Introduction
Parkinson's disease (PD) is the second most
prevalent neurodegenerative disease worldwide and is characterized
by bradykinesia, muscle rigidity, and reduced independence
(1–3). Oxidative stress, aging, infection,
genetic and environmental factors are considered major causes of PD
(4–7). However, the pathogenesis of the
disease is not fully understood.
The endoplasmic reticulum (ER) is a major
subcellular organelle and serves an important role in various
cellular functions, including protein folding, protein trafficking
and steroid and lipid metabolism (8–10).
Multiple factors, such as oxidative stress and ischemia-reperfusion
(I/R) injury, can induce the release of inflammatory cytokines and
lead to ER stress (11). ER
stress is a widely used marker of PD and is also closely related to
other neurological disorders, such as Huntington's disease and
Alzheimer's disease (AD) (10).
Investigation of the mechanism underlying ER stress in PD may lead
to the development of new therapeutic strategies.
Apelin, a neuropeptide that acts as a ligand for the
orphan G protein-coupled apelin receptor (APJ), has been extracted
from bovine stomach tissue and undergoes cleavage to generate
apelin-13, -17 and -36 (12–14). Apelin peptides are associated with
neuroprotection and cytoprotection. Apelin-13 reduces the cerebral
infarct volume in a middle cerebral artery occlusion rat model by
downregulating expression of inflammatory factors (15). In addition, the activity of
apelin-13 is higher compared with apelin-17 and -36 (14–16). Apelin-36, a long apelin peptide,
protects against ischemic brain injury by decreasing the levels of
caspase-3 and BCL2 associated X (Bax), which are well-established
apoptotic markers, thereby reducing neurological deficits (17). Furthermore, treatment with
apelin-13 markedly decreases brain edema and the total infarct
volume and suppresses apoptosis by reducing caspase-3 activation
(18). Our previous study has
demonstrated that apelin-13 protects against cerebral I/R injury
(15), consistent with other
literature. However, the mechanism by which apelin protects neurons
against 1-methyl-4-phenylpyridine (MPP+)-induced
apoptosis in a cellular model of PD remain unclear. The present
study investigated the effects of apelin-13 on
MPP+-treated SH-SY5Y cells, a cellular model of PD, and
the underlying molecular mechanism.
Materials and methods
Reagents and cell culture
Human apelin-13 [half maximal effective
concentration (EC50)=0.37 nM (19)] was obtained from Phoenix
Pharmaceuticals (St. Joseph, MO, USA). Its amino acid sequence is
Gln-Arg-Pro-Arg-Leu-Ser-His-L ys-Gly-Pro-Met-Pro-Phe (20). MPP+ iodide and the Cell
Counting Kit-8 (CCK-8) were obtained from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). Antibodies against p38 mitogen-activated
protein kinase (MAPK; cat. no. 8690), phosphorylated (phospho)-p38
MAPK (cat. no. 4511), extracellular signal-regulated kinase (ERK)
1/2 (cat. no. 4696), phospho-ERK1/2 (cat. no. 9101),
glucose-regulated protein 78 (GRP78, also known as BiP; cat. no.
3183), C/EBP homologous protein (CHOP; cat. no. 2895), and
caspase-12 (cat. no. 2202) were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Anti-β-actin primary antibody
(cat. no. TA-09), polyclonal goat anti-mouse (cat. no. ZB-2305) and
anti-rabbit secondary antibodies (cat. no. ZB-2301) were obtained
from OriGene Technologies, Inc. (Beijing, China) (21). The SH-SY5Y cell line was obtained
from the American Type Culture Collection (Manassas, VA, USA)
(22–24) and routinely cultured in DMEM
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and 100 U/ml
penicillin/streptomycin in the presence of 5% CO2 at
37°C.
Real-time cell analysis (RTCA)
Real-time cell analysis (RTCA) (25) was performed to study the effects
of MPP+ on SH-SY5Y cells. Each well of an E-Plate 16 was
incubated with 100 μl of culture media for 30 min at 37°C to
ensure substrate equilibrium; this background step was crucial.
Next, SH-SY5Y cells were digested with 0.25% trypsin/0.02% EDTA
solution, and 100 μl of culture media containing
5×104 cells was added to each well of the E-Plate 16 and
incubated overnight at 37°C. Finally, cells were treated with 0,
100, 250, 500, 750 or 1,000 μM MPP+ with or
without 100 nM apelin-13 and monitored for 36 h.
CCK-8 assay
The CCK-8 assay was used to measure the viability of
SH-SY5Y cells treated with MPP+ with or without
apelin-13. SH-SY5Y cells were digested as aforementioned and then
transferred to a 96-well transparent microplate in 100 μl of
DMEM containing 10% FBS (5×104 cells/well). Following
incubation overnight, the medium was replaced by fresh complete
medium containing the indicated drugs and cells were cultured for
~24 h. Thereafter, 10 μl of 5 mg/ml CCK-8 solution was added
to each well and plates were incubated in the presence of 5%
CO2 at 37°C for 0.5–4 h to allow dehydrogenases to
reduce WST-8. Absorbance of each well at 450 nm was measured using
a microplate reader. Cell viability was calculated using the
following formula: Cell viability (%)=(A–B)/(A′–B) ×100, where A is
the average absorbance of wells containing the indicated drugs,
SH-SY5Y cells, and CCK-8 solution; A′ is the average absorbance of
wells containing SH-SY5Y cells and CCK-8 solution; and B is the
average absorbance of wells containing medium and CCK-8
solution.
Flow cytometry
SH-SY5Y cells (5×105 cells/well) were
collected by centrifugation (800 × g for 5 min at room
temperature), treated with MPP+ for 24 h, and washed
with PBS. Thereafter, 500 μl of Annexin V binding buffer
(Biouniquer Technology, Beijing, China) was added to each cell
suspension followed by 5 μl of Annexin V-fluorescein
isothiocyanate (FITC) and 5 μl of propidium iodide for 5
min. A total of 10,000 cells were analyzed per sample by flow
cytometry, which was performed using a BD FACSCalibur flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA), using an
excitation wavelength of 488 nm and an emission wavelength of 530
nm. Data are representative of at least three independent
experiments. The results were analyzed using BD CellQuest Pro
software (version 5.1; BD Biosciences).
Western blotting
SH-SY5Y cells were transferred to 6-well plates and
incubated overnight in the presence of 5% CO2 at 37°C.
Thereafter, cells were treated with 0, 100, 250, 500, 750 or 1,000
μM MPP+ for 24 h with or without 100 nM
apelin-13, washed with ice-cold PBS, and lysed in RIPA buffer.
Cells were collected in clean 1.5 ml centrifuge tubes and cleared
by centrifugation at 1,200 × g for 35 min at 4°C. The protein
concentration was determined by the bicinchoninic acid assay, using
bovine serum albumin as a standard. Equal amounts (35 μg) of
cell extracts were analyzed by 10% SDS-PAGE. Separated proteins
were transferred to polyvinylidene fluoride membranes at 300 mA for
40–80 min. Membranes were blocked with TBS/0.1% Tween-20 (TBST)
containing 5% milk at room temperature for 1 h, rinsed thrice with
TBST for 10 min, and incubated overnight at 4°C with rabbit
anti-phospho-p38 (1:1,000), rabbit anti-phospho-ERK1/2 (1:2,000),
rabbit anti-GRP78 (1:1,000), mouse anti-CHOP (1:1,000), and rabbit
anti-caspase-12 (1:1,000) antibodies. Thereafter, membranes were
washed with TBST and incubated with appropriate secondary
antibodies [goat anti-rabbit-IgG (1:5,000) for phospho-ERK1/2,
phospho-p38, GRP78, and caspase-12, and goat anti-mouse-IgG
(1:5,000) for CHOP] at room temperature for 1 h. Protein bands were
visualized using an enhanced chemiluminescence detection system,
and their intensities were measured by densitometry, which was
analyzed using ImageJ software (version 1.46; National Institutes
of Health, Bethesda, MD, USA). To analyze differences in protein
loading between the lanes, blots initially probed with
anti-phospho-p38, anti-phospho-ERK1/2, and anti-GRP78 antibodies
were stripped and re-probed with mouse anti-p38 (1:1,000),
anti-ERK1/2 (1:2,000), and anti-actin (1:1,000) antibodies.
Subsequently, blots were incubated with goat anti-mouse-IgG
(1:5,000) antibodies at room temperature for 1 h and then
visualized as aforementioned.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Data are
presented as mean ± standard deviation of at least three
independent experiments. The Student's paired t-test was used to
assess differences between two groups. One-way analysis of variance
followed by Tukey's test was used to assess differences between
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Apelin-13 attenuates the
MPP+-induced decrease in the survival index of SH-SY5Y
cells
SH-SY5Y cells were incubated with 0, 100, 250, 500,
750, and 1,000 μM MPP+ for 36 h, and their growth
was monitored by RTCA. The results demonstrated that the cell index
of SH-SY5Y cells without MPP+ treatment (basal) was
gradually increased to ~2.5 at 36 h, while the cell index of
SH-SY5Y cells with MPP+ treatment for 36 h were ~1.25,
1.0, 0.35, 0.1 and 0.02 for 100, 250, 500, 750 and 1,000 μM
MPP+, respectively (Fig.
1A). The results indicated that MPP+ treatment
decreased the survival index of SH-SY5Y cells in a dose-dependent
manner. Next, the neuroprotective effect of apelin-13 was
investigated in SH-SY5Y cells treated with 500 μM
MPP+. Pretreatment with different concentrations of
apelin-13 (0.1, 1, 10, 100 and 1,000 nM) for 2 h dramatically
increased the survival of MPP+-treated SH-SY5Y cells in
a dose-dependent manner. The cell index of SH-SY5Y cells without
MPP+ or apelin-13 (basal) for 36 h was increased to
~4.1, while the cell index of SH-SY5Y cells with 100 nM apelin-13
treatment for 36 h was markedly increased to ~5.2 (Fig. 1B). The cell index of SH-SY5Y cells
with 500 μM MPP+ treatment for 36 h was reduced
to ~0.8 (Fig. 1B). However, the
cell indexes of SH-SY5Y cells with the pretreatment of 0.1, 1, 10,
100, and 1,000 nM apelin-13 for 2 h and then co-cultured with 500
μM MPP+ for 36 h were ~1.0, 1.49, 1.75, 2.1, and
2.52, respectively (Fig. 1B).
These data indicate that apelin-13 protected SH-SY5Y cells against
MPP+-induced neurotoxicity.
 | Figure 1Effects of different concentrations
of MPP+ and apelin-13 on the survival index of SH-SY5Y
cells determined by RTCA. (A) SH-SY5Y cells were grown for 24 h and
then treated with 0, 100, 250, 500, 750 and 1,000 μM
MPP+ for 36 h. The survival index was determined by
RTCA. (B) Cells were pretreated with 0, 0.1, 1, 10, 100 and 1,000
nM apelin-13 for 2 h and then treated with 500 μM
MPP+ for 36 h. MPP+,
1-methyl-4-phenylpyridine; RTCA, real-time cell analysis. |
Apelin-13 attenuates the
MPP+-induced decrease in the viability of SH-SY5Y
cells
To determine the optimal concentration of
MPP+ for subsequent experiments, SH-SY5Y cells were
treated with 0-1,000 μM MPP+ and their viability
was measured using the CCK-8 assay. MPP+ treatment
markedly decreased the viability of SH-SY5Y cells in a
dose-dependent manner, and treatment with 500 μM
MPP+ killed ~50% of the cells (Fig. 2A and B). Thus, the dose of 500
μM MPP+ was selected as the optimal concentration
for subsequent experiments. To evaluate the neuroprotective effect
of apelin-13, SH-SY5Y cells were pretreated with 0-1,000 nM
apelin-13 for 2 h, then treated with 500 μM MPP+,
and finally evaluated for viability with the CCK-8 assay. Apelin-13
dose-dependently attenuated the MPP+-induced decrease in
the viability of SH-SY5Y cells, and this effect plateaued at 100 nM
apelin-13 (Fig. 2C). Thus, the
dose of 100 nM apelin-13 was selected as the optimal concentration
for further experiments.
Next, the effect of pretreatment with 100 nM
apelin-13 was evaluated on SH-SY5Y cells treated with 500 μM
MPP+. Apelin-13 significantly protected SH-SY5Y cells
against MPP+-induced neurotoxicity compared with the
control (Fig. 2D). The ratio of
viable cells was 16% higher in the group pretreated with apelin-13
compared with the group treated with MPP+ alone
(P<0.05; Fig. 2D). These data
indicate that apelin-13 protected SH-SY5Y cells against
MPP+-induced neurotoxicity.
Apelin-13 inhibits
MPP+-induced apoptosis of SH-SY5Y cells
To further explore the effect of apelin-13 on
neuronal cell survival, apoptosis of SH-SY5Y cells was evaluated by
flow cytometry. The % of apoptotic SH-SY5Y cells was ~26% higher in
the group treated with 500 μM MPP+ compared with
the control group (P<0.001; Fig.
3), while only ~23% of SH-SY5Y cells were apoptotic in the
group pretreated with 100 nM apelin-13 (P<0.01 vs.
MPP+-treated alone group; Fig. 3).
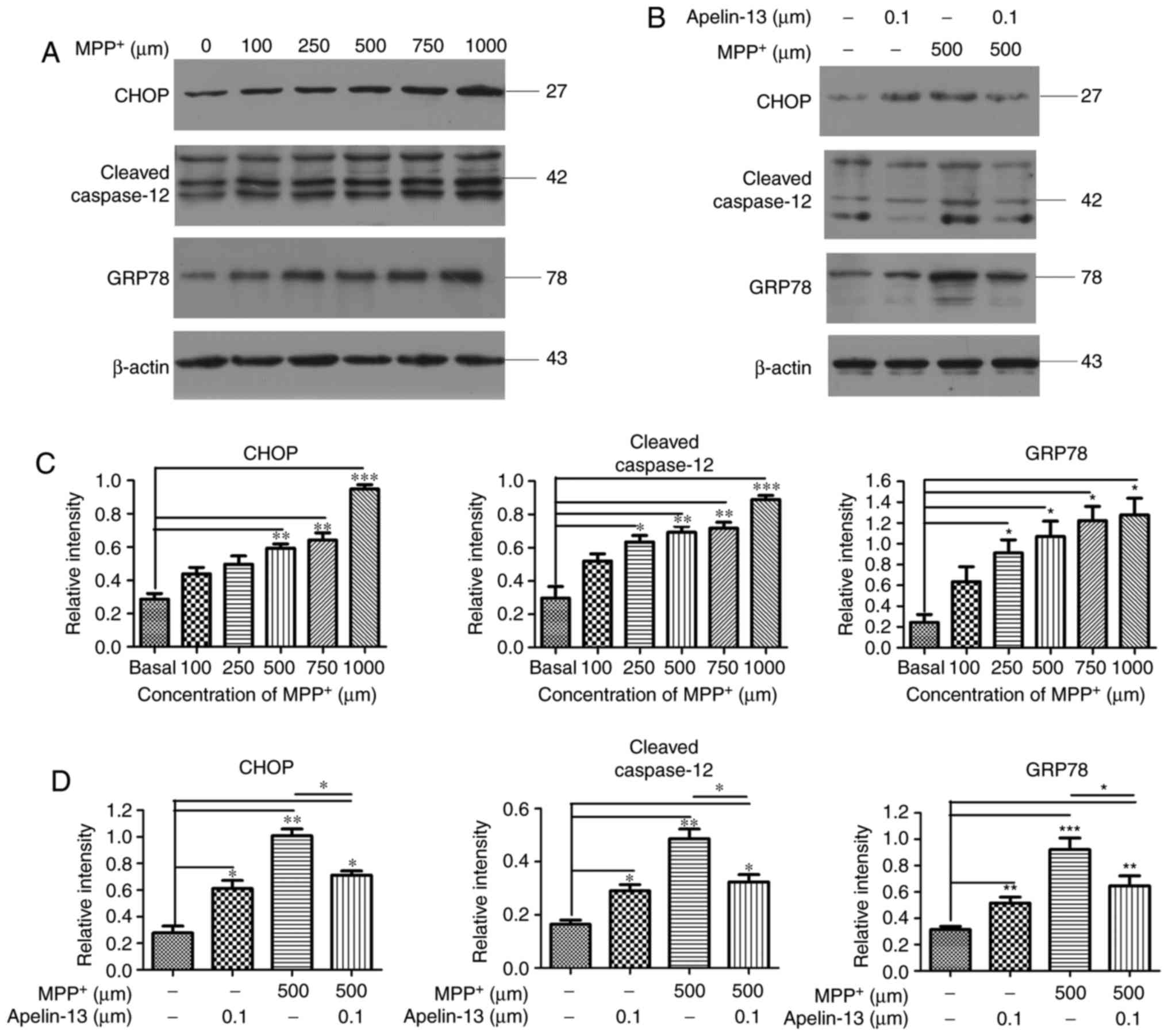
Apelin-13 attenuates GRP78, CHOP, and
cleaved caspase-12 upregulation in MPP+-treated SH-SY5Y
cells
GRP78 has a key role in multiple neurodegenerative
diseases, including AD and PD. To investigate whether GRP78 is
involved in the neurotoxic effect of MPP+, SH-SY5Y cells
were treated with various concentrations of MPP+ for 24
h (Fig. 4A). Western blot
analysis demonstrated that treatment with 100, 250, 500, 750 and
1,000 μM MPP+ increased the expression levels of
GRP78 in a dose-dependent manner (0.64±0.13, 0.91±0.12, 1.07±0.14,
1.22±0.13, and 1.27±0.16, respectively; Fig. 4C). To further investigate the
molecular mechanism by which apelin-13 elicits neuroprotective
effects in SH-SY5Y cells, cells were pretreated with 100 nM
apelin-13 for 2 h and then exposed to 500 μM MPP+
for 24 h (Fig. 4B). Pretreatment
with apelin-13 attenuated the MPP+-induced increase in
GRP78 expression (Fig. 4D).
Activated GRP78 increases the level of CHOP, which
causes ER stress and promotes apoptosis. To determine whether CHOP
is involved in the neuroprotective effect of apelin-13, the
expression levels of the CHOP protein were examined. Treatment with
100, 250, 500, 750 and 1,000 μM MPP+ increased
CHOP expression in a dose-dependent manner (0.44±0.03, 0.49±0.04,
0.59±0.03, 0.64±0.03, and 0.95±0.04, respectively; Fig. 4C). Pretreatment with apelin-13
attenuated the increase in CHOP expression induced by treatment
with 500 μM MPP+ (Fig. 4D).
Caspase-12 is activated during ER stress-induced
apoptosis, and it has an important role in neurodegenerative
diseases. To investigate whether apelin-13 treatment affected
caspase-12 activity, the expression levels of cleaved caspase-12
were examined by western blotting. Treatment with 100, 250, 500,
750 and 1,000 μM MPP+ increased cleaved
caspase-12 expression in a dose-dependent manner (0.52±0.03,
0.63±0.03, 0.69±0.04, 0.72±0.05 and 0.89±0.06, respectively;
Fig. 4C). Pretreatment with
apelin-13 attenuated the increase in cleaved caspase-12 expression
induced by treatment with 500 μM MPP+ (Fig. 4D). These results indicate that
apelin-13 prevented MPP+-induced apoptosis of SH-SY5Y
cells by reducing the expression of GRP78, CHOP and cleaved
caspase-12.
Apelin-13 upregulates phospho-ERK1/2
expression in MPP+-treated SH-SY5Y cells
MAPK family members p38 and ERK1/2 have important
roles in maintaining normal cellular functions. To investigate the
mechanism by which apelin-13 inhibited MPP+-induced
apoptosis of SH-SY5Y cells, immunoblotting for ERK1/2 and p38 was
performed. Treatment with 100, 250, 500, 750 and 1,000 μM
MPP+ increased expression of phospho-ERK1/2 (0.29±0.03,
0.49±0.03, 0.70±0.02, 0.73±0.04, and 0.80±0.05, respectively;
Fig. 5A) and phospho-p38
(0.7±0.02, 0.83±0.07, 0.91±0.05, 0.97±0.10, and 1.19±0.06,
respectively; Fig. 5A) in a
dose-dependent manner. Pretreatment with apelin-13 significantly
increased expression of phospho-ERK1/2 (Fig. 5B), but did not affect the
expression of phospho-p38 (Fig.
5B).
Discussion
Since apelin was discovered in 1998 (19), a number of studies have
demonstrated that the apelin/APJ signaling pathway has a crucial
role in the function and dysfunction of the cardiovascular system
(heart failure and hypertension), the urinary tract system (fluid
balance, diuresis, and vasopressin synthesis), and the
gastrointestinal and endocrine systems (obesity and insulin
resistance) (17,26,27). APJ and its endogenous ligand
apelin are widely distributed in numerous organs and tissues,
including the lung, adipose tissue, endothelium, heart, stomach,
pancreatic islands, and central nervous system (28). Recent studies have demonstrated
that apelin is neuroprotective in various neurological diseases,
such as insomnia, AD and PD; however, the underlying molecular
mechanism remains unknown (14).
Apelin-13, -17, -36, and -77 protect hippocampal neurons against
excitotoxic injury by inducing phosphorylation of AKT
serine/threonine (Akt) kinases and Raf-1 proto-oncogene/ERK1/2. In
addition, apelin protects hippocampal neurons against excitotoxic
injury induced by the N-methyl-D-aspartic acid (NMDA) receptor
(29).
Recent studies have demonstrated that, in addition
to its protective effect in hippocampal neurons, apelin may also
directly elicit protective effects in cultured mouse cortical
neurons by reducing NMDA-induced intracellular Ca2+
accumulation, cytochrome c release, mitochondrial
depolarization, caspase-3 activation, and reactive oxygen species
generation (30). Furthermore,
apelin protects human vascular smooth muscle cells and cultured rat
bone marrow mesenchymal stem cells against apoptosis by inhibiting
the phosphoinositide 3-kinase (PI3K)/Akt and MAPK/ERK1/2 signaling
pathways (31,32). In addition, apelin-13 and -36
protect against myocardial I/R injury by activating the reperfusion
injury salvage kinase pathway, which involves PI3K/Akt and p44/42
MAPK (33). These neuroprotective
effects of apelin are consistent with the present findings. In the
present study, the growth of control SH-SY5Y cells and cells
treated with MPP+ alone, or apelin-13 plus
MPP+ was monitored over 36 h by RTCA. Apelin-13
significantly attenuated MPP+-induced neurotoxicity and
improved the cell survival index. The CCK-8 assay demonstrated that
apelin-13 markedly increased the viability of
MPP+-treated SH-SY5Y cells, while flow cytometry
revealed that apelin-13 reduced the % of apoptotic SH-SY5Y cells
following MPP treatment. These results suggest that apelin-13
elicited neuroprotective effects in human SH-SY5Y cells, an in
vitro model of PD.
GRP78, a Hsp70 family ATPase, was discovered in
chicken embryo fibroblasts in 1997 (34–36). This protein is an important
molecular chaperone in the ER and is involved in multiple
processes, including assembly, folding, and trans-location of
nascent polypeptides, calcium homeostasis, and targeting of
misfolded proteins to the ER-associated protein degradation pathway
(37–39). A series of studies indicate that
GRP78 is not only involved in various aspects of tumor development
(such as cancer cell survival, proliferation, and metastasis,
angiogenesis, and chemoresistance), but also in many age-related
neurodegenerative disorders, including amyotrophic lateral
sclerosis, prion-related disorders, ischemic stroke, PD and AD
(40,41). Expression of GRP78 is
significantly higher in 3×Tg-AD mice, a triple transgenic AD model,
compared with control mice (42).
In addition, GRP78 is overexpressed in post-mortem human AD samples
and in in vitro models of AD, and ER stress increases its
expression in several tissues by ~3-fold (43,44). Recent studies indicate that
expression of GRP78 is increased and that this protein interacts
with α-synuclein in glucose-deprived SH-SY5Y cells, and similar
phenomena are observed in HEK293 cells transfected with SYN120
(amino acids 1–120 of α-synuclein) (45). Furthermore, there is evidence that
pretreatment with apelin reduces ER stress in diabetic Akita mice
by decreasing expression of GRP78 and other proteins, including
endoplasmic reticulum to nucleus signaling 1 (ERN1, also known as
IRE1α), protein kinase R-like endoplasmic reticulum kinase (PERK),
heat shock protein (Hsp) 70, and calnexin (46). CHOP and caspase-12 also have
important roles in ER stress. Apelin was reported to significantly
attenuate ER stress-induced expression of CHOP, cleaved caspase-12,
and GRP78 in the heart and thereby protect cells against I/R
injury-induced apoptosis (47). A
recent study indicates that apelin-36 protects neurons against
cerebral I/R injury by attenuating ER stress-induced elevations in
GRP78 and CHOP expression in rats following ischemic stroke
(48). There is evidence that
telmisartan elicits neuroprotective effects by suppressing ER
stress-induced increases in GRP78 and cleaved caspase-12 expression
in a rat model of PD (49).
However, the effect of apelin-13 on ER stress-induced upregulation
in GRP78, CHOP, and cleaved caspase-12 in a cellular model of PD
had not been explored to date. In the present study,
MPP+ treatment increased the GRP78, CHOP and cleaved
caspase-12 expression levels in human SH-SY5Y cells in a
dose-dependent manner, and this effect was attenuated by
pretreatment with apelin-13, suggesting that apelin-13 prevented ER
stress. As previously described, increased expression of GRP78,
CHOP, and cleaved caspase-12 is closely related to apoptosis
(47–49). Apelin-13 may mediate an
anti-apoptotic effect in the cellular model of PD by inhibiting ER
stress (Fig. 6). This is
consistent with the present findings that pretreatment with
apelin-13 markedly inhibited MPP+-induced apoptosis of
SH-SY5Y cells.
ERK1 and ERK2, which have molecular weights of 44
and 42 kDa respectively, transmit signals from the surface
receptors to the nucleus. Activated ERK1/2 serve important roles in
cell proliferation, cell differentiation, and maintenance of cell
morphology via modulating their targets, including ELK1, activating
transcription factor (ATF) and activator protein (AP)-1. MAPKs also
have important roles in cell proliferation (50,51). Several experiments demonstrate
that ERK1/2 are activated in APJ-transfected cells, including
HEK293T cells, mouse enteroendocrine cells, and CHO cells,
following treatment with apelin (26,52,53). However, apelin was reported to
promote the proliferation of human osteoblasts by activating the
Akt signaling pathway, not the MAPK pathway (p38, c-Jun N-terminal
kinase, and ERK1/2) (54). In the
present study, although MPP+ treatment dose-dependently
increased phosphorylation of ERK1/2 and p38 in SH-SY5Y cells,
apelin-13 significantly increased expression of pERK1/2, but did
not affect phopsho-p38. These results are consistent with our
previous finding that apelin-13 activates ERK1/2, but not
phopsho-p38, via coupling of the apelin receptor to Gi2-protein
(20). A recent study
demonstrated that apelin-13 protects the brain against I/R injury
by activating the ERK1/2 signaling pathway, and that PD98059, an
ERK1/2 inhibitor, markedly attenuates the elevation of BCL2
apoptosis regulator (Bcl-2) expression in the brain (55). These results indicate that
apelin-13 protects neurons against I/R injury-induced apoptosis
partly by activating the ERK1/2 signaling pathway and thereby
increasing Bcl-2 expression (55). In the present study, apelin-13 was
demonstrated to significantly upregulate phospho-pERK12 expression
and to inhibit GRP78/CHOP/cleaved-caspase-12 activation, resulting
in a decrease of MPP+-induced neuronal apoptosis in
SH-SY5Y cells. Expression of phosho-ERK1/2 is activated by
Gi2-protein and upregulated by β-arrestin protein, which may
explain why apelin-13 only increased the expression of
phospho-ERK1/2 and not phospho-p38. Apelin-13 may elicit
neuroprotective effects in the cellular model of PD via this
mechanism (Fig. 6), although
further studies will be required to confirm this.
In summary, the present study demonstrated that
apelin-13 protected neurons against ER stress-associated apoptosis
in an in vitro model of PD by downregulating GRP78, CHOP and
cleaved caspase-12 and by upregulating phospho-ERK1/2. However, the
precise mechanism needs to be further elucidated. Further
experiments, investigating the neuroprotective effects of apelin-13
in animal models of PD, are underway.
Acknowledgments
Not applicable.
Abbreviations:
|
AD
|
Alzheimer's disease
|
|
CC K-8
|
Cell Counting Kit-8
|
|
ER
|
endoplasmic reticulum
|
|
ERK1/2
|
extracellular signal-regulated kinase
1/2
|
|
GRP78
|
glucose-regulated protein 78
|
|
I/R
|
ischemia-reperfusion
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MPP+
|
1-methyl-4-phenylpyridine
|
|
NMDA
|
N-methyl-D- aspartic acid
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
Akt
|
AKT serine/threonine kinase 1
|
|
PD
|
Parkinson's disease
|
|
RTCA
|
real-time cell analysis
|
Funding
This work was supported by the National Nature
Science Foundation of China (grant no. 81671276), the Shandong
Province Natural Science Foundation (grant nos. ZR2014HL040,
ZR2017LC009 and ZR2018PC011), the Science and Technology Plan
Project of Shandong Higher Education Institutions (grant nos.
J17KA138 and J15LE19), the Supporting Found for Teachers' Research
of Jining Medical University (grant no. JY2017KJ035), and the
Science and Technology Planning Project of Shandong Province
University (grant no. 2016-56-85).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
YJ, BC and BB designed the experiments. YJ, HL, BJ,
ZW and CY performed the experiments. YJ, BJ, CW and YP analyzed the
data. YJ, HL, BC and BB wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singh A and Sen D: MicroRNAs in
Parkinson's disease. Exp Brain Res. 235:2359–2374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kader M, Ullén S, Iwarsson S, Odin P and
Nilsson MH: Factors contributing to perceived walking difficulties
in people with Parkinson's disease. J Parkinsons Dis. 7:397–407.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davie CA: A review of Parkinson's disease.
Br Med Bull. 86:109–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou C, Huang Y and Przedborski S:
Oxidative stress in Parkinson's disease: A mechanism of pathogenic
and therapeutic significance. Ann N Y Acad Sci. 1147:93–104. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim CY and Alcalay RN: Genetic forms of
Parkinson's disease. Semin Neurol. 37:135–146. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fengler S, Liepelt-Scarfone I, Brockmann
K, Schäffer E, Berg D and Kalbe E: Cognitive changes in prodromal
Parkinson's disease: A review. Mov Disord. 32:1655–1666. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Collier TJ, Kanaan NM and Kordower JH:
Aging and Parkinson's disease: Different sides of the same coin?
Mov Disord. 32:983–990. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sitia R and Braakman I: Quality control in
the endoplasmic reticulum protein factory. Nature. 426:891–894.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Claudio N, Dalet A, Gatti E and Pierre P:
Mapping the crossroads of immune activation and cellular stress
response pathways. EMBO J. 32:1214–1224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang HY, Wang ZG, Lu XH, Kong XX, Wu FZ,
Lin L, Tan X, Ye LB and Xiao J: Endoplasmic reticulum stress:
Relevance and therapeutics in central nervous system diseases. Mol
Neurobiol. 51:1343–1352. 2015. View Article : Google Scholar
|
|
11
|
Hotamisligil GS: Endoplasmic reticulum
stress and the inflammatory basis of metabolic disease. Cell.
140:900–917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
O'Carroll AM, Selby TL, Palkovits M and
Lolait SJ: Distribution of mRNA encoding B78/apj, the rat homologue
of the human APJ receptor, and its endogenous ligand apelin in
brain and peripheral tissues. Biochim Biophys Acta. 1492:72–80.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hosoya M, Kawamata Y, Fukusumi S, Fujii R,
Habata Y, Hinuma S, Kitada C, Honda S, Kurokawa T, Onda H, et al:
Molecular and functional characteristics of APJ. Tissue
distribution of mRNA and interaction with the endogenous ligand
apelin. J Biol Chem. 275:21061–21067. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheng B, Chen J, Bai B and Xin Q:
Neuroprotection of apelin and its signaling pathway. Peptides.
37:171–173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xin Q, Cheng B, Pan Y, Liu H, Yang C, Chen
J and Bai B: Neuroprotective effects of apelin-13 on experimental
ischemic stroke through suppression of inflammation. Peptides.
63:55–62. 2015. View Article : Google Scholar
|
|
16
|
Masri B, Morin N, Pedebernade L,
Knibiehler B and Audigier Y: The apelin receptor is coupled to Gi1
or Gi2 protein and is differentially desensitized by apelin
fragments. J Biol Chem. 281:18317–18326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gu Q, Zhai L, Feng X, Chen J, Miao Z, Ren
L, Qian X, Yu J, Li Y, Xu X and Liu CF: Apelin-36, a potent
peptide, protects against ischemic brain injury by activating the
PI3K/Akt pathway. Neurochem Int. 63:535–540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khaksari M, Aboutaleb N, Nasirinezhad F,
Vakili A and Madjd Z: Apelin-13 protects the brain against ischemic
reperfusion injury and cerebral edema in a transient model of focal
cerebral ischemia. J Mol Neurosci. 48:201–208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tatemoto K, Hosoya M, Habata Y, Fujii R,
Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, et
al: Isolation and characterization of a novel endogenous peptide
ligand for the human APJ receptor. Biochem Biophys Res Commun.
251:471–476. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bai B, Tang J, Liu H, Chen J, Li Y and
Song W: Apelin-13 induces ERK1/2 but not p38 MAPK activation
through coupling of the human apelin receptor to the Gi2 pathway.
Acta Biochim Biophys Sin (Shanghai). 40:311–318. 2008. View Article : Google Scholar
|
|
21
|
Wang C, Xu C, Liu M, Pan Y, Bai B and Chen
J: C-terminus of OX2R significantly affects downstream signaling
pathways. Mol Med. 16:159–166. 2017.
|
|
22
|
Zhong J, Yu H, Huang C, Zhong Q, Chen Y,
Xie J, Zhou Z, Xu J and Wang H: Inhibition of phosphodiesterase 4
by FCPR16 protects SH-SY5Y cells against MPP+-induced
decline of mitochondrial membrane potential and oxidative stress.
Redox Biol. 16:47–58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu JYD, Su P, Barber JEM, Nash JE, Le AD,
Liu F and Wong AHC: The neuroprotective effect of nicotine in
Parkinson's disease models is associated with inhibiting PARP-1 and
caspase-3 cleavage. Peer J. 5:e39332017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu L, Xu H, Cao L, Li T, Li R, Feng Y,
Chen J and Ma J: Salidroside protects against
MPP+-induced neuronal injury through DJ-1-Nrf2
antioxidant pathway. Evid Based Complement Alternat Med.
2017:53985422017.
|
|
25
|
Cai X, Bai B, Zhang R, Wang C and Chen J:
Apelin rerptor homodimer-oligomer revealed by single-molecule
imaging and novel Gprotein-dependent signaling. Sci Rep.
7:403352017. View Article : Google Scholar
|
|
26
|
Chen X, Bai B, Tian Y, Du H and Chen J:
Identification of serine 348 on the apelin receptor as a novel
regulatory phosphorylation site in apelin-13-induced G
protein-independent biased signaling. J Biol Chem. 289:31173–31187.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu H, He L, Li L and Chen L: Apelin/APJ
system as a therapeutic target in diabetes and its complications.
Mol Genet Metab. 119:20–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Susaki E, Wang G, Cao G, Wang HQ,
Englander EW and Greeley GH Jr: Apelin cells in the rat stomach.
Regul Pept. 129:37–41. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
O'Donnell LA, Agrawal A, Sabnekar P,
Dichter MA, Lynch DR and Kolson DL: Apelin, an endogenous neuronal
peptide, protects hippocampal neurons against excitotoxic injury. J
Neurochem. 102:1905–1917. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zeng XJ, Yu SP, Zhang L and Wei L:
Neuroprotective effect of the endogenous neural peptide apelin in
cultured mouse cortical neurons. Exp Cell Res. 316:1773–1783. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cui RR, Mao DA, Yi L, Wang C, Zhang XX,
Xie H, Wu XP, Liao XB, Zhou H, Meng JC, et al: Apelin suppresses
apoptosis of human vascular smooth muscle cells via APJ/PI3-K/Akt
signaling pathways. Amino Acids. 39:1193–1200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zeng X, Yu SP, Taylor T, Ogle M and Wei L:
Protective effect of apelin on cultured rat bone marrow mesenchymal
stem cells against apoptosis. Stem Cell Res. 8:357–367. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Simpkin JC, Yellon DM, Davidson SM, Lim
SY, Wynne AM and Smith CC: Apelin-13 and apelin-36 exhibit direct
cardioprotective activity against ischemia-reperfusion injury.
Basic Res Cardiol. 102:518–528. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shiu RP, Pouyssegur J and Pastan I:
Glucose depletion accounts for the induction of two
transformation-sensitive membrane proteinsin Rous sarcoma
virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci USA.
74:3840–3844. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoshida H, Haze K, Yanagi H, Yura T and
Mori K: Identification of the cisacting endoplasmic reticulum
stress response element responsible for transcriptional induction
of mammalian glucose-regulated proteins. Involvement of basic
leucine zipper transcription factors. J Biol Chem. 273:33741–33749.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Haas IG and Wabl M: Immunoglobulin heavy
chain binding protein. Nature. 306:387–389. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hendershot LM: The ER function BiP is a
master regulator of ER function. Mt Sinai J Med. 71:289–297.
2004.PubMed/NCBI
|
|
38
|
Otero JH, Lizak B and Hendershot LM: Life
and death of a BiP substrate. Semin Cell Dev Biol. 21:472–478.
2010. View Article : Google Scholar :
|
|
39
|
Gardner BM, Pincus D, Gotthardt K,
Gallagher CM and Walter P: Endoplasmic reticulum stress sensing in
the unfolded protein response. Cold Spring Harb Perspect Biol.
5:a0131692013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pfaffenbach KT and Lee AS: The critical
role of GRP78 in physiological and pathologic stress. Curr Opin
Cell Biol. 23:150–156. 2011. View Article : Google Scholar
|
|
41
|
Casas C: GRP78 at the centre of the stage
in cancer and neuroprotection. Front Neurosci. 11:1772017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Soejima N, Ohyagi Y, Nakamura N, Himeno E,
Iinuma KM, Sakae N, Yamasaki R, Tabira T, Murakami K, Irie K, et
al: Intracellular accumulation of toxic turn amyloid-β is
associated with endoplasmic reticulum stress in Alzheimer's
disease. Curr Alzheimer Res. 10:11–20. 2013.
|
|
43
|
Galán M, Kassan M, Kadowitz PJ, Trebak M,
Belmadani S and Matrougui K: Mechanism of endoplasmic reticulum
stress-induced vascular endothelial dysfunction. Biochim Biophys
Acta Mol Cell Res. 1843:1063–1075. 2014. View Article : Google Scholar
|
|
44
|
Li C, Wang L, Huang K and Zheng L:
Endoplasmic reticulum stress in retinal vascular degeneration:
Protective role of resveratrol. Invest Ophthalmol Vis Sci.
53:3241–3249. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bellucci A, Navarria L, Zaltieri M,
Falarti E, Bodei S, Sigala S, Battistin L, Spillantini M, Missale C
and Spano P: Induction of the unfolded protein response by
α-synuclein in experimental models of Parkinson's disease. J
Neurochem. 116:588–605. 2011. View Article : Google Scholar
|
|
46
|
Chen H, Zheng C, Zhang X, Li J, Li J,
Zheng L and Huang K: Apelin alleviates diabetes-associated
endoplasmic reticulum stress in the pancreas of Akita mice.
Peptides. 32:1634–1639. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tao J, Zhu W, Li Y, Xin P, Li J, Liu M, Li
J, Redington AN and Wei M: Apelin-13 protects the heart against
ischemia-reperfusion injury through inhibition of ER-dependent
apoptotic pathways in a time-dependent fashion. Am J Physiol Heart
Circ Physiol. 301:H1471–H1486. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Qiu J, Wang X, Wu F, Wan L, Cheng B, Wu Y
and Bai B: Low dose of Apelin-36 attenuates ER stress-associated
apoptosis in rats with ischemic stroke. Front Neurol. 8:5562017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tong Q, Wu L, Jiang T, Ou Z, Zhang Y and
Zhu D: Inhibition of endoplasmic reticulum stress-activated
IRE1α-TRAF2-caspase-12 apoptotic pathway is involved in the
neuroprotective effects of telmisartan in the rotenone rat model of
Parkinson's disease. Eur J Pharmacol. 776:106–115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Seger R and Krebs EG: The MAPK signaling
cascade. FASEB J. 9:726–735. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2012. View Article : Google Scholar
|
|
52
|
Masri B, Lahlou H, Mazarguil H, Knibiehler
B and Audigier Y: Apelin (65-77) activates extracellular
signal-regulated kinases via a PTX-sensitive G protein. Biochem
Biophys Res Commun. 290:539–545. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Masri B, Morin N, Cornu M, Knibiehler B
and Audigier Y: Apelin (65-77) activates p70 S6 kinase and is
mitogenic for umbilical endothelial cells. FASEB J. 18:1909–1911.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xie H, Tang SY, Cui RR, Huang J, Ren XH,
Yuan LQ, Lu Y, Yang M, Zhou HD, Wu XP, et al: Apelin and its
receptor are expressed in human osteoblasts. Regul Pept.
134:118–125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang Y, Zhang X, Cui H, Zhang C, Zhu C and
Li L: Apelin-13 protects the brain against ischemia/reperfusion
injury through activating PI3K/Akt and ERK1/2 signaling pathways.
Neurosci Lett. 568:44–49. 2014. View Article : Google Scholar : PubMed/NCBI
|