Introduction
Asthma, as a chronic inflammatory airway disease, is
a significant global disease which is a common source of morbidity
and a significant cause of preventable mortality (1,2).
Generally, it is caused due to the inhalation of allergens, house
dust, pollens, air pollutants, and inhalants, which are
characterized by airway hyper-responsiveness, eosinophilic airway
inflammation, as well as mucus hypersecretion. Inflammatory cells
secrete chemical regulators related to asthma development closely
(3,4). Asthma could result in
incompletely-reversible airway obstruction, especially at late
stages (5). Thus searching for
effective therapy that targets airway inflammation is required.
In the last few decades, exploring the efficacy of
natural compounds against various human metabolic diseases have
received increased attention among the scientific community
(6). Compounds from plants
belonging to different groups, including flavonoids, alkaloids and
polyphenols evaluated for their cancer preventive effects have
yielded promising data, thus offering a potential therapeutic
strategy against deadly diseases (7). The flavonol fisetin
(3,3′,4′,7-tetrahydroxyflavone), involved in fruits and vegetables
such as apples, strawberries, grapes, persimmon, cucumber, and
onion, was suggested to possess anti-inflammatory, anti-oxidant,
anti-microbial, and significantly anti-carcinogenic activity when
studied in various animal model systems and cell culture (8). Fisetin was able to ameliorate
inflammation response and oxidative stress in diseases, such as
bowel disease, oxidative skin damage, as well as lung injury
through inflammation suppression, and oxidative inhibition
(9). In addition, fisetin
possesses significant therapeutic effects against diabetic
complications and atherosclerosis (10). However, the role of fisetin in
regulating airway inflammation is not understood clearly and
requires more studies.
Nuclear factor-κB (NF-κB) signaling activity in
asthma is proved by upregulated NF-κB nuclear localization, IκB
phosphorylation, as well as IκB kinase-β (IKK-β) expression in the
airway tissue of asthmatics (11). Increased evidence has suggested
that NF-κB nuclear binding or staining is also observed in the
inflammatory cells of induced asthmatic sputum (12,13). Further, NF-κB phosphorylation in
the airways of allergen-challenged mice is ameliorated for
Toll-like receptor 2 (TLR2) or TLR4 gene deletion, indicating that
the innate immune system attributes to NF-κB activity in asthma
(14,15). However, up until now, the effect
of TLR5 on the progression of asthma is not clearly
illustrated.
Therefore, the present study, to our knowledge, is
the first time that fisetin was investigated for its the effects on
ovalbumin (OVA)-induced mice with asthma. Our results suggested
that fisetin was at least partly involced in improving progression
of airway inflammation via activation of MyD88 and NF-κB signaling
pathways. Fisetin is able to inhibit OVA-induced asthma by
inactivating MyD88 and NF-κB signaling pathway-regulated
inflammatory responses.
Materials and methods
Reagents and animal models
Forty male C57BL/6 mice weighing 20–25 g were
obtained from Nanjing Medical university (Nanjing, China). The mice
were maintained in a room temperature at 25±2°C and relative 50±5%
humidity-controlled environment with a standard cycle of 12 h
light/dark. The model animals were administered standard diet and
water ad libitum provided in the cages. The experimental
procedures of this study were approved by the ethics Committee on
Animal Research at Qinhuangdao First Hospital (Hebei, China). The
mice in the experiments were divided into 4 groups randomly as
follows: i) the control group (Con); ii) the OVA (Sigma-Aldrich,
St. Louis, MO, USA)-induced group (Mod); iii) 40 mg/kg
fisetin-treated OVA-induced group (FL); and iv) 50 mg/kg
fisetin-treated OVA-induced group (FH). OVA was purchased from
Sigma-Aldrich.
Asthmatic model establishment
The mice were arbitrarily divided into four groups
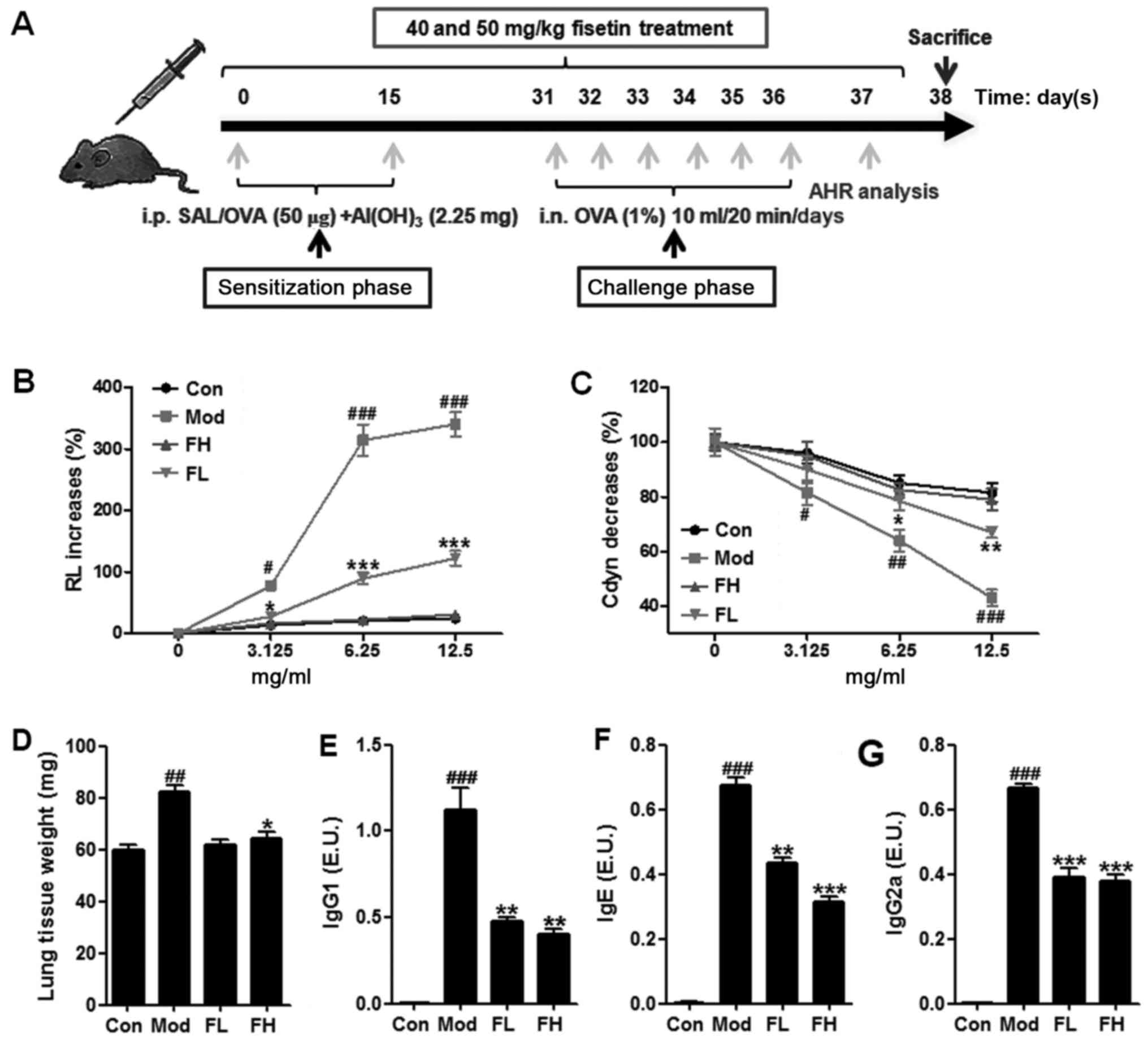
(n=10 each group) as described above. As shown in Fig. 1A, mice were sensitized with an
intraperitoneal injection of 0.2 ml saline containing 50 μg
OVA and 2.25 mg aluminum hydroxide. From day 31, the mice in Mod,
FL and FH groups were challenged for 6 consecutive days with a 1%
(w/v) OVA solution, which was administered with an ultrasonic
nebulizer (Yuyue, Jiangsu, China). The mice in the Con group were
sensitized and challenged with 0.09% saline (without 2.25 mg
aluminum hydroxide). At the same time, the OVA-induced mice were
treated with fisetin at different concentrations. Following the
animal model establishment, the blood samples were collected from
the mice immediately after the airway hyperresponsiveness (AHR)
measurements and stored at 4°C for 2 h before being centrifuged at
5,000 × g for 15 min at 4°C. The resulting serum was collected,
repackaged and stored at −80°C for the following studies. The whole
lung tissues were harvested on 4°C glacial table and weighed, and
either frozen in liquid nitrogen and kept at −80°C for
analysis.
Airway resistance and function
The airway resistance of treated animals was
determined as an increase in pulmonary resistance after the
challenges with 0, 3.125, 6.25 and 12.5 mg/ml aerosolized
β-methacholine (MCh) (Sigma, Shanghai, China). The resistance of
the 0.45 cm H2O.s.ml−1 orotracheal tube was
subtracted from all airway resistance measurements. Data are
presented as the pulmonary resistance (RL) and the lung dynamic
compliance (Cdyn) of the three independent experiments.
Bronchoalveolar lavage fluid (BALF)
collection
BALF collection was performed via lavaging the left
lung with 0.3 ml aliquots of phosphate-buffered saline (PBS)
(twice) through the tracheal cannula with total volume of 0.6 ml.
The resulting mixture was centrifuged at 1,000 × g for 10 min at
4°C. The supernatant was then collected and stored at −80°C for
further analysis. The pellet from the BALF was resuspended in 0.1
ml PBS for the cell count and classification analysis with an
automatic hematology analyzer (Mindray, Shenzhen, China).
Inflammatory cell counts in BALF
BALF was isolated as described above for analysis.
The animal models were sacrificed after the final challenge through
an intraperitoneal injection of 50 mg/kg pentobarbital (Hanlim
Pharm. Co., Seoul, Korea), then a tracheostomy was conducted. The
total number of inflammatory cells was calculated by counting the
cells in at least five fields of a hemocytometer after exclusion of
the dead cells through Trypan blue staining. The counts of
differential cells in BALF were calculated by Diff-Quik®
staining reagent (IMEB Inc., San Marcos, CA, USA) according to the
manufacturer's protocols. The number of neutrophils, lymphocytes,
and macrophages was evaluated through multiplying the percentages
from the total yield. Images of each slide were photographed with a
digital camera mounted on a microscope (Nikon, Tokyo, Japan).
Enzyme-linked immunosorbent assay
(ELISA)
Inflammatory mediators of cytokines in BALF and
serum, such as interleukin-1β (IL-1β), tumor necrosis factor-α
(TNF-α), IL-2, IL-4, IL-18, interferon-γ (IFN-γ) and IL-5, were
determined by ELISA kits according to the manufacturer's protocols.
ELISA (both from R&D Systems Inc., Minneapolis, MN, USA) was
also applied to determine the expression levels of OVA-specific
IgE, IgG2a and IgG1 in serum.
Determination of CD80 and CD86 on
DCs
The lungs were isolated from treated mice and
digested as previously described (16). Tissues were dissociated and single
cell suspensions were obtained. The following antibodies were
purchased from eBioscience (San Diego, CA, USA): 1:800 anti-CD80
Pe, 1:600 anti-CD86 PE, and isotype control antibodies. Lung DCs
were labeled with antibodies. After 30 min at 4°C, cells were
washed twice and fixed in 2% paraformaldehyde (PFA; Alfa Aesar,
Haverhill, MA, USA) diluted in PBS-BSA 0.2% for 15 min at 4°C.
Cells were washed, resuspended in PBS-BSA 0.2%, and analyzed by
flow cytometry. Finally, samples were collected and analyzed on a
FACSCalibur flow cytometer (BD Immunocytometry Systems, Franklin
Lakes, NJ, USA).
Immunochemical analysis
The lung tissue samples were collected, and fixed in
4% paraformaldehyde. Then, they were embedded in paraffin and cut
into 4 μm sections for histopathological analysis. Lung
sections were then stained with hematoxylin and eosin (H&e) and
periodic acid-Schiff (PAS) (IMEB Inc.) to calculate the
inflammatory changes. In eight animals from each group, five
arbitrarily selected fields of each mouse was photographed with an
optical microscope (Nikon) and the images were determined and
analyzed in detail. The airway inflammation degree in each group
was evaluated by three independent analysts. Histopathological
evaluation was based on the intensity of the inflammatory
infiltrate in randomly selected areas around the tissues and was
scored as 1 (no inflammatory cells), 2 (few cells), 3 (a few
cells), 4 (moderate cell infiltration), 5 (large number of
inflammatory cells) and 6 (completely inflammatory cells), assuming
a linear relationship between the amount of inflammation and
inflammatory score.
As for immunohistochemistry, IL-1β antigen was
calculated with a specific rabbit antibody (Cell Signaling
Technology, Danvers, MA, USA). The antigen-antibody complexes were
then visualized through the avidin-biotin-peroxidase complex kit
(elite kit; Vector Laboratories, Burlingame, CA, USA). The sections
were finally counterstained with hematoxylin and also mounted.
Images of sections were photographed with a digital camera
(Nikon).
Immunofluorescent analysis
Lung tissue samples were perfused with PBS, fixed
with 4% paraformaldehyde and then embedded. The 8 μm
thickness frozen sections were prepared for analysis and staining.
The slides were washed with PBS following methanol fixation for 10
min at −20°C and rinsed with PBS. Slides were then incubated in
blocking solution for 2 h and permeabilized by 0.3% Triton X-100 in
PBS for 30 min at room temperature. During incubation with
polyclonal rabbit anti-mouse TNF-α and IL-1β (Cell Signaling
Technology) in a humid chamber overnight at 4°C, the antigen
detection was carried out with Alexa 488 goat anti-rabbit IgG
(Invitrogen, Carlsbad, CA, USA) for 2 h. Negative control sections
were processed by omitting the specific primary antibody. The
sections were added with mounting media with
4′,6-diamidino-2-phenylindole (DAPI; Roche, Basel, Switzerland).
The Immunofluorescent labelings were analyzed by fluorescence
microscope (Zeiss Axio Imager).
Transmission electron microscope
observation
Lung tissues were cut into small pieces, fixed in
2.5% glutaraldehyde and osmium tetroxide. Tissues were rinsed with
PBS, and embedded after dehydration of ethonal and acetone. Samples
were then cut into thin slices with uranium acetate-lead citrate
double staining for observation.
Cell culture and treatment
The cells of TC-1 (ATCCCRL-2493) were obtained from
American Type Culture Collection (ATCC, Rockville, MD, USA). They
were cultured at the permissive temperature (37°C) in DMEM medium
containing 15% fetal bovine serum (FBS) and supplemented with 1%
penicillin-streptomycin-neomycin both provided by Gibco-BRL Life
Technologies (Grand Island, NY, USA) with a humidified incubator in
5% CO2 atmosphere. The cells could be used and treated
with 100 ng/ml lipopolysaccharide (LPS) for 24 h combined with or
without TLR5 siRNA (sc-40263; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) according to the manufacturer's instruction. Then,
cells were carefully harvested for further analysis.
Western blot analysis and reverse
transcription-quantitative PCR (RT-qPCR)
Proteins were extracted from the lung tissue or
cells using T-PER Tissue Protein extraction reagent kit (Thermo
Fisher Scientific, Waltham, MA, USA) according to the
manufacturer's instructions. Primary antibodies of TLR5, IKKα,
p-IKKα, IκBα, p-IκBα, NF-κB, p-NF-κB, IL-1β, TNF-α, IL-18 and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Cell Signaling
Technology) were used in the study. The immunoactive proteins were
detected by using an enhanced chemiluminescence western blot
detection kit. The bands were observed using an ECL western blot
analysis system (GE Healthcare, Pittsburgh, PA, USA) and exposed to
Kodak X-ray film.
Analysis of qPCR was performed as previously
described (17). Fold induction
values were calculated using the to 2−ΔΔCq method, where
ΔCq represents the differences in cycle threshold number between
the target gene and GAPDH. ΔΔCq represents the relative change in
the differences between the control and treatment groups. The
primers used in the study are shown in Table I.
 | Table IPrimer sequences of RT-PCR
analysis. |
Table I
Primer sequences of RT-PCR
analysis.
| Gene | Forward primers
(5′-3′) | Reverse primers
(5′-3′) |
|---|
| GAPDH |
CATTCAAGACCGGAAGAGG |
ACCTCAGCACCAGCATCACC |
| MyD88 |
AGTCGCACAAGAGTAGAGC |
GCATCTCGAGATTGGTTG |
| IL-1β |
CGGACGCAAATGGACT |
AGTCTGGATGGCTAGTTG |
| IL-18 |
GGAGCAGATGCAGTGGA |
CACTGTGCGGTTCCGTCTT |
| IRAK1 |
CCCAGTGCTGGGGTAAGC |
TGTTCGCTCTACTGCAGACT |
| TRAF6 |
ACAGAGGAAGTTAGCCG |
CTGTGCAGGCGATATGGTG |
| TNF-α |
GCATTTCCTACTCTCAATTCT |
TACTTAGAGTCGCCACAAT |
Statistical analysis
All data were calculated as means ± standard error
of the mean (±SD) and analyzed by SPSS software (version 17.0;
SPSS, Inc, Chicago, IL, USA). Groups were compared with Student's
t-test or one-way analysis of variance (ANOVA), followed by
Newman-Keuls post-hoc analysis. A p-value of <0.05 was
considered statistically significant.
Results
Fisetin decreased AHR in the OVA-induced
asthmatic mice
Following the sensitization and challenge protocol
(Fig. 1A), a well-established
animal model of OVA-induced asthma was used to calculate the effect
of fisetin on asthmatic mice. The results of this study showed that
short-term OVA challenge of mice with Mch resulted in a significant
increase in AHR mice of RL compared to mice in the control group
(Fig. 1B). Fisetin-treated mice
at different concentrations led to a significant decrease in the
AHR mice of RL towards Mch in comparison with the OVA-induced
asthmatic mice. In addition, in a Mch dose-dependent manner,
Fisetin administration showed significant inhibitory activity in
the AHR mice. Furthermore, short-term OVA challenge in the mice
also resulted in a significant downregulation of Cdyn towards
progressive doses of Mch compared to the mice in the control group
(Fig. 1C). Fisetin treatment
resulted in upregulated Cdyn in comparison to the OVA-induced
asthmatic mice. The data above suggested that fisetin had a
potential role in the regulation of AHR progression.
The lung weight of mice in the different groups was
measured (Fig. 1D). Higher weight
of mice was observed in mice with OVA induction than that in the
control group. However, mice with fisetin administration displayed
decreased weight of lungs, suggesting that fisetin may be effective
in AHR development in mice. After OVA treatment, the levels of
OVA-specific IgG1 (Fig. 1E), IgE
(Fig. 1F), IgG2a (Fig. 1G) in the Con group were too low to
be evaluated. OVA-treatment led to significant upregulation of
IgG1, Ige and IgG2a in BALF samples. Of note, fisetin
administration reversed the increased IgG1, IgE and IgG2a
significantly.
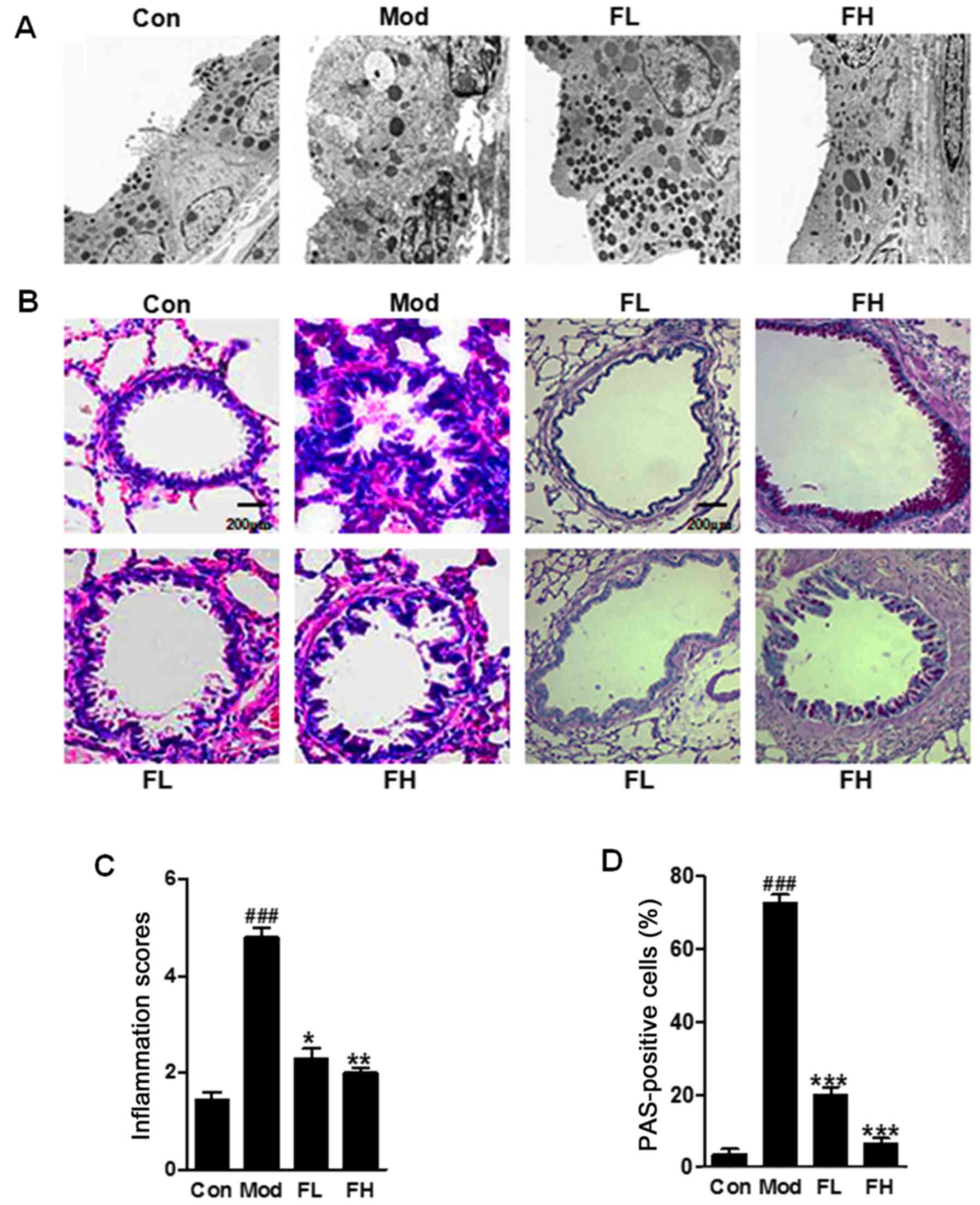
Fisetin attenuates airway inflammation in
the lung tissue samples from OVA-sensitized/challenged animal
model
The cell structure was injured seriously in the
OVA-induced lung tissue of mice (Fig.
2A). In the fisetin-induced group the cell structure was as
integrated as the control ones. The OVA-challenged mice displayed
marked infiltration of inflammatory cells into the peribronchial
and perivascular lesions in the lung tissue (Fig. 2B and C). However, in the
fisetin-treated mice, the cell infiltration was downregulated
significantly in the OVA-challenged animal model. In addition, the
lung sections with PAS staining showed that mucus was over-produced
in the bronchial airways in the OVA-induced mice. Notably, the
mucus production was reduced in the lung tissue samples of mice
with fisetin administration than that in the OVA-challenged mice
(Fig. 2B and D). Further,
morphological alterations of cells in lung tissue samples were
observed. The data above suggested that fisetin ameliorated airway
inflammation induced by OVA-sensitization and challenge in
mice.
Fisetin decreases inflammatory cell
counts and pro-inflammatory cytokine release
Inflammatory response is a major cause contributing
to OVA-sensitization and challenge-induced airway inflammation
(18). Thus, in this regard we
were attempted to explore whether fisetin could attenuate
inflammation response in OVA-induced asthmatic mice. The number of
inflammatory cells, such as macrophages, neutrophils, lymphocytes
and eosinophils, was upregulated significantly in the BALF of
OVA-sensitized/challenged mice in comparison to the wild-type
controls (Fig. 3A–D). However,
especially eosinophils, and the number of other cells, in the BALF
of OVA-induced mice was downregulated markedly in the
fisetin-treated mice compared to the OVA-sensitized/challenged
mice. Also, the total cells were calculated (Fig. 3E). Similarly, the total
inflammatory cells were accelerated in the mice with OVA-treatment,
which was decreased remarkably in OVA-induced asthmatic mice with
fisetin administration. Moreover, pro-inflammatory cytokines,
including IL-1β, TNF-α, IL-2, IL-4, IL-18, IFN-γ and IL-5, in BALF
and serum were calculated respectively (Fig. 3F and G). We found that cytokines
of IL-1β, TNF-α, IL-2, IL-4, IL-18 and IL-5 were stimulated highly
in the Mod group compared to the Con group. Fisetin reduced the
over-expression of these cytokines induced by OVA in mice. In
contrast, IFN-γ was downregulated in the OVA-induced wild-type
mice. Interestingly, it was upregulated due to fisetin treatment in
OVA-induced mice (Fig. 3F and G).
Also, these cytokines represent similar results in the serum of
mice. Taken together, the results further indicated that fisetin
may be useful in airway inflammation via inflammation
suppression.
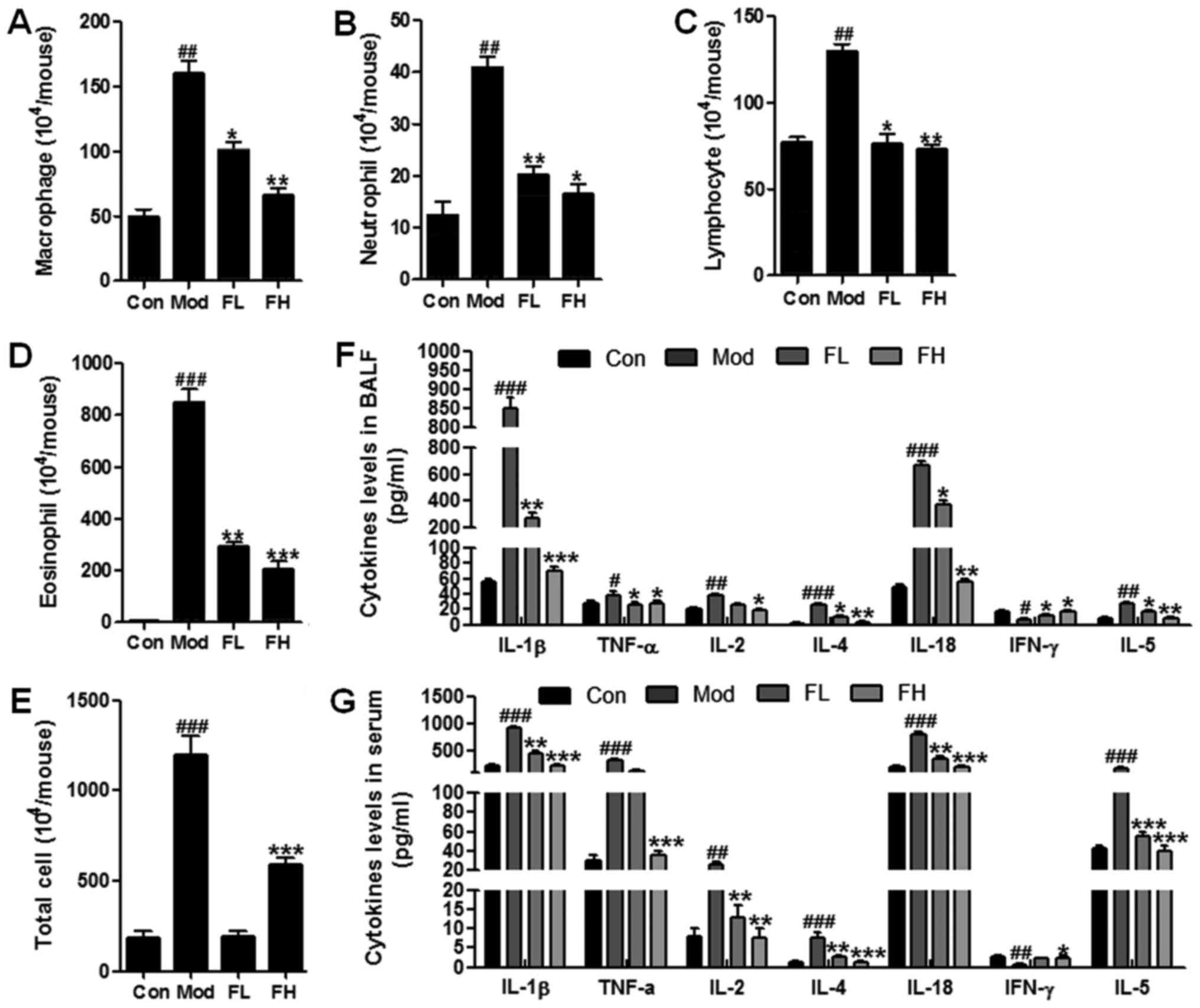 | Figure 3Fisetin decreased inflammatory cell
counts and pro-inflammatory cytokine release. Changes in the number
of (A) macrophages, (B) neutrophils, (C) lymphocytes, (D)
eosinophils, and (E) the total cells in the bronchoalveolar lavage
fluid (BALF) of mice. (F) Fisetin altered secretion of several
inflammatory cytokines in BALF, including interleukin-1β (IL-1β),
tumor necrosis factor-α (TNF-α), IL-2, IL-4, IL-18, interferon-γ
(IFN-γ) and IL-5. (G) Fisetin modulated release of several
inflammatory cytokines in serum, including IL-1β, TNF-α, IL-2,
IL-4, IL-18, IFN-γ and IL-5. The data are presented as the means ±
SD (n=10-15); #p<0.05, ##p<0.01 and
###p<0.001 vs. Con; *p<0.05,
**p<0.01 and ***p<0.001 vs. Mod. |
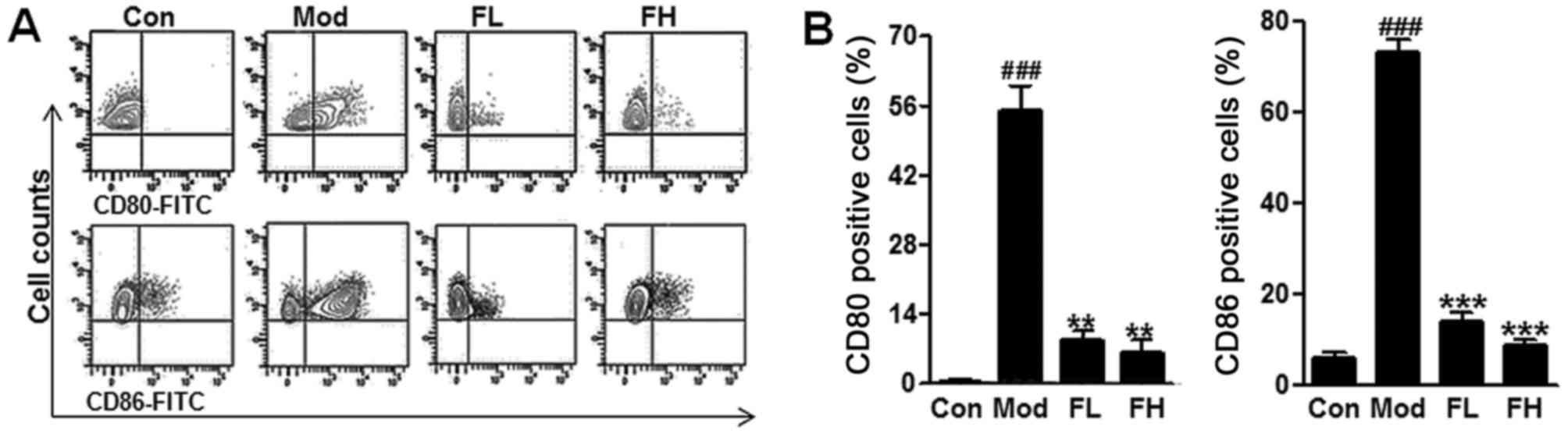
In addition, CD80 and CD86 are known to be
associated with AHR progression. Following the procedure of the
animal model, airway DCs isolated from lungs were analyzed for the
expression of costimulatory molecules, including CD80, and CD86 by
flow cytometry. In this study, we found that CD80 and CD86 positive
cells were higher in the OVA mice compared with the Control ones
via flow cytometry assays (Fig. 4A
and B), but fisetin reduced CD80 and CD86 positive cells in the
lung tissue samples of OVA-induced asthmatic mice. The data in this
regard further indicated that fisetin was, at least partly,
effective on the OVA-induced asthmatic mice.
TLR5-deficiency attenuates inflammation
response in OVA-induced asthmatic mice via MyD88 and NF-κB (p65)
signaling pathway suppression
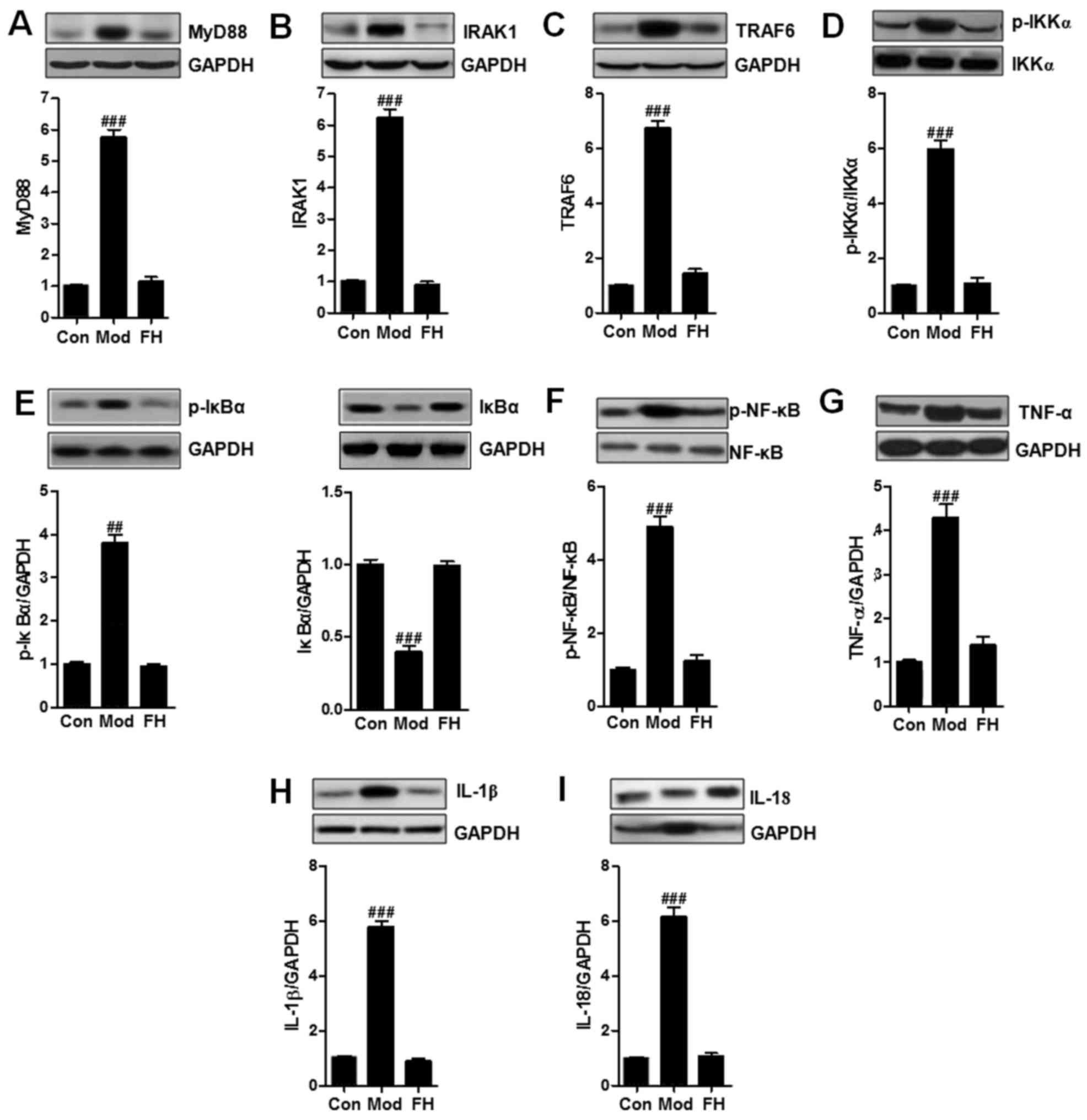
TLR5-modulated airway inflammation was supposed to
be linked with inflammation response. Thus, in this regard, the
possible mechanism by which TLR5 performed its role in asthmatic
mice was explored. TLR5-regulated signaling pathway through MyD88
activation is known to be related to NF-κB signaling pathway. NF-κB
activation is a key leading to pro-inflammatory cytokines secretion
and inflammatory response progression (19). Western blot analysis was used to
reveal the alterations of MyD88 (Fig.
5A), IRAK1 (Fig. 5B), TRAF6
(Fig. 5C), p-IKKα (Fig. 5D), p-IκBα (Fig. 5E), p-NF-κB (Fig. 5F), TNF-α (Fig. 5G), IL-1β (Fig. 5H), and IL-18 (Fig. 5I). MyD88 was upregulated in the
OVA group compared to that in the Con group. We considered that
fisetin downregulated MyD88 expression. Subsequently, the
downstream signals of IRAK1 and TRAF6 were also reduced
significantly in OVA-treated mice. Then, the phosphorylated IKKα,
IκBα, and NF-κB induced by OVA in OVA-induced mice was inactivated
due to fisetin administration. Finally, the releasing of typical
pro-inflammatory cytokines, such as TNF-α (Fig. 5G), IL-1β (Fig. 5H), and IL-18 (Fig. 5I), were reduced significantly in
the fisetin-treated mice.
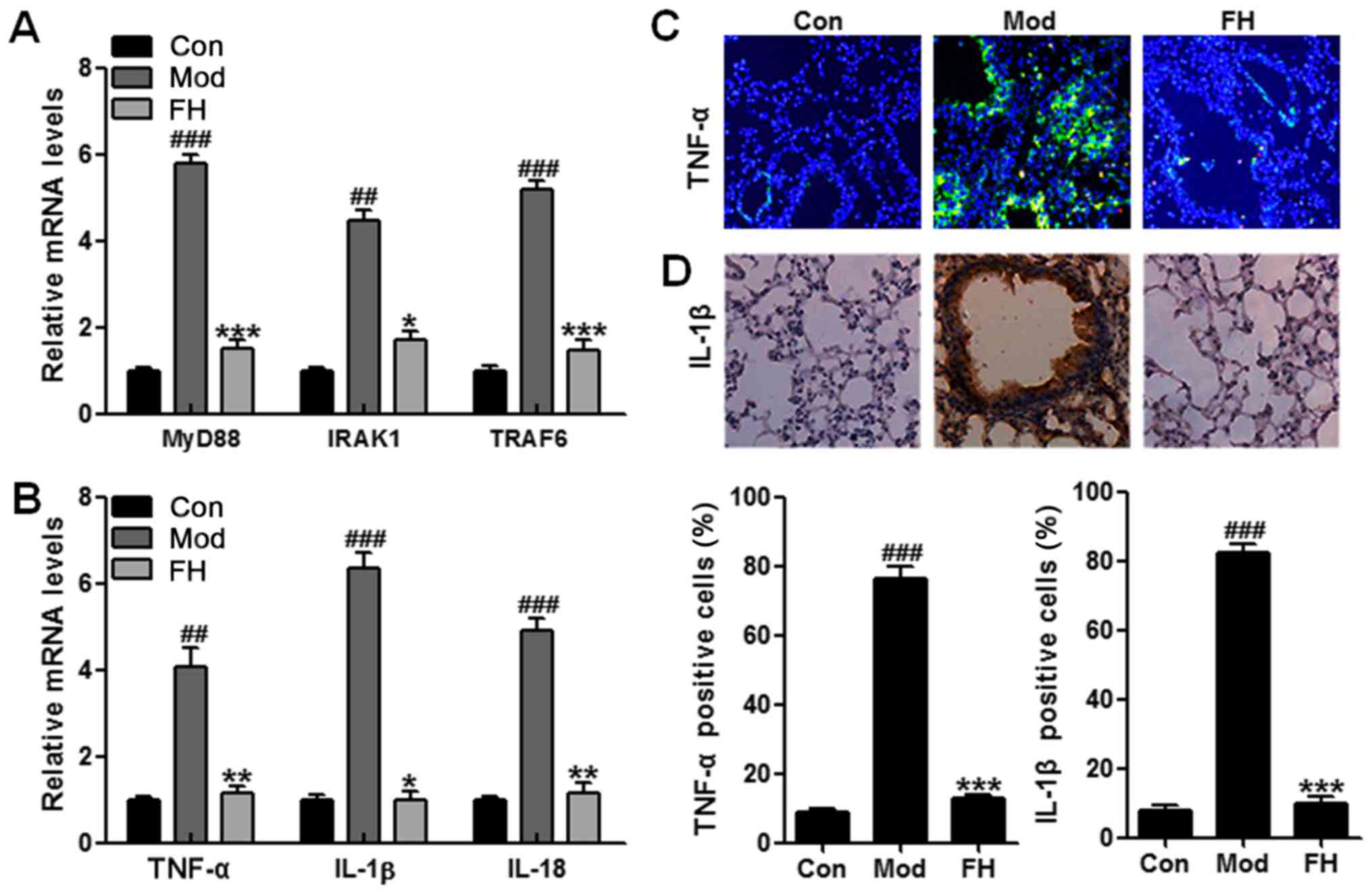
For further confirmation, RT-QPCR was applied to
analyze the gene mRNA levels. MyD88 signaling pathway was
inactivated for fisetin treatment in OVA-induced mice (Fig. 6A). Also, TNF-α, IL-1β and IL-18
mRNA levels were suppressed in the OVA-induced mice after fisetin
administration (Fig. 7B).
Moreover, immunofluorescence and immunochemical analysis further
proved that TNF-α and IL-1β were stimulated in OVA-induced mice,
which were downregulated significantly in OVA-treated mice with
fisetin, which was consistent with previous results (Fig. 6C and D). In conclusion, the data
suggested that fisetin could regulate AHR in the OVA-induced
asthmatic mice via MyD88- and NF-κB-dependent signaling
pathway.
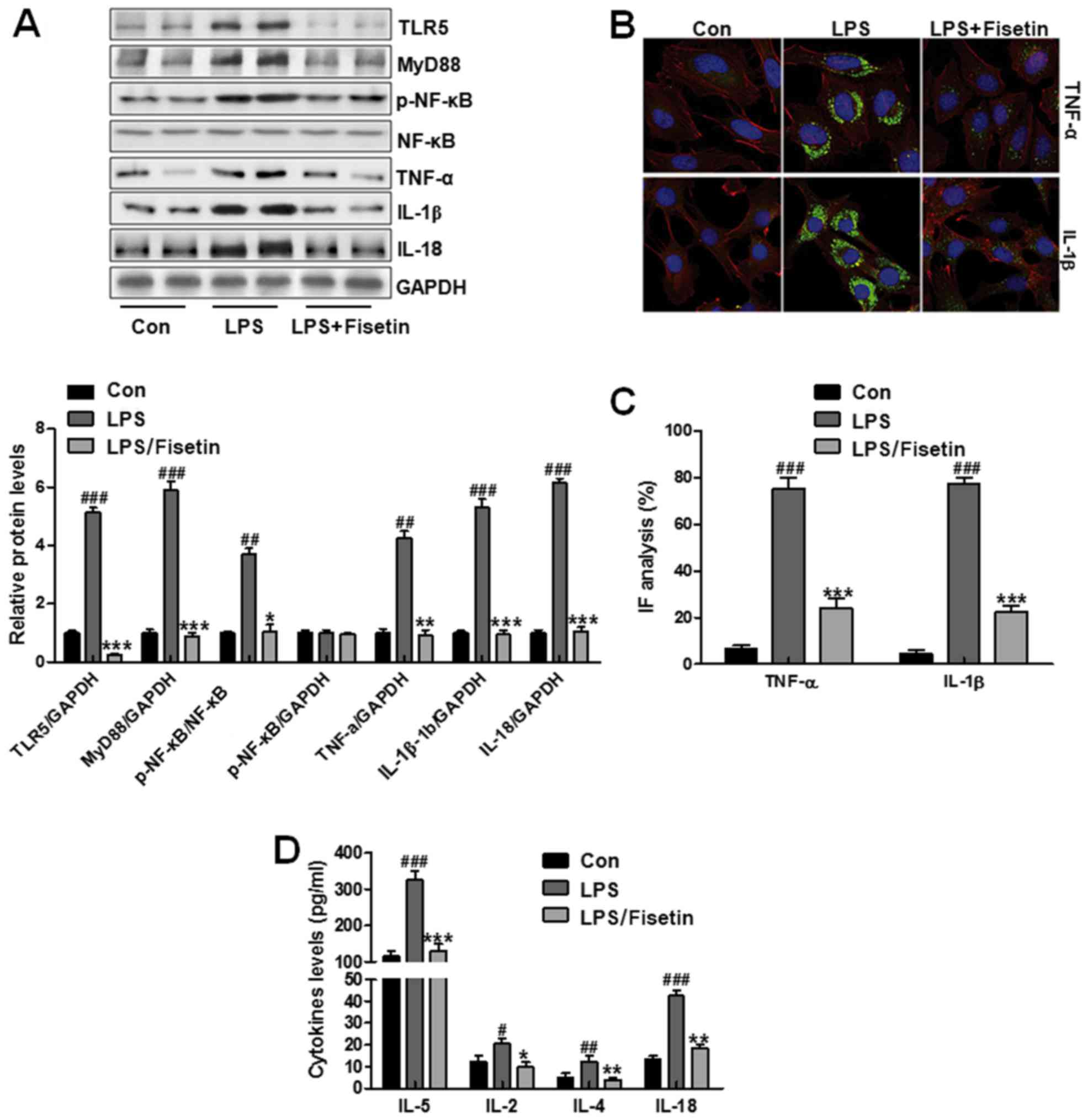
Fisetin ameliorated inflammtory response
in LPS-induced cells in vitro
Cell experiments were necessary to confirm the role
of fisetin in airway inflammation progression. TC1 cells were used
here, which were treated with LPS to produce inflammation response.
Then, 10 μM fisetin was performed to downregulate TLR5
expression and explore how inflammation response changed in
vitro. First, western blot analysis was used to assess TLR5
levels, which was expressed highly in LPS-induced groups. Of note,
in fisetin-treated groups, TLR5 expression levels were
downregulated significantly. Then, the down-streaming signal MyD88
was also reduced, causing inactivation of NF-κB and downregulation
of IL-18, TNF-α and IL-1β (Fig.
7A). In Fig. 7B and C,
immunofluorescent assays indicated that pro-inflammatory cytokines
of TNF-α and IL-1β were reduced in fisetin-treated groups compared
to that in the LPS-treated groups. Finally, other pro-inflammatory
cytokines, IL-5, IL-2, IL-4 and IL-8, in PBS-treated group were
higher compared to the control group, which was decreased in
fisetin-treated group (Fig. 7D).
The data further confirmed that fisetin was involved in airway
inflammation, and its downregulation had an inhibitory role in
pro-inflammatory cytokine secretion.
Discussion
The present study investigated the possible effect
of fisetin on allergic asthma and revealed the underlying mechanism
by which fisetin performed its role in ameliorating OVA-induced
mice. Allergic asthma is an inflammatory condition of the airway
which is caused by exacerbated responses to inhaled allergens and
characterized by reversible airway obstruction, infiltration of
eosinophils, and increased mucus production (5,20).
Some characteristic features of acute asthma are found in the
OVA-induced animal asthma model, which includes an accelerated
airway responding to Mch as well as eosinophil-rich airway
inflammation (21). Airway
neutrophils are clinically important due to their increasing
relation to asthma severity. Besides, sputum neutrophils have
negative relationship with airflow obstruction and lung function in
asthma (22). Hence, stimulation
that upregulates airway neutrophilia may lead to asthma
pathophysiology. In the present study, we found that OVA-treatment
induced high level of neutrophils, as well as other important
inflammatory cells, including macrophages, lymphocytes and
eosinophils. Fisetin reversed these cell counts. In addition,
short-term OVA inhalation led to a marked upregulation in RL, and a
pronounced downregulation in Cdyn towards the increased Mch doses.
Also, H&E and PAS staining of the lung slices suggested a
significant increase of inflammatory response in OVA-induced tissue
samples. The results above suggested fisetin may be considered as
an essential compound for prevention of airway inflammation.
Cytokine functions are characteristic features of
allergic asthma, which have been considered in AHR and eosinophilic
inflammation (14,23). IL-1β, IL-18 and TNF-α are very
well known as essential indicators of inflammation response
(24–26). IL-4 is important for the
development of AHR in animal models (27,28). IL-5 is involved in eosinophil
maturation, differentiation, recruitment, as well as survival
(29). Fisetin was originally
identified in a screen for flavonoids that could prevent oxidative
stress-induced nerve cell death (30). Fisetin is an agent that is
clinically effective with a broad range of anti-oxidative,
anti-inflammatory and anti-tumor activities against solid tumors,
such as breast, prostate, as well as colorectal tumors (31,32). Fisetin also has anti-inflammatory
activity both in vitro and in vivo that fisetin
reduced the level of inflammatory cytokines TNFα, IL-1β and IL-6 in
UVB-exposed skin (33). Fisetin
has been suggested to perform its role in suppressing tumor growth
by regulating DNA and apoptosis (34). Further, there are lower levels of
IFN-γ in asthma generally (35,36). IFN-γ is known to suppress
antigen-induced AHR in animals, suggesting that enhanced levels of
IFN-γ may be involved in the responses to AHR (37). This study also indicated similar
result that IFN-γ enhancement was related to fisetin-regulated
airway inflammation in OVA-induced mice.
The activation of NF-κB occurs when it dissociates
from IκBα, as the negative regulator for NF-κB, which is degraded
in the process. NF-κB phosphorylation involves IκBα activation,
which is catalyzed by IKK (38).
Liberation from IκB promotes NF-κB to translocate into the nucleus.
Then, it induces gene transcription through combination with NF-κB
responsive gene promoter. NF-κB has an essential role in airway
pathology via regulation of chemokines, cytokines as well as cell
adhesion molecules (39–41). These inflammatory mediators affect
the inflammatory cell type and quantity that infiltrate airway
tissue in the chronic airway obstructive diseases. NF-κB activation
in asthma occurs largely in response to inflammatory mediators such
as IL-1β and TNF-α or elicited by the activation of TLRs. In our
study, we found that MyD88/IRAK1/TRAF6 signaling pathway was
activated in the OVA-induced mice, leading to the NF-κB signaling
pathway activation and pro-inflammatory cytokine expression,
including IL-18, IL-1β and TNF-α. However, fisetin in vivo
reduced MyD88, IRAK1 and TRAF6 expression. Subsequently, NF-κB
phosphorylated levels were downregulated and the pro-inflammatory
cytokines were also reduced, suggesting that fisetin was at least
partly involved in airway inflammation through inhibition of MyD88
and NF-κB signaling pathways. In vitro experiments, in
fisetin treatment further confirmed that the relief of LPS-induced
cell inflammation was related to suppressed TLR5 activity through
MyD88 and NF-κB inhibition.
Altogether, this study revealed that OVA induced
airway inflammation development and progression by enhancing
inflammatory infiltration in mice via activation of
fisetin-mediated MyD88/NF-κB signaling pathways. These results
suggested potential association of the pathogenesis of airway
inflammation regarding how fisetin influenced asthma. Thus, use of
fisetin may be a possible strategy for asthma inhibition and
treatment.
Acknowledgments
Not applicable.
References
|
1
|
Wood LG, Garg ML and Gibson PG: A high-fat
challenge increases airway inflammation and impairs bronchodilator
recovery in asthma. J Allergy Clin Immunol. 127:1133–1140. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Masoli M, Fabian D, Holt S and Beasley R;
Global Initiative for Asthma (GINA) Program: The global burden of
asthma: executive summary of the GINA Dissemination Committee
report. Allergy. 59:469–478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sul B, Wallqvist A, Morris MJ, Reifman J
and Rakesh V: A computational study of the respiratory airflow
characteristics in normal and obstructed human airways. Comput Biol
Med. 52:130–143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blanc P, van Dyken S, Locksley R, Quinlan
PJ, Balmes JR, Iribarren C, Katz PP, Yelin EH, Trupin L and Eisner
MD: Chitin detection in home dust sampling. Am J Respir Crit Care
Med. 181:A46682010.
|
|
5
|
Zhu T, Zhang W, Wang DX, Huang NW, Bo H,
Deng W and Deng J: Rosuvastatin attenuates mucus secretion in a
murine model of chronic asthma by inhibiting the γ-aminobutyric
acid type A receptor. Chin Med J (Engl). 125:1457–1464. 2012.
|
|
6
|
Hendrich AB: Flavonoid-membrane
interactions: Possible consequences for biological effects of some
polyphenolic compounds. Acta Pharmacol Sin. 27:27–40. 2006.
View Article : Google Scholar
|
|
7
|
Cushnie TP and Lamb AJ: Recent advances in
understanding the antibacterial properties of flavonoids. Int J
Antimicrob Agents. 38:99–107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiruta C, Schubert D, Dargusch R and
Maher P: Chemical modification of the multitarget neuroprotective
compound fisetin. J Med Chem. 55:378–389. 2012. View Article : Google Scholar
|
|
9
|
Gelderblom M, Leypoldt F, Lewerenz J,
Birkenmayer G, Orozco D, Ludewig P, Thundyil J, Arumugam TV,
Gerloff C, Tolosa E, et al: The flavonoid fisetin attenuates
postischemic immune cell infiltration, activation and infarct size
after transient cerebral middle artery occlusion in mice. J Cereb
Blood Flow Metab. 32:835–843. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prakash D, Gopinath K and Sudhandiran G:
Fisetin enhances behavioral performances and attenuates reactive
gliosis and inflammation during aluminum chloride-induced
neurotoxicity. Neuromolecular Med. 15:192–208. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salter M, Biggadike K, Matthews JL, West
MR, Haase MV, Farrow SN, Uings IJ and Gray DW: Pharmacological
properties of the enhanced-affinity glucocorticoid fluticasone
furoate in vitro and in an in vivo model of respiratory
inflammatory disease. Am J Physiol Lung Cell Mol Physiol.
293:L660–L667. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hinz M and Scheidereit C: The IκB kinase
complex in NF-κB regulation and beyond. EMBO Rep. 15:46–61. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li M, Riddle SR, Frid MG, El Kasmi KC,
McKinsey TA, Sokol RJ, Strassheim D, Meyrick B, Yeager Me, Flockton
AR, et al: Emergence of fibroblasts with a proinflammatory
epigenetically altered phenotype in severe hypoxic pulmonary
hypertension. J Immunol. 187:2711–2722. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Edwards MR, Bartlett NW, Clarke D, Birrell
M, Belvisi M and Johnston SL: Targeting the NF-kappaB pathway in
asthma and chronic obstructive pulmonary disease. Pharmacol Ther.
121:1–13. 2009. View Article : Google Scholar
|
|
15
|
Li X, Chen Q, Chu C, You H, Jin M, Zhao X,
Zhu X, Zhou W and Ji W: Ovalbumin-induced experimental allergic
asthma is Toll-like receptor 2 dependent. Allergy Asthma Proc.
35:e15–e202014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sauer KA, Scholtes P, Karwot R and Finotto
S: Isolation of CD4+ T cells from murine lungs: A method
to analyze ongoing immune responses in the lung. Nat Protoc.
1:2870–2875. 2006. View Article : Google Scholar
|
|
17
|
Ji L, Xue R, Tang W, Wu W, Hu T, Liu X,
Peng X, Gu J, Chen S and Zhang S: Toll like receptor 2 knock-out
attenuates carbon tetrachloride (CCl4)-induced liver fibrosis by
downregulating MAPK and NF-κB signaling pathways. FEBS Lett.
588:2095–2100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hacha J, Tomlinson K, Maertens L,
Paulissen G, Rocks N, Foidart JM, Noel A, Palframan R, Gueders M
and Cataldo DD: Nebulized anti-IL-13 monoclonal antibody Fab'
fragment reduces allergen-induced asthma. Am J Respir Cell Mol
Biol. 47:709–717. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arakawa M, Mita T, Azuma K, Ebato C, Goto
H, Nomiyama T, Fujitani Y, Hirose T, Kawamori R and Watada H:
Inhibition of monocyte adhesion to endothelial cells and
attenuation of atherosclerotic lesion by a glucagon-like peptide-1
receptor agonist, exendin-4. Diabetes. 59:1030–1037. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liou CJ, Cheng PY, Huang WC, Chan CC, Chen
MC, Kuo ML and Shen JJ: Oral lovastatin attenuates airway
inflammation and mucus secretion in ovalbumin-induced murine model
of asthma. Allergy Asthma Immunol Res. 6:548–557. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reddy AT, Lakshmi SP and Reddy RC: Murine
model of allergen induced asthma. J Vis Exp. 63:e37712012.
|
|
22
|
Schepetkin IA and Quinn MT: Botanical
polysaccharides: Macrophage immunomodulation and therapeutic
potential. Int Immunopharmacol. 6:317–333. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lloyd CM and Saglani S: T cells in asthma:
Influences of genetics, environment, and T-cell plasticity. J
Allergy Clin Immunol. 131:1267–1274; quiz 1275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Redhu NS, Saleh A, Halayko AJ, Ali AS and
Gounni AS: Essential role of NF-κB and AP-1 transcription factors
in TNF-α-induced TSLP expression in human airway smooth muscle
cells. Am J Physiol Lung Cell Mol Physiol. 300:L479–L485. 2011.
View Article : Google Scholar
|
|
25
|
Hams E and Fallon PG: Innate type 2 cells
and asthma. Curr Opin Pharmacol. 12:503–509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Perkins C, Yanase N, Smulian G, Gildea L,
Orekov T, Potter C, Brombacher F, Aronow B, Wills-Karp M and
Finkelman FD: Selective stimulation of IL-4 receptor on smooth
muscle induces airway hyperresponsiveness in mice. J Exp Med.
208:853–867. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tanaka H, Nagai H and Maeda Y: Effect of
anti-IL-4 and anti-IL-5 antibodies on allergic airway
hyperresponsiveness in mice. Life Sci. 62:PL169–PL174. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Finkelman FD, Hogan SP, Hershey GK,
Rothenberg ME and Wills-Karp M: Importance of cytokines in murine
allergic airway disease and human asthma. J Immunol. 184:1663–1674.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fulkerson PC and Rothenberg ME: Targeting
eosinophils in allergy, inflammation and beyond. Nat Rev Drug
Discov. 12:117–129. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sakai E, Shimada-Sugawara M, Yamaguchi Y,
Sakamoto H, Fumimoto R, Fukuma Y, Nishishita K, Okamoto K and
Tsukuba T: Fisetin inhibits osteoclastogenesis through prevention
of RANKL-induced ROS production by Nrf2-mediated upregulation of
phase II antioxidant enzymes. J Pharmacol Sci. 121:288–298. 2013.
View Article : Google Scholar
|
|
31
|
Lee Se, Jeong SI, Yang H, Park CS, Jin YH
and Park YS: Fisetin induces Nrf2-mediated HO-1 expression through
PKC-δ and p38 in human umbilical vein endothelial cells. J Cell
Biochem. 112:2352–2360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Higa S, Hirano T, Kotani M, Matsumoto M,
Fujita A, Suemura M, Kawase I and Tanaka T: Fisetin, a flavonol,
inhibits TH2-type cytokine production by activated human basophils.
J Allergy Clin Immunol. 111:1299–1306. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen YC, Shen SC, Lee WR, Lin HY, Ko CH,
Shih CM and Yang LL: Wogonin and fisetin induction of apoptosis
through activation of caspase 3 cascade and alternative expression
of p21 protein in hepatocellular carcinoma cells SK-HEP-1. Arch
Toxicol. 76:351–359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Khan N, Afaq F, Syed DN and Mukhtar H:
Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle
arrest in human prostate cancer LNCaP cells. Carcinogenesis.
29:1049–1056. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pavord ID, Korn S, Howarth P, Bleecker ER,
Buhl R, Keene ON, Ortega H and Chanez P: Mepolizumab for severe
eosinophilic asthma (DREAM): A multicentre, double-blind,
placebo-controlled trial. Lancet. 380:651–659. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brooks GD, Buchta KA, Swenson CA, Gern JE
and Busse WW: Rhinovirus-induced interferon-gamma and airway
responsiveness in asthma. Am J Respir Crit Care Med. 168:1091–1094.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Akpinarli A, Guc D, Kalayci O, Yigitbas E
and Ozon A: Increased interleukin-4 and decreased interferon gamma
production in children with asthma: Function of atopy or asthma. J
Asthma. 39:159–165. 2002. View Article : Google Scholar
|
|
38
|
Yang SR, Yao H, Rajendrasozhan S, Chung S,
Edirisinghe I, Valvo S, Fromm G, McCabe MJ Jr, Sime PJ, Phipps RP,
et al: RelB is differentially regulated by IkappaB Kinase-α in B
cells and mouse lung by cigarette smoke. Am J Respir Cell Mol Biol.
40:147–158. 2009. View Article : Google Scholar
|
|
39
|
Gagliardo R, Chanez P, Profita M, Bonanno
A, Albano GD, Montalbano AM, Pompeo F, Gagliardo C, Merendino AM
and Gjomarkaj M: IκB kinase-driven nuclear factor-κB activation in
patients with asthma and chronic obstructive pulmonary disease. J
Allergy Clin Immunol. 128:635–45.e1, 2. 2011. View Article : Google Scholar
|
|
40
|
Bao Z, Guan S, Cheng C, Wu S, Wong SH,
Kemeny DM, Leung BP and Wong WS: A novel antiinflammatory role for
andrographolide in asthma via inhibition of the nuclear
factor-kappaB pathway. Am J Respir Crit Care Med. 179:657–665.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xiao M, Zhu T, Zhang W, Wang T, Shen YC,
Wan QF and Wen FQ: Emodin ameliorates LPS-induced acute lung
injury, involving the inactivation of NF-κB in mice. Int J Mol Sci.
15:19355–19368. 2014. View Article : Google Scholar : PubMed/NCBI
|