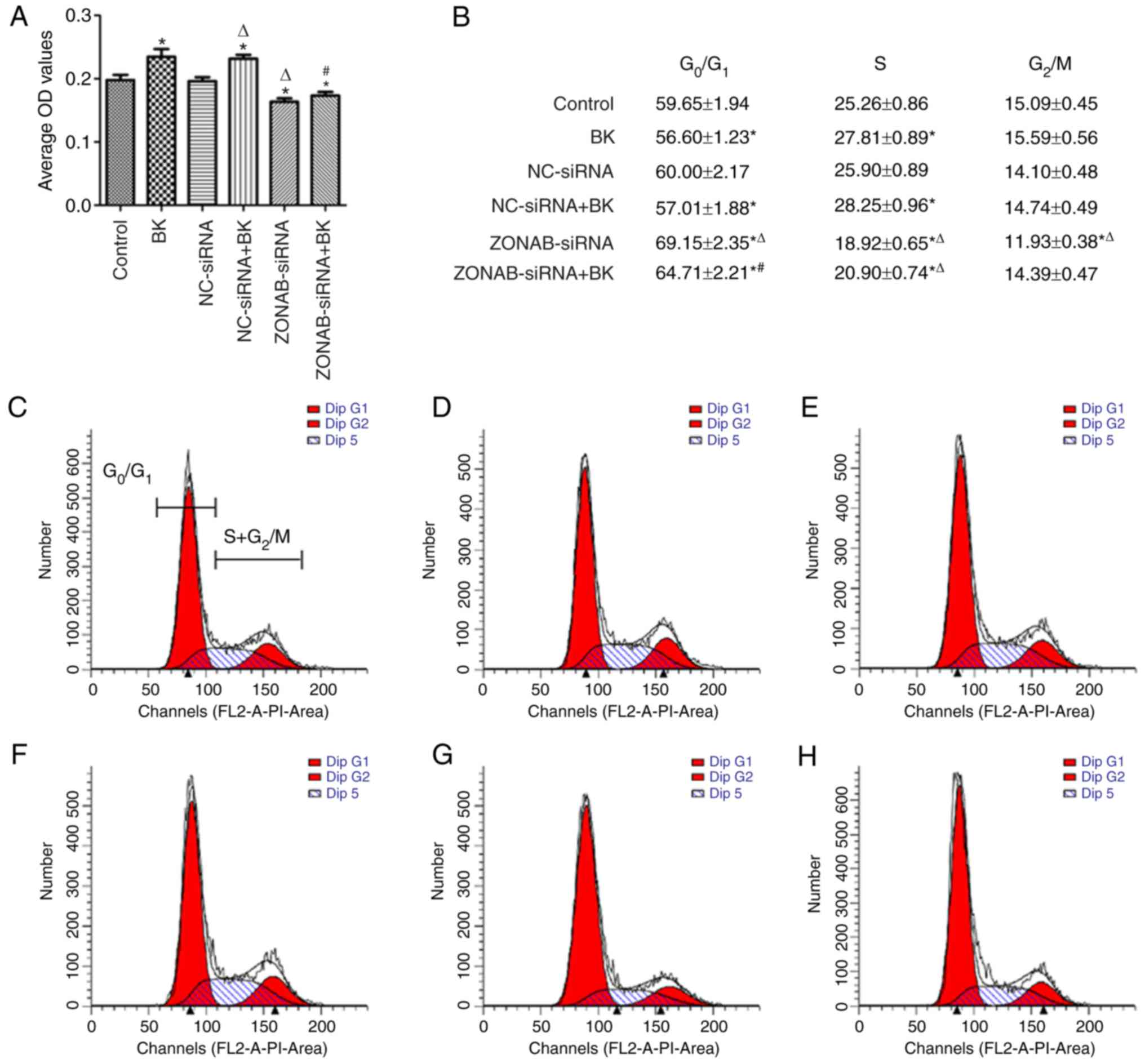
Knockdown with ZONAB siRNAs abolishes the
effect of BK on cell proliferation and cell cycle progression in
RCECs
According to the MTT assay data, cells transfected
with non-targeting control siRNA demonstrated proliferation
capacities that were similar to those of the control group
(P>0.05; Fig. 4A). Cells lines
transfected with ZONAB-siRNA exhibited lower cell proliferation and
OD values than those of the control group (P<0.05; Fig 4A). In contrast, a significant
increase of OD value and proliferation capacity was observed in the
BK-treated group compared with the control group (P<0.05;
Fig. 4A). However, proliferation
significantly decreased in cells transfected with ZONAB-siRNA in
combination with BK compared with those treated with BK alone
(P<0.05; Fig. 4A).
Furthermore, cell cycle distribution of RCECs was
quantified by flow cytometry (Fig.
4B–H). Cell cycle analysis revealed that a significantly
increased fraction of cells in the S phase and a significantly
lower percentage of cells in the G0/G1 phases
were present in cells treated with BK compared with the control
group (P<0.05; Fig. 4B–D). BK
pretreatment accelerated the G1- to S-phase switch and
enhanced DNA synthesis, thus inducing cell proliferation.
Conversely, there were a larger fraction of cells arrested in
G0/G1 phases and a reduction of the
proportion of cells distributed in S phase in ZONAB-siRNA
transfected cells compared with the control group (P<0.05;
Fig. 4B, C and G), and a similar
effect was observed in the ZONAB-siRNA+BK group compared with cells
treated with BK alone (P<0.05; Fig. 4B, D and H). These results
suggested that ZONAB-siRNA transfection induced cell cycle arrest
in G0/G1 phases and inhibited cell mitosis,
thus reversing BK-induced proliferation of RCECs.
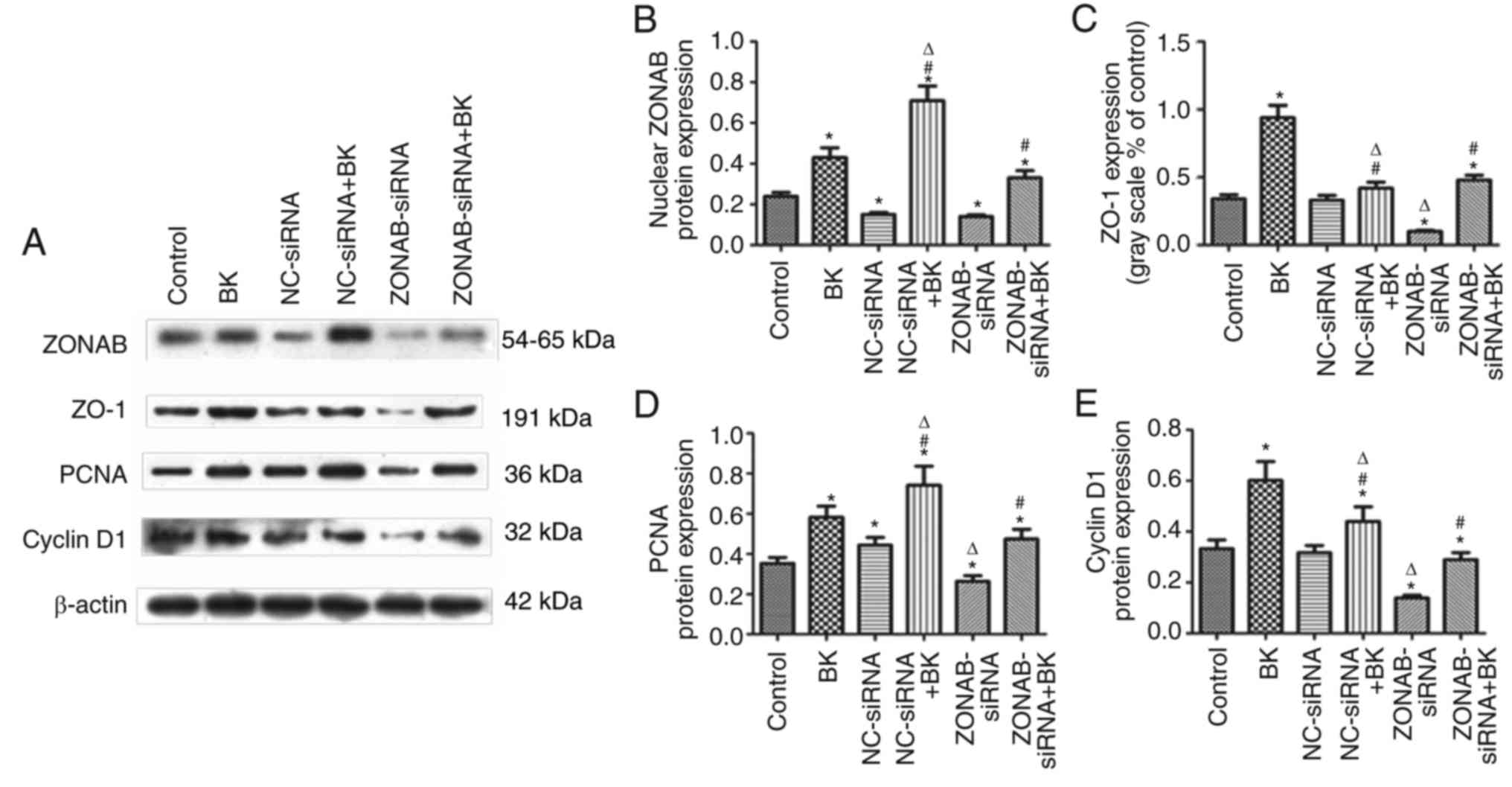
Involvement of the ZO-1/ZONAB pathway in
BK-induced proliferation of RCECs
Finally, the regulation of the ZO-1/ZONAB signaling
cascade itself, from the upstream molecules (ZO-1 and ZONAB) to the
downstream effectors (PCNA and cyclin D1) was investigated. PCNA
and cyclin D1 are used as markers regulating cell proliferation and
cell cycle progression in various types of cell (25,26). As demonstrated by western
blotting, transfection with ZONAB-siRNA resulted in knockdown of
ZONAB protein levels and inhibition of PCNA and cyclin D1
expression (P<0.05; Fig. 5),
whereas non-targeting siRNA transfection did not alter PCNA or
cyclin D1 protein levels (P>0.05; Fig. 5). BK pretreatment significantly
increased the expression of PCNA and cyclin D1. In turn,
transfection with ZONAB-siRNA inhibited BK-induced upregulation of
PCNA and cyclin D1 (P<0.05; Fig.
5), and subsequently blocked BK-stimulated cell
proliferation.
Discussion
The present study initially established the
involvement of the ZO-1/ZONAB pathway in BK-induced RCECs
proliferation. The data revealed that BK promoted cell
proliferation and cell cycle progression in RCECs. BK treatment
also resulted in the activation of signaling molecules in the
ZO-1/ZONAB pathway, including the upregulation of tight junction
ZO-1 and nuclear ZONAB, as well as PCNA and cyclin D1. Furthermore,
knockdown with ZONAB-siRNA inhibited cell proliferation, induced
cell cycle arrest and downregulated the PCNA and cyclin D1 protein
expression. Pre-treatment with siRNA to knockdown ZONAB blocked the
proliferation-promoting activity of BK. Taken together, these data
indicated that BK treatment increased RCECs proliferation, at least
in part due to the activation of the ZO-1/ZONAB pathway.
BK is a well-established mediator of exudative
corneal wound healing, ocular allergy and pro-inflammatory
responses on the ocular surface (13). BK and its receptors, the B1 and B2
receptor, are present in the tissue homogenates of rabbit, swine
and human eyes (27–29). BK interacts with its receptors on
the cell surface to mediate a variety of biological effects,
including cell proliferation. BK has been reported to induce
proliferation of various types of cell in ex vivo corneas
(8–12). However, the underlying mechanisms
by which BK stimulates the proliferation of ocular cells remain to
be fully understood. The majority of the biological functions of BK
are mediated by the B2 receptor, which leads to an increase of
intracellular Ca2+([Ca2+]i)
mobilization, and tyrosine kinase and protein kinase C (PKC)
activation via pertussis toxin (PTX)-insensitive G protein
(8,9,12,30,31). Previous reportshave suggested that
BK induces cell proliferation through stimulation of
phosphoinositide turnover,
[Ca2+]i-mobilization and diacylgylcerol
production, which lead to increased DNA synthesis in human corneal
epithelial cells and bovine CECs (8,9,12).
However, pretreatment with HOE-140, a specific B2 receptor
antagonist, attenuated the BK-induced increase in
[Ca2+]i, suggesting that B2 receptors serve a
crucial function in this process (8,9).
Multiple previous studies have demonstrated that BK induces cell
proliferation potentially through B2 receptor coupling
PTX-sensitive G protein/Ca2+/PKC and EFGR/p42/p44
mitogen activated protein kinase (MAPK)-dependent pathways in
various cell types (8–12,30–34). However, these mechanisms remained
to be verified in CECs.
Accumulating evidence has demonstrated that the
TJ-associated signaling proteins, ZO-1 and ZONAB, serve a vital
role in cell proliferation, gene expression and differentiation, as
reported in RPE cells and the renal proximal tubule (16–19,35–39). ZO-1 is a membrane-associated TJ
adaptor protein and possesses several PDZ domains, one SH3 domain,
and a domain homologous to yeast guanylate kinase (14,40). ZONAB is a Y-box transcription
factor that modulates cell proliferation through its interaction
with the SH3 domain of ZO-1. ZONAB is primarily distributed in the
nucleus or nuclear membrane of proliferating cells, and drives the
transcription of PCNA and cyclin D1 genes for the promotion of cell
proliferation and cell cycle progression (16–18). However, in slowly or
non-proliferating cells, nuclear ZONAB expression is reduced, and
binding of ZONAB to ZO-1 results in cytoplasmic sequestration and
inhibits the nuclear accumulation and transcriptional activity of
ZONAB, resulting in reduced proliferation (14–20). In the present study, with BK
pre-treatment, mRNA and protein levels of nuclear ZONAB were
significantly upregulated in a concentration-dependent manner,
suggesting the increase of ZONAB nuclear accumulation and
transcriptional activity, thus resulting in RCEC proliferation.
In the present study, the subcellular localization
of ZONAB was detected by immunofluorescence. High luminescence in
the nucleus indicated ZONAB expression following treatment with
0.1–1.0 µM BK or without BK in the control group, but not
following treatment with 0.01 µM BK. Treatment with 0.01
µM BK had no effect on ZONAB nuclear translocation, and the
concentration-dependent effect of BK on the nuclear accumulation of
ZONAB was not observed. Therefore, a wide range of BK
concentrations (0.0001–10 µM), previously reported in human
corneal epithelial cells (18),
should be selected to determine the effect of BK (<0.01
µM) on ZONAB nuclear distribution. In addition, the
subcellular localization of ZO-1 was also analyzed. Based on
previous immunofluorescence results, ZO-1 is primarily located at
intercellular junctions and in the cytoplasm (16–18). While specific ZO-1 staining at
intercellular junctions was not detected, ZO-1 was revealed to be
distributed in the cytoplasm and nucleus (data not shown). The
murine-derived monoclonal antibody against rabbit ZO-1 may have
been non-specific; alternatively, ZO-1 may be sparsely distributed
in the rabbit corneal endothelium. Even though the ZO-1 staining
results of the present study were not satisfactory, the data
suggested that the majority of ZONAB molecules bound to ZO-1 are
associated with TJ, and the expression of TJ-associated molecules,
ZO-1 and ZONAB, were affected by BK treatment.
Furthermore, the present study provided the evidence
supporting the involvement of the ZO-1/ZONAB pathway during RCEC
proliferation, as demonstrated by decreased cell proliferation, a
greater fraction of cells arrested in G0/G1
phase and the downregulation of PCNA and cyclin D1 following
ZONAB-siRNA transfection. These data are consistent with evidence
from previous transgenic experiments. A study by Balda et al
(16) demonstrated that depletion
of ZONAB by RNA interference or ZO-1 overexpression reduced
proliferation rates and final cell densities of Madin-darby canine
kidney cells, while overexpression of ZONAB resulted in increased
cell densities. Another investigation by Georgiadis et al
(20) revealed that
lentivirally-mediated overexpression of ZONAB or knockdown of ZO-1
resulted in an increased number of BrdU-positive cells and the
induction of RPE proliferation. Kampik et al (37) revealed that knockdown of ZO-1 led
to an average increase of 50% in human CECs density in corneal
samples from donors >60 years old, while overexpression of ZONAB
led to Ki67 upregulation but no significant increase in cell
density. Taken together, these data suggest that ZO-1 and ZONAB are
involved in signaling that modulates RCECs proliferation.
The present study investigated the function of the
ZO-1/ZONAB pathway during BK-induced cell proliferation. The data
revealed that transfection with ZONAB-siRNA reversed the
proliferation-promoting effect of BK. Significant knockdown of
ZONAB inhibited the transcriptional activity of the cell cycle
genes PCNA and cyclin D1, thus attenuating BK-induced cell
proliferation. Nevertheless, the exact mechanisms underlying
BK-induced activation of ZO-1/ZONAB signaling remain to be fully
elucidated. First, it is unclear whether the overexpression of
ZONAB and knockdown of ZO-1 increase proliferation in the RCECs
model utilized in the present study. Further transgenic research
targeting ZO-1 and ZONAB is required. Second, the effect of ZO-1
and ZONAB on CECs differentiation remains unclear, and cellular
mechanisms relevant to cell differentiation should be analyzed.
Third, bioinformatics analysis of ZONAB siRNA is required to
minimize off-target effects resulting from the introduction of
individual siRNAs. Finally, ongoing experiments by our group should
be replicated in human corneal endothelia, and focus on the
crosstalk between the ZO-1/ZONAB pathway and the BK-mediated
B2 receptor-G protein/Ca2+/PKC or
EFGR-p42/p44 MAPK-dependent pathway. Further research is required
to explore these areas.
In conclusion, the present study demonstrated that
BK promoted RCECs proliferation and cell cycle progression, and the
underlying mechanisms appeared to include the ZO-1/ZONAB pathway.
The signaling paradigm disclosed in the present study provide novel
insights and, potentially, novel therapeutic targets for cornea
regeneration and transplantation.
Acknowledgments
Not applicable.
References
|
1
|
Waring GO, Bourne WM, Edelhauser HF and
Kenyon KR: The corneal endothelium. Normal and pathologic structure
and function. Ophthalmology. 89:531–590. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Joyce NC: Proliferative capacity of the
corneal endothelium. Prog Retin Eye Res. 22:359–389. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bourne WM, Nelson LR and Hodge DO: Central
corneal endothelial cell changes over a ten-year period. Invest
Ophthalmol Vis Sci. 38:779–782. 1997.PubMed/NCBI
|
|
4
|
Saxena R, Boekhoorn SS, Mulder PG,
Noordzij B, van Rij G and Luyten GP: Long-term follow-up of
endothelial cell change after Artisan phakic intraocular lens
implantation. Ophthalmology. 115:608–613. 2008. View Article : Google Scholar
|
|
5
|
Kashuba E, Bailey J, Allsup D and Cawkwell
L: The kinin-kallikrein system: Physiological roles,
pathophysiology and its relationship to cancer biomarkers.
Biomarkers. 18:279–296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khan MM, Bradford HN, Isordia-Salas I, Liu
Y, Wu Y, Espinola RG, Ghebrehiwet B and Colman RW:
High-molecular-weight kininogen fragments stimulate the secretion
of cytokines and chemokines through uPAR, Mac-1, and gC1qR in
monocytes. Arterioscler Thromb Vasc Biol. 26:2260–2266. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Levy D and Zochodne DW: Increased mRNA
expression of the B1 and B2 bradykinin receptors and
antinociceptive effects of their antagonists in an animal model of
neuropathic pain. Pain. 86:265–271. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang SC, Chien C, Hsiao L, Wang C, Chiu
C, Liang K and Yang CM: Mechanisms of bradykinin-mediated
Ca2+ signaling in canine cultured corneal epithelial
cells. CellSignal. 13:565–574. 2001.
|
|
9
|
Wiernas TK, Davis TL, Griffin BW and
Sharif NA: Effects of bradykinin on signal transduction, cell
proliferation, and cytokine, prostaglandin E2 and
collagenase-1 release from human corneal epithelial cells. Br J
Pharmacol. 123:1127–1137. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng CY, Huang SC, Hsiao LD, Sun CC, Jou
MJ and Yang CM: Bradykinin-stimulated P42/44 MAPK activation
associated with cell proliferation in corneal keratocytes.
CellSignal. 16:535–549. 2004.
|
|
11
|
Cheng CY, Tseng HC and Yang CM:
Bradykinin-mediated cell proliferation depends on transactivation
of EGF receptor in corneal fibroblasts. J Cell Physiol.
227:1367–1381. 2012. View Article : Google Scholar
|
|
12
|
Yang SW, Lee WK, Lee EJ, Kim KY, Lim Y,
Lee KH, Rha HK and Hahn TW: Effect of bradykinin on cultured bovine
corneal endothelial cells. Ophthalmologica. 215:303–308. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Webb JG: The kallikrein/kinin system in
ocular function. J Ocul Pharmacol Ther. 27:539–543. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Balda MS and Matter K: Tight junctions and
the regulation of gene expression. Biochim Biophys Acta.
1788:761–767. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Terry S, Nie M, Matter K and Balda MS: Rho
signaling and tight junction functions. Physiology. 25:16–26. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Balda MS and Matter K: The tight junction
protein ZO-1 and an interacting transcription factor regulate
ErbB-2 expression. EMBO J. 19:2024–2033. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Balda MS, Garrett MD and Matter K: The
ZO-1-associated Y-box factor ZONAB regulates epithelial cell
proliferation and cell density. J Cell Bio1. 160:423–432. 2003.
View Article : Google Scholar
|
|
18
|
Lima WR, Parreira KS, Devuyst O, Caplanusi
A, N'kuli F, Marien B, Van Der Smissen P, Alves PM, Verroust P,
Christensen EI, et al: ZONAB promotes proliferation and represses
differentiation of proximal tubule epithelial cells. J Am Soc
Nephrol. 21:478–488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sourisseau T, Georgiadis A, Tsapara A, Ali
RR, Pestell R, Matter K and Balda MS: Regulation of PCNA and cyclin
D1 expression and epithelial morphogenesis by the ZO-1-regulated
transcription factor ZONAB/DbpA. Mol Cell Biol. 26:2387–2398. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Georgiadis A, Tschemutter M, Bainbridge
JW, Balaggan KS, Mowat F, West EL, Munro PM, Thrasher AJ, Matter K,
Balda MS, et al: The tight junction associated signaling proteins
ZO-1 and ZONAB regulate retinal pigment epithelium homeostasis in
mice. PloS One. 5:e157302010. View Article : Google Scholar
|
|
21
|
U.S. Office of Science and Technology
Policy: Technology, Laboratory animal welfare: U.S. government
principles for the utilization andcare of vertebrate animals used
in testing, research and training; notice. Fed Regist.
50:20864–20865. 1985.
|
|
22
|
Kay P, Nimni ME and Smith RE: Stability of
collagen phenotype in morphologically modulated rabbit corneal
endothelial cells. Invest Ophthalmol Vis Sci. 25:495–501.
1984.PubMed/NCBI
|
|
23
|
Kim TY, Kim WI, Smith RE and Kay ED: Role
of p27Kip1 in cAMP- and TGF-β2-mediated
antiproliferation in rabbit corneal endothelial cells. Invest
Ophthalmol Vis Sci. 42:3142–3149. 2001.PubMed/NCBI
|
|
24
|
Schäfer J, Höbel S, Bakowsky U and Aigner
A: Liposome-polyethylenimine complexes for enhanced DNA and siRNA
delivery. Biomaterials. 31:6892–6900. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park SY, Jeong MS, Han CW, Yu HS and Jang
SB: Structural and functional insight into proliferating cell
nuclear antigen. J Microbiol Biotechnol. 28:637–647. 2016.
View Article : Google Scholar
|
|
26
|
Qie S and Diehl JA: Cyclin D1, cancer
progression, and opportunities in cancer treatment. J Mol Med).
94:1313–1326. 2016. View Article : Google Scholar
|
|
27
|
Wiernas TK, Griffin BW and Sharif NA: The
expression of functionally-coupled B2-bradykinin
receptors in human corneal epithelial cells and their
pharmacological characterization with agonists and antagonists. Br
J Pharmacol. 121:649–656. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kuznetsova TP, Chesnokova NB and Paskhina
TS: Activity of tissue and plasma kallikrein and level of their
precursors in eye tissue structures and media of healthy rabbits.
Vopr Med Khim. 37:79–82. 1991.In Russian. PubMed/NCBI
|
|
29
|
Ma JX, Song Q, Hatcher HC, Crouch RK, Chao
L and Chao J: Expression and cellular localization of the
kallikrein-kinin system in human ocular tissues. Exp Eye Res.
63:19–26. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leeb-Lundberg LM: Bradykinin specificity
and signaling at GPR100 and B2 kinin receptors. Br J Pharmacol.
143:931–932. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dixon BS, Sharma RV, Dickerson T and
Fortune J: Bradykinin and angiotensin II: Activation of protein
kinase C in arterial smooth muscle. Am J Physiol. 266:C1406–1420.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mio T, Liu X, Toews ML, Adachi Y,
Romberger DJ, Spurzem JR and Rennard SI: Bradykinin augments
fibroblast-mediated contraction of released collagen gels. Am J
Physiol Lung Cell Mol Physiol. 281:L164–L171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bernier SG, Haldar S and Michel T:
Bradykinin-regulated interactions of the mitogen-activated protein
kinase pathway with the endothelial nitric-oxide synthase. J Biol
Chem. 275:30707–30715. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang CM, Lin MI, Hsieh HL, Sun CC, Ma YH
and Hsiao LD: Bradykinin-induced p42/44 MAPK phosphorylation and
cell proliferation via Src, EGF receptors, and PI3-K/Akt in
vascular smooth muscle cells. J Cell Physiol. 203:538–546. 2005.
View Article : Google Scholar
|
|
35
|
Arakawa Y, Kajino K, Kano S, Tobita H,
Hayashi J, Yasen M, Moriyama M, Arakawa Y and Hino O: Transcription
of dbpA, a Y box binding protein, is positively regulated by E2F1:
Implications in hepatocarcinogenesis. Biochem Biophys Res Commun.
322:297–302. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jayagopal A, Yang JL, Haselton FR and
Chang MS: Tight junction-associated signaling pathways modulate
cell proliferation in uveal melanoma. Invest Ophthalmol Vis Sci.
52:588–593. 2011. View Article : Google Scholar :
|
|
37
|
Kampik D, Basche M, Georgiadis A, Luhmann
UF, Smith AJ, Larkin F and Ali RR: Lentivirus mediated interference
with the ZO-1/ZONAB pathway induces cell cycle progression in human
corneal endothelial cells. Invest Ophthalmol Vis Sci.
53:60042012.
|
|
38
|
Spadaro D, Tapia R, Jond L, Sudol M,
Fanning AS and Citi S: ZO proteins redundantly regulate the
transcription factor DbpA/ZONAB. J Biol Chem. 289:22500–22511.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qiao X, Roth I, Féraille E and Hasler U:
Different effects of ZO-1, ZO-2 and ZO-3 silencing on kidney
collecting duct principal cell proliferation and adhesion. Cell
Cycle. 13:3059–3075. 2014. View Article : Google Scholar
|
|
40
|
Willott E, Balda MS, Fanning AS, Jameson
B, Van Itallie C and Anderson JM: The tight junction protein ZO-1
is homologous to the Drosophila discs-large tumor suppressor
protein of septate junctions. Proc Natl Acad Sci USA. 90:7834–7838.
1993. View Article : Google Scholar : PubMed/NCBI
|