Introduction
Percutaneous transluminal coronary angioplasty
(PTCA) is a commonly used clinical treatment for cardiovascular
diseases; however, the frequent complication of restenosis
following PTCA often limits the long-term beneficial effects of
PTCA (1–3). The three relatively independent
steps involved in the development of post-PTCA restenosis are as
follows: Thrombosis, intimal hyperplasia, and vascular remodeling
caused by post-injury vascular repair (3–5).
Various vascular diseases, including neointimal hyperplasia
following vascular intervention, are associated with abnormal
vascular smooth muscle cell (VSMC) proliferation and migration
(5,6–8).
Previous studies have also reported that differentiation and
activation of VSMCs and dysregulation of the extracellular matrix
(ECM) may have critical roles in intimal hyperplasia, thus
contributing to restenosis following balloon injury (9–11).
Therefore, it may be beneficial to identify an effective treatment
strategy against neointima formation and VSMC dysfunction following
vascular endothelial injury.
Formononetin (FMN) is a bioactive compound, which is
widely distributed in nature and has been reported to possess
numerous pharmacological activities, including antitumor, oxygen
free radical-scavenging, cell lipid peroxide-reducing and
cholesterol-reducing effects (12–15). In our preliminary experiments, it
was demonstrated that, in a rat model, FMN could attenuate
balloon-induced neointima formation and reduce platelet-derived
growth factor (PDGF) levels (Song et al, unpublished data).
PDGF is a potent growth factor associated with vascular
development, which is produced by platelets, SMCs and endothelial
cells in the injured vessel wall. At present, there are four known
isoforms of PDGF: PDGF-A, PDGF-B, PDGF-C and PDGF-D, among which
PDGF-A and PDGF-B can form homo- and heterodimers (16). The homodimer PDGF-BB is now
considered to be the strongest factor in stimulating the
proliferation of SMCs, and it can also induce production of ECM
proteins by pericytes, which are important for the basement
membrane of capillaries (17). It
has also been reported that PDGF-BB is closely associated with cell
migration in angiogenesis by regulating vascular endothelial growth
factor expression levels in mural cells and collagenases in
fibroblasts (18).
The present study aimed to investigate the effects
of FMN against balloon injury-induced neointima formation in
vivo, to determine its inhibitory effects on the proliferation
and migration of VSMCs induced by PDGF-BB in vitro, and to
assess the underlying mechanisms of these effects.
Materials and methods
Chemicals
FMN was obtained from Shanghai Aladdin Biochemical
Technology Co., Ltd. (Shanghai, China); the purity of the chemical
was 98%.
Animal grouping and treatment
Specific pathogen-free (SPF) grade healthy male
Sprague-Dawley rats (age, 2.5–3.5 months; weight, 250–350 g) were
purchased from Shanghai Laboratory Animal Center, Chinese Academy
of Sciences (Shanghai, China). Rats were maintained in a SPF grade,
quiet and well-ventilated animal room under the following
conditions: Temperature, 22–24°C; relative humidity, 50–60%; 12-h
light/dark cycle, for at least one week prior to the experiments.
Rats were allowed ad libitum access to food and water. The
present study was approved by the animal care committee of Linyi
Peoples’ Hospital Affiliated to Shandong University (Linyi, China),
and all experimental operations were conducted according to the
National Institutes of Health (NIH) Guide for the Care and Use of
Laboratory Animals (19).
A total of 30 rats were randomly divided into the
sham group (n=10) and model group (n=20). Rats in the sham group
were treated with intragastric administration of saline (10 ml/kg).
Rats in the experimental group with balloon-induced arterial injury
were divided into two groups (n=10/group): Model group
(intragastric administration of 10 ml/kg saline) and FMN group
(intragastric administration of 50 mg/kg FMN). Drug treatment was
initiated on the second day after modeling, and drugs were
administered once a day for 14 days.
Carotid artery injury model
A rat model of balloon-induced carotid artery injury
was generated as described previously (20). Briefly, Sprague-Dawley rats were
anesthetized with 10% chloral hydrate (300 mg/kg), fixed on an
operating table in the supine position, and the left common and
external carotid arteries were exposed and isolated. A 2 F balloon
catheter was introduced retrogradely into the common carotid artery
through the external carotid artery. The balloon was filled with
saline and pulled back and forth; this process was repeated three
times, in order to induce damage to the arterial endothelium. After
surgery, the balloon catheter was removed and then the dorsal end
of the external carotid artery was sutured to restore blood flow.
Rats in the sham group were subjected to a similar surgical
treatment; the left common and external carotid arteries were
exposed, but the rats did not undergo balloon injury.
A total of 2 weeks following surgery, rats were
euthanized and arterial segments were harvested for histological
staining, identification of intimal hyperplasia and western
blotting.
Histological observation
The carotid arteries were fixed with 10% neutral
formaldehyde solution for 48 h at 4°C (Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China) and were embedded in
paraffin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Carotid
artery sections (5 µm) from each group were stained with
hematoxylin and eosin. Briefly, after dewaxing with xylene and
rehydration through a graded series of ethanol (Sigma-Aldrich;
Merck KGaA), hematoxylin (Sigma-Aldrich; Merck KGaA) staining was
performed for 5 min at 25°C. After washing with tap water, tissue
sections were treated with ethanol containing hydrochloric acid
(Sigma-Aldrich; Merck KGaA). After immersion in tap water for 15
min, eosin (Sigma-Aldrich; Merck KGaA) staining was performed for 2
min. Subsequently, the slides were incubated in 95% anhydrous
ethanol for 5 min twice and in xylene solution for 10 min at 25°C,
after which the slides were mounted in neutral resin and observed
under an optical microscopy (BX51; Olympus Corporation, Tokyo,
Japan). Morphometric analysis was performed by an investigator who
was kept blind to the experimental procedure using three individual
sections of arterial segments from each group. The Image-Pro Plus
System 6.0 (Media Cybernetics, Inc., Rockville, MD, USA) was used
to measure the intima and media area of each group, and their
ratio. Intima area = inner elastic membrane surrounding area −
lumen area; media area = outer elastic membrane surrounding area −
inner elastic membrane surrounding area.
Proliferating cell nuclear antigen (PCNA)
staining
Carotid artery sections (5 µm) from each
group were stained with PCNA, according to the manufacturer’s
protocol. Sections were incubated with the following antibodies:
Anti-PCNA antibody (1:200, sc-53407; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) at 4°C overnight, after which biotinylated
goat anti-mouse secondary antibody (1:200, sc-2039; Santa Cruz
Biotechnology, Inc.) was added for 10 min at 37°C. Each section was
observed under an optical microscopy (BX51; Olympus Corporation),
and six fields of view were analyzed. The number of PCNA-positive
cells in the media and membranes of each vessel section were
counted using Image-Pro Plus System 6.0 (Media Cybernetics, Inc.),
the percentage was calculated, and the average value was determined
as the number of PCNA-positive cells.
Cells and cell culture
Aortic VSMCs were isolated from healthy control
rats, cultured and maintained as previously described (21). Cells were maintained and cultured
in Dulbecco’s modified Eagle’s medium (DMEM, 5 mM glucose)
supplemented with 10% fetal bovine serum (FBS) (both from Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1%
penicillin-streptomycin at 37°C in a humidified environment
containing 95% air and 5% CO2. Cells used in subsequent
experiments were obtained from passages 8 and 10.
MTT assay
Cells seeded in 96-well plates
(3×103/well) were divided into the following six groups:
Control group (without FMN or PDGF-BB treatment), FMN group
(treated with 20 µM FMN for 48 h at 37°C), PDGF-BB group
(treated with 40 ng/ml PDGF-BB for 48 h at 37°C), and FMN + PDGF-BB
groups (pretreated with 5,10 or 20 µM FMN for 24 h, followed
by treatment with PDGF-BB at a final concentration of 40 ng/ml for
24 h at 37°C). Subsequently, an MTT assay was performed by adding
20 µl MTT (5 mg/ml; Sigma-Aldrich; Merck KGaA) to the cells
for 4 h at 37°C. The supernatant was then discarded, 150 µl
dimethyl sulfoxide was added to each well, the plates were agitated
for 10 min and light absorbance was measured at 570 nm using a
microplate reader (PerkinElmer, Inc., Waltham, MA, USA).
Wound-healing assay
VSMCs were seeded into 6-well plates
(3.0×105 cells/well) and were treated as aforementioned
(cells in the FMN + PDGF-BB group were pretreated with 20 µM
FMN for 24 h, and were then treated with PDGF-BB at a final
concentration of 40 ng/ml for 24 h). Once the cells adhered to the
dishes and just before PDGF-BB was added, a sterile plastic
10-µl micropipette tip was used to generate one homogeneous
wound through the cell layer, and the cells were washed twice with
PBS. The wound width in each group was measured under a microscope
(IX51; Olympus Corporation) and images were captured at 100×
magnification; images of the initial wound width and the wound
width after 24 h were obtained. Wound healing percentage (%) =
(initial wound width − 24 h wound width)/initial wound width ×
100.
Cell migration assay
VSMCs were seeded into the upper well of a Transwell
plate (105/well) in 200 µl serum-free DMEM, and
the bottom chambers were filled with 600 µl DMEM containing
10% FBS with saline, FMN and/or PDGF-BB as aforementioned. Cells
were incubated for 24 h at 37°C after all the treatments were
implemented. Subsequently, the chambers were washed gently with
PBS, cells were gently removed from the upper surface of the
chamber with a cotton swab, and the remaining cells in the lower
surface of the chamber were fixed in methanol for 20 min and
stained with crystal violet. Cells that had migrated and remained
on the lower surface of the chamber were counted under a microscope
(BX51; magnification, ×100; Olympus Corporation).
Immunohistochemistry
Carotid artery sections (5 µm) from each
group were dewaxed and dehydrated. Subsequently, the sections were
incubated with 3% hydrogen peroxide in methanol to block endogenous
peroxidase and nonspecific binding was blocked with 5% bovine serum
albumin at room temperature for 10 min (Gibco; Thermo Fisher
Scientific, Inc.). The sections were then incubated with
anti-transforming growth factor (TGF)-β1 primary antibody (1:200,
ab92486; Abcam, Cambridge, UK) at 4°C overnight. An SABC kit
(SA1025) and a DAB kit (AR1022) (both from Wuhan Boster Biological
Technology, Ltd., Wuhan, China) were used for subsequent reactions,
according to the manufacturer’s protocols. Five sections of each
tissue were observed under an optical microscope (magnification,
×400; Olympus Corporation), and Image-Pro Plus System 6.0 (Media
Cybernetics, Inc.) was used to analyze positive cell counts.
Immunocytochemistry
VSMCs were seeded on cover slips in the presence of
DMEM containing 10% FBS in 6-well plates, and were treated as
aforementioned. The expression levels of TGF-β1 in the cells were
detected using an anti-TGF-β1 primary antibody (1:100, ab92486;
Abcam) at 4°C overnight. Subsequently, cells were incubated with a
biotinylated goat anti-rabbit secondary antibody (1:200, ab97049;
Abcam) at 37°C for 10 min. DAB kit (AR1000; Wuhan Boster Biological
Technology, Ltd.) was used for subsequent reactions, according to
the manufacturer’s protocols. The results were analyzed in the same
manner as immunohistochemistry staining.
Western blotting
Western blotting was applied to measure the
expression levels of SMAD family member 3 (Smad3) and
phosphorylated (p)-Smad3 in carotid arteries from rats in each
group, and in VSMCs exposed to various treatments in vitro.
For the in vitro and in vivo assays, VSMCs and
carotid artery tissues were harvested and lysed with
Radioimmunoprecipitation Assay Lysis Buffer (Beyotime Institute of
Biotechnology, Haimen, China). The protein samples were quantified
using a bicinchoninic acid protein assay kits (Beyotime Institute
of Biotechnology) and were separated by 10% SDS-PAGE (30 µg
protein/lane). Following electrophoresis, proteins were
electro-transferred onto polyvinylidene fluoride (PVDF) membranes
(EMD Millipore, Billerica, MA, USA). The PVDF membranes were
blocked with 5% skim milk powder solution for 1.5 h at room
temperature. Protein levels were detected using the following
primary antibodies: Rabbit anti-Smad3 (1:500, ab40854; Abcam),
rabbit anti-p-Smad3 (1:200, ab52903; Abcam) and mouse anti-β-actin
(1:5,000, ab6276; Abcam), which was used as an internal control.
Following incubation with primary antibodies at room temperature
for 2 h, PVDF membranes were washed in 0.05% Tween-20/Tris-buffered
saline, and incubated with horseradish peroxidase-conjugated
secondary antibodies (goat anti-rabbit: 1:2,000, ZB-2301; goat
anti-mouse: 1:5,000, ZDR-5307; OriGene Technologies, Inc., Beijing,
China) at room temperature for 1 h. The bound antibodies were
visualized using an enhanced chemiluminescence reagent (EMD
Millipore). Relative protein expression levels were semi-quantified
by densitometric analysis using the ChemiDoc XRS+ image analyzer
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All data from at least three independent experiments
were expressed as the means ± standard deviation. SPSS 19.0
statistical software (IBM Corp., Armonk, NY, USA) was used to
analyze the variance in data. Data were analyzed using one-way
analysis of variance followed by the least significant difference
post-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
FMN prevents neointima formation in a
balloon-induced carotid artery injury model
Typical histological analysis using hematoxylin and
eosin staining detected marked structural alterations to the
vasculature and narrowing of the vessel cavity in the model group
compared with in the sham group. As shown in Fig. 1, in the model group, serious
intimal hyperplasia was observed, alongside thickened intima and
matrix accumulation, incomplete intimal repair, discontinuous
internal elastic lamina and roughened inner surface of blood
vessels. In addition, VSMCs exhibited abnormal proliferation and
migration from media to the intima, thus indicating successful
establishment of the carotid artery injury model. Conversely, in
the FMN treatment group, abnormal proliferation of VSMCs in
neointima was markedly reduced, and alterations to the vascular
structure were attenuated. Quantitative analysis of neointima
formation demonstrated that, in the FMN treatment group, intima
area and intima/media area ratio were decreased compared with in
the model group (Table I). These
findings suggested that FMN may significantly prevent abnormal
neointimal hyperplasia in a balloon-induced carotid artery injury
model.
 | Table IQuantitative analysis of neointima
formation. |
Table I
Quantitative analysis of neointima
formation.
| Group | Intima area
(mm2) | Media area
(mm2) | Intima/media area
ratio |
|---|
| Model | 19.2±3.4 | 9.8±1.6 | 1.96±0.27 |
| Formononetin | 3.3±0.4a | 7.2±1.1 | 0.45±0.11a |
VSMC proliferation in the intima of a
balloon-induced carotid artery injury model
The results of carotid artery PCNA staining are
presented in Fig. 2; positive
cell nuclei were stained brown. Large amounts of cell proliferation
were detected in the intima and media of the model group (Fig. 2A). The positive cell rate was
significantly increased compared with in the sham group
(P<0.01). After FMN treatment, the number of PCNA-positive cells
was significantly reduced compared with in the model group
(Fig. 2B). These results
indicated that FMN treatment may inhibit abnormal intimal
hyperplasia.
FMN inhibits PDGF-BB-induced VSMC
proliferation
The present results demonstrated that PDGF-BB
treatment alone could stimulate the proliferation of VSMCs, as
revealed by a significantly increased optical density value
compared with in the control group. Conversely, pretreatment with
FMN effectively inhibited PDGF-BB-stimulated proliferation of
VSMCs, and the maximum inhibitory effect was observed in the 20
µM FMN pretreatment group; the findings were not
significantly different compared with in the control group. In
addition, no notable cytotoxicity of FMN was detected, and
treatment with 20 µM FMN alone did not significantly affect
the cell proliferation of VSMCs compared with in the control group
(P>0.05; Fig. 3).
FMN regulates PDGF-BB-induced VSMC
migration
The inhibitory effects of FMN on SMC migration were
examined using a Transwell plate with filters (pore size, 8
µm). The results demonstrated that PDGF-BB could
significantly induce SMC migration (Fig. 4), which was attenuated by
treatment with FMN. The number of migrated cells in the FMN
treatment group was slightly decreased; however, there was no
statistically significant difference compared with in the control
group (P>0.05). Subsequently, the migratory ability of VSMCs was
confirmed using a wound-healing assay; similar trends were observed
as in the Transwell assay. These data suggested that FMN may
significantly decrease the migratory ability of VSMCs induced by
PDGF-BB.
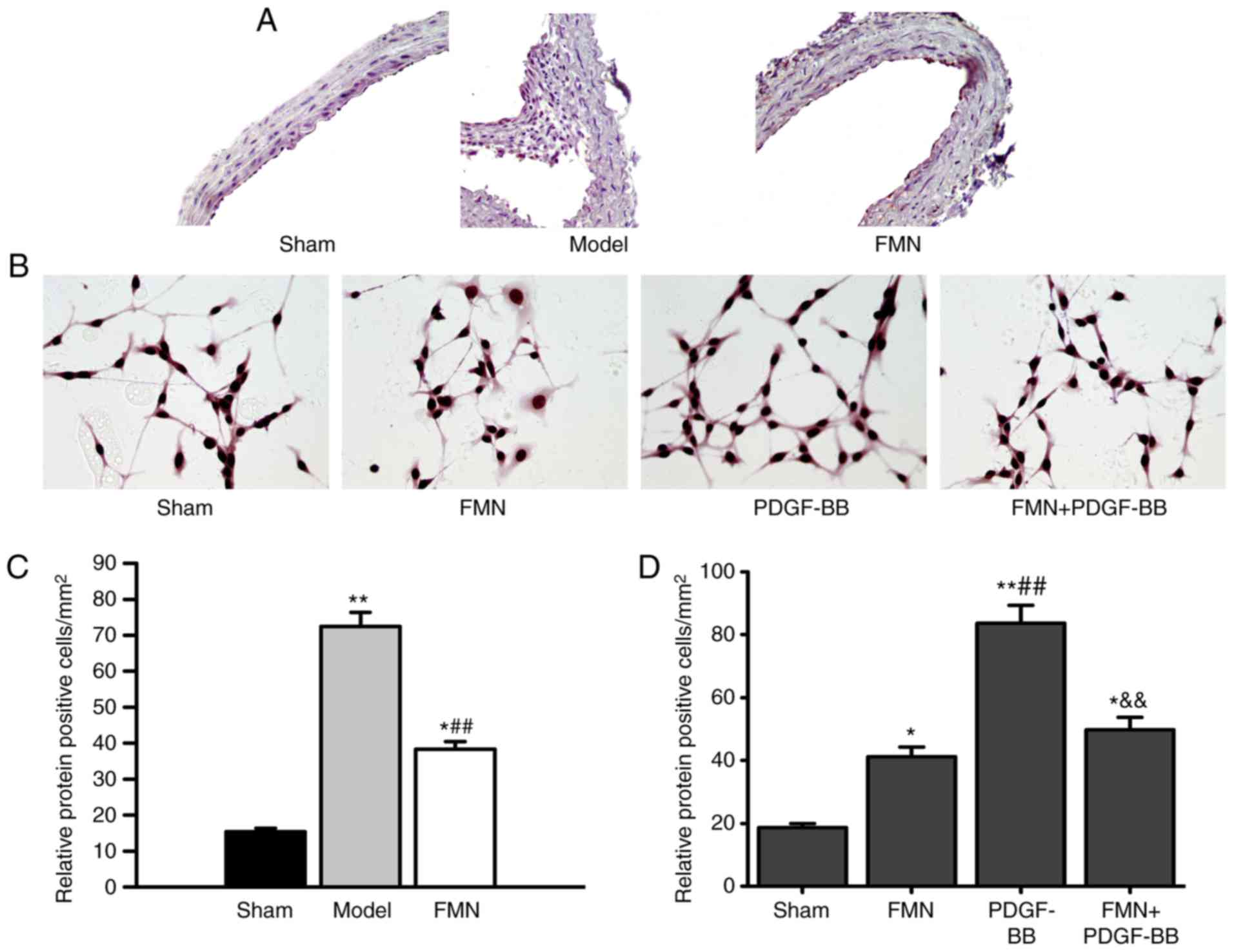
TGF-β1 expression is decreased by FMN
treatment in vivo and in vitro
The expression levels of TGF-β1 in carotid artery
tissues and PDGF-BB-treated cells were detected by
immunohistochemistry and immunocytochemistry, respectively
(Fig. 5). As shown in Fig. 5A and C, the expression levels of
TGF-β1 in the carotid artery tissues of the model group were
markedly elevated compared with in the sham group. FMN treatment
significantly attenuated the increased expression of TGF-β1 in the
carotid artery tissues. To confirm whether FMN could modulate
TGF-β1 expression following artery injury, PDGF-BB stimulation in
VSMCs was used to imitate injury in vitro. The data
indicated that treatment with PDGF-BB markedly increased the
expression levels of TGF-β1 in VSMCs, whereas FMN was able to
neutralize the effects of PDGF-BB (Fig. 5B and D).
FMN regulates Smad3 expression in vivo
and in vitro
To further investigate whether FMN may modulate
TGF-β1/Smad3 signaling, Smad3 and p-Smad3 expression was detected
in carotid artery tissues and VSMCs. As shown in Fig. 6A, in rats with balloon-induced
carotid artery injury, Smad3 and p-Smad3 expression levels were
elevated, whereas FMN treatment significantly reduced the
expression levels. In addition, increased Smad3 and p-Smad3
expression was detected in VSMCs treated with PDGF-BB, whereas FMN
was able to block the stimulatory effects of PDGF-BB. In the FMN
treatment group, Smad3 and p-Smad3 expression was slightly reduced
compared with in the control group; however, this was not
statistically significant (P>0.05).
Discussion
Restenosis is a major limitation of the application
of arterial reconstruction procedures, including balloon
angioplasty, stenting and coronary artery bypass; during the
process of restenosis formation, SMCs migrate from the media toward
the intima, where they abnormally proliferate and undergo
phenotypic changes (22,23). The main findings of the present
study were as follows: i) Marked neointima formation was detected
in a rat model of balloon-induced carotid artery injury, and FMN
treatment significantly prevented neointima formation; ii) in
vitro experiments confirmed that FMN markedly inhibited the
abnormal proliferation of PDGF-BB-induced VSMCs; iii) the
mechanisms underlying the effects of FMN on the inhibition of
neointima formation in a rat model of balloon-induced carotid
artery injury, and the proliferation of PDGF-BB-induced VSMCs, may
be associated with regulation of the TGF-β/Smad3 signaling
pathway.
FMN is a natural isoflavone compound that exists in
numerous medicinal plants, including Caulis Spatholobi,
Astragalus membranaceus and Trifolium pretense
(24–26). Previous studies have revealed that
FMM and its derivatives exhibit cardioprotective (27), vasorelaxation (28,29) and anti-hypertensive effects
(29). Furthermore, it has been
suggested that FMN, as a phytoestrogen, may inhibit vascular
remodeling and neointima formation, thus protecting the
cardiovascular system, which may be clinically useful in preventing
cardiovascular disease in women and men as a safer substitute for
feminizing estrogens (30). Based
on the existing literature, it may be hypothesized that FMN
possesses the ability to ameliorate post-PTCA restenosis.
In our preliminary experiments, it was observed that
FMN could attenuate balloon-induced neointima formation, inhibit
platelet aggregation and reduce PDGF levels in a rat model of
balloon-induced carotid artery injury. In the present study, the
inhibitory effects of FMN were confirmed on neointima formation in
a rat model, as revealed by attenuated alterations in vascular
structure in the injured carotid artery. In addition, FMN-induced
inhibition of PDGF-BB-stimulated SMC proliferation and migration
further verified the potential bioactivity of FMN. The effects of
FMN on proliferation were slightly larger, as cell proliferation
may be faster than migration.
To understand these effects, the present study
further investigated the mechanisms underlying the activity of FMN.
It has been well documented that PDGF and TGF-β1 are critical
growth factors that have important roles in regulating SMC
migration and proliferation under pathological conditions (31). TGF-β1, which is a highly complex
polypeptide and an important member of the TGF-β superfamily, is
believed to have multifunctional properties that serve critical
roles in the pathophysiology of major diseases, including
cardiovascular diseases (32,33). In a previous study, it was
revealed that TGF-β1 expression is observed at an early stage of
acute injury until day 14 after injury, and is decreased by 21 days
(34). The present study detected
elevated secretion of TGF-β1 in the rat model, which could be
reversed by FMN treatment. Furthermore, in the VSMCs in
vitro assay, a similar expression profile of TGF-β1 was
detected after PDGF-BB stimulation as that detected in
vivo.
Previous studies have reported that the TGF-β1/Smad3
signaling pathway is closely associated with neointima formation
following vascular injury (34–36). It has been revealed that TGF-β1
may be involved in restenosis by recruiting mesenchymal stem cells
following arterial injury (34),
and stimulating fibronectin and collagen I/III synthesis in VSMCs
via the Smad3 signaling pathway, thus resulting in ECM deposition
in the neointima (35) and
restenosis (36). It has also
been reported that inhibition of Smad3 expression may compromise
the proliferation of VSMCs (37).
Furthermore, it has been reported that blocking TGF-β1/Smad3 signal
transduction in a rat model of balloon-induced carotid artery
injury could inhibit intimal hyperplasia (38). In the present study, it was
revealed that FMN could alter the TGF-β1/Smad3 signaling pathway
in vivo and in vitro. These results indicated that
FMN may exert its effects via affecting the TGF-β1/Smad3 signaling
pathway, which in turn may markedly inhibit VSMCs proliferation and
migration.
In conclusion, in addition to its previously
demonstrated pharmacological activities, the present study
confirmed the FMN affects neointima formation in a rat model of
balloon-induced carotid artery injury in vivo and
PDGF-BB-induced VSMC proliferation and migration in vitro;
this study provided pre-clinical data to suggest a novel clinical
use for the natural isoflavone FMN. Furthermore, to the best of our
knowledge, the present study was the first to demonstrate that FMN
could adjust PDGF/TGF-β1/Smad3 signaling in vitro and in
vivo, which may be a possible mechanism underlying the effects
of FMN on neointima formation following intimal injury. The
detailed mechanisms regarding how FMN regulates PDGF/TGF-β1/Smad3
signaling transduction require further in-depth investigation.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors’ contributions
TS and YL participated in the design of the study
and performed the statistical analysis. TS, YL, JZ, TJ, XJ and XL
carried out the immunoassays and conceived the study, and
participated in its design and coordination. TS, YL and XL drafted
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by Linyi Peoples’ Hospital
affiliated to Shandong University animal care committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rittger H, Waliszewski M, Brachmann J,
Hohenforst-Schmidt W, Ohlow M, Brugger A, Thiele H, Birkemeyer R,
Kurowski V, Schlundt C, et al: Long-term outcomes after treatment
with a paclitaxel-coated balloon versus balloon angioplasty:
Insights from the PEPCAD-DES study (Treatment of drug-eluting stent
(DES) in-stent restenosis with sequent please paclitaxel-coated
percutaneous transluminal coronary angioplasty (PTCA) catheter).
JACC Cardiovasc Interv. 8:1695–700. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim MS and Dean LS: In-stent restenosis.
Cardiovasc Ther. 29:190–198. 2011. View Article : Google Scholar
|
|
3
|
Renna NF, de Las Heras N and Miatello RM:
Pathophysiology of vascular remodeling in hypertension. Int J
Hypertens. 2013:8083532013.PubMed/NCBI
|
|
4
|
Lee T, Chauhan V, Krishnamoorthy M, Wang
Y, Arend L, Mistry MJ, El-Khatib M, Banerjee R, Munda R and
Roy-Chaudhury P: Severe venous neointimal hyperplasia prior to
dialysis access surgery. Nephrol Dial Transplant. 26:2264–2270.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Curcio A, Torella D and Indolfi C:
Mechanisms of smooth muscle cell proliferation and endothelial
regeneration after vascular injury and stenting: Approach to
therapy. Circ J. 75:1287–1296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qu A, Jiang C, Xu M, Zhang Y, Zhu Y, Xu Q,
Zhang C and Wang X: PGC-1alpha attenuates neointimal formation via
inhibition of vascular smooth muscle cell migration in the injured
rat carotid artery. Am J Physiol Cell Physiol. 297:C645–C653. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berk BC: Vascular smooth muscle growth:
Autocrine growth mechanisms. Physiol Rev. 81:999–1030. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Newby AC and Zaltsman AB: Molecular
mechanisms in intimal hyperplasia. J Pathol. 190:300–309. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nili N, Cheema AN, Giordano FJ, Barolet
AW, Babaei S, Hickey R, Eskandarian MR, Smeets M, Butany J,
Pasterkamp G and Strauss BH: Decorin inhibition of PDGF-stimulated
vascular smooth muscle cell function: Potential mechanism for
inhibition of intimal hyperplasia after balloon angioplasty. Am J
Pathol. 163:869–878. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Strauss BH, Chisholm RJ, Keeley FW,
Gotlieb AI, Logan RA and Armstrong PW: Extracellular matrix
remodeling after balloon-angioplasty injury in a rabbit model of
restenosis. Circ Res. 75:650–658. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schwartz RS, Holmes DR Jr and Topol EJ:
The restenosis paradigm revisited: An alternative proposal for
cellular mechanisms. J Am CollCardiol. 20:1284–1293. 1992.
View Article : Google Scholar
|
|
12
|
Auyeung KK, Law PC and Ko JK: Novel
anti-angiogenic effects of formononetin in human colon cancer cells
and tumor xenograft. Oncol Rep. 28:2188–2194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu T, Liu QM, He XW, Huang F, Zhang MW and
Jiang JG: Identification of bioactives from Astragalus chinensis
L.f. and their antioxidant, anti-inflammatory and
anti-proliferative effects. J Food Sci Technol. 54:4315–4323. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vaya J, Belinky PA and Aviram M:
Antioxidant constituents from licorice roots: Isolation, structure
elucidation and antioxidative capacity toward LDL oxidation. Free
Radic Biol Med. 23:302–313. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clifton-Bligh PB, Nery ML, Clifton-Bligh
RJ, Visvalingam S, Fulcher GR, Byth K and Baber R: Red clover
isoflavones enriched with formononetin lower serum LDL
cholesterol-a randomized, double-blind, placebo-controlled study.
Eur J Clin Nutr. 69:134–142. 2015. View Article : Google Scholar
|
|
16
|
Saik JE, Gould DJ, Watkins EM, Dickinson
ME and West JL: Covalently immobilized platelet-derived growth
factor-BB promotes angiogenesis in biomimetic poly(ethylene glycol)
hydrogels. Acta Biomater. 7:133–143. 2011. View Article : Google Scholar :
|
|
17
|
Salabei JK and Hill BG: Implications of
autophagy for vascular smooth muscle cell function and plasticity.
Free Radic Biol Med. 65:693–703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heldin CH and Westermark B: Mechanism of
action and in vivo role of platelet-derived growth factor. Physiol
Rev. 79:1283–1316. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
National Institutes of Health Pub.
National Institutes of Health Guide for the Care and Use of
Laboratory Animals. No. 85–23, revised 1996.
|
|
20
|
Clowes AW, Reidy MA and Clowes MM:
Kinetics of cellular proliferation after arterial injury. I. Smooth
muscle growth in the absence of endothelium. Lab Investig.
49:327–333. 1983.PubMed/NCBI
|
|
21
|
Keller AC, Knaub LA, McClatchey PM, Connon
CA, Bouchard R, Miller MW, Geary KE, Walker LA, Klemm DJ and Reusch
JE: Differential mitochondrial adaptation in primary vascular
smooth muscle cells from a diabetic rat model. Oxid Med Cell
Longev. 2016:85242672016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zargham R: Preventing restenosis after
angioplasty: A multistage approach. Clin Sci. 114:257–264. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hao H, Gabbiani G and Bochaton-Piallat ML:
Arterial smooth muscle cell heterogeneity: Implications for
atherosclerosis and restenosis development. Arterioscler Thromb
Vasc Biol. 23:1510–1520. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Song T and Jin X: UPLC-MS/MS assay
for simultaneous determination of four compounds in rat plasma:
Application to pharmacokinetic study after oral administration of
Caulis Spatholobi extract. Biomed Chromatogr. 30:1714–1720. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li W, Sun YN, Yan XT, Yang SY, Kim S, Lee
YM, Koh YS and Kim YH: Flavonoids from Astragalusmembranaceus and
their inhibitory effects on LPS-stimulated pro-inflammatory
cytokine production in bone marrow-derived dendritic cells. Arch
Pharm Res. 37:186–192. 2014. View Article : Google Scholar
|
|
26
|
Tava A, Pecio Ł, Stochmal A and Pecetti L:
Clovamide and flavonoids from leaves of Trifoliumpratense and T.
pratense subsp nivale grown in Italy. Nat Prod Commun. 10:933–936.
2015.PubMed/NCBI
|
|
27
|
Zhang S, Tang X, Tian J, Li C, Zhang G,
Jiang W and Zhang Z: Cardioprotective effect of sulphonated
formononetin on acute myocardial infarction in rats. Basic Clin
Pharmacol Toxicol. 108:390–395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu JH, Li Q, Wu MY, Guo DJ, Chen HL, Chen
SL, Seto SW, Au AL, Poon CC, Leung GP, et al: Formononetin, an
isoflavone, relaxes rat isolated aorta through
endothelium-dependent and endothelium-independent pathways. J Nutr
Biochem. 21:613–620. 2010. View Article : Google Scholar
|
|
29
|
Sun T, Liu R and Cao YX: Vasorelaxant and
antihypertensive effects of formononetin through
endothelium-dependent and -independent mechanisms. Acta Pharmacol
Sin. 32:1009–1018. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dubey RK, Gillespie DG, Imthurn B,
Rosselli M, Jackson EK and Keller PJ: Phytoestrogens inhibit growth
and MAP kinase activity in human aortic smooth muscle cells.
Hypertension. 33:177–182. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zeng L, Li Y, Yang J, Wang G, Margariti A,
Xiao Q, Zampetaki A, Yin X, Mayr M, Mori K, et al: XBP 1-deficiency
abrogates neointimal lesion of injured vessels via cross talk with
the PDGF signaling. Arterioscler Thromb Vasc Biol. 35:2134–2144.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kitisin K, Saha T, Blake T, Golestaneh N,
Deng M, Kim C, Tang Y, Shetty K, Mishra B and Mishra L: Tgf-Beta
signaling in development. Sci STKE. 2007:cm12007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Serralheiro P, Soares A, Costa Almeida CM
and Verde I: TGF-β1 in vascular wall pathology: Unraveling chronic
venous insufficiency pathophysiology. Int J Mol Sci. 18:E25342017.
View Article : Google Scholar
|
|
34
|
Pang LJ, Wei CL, Duan JC, Zou H, Cao WW,
Qi Y, Jia W, Hu JM, Zhao W, Jiang JF, et al: TGF-β1/Smad signaling,
MMP-14, and MSC markers in arterial injury: Discovery of the
molecular basis of restenosis. Int J Clin Exp Pathol. 7:2915–2924.
2014.
|
|
35
|
Ryer EJ, Hom RP, Sakakibara K, Nakayama
KI, Nakayama K, Faries PL, Liu B and Kent KC: PKC delta is
necessary for Smad3 expression and transforming growth factor
beta-induced fibronectin synthesis in vascular smooth muscle cells.
Arterioscler Thromb Vasc Biol. 26:780–786. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu P, Wang S, Cai W and Sheng C: Role of
TGF-β1/Smad3 signaling pathway in secretion of type I and III
collagen by vascular smooth muscle cells of rats undergoing balloon
injury. J Biomed Biotechnol. 2012:9659532012. View Article : Google Scholar
|
|
37
|
Cheng ZH, Cai WW, Lu P, Ma SJ, Chen Y and
Sheng J: Effects of signal transduction interruption of
transforming growth factor-β1 by anti-Smad3 on proliferation of
vascular smooth muscle cells. J Shanghai Jiaotong Univ. 29:935–937.
2009.In Chinese.
|
|
38
|
Lu P, Wang S, Cai W and Sheng J: TGF-beta
1/Smad3 expression and its effects on carotid intimal hyperplasia.
Front Biosci. 4:2022–2028. 2012. View
Article : Google Scholar
|