Introduction
Anorectal malformations (ARMs) are common congenital
malformations of the digestive tract manifesting as ectopic anus,
anal stenosis and/or an abnormal fistula between the rectum and
urinary tract, with an incidence of 2-6/10,000 (1-4).
ARMs are usually combined with malformations of other systems, and
although the majority of patients with ARMs require surgery, their
quality of life is likely to be impaired postoperatively (5). Although earlier studies have
revealed that multiple genes and signaling pathways are important
in the pathophysiology of ARMs (3,6),
the specific mechanisms remain to be fully elucidated. Therefore,
it is necessary to investigate the pathogenesis of ARMs.
MicroRNAs (miRNAs) regulate gene expression at the
post-transcriptional level by binding to the 3′-untranslated
regions (3′-UTRs) of target mRNAs, and have been reported to be
important in various congenital diseases and in the embryonic
development of various systems (7-10).
Previous studies have investigated the functions of miRNAs in
gastrointestinal development in various species. For example, miRNA
(miR)-143 was reported to regulate genes involved in the
differentiation of connective tissue cells and to induce the
proliferation and differentiation of smooth muscle cells, thus
influencing bovine gut development (8). As the main effector of miR-17-92
cluster components, a high expression of miR-17-5p in the crypt
progenitor compartment is potentially involved in controlling cell
differentiation and proliferation in human colon development
(11). However, current
understanding of the functions of miRNAs in the development of ARMs
remains limited. An increased understanding of miRNA expression
profiles may reveal their functional roles in ARM development, thus
providing a valuable basis for investigating the mechanisms
responsible for this condition.
Ethylenethiourea (ETU)-induced ARM rat fetuses are
the most commonly used animal model for investigating this disease,
and the key abnormalities in the experimental embryos include the
following: i) no fusion between the urorectal septum and cloacal
membrane; ii) delay of tailgut regression; iii) abnormal apoptosis
in the cloacal wall; iv) underdevelopment of the dorsal cloaca and
its membrane (12,13). In the present study, miRNA
expression patterns in the hindgut of rat fetuses with ETU-induced
ARMs were profiled between gestational day (GD)14 and GD16, which
are critical time-points during anorectal development (4,12-14). Additionally, advanced
bioinformatics analysis was performed, including target gene
prediction, construction of regulatory networks and functional
enrichment analyses to clarify the molecular mechanisms involved in
the development of ARMs.
Materials and methods
Ethics statement
The present study was approved by the China Medical
University Ethics Committee (Shenyang, China; no. 2015 PS213K) and
all procedures involving animals were performed in accordance with
the guidelines for the care and use of laboratory animals (15).
Animal models and tissue preparation
Mature female Wistar rats (n=36; age, 7-9 weeks-old;
body weight, 250-300 g) were provided by the Experimental Animal
Center of Shengjing Hospital of China Medical University (Shenyang,
China) and housed in a specific-pathogen-free animal laboratory
(room temperature, 22±2°C; humidity, 55±5%; 12 h light/dark cycle
and ad libitum access to water and food) at The Key
Laboratory of Health Ministry for Congenital Malformations
(Shenyang, China). The surgical procedures were performed following
sacrifice by intraperitoneal injection of sodium pentobarbital, and
all efforts were made to minimize animal suffering.
ARM was induced in fetal rats as described in
earlier reports (4). Briefly, 18
pregnant rats were gavage-fed a single dose of 125 mg/kg of 1% ETU
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) on GD10,
whereas the remaining 18 pregnant rats received corresponding doses
of ETU-free saline as a control. The rat fetuses were obtained by
cesarean delivery on GD14-16, and the presence of ARMs was
determined by light microscopy.
No malformations were observed in the 258 embryos of
the normal rats. Among the ETU-treated embryos, all 236 embryos had
a short or absent tail, and 14 died in utero. The incidence
of ARMs in the ETU-treated embryos was 85.6% (202/236).
miRNA profiling and microarray
analysis
Assessment of RNA quality
Total RNA was isolated using an miRNeasy Mini kit
(Qiagen GmbH, Hilden, Germany) according to the manufacturer's
protocol. RNA quality was assessed using a K5500
micro-spectrophotometer (Shanghai Drawell Scientific Instrument
Co., Ltd., Shanghai, China) and formaldehyde agarose gel
electrophoresis. Values of absorbance (A)260/A280 ≥1.5 and
A260/A230 ≥1 indicated acceptable RNA purity, and an RNA integrity
number ≥7 from the Agilent 2200 RNA assay (Agilent Technologies,
Inc., Santa Clara, CA, USA) indicated acceptable RNA integrity.
miRNA microarray assay
miRNA profiling was performed using the RiboArray
platform (Ribobio Co., Ltd., Guangzhou, China). In brief, l
μg of total RNA was labeled with Cy3 using the ULS™ microRNA
labeling kit (Kreatech; Leica Microsystems, Inc., Buffalo Grove,
IL, USA) and hybridized on the microarray. The microarray contained
761 specific oligos for rat miRNAs, based on the Sanger miRBase
21.0 database (www.mirbase.org/).
Data normalization and analysis
Firstly, chip images were examined to exclude
impurity, scratch marks and high background intensity. Following
background adjustment by subtraction of background from foreground,
the microarray data were normalized using a quantile (median)
method and box-plots were generated based on the relative logarithm
expression using the limma package in R software (version 3.3.0;
www.r-project.org). Three-dimensional principal
component analysis was performed using the scatter-plot3d package
in R. Analysis of the differentially expressed miRNAs was performed
using the limma package in R. Standard selection criteria for
identifying the differentially expressed miRNAs were established as
a fold change (FC) >2 or <−2, and P<0.05. Hierarchical
clustering analysis of the miRNAs was performed and graphs were
generated using the gplots package in R software.
Target gene prediction, Gene Ontology
(GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG)
analyses
miRNAs with |log2(FC)| >4.25 were
selected for further analysis. Target genes of these miRNAs were
predicted using the target gene-prediction platforms miRWalk 3.0
(mirwalk.umm.uni-heidelberg.de/), TargetScan 7.1 (www.targetscan.org/vert_71/), miRBase 22
(www.mirbase.org/), miRDB (mirdb.org/miRDB/), and
DIANA v5.0 (diana.imis.athena-innovation.
gr/DianaTools/index.php?r=site/page&view=software). Those
miRNAs predicted by at least three databases were considered as
predicted target genes. Experimentally validated target genes were
identified using miRWalk 3.0.
The biological functions of the key miRNAs were
determined by GO clustering, including the three domains
'biological process' (BP), 'cellular component' (CC) and 'molecular
function' (MF), and KEGG pathway analysis of the target genes using
the Database for Annotation, Visualization and Integrated Discovery
Bioinformatics Resources 6.7 (david-d.ncifcrf.gov/); corresponding
results were displayed using the ggplot2 and gcookbook packages in
R software.
Regulatory networks of the miRNA-target genes and
miRNA-target gene signaling pathways were constructed and
visualized using Cytoscape 3.4.0 (www.cytoscape.org/) in the Java Environment (16).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT was performed using a PrimeScript RT Reagent kit
(Takara Biotechnology Co., Ltd., Dalian, China). Stem-loop RT
primers and PCR primers for each miRNA were purchased from Ribobio
Co., Ltd. (cat. nos. U6 MQP-0202, rno-mir-125b-2-3p
miRQ0004731-1-1, rno-mir-187-5p miRQ0017144-1-1, rno-mir-3542
miRQ0017796-1-1, rno-mir-92a-2-5p miRQ0017108-1-1 and
rno-mir-99a-5p miRQ0000820-1-1). Fluorescence qPCR was performed on
an ABI 7500 detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Each 20 μl reaction
mixture contained 2 μl template cDNA, 0.4 μM of each
gene-specific primer, 10 μl SYBR Premix Ex Taq II (Tli
RNaseH Plus, 2X), 0.4 μl ROX Reference Dye II (50X), and 6
μl sterilized Rnase-free water. The reactions were incubated
at 50°C for 2 min, 95°C for 2 min, followed by 40 cycles of 95°C
for 15 sec, and 60°C for 60 sec. The reactions were incubated at
50°C for 2 min, 95°C for 2 min, followed by 40 cycles of 95°C for
15 sec, and 60°C for 60 sec. With stable expression in the normal
and ARM groups, U6 was selected as an endogenous reference gene for
qPCR normalization purposes and the relative expression level of
each miRNA was calculated using the 2−ΔΔCq method
(17). RT-qPCR analysis was
performed with three replicates.
Statistical analysis
Statistical analysis was performed using SPSS 21.0
software (IBM SPSS, Armonk, NY, USA). Statistically significant
differences were identified using Student's t-test (two-tailed).
All values are presented as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Screening of differentially expressed
miRNAs and clustering analysis
The miRNAs potentially involved in ARM development
were investigated using microarray screening of differentially
expressed miRNAs at key time-points during anus formation (GD14,
GD15 and GD16). A heatmap of the 761 miRNAs at the three
time-points is shown in Fig. 1A.
The results of the microarray screening revealed different
expression patterns between the normal and ARM groups, and the
majority of miRNAs exhibited increased expression in the ARM group.
In the normal group, a higher expression level of miRNAs was
detected at GD15 compared with GD14 and GD16, and the expression
trend was opposite to that in the ARM group.
A total of 38 miRNAs were upregulated on GD14 in the
ARM group compared with the normal group; 32 were upregulated and
17 were downregulated at GD15 in the ARM group compared with the
normal group; and 42 were upregulated at GD16 in the ARM group
compared with the normal group. The overlap of these miRNAs between
the three time-points is shown in Fig. 1B. The detailed information and
expression patterns of the differentially expressed miRNAs at the
three time-points are shown in Table
I and Fig. 1C.
 | Table IDifferentially expressed miRNAs at
GD14, GD15 and GD16. |
Table I
Differentially expressed miRNAs at
GD14, GD15 and GD16.
| miRNA | lgFC | FDR |
|---|
| G14; N vs. A
(38) | | |
| miR-125b-2-3p | 5.6523 | 0.0181 |
| miR-92a-2-5p | 4.9863 | 0.0033 |
| miR-99a-5p | 4.7777 | 0.0207 |
| miR-331-3p | 4.7257 | 0.0171 |
| miR-410-3p | 4.5907 | 0.0124 |
| miR-26b-3p | 4.2617 | 0.0215 |
| miR-190a-3p | 4.2564 | 0.0052 |
| miR-212-5p | 4.1900 | 0.0495 |
| miR-802-5p | 4.1618 | 0.0230 |
| miR-3586-3p | 4.1185 | 0.0394 |
| miR-143-5p | 4.1014 | 0.0133 |
| miR-132-3p | 4.0487 | 0.0068 |
| miR-200a-3p | 3.9410 | 0.0020 |
| miR-6319 | 3.8708 | 0.0209 |
| miR-497-3p | 3.8449 | 0.0105 |
| miR-29a-3p | 3.7200 | 0.0008 |
| miR-133a-5p | 3.6877 | 0.0338 |
| miR-501-5p | 3.4335 | 0.0379 |
| miR-369-5p | 3.4053 | 0.0480 |
| miR-322-5p | 3.3726 | 0.0006 |
| miR-146a-3p | 3.1359 | 0.0068 |
| miR-9b-3p | 3.0811 | 0.0077 |
| miR-31b | 3.0555 | 0.0216 |
| miR-301b-3p | 3.0376 | 0.0278 |
| let-7i-3p | 3.0366 | 0.0087 |
| miR-291b | 3.0339 | 0.0077 |
| miR-490-3p | 2.9198 | 0.0040 |
| miR-30e-3p | 2.8834 | 0.0112 |
| miR-421-3p | 2.8269 | 0.0162 |
| miR-6329 | 2.6684 | 0.0479 |
| miR-411-5p | 2.4638 | 0.0261 |
| miR-344i | 2.2869 | 0.0288 |
| miR-326-3p | 1.8954 | 0.0491 |
| miR-376b-5p | 1.8833 | 0.0128 |
| miR-666-3p | 1.8752 | 0.0217 |
| miR-192-3p | 1.7802 | 0.0307 |
| miR-3557-5p | 1.6589 | 0.0399 |
| miR-376b-3p | 1.0623 | 0.0007 |
| G15; N vs. A
(49) | | |
| miR-741-3p | 4.9521 | 0.0013 |
| miR-935 | 4.9112 | 0.0077 |
| miR-542-5p | 3.3498 | 0.0025 |
| miR-1249 | 3.2639 | 0.0007 |
| miR-5132-3p | 2.1773 | 0.0133 |
| miR-129-2-3p | 2.0751 | 0.0011 |
| miR-409b | 1.9867 | 0.0165 |
| miR-7a-1-3p | 1.8004 | 0.0432 |
| miR-3550 | 1.7798 | 0.0096 |
| miR-349 | 1.6717 | 0.0172 |
| miR-874-5p | 1.6689 | 0.0232 |
| miR-3547 | 1.6623 | 0.0007 |
| miR-551b-5p | 1.6278 | 0.0027 |
| miR-361-3p | 1.5764 | 0.0205 |
| miR-3546 | 1.5650 | 0.0199 |
| miR-455-3p | 1.5239 | 0.0484 |
| miR-212-3p | 1.5230 | 0.0169 |
| miR-743a-3p | 1.4840 | 0.0116 |
| miR-196a-3p | 1.3656 | 0.0000 |
| miR-28-5p | 1.3441 | 0.0328 |
| miR-194-3p | 1.3365 | 0.0083 |
| miR-15b-5p | 1.3145 | 0.0170 |
| miR-191b | 1.2964 | 0.0156 |
| miR-540-3p | 1.2545 | 0.0328 |
| let-7b-3p | 1.2488 | 0.0363 |
| miR-191a-5p | 1.2430 | 0.0180 |
| miR-6315 | 1.1330 | 0.0307 |
| miR-125b-5p | 1.1254 | 0.0038 |
| miR-125a-5p | 1.1246 | 0.0073 |
| miR-210-3p | 1.1245 | 0.0308 |
| miR-652-5p | 1.0856 | 0.0302 |
| miR-20b-5p | 1.0384 | 0.0139 |
| miR-144-5p | −6.6610 | 0.0130 |
| miR-539-3p | −5.9386 | 0.0043 |
| miR-708-5p | −5.4602 | 0.0468 |
| miR-412-5p | −5.1433 | 0.0053 |
| miR-187-5p | −4.9449 | 0.0021 |
| miR-3542 | −4.7610 | 0.0261 |
| miR-224-3p | −4.2339 | 0.0112 |
| miR-101a-5p | −4.2198 | 0.0133 |
| miR-200c-5p | −4.0638 | 0.0208 |
| miR-19b-2-5p | −3.4133 | 0.0476 |
| miR-384-5p | −3.2683 | 0.0244 |
| miR-135a-5p | −2.8032 | 0.0037 |
| miR-204-3p | −2.7952 | 0.0009 |
| miR-741-5p | −2.7242 | 0.0060 |
| let-7f-5p | −2.2251 | 0.0124 |
| miR-702-5p | −1.4132 | 0.0039 |
| miR-449c-3p | −1.2088 | 0.0303 |
| G16; N vs. A
(42) | | |
| miR-409a-5p | 6.3869 | 0.0271 |
| miR-92a-2-5p | 4.8504 | 0.0128 |
| miR-484 | 4.7546 | 0.0084 |
| miR-673-3p | 4.5965 | 0.0110 |
| miR-125b-2-3p | 4.3396 | 0.0171 |
| miR-3542 | 4.2864 | 0.0007 |
| miR-127-5p | 4.2316 | 0.0246 |
| miR-6325 | 4.1901 | 0.0126 |
| miR-23b-5p | 4.1463 | 0.0001 |
| miR-3068-3p | 3.9677 | 0.0370 |
| miR-99a-5pa | 3.8542 | 0.0053 |
| miR-143-5p | 3.7453 | 0.0142 |
| miR-6318 | 3.7171 | 0.0420 |
| miR-9a-5p | 3.6453 | 0.0070 |
| miR-871-3p | 3.5678 | 0.0002 |
| miR-29b-1-5p | 3.5150 | 0.0137 |
| miR-219a-5p | 3.4518 | 0.0295 |
| miR-1912-5p | 3.2806 | 0.0171 |
| miR-187-5pa | 3.2137 | 0.0074 |
| miR-9a-3p | 3.1949 | 0.0292 |
| miR-296-5p | 2.8674 | 0.0025 |
| miR-483-3p | 2.6111 | 0.0454 |
| miR-500-3p | 2.5249 | 0.0157 |
| miR-881-3p | 2.4781 | 0.0438 |
| miR-665 | 2.4316 | 0.0108 |
| miR-3552 | 2.3654 | 0.0418 |
| miR-351-5p | 2.1768 | 0.0025 |
| miR-742-5p | 2.0011 | 0.0080 |
| miR-708-3p | 1.9967 | 0.0259 |
| miR-324-5p | 1.9943 | 0.0130 |
| miR-3573-3p | 1.9430 | 0.0298 |
| miR-29b-5p | 1.8790 | 0.0485 |
| miR-125a-5p | 1.8771 | 0.0059 |
| miR-485-5p | 1.7577 | 0.0154 |
| miR-129-2-3p | 1.6690 | 0.0001 |
| miR-191a-5p | 1.6207 | 0.0372 |
| miR-204-3p | 1.6186 | 0.0182 |
| let-7d-5p | 1.6180 | <0.0001 |
| miR-3084b-5p | 1.5984 | 0.0362 |
| miR-125b-5p | 1.2629 | 0.0247 |
| miR-702-5p | 1.2500 | 0.0419 |
| miR-30c-1-3p | 1.1605 | 0.0216 |
Predicted and validated target genes of
miRNAs
There were 18 differentially expressed miRNAs with
|log2(FC)| >4.25 (miR-125b-2-3p, miR-144-5p,
miR-187-5p, miR-190a-3p, miR-26b-3p, miR-331-3p, miR-3542,
miR-409a-5p, miR-410-3p, miR-412-5p, miR-484, miR-539-3p,
miR-673-3p, miR-708-5p, miR-741-3p, miR-92a-2-5p, miR-935 and
miR-99a-5p), which were selected for further analysis. The numbers
of predicted target genes for the above miRNAs were 229, 27, 104,
81, 177, 54, 219, 19, 57, 1, 112, 66, 17, 43, 143, 642, 148 and 5,
respectively. Bone morphogenetic protein receptor type 2 (BMPR2)
was the only experimentally validated target gene of miR-99a-5p in
rats (18). The predicted and
experimentally validated target genes of the 18 miRNAs were
subjected to further analysis. The regulatory network between the
miRNAs and their target genes is presented in Fig. 1D.
GO and KEGG enrichment analyses, and
construction of the regulatory network
According to GO analysis of the target genes of the
18 miRNAs, the functions of DNA binding and gene regulation,
metabolic and biosynthetic process of biomolecule, differentiation
and projection of neuron were enriched. The top 15 enriched terms
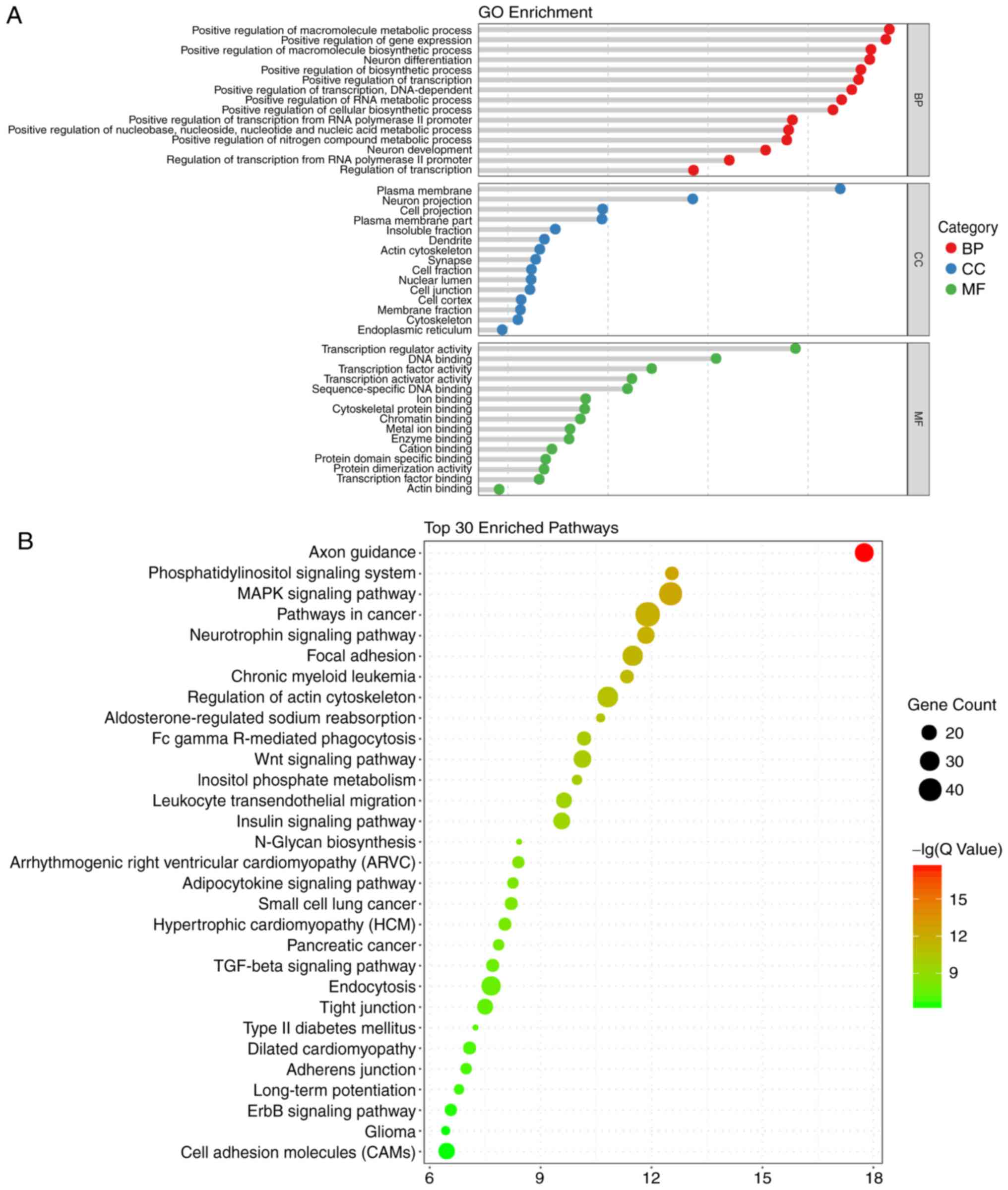
in the BP, CC and MF terms are presented in Fig. 2A.
In terms of the KEGG analysis, a total of 49
signaling pathways were enriched, and the top 30 are listed in
Fig. 2B. Among those pathways,
'axon guidance' was enriched the most, followed by
'phosphatidylinositol signaling system'. Notably, the enriched
pathways included the Wingless Type MMTV integration site family
(Wnt), mitogen-activated protein kinase (MAPK), and transforming
growth factor-β (TGF-β) pathways, which are known to be closely
associated with the process of embryonic development and ARM
(6,19-21). Therefore, miRNA target
gene-pathway networks were constructed for the miRNAs and target
genes contributing to these three signaling pathways (Fig. 3A). A total of 15 core miRNAs
associated with the signaling pathways of interest were screened
out: miR-92a-2-5p, miR-125b-2-3p, miR-935, miR-190a-3p, miR-741-3p,
miR-26b-3p and miR-539-3p were potentially associated with all
three pathways; miR-484, miR-3542, miR-708-5p and miR-187-5p were
potentially associated with two pathways; and miR-410-3p,
miR-331-3p, miR-673-3p and miR-99a-5p were potentially associated
with only one pathway. As demonstrated by the miRNA target
gene-signaling pathway network, miR-92a-2-5p exhibited the most
extensive connection with target genes in the relevant
pathways.
Validation of important miRNAs
Among the 15 core differentially expressed miRNAs,
five (miR-125b-2-3p, miR-92a-2-5p, miR-99a-5p, miR-187-5p and
miR-3542) were differentially expressed at two time-points, and
were selected for RT-qPCR validation (Fig. 3B). For all miRNAs, the relative
expression levels were consistent with the microarray results
(Fig. 3C). There were significant
differences between the normal and ARM group for three miRNAs
(miR-125-2-3p, miR-92a-2-5p and miR-99a-5p).
Discussion
The role of miRNAs in embryonic development and
congenital deformities of gastrointestinal system have been
described, however, the association between miRNAs and ARMs remains
to be fully elucidated (8,10,11,22-24).
Examining miRNA expression patterns may assist in identifying the
pathogenesis of ARM. In the present study, miRNAs that may be
involved in the course of ARM were profiled and bioinformatics
analyses were performed to reveal the potential mechanism.
According to previous morphological investigations,
rectourethal fistula and a common cloaca were present in all ARM
rat fetuses, as a result of failed fusion of the urorectal septum
and cloacal membrane at GD15 (4,13,25). Therefore, the present study
detected aberrantly expressed miRNAs in ARM fetuses compared with
normal rat fetuses at key time-points for anus formation (GD14,
GD15 and GD16). The number of differentially expressed miRNAs at
each time-point was 38, 49 and 42, respectively. Hierarchical
analysis revealed that the increases of miRNAs in the ARM group
were predominant, suggesting that increases in miRNAs may be one of
the factors causing ARMs. In the normal group, there were
differences in miRNA expression between the three time-points,
which may be critical for embryonic development and
organogenesis.
From the microarray results, 18 miRNAs with the
highest significant FC values were selected for further
bioinformatics analysis. By predicting target genes and searching
for experimentally validated targets, an miRNA-target gene
regulatory network was constructed. In a previous study by Jin
et al (9), aberrantly
expressed miRNAs in the terminal hindgut following the formation of
ARM in Sprague-Dawley rat fetuses were profiled by the miRNA
microarray, and miR-193 was screened out with |FC| >2. The
present study focused on differentially expressed miRNAs prior to
ARM having fully developed (GD14 and GD15), and the inclusion
criteria of miRNAs selected for further bioinformatics analysis was
|log2(FC)| >4.25; therefore, none of the miR-193
family members, including miR-193a-3p, miR-193a-5p, miR-193b-3p and
miR-193b-5p, were included in the miRNAs selected for further
analysis. Differences in the chip array and the samples used may
also be factors in the discrepancy between these two studies.
Further functional analysis indicated that the
target genes of the differentially expressed miRNAs were enriched
in processes, including gene regulation and neuron development, and
KEGG pathway analysis indicated that 49 significant signaling
pathways were enriched, including the Wnt, MAPK and TGF-β signaling
pathways, which have also previously been reported to be closely
associated with ARM (6,19-21).
The neuron-associated terms that were enriched in
the GO and KEGG functional analyses indicated that these miRNAs,
were not only potentially associated with the development of ARMs,
but may also be involved in neurode-velopmental abnormalities,
which often appear concurrently with ARMs (26,27). In addition to the Wnt, MAPK and
TGF-β pathways, the BMP signaling pathway is also important in ARMs
(28-30). Wnt/β-catenin signal transduction
dysregulation can lead to an ARMs phenotype (31). Studies have revealed that this may
be caused by abnormal BMP signaling, as BMP4 and BMP7 were
abnormally increased in the β-catenin-induced ARM phenotype, and
furthermore, knockout of the BMP receptor, BMPR1A, partially
rescued the malformation (32,33). However, in the previous study,
only the BMRP1A receptor was knocked out, whereas the other BMP
receptor, BMPR2, was not altered. Therefore, whether or not the
knockout of BMPR2 and BMPR1A together is able to rescue the
deformity to a greater degree, remains to be elucidated.
In the present study, the potential regulatory roles
of the miRNAs and their corresponding target genes included in the
enriched Wnt, MAPK and TGF-β signaling pathways were examined by
constructing miRNA-target gene-pathway regulatory networks, which
identified 15 core miRNAs indirectly associated with the these
three signaling pathways. Among the 15 core miRNAs, five miRNAs
(miR-125b-2-3p, miR-92a-2-5p, miR-99a-5p, miR-187-5p and miR-3542)
were selected for RT-qPCR validation, as they were differentially
expressed at two time-points in the microarray. The results
revealed that the relative expression levels of miR-125b-2-3p,
miR-92a-2-5p and miR-99a-5p were consistent with the microarray,
with statistical significance.
miR-92 is a member of the miR-17-92 cluster, which
can regulate fetal development at the early stages, and may promote
proliferation and inhibit differentiation during embryogenesis
(11,34). miR-125, which is a highly
conserved miRNA family, can regulate early embryonic development by
affecting cell fate and differentiation (35-37). Previously, GO analysis
demonstrated that miR-125 controls axon guidance pathways, synaptic
plasticity and catabolic processes (38). This GO and KEGG analysis was
similar to the functional enrichment results in the present study,
which suggests that miR-125 may be crucial in the pathogenesis of
ARM. Regarding the miR-99a/100-125b cluster, different members can
exert homogenous functions. RNAs of the miR-125 family can directly
bind to the 3′-UTR of p53, B cell lymphoma-2 (Bcl-2), Bcl-2-like 12
and Mcl-1, and regulate cell proliferation and apoptosis (39,40). Similarly, miR-99 family members
can reduce the expression of homeobox A1, which downregulates the
expression of the downstream Bcl-2 gene, and leads to reduced cell
survival and enhanced apoptosis (41). In hematopoietic stem and
progenitor cells, miR-99a/100-125b can regulate homeostasis by
shifting the balance between TGF-β and Wnt signaling (42); and in early chondrogenic
differentiation in rats, miR-99a and miR-125b also have critical
regulatory roles, with miR-99a being particularly important and
negatively regulating chon-drogenic differentiation by directly
targeting BMPR2 (18). These
previous studies revealed that the dysregulation of
apoptosis-associated genes, BMP family genes and homeobox genes are
associated with ARM; however, the functions exerted by miRNAs in
the disease remain to be fully elucidated, and further
investigation is required.
In conclusion, the dynamic changes in differentially
expressed miRNAs were investigated in normal and ARM rat fetuses at
key time-points during anorectal development (GD14-16). The
aberrant expression of miR-125b-2-3p, miR-92a-2-5p and miR-99a-5p
during this period suggested that these miRNAs may be involved in
ARM in rats. However, the result of this investigation into ARMs
are preliminary, and further investigations are required to
investigate the potential target genes which may contribute to ARM
pathogenesis. In addition, normal and ARM fetuses were compared to
screen miRNAs that may function in the course of this disease,
however, the study did not evaluate miRNA expression in fetuses
without the ARM phenotype following ETU treatment, which may assist
in revealing the protective mechanisms that occur to avoid ARM
following ETU stimulation. Further in vivo and in
vitro investigations are also required to clarify the specific
mechanisms responsible for the development of ARMs.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81470788 and 81770511), the
Project of Key Laboratory of the Education Department of Liaoning
Province (grant no. LS201601) and the Outstanding Scientific Fund
of Shengjing Hospital (grant no. 201502).
Availability of data and materials
Data sharing is not applicable, as no datasets were
generated or analyzed during the study.
Authors' contributions
YB and XT conceived and designed the experiments; CL
performed the experiments; CL analyzed the data; ZY and WW
contributed to data interpretation; CL wrote the manuscript.
Ethics approval and consent to
participate
The present study was approved by the China Medical
University Ethics Committee (no. 2015 PS213K).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
miRNAs
|
microRNAs
|
|
ARMs
|
anorectal malformations
|
|
ETU
|
ethylenethiourea
|
|
GD
|
gestational day
|
|
FC
|
fold change
|
|
3′-UTRs
|
3′-untranslated regions
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genome
|
|
Wnt
|
Wingless type MMTV integration site
family
|
|
MAPK
|
mitogen-activated protein kinase
|
|
TGF-β
|
transforming growth factor-β
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
BMP
|
bone morphogenetic protein
|
Acknowledgments
The authors would like to thank Professor Jie Liu
(Ph.D., Science Experiment Center of China Medical University.
Shenyang, China) and Mr. Liangcai Wu (M.M., Shanghai First
Maternity and Infant Hospital, First Maternity and Infant Hospital
Affiliated to Tongji University, Shanghai, China) for their
assistance in discussions during the course of the investigations.
Additionally, they are acknowledged for their critical and
insightful comments on the manuscript received from reviewers.
References
|
1
|
Cuschieri A; EUROCAT Working Group:
Descriptive epidemiology of isolated anal anomalies: A survey of
4.6 million births in Europe. Am J Med Genet. 103:207–215. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Blaauw I, Wijers CH, Schmiedeke E,
Holland-Cunz S, Gamba P, Marcelis CL, Reutter H, Aminoff D,
Schipper M, Schwarzer N, et al: First results of a European
multi-center registry of patients with anorectal malformations. J
Pediatr Surg. 48:2530–2535. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wijers CH, van Rooij IA, Marcelis CL,
Brunner HG, de Blaauw I and Roeleveld N: Genetic and nongenetic
etiology of nonsyndromic anorectal malformations: A systematic
review. Birth Defects Res C Embryo Today. 102:382–400. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bai Y, Chen H, Yuan ZW and Wang W: Normal
and abnormal embryonic development of the anorectum in rats. J
Pediatr Surg. 39:587–590. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grano C, Bucci S, Aminoff D, Lucidi F and
Violani C: Quality of life in children and adolescents with
anorectal malformation. Pediatr Surg Int. 29:925–930. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Draaken M, Prins W, Zeidler C, Hilger A,
Mughal SS, Latus J, Boemers TM, Schmidt D, Schmiedeke E, Spychalski
N, et al: Involvement of the WNT and FGF signaling pathways in
non-isolated anorectal malformations: Sequencing analysis of WNT3A,
WNT5A, WNT11, DACT1, FGF10, FGFR2 and the T gene. Int J Mol Med.
30:1459–1464. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dong R, Shen Z, Zheng C, Chen G and Zheng
S: Serum microRNA microarray analysis identifies miR-4429 and
miR-4689 are potential diagnostic biomarkers for biliary atresia.
Sci Rep. 6:210842016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang G, Malmuthuge N, McFadden TB, Bao H,
Griebel PJ, Stothard P and Guan le L: Potential regulatory role of
microRNAs in the development of bovine gastrointestinal tract
during early life. PLoS One. 9:e925922014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin S, Wang J, Chen H and Xiang B:
Differential miRNA expression analysis during late stage terminal
hindgut development in fetal rats. J Pediatr Surg. 52:1516–1519.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li S, Wang S, Guo Z, Wu H, Jin X, Wang Y,
Li X and Liang S: miRNA profiling reveals dysregulation of RET and
RET-regulating pathways in Hirschsprung's disease. PLoS one.
11:e01502222016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Monzo M, Navarro A, Bandres E, Artells R,
Moreno I, Gel B, Ibeas R, Moreno J, Martinez F, Diaz T, et al:
Overlapping expression of microRNAs in human embryonic colon and
colorectal cancer. Cell Res. 18:823–833. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Macedo M, Martins JL and Meyer KF:
Evaluation of an experimental model for anorectal anomalies induced
by ethyl-enethiourea. Acta Cir Bras. 22:130–136. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qi BQ, Beasley SW and Frizelle FA:
Clarification of the processes that lead to anorectal malformations
in the ETU-induced rat model of imperforate anus. J Pediatr Surg.
37:1305–1312. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Faria DJ, Simões Mde J and Martins JL: Is
it possible folic acid reduce anorectal malformations
ethylenethiourea induced in rats? Acta Cir Bras. 30:517–522. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press; Washington, DC: 2011
|
|
16
|
Kohl M, Wiese S and Warscheid B:
Cytoscape: Software for visualization and analysis of biological
networks. Methods Mol Biol. 696:291–303. 2011. View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Zhou X, Wang J, Sun H, Qi Y, Xu W, Luo D,
Jin X, Li C, Chen W, Lin Z, et al: MicroRNA-99a regulates early
chondrogenic differentiation of rat mesenchymal stem cells by
targeting the BMPR2 gene. Cell Tissue Res. 366:143–153. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang C, Li L and Cheng W: Anorectal
malformation: The etiological factors. Pediatr Surg Int.
31:795–804. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wong EH, Ng CL, Lui VC, So MT, Cherny SS,
Sham PC, Tam PK and Garcia-Barceló MM: Gene network analysis of
candidate loci for human anorectal malformations. PLoS One.
8:e691422013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakamura T, Tsuchiya K and Watanabe M:
Crosstalk between Wnt and Notch signaling in intestinal epithelial
cell fate decision. J Gastroenterol. 42:705–710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lei H, Tang J, Li H, Zhang H, Lu C, Chen
H, Li W, Xia Y and Tang W: MiR-195 affects cell migration and cell
proliferation by downregulating DIEXF in Hirschsprung's disease.
BMC Gastroenterol. 14:1232014. View Article : Google Scholar
|
|
23
|
Park C, Yan W, Ward SM, Hwang SJ, Wu Q,
Hatton WJ, Park JK, Sanders KM and Ro S: MicroRNAs dynamically
remodel gastrointestinal smooth muscle cells. PLoS One.
6:e186282011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang W, Tang J, He J, Zhou Z, Qin Y, Qin
J, Li B, Xu X, Geng Q, Jiang W, et al:
SLIT2/ROBO1-miR-218-1-RET/PLAG1: A new disease pathway involved in
Hirschsprung's disease. J Cell Mol Med. 19:1197–1207. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matsumaru D, Murashima A, Fukushima J,
Senda S, Matsushita S, Nakagata N, Miyajima M and Yamada G:
Systematic stereoscopic analyses for cloacal development: The
origin of anorectal malformations. Sci Rep. 5:139432015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang W, Jia H, Zhang H, Chen Q, Zhang T,
Bai Y and Yuan Z: Abnormal innervation patterns in the anorectum of
ETU-induced fetal rats with anorectal malformations. Neurosci Lett.
495:88–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guan K, Li H, Fan Y, Wang Y and Yuan Z:
Defective development of sensory neurons innervating the levator
ani muscle in fetal rats with anorectal malformation. Birth Defects
Res A Clin Mol Teratol. 85:583–587. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mandhan P, Quan QB, Beasley S and Sullivan
M: Sonic hedgehog, BMP4, and Hox genes in the development of
anorectal malformations in Ethylenethiourea-exposed fetal rats. J
Pediatr Surg. 41:2041–2045. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang J, Tang XB, Wang WL, Yuan ZW and Bai
YZ: Spatiotemporal expression of BMP7 in the development of
anorectal malformations in fetal rats. Int J Clin Exp Pathol.
8:3727–3734. 2015.PubMed/NCBI
|
|
30
|
Pyati UJ, Cooper MS, Davidson AJ,
Nechiporuk A and Kimelman D: Sustained Bmp signaling is essential
for cloaca development in zebrafish. Development. 133:2275–2284.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ng RC, Matsumaru D, Ho AS, Garcia-Barceló
MM, Yuan ZW, Smith D, Kodjabachian L, Tam PK, Yamada G and Lui VC:
Dysregulation of Wnt inhibitory factor 1 (Wif1) expression resulted
in aberrant Wnt-β-catenin signaling and cell death of the cloaca
endoderm, and anorectal malformations. Cell Death Differ.
21:978–989. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miyagawa S, Harada M, Matsumaru D, Tanaka
K, Inoue C, Nakahara C, Haraguchi R, Matsushita S, Suzuki K,
Nakagata N, et al: Disruption of the temporally regulated cloaca
endodermal β-catenin signaling causes anorectal malformations. Cell
Death Differ. 21:990–997. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stevens ML, Chaturvedi P, Rankin SA,
Macdonald M, Jagannathan S, Yukawa M, Barski A and Zorn AM: Genomic
integration of Wnt/β-catenin and BMP/Smad1 signaling coordinates
foregut and hindgut transcriptional programs. Development.
144:1283–1295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jevnaker AM, Khuu C, Kjøle E, Bryne M and
Osmundsen H: Expression of members of the miRNA17-92 cluster during
development and in carcinogenesis. J Cell Physiol. 226:2257–2266.
2011. View Article : Google Scholar
|
|
35
|
Ouchi Y, Yamamoto J and Iwamoto T: The
heterochronic genes lin-28a and lin-28b play an essential and
evolutionarily conserved role in early zebrafish development. PLoS
One. 9:e880862014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim KH, Seo YM, Kim EY, Lee SY, Kwon J, Ko
JJ and Lee KA: The miR-125 family is an important regulator of the
expression and maintenance of maternal effect genes during
preimplantational embryo development. Open Biol. 6:1601812016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ambros V: MicroRNAs and developmental
timing. Cur Opin Genet Dev. 21:511–517. 2011. View Article : Google Scholar
|
|
38
|
Malmevik J, Petri R, Klussendorf T, Knauff
P, Åkerblom M, Johansson J, Soneji S and Jakobsson J:
Identification of the miRNA targetome in hippocampal neurons using
RIP-seq. Sci Rep. 5:126092015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yin H, Sun Y, Wang X, Park J, Zhang Y, Li
M, Yin J, Liu Q and Wei M: Progress on the relationship between
miR-125 family and tumorigenesis. Exp Cell Res. 339:252–260. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tong Z, Liu N, Lin L, Guo X, Yang D and
Zhang Q: miR-125a-5p inhibits cell proliferation and induces
apoptosis in colon cancer via targeting BCL2, BCL2L12 and MCL1.
Biomed Pharmacother. 75:129–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen D, Chen Z, Jin Y, Dragas D, Zhang L,
Adjei BS, Wang A, Dai Y and Zhou X: MicroRNA-99 family members
suppress Homeobox A1 expression in epithelial cells. PLoS One.
8:e806252013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Emmrich S, Rasche M, Schöning J, Reimer C,
Keihani S, Maroz A, Xie Y, Li Z, Schambach A, Reinhardt D and
Klusmann JH: miR-99a/100-125b tricistrons regulate hematopoietic
stem and progenitor cell homeostasis by shifting the balance
between TGFbeta and Wnt signaling. Genes Dev. 28:858–874. 2014.
View Article : Google Scholar : PubMed/NCBI
|