Introduction
Intrauterine adhesions (IUAs), also referred to as
Asherman's syndrome, are mainly characterized by spanomenorrhea,
amenorrhea, infertility, recurrent miscarriage, abdominal pain and
other complications later in pregnancy (1). Those clinical symptoms are
associated with major health concerns, particularly for women of
childbearing age. As the pathogenesis of IUAs has not been fully
elucidated, the successful pregnancy rate remains low, despite
advances in therapeutic modalities.
IUAs usually develop following intrauterine surgery
and infection. It has been made explicit that normal repair
following trauma is regulated by a complex set of interactions in a
network of pro- and anti-fibrotic cytokines. Once an insult has
been delivered to the tissues, a fibrotic process is initiated with
the activation of matrix-producing fibroblasts and accumulation of
extracellular matrix (ECM) coupled with tissue regeneration. Any
deregulation of the self-limited wound healing process and
excessive accumulation of ECM may lead to abnormal formation of
fibrous tissue (fibrosis) rather than normal tissue restoration
(2). During this fibrous
response, transforming growth factor (TGF)-β1, a multifunctional
cytokine that regulates cell growth, adhesion, migration, apoptosis
and differentiation, plays a crucial role in the canonical
TGF-β/Smad signaling pathway (3,4).
Furthermore, it may also regulate other downstream cellular
responses, induce epithelial-to-mesenchymal transition (EMT) and
mediate fibroblast activation, responses involved in facilitating
fibrotic diseases (5,6). Matrix metalloproteinase (MMP)-9, the
downstream target gene of TGF-β1, has been identified as an
anti-fibrotic factor due to its proteolytic degradation of ECM that
is usually downregulated in a number of fibrotic diseases. By
contrast, MMP-9 is occasionally significantly upregulated during
EMT, promoting fibrous tissue proliferation, leading to chronic
kidney disease and skin wound healing (7,8).
However, the role of MMP-9 in the pathological process underlying
the development of IUAs remains uncertain.
In addition, recent studies have demonstrated that
endometrial stem cells are involved in endometrial regeneration,
which may be crucial for the treatment of IUAs (9–11).
Bone marrow stem cells (BMSCs) have been hypothesized to be
important for endometrial regeneration and repair, but the
underlying mechanism has not been reported in detail (12,13). Several lines of evidence indicate
that the stromal derived factor (SDF)-1/C-X-C chemokine receptor
type 4 (CXCR-4) axis plays a crucial role during BMSC homing
(14,15). ERα is responsible for the estrogen
involvement in cell growth and proliferation (16). Our previous cell study has
demonstrated that ERα was able to effectively promote BMSC
proliferation and migration via SDF-1/CXCR-4 signaling (17). The aim of the present study was to
investigate the expressions of ERα and the SDF-1/CXCR-4 axis in
human and rat endometrium with IUAs.
Materials and methods
Patient samples
A total of 76 patients were admitted to the First
Affiliated Hospital of Chongqing Medical University between June
2015 and February 2016, and 56 cases (mild-moderate, n=40; and
severe, n=16) were diagnosed with IUAs by hysteroscopy according to
the standards of IUA diagnosis published by the American Fertility
Society (AFS) (1,18). A total of 20 samples of normal
endometrium were obtained following septate uterus excision and
were used as the control group. All the endometrial biopsy samples
were acquired from subjects during the luteal phase, in an attempt
to avoid the influence of hormones, using disposable hysteroscopic
endometrial biopsy catheters. The patients were aged 18–42 years,
with a mean age of 27 years. Patients with additional endometrial
complications, including dysfunctional uterine bleeding, polycystic
ovary syndrome, adenomyosis, or other hormone-dependent conditions,
were excluded. All the patients had regular menstrual cycles, with
no hormone therapy at least 3 months prior to surgery; no pregnant
or lactating patients were included. The study was approved by the
Ethics Commission of Chongqing Medical University, and informed
consent was obtained from all patients.
Experimental animals
A total of 100 female adult Sprague-Dawley rats,
aged 6–8 weeks and weighing 220–280 g, provided by the Central
Laboratory of Southwest Hospital, the First Affiliated Hospital of
Third Military Medical University, were employed in this
experiment. The rats were housed in a comfortable environment with
controlled temperature, with free access to food and water. A 12-h
dark and light cycle was maintained. Vaginal smears were collected
at 8:00 a.m. and 3:00 p.m. daily to determine whether the animals
had normal and regular estrous cycles. Through vaginal cytology
assessment during two successive estrous cycles, a total of 80 rats
that met the standards were selected and divided into 8 groups,
including the control group (n=10) and 7 experimental groups (n=10
per group). The follow-up animal operations were all conducted
during the estrus stage. All animal procedures in the subsequent
experiments were approved by the Ethics Committee of the Third
Military Medical University.
Animal model
Rat IUA models were constructed according to Jing
et al and Hunter et al (12,19,20). Anesthesia was performed with 10%
chloral hydrate (3 ml/kg, intramuscular injection) as previously
described (21). All the animals
were capable of breathing on their own during the entire procedure.
The animals were then placed in a supine position and the lower
abdomen was shaved and sterilized with 70% ethanol on the operating
table. When the rats were confirmed to be sufficiently
anesthetized, without righting or corneal reflexes, a vertical
incision (2.5–3 cm) was performed until the bilateral uterine horns
were exposed. After normal anatomy was verified, the junctions of
the uterine horns and the proximal uterus were closed with clamps.
Subsequently, ~0.5 ml of 95% ethanol was instilled into the lumen
of the uterine horns for ~5 min using a 1-ml syringe. Before the
abdomen was closed, the uterine cavity and peritoneal cavity were
thoroughly rinsed with physiological saline. A comfortable
environment was prepared for all rats postoperatively.
Animal specimen collection
To evaluate the IUA characteristics and development
process, rat bilateral uterine horn tissues were collected from the
control group (normal endometrium) and the experimental groups (at
postoperative days 1 and 3 and the first, second, third, fourth and
fifth estrus phase) and preserved in 10% paraformaldehyde and/or at
−80°C for the following experiments.
Hematoxylin and eosin (H&E) and
Masson's trichrome staining
After fixation in 10% paraformaldehyde, endometrial
samples from patients and uterine sections from rats were
dehydrated in graded ethanol solutions, embedded in paraffin and
cut into 6-µm transverse sections. H&E staining was
applied to observe the morphological variations of the endometrium
and confirm whether the thin endometrium of IUAs was successfully
formed: First, the thickness of the endometrium was measured using
a light microscope (XS-71; Leica Microsystems GmbH, Wetzlar,
Germany) and an imaging analysis system (AxioVision rel.4.8; Carl
Zeiss, Jena, Germany). In addition, endometrial gland count, gland
density and other morphological variations were observed and
evaluated under a light microscope at a magnification of ×200
and/or ×400, and the differences between the control and IUA groups
were evaluated and compared with the Student's t-test and/or
analysis of variance. Masson's trichrome staining was employed to
confirm the degree of endometrial fibrosis in animal samples.
Immunohistochemistry
Slices were prepared by H&E staining. After the
sections were dewaxed in xylene and hydrated with descending
ethanol concentrations, the sections were heated in citrate buffer
(pH 6.0; ZL1-9065; Zhongshan Jinqiao, Beijing, China) in a
microwave oven for 20 min for antigen retrieval and then cooled
naturally to room temperature. Washing the sections was performed
in PBS for 3 min x 3 cycles prior to incubation in 3%
H2O2 for 15 min at room temperature; then
washing was performed with PBS for 5 min x 3 cycles. After the
sections were blocked in 10% rabbit serum for 30 min at room
temperature, they were incubated overnight at 4°C with rabbit
anti-vimentin (dilution, 1:300; bs-8533R, Bioss, Beijing, China),
rabbit anti-cytokeratin (dilution, 1:50; ab41825, Abcam, Shanghai,
China), rabbit anti-CD34 (dilution, 1:300; Abcam), rabbit anti-ERα
(dilution, 1:100; MAB57151, R&D Systems, Shanghai, China),
mouse anti-TGF-β1 (dilution, 1:200; ab92486, Abcam), rabbit
anti-MMP-9 (dilution, 1:100; ab76003, Abcam), and rabbit
anti-CXCR-4 (dilution, 1:100; ab124824, Abcam) antibodies. Negative
control included omission of the primary antibody and use of
irrelevant primary antibodies. The sections were then incubated
with the corresponding-secondary antibodies for 30 min at 37°C. The
slides were washed in PBS for 5 min for 3 cycles prior to
incubation in horseradish enzyme labeled chain avidin solution for
30 min at 37°C and washed in PBS for 5 min x 3 cycles. The sections
were visualized by diaminobenzidine followed by counterstaining,
dehydration, clearing and sealing. The slides were evaluated
independently by 3 pathologists for distribution and intensity of
signal. Intensity was scored from 0 to 3 as follows: 0, absent
immunopositivity; 1, low immunopositivity; 2, moderate
immunopositivity; and 3, intense immunopositivity. A mean of 22
fields was observed for each tissue. All values are represented as
the mean ± standard error of the mean (SEM) (3,22).
Reverse transcription
quantitative-polymerase chain reaction (RT-qPCR) assay
Total RNA was extracted using a high-purity total
RNA rapid extraction kit (RP1201, BioTeke, Beijing, China)
according to the manufacturer's instructions. cDNA was synthesized
using the Rever Tre Ace-a kit (Toyobo Co., Ltd., Shanghai, China).
The primers used for amplifying ERα, TGF-β1, MMP-9, SDF-1 and
CXCR-4 were purchased from Jinmai Co., Ltd. (Chongqing, China).
qPCR was performed using the ABI 7500 Real-Time PCR System (Applied
Biosystems, Shanghai, China) according to the manufacturer's
instructions using the SYBR-Green Premix Ex Taq kit (Toyobo Co.,
Ltd.). The PCR conditions were 96°C for 30 sec, 57°C for 30 sec,
and 72°C for 30 sec. The experiments were performed in triplicate
for each sample. Relative quantification of mRNA was performed
using the comparative threshold cycles (CT) method. This value was
used to plot the gene expression employing the formula
2−ΔΔCq.
ERα, F: 5′-AACCACCTTTGATCTATTC-3′ and R:
5′-GCGCCAGACCAGACCAATCATC-3′; TGF-β1, F:
5′-GACCGCAACAACGCAATCTATG-3′ and R: 5′-CTCCACAGTTGACTTGAATC-3′;
MMP-9, F: 5′-CCCTACTGCTGGTCCTTCTG-3′ and R:
5′-GACCGTCCTTGAAGAAATGCAG-3′; SDF-1, F:
5′-TGCACAATGGAGCTTTTATAAC-3′ and R: 5′-AAAGCAAACCGAATACAGAC-3′; and
CXCR-4, F: 5′-AGGCCGTCTATGTGGGTGTCTGG-3′ and R:
5′-GAGGGCCTTGCGCTTCTGG-3′.
Western blot analysis
Tissues were lysed with RIPA buffer containing
protease inhibitors (Roche, Shanghai, China) following grinding in
a mortar. The cell lysates were centrifuged at 16,000 × g for 15
min at 4°C and the supernatants were collected and the protein
contents were determined using a bicinchoninic acid protein assay
kit. Protein extracts were loaded on a 10% sodium dodecyl
sulfate-polyacrylamide gel for electrophoresis and transferred onto
polyvinylidene fluoride membrane. The blots were blocked in 5%
skimmed milk in Tris-buffered saline containing 0.1% Tween-20 for 2
h at room temperature, and then incubated with the primary antibody
at 4°C overnight. Anti-ERα mouse polyclonal antibody (1:1,000),
anti-TGF-β1 mouse polyclonal (1:1,000); anti-CXCR4 rabbit
polyclonal antibody (1:500), anti-SDF-1 rabbit polyclonal antibody
(1:1,000) or anti-MMP-9 rabbit polyclonal antibody (1:1,000) was
incubated with the membrane for 2 h at 37°C. Secondary antibodies
that were conjugated to horseradish peroxidase were incubated with
the membrane for 1 h at 37°C. The proteins that were revealed by
western blot were visualized using chemiluminescence (Biyuntian
Company, Shanghai, China). The densities of the bands were analyzed
using a gel imaging system and calculated compared with the
internal control.
Statistical analysis
All data were analyzed with SPSS software version
22.0 (IBM Corp., Armonk, NY, USA). Histological and
immunohistochemical data are presented as mean ± SEM. Other data
are reported as mean ± standard deviation. Each experiment was
performed in triplicate. Student's t-test was employed for
within-group comparisons and analysis of variance for multiple
groups. Statistical significance was defined as a P-value of
<0.05.
Results
Immunohistochemical findings
Hematoxylin and eosin staining revealed the
following: Compared with the control group, the thickness and
number of glands of the IUA endometrium were significantly reduced,
whereas the cuboid epithelial cells of the endometrium were
gradually replaced by low columnar cells or were absent (P<0.05;
Fig. 1A and B). The IUA
endometrium was thinner and less continuous, with irregularly
structured, sparse glands. Endometrial epithelial cells and
glandular epithelial cells were transformed into low columnar or
even flat cells (Fig. 1A).
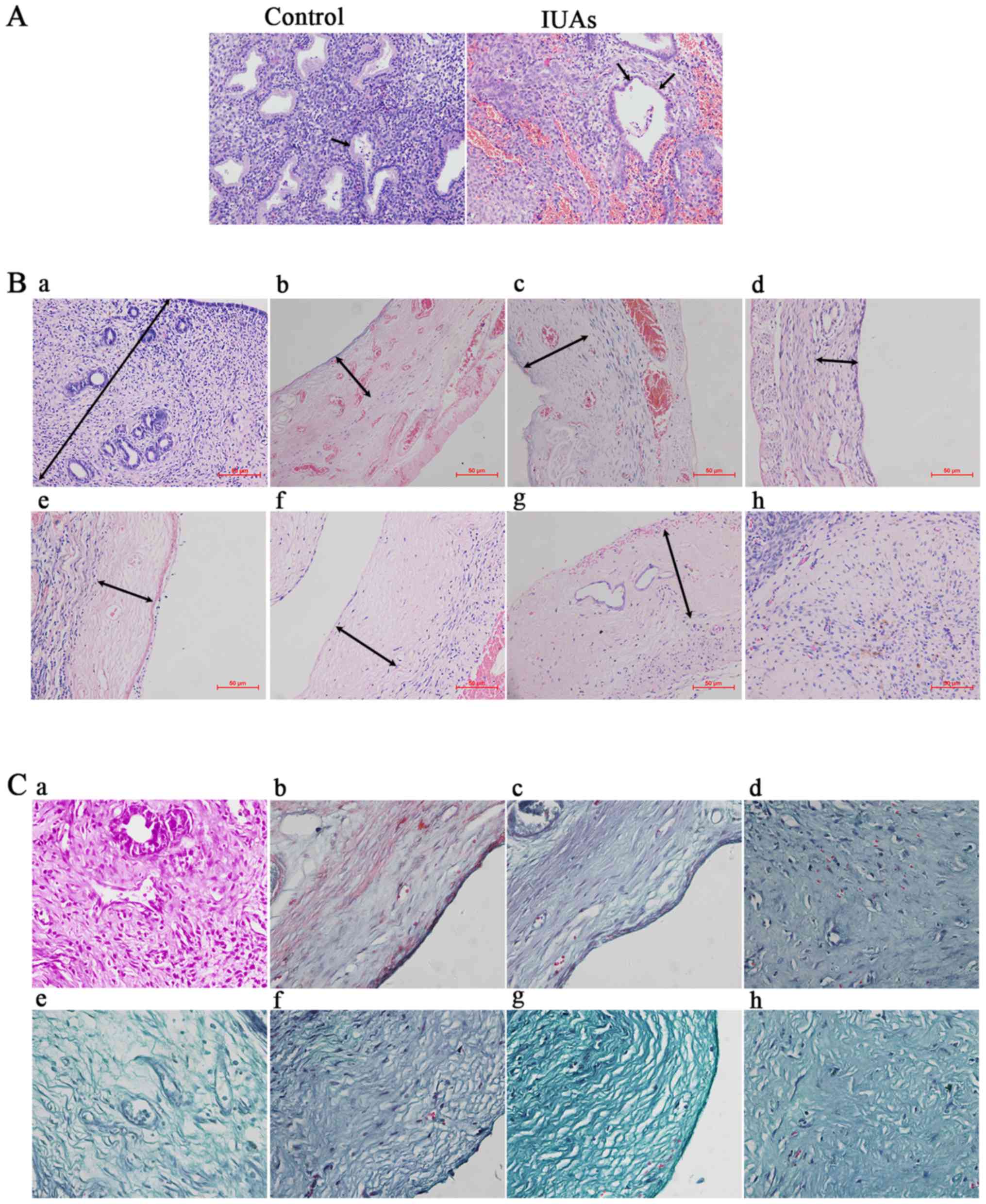 | Figure 1Hematoxylin and eosin staining
(H&E) and Masson's trichrome staining for patients and rats
with intrauterine adhesions (IUAs). (A) H&E staining of patient
endometrium with IUAs (×200). Black arrow in control group
indicates cuboid epithelial cells; black arrows in the IUAs group
indicate low columnar and flat glandular epithelial cells. (B)
H&E staining of rat endometrium (×200). a, Control group
endometrium. Black arrow indicates the thickness of normal
endometrium; b→h, experimental groups on postoperative days 1 and
3, and in the first, second, third, fourth and fifth estrous
cycles, respectively. Black arrows indicate the reduced thickness
of the endometrium. (C) Masson's trichrome staining for endometrial
fibrosis of rat IUAs (magnification, ×400). a, Control group
endometrium; b→h, representative fibrotic endometrium on days 1 and
3, and at the first, second, third, fourth and fifth estrous cycles
after the operation, respectively. |
In order to determine the fibrotic characteristics
in rat IUAs, Masson's trichrome staining was employed to detect
fibrosis. It was observed that, as the time progressed
postoperatively, the endometrium was gradually replaced or covered
by fibrous scar tissue (Fig.
1C).
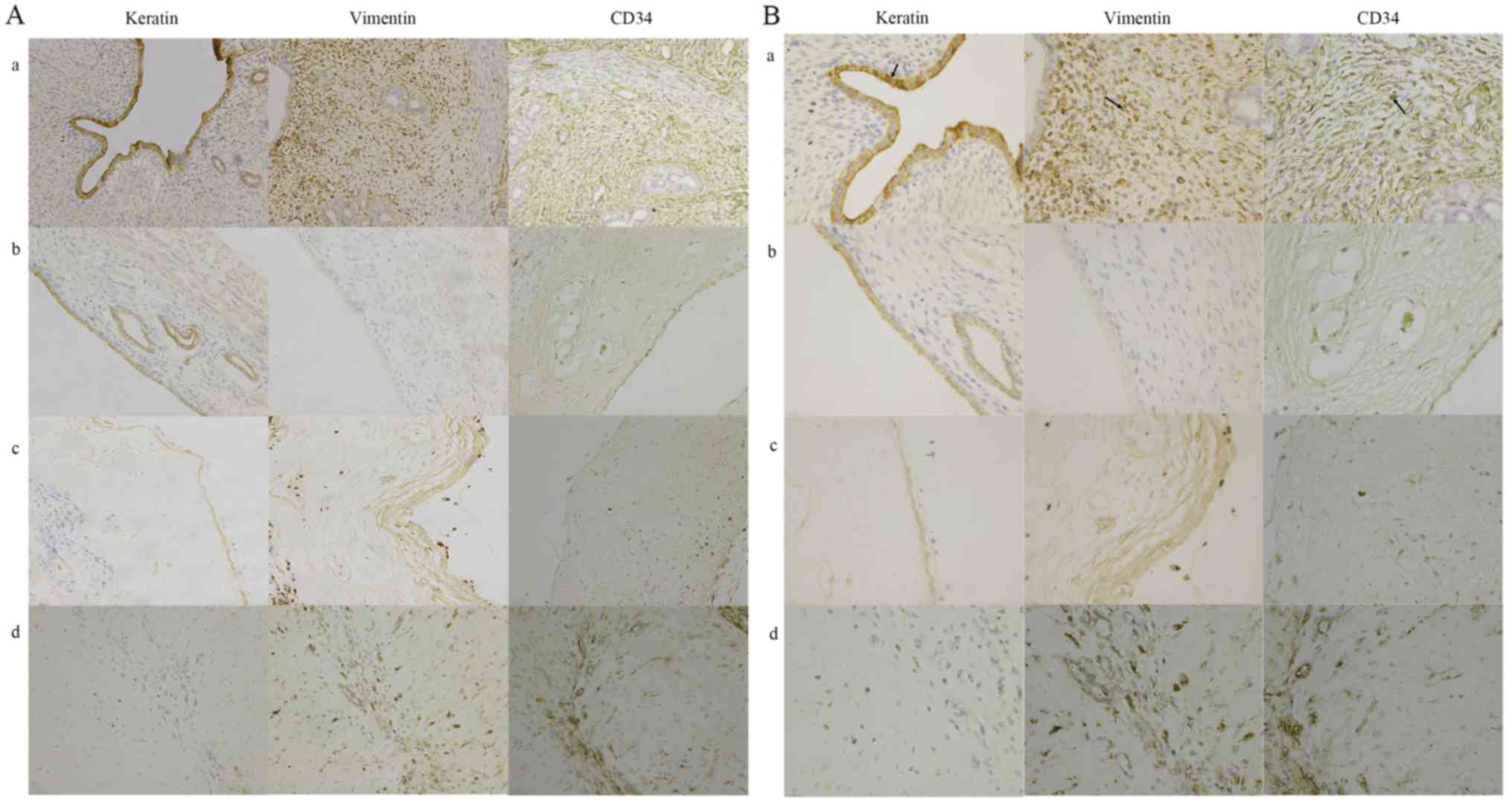
The protein expression levels of keratin, vimentin
and CD34 in rats were detected by immunohistochemistry. Owing to
reduction of cells lining the endometrial cavity/glandular
epithelial cells, keratin protein expression in rat experimental
groups was significantly decreased compared with the control group
(P<0.05). Due to the reduction of stromal cells and capillaries,
vimentin and CD34 protein expression were significantly decreased
in rat experimental groups compared with the control group
(P<0.05; Fig. 2).
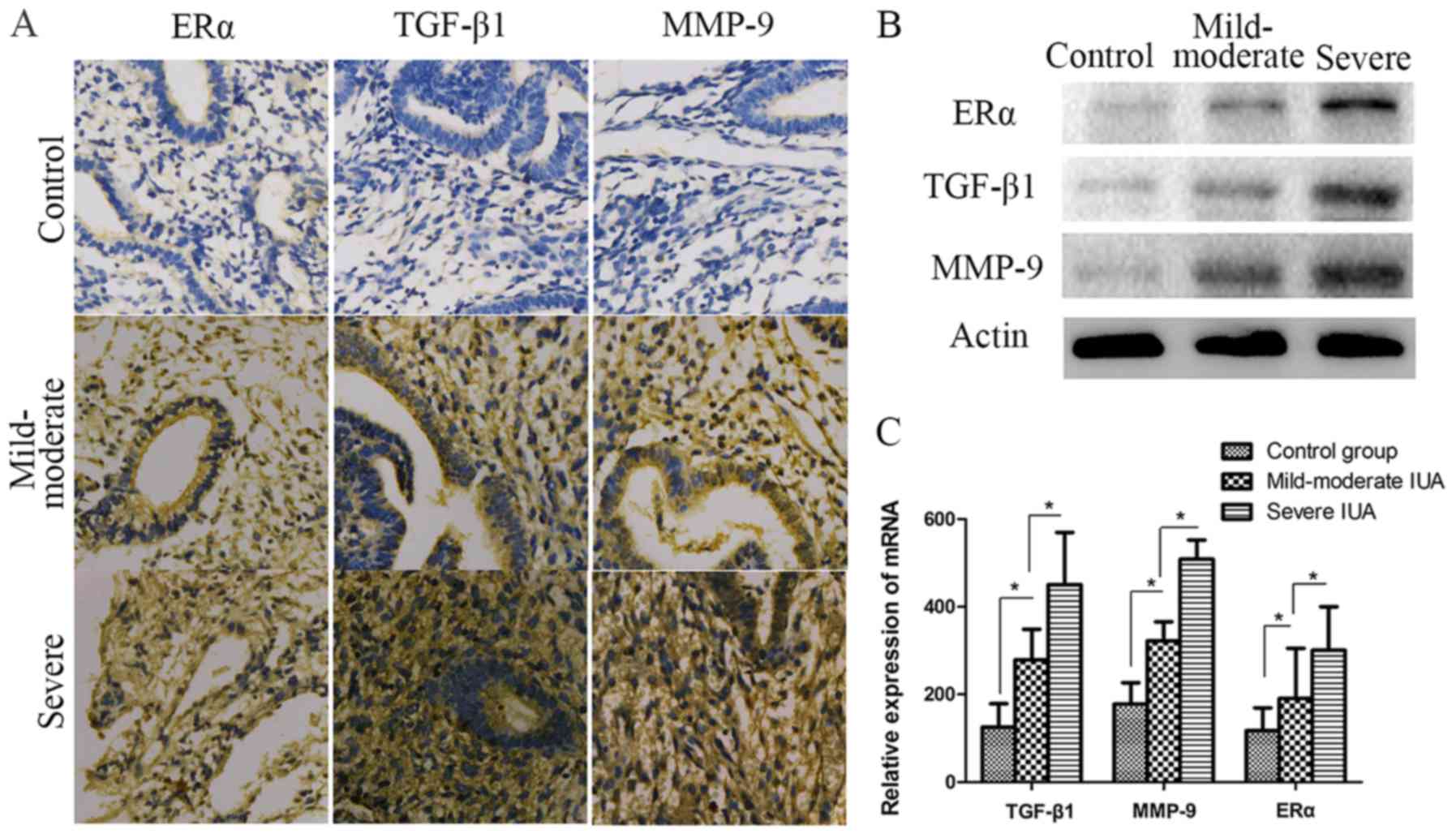
The expression levels of ERα, TGF-β1 and MMP-9 were
significantly increased in patients with IUAs. To investigate the
association among fibrosis, ERα and IUA endometrium development and
progression, TGF-β1 and MMP-9 expression was detected in patient
endometrium with different degrees of IUAs. As shown in Fig. 3, the results of protein and mRNA
analysis revealed that TGF-β1 and MMP-9 were
significantly increased in the IUA groups compared with the control
group (P<0.05) (Fig. 3).
Furthermore, TGF-β1 and MMP-9 in severe IUA endometrium were
significantly higher compared with those in mild-to-moderate IUA
endometrium (P<0.05; Fig. 3).
For ERα, the expression levels detected by western blotting,
immunohistochemistry and RT-qPCR in the IUA groups were
significantly higher compared with those in the control group;
similar to TGF-β1 and MMP-9, ERα expression in the severe IUA group
was significantly higher compared with that in the mild-to-moderate
group. (P<0.05; Fig. 3).
The expression of ERα, TGF-β1 and MMP-9 was
significantly increased in rat endometrium with IUAs. To identify
the association of ERα, TGF-β1 or MMP-9 with IUA formation and
degree of progression, rat uterine tissues were collected after
surgery at different time points, as previously mentioned (at
postoperative days 1 and 3 and at the first, second, third, fourth
and fifth estrous cycles), for protein and mRNA determination.
Compared with the control group, ERα and TGF-β1 were significantly
reduced on postoperative days 1 and 3, reaching their lowest level
on postoperative day 3; subsequently, over time, the expression
levels of ERα and TGF-β1 started to increase at the first
postoperative estrous cycle, and then significantly exceeded the
ones in the control group from the second postoperative estrous
cycle onwards (P<0.05; Fig.
4).
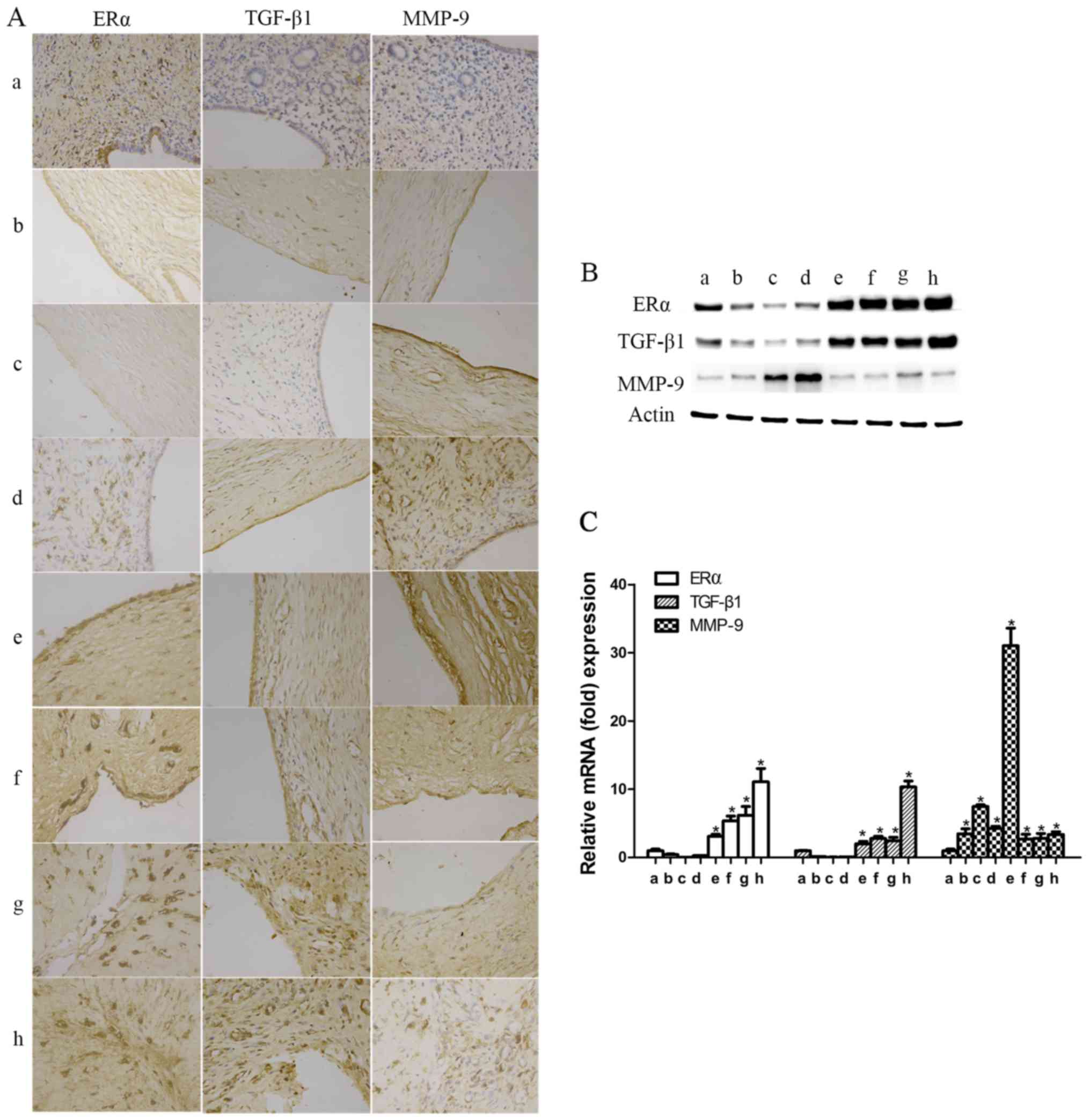 | Figure 4Expression levels of estrogen receptor
α (ERα), transforming growth factor-β1 (TGF-β1), matrix
metalloproteinase-9 (MMP-9) in rats with intrauterine adhesions
(IUAs). (A) The expression levels of ERα, TGF-β1 and MMP-9 in rat
endometrial tissues were detected by immunochemistry
(magnification, 400). (B) The relative protein expression was
detected by western blotting, and the results were in accordance
with those of immunochemistry. (C) The mRNA expression levels of
ERα, TGF-β1 and MMP-9 in rat endometrial tissues were detected by
reverse transcription-quantitative polymerase chain reaction. a,
ERα, TGF-β1 and MMP-9 in the endometrium of the control group; b→h,
ERα, TGF-β1 and MMP-9 in the endometrium of the experimental groups
on postoperative days 1 and 3, and in the first, second, third,
fourth and fifth estrous cycles (*P<0.05). |
The expression of MMP-9 during the early phase was
significantly increased from day 1 after surgery, reaching its
highest level at the second postoperative estrous cycle (P<0.05;
Fig. 4). Similar to humans, MMP-9
expression was found to be significantly upregulated at the fifth
estrous cycle (P<0.05; Fig.
4). These findings suggest that TGF-β1, MMP-9 and ERα were
involved in IUA development and progression, but the underlying
mechanism has not been reported in detail.
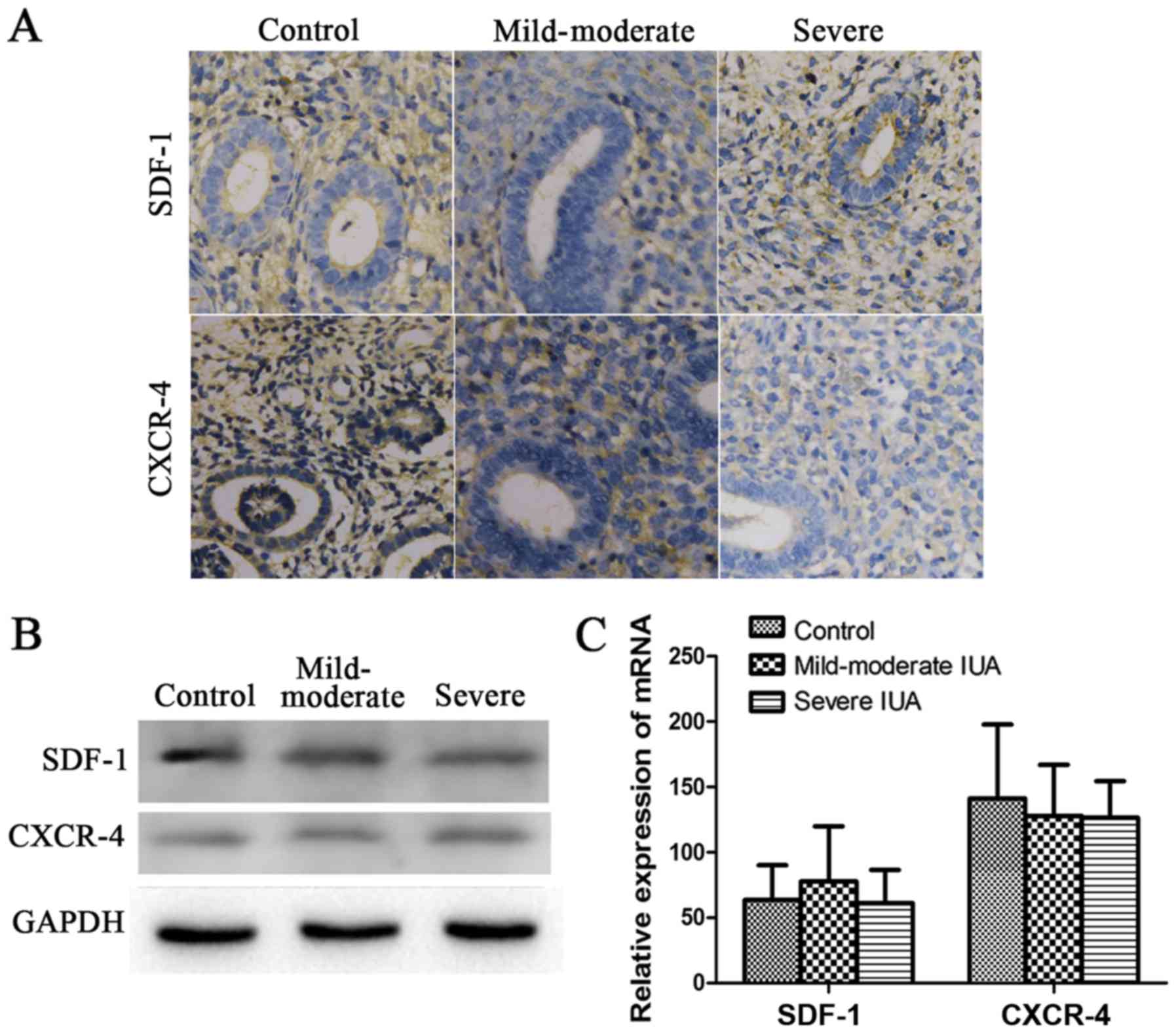
To determine whether the SDF-1/CXCR-4 axis affects
the pathogenesis of IUAs, the expression of SDF-1 and CXCR-4 was
measured in the endometrium of patients and rats with IUAs. In the
human experiments, the expression of SDF-1 and CXCR-4 at the
protein and mRNA level, whether in the mild-to-moderate or the
severe IUA group, did not differ significantly compared with the
control group (P>0.05; Fig.
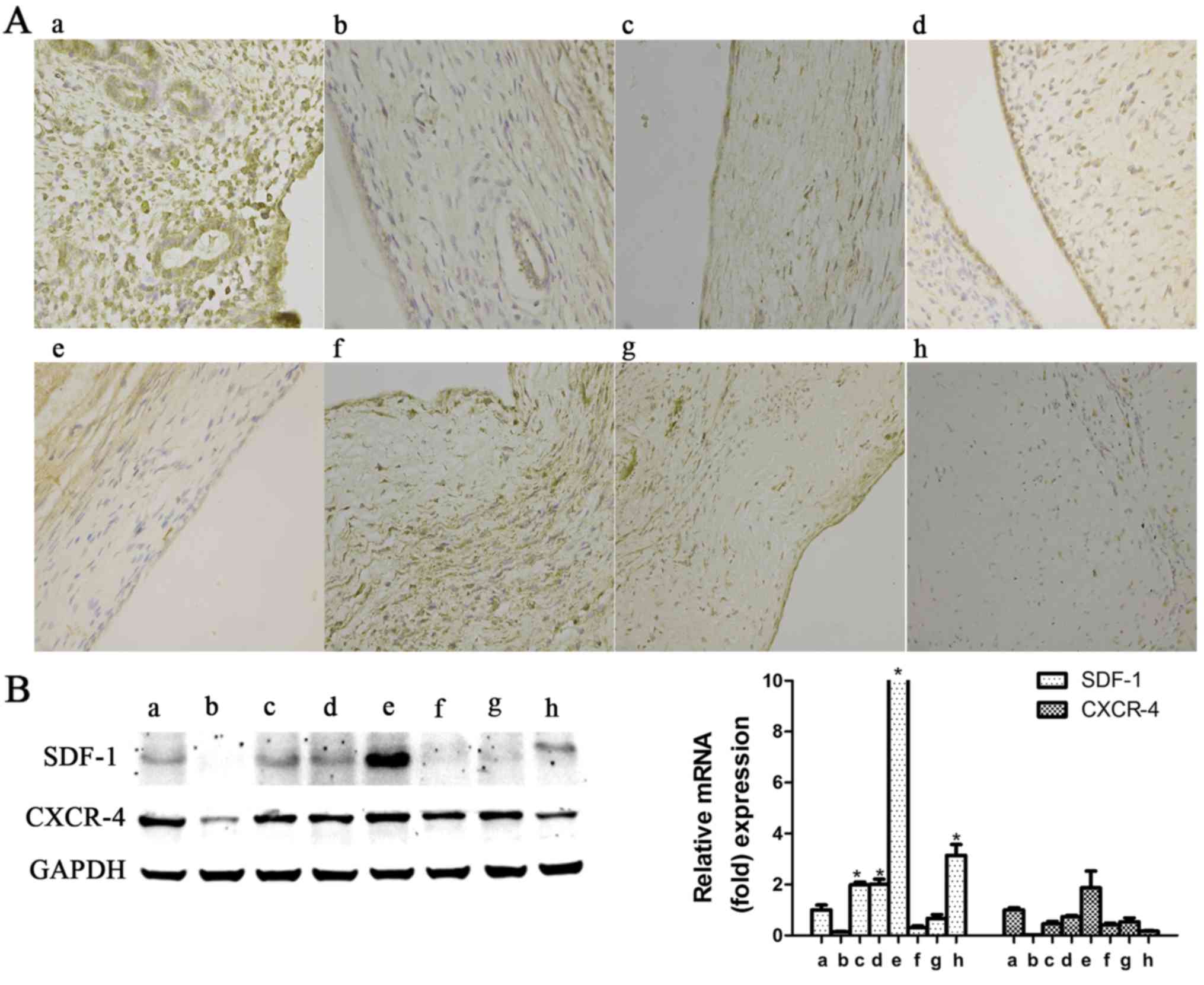
5). In the rat experiments, with progressing time after
surgery, SDF-1 expression exhibited an increasing tendency in the
early phase, reaching its highest level at the second postoperative
estrus phase (P<0.05), after which time it again decreased
(Fig. 6); as regards CXCR-4,
there was no significant upregulation observed at any of the
detection time points (P>0.05; Fig. 6).
Discussion
In the present study, significant upregulation of
TGF-β1, MMP-9 and ERα expression was detected in patients and rats
with IUAs, despite a minor fluctuation of the MMP-9 level in rats.
In addition, we found that SDF-1 and CXCR-4 did not differ
significantly in the endometrium of the patients, whereas in the
endometrium of the rats, SDF-1 expression was significantly
increased during the early postoperative phase and then sharply
declined; however, CXCR-4 expression in rat endometrium did not
differ significantly after surgery. These results suggest that the
formation and development of IUAs is mainly associated with
excessive fibrosis and insufficient restoration of the endometrium
induced by various cytokines and growth factors.
At high-power magnification, it was observed that,
along with the development and progression of IUAs, fibrotic
tissues gradually covered or replaced the normal endometrium and
promoted the formation of IUAs (Figs.
1 and 2). This outcome
suggests that fibrosis plays a key role in IUAs, which was also
demonstrated by previous studies (3,4).
TGF-β1, as a pivotal mediator and indicator of fibrogenesis, has
been found to be implicated in the pathogenesis of numerous
fibrotic diseases, such as cardiac fibrotic and hypertrophic
remodeling, hepatic fibrosis and chronic kidney diseases (23,24). An increased TGF-β1 level is often
present in tissues exhibiting an uncontrolled fibrotic response. In
the present study, animal and human subjects were used to
investigate the expression of TGF-β1 in endometrium with IUAs, and
it was observed that, compared with the control group, the mRNA and
protein levels of TGF-β1 were significantly upregulated in the
experimental groups, and the degree of endometrial fibrosis was
consistent with the expression level of TGF-β1 during the formation
of IUAs (Figs. 3 and 4). Based on these results, it may be
argued that TGF-β1 contributes to fibrosis development and
progression of IUAs, which was also supported by Salma et al
(4) and other scholars (25,26). Moreover, as a downstream target
gene of TGF-β1, MMP-9 has been previously considered to be an
anti-fibrotic factor due to its ability to degrade and remodel the
ECM (7,27). It was previously reported that
MMP-9 expression in IUAs was inversely correlated with endometrial
fibrosis (26). However, in the
present study, we found that MMP-9 was involved in the development
and progression of IUAs with its pro-fibrotic function,
particularly at the early phase. This result is consistent with
previous findings indicating that MMP-9 inhibitors could
effectively alleviate chronic kidney fibrotic diseases,
particularly at the early stages (28). Therefore, it is hypothesized that,
in addition to their contribution to the generation of
myofibroblasts through EMT and involvement in the pro-fibrotic role
of interstitial macrophages, MMPs are also dysregulated and
involved in every aspect of inflammation and tissue repair
(29).
ERα is a well-known nuclear transcription factor,
proven to promote the proliferation or metabolism of endometrial
epithelial cells after combining with estrogen, and increasing the
synthesis of intracellular DNA and protein (30). Estrogen is usually included in the
clinical therapy of IUAs to promote the proliferation and repair of
the endometrium. A successful case of prolonged estrogen
supplementation prior to conventional controlled ovarian
hyperstimulation in a woman who had experienced repeated
implantation failure due to an unresponsive thin endometrium was
presented by Shen et al (31). In addition, Cai et al
(32) also demonstrated that the
administration of estrogen exerts a preventive effect on the
development of endometrial fibrosis in rats and rabbits. The TGF-β1
signaling pathway may be interposed by functional coadjutant
interactions between Smad and other types of transcriptional
factors, kinase receptors and nuclear receptors. Inhibition of
ERα-dependent TGF-β1/Smad signaling may involve the regulation of
renal fibroblast activation with its potential preventive mechanism
(33).
In view of those findings, we examined endometrial
tissues of humans and rats with IUAs and observed that the
expression of ERα in the experimental groups was significantly
higher compared with that in the control group, and that ERα
expression in severe IUA endometrium was significantly higher
compared with that in mild-to-moderate IUA endometrium (Figs. 3 and 4). Another notable finding is that
attracted the expression tendency of ERα corresponded to the
expression of TGF-β1 in human and rat IUAs. These results suggest
that the formation of IUAs is possibly associated with abnormal
upregulation of ERα, which is consistent with the findings in
cardiac failure (34). In the
present study, the active upregulation of ERα may have been derived
from lack of estrogen in the endometrium with IUAs. The activation
of endometrial excessive fibrosis unconventionally stimulated by
TGF-β1 in IUAs induced ERα upregulation. ERα in endometrium with
IUAs is prevented from interacting with estrogen, resulting in a
relative lack of estrogen at the endometrium rather than a shortage
of estrogen in the circulation, with further abnormal upregulation
of ERα in the IUA endometrium. Estrogen superabundance is
detrimental to endocrine organ function and even aggravates
fibrosis to some degree. Therefore, for IUAs patients,
individualizing the dose of estrogen is advocated in current
clinical practice.
BMSCs, a category of CXCR-4-expressing bone-derived
multifunctional stem cells that are capable of differentiating into
lineages of cells, have been investigated as a therapy for a number
of diseases. Zhao et al (12,13) and Alawadhi et al (35) have also demonstrated that
ectogenic BMSCs administered systemically or locally have the
potential to selectively migrate to and repair the injury, but the
underlying mechanism has not been reported in detail. It is well
known that chemokine SDF-1 and its special receptor CXCR-4 play a
crucial role in BMSC homing (14,15,36). Our previous cell study
demonstrated that ERα may promote BMSC proliferation and migration
via SDF-1/CXCR-4 (17). In the
present study, the expressions of SDF-1 and CXCR-4 exhibited no
obvious change in patient endometrium (Fig. 5). SDF-1 is a dynamically altered
chemokine secreted by damaged tissues. Samples of endometrium with
IUAs, particularly in patients with fertility requirements, cannot
be frequently collected for investigation. In the present study,
animal groups with different sampling time points were established
to monitor variations in SDF-1 and CXCR-4 after surgery in rats.
There was no significant upregu-lation of CXCR-4 expression in the
endometrium with IUAs, while SDF-1 exhibited an increasing tendency
at the early phase (Fig. 6). In
view of this finding, it was hypothesized that SDF-1 may be one of
inflammatory mediators of short-term endometrial damage, but not a
BMSC-specific chemokine. In addition, it may also be hypothesized
that autologous CXCR-4-expressing BMSCs are incapable of homing and
differentiation to endometrial cells for a short time following
endometrial damage; however, the recovery effect of SDF-1/CXCR-4
axis-mediated BMSC homing for IUAs in the long-term requires
further investigation.
In conclusion, the present study investigated the
expression of fibrotic factors, ERα and SDF-1/CXCR-4 axis in IUAs.
It is recommended that the dose of estrogen should be
individualized in the treatment of IUAs. In addition, it may be
hypothesized that the formation and development of IUAs are closely
associated with an abnormal endometrial fibrosis microenvironment
(niche) involved with TGF-β1 and MMP-9, and inadequent normal
endometrial regeneration involved with various growth factors and
cytokines. Thus, specific interventions are crucial for suppressing
excessive fibrosis and providing a protective effect by promoting
endometrial restoration during the early stages of endometrial
injury. However, the specific underlying mechanisms require further
elucidation, and whether SDF-1/CXCR-4 axis-mediated BMSC homing and
differentiation is required for endometrial restoration requires
verification in future studies.
Acknowledgments
Not applicable.
References
|
1
|
Yu D, Wong YM, Cheong Y, Xia E and Li TC:
Asherman syndrome-one century later. Fertil Steril. 89:759–779.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park JO, Lee BH, Kang YM, Kim TH, Yoon JY,
Kim H, Kwon UH, Lee KI, Lee HM and Moon SH: Inflammatory cytokines
induce fibrosis and ossification of human ligamentum flavum cells.
J Spinal Disord Tech. 26:E6–E12. 2013. View Article : Google Scholar
|
|
3
|
Hu J, Zeng B, Jiang X, Hu L, Meng Y, Zhu Y
and Mao M: The expression of marker for endometrial stem cell and
fibrosis was increased in intrauterine adhesious. Int J Clin Exp
Pathol. 8:1525–1534. 2015.PubMed/NCBI
|
|
4
|
Salma U, Xue M, Ali Sheikh MS, Guan X, Xu
B, Zhang A, Huang L and Xu D: Role of transforming growth factor-β1
and smads signaling pathway in intrauterine adhesion. Mediators
Inflamm. 2016:41582872016. View Article : Google Scholar
|
|
5
|
Rahimi RA and Leof EB: TGF-beta signaling:
A tale of two responses. J Cell Biochem. 102:593–608. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Islam SS, Mokhtari RB, El Hout Y, Azadi
MA, Alauddin M, Yeger H and Farhat WA: TGF-β1 induces EMT
reprogramming of porcine bladder urothelial cells into collagen
producing fibroblasts-like cells in a Smad2/Smad3-dependent manner.
Cell Commun Signal. 8:39–58. 2014. View Article : Google Scholar
|
|
7
|
Zhao H, Dong Y, Tian X, Tan TK, Liu Z,
Zhao Y, Zhang Y, Harris D and Zheng G: Matrix metalloproteinases
contribute to kidney fibrosis in chronic kidney diseases. World J
Nephrol. 2:84–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kramann R, Dirocco DP, Maarouf OH and
Humphreys BD: Matrix producing cells in chronic kidney disease:
Origin, regulation, and activation. Curr Pathobiol Rep. 1:2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maruyama T, Masuda H, Ono M, Kajitani T
and Yoshimura Y: Human uterine stem/progenitor cells: Their
possible role in uterine physiology and pathology. Reproduction.
140:11–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kato K, Yoshimoto M, Kato K, Adachi S,
Yamayoshi A, Arima T, Asanoma K, Kyo S, Nakahata T and Wake N:
Characterization of side-population cells in human normal
endometrium. Hum Reprod. 22:1214–1223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gargett CE and Ye L: Endometrial
reconstruction from stem cells. Fertil Steril. 98:11–20. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jing Z, Qiong Z, Yonggang W and Yanping L:
Rat bone marrow mesenchymal stem cells improve regeneration of thin
endometrium in rat. Fertil Steril. 101:587–594. 2014. View Article : Google Scholar
|
|
13
|
Zhao J, Zhang Q, Wang Y and Li Y: Uterine
infusion with bone marrow mesenchymal stem cells improves
endometrium thickness in a rat model of thin endometrium. Reprod
Sci. 22:181–188. 2015. View Article : Google Scholar :
|
|
14
|
Wang L, Guo S, Zhang N, Tao Y, Zhang H, Qi
T, Liang F and Huang Z: The role of SDF-1/CXCR4 in the
vasculogenesis and remodeling of cerebral arteriovenous
malformation. Ther Clin Risk Manag. 11:1337–1344. 2015.PubMed/NCBI
|
|
15
|
Yang D, Sun S, Wang Z, Zhu P, Yang Z and
Zhang B: Stromal cell-derived factor-1 receptor
CXCR4-overexpressing bone marrow mesenchymal stem cells accelerate
wound healing by migrating into skin injury areas. Cell Reprogram.
15:206–215. 2013.PubMed/NCBI
|
|
16
|
Tica AA, Tica OS, Georgescu CV, Pirici D,
Bogdan M, Ciurea T, Mogoanta SS, Georgescu CC, Comanescu AC,
Balseanu TA, et al: GPER and ERα expression in abnormal endometrial
proliferations. Rom J Morphol Embryol. 57:413–418. 2016.
|
|
17
|
Hu H and Yuan R: Estrogen receptor ESR1
promotes BMSCs cell proliferation and migration via regulation of
SDF-1/CXCR4 signaling. Int J Clin Exp Med. 9:21092–21099. 2016.
|
|
18
|
The American Fertility Society
classifications of adnexal adhesions, distal tubal occlusion, tubal
occlusion secondary to tubal ligation, tubal pregnancies, mullerian
anomalies and intrauterine adhesions. Fertil Steril. 49:944–955.
1988. View Article : Google Scholar
|
|
19
|
Zhao J, Gao H and Li Y: Development of an
animal model for thin endometrium using 95% ethanol. J Fert In
Vitro. 2:42012.
|
|
20
|
Hunter RK II, Nevitt CD, Gaskins JT,
Keller BB, Bohler HC Jr and LeBlanc AJ: Adipose-derived stromal
vascular fraction cell effects on a rodent model of thin
endometrium. PLoS One. 10:e01448232015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harvey BK, Airavaara M, Hinzman J, Wires
EM, Chiocco MJ, Howard DB, Shen H, Gerhardt G, Hoffer BJ and Wang
Y: Targeted overexpression of glutamate transporter 1 (GLT-1)
reduces ischemic brain injury in a rat model of stroke. PLoS One.
6:e221352011. View Article : Google Scholar
|
|
22
|
Chen Y, Chang Y and Yao S: Role of
angiogenesis in endometrial repair of patients with severe
intrauterine adhesion. Int J Clin Exp Pathol. 6:1343–1350.
2013.PubMed/NCBI
|
|
23
|
Sui X, Wei H and Wang D: Novel mechanism
of cardiac protection by valsartan: Synergetic roles of TGF-β1 and
HIF-1α in Ang II-mediated fibrosis after myocardial infarction. J
Cell Mol Med. 19:1773–1782. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harris WT, Kelly DR, Zhou Y, Wang D,
MacEwen M, Hagood JS, Clancy JP, Ambalavanan N and Sorscher EJ:
Myofibroblast differentiation and enhanced TGF-B signaling in
cystic fibrosis lung disease. PLoS One. 8:e701962013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tao Z and Duan H: Expression of
adhesion-related cytokines in the uterine fluid after transcervical
resection of adhesion. Zhonghua Fu Chan Ke Za Zhi. 47:734–737.
2012.
|
|
26
|
Hu S, Li Y, Meng WJ and Tan SQ: Effects of
Fukang oral liquid on the prevention of intrauterine adhesion and
expressions of TGF-beta1, PAI-1 and MMP-9 in endometrium of rats.
Sichuan Da Xue Xue Bao Yi Xue Ban. 44:540–544. 2013.PubMed/NCBI
|
|
27
|
Musial K and Zwolinska D: Matrix
metalloproteinases (MMP-2,9) and their tissue inhibitors (TIMP-1,2)
as novel markers of stress response and atherogenesis in children
with chronic kidney disease (CKD) on conservative treatment. Cell
Stress Chaperones. 16:97–103. 2011. View Article : Google Scholar :
|
|
28
|
Zeisberg M, Khurana M, Rao VH, Cosgrove D,
Rougier JP, Werner MC, Shield CF III, Werb Z and Kalluri R:
Stage-specific action of matrix metalloproteinases influences
progressive hereditary kidney disease. PLoS Med. 3:e1002006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Catania JM, Chen G and Parrish AR: Role of
matrix metal-loproteinases in renal pathophysiologies. Am J Physiol
Renal Physiol. 292:F905–F911. 2007. View Article : Google Scholar
|
|
30
|
Lindberg MK, Weihua Z, Andersson N,
Moverare S, Gao H, Vidal O, Erlandsson M, Windahl S, Andersson G,
Lubahn DB, et al: Estrogen receptor specificity for the effects of
estrogen in ovariectomized mice. J Endocrinol. 174:167–178. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shen MS, Wang CW, Chen CH and Tzeng CR:
New horizon on successful management for a woman with repeated
implantation failure due to unresponsive thin endometrium: Use of
extended estrogen supplementation. J Obstet Gynaecol Res.
39:1092–1094. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cai H, Li H and He Y: Interceed and
estrogen reduce uterine adhesions and fibrosis and improve
endometrial receptivity in a rabbit model of intrauterine
adhesions. Reprod Sci. 23:1208–1216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim D, Lee AS, Jung YJ, Yang KH, Lee S,
Park SK, Kim W and Kang KP: Tamoxifen ameliorates renal
tubulointerstitial fibrosis by modulation of estrogen receptor
α-mediated transforming growth factor-β1/Smad signaling pathway.
Nephrol Dial Transplant. 29:2043–2053. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mahmoodzadeh S, Eder S, Nordmeyer J, Ehler
E, Huber O, Martus P, Weiske J, Pregla R, Hetzer R and
Regitz-Zagrosek V: Estrogen receptor alpha upregulation and
redistribution in human heart failure. FASEB J. 20:926–934. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Alawadhi F, Du H, Cakmak H and Taylor HS:
Bone Marrow-Derived Stem Cell (BMDSC) transplantation improves
fertility in a murine model of Asherman's syndrome. PLoS One.
9:e966622014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang X, Gao Y, Shi H, Liu N, Zhang W and
Li H: Influence of the intensity and loading time of direct current
electric field on the directional migration of rat bone marrow
mesenchymal stem cells. Front Med. 10:286–296. 2016. View Article : Google Scholar : PubMed/NCBI
|