Introduction
Breast cancer is the most frequently diagnosed
cancer and the leading cause of mortality among women worldwide
(1,2), accounting for 23% of total cancer
cases and 14% of cancer-associated mortality, according to the
global cancer statistics for 2011 (3). Triple negative breast cancer (TNBC)
is a subtype of breast cancer lacking the expression of estrogen
receptor (ER), human epidermal growth factor receptor type 2 (HER2)
and progesterone receptor (4),
and accounts for ~15% of all breast cancer cases (5). Women with TNBC have relatively poor
prognoses, and specific treatment guidelines are not available for
these patients. In addition, due to its specific molecular
expression features, TNBC is resistant to existing endocrine
therapies and HER2-targeted therapies, including trastuzumab
(6). Therefore, the development
of possible therapeutic targeted agents for TNBC is urgently
required.
Substantial effort has focused on deconstructing the
molecular mechanisms underlying the development and progression of
TNBC, and developing more efficient and effective therapies. A
growing body of evidence indicates that adipose tissue, and more
specifically adipocytes, which represent the most abundant cell
type of the breast cancer microenvironment, are involved in the
initiation, growth and metastasis of breast cancer (7–9).
In previous studies, cancer cell growth and metastasis were found
to occur predominantly in adipocyte-rich microenvironments. All
adipocyte-derived factors, including adipokines, proinflammatory
cytokines, chemokines, growth factors, hormones, proangiogenic
factors and extracellular matrix constituents, may be considered as
contributing factors for the growth, development and metastasis of
breast cancer (10–12). CC-chemokine ligand 5 (CCL5), which
is upregulated in breast cancer and peritumoral adipose tissue, has
been demonstrated to be associated with metastasis and poorer
overall survival rates in patients with TNBC (13–16). Accumulated evidence suggests that
adipocytes promote the proliferation, migration and invasion of
TNBC cells by inducing the expression of mesenchymal markers and
promoting the epithelial-mesenchymal transition (EMT) of TNBC cells
(17). E-cadherin is an
epithelial-related protein, and vimentin and snail are
mesenchymal-related proteins. The phosphorylation of protein kinase
B (AKT) can inhibit glycogen synthase kinase-3β (GSK3β) and
stabilize β-catenin, which can translocate to the nucleus and
engage the transcription factors lymphoid enhancer-binding factor 1
(LEF) and T-cell factor (TCF), promoting the transcription of a
series of EMT-related genes (18).
Emodin (1,3,8-trihydroxy-6-methylanthraquinone) is a
biologically active anthraquinone derivative isolated from the
roots and bark of Rheum palmatum, Polygonum
cuspidatum medicine, and other Chinese herbs (19). Emodin has been widely investigated
for its anti-atherogenic, antibacterial, anti-inflammatory,
diuretic and immunosuppressive properties (20,21), in addition to its anticancer
effects against a variety of cancer types, including breast
(22–24), pancreatic (25,26), lung (27), liver (28), prostate (29), ovarian (30), colon (31) and gallbladder (32) cancer, among others. It has been
demonstrated that emodin induces the apoptosis of human breast
cancer MDA-MB-231 (33) and
MDA-MB-453 (34) cells, although
the potential mechanisms remain to be elucidated. In addition, the
effects of emodin on the migration and invasion of TNBC cells
require elucidation.
In the present study, MDA-MB-231 and MDA-MB-453 TNBC
cells were co-cultured with human adipocytes and treated with
emodin. The results revealed that emodin inhibited the secretion of
CCL5 from adipocytes, suppressed EMT of TNBC cells, and inhibited
TNBC cell proliferation and metastasis in vitro and in
vivo. These observations suggest that emodin may serve as a
potential therapeutic agent for TNBC.
Materials and methods
Reagents
CCL5 protein was purchased from R&D systems,
Inc. (Minneapolis, MN, USA; cat. no. 278-RN-050), Rabbit anti-mouse
immunoglobulin G (IgG) H&L horseradish peroxidase (HRP; cat.
no. ab6728; 1:5,000), goat anti-rabbit IgG H&L HRP (cat. no.
ab6721; 1:5,000), goat anti-mouse IgG H&L (Alexa
Fluor® 594; cat. no. ab150116; 1:5,000) CCL5 (cat. no.
ab56709; 1:1,000), phosphorylated (p-)CCR5 (cat. no. ab63411;
1:1,000), and CCR5 (cat. no. ab65850; 1:1,000) antibodies were
purchased from Abcam (Cambridge, UK), p-AKT (cat. no. 4060;
1:2,000), AKT (cat. no. 9272), p-extracellular signal-regulated
kinase (Erk) (cat. no. 4370; 1:1,000), Erk (cat. no. 4695;
1:1,000), matrix metalloproteinase (MMP)-2 (cat. no. 87809;
1:1,000), MMP-9 (cat. no. 13667; 1:1,000), E-cadherin (cat. no.
14472; 1:1,000) and vimentin (cat. no. 5741; 1:1,000) antibodies
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA).
Cell culture
MDA-MB-231 and MDA-MB-453 TNBC cells, purchased from
the Cell Bank of the Chinese Science Institute (Shanghai, China)
were cultured in 1640 medium supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and 1% penicillin-streptomycin and maintained at 37°C and 5%
CO2. Human adipocytes were cultured in DMEM (Gibco;
Thermo Fisher Scientific, Inc.).
Human adipose tissue
Human adipose tissue samples were obtained from
mammary adipose tissue biopsies of healthy women (n=12; age 25–63
years; body mass index 24.2–29.0) undergoing surgical mammary
reduction. All women were otherwise healthy and free of metabolic
or endocrine diseases. Informed consent was obtained from every
patient prior to the surgical procedure. The Ethics Committee of
Longhua Hospital Affiliated to Shanghai University of Traditional
Chinese Medicine (Shanghai, China) approved the present study. The
adipose tissue was digested with collagenase, and adipose-derived
stromal vascular fraction (SVF) cells were isolated and
differentiated as previously reported (35). Informed consent was obtained from
the patients for participation in the study.
MTT assay
An MTT assay was used to detect the effect of human
adipocytes on TNBC cell viability. The supernatants of the SVF
cells or adipocytes were collected, and MDA-MB-231 or MDA-MB-453
cells were incubated with the supernatant in 96-well plates at a
density of 5,000 cells per well. After 24 h, epirubicin at
concentrations of 0.01, 0.1, 0.5, 1 or 10 μM or emodin at
concentrations of 1, 10, 50, 100 or 200 μM were added to the cell
supernatant at 37°C, using three wells per concentration. After 24
h, MTT solution (10 μl) at a concentration of 5 mg/ml was added to
90 μl of culture media and incubated for 4 h at 37°C in a
humidified atmosphere of 5% CO2. The supernatant was
removed and 100 μl per well of DMSO was added to the plates. The
optical density was measured at 492 nm in a microplate reader. The
experiment was performed in triplicate.
Transwell invasion assay
The invasive capacity of the cells was evaluated
with a Transwell assay in 24-well plates (Corning Life Sciences,
Corning, NY, USA) according to the manufacturer’s protocol.
Briefly, the upper chambers were coated with Matrigel (BD
Biosciences, Franklin Lakes, NJ, USA). The MDA-MB-231 or MDA-MB-453
cells were seeded in the upper chamber of the Matrigel-coated
Transwell culture system in 200 μl serum free-medium, and human SVF
cells or adipocytes were seeded in the lower chamber in 500 μl
medium with 10% FBS. The cells were then allowed to invade the
Matrigel matrix for 24 h. Subsequently, the transmigrated cells
were fixed and stained with crystal violet and counted in five
randomly selected microscopic fields using an IX71 light microscope
(Olympus Corporation, Tokyo, Japan). All experiments were performed
in triplicate.
Wound healing assay
The MDA-MB-231 or MDA-MB-453 cells were seeded in
the lower chamber of a Transwell culture system, with human SVF
cells or adipocytes in the upper chamber. When cellular confluence
reached ~90%, wounds were created in the confluent cells using
10-μl pipette tips. The cells were then rinsed with medium to
remove any free-floating cells and debris. Wound healing was
observed at 12 and 24 h within the scrape line, and representative
images of the scrape lines were captured.
ELISA
The levels of interleukin-8 (IL-8), insulin-like
growth factor-1 (IGF-1) and CCL5 in the supernatant of the SVF
cells, adipocytes, MDA-MB-231 and MDA-MB-453 cells were detected by
ELISA according to the manufacturer’s protocol (RayBiotech,
Norcross, GA, USA). The TNBC cells co-cultured with human
adipocytes were treated for 24 h with 0.50 μM epirubicin and 50 μM
emodin (for MDA-MB-231) or with 0.25 μM epirubicin and 25 μM emodin
(for MDA-MB-453). The levels of CCL5 in the supernatant of the SVF
cells, adipocytes, and co-cultures were detected using ELISA.
Western blot analysis
The MDA-MB-231 cells were seeded in the lower
chamber of a Transwell culture system, with human SVF cells or
adipocytes in the upper chamber. When cellular confluence reached
~90%, the MDA-MB-231 cells were treated with 6 μg/ml CCL5 antibody
(cat. no. 2988; Cell Signaling Technology, Inc.), 100 pg/ml CCL5
(cat. no. 96-300-06; PeproTech, Inc., Rocky Hill, NJ, USA), and
0.50 μM epirubicin or 50 μM emodin for 24 h at 37°C and 5%
CO2. The protein lysates were obtained from the
MDA-MB-231 cells treated with a lysis buffer (cat. no. 78501;
Thermo Fisher Scientific, Inc.) with a protease inhibitor cocktail.
The proteins were quantified using a BCA Kit (cat. no. 23225;
Thermo Fisher Scientific, Inc.) and 10 μg protein was loaded and
separated by 8–12% SDS-PAGE according to the protein weight and
blotted onto a PVDF nitrocellulose membrane (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The membrane was blocked in 5% milk in
PBST for 1 h and then probed with primary antibodies overnight at
4°C, followed by incubation with secondary antibodies for 1 h at
room temperature. The signal was then visualized with enhanced
chemiluminescence reagent, following the manufacturer’s protocol
(cat. no. 34080; Thermo Fisher Scientific, Inc.).
Tumor xenograft experiments
All mouse experiments were performed in accordance
with approved protocols from the Shanghai Medical Experimental
Animal Care Commission (Shanghai, China). A total of 72 female
Balb/C nude mice (age, 4–6 weeks; weight, 18–21 g) were obtained
from Shanghai BiKai Bio-Technology (Shanghai, China) and housed
under specific pathogen-free conditions at constant temperature
(22±2°C) and humidity (55±5%) in a 12 h light/dark schedule with
ad libitum access to water and standard laboratory chow.
These mice were divided into six groups. Of the mice, 36 were fed
with a basic diet and 36 received a high fat and sugar diet for 20
days. All mice were inoculated subcutaneously with MDA-MB-231 cells
(1×107) in 100 μl of 1640-Matrigel mixture (1:1 ratio)
in the thoracic fat pad. When the tumor volumes reached an average
of ~100 mm3, the mice were treated with saline orally
(p.o.), three times per week, 40 mg/ml emodin p.o., once a day for
21 days, or 5 mg/ml epirubicin intravenously (i.v.) weekly for 3
weeks. The tumor volumes were measured every 5 days in two
dimensions with vernier calipers. The tumor volumes were calculated
using the following formula: Length × width2 × 0.5.
There were 12 mice were in each group, 6 of the 12 tumor samples
were immersed in 4% paraformaldehyde for hematoxylin and eosin
(H&E) staining, and 6 of 12 tumor samples were separated from
liver and lung tissues and weighed. Tumor tissues from the in
vivo experiments were collected for immunohistochemical
analysis. The experiments were approved by the Ethics Committee of
Longhua Hospital Affiliated to Shanghai University of Traditional
Chinese Medicine.
Cellular immunofluorescence
The cells were cultured overnight on coverslips in
6-well plates. The coverslips were then removed and maintained in
4% polyformaldehyde for 15 min, and then washed three times in PBS
solution. The coverslips were then treated with 0.2% Triton X-100
for 15 min, and then blocked with 3% goat serum (Gibco; Thermo
Fisher Scientific, Inc.) at room temperature for 1 h. The
coverslips were then incubated with E-cadherin antibody (1:1,000)
at 4°C for 2 h, and then with secondary antibody linked with Alexa
Fluor 594 (1:200) for 1 h. Following staining with DAPI, the cells
on the coverslips were observed under a confocal microscope.
H&E staining
Tissues were immersed in 4% paraformaldehyde for 4
h, and then transferred to 70% ethanol. Individual lobes of the
tissues biopsy materials were placed in processing cassettes,
dehydrated through a serial alcohol gradient, and then stained with
H&E. Following staining, the sections were dehydrated through
increasing concentrations of ethanol and xylene.
Statistical analysis
All statistical analyses were performed using SPSS
16.0 software (SPSS, Inc., Chicago, IL, USA). All data are
presented as the mean ± standard deviation. Statistical
significance was determined using a paired or unpaired Student’s
t-test in cases of standardized expression data. One-way analysis
of variance was performed for multiple group comparisons, and
comparisons between two groups were made using the least
significant difference method. P<0.05 was considered to indicate
a statistically significant difference.
Results
Emodin inhibits TNBC cell proliferation
and metastasis more efficiently than epirubicin under
adipocyte-rich conditions
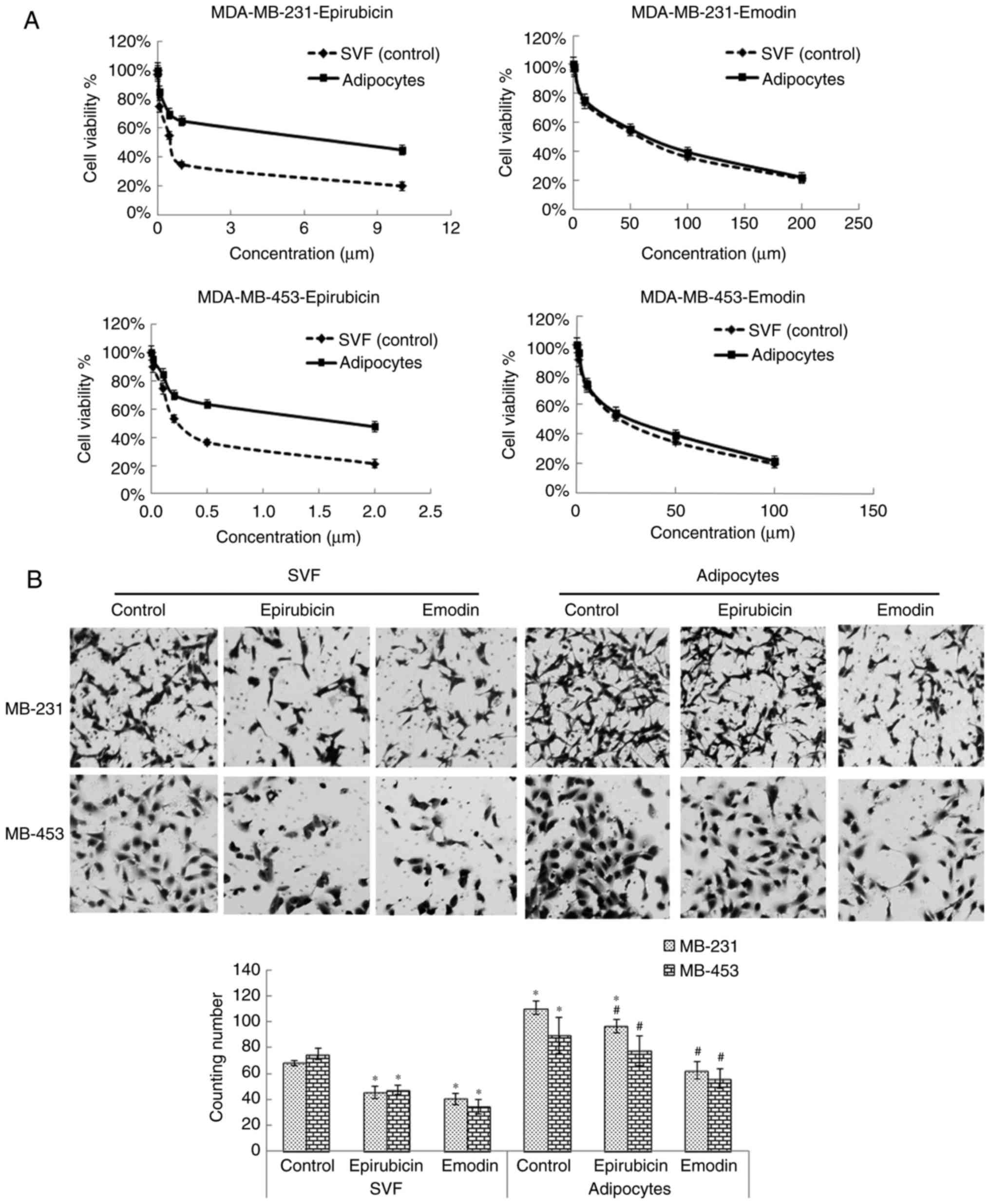
The present study investigated the effects of
epirubicin and emodin on TNBCs in the supernatant of SVF cells or
adipocytes. The MTT assay results suggested that the
IC50 values of epirubicin and emodin against MDA-MB-231
were 0.55 and 55.2 μM respectively, and when the MDA-MB-231 cells
were incubated in the supernatant of adipocytes, the
IC50 values were 5.1 and 67.1 μM, respectively. Similar
trends were observed with the MDA-MB-453 cells, demonstrating that,
when co-cultured with adipocytes, the IC50 of epirubicin
was ~9.27 times higher, compared with the value obtained without
adipocytes, whereas the IC50 of emodin was only ~1.21
times higher, a finding which suggested that adipocytes led to
resistance of TNBC to epirubicin, but not to emodin (Fig. 1A). The Transwell and wound healing
assays demonstrated that adipocytes assisted in human TNBC
MDA-MB-231 and MDA-MB-453 cell migration and invasion, and emodin
caused more marked inhibition of TNBC cell migration and invasion,
compared with epirubicin in the supernatant of adipocytes (Fig. 1B and C). Taken together, these
findings showed that emodin inhibited TNBC cell proliferation and
metastasis more efficiently than epirubicin in the supernatant of
adipocytes.
Emodin inhibits the release of
cytokines/chemokines and growth factors by human mammary
adipocytes
It has been reported that the secretion of IL-8,
CCL5 and IGF-1 by adipocytes can promote the migration of TNBC
cells (16,17). In the present study, the results
of the ELISA showed that the levels of IL-8, CCL5 and IGF-1 were
higher in the cell supernatant of the adipocytes. The levels of
these proteins were also measured in the cell culture supernatants
following treatment with emodin or epirubicin. The results
suggested that only CCL5 was downregulated following emodin
treatment (Fig. 2A-F); however,
no changes were observed following epirubicin treatment, indicating
that emodin may inhibit TNBC proliferation and migration by
decreasing the release of CCL5.
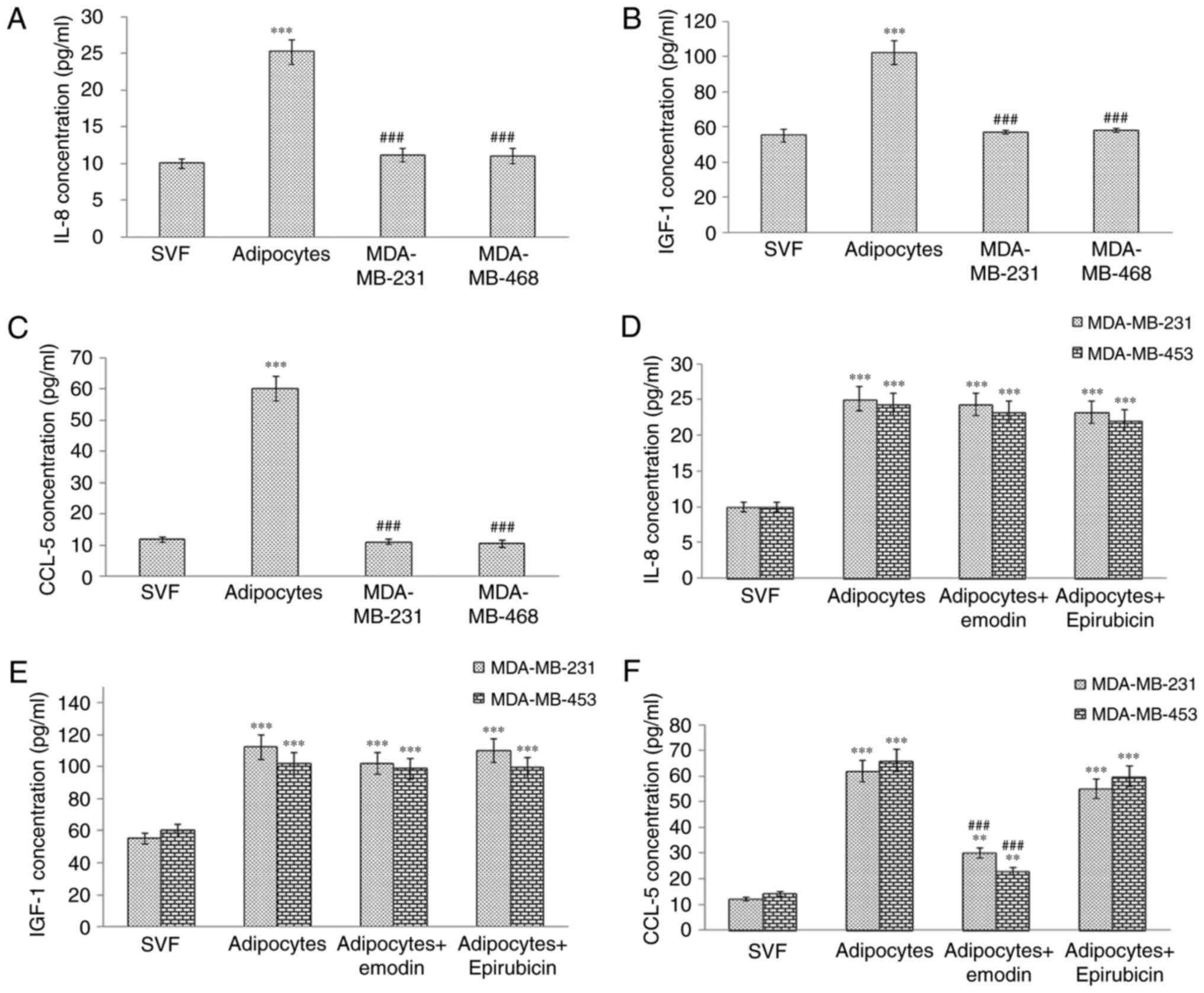 | Figure 2Levels of IL-8, IGF-1 and CCL5 in
cell culture supernatants detected by ELISA. Levels of (A) IL-8,
(B) IGF-1 and (C) CCL5 in supernatant of SVF cells, adipocytes,
MDA-MB-231 and MDA-MB-453 cells were detected using ELISA. Triple
negative breast cancer cells co-cultured with human adipocytes were
treated with 0.50 μM epirubicin and 50 μM emodin (for MDA-MB-231
cells) or with 0.25 μM epirubicin and 25 μM emodin (for MDA-MB-453
cells) for 24 h, and levels of (D) IL-8, (E) IGF-1 and (F) CCL5 in
the supernatant of SVF cells, adipocytes or co-cultures were
detected using ELISA. Results are presented as the mean ± standard
deviation of independent experiments performed in triplicate.
**P<0.01 and ***P<0.001, vs. control;
and ###P<0.001, vs. adipocyte group. IL-8,
interleukin-8; IGF-1, insulin-like growth factor-1; CCL5,
CC-chemokine ligand 5; SVF, adipose-derived stromal vascular
fraction. |
Emodin inhibits TNBC invasion by
antagonizing the secretion of CCL5 from adipocytes
To investigate whether emodin inhibited TNBC cell
invasiveness by antagonizing the secretion of CCL5 from adipocytes,
TNBC cells were treated with 6 μg/ml CCL5 antibody, CCL5 100 pg/ml,
and 0.5 μM epirubicin or 50 μM emodin for MDA-MB-231 cells, or 0.25
μM epirubicin or 25 μM emodin for MDA-MB-453 cells. The MTT assay
results suggested that CCL5 antibody at a concentration of 6 μg/ml
inhibited cell proliferation and that CCL5 reversed the inhibitory
effect of emodin treatment (Fig.
3A). The Transwell assay suggested that CCL5 antibody inhibited
cell invasion (Fig. 3B); however,
CCL5 reversed the inhibitory effect of emodin treatment. The above
results suggested that emodin may inhibit the proliferation and
invasion of TNBCs by partially downregulating the secretion of
CCL5.
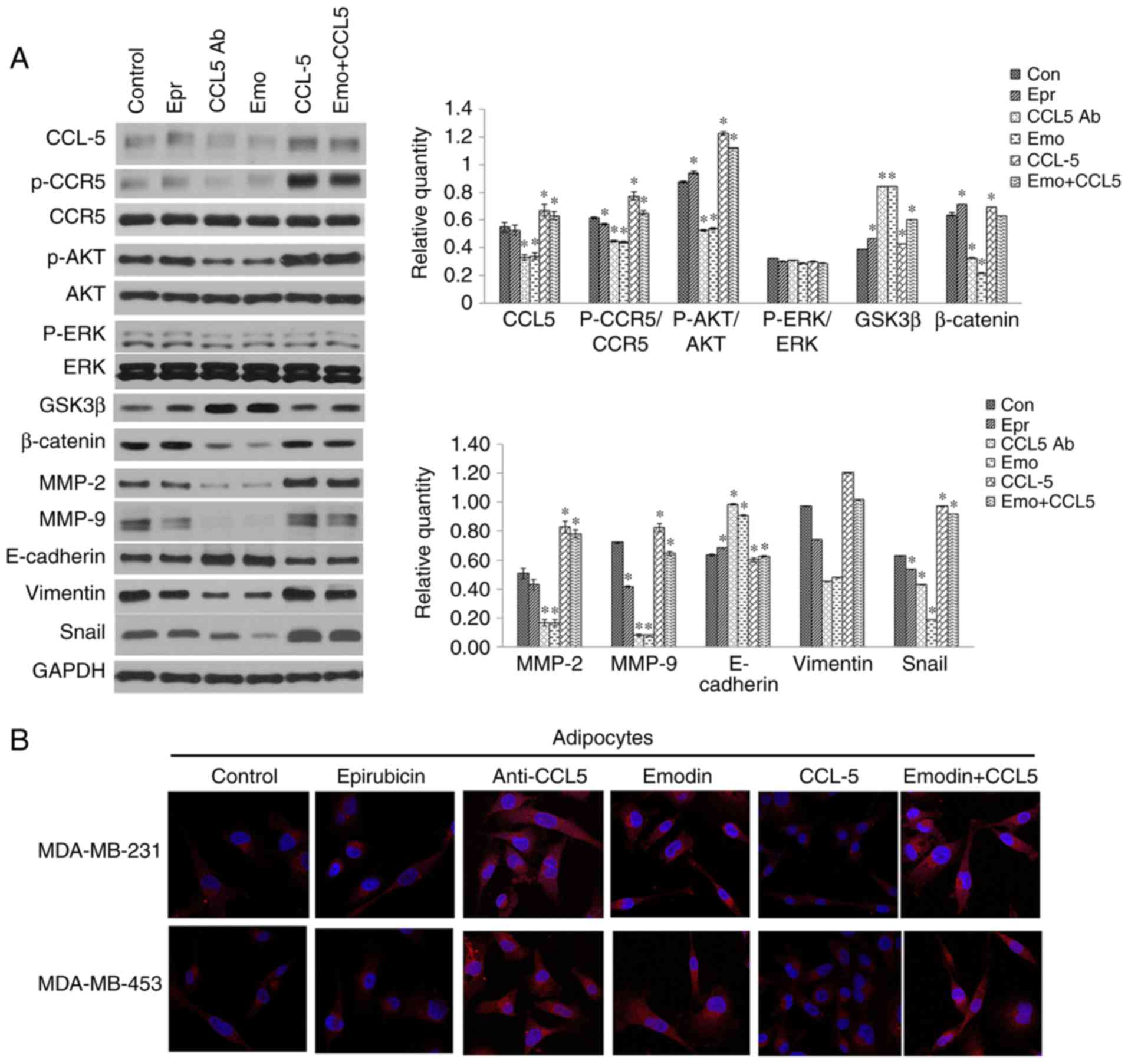
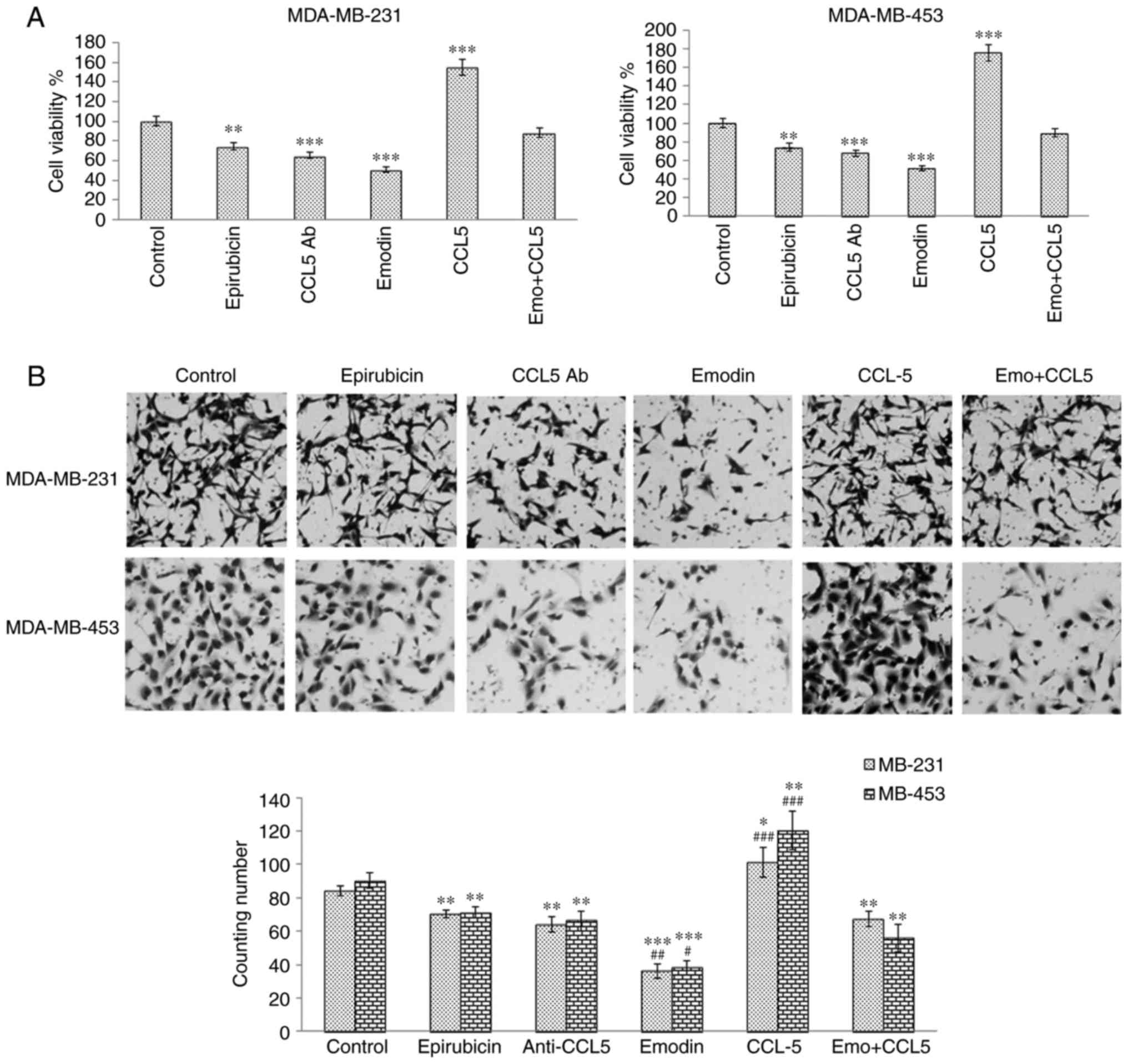 | Figure 3Emodin inhibits TNBC cell viability
and invasion by antagonizing secretion of CCL5 from adipocytes. (A)
Cell viability of TNBC cells treated with 6 μg/ml CCL5 antibody, 50
pg/ml CCL5, 0.50 μM epirubicin, or 50 μM emodin (for MDA-MB-231),
or 0.25 μM epirubicin or 25 μM emodin (for MDA-MB-453) was detected
using an MTT assay. (B) Invasion of TNBC cells co-cultured with
human adipocytes was detected using a Transwell assay. The images
were observed by light microscopy and images were captured at
magnification, ×200. Results are presented as the mean ± standard
deviation of independent experiments performed in triplicate.
*P<0.05, **P<0.01,
***P<0.001, vs. control; #P<0.05,
##P<0.01 and ###P<0.001, vs.
emodin+CCL5 group. TNBC, triple negative breast cancer; CCL5,
CC-chemokine ligand 5; Emo, emodin; Ab, antibody. |
Downstream signaling pathways of CCR5 and
EMT-associated markers
To investigate the mechanism underlying the
inhibitory effect of emodin on TNBC proliferation, migration and
invasion via the CCL5/CCR5 pathway, the TNBC cells were treated
with CCL5 antibody, emodin, or epirubicin, and the downstream
signaling pathways of CCR5 and EMT-associated markers were analyzed
by western blot or immunofluorescence assays. The results suggested
that emodin inhibited the phosphorylation of AKT, activated GSK3β,
downregulated the expression of β-catenin and mesenchymal-related
proteins vimentin and snail, and upregulated the expression of
epithelial-related protein E-cadherin. Epirubicin inhibited the
phosphorylation of CCR5, promoted the phosphorylation of AKT,
downregulated the expression of MMP-9 and snail, and upregulated
the expression of GSK-β, β-catenin and E-cadherin. CCL-5 antibody
downregulated the expression of CCL5, β-catenin, MMP-2, MMP-9 and
snail, upregulated the expression of GSK3β and E-cadherin, and
inhibited the phosphorylation of CCR5 and AKT. CCL5 promoted the
phosphorylation of CCR5 and AKT, and upregulated the expression of
CCL5, β-catenin, MMP-2, MMP-9 and snail. Emodin+CCL5 upregulated
the expression of CCL5, activated GSK3b, MMP-2 and snail, and
promoted the phosphorylation of CCR5 and AKT (Fig. 4A and B). These results indicated
that emodin negatively regulated the downstream signaling pathways
of CCR5 and EMT-associated markers, thereby inhibiting invasion and
migration.
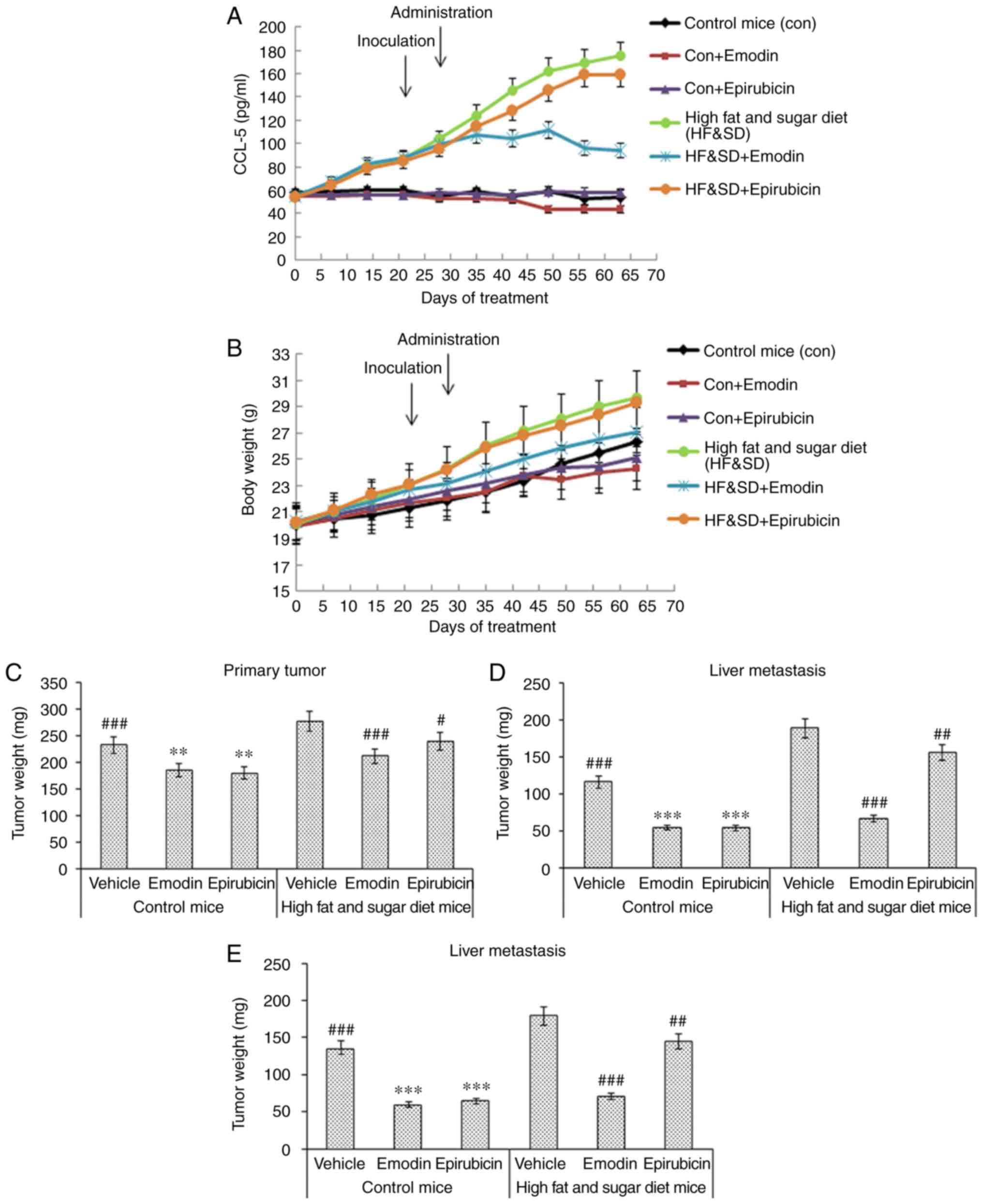
In vivo evaluation of the effect of
emodin on TNBC tumor growth and metastasis
The in vivo assay showed that emodin
inhibited the tumor growth and metastasis of TNBC cells by
decreasing levels of CCL5 in the serum of mice fed a high fat and
sugar diet. The nude mice were fed a high fat and sugar diet for 20
days and then inoculated with TNBC cells in the fat pad. After 7
days, the mice were treated with 40 mg/ml emodin p.o. once a day
for 21 days, or 5 mg/ml epirubicin i.v. weekly for 3 weeks. The
results suggested that emodin downregulated the level of CCL5,
whereas epirubicin did not affect the level of CCL5 (Fig. 5A). Emodin had a similar effect on
control nude mice bearing MDA-MB-231 with epirubicin; however, the
efficacy of emodin was significantly higher on the nude mice fed a
high fat and sugar diet than epirubicin according to the body
weight (Fig. 5B), primary tumor
(Fig. 5C), liver metastasis
(Fig. 5D) and lung metastasis
(Fig. 5E) tumor weight. The
H&E staining data suggested that compared with the vehicle and
epirubicin groups the emodin reduced lung (Fig. 5F) and liver metastasis (Fig. 5G) of the TNBC tumor. The above
results indicated that emodin inhibited tumor growth and the lung
and liver metastasis of TNBC cells by decreasing levels of CCL5 in
mice fed a high fat and sugar diet (Fig. 5).
Discussion
Breast cancer is the most common malignant tumor in
women worldwide. The majority of patients with advanced breast
cancer have serious systemic metastasis, which results in a high
mortality rate (1,2). Adipose tissue represents a major
component of the tumor microenvironment, particularly for breast
cancer (7,8). In addition to providing an
insulating and mechanically supportive site for energy storage,
adipose tissue has endocrine functions, regulating systemic energy
and metabolic homeostasis through a complex network of signals
(8,36). Data from previous investigations
have suggested that the release of CCL5 by adipocytes contributes
to increase motility and invasiveness of breast cancer cells. CCL5
is detectable in peritumoral adipose tissue of TNBCs, and
correlates with lymph node and distant metastases, and reduced
overall survival rates (16). The
process of EMT is involved in embryogenesis, underlying mesoderm
formation and neural crest development (37), and is known to be involved in
wound healing, fibrosis and tumor progression by enhancing tumor
cell migration and invasion (9,38).
Adipocytes may contribute to EMT in breast cancer cells through the
secretion of CCL5 (16).
The results of the present study suggested that
emodin inhibited TNBC proliferation, migration and metastasis more
effectively than epirubicin in adipocyte co-culture conditions.
Therefore, the present study investigated the underling mechanisms.
The ELISA results showed that emodin inhibited the release of CCL5
from human adipocytes, leading to the investigation of whether
emodin inhibited TNBC proliferation, migration and invasion by
downregulating the secretion of CCL5. The results of the
investigations confirmed this.
In the present study, TNBC cells co-cultured with
adipocytes were treated with CCL5 antibody, emodin, or epirubicin,
and the downstream signaling pathways of CCR5- and EMT-associated
markers were analyzed by western blot analysis and confocal
microscopy. The phosphorylation of AKT can inhibit GSK3h, which may
stabilize β-catenin. The latter may translocate to the nucleus and
engage the transcription factors TCF and LEF1, and promote
transcription of a series of EMT-related genes (18). The data in the present study
suggested that emodin inhibited the phosphorylation of CCR5 and
AKT, downregulated the expression of β-catenin, vimentin and snail,
and upregulated the expression of E-cadherin. The above results
indicated that emodin inhibited invasion and migration by
suppressing the downstream signaling pathways of CCR5- and
EMT-associated markers.
The in vivo data showed that a high fat and
sugar diet contributed to tumor growth and metastasis. Emodin
inhibited tumor growth, and inhibited the lung and liver metastasis
of TNBC cells in vivo by decreasing the secretion of CCL5 in
the serum of mice fed a high fat and sugar diet.
In conclusion, emodin was shown to antagonize the
secretion of CCL5 from adipocytes and inhibit EMT of TNBC cells,
further inhibiting tumor growth, and lung and liver metastasis.
However, the mechanism underlying the negative regulation of CCL5
release by emodin requires elucidation in future investigations.
The above data suggest a novel therapeutic agent for preventing
TNBC metastasis.
Acknowledgments
Not applicable.
References
|
1
|
Siegel RL, Naishadham D and Jemal A:
Cancer statistics, 2013. CA Cancer J Clin. 63:11–30. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brenton JD, Carey LA, Ahmed AA and Caldas
C: Molecular classification and molecular forecasting of breast
cancer: Ready for clinical application? J Clin Oncol. 23:7350–7360.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cleator S, Heller W and Coombes RC:
Triple-negative breast cancer: Therapeutic options. Lancet Oncol.
8:235–244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Foulkes WD, Smith IE and Reisfilho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calle EE and Kaaks R: Overweight, obesity
and cancer: Epidemiological evidence and proposed mechanisms. Nat
Rev Cancer. 4:579–591. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nieman K, Romero IL, Van Houten B and
Lengyel E: Adipose tissue and adipocytes support tumorigenesis and
metastasis. Biochim Biophys Acta. 1831:1533–1541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bifulco M and Pisanti S: ‘Adiponcosis’: A
new term to name the obesity and cancer link. J Clin Endocrinol
Metab. 98:4664–4665. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iyengar P, Combs TP, Shah SJ, Gouon-Evans
V, Pollard JW, Albanese C, Flanagan L, Tenniswood MP, Guha C,
Lisanti MP, et al: Adipocyte-secreted factors synergistically
promote mammary tumorigenesis through induction of anti-apoptotic
transcriptional programs and proto-oncogene stabilization.
Oncogene. 22:6408–6423. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park J, Morley TS, Kim M, Clegg DJ and
Scherer PE: Obesity and cancer-mechanisms underlying tumour
progression and recurrence. Nat Rev Endocrinol. 10:455–465. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
D’Esposito V, Passaretti F, Hammarstedt A,
Liguoro D, Terracciano D, Molea G, Canta L, Miele C, Smith U,
Beguinot F and Formisano P: Adipocyte-released insulin-like growth
factor-1 is regulated by glucose and fatty acids and controls
breast cancer cell growth in vitro. Diabetologia. 55:2811–2822.
2012. View Article : Google Scholar :
|
|
13
|
Luboshits G, Shina S, Kaplan O, Engelberg
S, Nass D, Lifshitz-Mercer B, Chaitchik S, Keydar I and Ben-Baruch
A: Elevated expression of the CC chemokine regulated on activation,
normal T cell expressed and secreted (RANTES) in advanced breast
carcinoma. Cancer Res. 59:4681–4687. 1999.PubMed/NCBI
|
|
14
|
Balkwill FR: Cancer and the chemokine
network. Nat Rev Cancer. 4:540–550. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Azenshtein E, Luboshits G, Shina S,
Neumark E, Shahbazian D, Weil M, Wigler N, Keydar I and Ben-Baruch
A: The CC chemokine RANTES in breast carcinoma progression:
Regulation of expression and potential mechanisms of promalignant
activity. Cancer Res. 62:1093–1102. 2002.PubMed/NCBI
|
|
16
|
D’Esposito V, Liguoro D, Ambrosio MR,
Collina F, Cantile M, Spinelli R, Raciti GA, Miele C, Valentino R,
Campiglia P, et al: Adipose microenvironment promotes triple
negative breast cancer cell invasiveness and dissemination by
producing CCL5. Oncotarget. 7:24495–24509. 2016.
|
|
17
|
Lee Y, Jung WH and Koo JS: Adipocytes can
induce epithelial-mesenchymal transition in breast cancer cells.
Breast Cancer Res Treat. 153:323–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang L, Lin C and Liu ZR: P68 RNA helicase
mediates PDGF-induced epithelial mesenchymal transition by
displacing Axin from beta-catenin. Cell. 127:139–155. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shang X and Yuan Z: Determination of six
components in Rhubarb by cyclodextrin-modified micellar
electrokinetic chromatography using a mixed micellar system of
sodium cholate and sodium taurocholate. Analytica Chimica Acta.
456:183–188. 2002. View Article : Google Scholar
|
|
20
|
Xia XM, Li BK, Xing SM and Ruan HL: Emodin
promoted pancreatic claudin-5 and occludin expression in
experimental acute pancreatitis rats. World J Gastroenterol.
18:2132–2139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou M, Xu H, Pan L, Wen J, Guo Y and Chen
K: Emodin promotes atherosclerotic plaque stability in fat-fed
apolipoprotein E-deficient mice. Tohoku J Exp Med. 215:61–69. 2008.
View Article : Google Scholar
|
|
22
|
Guo J, Li W, Shi H, Xie X, Li LR, Tang H,
Wu M, Kong Y, Yang L, Gao J, et al: Synergistic effects of curcumin
with emodin against the proliferation and invasion of breast cancer
cells through upregulation of miR-34a. Mol Cell Biochem.
382:103–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang Z, Chen G and Shi P: Effects of
emodin on the gene expression profiling of human breast carcinoma
cells. Cancer Detect Prev. 32:286–291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zu C, Zhang M, Xue H, Cai X, Zhao L, He A,
Qin G, Yang C and Zheng X: Emodin induces apoptosis of human breast
cancer cells by modulating the expression of apoptosis-related
genes. Oncol Lett. 10:2919–2924. 2015. View Article : Google Scholar
|
|
25
|
Lin SZ, Wei WT, Chen H, Chen KJ, Tong HF,
Wang ZH, Ni ZL, Liu HB, Guo HC and Liu DL: Antitumor activity of
emodin against pancreatic cancer depends on its dual role:
Promotion of apoptosis and suppression of angiogenesis. PLoS One.
7:e421462012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu A, Sha L, Shen Y, Huang L, Tang X and
Lin S: Experimental study on anti-metastasis effect of emodin on
human pancreatic cancer. Zhongguo Zhong Yao Za Zhi. 36:3167–71.
2011.In Chinese.
|
|
27
|
He L, Bi JJ, Guo Q, Yu Y and Ye XF:
Effects of emodin extracted from Chinese herbs on proliferation of
non-small cell lung cancer and underlying mechanisms. Asian Pac J
Cancer Prev. 13:1505–1510. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu JQ, Bao W and Lei JC: Emodin regulates
apoptotic pathway in human liver cancer cells. Phytother Res.
27:251–257. 2013. View
Article : Google Scholar
|
|
29
|
Cha TL, Qiu L, Chen CT, Wen Y and Hung MC:
Emodin down-regulates androgen receptor and inhibits prostate
cancer cell growth. Cancer Res. 65:2287–2295. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xue H, Chen Y, Cai X, Zhao L, He A, Guo K
and Zheng X: The combined effect of survivin-targeted shRNA and
emodin on the proliferation and invasion of ovarian cancer cells.
Anticancer Drugs. 24:937–944. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma YS, Weng SW, Lin MW, Lu CC, Chiang JH,
Yang JS, Lai KC, Lin JP, Tang NY, Lin JG and Chung JG: Antitumor
effects of emodin on LS1034 human colon cancer cells in vitro and
in vivo: Roles of apoptotic cell death and LS1034 tumor xenografts
model. Food Chem Toxicol. 50:1271–1278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang W, Sun Y, Li X, Li H, Chen Y, Tian Y,
Yi J and Wang J: Emodin potentiates the anticancer effect of
cisplatin on gallbladder cancer cells through the generation of
reactive oxygen species and the inhibition of survivin expression.
Oncol Rep. 26:1143–1148. 2011.PubMed/NCBI
|
|
33
|
Sun Y, Wang X, Zhou Q, Lu Y, Zhang H, Chen
Q, Zhao M and Su S: Inhibitory effect of emodin on migration,
invasion and metastasis of human breast cancer MDA-MB-231 cells in
vitro and in vivo. Oncol Rep. 33:338–346. 2015. View Article : Google Scholar
|
|
34
|
Yan Y, Su X, Liang Y, Zhang J, Shi C, Lu
Y, Gu L and Fu L: Emodin azide methyl anthraquinone derivative
triggers mitochondrial-dependent cell apoptosis involving in
caspase-8-mediated Bid cleavage. Mol Cancer Ther. 7:1688–1697.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Isakson P, Hammarstedt A, Gustafson B and
Smith U: Impaired preadipocyte differentiation in human abdominal
obesity: Role of Wnt, tumor necrosis factor-alpha, and
inflammation. Diabetes. 58:1550–1557. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Poulos SP, Hausman DB and Hausman GJ: The
development and endocrine functions of adipose tissue. Mol Cell
Endocrinol. 323:20–34. 2010. View Article : Google Scholar
|
|
37
|
Duband JL, Monier F, Delannet M and
Newgreen D: Epithelium-mesenchyme transition during neural crest
development. Acta Anat. 154:63–78. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kalluri R and Neilson EG:
Epithelial-mesenchymal transition and its implications for
fibrosis. J Clin Invest. 112:1776–1784. 2003. View Article : Google Scholar : PubMed/NCBI
|