Introduction
Angiogenesis, the formation of new blood vessels
from preexisting ones, is a dynamic complex process including cell
proliferation, migration and capillary-like tube formation
(1). It plays an important role
in a wide range of pathophysiological events, including diabetic
retinopathy, peripheral vascular disease, endometriosis, tissue
regeneration, atherosclerosis, obesity, rheumatoid arthritis, and
cancer (2-4). Scientists have found that
angiogenesis has close relationship with the development of cancer
(5-7). Tumor growth needs blood vessels to
provide nutrients and discharge metabolic waste (8). Inhibition of angiogenesis has been
called the fourth modality of anticancer therapy (9). Vascular endothelial growth factors
(VEGFs) are crucial regulators of vascular development during
embryogenesis (vasculogenesis) as well as blood vessel formation
(angiogenesis) (10,11). Binding of VEGF to vascular
endothelial growth factor receptor 1 (VEGFR1) (Flt-1) and VEGFR2
(KDR/Flk-1), two receptors for VEGF with intrinsic tyrosine kinase
activity, lead to activation of phospholipase C (PLC), PKC and
MAP-kinase. VEGFR2 is the major mediator which plays proangiogenic
effect induced by VEGF (12). The
inhibitors of VEGFR2 such as sorafenib and sunitinib have been used
in clinical treatment of cancer (13-15). Sorafenib inhibited tumor growth by
49% at the dose of 10 mg/kg, which inhibited the phosphorylation of
both ERK and eIF4E, reduced the microvessel area, and induced tumor
cell apoptosis in the PLC/PRF/5 xenograft model. Sunitinib is a
novel oral small-molecule multitargeted receptor tyrosine kinase
inhibitor that has shown direct antitumor activity and
antiangiogenic action.
Pedicellus Melo from dry stems of the
cucurbitaceous plant Cucumis melo L. Pedicellus Melo
is widely used in traditional Chinese medicine to treat digestive
system and hepatic diseases, including dyspepsia, jaundice, acute
and chronic hepatitis, hepatic cirrhosis and liver cancer. However,
its pharmacological mechanism of antitumor effect and the medicinal
material basis have not been well studied. Chemical studies have
shown that Pedicellus Melo consists of abundant
cucurbitacins, which have become a key point of study as promising
therapeutic antitumor drugs (16-18). According to the chemical
structure, cucurbitacins are divided into twelve categories
(19). Cucurbitacin B,
cucurbitacin D, cucurbitacin E and cucurbitacin I have been proven
to have antitumor effect through inducing cellular apoptosis
(20,21). Cucurbitacin B (CuB) is the most
abundant component in the cucurbitacins of Pedicellus Melo.
In vitro experiments also proved the inhibitory effect of
CuB on the growth of human cancer cell lines and tumor xenografts
including HepG2 cells (22), and
SW480 cells (23). CuB has
antitumor effect through different mechanisms. The antitumor effect
of CuB on HepG2 cells was due to the induction of cell cycle arrest
as well as apoptosis. CuB suppresses non-small-cell lung cancer
growth (24), which significantly
altered the actin cytoskeletal assembly, induced G2/M cell cycle
arrest and mitochondrial apoptosis by targeting cellular thiols.
CuB may inhibit the proliferation of human breast cancer cells
through disruption of the microtubule network and downregulation of
c-Myc. At present, it is considered that inhibition of angiogenesis
is an important means in anticancer therapy. It remains unknown
whether CuB inhibits pathological angiogenesis especially tumor
angiogenesis, and its underlying mechanism has not been
revealed.
In this study, we found that CuB inhibited human
umbilical vascular endothelial cell (HUVEC) proliferation,
migration and tubulogenesis in vitro. We also demonstrated
that CuB suppressed VEGF-induced angiogenesis in chick embryo
chorioallantoic membrane (CAM) assay in vivo. Furthermore,
our results showed that CuB induced HUVEC apoptosis, and may induce
apoptosis by triggering the mitochondrial apoptotic pathways.
Finally, we found that CuB inhibiting angiogenesis was associated
with inhibition of the activity of VEGFR2. Our studies suggested
that CuB is a novel tumor angiogenesis inhibitor and could be a
potential drug candidate for angiogenesis related diseases.
Materials and methods
Cells and reagents
HUVECs and HepG2 cells were obtained from Allcells,
LLC (Shanghai, China). CuB (purity 99%) was purchased from Chengdu
Herbpurify Co., Ltd. (Chengdu, China). DMEM/F-12, fetal bovine
serum (FBS), was from Gibco (Carlsbad, CA, USA). EGM-2 complete
medium was purchased from Lonza Technology (Basel, Switzerland).
Twenty-four-well plate with Matrigel was from BD Biosciences
(Franklin Lakes, NJ, USA). The Annexin V-FITC/propidium iodide (PI)
kit and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) reagents were purchased from Beyotime Institute of
Biotechnology (Shanghai, China). Fertilized chicken eggs were from
Harbin Veterinary Research Institute, CAAS (China). VEGF was
purchased from PeproTech (Rocky Hill, NJ, USA). Antibodies against
VEGFR2 was purchased from Sigma (St. Louis, MO, USA). Antibodies
against phosphated-VEGFR2 (Tyr1175) was purchased from Cell
Signaling Technology (Beverly, MA, USA). Antibodies for Bcl-2, Bax,
caspase-3 and cleaved caspase-3 were purchased from Cell Signaling
Technology. CuB was prepared as a 10 mM stock solution in
phosphate-buffered saline (PBS) at pH 7.4. This stock solution was
further diluted to the desired concentration with culture medium.
Fresh CuB stock solution was prepared before use.
Cell culture
HUVEC was cultured in DMEM/F-12 with 10% FBS, 10
U/ml penicillin and 10 mg/ml streptomycin or cultured in complete
endothelial cell medium (EGM-2) with grow factors and 2% FBS in a
humidified 5% CO2 atmosphere at 37°C. Cells were
dissociated with 0.25% trypsin just before transferring for
experiments and counted using a hemocytometer.
Cell viability assay
The effects of extracts isolated from Pedicellus
Melo on the proliferation of HUVEC were determined by MTT
assay. Briefly, HUVEC was seeded in a 96-well plate in a final
volume of 200 µl/well of culture medium, at 1×104
cells/well 24 h prior to treatment. The cells were treated with
different concentrations of extractions and compounds isolated from
Pedicellus Melo. Then 20 µl of 5 mg/ml MTT was added
to each well. After 4 h incubation at 37°C, 150 µl dimethyl
sulfoxide (DMSO) was added and rocked at room temperature for 15
min. The absorbance was measured at 490 nm. The percentage of the
absorbance was calculated against untreated cells. All experiments
were repeated three times.
In vitro migration assays
Monolayer HUVEC migration was modified from the
method described previously (25). Briefly, HUVECs were allowed to
grow to confluence on 6-well plates. After 1 h of mitomycin C (2
µg/ml) treatment, monolayer cells were scratched with
pipette tip and washed three times with PBS. Fresh media with or
without 10 ng/ml VEGF and various concentrations of CuB were added
into the wells. Cells were further cultured for 24 h, and the
HUVECs migration in culture was determined by measuring wound
distance in cell monolayers. Three different images from each well
along the wound were captured by a digital camera under a
microscope (×200). Wound distance was measured and analyzed by
Image-Pro Plus software.
Capillary-like structure formation
assay
HUVECs (1×105) were cultured in EGM-2
medium in a Matrigel-precoated 24-well plate at 37°C. Immediately
following cell addition, CuB was added and the plate tapped gently.
The plate was incubated overnight at 37°C, in 5% CO2
humidified incubator. After treatment, the medium was aspirated and
the plate washed with PBS twice. Then cells were stained with Green
Cell Tracker at 10 µM dissolved in PBS and incubated for 60
min at 37°C, in 5% CO2 humidified incubator. Each well
was washed two times with 1 ml PBS and 200 µl PBS was added
to each well. Pictures were taken with 10× objective lens. The
level of tube formation was quantified by measuring the number of
tubes in three randomly chosen fields from each well and was
calculated against untreated groups.
In vivo chick embryo chorioallantoic
membrane assay
According to a previous method (10), fertilized chick eggs were
incubated at 38.5-39°C with relative humidity at 65-70%. Five days
later, a 1-2 cm2 window was opened with forcep and the
shell membrane was removed to expose the chorioallantoic membrane.
Sterilized 5-mm diameter filter (Whatman, Maidstone, UK) disks
absorbed CuB or dissolvant alone as control were put onto the
avascular area of CAM. Then the window was sealed with filter
plastic tape and eggs were incubated for 4 days. The CAM was
observed under stereomicroscope and the neovascularization was
quantified using Image-Pro Plus software. All animal procedures and
experiments were approved by the Institutional Animal Care and Use
Committee of Harbin Medical University.
Assessment of apoptosis by Annexin
V-FITC/PI staining
Apoptosis analyses of HUVEC cells were carried out
by flow cytometry. HUVEC cells were treated with various
concentrations of CuB. After the treatment, the cells were
harvested by 0.25% trypsin and washed with PBS and then resuspended
in 200 µl binding buffer with an addition of 5 µl
Annexin V-fluorescein isothiocyanate and 10 µl PI. The
mixture was kept in the dark for 15 min at 4°C and then 300
µl of binding buffer was added and analyzed with flow
cytometry.
Western blotting
To determine the effects of CuB on VEGF-dependent
angiogenesis and HUVEC apoptosis, western blot analysis was
performed. Starved HUVECs were treated with CuB for 2 h followed by
inducing with 100 ng/ml VEGF165. The apoptosis-related proteins
were prepared after treated with indicated concentration of CuB for
12 h. HUVECs lysates were suspended in RIPA buffer (10 mmol/l Tris
(pH 7.4), 150 mmol/l NaCl, 1% Triton X-100, 1% deoxycholic acid,
0.1% SDS, 5 mmol/l EDTA (pH 8.0), 1 mmol/l PMSF). Protein
concentration was determined using the BCA assay and equalized
before loading. Proteins (80 µg) were resolved on 12% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and transferred
onto nitrocellulose membranes. The membranes were blocked with 5%
defatted milk in TBS and 0.1% Tween-20 and probed antibodies
overnight at 4°C. Signals were taken by LI-COR Infrared Imaged
Odyssey.
Statistical analysis
Results of cell proliferation, apoptosis and tube
formation were expressed as the mean of at least triplicate
determinations. Statistical comparisons between drug-treated group
and untreated group were performed by SPSS 12.0. Data are presented
as mean ± standard deviations. The bar graphs were prepared using
the GraphPad Prism 5. p-values <0.05 were considered
statistically significant.
Results
CuB inhibits proliferation in HUVEC
Proliferation of endothelial cells is an important
step in the processes of angiogenesis and tumor cells proliferation
is required for tumor growth (26). To investigate the inhibitory
effects of CuB on cell proliferation in both endothelial cells and
HepG2 cells, we performed the MTS assay with the two types of
cells. CuB inhibited the proliferation of HUVEC and HepG2 cells in
a dose-dependent manner, and the inhibitory effect of CuB on the
HUVEC was greater than that of HepG2 cells (Fig. 1).
CuB inhibits HUVECs migration
To investigate the potential roles of CuB in
angiogenesis in vitro, we performed migration assays to test
the effects of CuB on HUVECs. Cells in 6-well plates were
scratched, washed with phosphate-buffered saline (PBS) and
incubated for 8-12 h (Fig. 2).
Cells migrated toward the wound regions were analyzed. We found
that CuB inhibited VEGF induced HUVECs migration in wound-healing
assay in a dose-dependent manner.
CuB inhibited tube formation in vitro and
angiogenesis in vivo
Endothelial cell tube formation is a key step
involved in tumor angiogenesis (27). To study the effect of CuB on
endothelial cell angiogenesis, we added HUVEC with different
concentrations of CuB onto Matrigel and examined the formation of
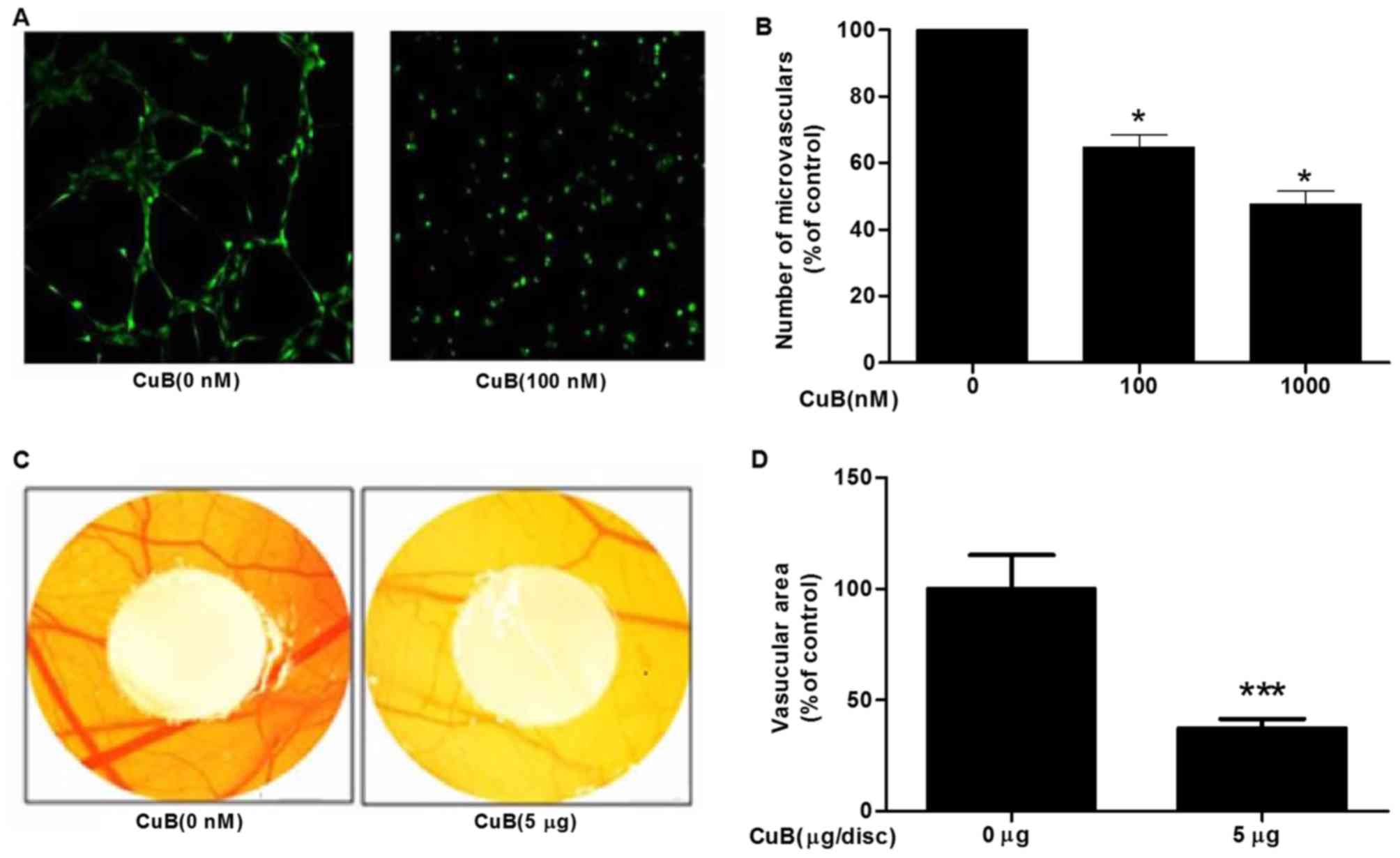
capillary-like structure by HUVECs. Our results showed that the
number of tubular capillary-like network on Matrigel was inhibited
by CuB in a dose-dependent manner (Fig. 3A and B). To directly evaluate the
effects of CuB on angiogenesis and vascular development in
vivo, we examined the inhibitory effects of CuB on the chick
chorioallantoic membrane to measure the antiangiogenesis property
of CuB in vivo. After 2 days treatment of CuB. The formation
of novel blood vessels was obviously blocked by CuB (5
µg/disc), suggesting that CuB inhibited chick embryo
chorioallantoic membrane angiogenesis, and decreased 62.8% in
branching patterns of the blood vessels (Fig. 3C and D).
CuB induced apoptosis in HUVECs
We further tested the effect of CuB on cell
apoptosis by flow cytometry with PI staining. We evaluated the
effect of CuB on HUVEC apoptosis with Annexin V/PI assay (Fig. 4). The percentage of apoptotic
cells in HUVECs was ~50 and 80% at the concentration of 100 and
1,000 nM of CuB, respectively. These data suggested that CuB can
induce apoptosis of endothelial cells.
CuB triggers the mitochondrial signaling
pathway
CuB inhibited the expression of Bcl-2 and increased
the expression of Bax (Fig. 5).
On the other hand, we also found that the expression of caspase-3
was downregulated and cleaved caspase-3 was upregulated in HUVEC.
The above results suggested that CuB inhibited the mitochondrial
signaling pathway.
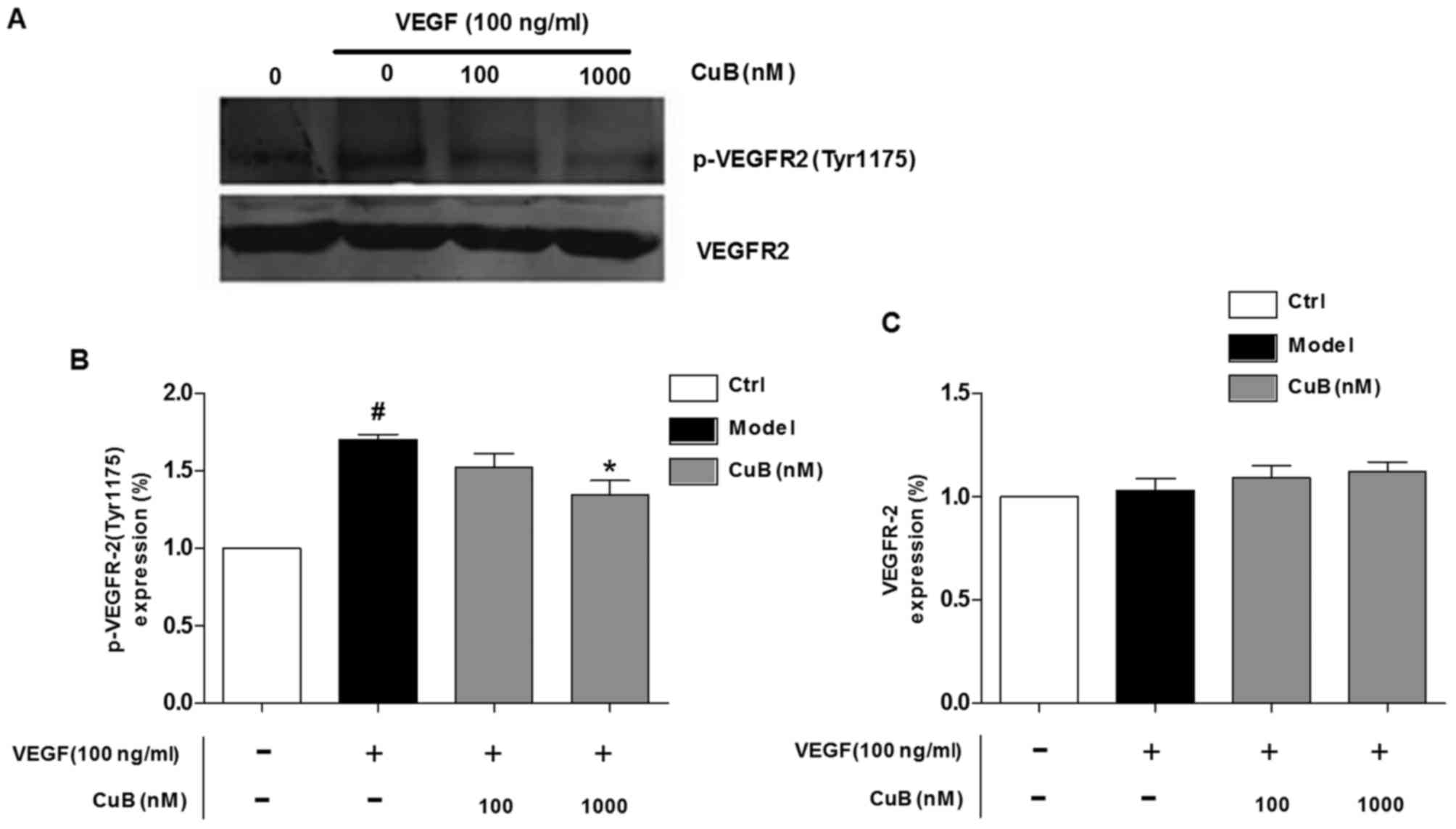
CuB suppresses VEGF activated VEGFR2
phosphorylation
VEGFR2 is the major receptor of VEGF in
angiogenesis, and VEGF/VEGFR2 pathway plays a central role in
angiogenesis. To clarify the underlying molecular mechanism of CuB
on angiogenesis, we studied the effects of CuB on the
phosphorylation of VEGFR2 in HUVECs. CuB decreased the VEGF
activated VEGFR2 phosphorylation in a dose-dependent manner
(Fig. 6). CuB (1000 nM)
dramatically inhibited the phosphorylation of VEGFR2, suggesting
that CuB is a potent inhibitor of VEGFR in HUVECs.
Discussion
Tumor growth depends on angiogenesis and
antiangiogenic therapy is a new target for cancer treatment
(28). Identification of
angiogenesis inhibitors will benefit drug discovery for tumor and
other diseases associated with angiogenesis (29). In this in vivo angiogenesis
study, a standard chick embryo CAM assay of angiogenesis was used.
We found that CuB inhibited CAM angiogenesis in a dose-dependent
manner. In angiogenesis study in vitro, CuB suppressed key
steps involved in angiogenesis, including proliferation, migration,
tubulogenesis, and induced apoptosis in endothelial cells. Further
studies illuminated that CuB induced endothelial cell apoptosis via
suppressing the mitochondrial pathway.
Pedicellus Melo L. belongs to the
Cucurbitaceae family which consists of various cucurbitacins used
as a natural medicine in China for centuries. There is increasing
evidence showing that cucurbitacins not only inhibited cancer cell
proliferation and induced cell apoptosis, but also showed the
synergistic effect with known chemotherapeutic agents, such as
doxorubicin and gemcitabine. The reported molecular mechanisms of
action of cucurbitacins in human cancer cells not only inhibited
signal transducers and activators of transcription-3 (STAT3), but
also affected other signaling pathways, such as the MAPK pathway
(30). Cucurbitacins as potential
anticancer drugs have attracted the attention of the drug industry.
It has been reported that cucurbitacins especially CuB is widely
utilized for antitumor activity in traditional Chinese medicine
(31,32). In this study, we demonstrated that
CuB significantly inhibited proliferation in HUVEC. Interestingly,
CuB inhibited much stronger proliferation in endothelial cells than
that in HepG2 cells. These results suggested whether CuB inhibited
tumor growth mainly through targeting endothelial cells other than
tumor cells. With the development of the pharmacological mechanism
in cucurbitacins, cucurbitacin E has been proven to be an
anti-angiogenic agent by inhibiting VEGFR2-mediated Jak-STAT3 and
mitogen-activated protein kinases signaling pathways. However,
whether the same kind of compound as CuB, can also inhibit tumor
angiogenesis is still unknown. Whether the anti-angiogenisis will
be a new therapeutic target for cucurbitacins need to be further
studied.
Angiogenesis is a very complex process, it is the
result of the interaction of different factors in time and space.
Angiogenesis begins with the degradation of the basement membrane
by activated endothelial cells that will migrate and proliferate,
leading to the formation of solid endothelial cell sprouts in the
stromal space. The activation and proliferation of endothelial
cells is the first step in this process. In this study, we
demonstrated that CuB significantly inhibited proliferation in
HUVEC. The migration and new vessel formation of endothelial cells
is also the key steps in the angiogenesis. In our results, CuB
obviously inhibited the two steps in angiogenesis. The chick CAM
assay is a typical and widely used experiment in angiogenesis
study. In this in vivo experiment, the formation of novel
blood vessels was obviously blocked by CuB. It suggested that CuB
may suppress angiogenesis in vivo and in vitro.
In the process of angiogenesis, endothelial cell
apoptosis is an important factor for the inhibition of
angiogenesis. There is a dynamic balance between the formation of
new blood vessels and apoptosis, if it can promote the apoptosis,
angiogenesis will be inhibited. Increasing number of studies have
shown that mitochondria played a crucial role in cellular apoptotic
pathway (33). Bcl-2 protein
family plays an essential role in the regulation of mitochondrial
apoptosis pathway (34). The
pro-apoptotic Bcl-2 family protein Bax has been shown to be
required for the disruption of mitochondrial of cancer cells,
whereas the antiapoptotic Bcl-2 can prevent cell death by
interfering with the activation of Bax (35). In the present study, we found that
the expression level of Bcl-2 was suppressed and the expression of
Bax was upregulated in HUVEC. To further characterize the molecular
mechanisms of CuB-induced apoptotic pathways, we examined the
expression of caspase-3 and cleaved caspase-3. Compared with
control group, the CuB could downregulate the expression of
caspase-3 and upregulate the expression of cleaved caspase-3. Thus,
we concluded that CuB may induce apoptosis in endothelia cells by
triggering the mitochondrial apoptotic pathways.
VEGFs are critical regulators of vasculogenesis and
angiogenesis by binding to their cognate receptor. Among the three
receptors (VEGFR1,2,3), VEGFR2 is the main receptor of VEGF
involved in angiogenesis (36).
Activation of the VEGF signaling pathway triggers a network of
signaling processes that promote endothelial cell growth, migration
and tube formation. In this study, we found that the activity of
VEGFR2 was inhibited by CuB in a dose-dependent manner. This result
showed that CuB inhibited VEGF mediated angiogenesis through VEGFR2
inhibition.
Angiogenesis is a complex multistep process.
Inhibition of any step of these processes may result in the damage
of angiogenesis and can serve as a potential antitumor therapy. We
found CuB inhibited angiogenesis effects of tumors by triggering
the mitochondrial apoptotic pathways in endothelia cells. Shukla
et al also reported that CuB inhibited metastasis and
angiogenesis of non-small cell lung cancer which was related with
the downregulation of Wnt/β-catenin signaling axis (37). This shows that CuB inhibited
angiogenesis possibly through different mechanisms. The present
study suggests that CuB as a VEGFR2 inhibitor is a potential drug
candidate for diseases associated with pathological
angiogenesis.
In conclusion, our studies showed that CuB
significantly inhibited human umbilical vascular endothelial cell
(HUVEC) proliferation, migration, tubulogenesis in vitro,
and blocked angiogenesis in chick embryo chorioallantoic membrane
assay in vivo. Furthermore, CuB induced HUVEC apoptosis and
may induce apoptosis by triggering the mitochondrial apoptotic
pathway. Finally, we found that CuB inhibiting angiogenesis was
associated with inhibition of the activity of VEGFR2. Our new
finding of CuB in angiogenesis suggested a novel role of CuB as an
antiangiogenesis agent.
Acknowledgments
Not applicable.
Funding
This study was supported by the Heilongjiang
Postdoctoral fund, Heilongjiang Province Science Foundation for
Youths (no. QC2013C081) the funding from Key Laboratory of
Cardiovascular Medicine Research (Harbin Medical University), the
National Natural Science Foundation of China (no. 81703760) and the
Central Guide Local Project of Science and Technology Development
(grant no. ZY16A07).
Availability of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XMP was mainly responsible for the design and
writing of the article, and performed some experimental operations.
FG performed the cell viability assay and capillary-like structure
formation assay. JXZ and LJW performed the western blotting
experiment. XZ was a major contributor in data analysis. XL was in
charge of making diagrams. MMS participated in other in
vitro experiments. YZ was mainly responsible for the conception
and revision of the article. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All animal procedures and experiments were approved
by the Institutional Animal Care and Use Committee of Harbin
Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang K, Lu J, Mori T, Smith-Powell L,
Synold TW, Chen S and Wen W: Baicalin increases VEGF expression and
angiogenesis by activating the ERR{α}/PGC-1{α} pathway. Cardiovasc
Res. 89:426–435. 2011. View Article : Google Scholar
|
|
2
|
Carmeliet P: Angiogenesis in life, disease
and medicine. Nature. 438:932–936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Folkman J: Angiogenesis: An organizing
principle for drug discovery? Nat Rev Drug Discov. 6:273–286. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cao Y: Adipose tissue angiogenesis as a
therapeutic target for obesity and metabolic diseases. Nat Rev Drug
Discov. 9:107–115. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li WW, Li VW, Hutnik M and Chiou AS: Tumor
angiogenesis as a target for dietary cancer prevention. J Oncol.
2012:8796232012. View Article : Google Scholar
|
|
6
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grothey A and Galanis E: Targeting
angiogenesis: Progress with anti-VEGF treatment with large
molecules. Nat Rev Clin Oncol. 6:507–518. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tammali R, Reddy AB, Srivastava SK and
Ramana KV: Inhibition of aldose reductase prevents angiogenesis in
vitro and in vivo. Angiogenesis. 14:209–221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Folkman J: Endogenous angiogenesis
inhibitors. APMIS. 112:496–507. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yi ZF, Cho SG, Zhao H, Wu YY, Luo J, Li D,
Yi T, Xu X, Wu Z and Liu M: A novel peptide from human
apolipoprotein(a) inhibits angiogenesis and tumor growth by
targeting c-Src phosphorylation in VEGF-induced human umbilical
endothelial cells. Int J Cancer. 124:843–852. 2009. View Article : Google Scholar :
|
|
11
|
Yi T, Cho SG, Yi Z, Pang X, Rodriguez M,
Wang Y, Sethi G, Aggarwal BB and Liu M: Thymoquinone inhibits tumor
angiogenesis and tumor growth through suppressing AKT and
extracellular signal-regulated kinase signaling pathways. Mol
Cancer Ther. 7:1789–1796. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang N, Wang L, Esko J, Giordano FJ, Huang
Y, Gerber HP, Ferrara N and Johnson RS: Loss of HIF-1alpha in
endothelial cells disrupts a hypoxia-driven VEGF autocrine loop
necessary for tumorigenesis. Cancer Cell. 6:485–495. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu L, Cao Y, Chen C, Zhang X, McNabola A,
Wilkie D, Wilhelm S, Lynch M and Carter C: Sorafenib blocks the
RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor
cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer
Res. 66:11851–11858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kane RC, Farrell AT, Saber H, Tang S,
Williams G, Jee JM, Liang C, Booth B, Chidambaram N, Morse D, et
al: Sorafenib for the treatment of advanced renal cell carcinoma.
Clin Cancer Res. 12:7271–7278. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cabebe E and Wakelee H: Sunitinib: A newly
approved small-molecule inhibitor of angiogenesis. Drugs Today
(Barc). 42:387–398. 2006. View Article : Google Scholar
|
|
16
|
Alghasham AA: Cucurbitacins - a promising
target for cancer therapy. Int J Health Sci (Qassim). 7:77–89.
2013. View
Article : Google Scholar
|
|
17
|
Molavi O, Ma Z, Mahmud A, Alshamsan A,
Samuel J, Lai R, Kwon GS and Lavasanifar A: Polymeric micelles for
the solubilization and delivery of STAT3 inhibitor cucurbitacins in
solid tumors. Int J Pharm. 347:118–127. 2008. View Article : Google Scholar
|
|
18
|
Bishayee A, Ahmed S, Brankov N and Perloff
M: Triterpenoids as potential agents for the chemoprevention and
therapy of breast cancer. Front Biosci (Landmark Ed). 16:980–996.
2011. View Article : Google Scholar
|
|
19
|
Chen JC, Chiu MH, Nie RL, Cordell GA and
Qiu SX: Cucurbitacins and cucurbitane glycosides: Structures and
biological activities. Nat Prod Rep. 22:386–399. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duangmano S, Sae-Lim P, Suksamrarn A,
Domann FE and Patmasiriwat P: Cucurbitacin B inhibits human breast
cancer cell proliferation through disruption of microtubule
polymerization and nucleophosmin/B23 translocation. BMC Complement
Altern Med. 12:1852012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dakeng S, Duangmano S, Jiratchariyakul W,
U-Pratya Y, Bögler O and Patmasiriwat P: Inhibition of Wnt
signaling by cucurbitacin B in breast cancer cells: Reduction of
Wnt-associated proteins and reduced translocation of
galectin-3-mediated β-catenin to the nucleus. J Cell Biochem.
113:49–60. 2012. View Article : Google Scholar
|
|
22
|
Liu T, Zhang M, Zhang H, Sun C and Deng Y:
Inhibitory effects of cucurbitacin B on laryngeal squamous cell
carcinoma. Eur Arch Otorhinolaryngol. 265:1225–1232. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yasuda S, Yogosawa S, Izutani Y, Nakamura
Y, Watanabe H and Sakai T: Cucurbitacin B induces G2 arrest and
apoptosis via a reactive oxygen species-dependent mechanism in
human colon adenocarcinoma SW480 cells. Mol Nutr Food Res.
54:559–565. 2010. View Article : Google Scholar
|
|
24
|
Kausar H, Munagala R, Bansal SS, Aqil F,
Vadhanam MV and Gupta RC: Cucurbitacin B potently suppresses
non-small-cell lung cancer growth: Identification of intracellular
thiols as critical targets. Cancer Lett. 332:35–45. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo D, Luo Y, He Y, Zhang H, Zhang R, Li
X, Dobrucki WL, Sinusas AJ, Sessa WC and Min W: Differential
functions of tumor necrosis factor receptor 1 and 2 signaling in
ischemia-mediated arteriogenesis and angiogenesis. Am J Pathol.
169:1886–1898. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lynch CN, Wang YC, Lund JK, Chen YW, Leal
JA and Wiley SR: TWEAK induces angiogenesis and proliferation of
endothelial cells. J Biol Chem. 274:8455–8459. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaneko T, Nagata I, Miyamoto S, Kubo H,
Kikuchi H, Fujisato T and Ikada Y: Effects of nicardipine on tube
formation of bovine vascular endothelial cells in vitro. Stroke.
23:1637–1642. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Z and Han ZC: STAT3: A critical
transcription activator in angiogenesis. Med Res Rev. 28:185–200.
2008. View Article : Google Scholar
|
|
29
|
Zhang X, Song Y, Wu Y, Dong Y, Lai L,
Zhang J, Lu B, Dai F, He L, Liu M, et al: Indirubin inhibits tumor
growth by antitumor angiogenesis via blocking VEGFR2-mediated
JAK/STAT3 signaling in endothelial cell. Int J Cancer.
129:2502–2511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Boykin C, Zhang G, Chen YH, Zhang RW, Fan
XE, Yang WM and Lu Q: Cucurbitacin IIa: A novel class of anticancer
drug inducing non-reversible actin aggregation and inhibiting
survivin independent of JAK2/STAT3 phosphorylation. Br J Cancer.
104:781–789. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chan KT, Li K, Liu SL, Chu KH, Toh M and
Xie WD: Cucurbitacin B inhibits STAT3 and the Raf/MEK/ERK pathway
in leukemia cell line K562. Cancer Lett. 289:46–52. 2010.
View Article : Google Scholar
|
|
32
|
Gupta P and Srivastava SK: Inhibition of
Integrin-HER2 signaling by cucurbitacin B leads to in vitro and in
vivo breast tumor growth suppression. Oncotarget. 5:1812–1828.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Onishi Y, Kawamoto T, Ueha T, Kishimoto K,
Hara H, Fukase N, Toda M, Harada R, Minoda M, Sakai Y, et al:
Transcutaneous application of carbon dioxide (CO2) induces
mitochondrial apoptosis in human malignant fibrous histiocytoma in
vivo. PLoS One. 7:e491892012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fesik SW: Promoting apoptosis as a
strategy for cancer drug discovery. Nat Rev Cancer. 5:876–885.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiang L, Luo M, Liu D, Chen B, Zhang W,
Mai L, Zeng J, Huang N, Huang Y, Mo X, et al: BAD overexpression
inhibits cell growth and induces apoptosis via
mitochondrial-dependent pathway in non-small cell lung cancer.
Cancer Cell Int. 13:532013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Olsson AK, Dimberg A, Kreuger J and
Claesson-Welsh L: VEGF receptor signalling - in control of vascular
function. Nat Rev Mol Cell Biol. 7:359–371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shukla S, Sinha S, Khan S, Kumar S, Singh
K, Mitra K, Maurya R and Meeran SM: Cucurbitacin B inhibits the
stemness and metastatic abilities of NSCLC via downregulation of
canonical Wnt/β-catenin signaling axis. Sci Rep. 6:218602016.
View Article : Google Scholar
|