Introduction
The prevalence of diabetes has been increasing in
recent decades (1). Additionally,
insulin resistance (IR) has been demonstrated to serve a crucial
function in the development of type-2 diabetes mellitus (2). Fabbrini et al (3) recently reported that hepatic fatty
acid metabolism disorder was associated with IR, and decreasing
liver triglyceride (TG) accumulation in obese animals was
associated with improved insulin sensitivity (4), strengthening the association between
hepatic fatty acid metabolism and IR.
Farnesoid X receptor (FXR) is a member of the
nuclear hormone receptor superfamily (5), which controls bile acid (BA)
synthesis and transport, as well as lipid metabolism, through
actions on the liver and intestines (6). In addition, Duran-Sandoval et
al (7) using FXR knockout
(KO) mice have identified a critical role of FXR in peripheral
insulin signaling and hepatic glucose metabolism. FXR KO mice
display glucose intolerance and insulin insensitivity; activation
of FXR with the GW4064 agonist, or with a virus overexpressing a
constitutively active form of FXR in the liver; lowered blood
glucose levels in both db/db and wild-type mice by repressing
gluconeogenic genes in the liver; and activating hepatic glycogen
synthesis (8,9). However, the underlying mechanisms
through which FXR alters hepatic fatty acid metabolism remain
undetermined.
Fexaramine (Fex) is a selective agonist of FXR. It
is a non-BA synthetic activator with marked selectivity for FXR
over other BA receptors, including pregnane X receptor,
constitutive androstane receptor, and vitamin D receptor (10). In the present study, db/db mice
were treated with Fex, in order to evaluate the regulatory effects
of activation of FXR on hepatic glucose and fatty acid metabolism,
and to elucidate the mechanisms of this.
Materials and methods
Animal models
Male db/db mice (n=20; age, 5 weeks; weight, 18–23
g) were purchased from the Nanjing Biomedical Research Institute of
Nanjing University (Nanjing, China). Mice (n=5/cage) were
maintained at a constant temperature (23±2°C) and humidity (50±10%)
in a 12-h light/dark cycle. Mice had ad libitum access to
standard chow (consisting of 10% fat, 70% carbohydrate and 20%
protein) and water. Following one week of acclimatization, mice
were randomly divided into two experimental groups (n=10/group):
Fex group and control (Con) group. The Fex group was administered
50 mg/kg body weight Fex [in corn oil, as detailed by Fang et
al (11); MedchemExpress,
Monmouth Junction, NJ, USA] by gavage daily for 8 weeks, whereas
the control group received corn oil. A glucose tolerance test (GTT)
was performed during the final week of feeding as described below.
All experiments involving animals were performed according to the
procedures approved by the Institutional Animal Care and Use
Committee of the Institute of Zoology, Hebei General Hospital
(Shijiazhuang, China). The mice were euthanized via cervical
dislocation.
Biological assays
The body weight of each mouse was recorded every
day; the quantity of food consumed by each cage was recorded twice
a week, and the mean food consumption per mouse was calculated.
Following the experimental period, the animals were fasted
overnight and sacrificed. Liver tissue was surgically removed from
each mouse, weighed, snap frozen in liquid nitrogen, and stored at
−80°C for future analysis. Blood samples were collected from the
orbital vein for the measurement of serum indexes. Blood glucose
levels were measured using an Accuchek Active Meter (Roche Applied
Science, Penzberg, Germany) according to the manufacturer's
instructions. Enzyme kits were used to measure the total
cholesterol (TC; A110-1), TG (A111-1), free fatty acid (FFA;
A042-2; all from Nanjing Jiancheng Bioengineering Institute,
Nanjing, China), aspartate aminotransferase (AST) and alanine
aminotransferase (ALT) (both from Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany).
Intraperitoneal (i.p.) GTT and serum
insulin
Following 8 weeks of treatment, mice were subjected
to an i.p. GTT. Mice received i.p. injection of 2 g/kg glucose
following 12 h fasting. Glucose levels were measured from blood
collected from the tail using an Accuchek Active Meter immediately
prior to and 15, 30, 60 and 120 min following i.p. glucose
injection. For insulin, serum was prepared from blood by
centrifugation (at 2,000 × g for 10 min at 37°C), and measured with
insulin ELISA kits (ALPCO Diagnostics, Salem, NH, USA), according
to the manufacturer's guidelines.
Assay of TG in liver tissue
Liver TG content was measured as follows: Liver
samples were weighed and homogenized with anhydrous ethanol (1 g; 9
ml) and centrifuged at 1,400 × g for 10 min at 4°C. The supernatant
was collected to determine the total amount of tissue lipids, using
the ELISA kit according to the manufacturer's protocol (Nanjing
Jiancheng Bioengineering Institute).
Hematoxylin and eosin (H&E) staining
and Oil-Red-O staining
Liver samples were collected from mice following 8
weeks of treatment. Samples were frozen and cut into 5-mm sections.
The sliced sections were treated with H&E and also stained with
Oil Red O using a light micoscope as previously described (12).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from liver tissue using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The mRNA concentration was quantified using a Nano Drop
spectrophotometer (Thermo Fisher Scientific, Inc.). The diluted
mRNA (0.5 mg/ml) was reverse-transcribed using the Total RevertAid
First Strand cDNA synthesis kit (Thermo Fisher Scientific, Inc.)
according to the manu facturer's protocol, and the gene expression
levels were determined on ABI 7300 Real-Time PCR System (Applied
Biosystems, USA) using the Syber Green I Go Taqs Qpcr Detection kit
(Tiangen Biotech Co., Ltd., Bejing, China) and normalized to GAPDH
expression using the 2−ΔΔCq method (13). The standard cycling conditions
were as follows: 95°C for 3 min, followed by 40 cycles at 95°C for
5 sec, 60°C for 10 sec and 72°C for 15 sec. The primer sequences
for each gene are listed in Table
I.
 | Table IPrimers used for gene expression
analysis. |
Table I
Primers used for gene expression
analysis.
| Gene | Forward primer
sequence (5′-3′) | Reverse primer
sequence (5′-3′) |
|---|
| GAPDH |
ACCCAGAAGACTGTGGATGG |
GGAGACAACCTGGTCCTCAG |
| FXR |
GCCATCAAGGACGTCAGCA |
CTTCCTCCGAGTAGCGAATCAG |
| SHP |
CGATCCTCTTCAACCCAGATG |
AGGGCTCCAAGACTTCACACA |
| AMPK |
ATCCAAACACCAAGGCGT |
TTCCATTCATAGTCCAACTGCT |
| CPT1-α C |
GCACGGAAGGAAAATG |
TGTGCCCAATATTCCTGG |
| PGC-1α |
ACCAAACCCACAGAGAACAG |
GGGTCAGAGGAAGAGATAAAGTTG |
| ACC |
TGACAGACTGATCGCAGAGAAAG |
TGGAGAGCCCCACACACA |
| SREBP-1c |
GGAGCCATGGATTGCACATT |
GCTTCCAGAGAGGAGGCCAG |
| Fas |
GCTGCGGAAACTTCAGGAAAT |
AGAGACGTGTCACTCCTGGACTT |
Western blotting
The nuclear, cytosolic and membrane fractions were
extracted from liver tissue as previously described (14). To obtain total proteins, liver
tissues from the mice were lysed in a buffer containing 50 mM
Tris-HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 0.1% SDS and 1 mM
phenylmethylsulfonyl fluoride. The protein concentration was
measured using a bicinchoninic acid assay method (Beijing Solarbio
Science and Technology, Beijing, China). Lysates of 10–15 µg
protein were separated by 10% SDS-PAGE gel, transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA), blocked with 5% skimmed dry milk at room temperature for 1 h,
and probed with primary antibodies at 4°C overnight. Antibodies
against FXR (ab129089, anti-rabbit; 1:1,000), small heterodimer
partner (SHP; ab32559, anti-rabbit; 1:1,000), AMP-activated protein
kinase (AMPK; ab32047, anti-rabbit; 1:1,000), phosphorylated
(p)-AMPK (ab133448, anti-rabbit; 1:1,000), acetyl coenzyme A
carboxylase (ACC; ab45174, anti-rabbit; 1:2,000), p-ACC (ab169768,
anti-rabbit; 1:1,000), carnitine palmitoyl transferase 1α (CPT1-α;
ab128568, anti-mouse; 1:800), peroxisome proliferator-activated
receptor-coactivator-1α (PGC-1α; ab54481, anti-rabbit; 1:1,000),
sterol-regulatory element binding protein (SREBP)-1c (ab28481,
anti-rabbit; 1:800) and fatty acid synthase (Fas; ab82419,
anti-rabbit; 1:1,000) were all purchased from Abcam (Cambridge,
UK). The blots were subsequently incubated with horseradish
peroxidase-conjugated secondary antibodies, the secondary
antibodies used were peroxidase-conjugated rabbit (sc2031) or mouse
(sc2032) antibodies (1:10,000; both from GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA) followed by detection via
enhanced chemiluminescence (sc2048; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). The protein bands were quantified by densitometry
using ImageJ software (version 1.51k; National Institutes of
Health, Bethesda, MD, USA). Normalization was performed by blotting
the same samples with an antibody against GAPDH (ab54481,
anti-rabbit; 1:10,000; Abcam).
Statistical analysis
All data are presented as the mean ± standard error
of the mean. Student's t-test was performed for comparisons between
two groups. SPSS version 21.0 software (IBM Corp., Armonk, NY, USA)
was used for the statistical analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Body weight and weight gain decreases
significantly with fexaramine treatment in db/db mice
Following 8 weeks of treatment, mice in the Fex
group exhibited a significant decrease in body weight and weight
gain compared with the Con group, whereas there was no significant
difference in mean food intake per day between the two groups
(Fig. 1A–D). In order to exclude
the idea that the lower body weight did not result from the
pathological effects of Fex, ALT and AST levels in the serum were
also detected, and the results indicated that there was no
statistically significant difference in ALT and AST levels between
the Con and Fex groups (Fig. 1E).
This indicated that the reduction in body weight was a result of
Fex and was independent of food intake and the pathological effect
of Fex on the liver.
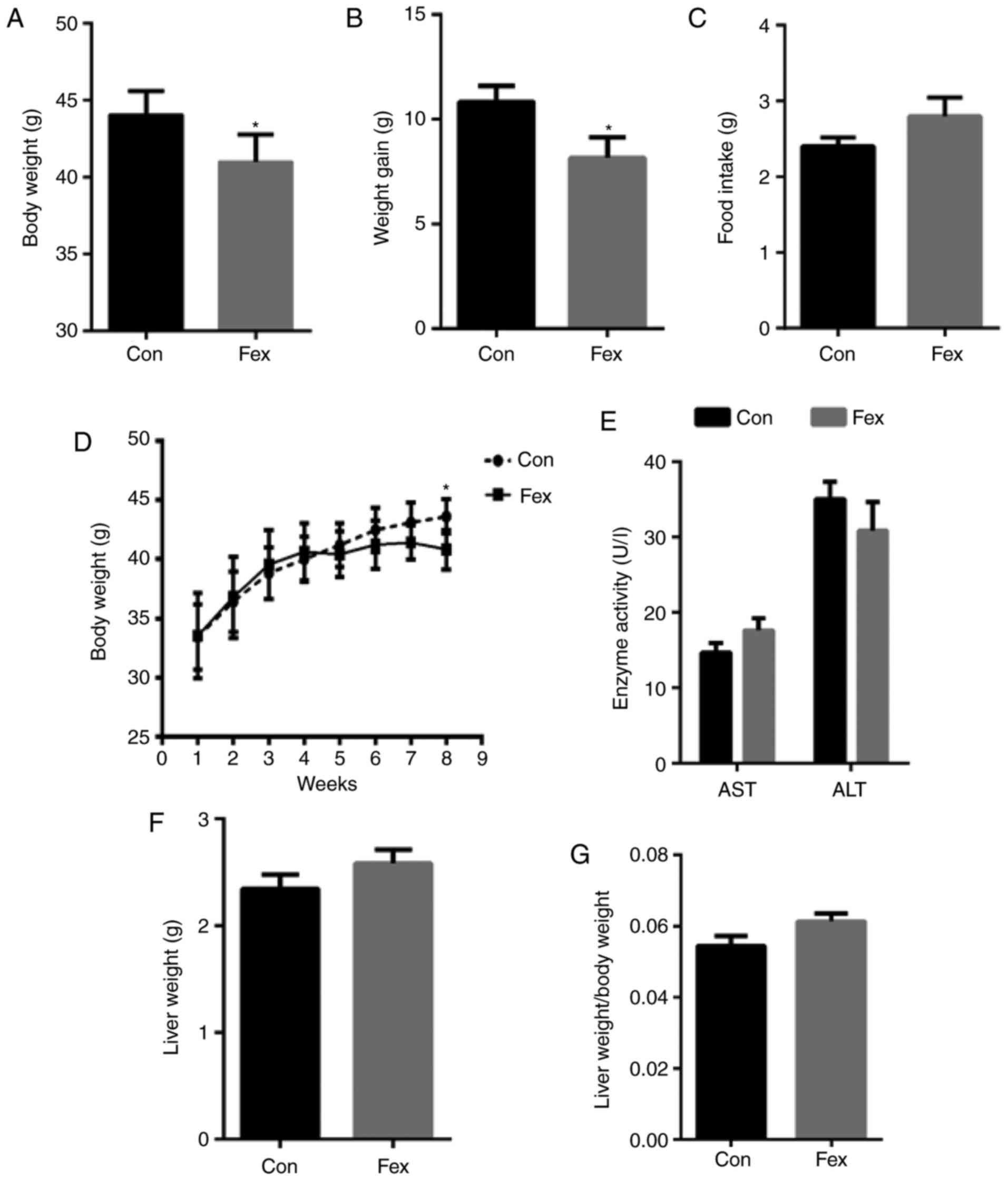 | Figure 1Comparison of (A) body weight, (B)
weight gain, (C) food intake, (D) body weight growth (from the
beginning of the intervention to the end), (E) AST and ALT, (F)
liver weight, and (G) the ratio of liver weight to body weight
between the Con and Fex groups following 8 weeks of treatment. Data
are presented as the mean + or ± standard error of the mean,
(n=10). *P<0.05 vs. Con group. AST, aspartate
aminotransferase; ALT, alanine aminotransferase; Con, control; Fex,
fexaramine. |
To further investigate the effects of Fex on liver
weight, liver weight was also recorded. There were no significant
differences in the liver weight or the liver/body weight ratio
between the Fex group and the Con group at the end of the
experiment (Fig. 1F and G).
Effects of Fex on blood glucose, serum
insulin and insulin sensitivity
To determine the effects of Fex on blood glucose and
insulin, fasting blood glucose and serum insulin were measured, and
the results indicated that both fasting blood glucose and serum
insulin were not significantly different between the two groups
(Fig. 2A and B); the authors
speculate that this was likely due to the short intervention time
of Fex. To test whether Fex improved insulin sensitivity, an i.p.
GTT was performed following 8 weeks of Fex treatment. I.p. GTT
indicated significantly reduced blood glucose levels following
glucose loading at 15 min in the Fex group, whereas glucose levels
at 0, 30, 60 and 120 min did not significantly differ between the
two groups (Fig. 2C). The area
under the curve (AUC) was then calculated. In accordance with the
above results, the AUC of the Fex group was markedly lower than
that of the Con group (Fig. 2D),
thus suggesting improved insulin sensitivity with Fex
treatment.
Improved liver and serum lipid profile in
Fex-treated mice
Following 8 weeks of treatment with Fex, serum and
liver TG levels were significantly decreased in the Fex group
compared with the Con group (Fig. 3A
and B). Serum TC in the Fex group exhibited no significant
difference compared with the Con group (Fig. 3C). Serum FFA was significantly
decreased in the Fex group, compared with controls (Fig. 3D). As revealed by H&E
staining, the number and size of lipid droplets in the livers of
the mice treated with Fex were also markedly reduced (Fig. 3E and F).
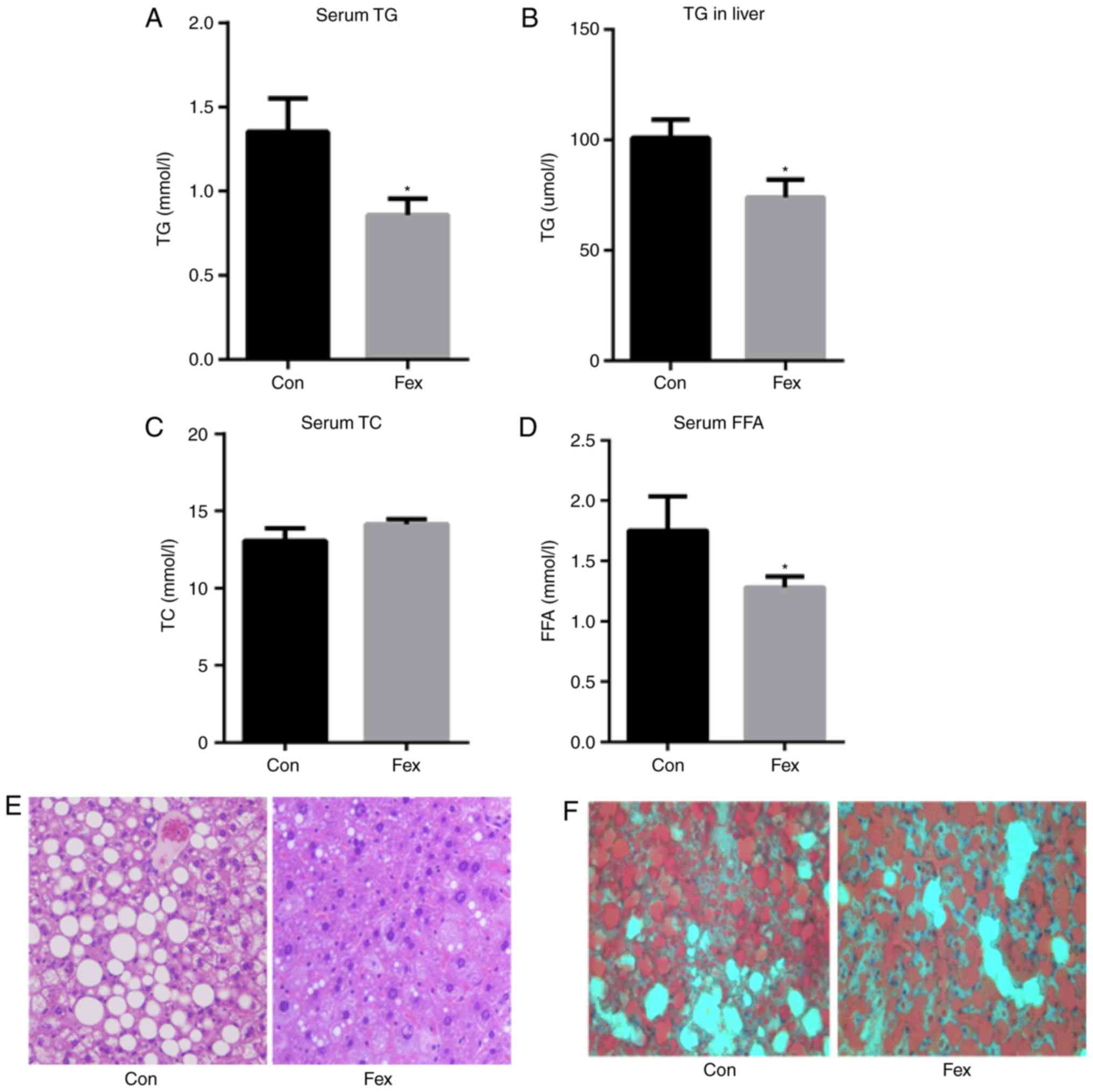 | Figure 3Effect of Fex on (A) serum TG, (B)
hepatic TG, and serum (C) TC and (D) FFA following 8 weeks of
treatment. Effect of Fex on liver lipid generation assessed via (E)
hematoxylin and eosin, and (F) Oil-Red-O staining (original
magnification, ×400). Data are presented as the mean + standard
error of the mean, (n=10). *P<0.05 vs. Con group.
Fex, fexaramine; TG, triglycerides; TC, total cholesterol; FFA,
free fatty acids; Con, control. |
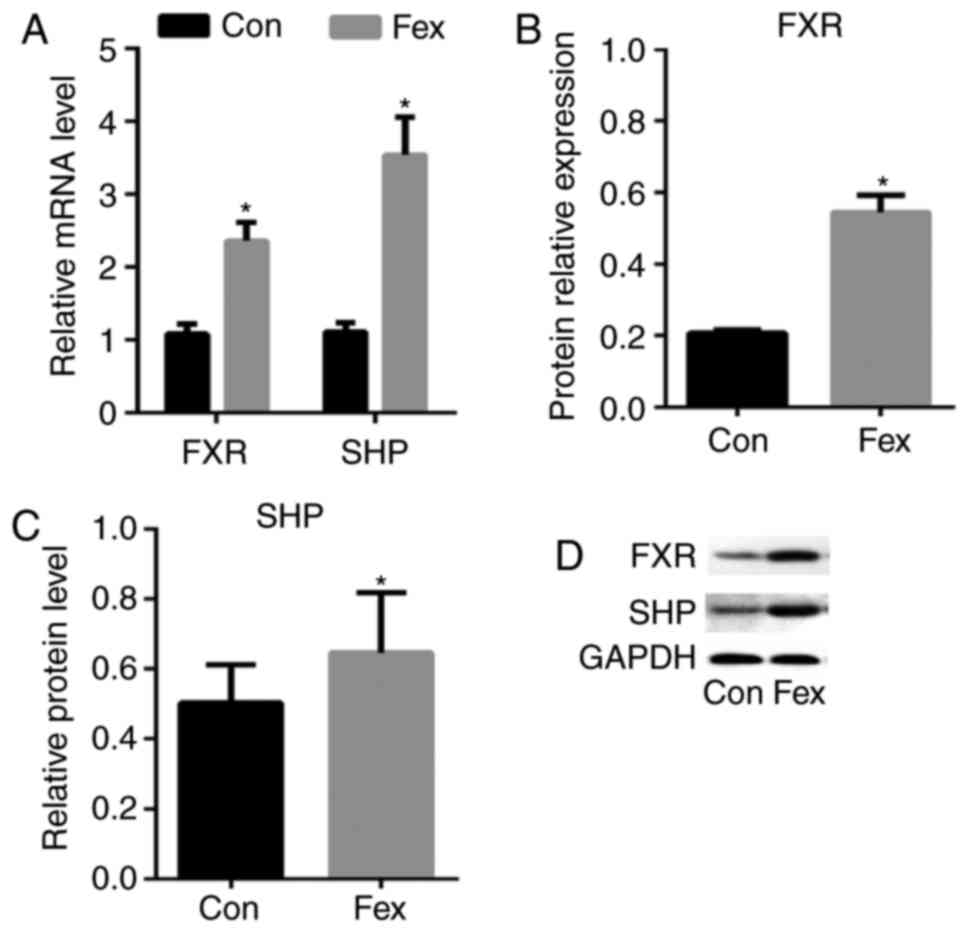
Effect of Fex on genes associated with
hepatic fatty acid metabolism
RT-qPCR and western blotting were used to measure
the mRNA and protein expression level, respectively, of certain
genes associated with fatty acid metabolism in the livers of mice
treated with or without Fex. Both mRNA and protein expression
levels of FXR were significantly higher in the livers of the Fex
group than in the Con group, and SHP, a downstream gene of FXR
exhibited a similar change (Fig.
4), which indicated that Fex can activate hepatic FXR
signaling. Lipogenesis and fatty acid oxidation are two key
metabolic pathways that regulate hepatic triacylglycerol metabolism
(15). The protein and mRNA
expression levels of the genes associated with these two pathways
were evaluated. The mRNA expression levels of AMPK, CPT1-α and
PGC-1α and protein expression of p-AMPK/AMPK, p-ACC, CPT1-α and
PGC-1α, which are associated with fatty acid oxidation, were
significantly increased by Fex treatment, whereas the total ACC did
not markedly differ between the two groups (Fig. 5). SREBP-1c and Fas, which were
associated with lipogenesis, did not significantly differ between
the two groups both in mRNA and protein levels (Fig. 6). These results indicated that
hepatic FXR signaling can reduce serum lipid, probably by
activating fatty acid oxidation.
 | Figure 5Effect of Fex on fatty acid oxidation
in the liver. (A) The mRNA expression levels of AMPK, CPT1-α and
PGC-1α. (B–E) The protein expression levels of p-AMPK/AMPK,
p-ACC/ACC, CPT1-α and PGC-1α were examined by western blotting and
densitometric analysis of protein expression. Data are presented as
the mean + standard error of the mean, (n=5). *P<0.05
vs. Con group. Fex, fexaramine; AMPK, AMP-activated protein kinase;
ACC, acetyl coenzyme A carboxylase; CPT1-α, carnitine palmitoyl
transferase 1α; PGC-1α, peroxisome proliferator-activated
receptor-coactivator-1α; p-, phosphorylated; Con, control. |
Discussion
A previous study demonstrated that Fex could protect
against diet-induced weight gain (11). Ryan et al (6) had demonstrated that the impact of
vertical sleeve gastrectomy on body weight and glucose tolerance
appeared lesser in FXR deficient mice, suggesting an association
between FXR and body weight. In the present study, it was
demonstrated that mice fed with Fex exhibited a significant
decrease in body weight and weight gain compared with the Con
group, and the change was independent of food intake and the
pathological effect of Fex on the liver.
Hepatic steatosis is closely associated with liver
weight and de Oliveira et al (16) previously demonstrated that a
high-fat diet can result in significant body weight gain in C57BL
mice. The current study revealed that Fex intervention for 8 weeks
could significantly reduce body weight compared with the animals of
the model group. However, there was no significant difference in
the liver weight and liver/body weight ratio between the Fex group
and the Con group animals in the present study. The db/db mouse is
a diabetes animal model associated with leptin deficiency, and does
not require a high-fat diet (17); therefore, the liver weight and
steatosis is not very clear compared with the high-fat diet-induced
model. In the present study, db/db mice were used as a diabetes
animal model, which may be the main reason why liver weight was not
changed by Fex intervention. Another reason for this result may be
the short duration of the drug intervention; it may be speculated
that a long term Fex treatment for 12 weeks might induce more
notable changes in db/db mice.
In diabetic animals, FXR expression is diminished,
suggesting a link between FXR and glucose metabolism (18). Accordingly, it has been
demonstrated that FXR activation in db/db mice could alleviate
hyperglycemia, enhancing insulin response (8). Consistently, mice with FXR
deficiency displayed peripheral insulin resistance and defects in
their glucose disposal rate (19). In the current study, Fex was used,
an FXR-specific agonist to activate FXR in the liver, and
demonstrated that activation of FXR could result in both
hypoglycemia and an improvement in insulin sensitivity. The effects
of Fex treatment, such as hypoglycemia and improved insulin
sensitivity, can be illustrated by the decreased blood glucose
levels following glucose loading in 15 min, and the AUC in the Fex
group. However, fasting blood glucose and serum insulin did not
differ significantly between the two groups; therefore, the reason
for this phenomenon requires further study to be elucidated.
Lambert et al (20) used FXR KO mice to verify the
functional role of FXR. They demonstrated that FXR KO mice
exhibited higher serum levels of BA, TG and FFAs in close
association with increased high-density lipoprotein cholesterol and
lipoprotein lipase activity. Furthermore, another study
demonstrated that FXR KO mice are characterized by elevated plasma
bile salt, TG and cholesterol levels, as well as increased hepatic
TG and cholesterol levels (21).
These findings all demonstrated that there is a close association
between FXR and lipid metabolism. In the present study, it was
demonstrated that TG levels in the serum and liver of mice gavaged
with Fex were significantly decreased, and lipid droplets in the
liver also appeared to be reduced in both number and size. Based on
these findings, it was speculated that activation of FXR exerted an
effect of lowering TG levels in both the plasma and the liver.
AMPK has a central role in controlling lipid
metabolism through modulating the downstream ACC and carnitine
palmitoyl transferase 1 (CPT1) pathway (22). The phosphorylation of ACC at Ser79
by AMPK leads to the inactivation of ACC (23), and the level of Ser79
phosphorylation is commonly used as an in-vivo
measure of AMPK signaling in response to various stimuli (24). CPT1 catalyzes the first
rate-limiting step in the β oxidation of long-chain fatty acids in
mitochondria and it can be inhibited by unphosphorylated ACC
(25). There are three isoforms
of CPT1: CPT1α, CPT1β and CPT1c. Of the three isoforms of CPT1,
only CPT1α localizes in the mitochondria (26). AMPK extracellular signaling is the
regulated kinase pathway downstream of FXR (27). AMPK activity was determined by
measuring the phosphorylation level of the AMPKa subunit at Thr172,
which reflects the activation of AMPK (28). A previous study also demonstrated
that FXR can upregulate the expression of SHP (29). In the current study, it was
demonstrated that, upon FXR activation, SHP expression was
upregulated, and the expression of p-AMPK/AMPK, ACC and CPT1α was
increased, which induces fatty acid β oxidation, thereby improving
lipid metabolism. Based on the aforementioned results, it was
hypothesized that FXR may induce fatty acid β oxidation through the
AMPK-ACC-CPT1α pathway.
PGC-1α is a transcription co-factor that interacts
with numerous transcription factors, and has been demonstrated to
be a potent activator of mitochondrial biogenesis and fatty acid
oxidation (30). Hepatic PGC-1α
protein expression and transcriptional activation of mitochondrial
biogenesis have been reported to be lower in a rodent model with
hepatic steatosis (31).
Furthermore, an elevation in hepatic PGC-1α expression results in
increased mitochondrial content and/or function, increased complete
fatty acid oxidation and TCA cycle flux, and reduced hepatic
triacylglycerol content and secretion (32). Therefore, it was further
investigated whether PGC-1α mediates the lipid-lowering effect of
FXR. The results demonstrated that PGC-1α was significantly
increased by Fex treatment, which indicated that FXR may increase
the expression of PGC-1α in the liver to exert a lipid-lowering
effect.
Previous studies have reported that activation of
FXR lowers plasma triglyceride levels by a mechanism that involves
the repression of hepatic SREBP-1c expression (33,34). However, Matsukuma et al
(35) reported that although
activation of FXR represses SREBP-1c expression, activation of FXR
does not suppress SREBP-1c target genes, including fatty acid
synthase. In the current study, it was demonstrated that the
relative protein expression of both SREBP-1c and Fas did not
significantly differ between the Fex group and the Con group, and
the inconsistencies of these studies require further
investigation.
In conclusion, the present study indicated that
db/db mice treated with Fex exhibited lower TG and FFA levels and
improved lipid metabolism, and this may be achieved via sequential
events involving the activation of FXR by Fex, and activation of
the FXR downstream signaling pathway, AMPK-ACC-CPT1α, which results
in increase of fatty acid β oxidation, ultimately achieving
lipid-lowering effects. These findings may provide a mechanistic
basis for targeting FXR as a potential therapeutic strategy to
combat dyslipidemia in diabetic patients.
Acknowledgments
Not applicable.
Funding
The present study was funded by the International
Cooperation Program of Hebei Province (grant no. 15397750D).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL collected and analyzed the majority of the data
and wrote the manuscript. HM collected and analyzed data and edited
the manuscript. AS, XY, YZ, WC and LY collected data. CW provided
input regarding data analysis and statistics. HM was involved in
study design, oversaw data collection and analysis, and wrote and
edited the manuscript.
Ethics approval and consent to
participate
All experiments involving animals were performed
according to the procedures approved by the Institutional Animal
Care and Use Committee of the Institute of Zoology, Hebei General
Hospital (Shijiazhuang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guariguata L, Whiting DR, Hambleton I,
Beagley J, Linnenkamp U and Shaw JE: Global estimates of diabetes
prevalence for 2013 and projections for 2035. Diabetes Res Clin
Pract. 103:137–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Samuel VT, Petersen KF and Shulman GI:
Lipid-induced insulin resistance: Unravelling the mechanism.
Lancet. 375:2267–2277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fabbrini E and Magkos F: Hepatic steatosis
as a marker of metabolic dysfunction. Nutrients. 7:4995–5019. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Monsénégo J, Mansouri A, Akkaoui M, Lenoir
V, Esnous C, Fauveau V, Tavernier V, Girard J and Prip-Buus C:
Enhancing liver mitochondrial fatty acid oxidation capacity in
obese mice improves insulin sensitivity independently of hepatic
steatosis. J Hepatol. 56:632–639. 2012. View Article : Google Scholar
|
|
5
|
Forman BM, Goode E, Chen J, Oro AE,
Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW,
et al: Identification of a nuclear receptor that is activated by
farnesol metabolites. Cell. 81:687–693. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ryan KK, Tremaroli V, Clemmensen C,
Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE,
Sandoval DA, Kohli R, Bäckhed F and Seeley RJ: FXR is a molecular
target for the effects of vertical sleeve gastrectomy. Nature.
509:183–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duran-Sandoval D, Cariou B, Percevault F,
Hennuyer N, Grefhorst A, van Dijk TH, Gonzalez FJ, Fruchart JC,
Kuipers F and Staels B: The farnesoid X receptor modulates hepatic
carbohydrate metabolism during the fasting-refeeding transition. J
Biol Chem. 280:29971–29979. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Lee FY, Barrera G, Lee H, Vales
C, Gonzalez FJ, Willson TM and Edwards PA: Activation of the
nuclear receptor FXR improves hyperglycemia and hyperlipidemia in
diabetic mice. Proc Natl Acad Sci USA. 103:1006–1011. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cariou B, van Harmelen K, Duran-Sandoval
D, van Dijk TH, Grefhorst A, Abdelkarim M, Caron S, Torpier G,
Fruchart JC, Gonzalez FJ, et al: The farnesoid X receptor modulates
adiposity and peripheral insulin sensitivity in mice. J Biol Chem.
281:11039–11049. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Downes M, Verdecia MA, Roecker AJ, Hughes
R, Hogenesch JB, Kast-Woelbern HR, Bowman ME, Ferrer JL, Anisfeld
AM, Edwards PA, et al: A chemical, genetic, and structural analysis
of the nuclear bile acid receptor FXR. Mol Cell. 11:1079–1092.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fang S, Suh JM, Reilly SM, Yu E, Osborn O,
Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Y, et al:
Intestinal FXR agonism promotes adipose tissue browning and reduces
obesity and insulin resistance. Nat Med. 21:159–165. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kong B, Luyendyk JP, Tawfik O and Guo GL:
Farnesoid X receptor deficiency induces nonalcoholic
steatohepatitis in low-density lipoprotein receptor-knockout mice
fed a high-fat diet. J Pharmacol Exp Ther. 328:116–122. 2009.
View Article : Google Scholar :
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Das M, Das S, Lekli I and Das DK: Caveolin
induces cardio-protection through epigenetic regulation. J Cell Mol
Med. 16:888–895. 2012. View Article : Google Scholar
|
|
15
|
Kong Q, Zhang H, Zhao T, Zhang W, Yan M,
Dong X and Li P: Tangshen formula attenuates hepatic steatosis by
inhibiting hepatic lipogenesis and augmenting fatty acid oxidation
in db/db mice. Int J Mol Med. 38:1715–1726. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Oliveira PR, da Costa CA, de Bem GF, de
Cavalho LC, de Souza MA, de Lemos Neto M, da Cunha Sousa PJ, de
Moura RS and Resende AC: Effects of an extract obtained from fruits
of Euterpe oleracea Mart. in the components of metabolic syndrome
induced in C57BL/6J mice fed a high-fat diet. J Cardiovasc
Pharmacol. 56:619–626. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bogdanov P, Corraliza L, Villena JA,
Carvalho AR, Garcia-Arumí J, Ramos D, Ruberte J, Simó R and
Hernández C: The db/db mouse: A useful model for the study of
diabetic retinal neurodegeneration. PLoS One. 9:e973022014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duran-Sandoval D, Mautino G, Martin G,
Percevault F, Barbier O, Fruchart JC, Kuipers F and Staels B:
Glucose regulates the expression of the farnesoid X receptor in
liver. Diabetes. 53:890–898. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han CY, Kim TH, Koo JH and Kim SG:
Farnesoid X receptor as a regulator of fuel consumption and
mitochondrial function. Arch Pharm Res. 39:1062–1074. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lambert G, Amar MJ, Guo G, Brewer HB Jr,
Gonzalez FJ and Sinal CJ: The farnesoid X-receptor is an essential
regulator of cholesterol homeostasis. J Biol Chem. 278:2563–2570.
2003. View Article : Google Scholar
|
|
21
|
Schonewille M, de Boer JF and Groen AK:
Bile salts in control of lipid metabolism. Curr Opin Lipidol.
27:295–301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ronnett GV, Kleman AM, Kim EK, Landree LE
and Tu Y: Fatty acid metabolism, the central nervous system, and
feeding. Obesity (Silver Spring). 14(Suppl 5): S201–S207. 2006.
View Article : Google Scholar
|
|
23
|
Hardie DG and Pan DA: Regulation of fatty
acid synthesis and oxidation by the AMP-activated protein kinase.
Biochem Soc Trans. 30:1064–1070. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carling D, Zammit VA and Hardie DG: A
common bicyclic protein kinase cascade inactivates the regulatory
enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett.
223:217–222. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ruderman NB, Saha AK and Kraegen EW:
Minireview: Malonyl CoA, AMP-activated protein kinase, and
adiposity. Endocrinology. 144:5166–5171. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sierra AY, Gratacós E, Carrasco P, Clotet
J, Ureña J, Serra D, Asins G, Hegardt FG and Casals N: CPT1c is
localized in endoplasmic reticulum of neurons and has carnitine
palmitoyl-transferase activity. J Biol Chem. 283:6878–6885. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Noh K, Kim YM, Kim YW and Kim SG:
Farnesoid X receptor activation by chenodeoxycholic acid induces
detoxifying enzymes through AMP-activated protein kinase and
extracellular signal-regulated kinase 1/2-mediated phosphorylation
of CCAAT/enhancer binding protein β. Drug Metab Dispos.
39:1451–1459. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hawley SA, Davison M, Woods A, Davies SP,
Beri RK, Carling D and Hardie DG: Characterization of the
AMP-activated protein kinase kinase from rat liver and
identification of threonine 172 as the major site at which it
phosphorylates AMP-activated protein kinase. J Biol Chem.
271:27879–27887. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gardès C, Chaput E, Staempfli A, Blum D,
Richter H and Benson GM: Differential regulation of bile acid and
cholesterol metabolism by the farnesoid X receptor in Ldlr −/− mice
versus hamsters. J Lipid Res. 54:1283–1299. 2013. View Article : Google Scholar
|
|
30
|
Holloszy JO: Regulation by exercise of
skeletal muscle content of mitochondria and GLUT4. J Physiol
Pharmacol. 59(Suppl 7): S5–S18. 2008.
|
|
31
|
Aharoni-Simon M, Hann-Obercyger M, Pen S,
Madar Z and Tirosh O: Fatty liver is associated with impaired
activity of PPARγ-coactivator 1α (PGC1α) and mitochondrial
biogenesis in mice. Lab Invest. 91:1018–1028. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morris EM, Meers GM, Booth FW, Fritsche
KL, Hardin CD, Thyfault JP and Ibdah JA: PGC-1α overexpression
results in increased hepatic fatty acid oxidation with reduced
triacylglycerol accumulation and secretion. Am J Physiol
Gastrointest Liver Physiol. 303:G979–G992. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Watanabe M, Houten SM, Wang L, Moschetta
A, Mangelsdorf DJ, Heyman RA, Moore DD and Auwerx J: Bile acids
lower triglyceride levels via a pathway involving FXR, SHP, and
SREBP-1c. J Clin Invest. 113:1408–1418. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Y, Castellani LW, Sinal CJ, Gonzalez
FJ and Edwards PA: Peroxisome proliferator-activated receptor-gamma
coactivator 1alpha (PGC-1alpha) regulates triglyceride metabolism
by activation of the nuclear receptor FXR. Genes Dev. 18:157–169.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matsukuma KE, Bennett MK, Huang J, Wang L,
Gil G and Osborne TF: Coordinated control of bile acids and
lipogenesis through FXR-dependent regulation of fatty acid
synthase. J Lipid Res. 47:2754–2761. 2006. View Article : Google Scholar : PubMed/NCBI
|