Introduction
Osteosarcoma is the most common primary malignant
bone tumor in children and young adults in the USA (1). In the past decades, several studies
have reported improved outcomes for patients with osteosarcoma with
the addition of neoadjuvant and adjuvant chemotherapy, and
long-term survival for localized patients now approaches 70%
(2,3). Despite the great progress in
treating osteosarcoma, resistance to chemotherapy often results in
metastasis and recurrence in patients with osteosarcoma, which
leads to poor prognoses (4).
Cisplatin is a standard osteosarcoma therapeutic agent that
interacts with nucleophilic N7 sites of purine bases in
DNA to induce DNA damage and subsequent cell death (5). However, the development of
chemoresistance to cisplatin in osteosarcoma limits the
effectiveness of chemotherapy drugs and the long-term survival rate
for osteosarcoma patients with metastatic or recurrent disease
remains poor (6).
Chemoresistance is acquired by tumors during
chemotherapy treatment, even in tumors which were initially
sensitive to the drug. This adaptive response could be due to
regulation of expression of one or several genes and activation of
related signaling pathways (7).
Novel agents that target specific molecular alterations in tumors
have been developed during recent decades, and these drugs have
demonstrated encouraging results in restoring chemosensitivity
(7).
Transglutaminase 2 (TGM2) is a member of the
transglutaminase family. TGM2 is a multifunctional protein which is
involved in cell apoptosis and extracellular matrix degradation
(8,9). Aberrant expression of TGM2 has been
observed in multiple types of cancer cells and is associated with
poor drug response and poor patient survival (10,11). In vitro and in vivo
experiments have demonstrated that TGM2 may be a potential
therapeutic target for chemoresistant ovarian cancer (12). A recent study suggested a new
antiapoptotic function of TGM2, with TGM2 inhibiting osteosarcoma
cell apoptosis via regulating expression of BCL2 associated X (Bax)
and the release of cytochrome C under hypoxia conditions (13). However, the role of TGM2 may
differ in different tumor cell lines, and whether it is associated
with the chemoresistance of osteosarcoma cells remains unknown
(14,15).
To better understand the effect of TGM2 on
chemoresistance, and provide a potential novel method of
osteosarcoma treatment, in vitro and in vivo models
were used to investigate the role of TGM2 on chemoresistance by
regulating the AKT serine/threonine kinase (Akt) and
mitogen-activated protein kinase (MAPK) pathways. Knockdown of TGM2
was also demonstrated to successfully reverse chemoresistance to
cisplatin in osteosarcoma.
Materials and methods
Cell culture and establishment of
cisplatin-resistant cells
The human osteosarcoma cell line Saos2 was purchased
from the American Type Cell Culture (Manassas, VA, USA). Cells were
cultured in DMEM medium (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS;
HyClone; GE Healthcare Life Sciences) at 37°C in a 95% humidified
and 5% CO2 incubator. Cells were incubated with
different concentrations of cisplatin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). The concentration was stepwisely increasing to
establish cisplatin-resistant cells (from 0.1 to 1 µg/ml).
Cells were cultured under each concentration of cisplatin for 7
passages. After 1 year, cells growing in 1 µg/ml cisplatin
were designated as cisplatin-resistant cells and termed
Saos2-CIS-R.
TGM2 knockdown and overexpression
To downregulate the expression of TGM2, Saos2-CIS-R
cells were transduced with lentiviral particles containing short
hairpin RNA (shRNA) against TGM2 (sh-TGM2; purchased from GeneChem
Co., Ltd., Shanghai, China). A green fluorescent protein-tagged
lentiviral vector containing a scrambled shRNA was transduced as a
negative control. For TGM2 overexpression, Saos2 cells were
transduced with lentiviral particles encoding for the full-length
TGM2 gene (TGM2; purchased from GeneChem Co., Ltd.). Vectors were
propagated on HEK293T cells (American Type Culture Collection),
purified and titered, then stored at −80°C prior to use. All
transduced cells were subsequently exposed to 3 µg/ml
puromycin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 4
weeks post-infection to remove non-transduced cells, and then
maintained in 1 µg/ml puromycin. The expression of TGM2 in
all cells was confirmed by western blotting.
Cell viability and cisplatin sensitivity
assay
Cells were seeded on 96-well plates at a density of
5×103 cells per well. Cell Counting Kit-8 (CCK-8;
Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was used to
measure cell viability following treating cells with different
concentrations of cisplatin. After treatments, the culture medium
was removed, and cells were washed with PBS. A total of 100
µl fresh medium with 10 µl of CCK-8 solution was
added to each well for 2 h at 37°C. Optical density (OD) was
measured at 450 nm using a microplate reader (BioTek Instruments,
Inc., Winooski, VT, USA). The OD readings of the treatment groups
were divided by their corresponding control readings to obtain the
ratio of cell viability.
Cell apoptosis assay
Cells were seeded in 6-well plates at a density of
1.5×105 cells per well. Then cells were treated with
different concentrations of cisplatin for 24 h, as indicated. Cells
were harvested following cisplatin treatments, washed twice with
cold PBS and centrifuged. The supernatants were discarded and the
cells were resuspended in 1X Annexin-binding buffer. A total of 5
µl Annexin V-allophycocyanin (APC) or Annexin
V-allophycocyanin (FITC) solution (BD Biosciences, Franklin Lakes,
NJ, USA) was added to the cells at room temperature for 15 min,
then 5 µl propidium iodide (PI) solution (BD Biosciences)
was added. The ratio of apoptotic cells (% Annexin V-APC or Annexin
V-FITC positive cells per total) was measured by flow cytometry (BD
FACSDiva version 8.0.1; BD Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol reagent (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) following the
manufacturer's instructions. cDNA was generated by reverse
transcription of 1 µg total RNA using the 1st Strand cDNA
Synthesis kit (Takara Biotechnology Co., Ltd., Dalian, China).
Relative gene expression was determined by qPCR using the SYBR
Premix Ex Taq kit (Takara Biotechnology Co., Ltd.). The cycling
conditions were 40 cycles of 95°C for 5 sec and 60°C for 34 sec.
The ABI Prism 7500 Fast Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) was used to perform the PCR
experiments and to analyze the results. Primers were designed and
selected using BLAST (National Institutes of Health, Bethesda, MD,
USA), and are listed in Table I.
Relative fold changes in mRNA expression were calculated using the
2−ΔΔCq method (16).
 | Table ISequences of primers used in the
quantitative polymerase chain reaction analysis. |
Table I
Sequences of primers used in the
quantitative polymerase chain reaction analysis.
| Gene | Primer | Sequence
('5-3') |
|---|
| Bcl-2 | Forward |
GAACTGGGGGAGGATTGTGG |
| Bcl-2 | Reverse CC |
GTACAGTTCCACAAAGGC |
| Bax | Forward CC |
AGAGGCGGGGTTTCAT |
| Bax | Reverse |
GGAAAAAGACCTCTCGGGGG |
| GAPDH | Forward |
ACCACCATGGAGAAGGCTGG |
| GAPDH | Reverse |
CTCAGTGTAGCCCAGGATGC |
Western blot analysis
Cells were washed twice with cold PBS, then total
protein was extracted with RIPA lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China). Protein concentration was
quantified by a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology, Shanghai, China). A total of 20
µg protein (for each sample) was loaded and separated by 10%
or 12.5% SDS-PAGE, then transferred to polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
blocked with 5% fat-free milk for 1 h at room temperature and
incubated with primary antibodies overnight at 4°C (anti-TGM2,
anti-Bcl-2, anti-Bax, anti-akt, anti-p-akt, anti-caspase-3.
anti-cleave-caspase-3 and the MAPK family antibody kits; cat. nos.
3557S, 3498, 5023, 8202S, 9926T, 9910T and 4060S; 1:1,000 dilution;
Cell Signaling Technology, Inc., Danvers, MA, USA). Following three
washes with TBS/0.1% Tween-20 (TBST), the membranes were incubated
with anti-rabbit or anti-mouse immunoglobulin G for 1 h at room
temperature (cat. nos. 7076 and 7074; 1:5,000 dilution; Cell
Signaling Technology, Inc.) and visualized using an enhanced
chemiluminescence system (PerkinElmer, Inc., Waltham, MA, USA).
Positive immunoreactive bands were densitometrically quantified
(Quantity One 1-D, version 4.6.9; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and normalized to GAPDH.
Animal experiments
All animal operations were approved by the Animal
Ethics Committee of Linyi People's Hospital (Linyi, China; Approval
no. 2016-A036). A total of 40 four-weeks-old female nude mice
(BALB/c, nu/nu; SIPPR-BK Laboratory Animal Co. Ltd, Shanghai,
China) were housed under pathogen-free conditions at 18~22°C and
50% humidity; The mice had free access to food and water and
exposed to the natural light-dark cycle. Cells (Saos2-CIS-R,
scramble and sh-TGM2) were cultured, counted and resuspended in PBS
at a final concentration of 2×108 cells/ml. Mice were
anesthetized with 1.5% pentobarbital sodium (40 mg/kg) and then
injected with 50 µl of cell suspension into the proximal
tibia. When the tumor size reached 100 mm3, the
experimental groups were intraperitoneally injected with cisplatin
twice a week at a dosage of 15 mg/kg. The control group was
injected with the same volume of normal saline. After 4 weeks of
cisplatin treatment, the tumors were removed and their volumes were
calculated, then fixed with 4% paraformaldehyde for the following
experiments.
Immunohistochemistry
Tumor tissues were fixed in 4% paraformaldehyde for
48 h at room temperature, embedded in paraffin for 48 h at room
temperature and then 5-µm thick sections were obtained.
After deparaffinating, sections were stained with hematoxylin and
eosin (H&E) under standard H&E staining procedures. The
expression of Ki-67 and cleaved caspase-3 was detected using
monoclonal antibodies (anti-Ki-67 and anti-cleave-caspase-3; cat.
nos. ab15580 and ab2302; 1:200; Abcam, Cambridge, MA, USA) and a
horseradish peroxidase-conjugated anti-mouse or anti-rabbit
secondary antibody (cat. nos. ab205719 and ab6721; 1:100; Abcam),
followed by color development with diaminobenzidine
tetrahydrochloride (DAB; Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA). The sections were counterstained with hematoxylin
following DAB staining. The images were observed and analyzed under
a microscope (Leica Microsystems GmbH, Wetzlar, Germany) with
Image-Pro Plus version 6.0 (Media Cybernetics, Inc., Rockville, MD,
USA). The magnification of H&E sections was ×40 and for
immuohistochemical staining ×200. The positive staining in images
was quantified as integral optical density (IOD)/area, which was
expressed as mean density. For each section, 3 fields were
calculated and the average taken, and 6 sections were imaged in
each group.
Statistical analysis
Results were expressed as the mean ± standard
deviation from three independent repeats, including the animal
experiments. Statistical analyses were performed with Student's
t-test or one-way analysis of variance followed by Duncan's post
hoc test using SPSS 24.0 (IBM Corp., Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
TGM2 is upregulated in
cisplatin-resistant osteosarcoma cells
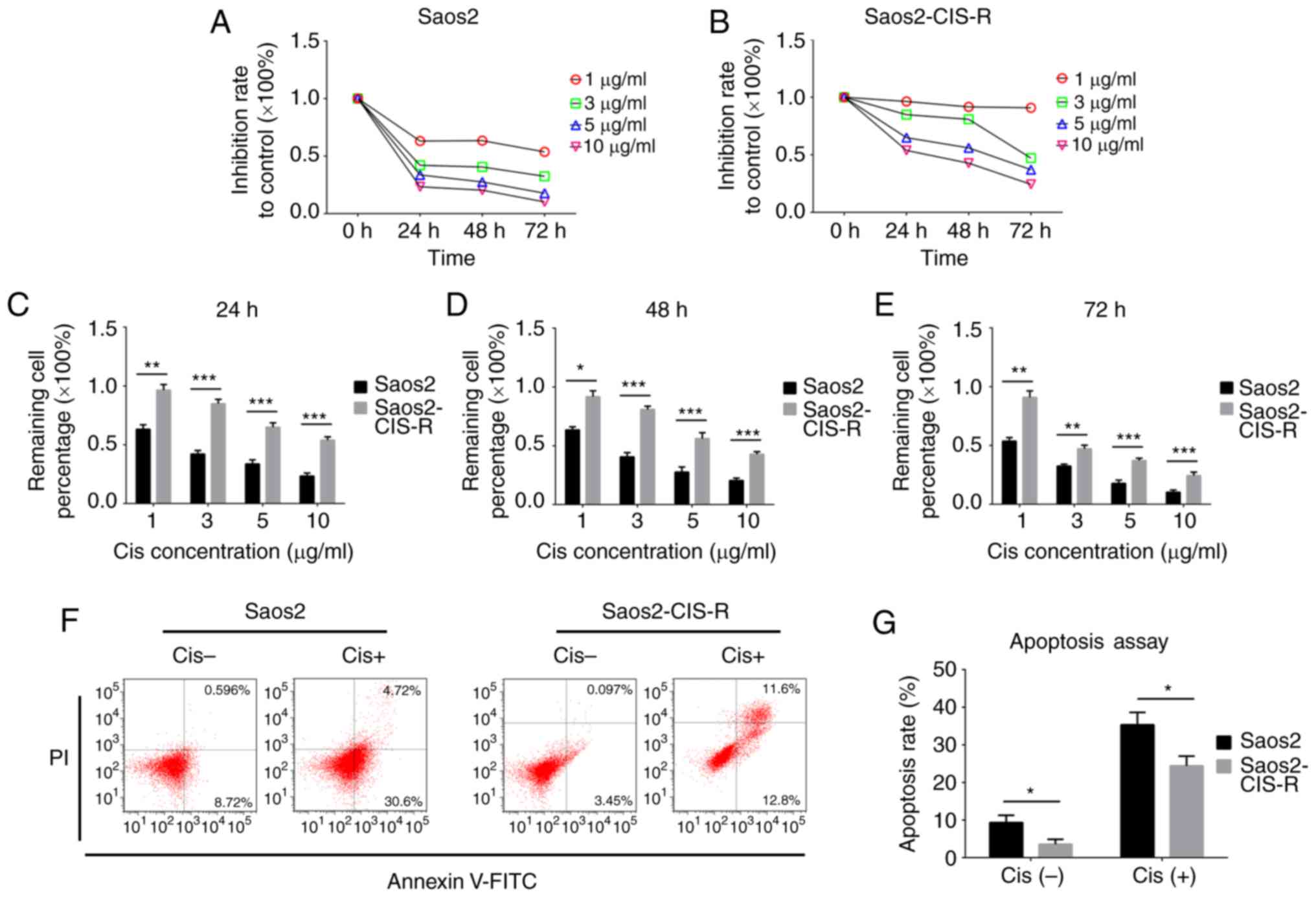
First, a cisplatin-resistant osteosarcoma cell line
(termed here Saos2-CIS-R) was established based on the parental
Saos2 osteosarcoma cells. To verify the chemoresistance of these
cells, a cell viability assay was performed following treatment
with different concentrations of cisplatin. The results
demonstrated that Saos2-CIS-R cells had developed chemoresistance
to cisplatin (Fig. 1). In the
parental Saos2 cells, cell viability was gradually reduced with the
increase of concentration of cisplatin and treating time (Fig. 1A and C–E). The cell viability of
Saos2-CIS-R cells was significantly higher compared with Saos2
cells, and the Saos2-CIS-R cells exhibited almost complete
resistance to 1 µg/ml cisplatin (Fig. 1B–E). The apoptosis rate was
detected using flow cytometry following treatment with 5
µg/ml cisplatin for 24 h. The results indicated that
Saos2-CIS-R cells had a lower apoptosis rate following cisplatin
treatment compared with the parental Saos2 cells (Fig. 1F and G).
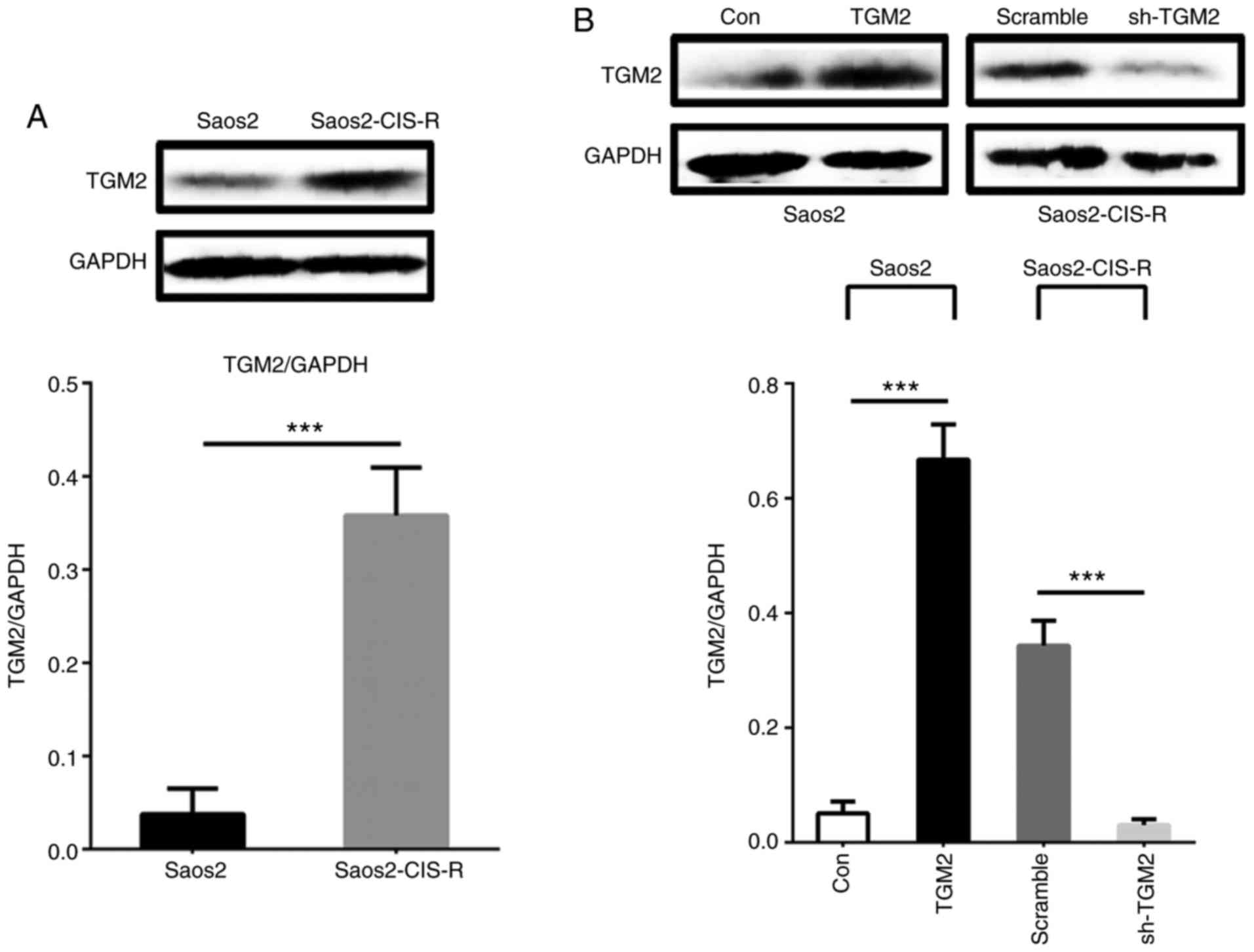
Next, TGM2 protein expression was measured by
western blot analysis. As illustrated in Fig. 2A, TGM2 protein expression levels
were significantly increased in Saos2-CIS-R cells compared with
Saos2 cells. The knockdown of TGM2 by shRNA (sh-TGM2) in
Saos2-CIS-R cells and the overexpression of TGM2 (TGM2) in the
parental Saos2 cells were also confirmed by western blot analysis
(Fig. 2B).
TGM2 regulates the chemosensitivity of
osteosarcoma cells to cisplatin
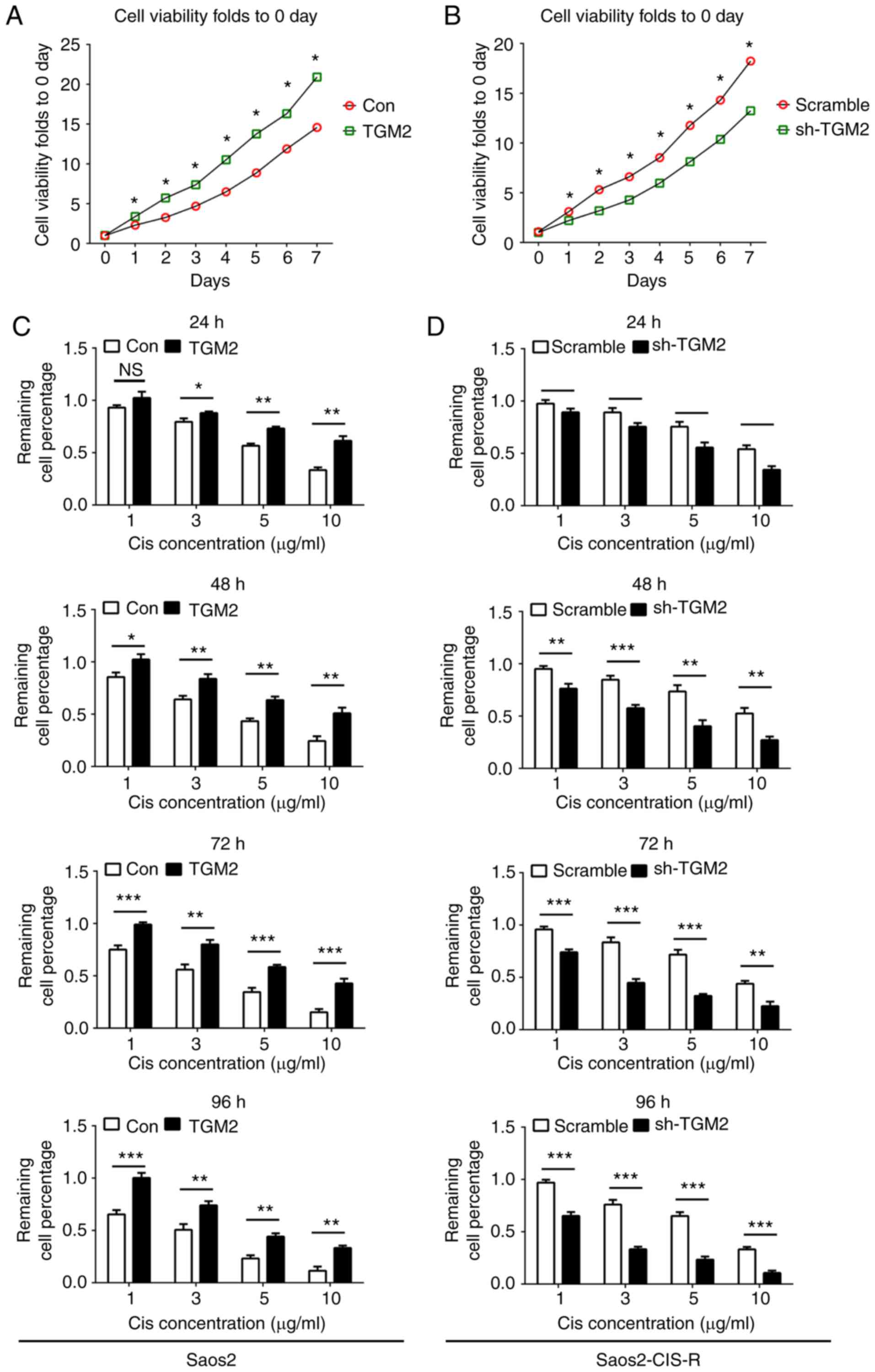
The abnormal expression of TGM2 in the Saos2-CIS-R
and Saos2 cell lines was hypothesized to be associated with the
chemosensitivity. To examine the role of TGM2 in cisplatin
sensitivity, a lentivirus-based gene knockdown and overexpression
system was used in vitro. Cells were cultured for 7 days and
the cell viability was measured over time in order to detect cell
proliferation rates. In Saos2 cells, overexpression of TGM2
significantly promoted cell proliferation at all time points tested
compared with control cells (Fig.
3A). In Saos2-CIS-R cells, knockdown of TGM2 significantly
reduced cell proliferation from day 1 to 7 compared with scramble
control (Fig. 3B). Following
treatment with different concentrations of cisplatin for 24, 48, 72
and 96 h, cell viability results indicated that overexpression of
TGM2 increased the chemoresistance of Saos2 cells to cisplatin
compared with control Saos2 cells (Fig. 3C). Similarly, knockdown of TGM2 in
Saos2-CIS-R cells enhanced the chemosensitivity to cisplatin from
24 to 96 h compared with scramble control (Fig. 3D). Flow cytometry analysis further
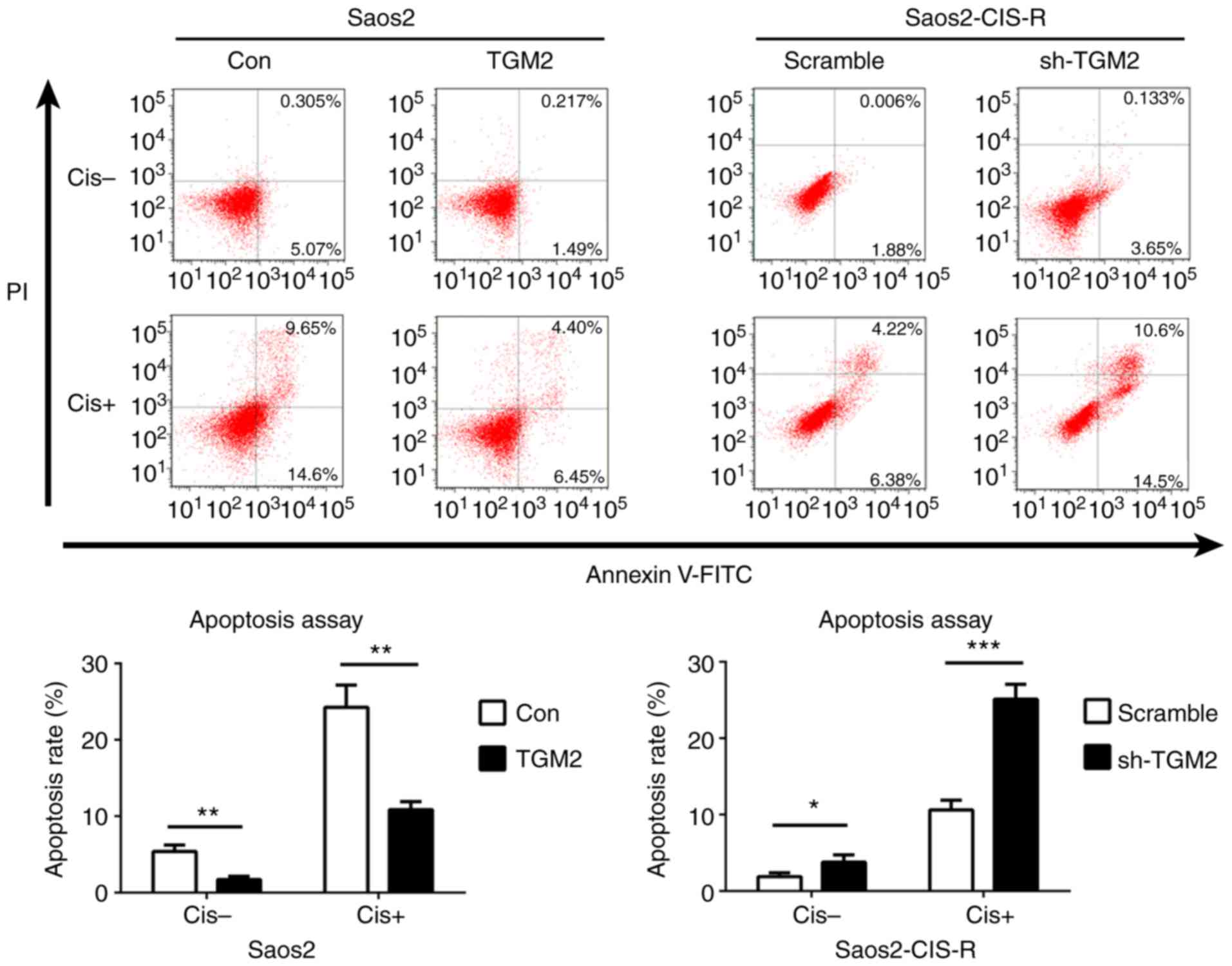
confirmed that overexpression of TGM2 in Saos2 cells reduced
cisplatin-induced cell apoptosis, while knockdown of TGM2 in
Saos2-CIS-R cells significantly increased cisplatin-induced cell
apoptosis compared with scramble control (Fig. 4).
TGM2 regulates the MAPK pathway and
caspase-3 activity in osteosarcoma cells
To further investigate the potential mechanism of
TGM2 in regulating osteosarcoma cell chemo-sensitivity, the gene
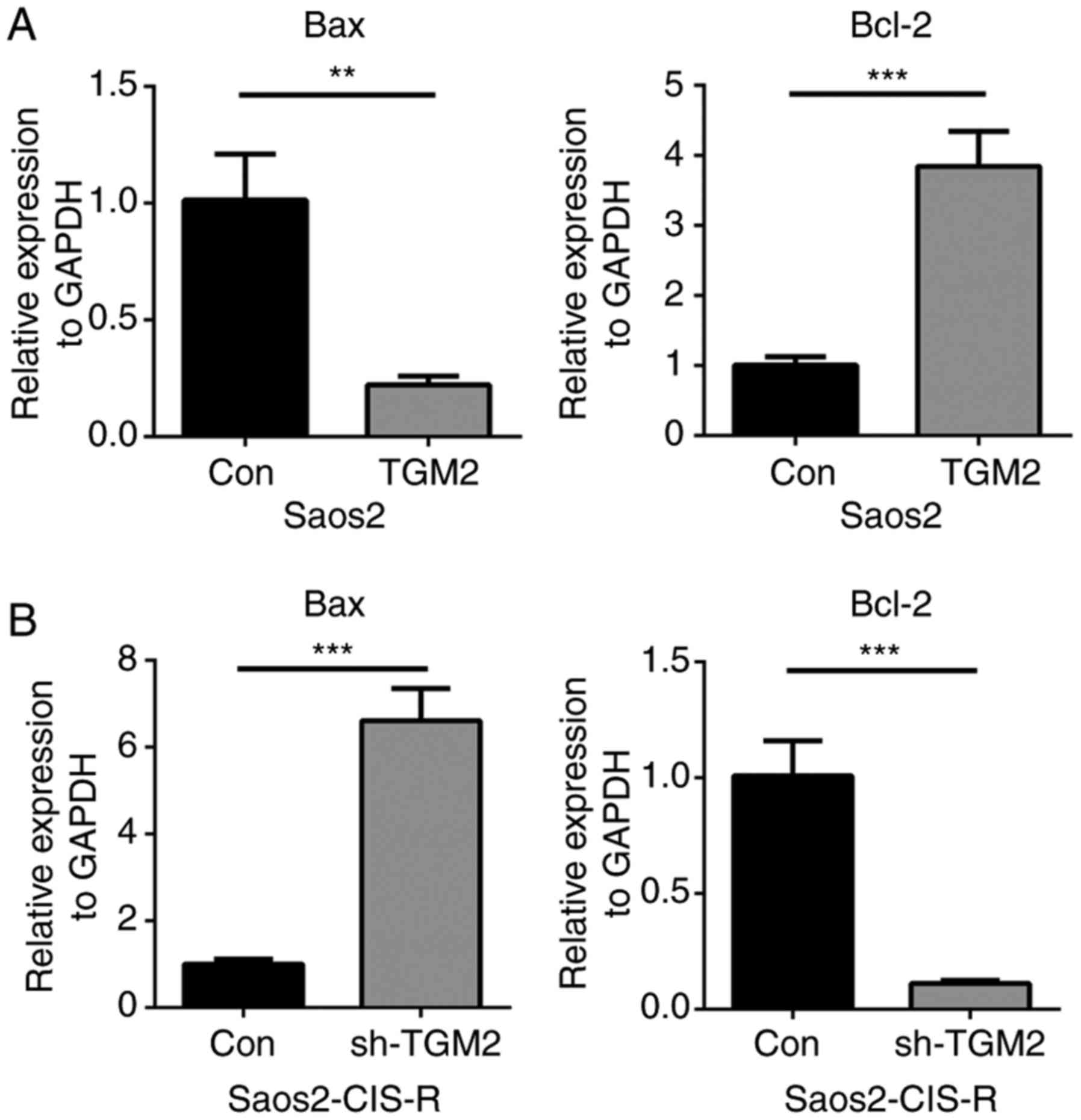
expression levels of Bax and BCL2 apoptosis regulator (Bcl-2) were
measured in Saos2 and Saos2-CIS-R cells. Following treatment with 5
µg/ml cisplatin for 24 h, Bax gene expression was
downregulated, while Bcl-2 gene expression was upregulated, in the
TGM2-overexpressing Saos2 cells compared with control Saos2 cells
(Fig. 5A). On the contrary, in
sh-TGM2 cells, Bax gene expression was upregulated and Bcl-2 was
downregulated compared with scramble control (Fig. 5B). Western blot analysis confirmed
these results on the protein level; Bax protein expression was
reduced in TGM2-overexpressing Saos2 cells and increased in
TGM2-knockdown Saos2-CIS-R cells; Bcl-2 expression exhibited the
opposite results (Fig. 6A). The
Akt and the MAPK signaling pathways and the caspase family of
proteins are also known to have important roles in cell apoptosis.
Therefore, the protein expression levels of Akt/phosphorylated (p-)
Akt, caspase-3/cleaved caspase-3, p38/p-p38, extracellular
signal-regulated kinase (ERK)/p-ERK and c-Jun N-terminal kinase
(JNK)/p-JNK were analyzed by western blotting. The results
indicated that, overexpression of TGM2 in Saos2 cells activated the
Akt and MAPK pathways but inhibited the activity of caspase-3
(Fig. 6). By contrast, knockdown
of TGM2 in the Saos2-CIS-R cells promoted the expression of cleaved
caspase-3 and inhibited the activation of Akt and MAPK pathways
(Fig. 6).
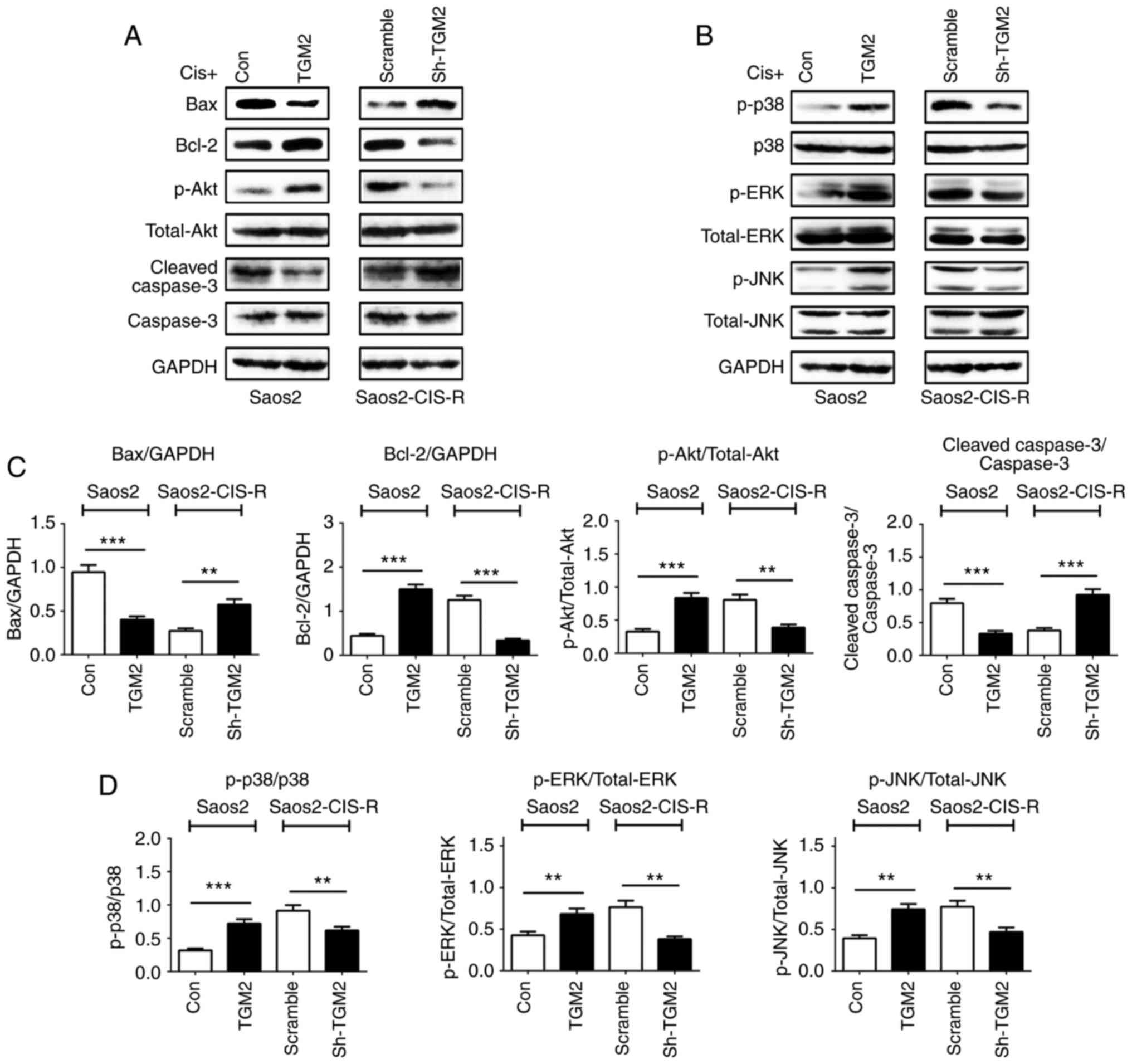 | Figure 6Expression of pathway-related
proteins and caspase-3 in osteosarcoma cells. (A and B) Following
treatment with 5 µg/ml cisplatin for 24 h, protein
expression levels of Bax, Bcl-2, Akt, p-Akt, cleaved caspase-3,
caspase-3, p-p38, p38, p-ERK, ERK, p-JNK, JNK and GAPDH were all
measured by western blotting. (C and D) Densitometry analysis of
western blot results. Data are presented as the mean ± standard
deviation from at least three independent experiments.
**P<0.005 and ***P<0.0005, with
comparisons indicated by lines. Bax, BCL2 associated X; Bcl-2, BCL2
apoptosis regulator; Akt, AKT serine/threonine kinase; p-,
phosphorylated; ERK, extracellular signal-regulated kinase; JNK,
c-Jun N-terminal kinase; Cis, cisplatin; TGM2, transglutaminase 2;
Con, control; sh, short hairpin. |
TGM2 knockdown enhances the
chemosensitivity of osteosarcoma to cisplatin in vivo
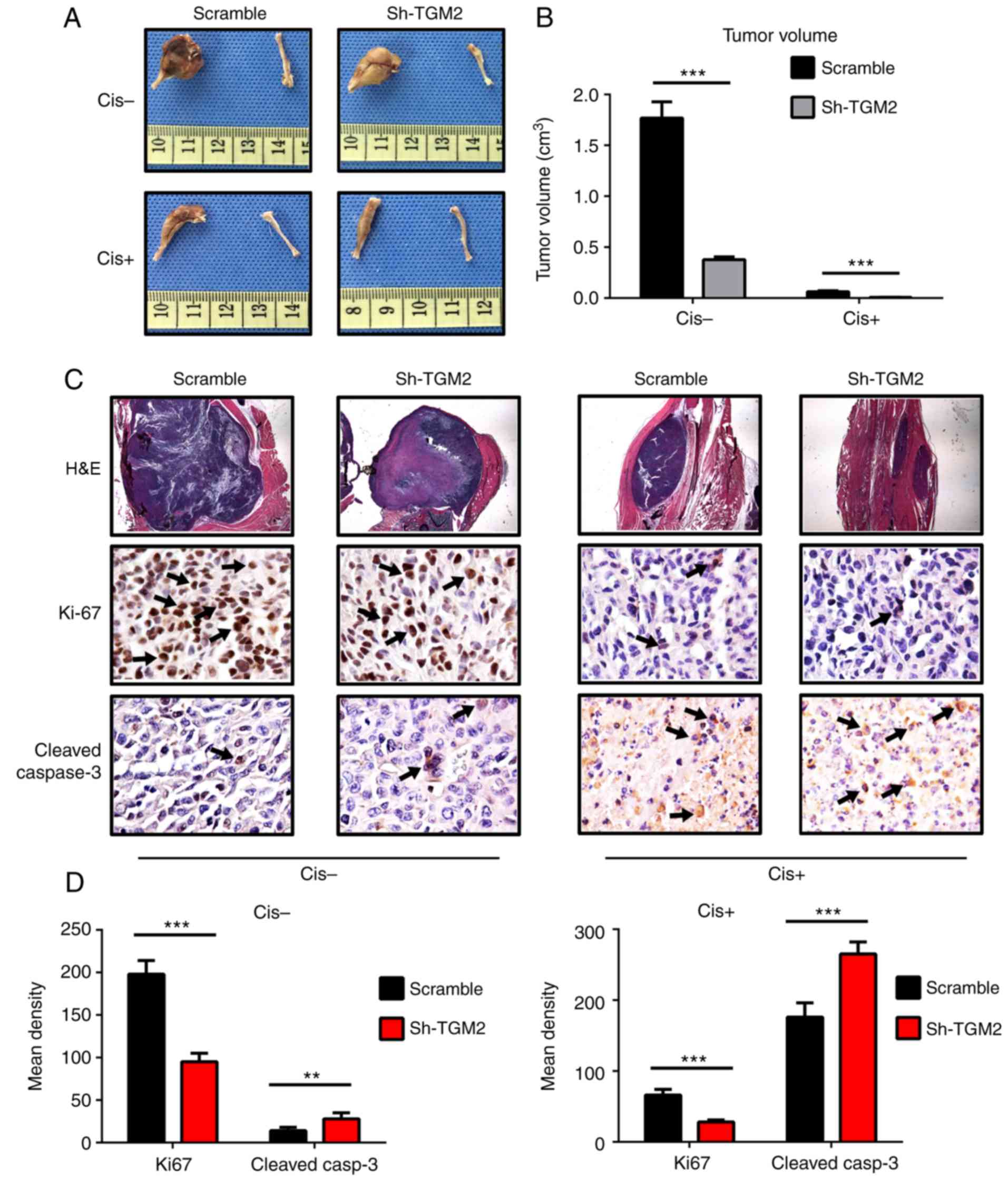
Given that the in vitro experiments revealed
encouraging results, the effect of TGM2 on chemosensitivity to
cisplatin was further investigated in a mouse model of
osteosarcoma. At the end of in vivo treatments, tumors were
removed and their volumes were calculated. Tumor volumes were
decreased in all groups following treatment with cisplatin. In the
sh-TGM2 group, cisplatin treatment almost completely eliminated the
tumors, and the femur remained intact (Fig. 7A). In the scramble control group,
the tumors were still obvious, and the proximal femur was deformed,
which suggests that these cells were chemoresistant. Quantification
of the tumor volumes revealed that TGM2 knockdown resulted in
significantly reduced tumor volumes compared with the scramble
control group (Fig. 7B).
H&E staining and immunohistochemistry analysis
also indicated that knockdown of TGM2 enhanced the
chemo-sensitivity to cisplatin. The H&E staining revealed that
the tumor mass was significantly decreased in the femurs of the
sh-TGM2 group compared with scramble group (Fig. 7C). In addition, the scramble group
displayed an increase of positive Ki-67 staining compared with the
sh-TGM2 group without cisplatin treatment. However, following
cisplatin treatment, Ki-67 staining was markedly reduced, and there
was almost no positive staining in the sh-TGM2 group, compared with
little staining in the scramble group (Fig. 7C; Ki-67 staining represented by
arrows). Quantitative analysis of the results demonstrated that the
sh-TGM2 group had significantly reduced Ki-67 staining with or
without cisplatin treatment compared with the scramble control
(Fig. 7D). Cleaved caspase-3
staining was barely obvious before cisplatin treatment (Fig. 7C). In the sh-TGM2 group, a small
amount of positive staining was observed (Fig. 7C; represented by arrows), while
almost no positive staining was observed in the scramble group.
Following cisplatin treatment, cleaved caspase-3 staining was
remarkably increased, and there was more positive staining in the
sh-TGM2 group (Fig. 7C).
Quantitative analysis of the immunohistochemistry results confirmed
that the sh-TGM2 group had significantly increased cleaved
caspase-3 staining compared with the scramble group (Fig. 7D). These results demonstrated that
knockdown of TGM2 in the Saos2-CIS-R cells suppressed the
osteosarcoma cell proliferation and promoted cell apoptosis, thus
enhancing the chemosensitivity to cisplatin.
Discussion
Chemotherapy is one of the principal modes of
treatment for cancer, and cisplatin is a standard chemotherapy
agent for osteosarcoma. However, chemoresistance of cisplatin has
become a growing problem (17).
Several mechanisms have been reported to be involved in drug
chemoresistance, including mutation of drug targets and alterations
in drug metabolism, while the detailed mechanisms have yet to be
fully elucidated (18).
Considering the complicated mechanisms of chemoresistance, the
establishment of resistant cancer cell lines is crucial for
studying the underlying mechanisms. In the present study, a
cisplatin-resistant osteosarcoma cell line was established by
exposure of osteosarcoma cells to a stepwisely increasing
concentration of cisplatin (19).
The deregulation of apoptosis is one of the most
important downstream mechanisms of chemoresistance in cancer.
Changes in expression of apoptosis-related proteins, such as the
Bcl-2 family and the caspase family of proteins, are associated
with various cancers and responsible for resistance to chemotherapy
(20). According to Zhao et
al (21), Bcl-2 was markedly
downregulated in a dose-dependent manner in cisplatin-treated Saos2
cells. Another study reported that gankyrin (P28GANK) conferred
multidrug resistance by modulating expression of multidrug
resistance protein 1 (MDR1), Bcl-2, and Bax in osteosarcoma cells
(22). These studies indicated
that regulation of Bax and Bcl-2 expression was an important
mechanism of developing chemoresistance in osteosarcoma. Caspase-3
is a key protein in the regulation of cell apoptosis and is
involved in the formation of chemoresistance (23). TGM2 is also reported to have
crucial roles in many processes including cell apoptosis (24). In multiple cancer cell lines,
expression of TGM2 has been demonstrated to be associated with
cisplatin and doxorubicin chemoresistance, and knockdown of TGM2
could increase chemosensitivity (25-27). A previous study indicated that
TGM2 regulated cell apoptosis in a complicated manner: It not only
promoted apoptosis but could also inhibit apoptosis (27). Recently, Wang et al
(13) studied the role of TGM2 in
the U2OS cell line. As their results demonstrated, TGM2 inhibited
tumor cell apoptosis through downregulation of Bax and prevention
of cytochrome C release (13).
These results are also consistent with the experiments in the
present study (Fig. 5). According
to another study, increased expression of TGM2 was associated with
epithelial-mesenchymal transition (EMT) in breast cancer and
downregulation of TGM2 could reverse EMT and increase the
chemosensitivity of breast cancer to docetaxel (28). In the present study, the gene
expression of TGM2 was upregulated in Saos2-CIS-R cells, suggesting
that gene regulation of TGM2 might be one of the mechanisms
inducing chemoresistance. Furthermore, knockdown of TGM2 reversed
the chemosensitivity of Saos2-CIS-R cells to cisplatin, and induced
upregulation of Bax and caspase-3, and downregulation of Bcl-2.
These results were consistent with previous reports (13,25,26), indicating that TGM2 enhances
chemoresistance via regulation of Bax, Bcl-2 and caspase-3.
Activation of the Akt and MAPK pathways has been
observed in many malignancies and is considered to contribute to
tumor cell survival (29,30). A previous study has demonstrated
that inhibition of the Akt pathway leads to the restoration of
chemosensitivity in osteosarcoma cells (31). In addition, in vitro and
in vivo studies have demonstrated that the Akt pathway has
an important role in the adaptive resistance of tumors to
chemotherapies, which is termed as 'oncogenic bypass' (32,33). The MAPK pathway has also been
demonstrated to have a key role in tumor cell survival and
migration (34). Several drugs
targeting the MAPK pathway have been reported to inhibit cell
proliferation in osteosarcoma (35). The present study indicated that
the Akt and MAPK pathways were activated in the drug-resistant cell
line, and their activation was significantly inhibited following
knockdown of TGM2. These results revealed that knockdown of TGM2
enhanced the chemosensitivity of osteosarcoma to cisplatin by
inhibiting the activation of Akt and MAPK signaling.
In conclusion, our in vivo and in
vitro experiments revealed that TGM2 was associated with the
chemoresistance to cisplatin in osteosarcoma, and that knockdown of
TGM2 enhanced osteosarcoma cell chemosensitivity. TGM2 might affect
the chemosensitivity of osteosarcoma via regulation of the
activation of MAPK and Akt pathways. Expression of Bcl-2, Bax and
caspase-3 was also involved in the developing chemoresistance in
osteosarcoma. The present findings suggest a potentially important
role of TGM2 in the regulation of osteosarcoma chemosensitivity.
TGM2 may therefore serve as a potential therapeutic target for
osteosarcoma.
Acknowledgments
Not applicable.
Funding
No funding was received
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
CY conceived and designed the experiments, performed
the experiments, analyzed the data, contributed
reagents/materials/analytical tools, wrote the paper, prepared
figures and tables, and reviewed drafts of the paper. JC performed
the experiments, analyzed the data, contributed
reagents/materials/analysis tools, and prepared figures and tables.
FG performed the experiments. GW conceived and designed the
experiments and reviewed drafts of the paper.
Ethics approval and consent to
participate
All animal operations were approved by the Animal
Ethics Committee of Linyi People's Hospital (Linyi, China; Approval
no. 2016-A036).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bian ZY, Fan QM, Li G, Xu WT and Tang TT:
Human mesenchymal stem cells promote growth of osteosarcoma:
Involvement of interleukin-6 in the interaction between human
mesenchymal stem cells and Saos-2. Cancer Sci. 101:2554–2560. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tu B, Du L, Fan QM, Tang Z and Tang TT:
STAT3 activation by IL-6 from mesenchymal stem cells promotes the
proliferation and metastasis of osteosarcoma. Cancer Lett.
325:80–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dieudonné FX, Marion A, Haÿ E, Marie PJ
and Modrowski D: High Wnt signaling represses the proapoptotic
proteoglycan syndecan-2 in osteosarcoma cells. Cancer Res.
70:5399–5408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He H, Ni J and Huang J: Molecular
mechanisms of chemoresistance in osteosarcoma (Review). Oncol Lett.
7:1352–1362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Longley DB and Johnston PG: Molecular
mechanisms of drug resistance. J Pathol. 205:275–292. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsieh YF, Liu GY, Lee YJ, Yang JJ, Sándor
K, Sarang Z, Bononi A, Pinton P, Tretter L, Szondy Z and Tsay GJ:
Transglutaminase 2 contributes to apoptosis induction in Jurkat T
cells by modulating Ca2+ homeostasis via cross-linking
RAP1GDS1. PLoS One. 8:e815162013. View Article : Google Scholar
|
|
9
|
Lauzier A, Charbonneau M, Paquette M,
Harper K and Dubois CM: Transglutaminase 2 cross-linking activity
is linked to invadopodia formation and cartilage breakdown in
arthritis. Arthritis Res Ther. 14:R1592012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singer CF, Hudelist G, Walter I,
Rueckliniger E, Czerwenka K, Kubista E and Huber AV: Tissue
array-based expression of transglutaminase-2 in human breast and
ovarian cancer. Clin Exp Metastasis. 23:33–39. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park KS, Kim HK, Lee JH, Choi YB, Park SY,
Yang SH, Kim SY and Hong KM: Transglutaminase 2 as a cisplatin
resistance marker in non-small cell lung cancer. J Cancer Res Clin
Oncol. 136:493–502. 2010. View Article : Google Scholar
|
|
12
|
Hwang JY, Mangala LS, Fok JY, Lin YG,
Merritt WM, Spannuth WA, Nick AM, Fiterman DJ, Vivas-Mejia PE,
Deavers MT, et al: Clinical and biological significance of tissue
transglutaminase in ovarian carcinoma. Cancer Res. 68:5849–5858.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang W, Li X, Han XZ, Meng FB, Wang ZX,
Zhai YQ and Zhou DS: Transglutaminase-2 is involved in cell
apoptosis of osteosarcoma cell line U2OS under hypoxia condition.
Cell Biochem Biophys. 72:283–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mehta K: High levels of transglutaminase
expression in doxorubicin-resistant human breast carcinoma cells.
Int J Cancer. 58:400–406. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Antonyak MA, Jansen JM, Miller AM, Ly TK,
Endo M and Cerione RA: Two isoforms of tissue transglutaminase
mediate opposing cellular fates. Proc Natl Acad Sci USA.
103:18609–18614. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔC T method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Li S, Sun W, Wang H, Zuo D, Hua Y and Cai
Z: Research progress on the multidrug resistance mechanisms of
osteosarcoma chemotherapy and reversal. Tumour Biol. 36:1329–1338.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Asada N, Tsuchiya H, Ueda Y and Tomita K:
Establishment and characterization of an acquired
cisplatin-resistant subline in a human osteosarcoma cell line.
Anticancer Res. 18:1765–1768. 1998.PubMed/NCBI
|
|
20
|
Letai AG: Diagnosing and exploiting
cancer's addiction to blocks in apoptosis. Nat Rev Cancer.
8:121–132. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao Z, Tao L, Shen C, Liu B, Yang Z and
Tao H: Silencing of Barkor/ATG14 sensitizes osteosarcoma cells to
cisplatin-induced apoptosis. Int J Mol Med. 33:271–276. 2014.
View Article : Google Scholar :
|
|
22
|
Wang G, Rong J, Zhou Z and Duo J: Novel
gene P28GANK confers multidrug resistance by modulating the
expression of MDR-1, Bcl-2 and Bax in osteosarcoma cells. Mol Biol.
44:1010–1017. 2010. View Article : Google Scholar
|
|
23
|
Botham RC, Roth HS, Book AP, Roady PJ, Fan
TM and Hergenrother PJ: Small-molecule procaspase-3 activation
sensitizes cancer to treatment with diverse chemotherapeutics. ACS
Cent Sci. 2:545–559. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee HJ and Lee CH: Transglutaminase-2 is
involved in expression of osteoprotegerin in MG-63 osteosarcoma
cells. Biomol Ther. 21:204–209. 2013. View Article : Google Scholar
|
|
25
|
Cao L, Petrusca DN, Satpathy M, Nakshatri
H, Petrache I and Matei D: Tissue transglutaminase protects
epithelial ovarian cancer cells from cisplatin-induced apoptosis by
promoting cell survival signaling. Carcinogenesis. 29:1893–1900.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han JA and Park SC: Reduction of
transglutaminase 2 expression is associated with an induction of
drug sensitivity in the PC-14 human lung cancer cell line. J Cancer
Res Clin Oncol. 125:89–95. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nurminskaya MV and Belkin AM: Cellular
functions of tissue transglutaminase. Int Rev Cell Mol Biol.
294:1–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He W, Sun Z and Liu Z: Silencing of TGM2
reverses epithelial to mesenchymal transition and modulates the
chemosensitivity of breast cancer to docetaxel. Exp Ther Med.
10:1413–1418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zöllinger A, Stühmer T, Chatterjee M,
Gattenlöhner S, Haralambieva E, Müller-Hermelink HK, Andrulis M,
Greiner A, Wesemeier C, Rath JC, et al: Combined functional and
molecular analysis of tumor cell signaling defines 2 distinct
myeloma subgroups: Akt-dependent and Akt-independent multiple
myeloma. Blood. 112:3403–3411. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peng DJ, Wang J, Zhou JY and Wu GS: Role
of the Akt/mTOR survival pathway in cisplatin resistance in ovarian
cancer cells. Biochem Biophys Res Commun. 394:600–605. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang H, Luo QF, Peng AF, Long XH, Wang TF,
Liu ZL, Zhang GM, Zhou RP, Gao S, Zhou Y, et al: Positive feedback
regulation between Akt phosphorylation and fatty acid synthase
expression in osteosarcoma. Int J Mol Med. 33:633–639. 2014.
View Article : Google Scholar
|
|
32
|
Wheeler DL, Huang S, Kruser TJ,
Nechrebecki MM, Armstrong EA, Benavente S, Gondi V, Hsu KT and
Harari PM: Mechanisms of acquired resistance to cetuximab: Role of
HER (ErbB) family members. Oncogene. 27:3944–3956. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sergina NV, Rausch M, Wang D, Blair J,
Hann B, Shokat KM and Moasser MM: Escape from HER-family tyrosine
kinase inhibitor therapy by the kinase-inactive HER3. Nature.
445:437–441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liao YX, Zhou CH, Zeng H, Zuo DQ, Wang ZY,
Yin F, Hua YQ and Cai ZD: The role of the CXCL12-CXCR4/CXCR7 axis
in the progression and metastasis of bone sarcomas (Review). Int J
Mol Med. 32:1239–1246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng DD, Zhu B, Li SJ, Yuan T, Yang QC
and Fan CY: Down-regulation of RPS9 inhibits osteosarcoma cell
growth through inactivation of MAPK signaling pathway. J Cancer.
8:2720–2728. 2017. View Article : Google Scholar : PubMed/NCBI
|