Introduction
Bladder cancer is one of the most common types of
cancer in humans worldwide and causes a large number of cases of
cancer-associated mortality each year (1,2).
Rapid tumour growth and metastasis are the main reasons for the
high mortality rates of patients with bladder cancer (2,3).
Therefore, investigating the regulatory mechanisms underlying the
proliferation, migration and invasion of bladder cancer cells may
assist in developing novel therapeutic strategies for this disease
(2,3).
As a class of non-coding RNAs greater than 200
nucleotides in length, long non-coding RNAs (lncRNAs) function
mainly through their interaction with mRNAs, microRNAs (miRs) or
proteins (4). LncRNAs have been
implicated in a variety of cellular biological processes, including
cell proliferation, apoptosis, differentiation, motility, and
tumourigenesis (5-7). In addition, certain lncRNAs regulate
the expression of genes involved in tumour-related signalling
pathways, including Wnt/β-catenin and mammalian target of
rapamycin/phosphoinositide 3-kinase (8,9).
In previous years, an increasing number of studies have reported
that lncRNAs, including MALAT1 (10), XIST (11), ATB (12) and GAS5, are key in the development
and malignant progression of various types of human cancer,
including bladder cancer (13).
The small nucleolar RNA host gene 20 (SNHG20) lncRNA
is located on 17q25.2 and contains 2,183 nucleotides. Several
studies have reported that SNHG20 is involved in promoting several
common types of human cancer, including hepatocellular carcinoma
(HCC) (14,15), non-small cell lung cancer (NSCLC)
(16), colorectal cancer
(17), ovarian cancer (18), gastric cancer (19) and breast cancer (20). For example, SNHG20 was found to be
significantly upregulated in HCC and colorectal cancer, and the
high expression of SNHG20 was a predictor of poor prognosis
(15,17). Chen et al reported that
SNHG20 promoted NSCLC cell proliferation and migration by
epigenetically silencing the expression of P21 (16). Liu et al found that SNHG20
promoted gastric cancer progression by inhibiting the expression of
p21 and regulating the glycogen synthase kinase-3β/β-catenin
signalling pathway (19).
However, the expression and function of SNHG20 in bladder cancer
remains to be elucidated.
In the present study, the clinical significance of
the expression of SNHG20 in bladder cancer was investigated, and
the function and molecular mechanism of SNHG20 in regulating the
malignant phenotypes of bladder cancer cells were examined.
Materials and methods
Tissue sample collection
The present study was approved by the Ethics
Committee of The First People's Hospital of Jining City (Jining,
China). Primary bladder cancer tissues and paired adjacent tumour
tissues were collected from 54 patients with bladder cancer at The
First People's Hospital of Jining City between March 2011 and
September 2012. These patients included 33 men and 21 women, who
ranged in age between 43 and 69 years old with a mean age of 60.5
years old. The patients were not exposed to chemotherapy or
radiotherapy prior to surgery, and these tissues were confirmed by
histopathological evaluation. Informed consent was obtained from
all patients. The fresh tissues were stored at 80°C until use.
Plasmid construction
To generate the SNHG20 short hairpin (sh)RNA
plasmid, self-complementary hairpin DNA oligonucleotides (forward,
5′-GAT CCG GCC CAG ATT GGT ACA TTT-3′ and reverse, 5′-AGC TTA AAT
GTA CCA ATC TGG GCC-3′) were annealed and subcloned into the
pRNAT-U6.1/Neo vector (GenScript, Nanjing, China). A negative
control was also subcloned into the pRNAT-U6.1/Neo vector (NC
shRNA).
Cell culture and transfection
The HT-1376, RT112, 253J, and T24 bladder cancer
cell lines and the SV-HUC-1 normal urinary tract epithelial cell
line were purchased from the Chinese Academy of Sciences Cell Bank
(Shanghai, China). The cells were cultured in Dulbecco's modified
Eagle's medium (DMEM, Thermo Fisher Scientific, Inc., Waltham, USA)
with 10% foetal bovine serum (FBS, Thermo Fisher Scientific, Inc.)
and incubated at 37°C in a 5% CO2 humidified incubator.
For cell transfection, the 253J and T24 cells were cultured to 70%
confluence and transfected with NC shRNA or SNHG20 shRNA using
Lipofectamine 2000 (Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol. The T24 cells were stably transfected with
NC shRNA or SNHG20 shRNA using 400 µg/ml neomycin
selection.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the cells and tissues
using a TRIzol kit (Thermo Fisher Scientific, Inc.), and cDNA was
synthesised using a reverse transcription kit (Thermo Fisher
Scientific, Inc.). qPCR was performed using a fluorescence
quantitative PCR kit (Thermo Fisher Scientific, Inc.) using 1
µg cDNA. The reaction conditions for all qPCR experiments
were as follows: 95°C for 5 min, followed by 40 cycles of 95°C for
10 sec, 60°C for 30 sec and 72°C for 30 sec. GAPDH was used as the
internal reference. The relative expression was analysed using the
2−ΔΔCq method (21).
The sequences of the SNHG20 primers were as follows: Sense, 5′-ATG
GCT ATA AAT AGA TAC ACG C-3′ and antisense, 5′-GGT ACA AAC AGG GAG
GGA-3′; the sequences of the GAPDH primers were as follows: Sense,
5′-TGT TCG TCA TGG GTG TGA AC-3′ and antisense, 5′-ATG GCA TGG ACT
GTG GTC AT-3′.
Cell survival assay
The transfected cells (5,000 cells/well) were seeded
in a 96-well plate and incubated for 0, 24, 48 or 72 h.
Subsequently, 10 µl MTT solution (5 mg/ml) was added. The
cells were incubated at 37°C for 4 h. Following this, the
supernatant was removed, and 100 µl of dimethyl sulfoxide
was added. The optical density (OD) value at 570 nM was measured on
a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Cell proliferation assay
Cell proliferation was examined using Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto,
Japan). At 48 h post-transfection, the cells (3,000 cells per well)
were seeded into 96-well plates and cultured for 0, 24, 48 and 72
h. The OD value at 450 nM was measured on a microplate reader
(Bio-Rad Laboratories, Inc.).
Colony formation assay
The transfected cells (500 cells/well) were added to
6-well plates to culture for 14 days and stained with 0.5% crystal
violet (Beyotime Institute of Biotechnology, Haimen, China) at room
temperature for 10 min. The cells were then counted and images were
captured under an inverted microscope.
Cell apoptosis assay
Following transfection for 48 h, the cells were
collected by centrifugation at 1,500 × g for 10 min at room
temperature, and incubated with 500 µl binding buffer, 5
µl FITC Annexin V and 5 µl propidium iodide. The
fluorescence of the stained cells was then analysed using flow
cytometry (BD FACSCalibur, BD Biosciences, Franklin Lakes, NJ,
USA).
Wound healing assay
The bladder cancer cells were grown in 6-well plates
with DMEM with 10% FBS. Wounds were created by scratching the cell
surface with a 10-µl pipette tip. Then, cells were washed by
PBS and cultured at 37°C with 5% CO2 for 48 h. Cells
cultured with DMEM served as the blank control group. After 24 h,
the cells were observed under an inverted microscope.
Cell invasion assay
Matrigel pre-coated Transwell chambers (BD
Biosciences) were used to examine cell invasion. The cell
suspension (1×105 cells per ml) was prepared in DMEM,
following which, 300 µl of DMEM with 10% FBS was added into
the lower chamber, and 300 µl of cell suspension was added
into the upper chamber. The cells were then cultured at 37°C for 24
h, and cells that did not invade through the membrane in the filter
were removed by wiping. The cells that had invaded through the
membrane were fixed, stained with crystal violet (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), and counted under an inverted
microscope.
Tumour formation assay
BALB/c mice (n=8, male, 20-22 g, 8-week-old, Hunan
SJA Laboratory Animal Co., Ltd, Changsha, China) were maintained
under specific pathogen-free conditions: Free access to food and
water at 22-25°C under a 12 h light/dark cycle. T24 cells were
stably transfected with SNHG20 shRNA or NC shRNA, and a cell
suspension containing 107 cells was subcutaneously
injected into the posterior flank of each animal. The tumour
volumes were determined at different time points (tumour volume =
length × width2 × 0.5). At 30 days following injection,
all animals were sacrificed, and tumour tissues were obtained.
Western blot analysis
Total proteins were extracted from cells using RIPA
lysis buffer (Thermo Fisher Scientific, Inc.) and protein
concentration was determined using the Pierce BCA Protein Assay kit
(Thermo Fisher Scientific, Inc.). The proteins (50 µg per
lane) were separated on 10% SDS-PAGE gels and then transferred onto
PVDF membranes (Thermo Fisher Scientific, Inc.). The membranes were
blocked with 5% non-fat dry milk at room temperature for 3 h and
then incubated with primary antibodies, including antibodies
targeting Caspase-3 (1:200, cat. no. ab13847, Abcam, Cambridge, MA,
USA), Caspase-9 (1:200, cat. no. ab32539, Abcam), B-cell lymphoma 2
(Bcl2; 1:500, cat. no. ab32124, Abcam), matrix metalloproteinase
(MMP)2 (1:200, cat. no. ab92536, Abcam), MMP9 (1:500, cat. no.
ab76003, Abcam), c-Myc (1:200, cat. no. ab32072, Abcam), β-catenin
(1:200, cat. no. ab16051, Abcam), and GAPDH (1:200, cat. no.
ab9485, Abcam) at room temperature for 3 h. Subsequently, the
membranes were incubated with HRP-conjugated secondary antibody
(1:5,000, cat. no. ab6721, Abcam) for 1 h at room temperature.
Chemiluminescence was examined using SuperSignal West Femto Maximum
Sensitivity substrate (Thermo Fisher Scientific, Inc.). The
quantities of protein were analysed using ImageJ software 1.46
(National Institutes of Health, Bethesda, MD, USA). GAPDH was used
as the internal control.
Statistical analysis
Data are expressed as the mean ± standard deviation.
SPSS 20.0 software (IBM SPSS, Armonk, NY, USA) was used for
statistical analysis. Student's t-test was used for comparisons
between two groups, and one-way analysis of variance followed by
Tukey's post hoc test was used for comparisons of more than two
groups. The Kaplan-Meier method was used for survival analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
SNHG20 is upregulated in bladder cancer
tissues
In the present study, the expression of SNHG20 was
first examined in bladder cancer tissues and matched adjacent
non-tumour tissues. The RT-qPCR data revealed that SNHG20 was
significantly upregulated in the bladder cancer tissues compared
with the matched adjacent non-tumour tissues (Fig. 1A). The association between the
expression of SNHG20 and clinico-pathological characteristics in
patients with bladder cancer was then examined. Based on the mean
expression value of SNHG20 in bladder cancer tissues, the patients
were divided into a low expression group and high expression group.
As shown in Table I, a high
expression of SNHG20 was significantly associated with advanced
tumour-node-metastasis (TNM) stage and lymph node metastasis in
bladder cancer. In addition, it was observed that patients with
bladder cancer with a high expression of SNHG20 exhibited reduced
survival rate compared with those with a low expression of SNHG20
(Fig. 1B). In addition, the
expression of SNHG20 was detected in bladder cancer cell lines
(HT-1376, RT112, 253J, and T24) and the SV-HUC-1 normal urinary
tract epithelial cell line. The data indicated that the expression
of SNHG20 was increased in the bladder cancer cell lines compared
with its expression in the SV-HUC-1 cells (Fig. 1C). Therefore, it was suggested
that SNHG20 is upregulated in bladder cancer, contributing to its
malignant progression and poor prognosis.
 | Table IAssociation between the expression of
SNHG20 and clinicopathological characteristics in bladder
cancer. |
Table I
Association between the expression of
SNHG20 and clinicopathological characteristics in bladder
cancer.
| Characteristic | Cases (n=54) | Expression of
SNHG20
| P-value |
|---|
| High (n=28) | Low (n=26) |
|---|
| Age (years) | | | | 0.574 |
| <55 | 20 | 9 | 11 | |
| ≥55 | 34 | 19 | 15 | |
| Sex | | | | 0.586 |
| Male | 33 | 16 | 17 | |
| Female | 21 | 12 | 9 | |
| Lymph node
metastasis | | | | 0.024a |
| Negative | 35 | 14 | 21 | |
| Positive | 19 | 14 | 5 | |
| Stage | | | | 0.014a |
| I-II | 27 | 9 | 18 | |
| III-IV | 27 | 19 | 8 | |
SNHG20 knockdown inhibits bladder cancer
cell proliferation and survival, and induces cell apoptosis
To investigate the function of SNHG20 in bladder
cancer, the T24 and 253J bladder cancer cells were transfected with
SNHG20 shRNA or NC shRNA, separately. Following transfection, the
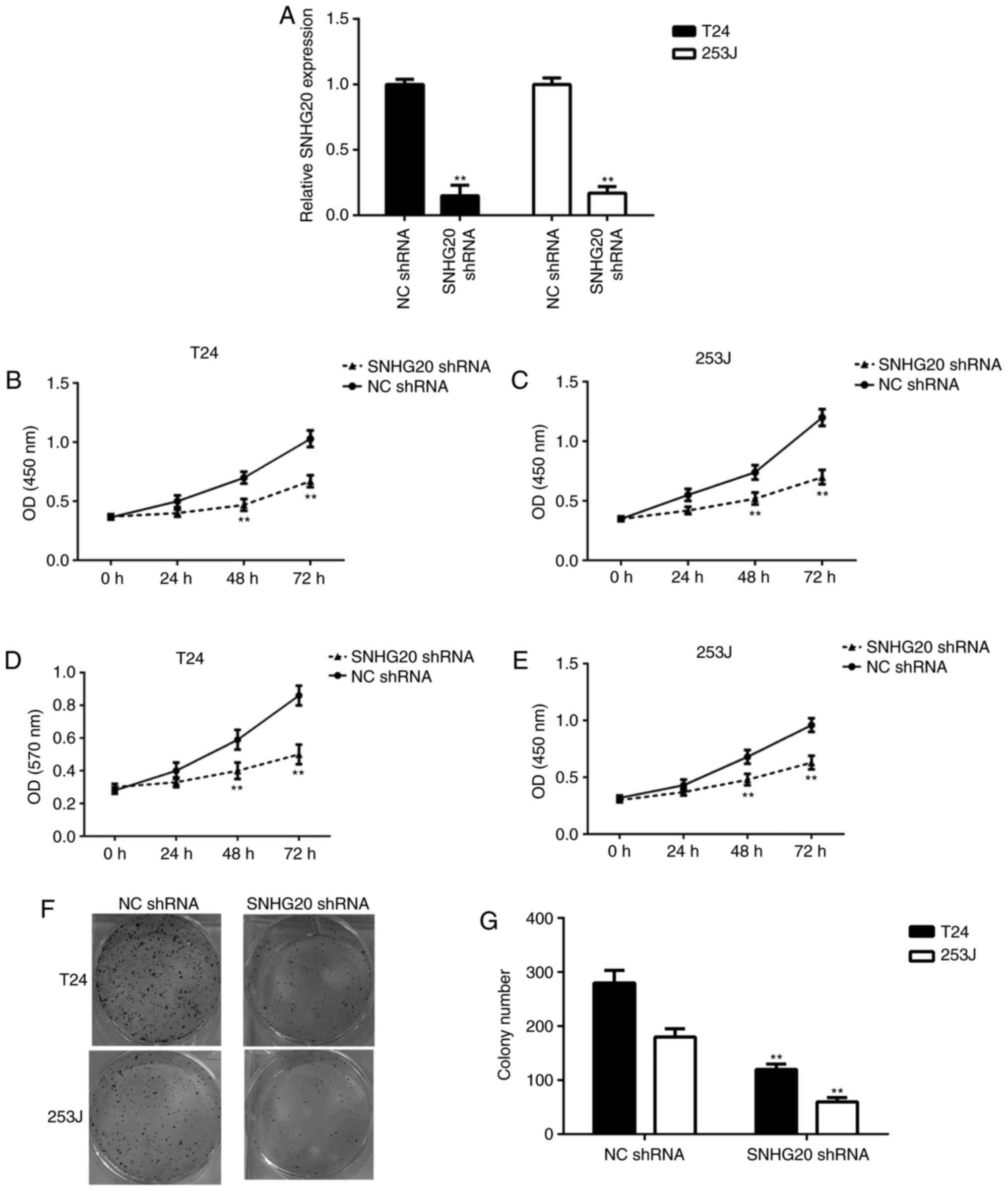
RT-qPCR data showed that the expression of SNHG20 was significantly
reduced in the SNHG20 shRNA group compared with that in the NC
shRNA group (Fig. 2A). The CCK-8
assay and MTT assay data showed that the knockdown of SNHG20
significantly reduced the proliferation and survival of bladder
cancer cells (Fig. 2B-E). In
addition, the downregulation of SNHG20 reduced the colony formation
ability of the bladder cancer cells (Fig. 2F and G). Therefore, inhibiting the
expression of SNHG20 reduced bladder cancer cell proliferation and
survival.
The present study then investigated the effects of
SNHG20 on bladder cancer cell apoptosis. Flow cytometric assay data
indicated that SNHG20 knockdown significantly induced bladder
cancer cell apoptosis compared with the cells transfected with the
NC shRNA (Fig. 3A and B).
Consistently, SNHG20 knockdown increased the protein expression
levels of Caspase-3 and Caspase-9 and inhibited the protein
expression of Bcl2 in the bladder cancer cells (Fig. 3C and D).
Inhibition of the expression of SNHG20
decreases the migration and invasion of bladder cancer cells
Tumour cell migration and invasion are key processes
during cancer metastasis. Therefore, the present study examined
whether SNHG20 affected the migration and invasion of bladder
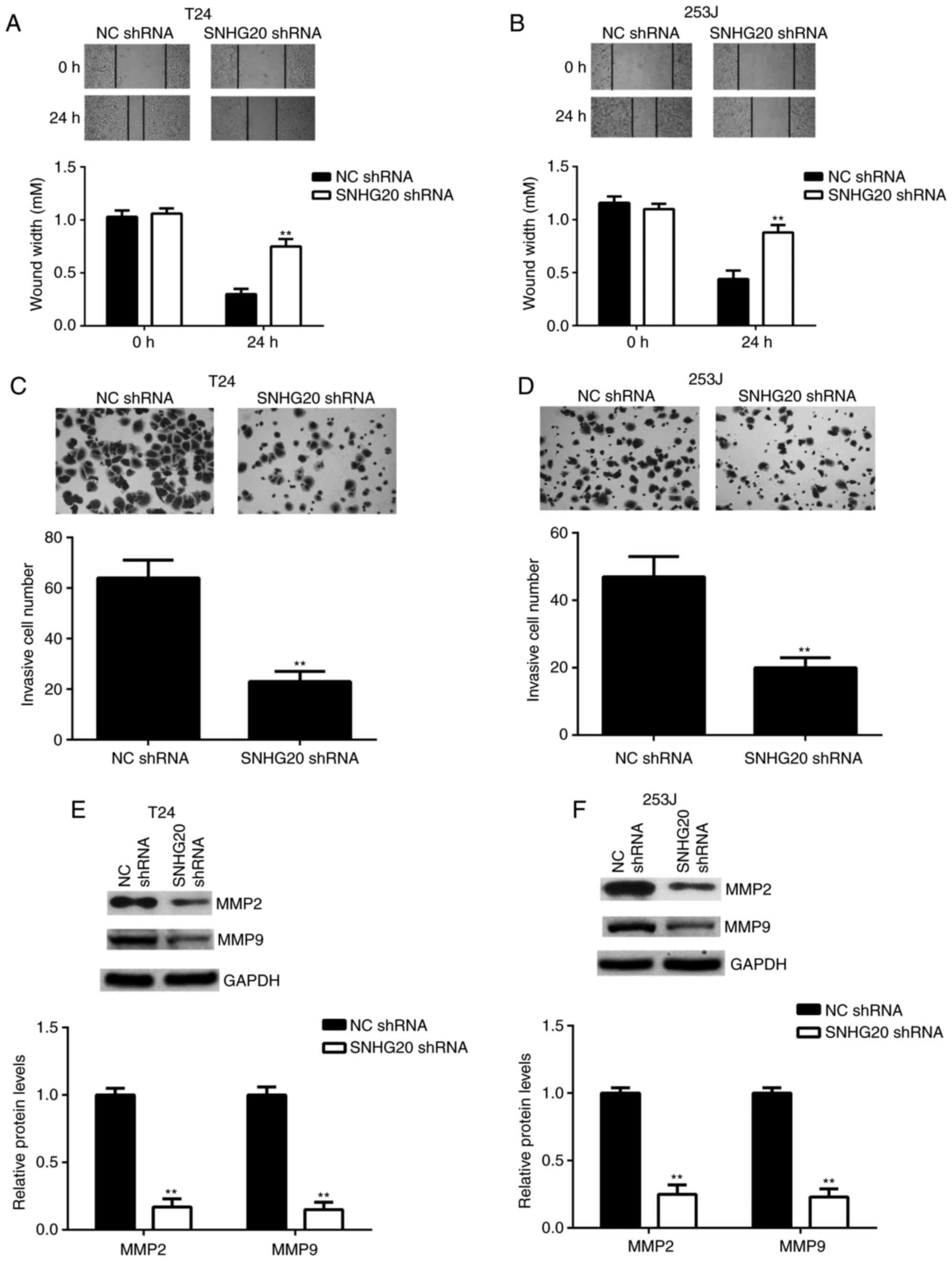
cancer cells. The wound healing assay data revealed that bladder
cancer cell migration was significantly repressed in the SNHG20
shRNA group compared with that in the NC shRNA group (Fig. 4A and B). The Transwell assay data
indicated that bladder cancer cell invasion was also significantly
decreased in the SNHG20 shRNA group compared with that in the NC
shRNA group (Fig. 4C and D).
Consistently, the protein levels of MMP2 and MMP9, two key factors
in tumour metastasis, were significantly downregulated following
SNHG20 knockdown (Fig. 4E and F).
These findings demonstrated that inhibition of the expression of
SNHG20 decreased the migration and invasion of bladder cancer cells
and suggested that SNHG20 may be involved in promoting cancer
metastasis.
Knockdown of SNHG20 inhibits
Wnt/β-catenin signalling pathway activity
Wnt/β-catenin signalling is key in the pathogenesis
of bladder cancer. Therefore, the present study examined the
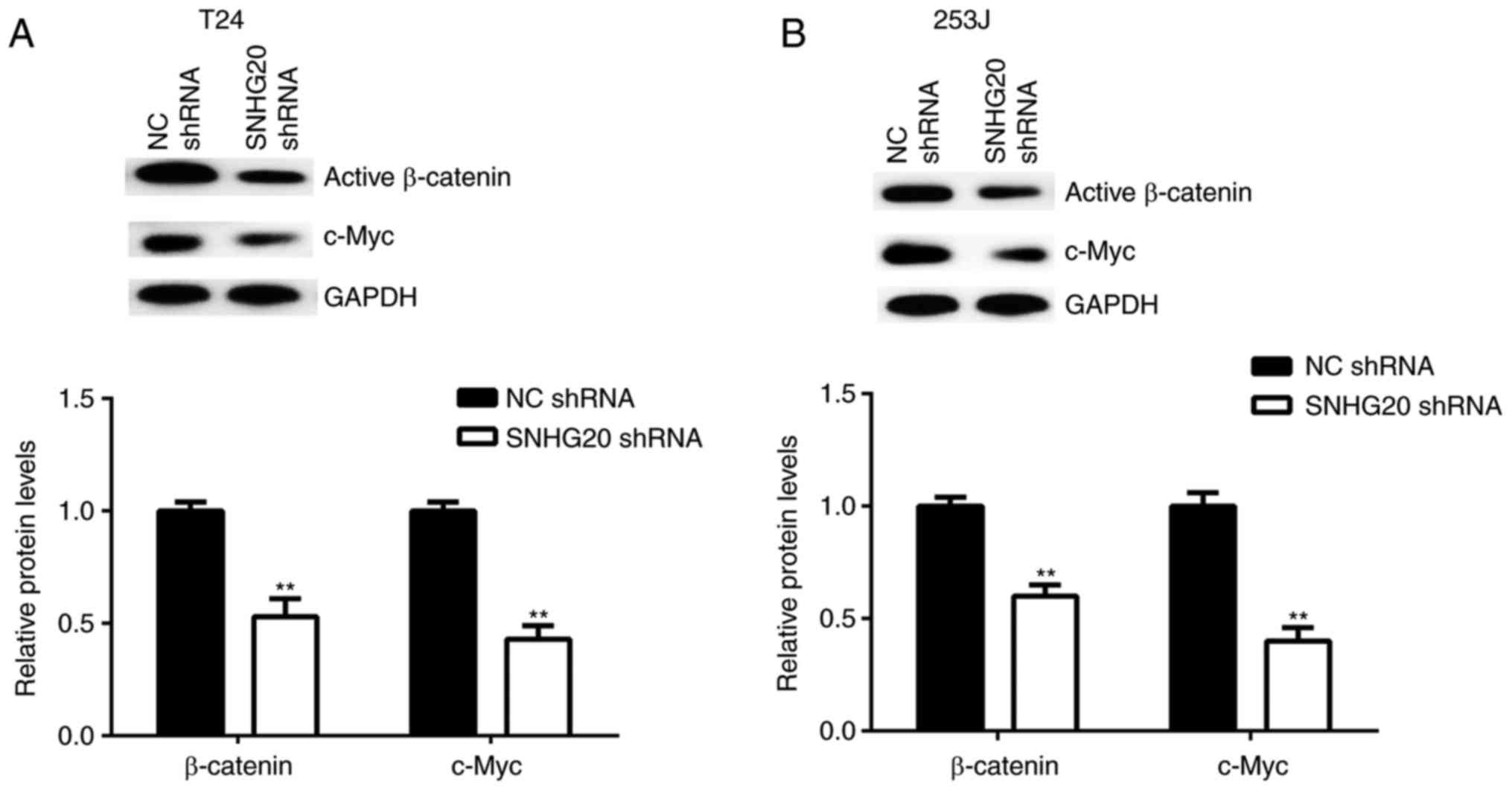
effects of the downregulation of SNHG20 on Wnt/β-catenin signalling
activity in bladder cancer cells. The protein levels of active
β-catenin and c-Myc, a key target gene of Wnt/β-catenin signalling,
were examined in bladder cancer cells following SNHG20 knockdown.
The western blot data showed that the protein levels of active
β-catenin and c-Myc were significantly reduced in the SNHG20 shRNA
group compared with the levels in the NC shRNA group (Fig. 5A and B). Therefore, the knockdown
of SNHG20 inhibited Wnt/β-catenin signalling pathway activity.
SNHG20 knockdown inhibits tumour growth
of bladder cancer cells in vivo
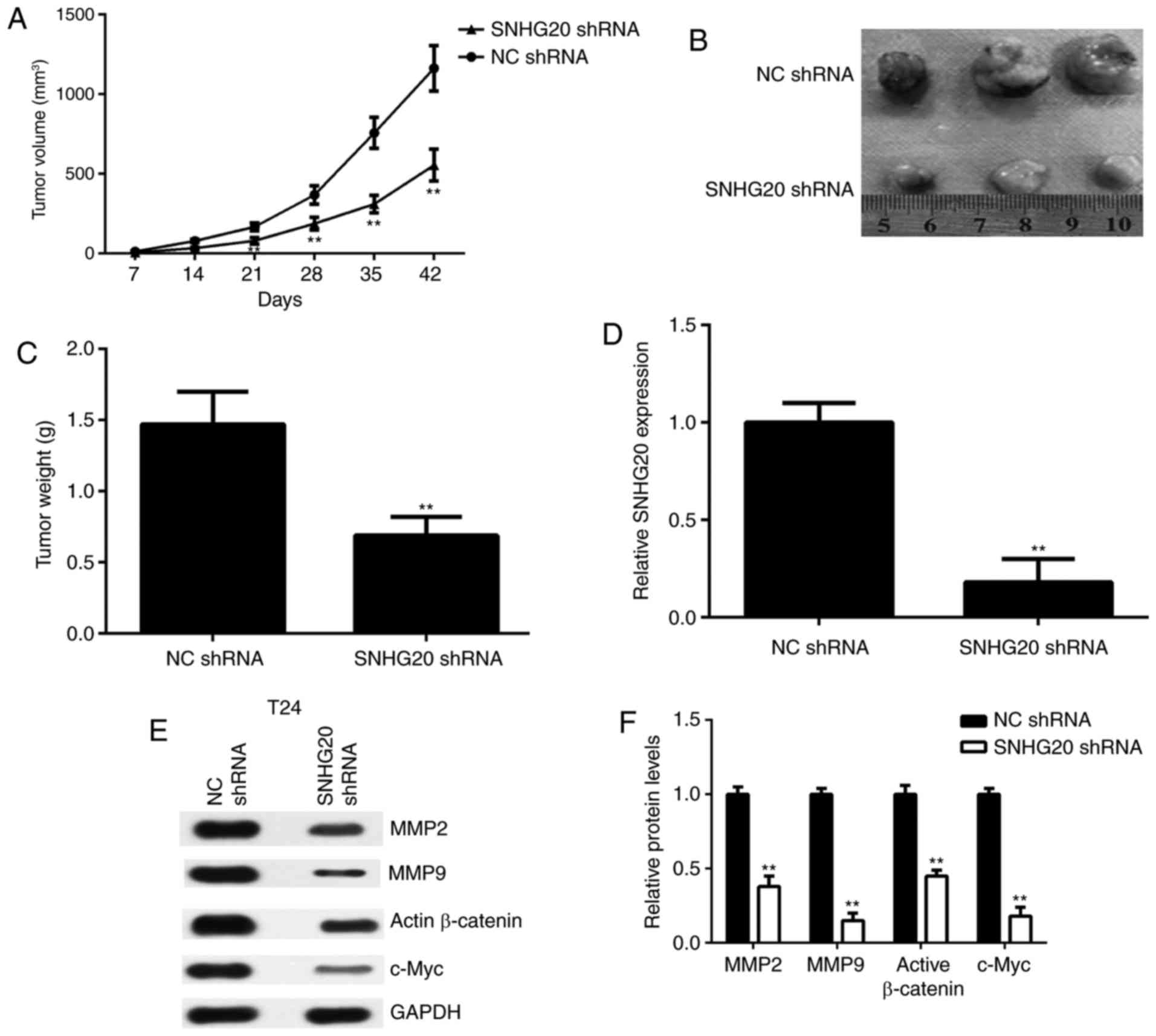
Finally, the effects of SNHG20 in bladder cancer
were examined in vivo. A BALB/c nude mouse xenograft model
was established using T24 cells that were stably transfected with
SNHG20 shRNA. The data revealed that the tumour volumes and weights
were significantly reduced in the SNHG20 shRNA group compared with
those in the NC shRNA group (Fig.
6A-C). Subsequently, xenograft tissues were obtained and the
expression of SNHG20 was examined in each group. The RT-qPCR data
confirmed that the levels of SNHG20 were reduced in the SNHG20
shRNA group compared with those in the NC shRNA group (Fig. 6D). In addition, the protein levels
of MMP2, MMP9, active β-catenin and c-Myc were reduced in the
SNHG20 shRNA group compared with those in the NC shRNA group
(Fig. 6E and F). These data
indicated that SNHG20 knockdown inhibited the tumour growth of
bladder cancer cells in a mouse xenograft model and was associated
with reduced Wnt/β-catenin signalling activity.
Discussion
Investigating the regulatory mechanisms underlying
bladder cancer growth and metastasis may be beneficial for the
development of promising therapeutic strategies for bladder cancer.
In the present study, it was found that SNHG20 was significantly
upregulated in bladder cancer tissues and cell lines, compared with
its expression in adjacent non-tumour tissues and the SV-HUC-1
normal urinary tract epithelial cell line, respectively. In
addition, the high expression of SNHG20 was associated with
advanced clinical stage, lymph node metastasis, and reduced
survival rate of patients. SNHG20 knockdown caused a significant
reduction in cancer cell survival, proliferation, colony formation,
migration and invasion, and induced cell apoptosis. The inhibition
of SNHG20 also reduced tumour growth in vivo. In addition,
the inhibition of SNHG20 suppressed the activation of Wnt/β-catenin
signalling and the expression of certain key genes in bladder
cancer cells.
In previous years, several lncRNAs have been
demonstrated to be dysregulated in bladder cancer and involved in
its malignant progression (22,23). For example, lncRNA ATB promotes
the proliferation, migration and invasion of bladder cancer cells
by suppressing miR-126 (12).
LncRNA SPRY4-IT1 sponges miR-101-3p to promote the proliferation
and metastasis of bladder cancer cells through increasing the
expression of enhancer of zeste homolog 2 (EZH2) (24). However, the expression and exact
role of SNHG20 in bladder cancer remains to be fully elucidated. In
the present study, it was found that the expression levels of
SNHG20 were significantly higher in bladder cancer tissues than in
matched adjacent non-tumour tissues, and its increased expression
was significantly associated with advanced TNM stage, lymph node
metastasis, and reduced survival rates of patients with bladder
cancer. These findings suggested that the upregulation of SNHG20
may contribute to the malignant progression of bladder cancer and
that SNHG20 may serve as a potential predicator for the prognosis
of patients with bladder cancer.
As SNHG20 was significantly upregulated in bladder
cancer, bladder cancer cells were transfected with SNHG20 shRNA to
knock down its expression. Further investigation revealed that
SNHG20 knockdown markedly inhibited the proliferation, survival and
colony formation of the bladder cancer cells. In addition, SNHG20
knockdown caused a significant reduction in the tumour growth of
bladder cancer cells in a xenograft mouse model. These findings
suggested that SNHG20 promoted the proliferation of bladder cancer
cells in vitro and in vivo. The effects of the
inhibition of SNHG20 on bladder cancer cell apoptosis were then
examined. The flow cytometry results revealed that the
downregulation of SNHG20 notably induced bladder cancer cell
apoptosis. Consistent with these findings, SNHG20 knockdown
increased the protein levels of two key apoptotic biomarkers,
Caspase-3 and Caspase-9, but decreased the expression of Bcl2, an
important anti-apoptotic protein (25,26).
Tumour cell migration and invasion have been shown
to promote tumour growth and enhance cancer invasion and metastasis
(27,28). Therefore, the present study
examined the role of SNHG20 in the regulation of bladder cancer
cell migration and invasion. The findings showed that inhibiting
the expression SNHG20 significantly reduced cell migration and
invasion, accompanied with decreased expression levels of MMP2 and
MMP9, two key factors associated with extracellular matrix
degradation and tumour invasion and metastasis (29). These findings suggested that
SNHG20 may be involved in promoting bladder cancer metastasis.
It has been widely reported that the expression
levels of Wnt factors are significantly upregulated in bladder
cancer (30,31), and the Wnt/β-catenin signalling is
important in the malignant progression of bladder cancer (32,33). For example, Shen et al
showed that the levels of β-catenin in human bladder cancer tissues
were upregulated with increasing grade of malignancy (30). Schmitz-Drager et al
investigated a total of 185 paraffin-embedded bladder cancer tissue
specimens immunohistochemically for the overexpression of c-myc
(31). Mao et al reported
that activation of the Wnt/β-catenin signalling pathway induced
epithelial-mesenchymal transition and promote bladder cancer
metastasis (34). In addition,
the Wnt signalling has been suggested as a molecular target for
bladder cancer (35,36). For example, Guo et al
showed that the downregulation of miR-144 increased bladder cancer
cell proliferation by targeting EZH2 and regulating Wnt signalling
(35). Costa et al
reported that the epigenetic deregulation of Wnt pathway inhibitors
contributed to aberrant activation of the Wnt signalling pathway in
bladder (36). He et al
reported that SNHG20 was involved in promoting ovarian cancer
progression by activating the Wnt/β-catenin signalling pathway
(18). Therefore, the present
study investigated whether SNHG20 functioned in bladder cancer
through regulating the Wnt/β-catenin signalling pathway. The data
obtained in the present study showed that inhibiting the expression
of SNHG20 in bladder cancer cells caused a significant reduction in
the expression levels of active β-catenin and c-Myc, a target gene
of the Wnt/β-catenin signalling (37). These findings suggested that
SNHG20 also activates the Wnt/β-catenin signalling pathway in
bladder cancer cells.
In conclusion, to the best of our knowledge, the
present study is the first report of SNHG20 being significantly
upregulated in bladder cancer and that this was associated with its
malignant progression and poor patient prognosis. In addition,
SNHG20 was found to activate the Wnt/β-catenin pathway and has a
promoting role in bladder cancer. These findings suggested that
SNHG20 may become a potential therapeutic target for bladder cancer
treatment. Further investigations are required to clarify the
function of SNHG20 in bladder cancer metastasis in vivo
using animal experiments.
Funding
No funding was received.
Availability of data and materials
All data generated or analysed during the present
study are included within this manuscript.
Authors' contributions
QZ and SG collected clinical tissues and performed
experiments. QD performed statistical analysis. QZ wrote the
manuscript. YL designed the study and revised the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The First People's Hospital of Jining City, and written informed
consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Skeldon SC and Larry Goldenberg S: Bladder
cancer: A portal into mens health. Urol Onco. 33:40–44. 2015.
View Article : Google Scholar
|
|
3
|
Sathe A and Nawroth R: Targeting the
PI3K/AKT/mTOR pathway in bladder cancer. Methods Mol Biol.
1655:335–350. 2018. View Article : Google Scholar
|
|
4
|
Li J, Zi Y, Wang W and Li Y: LncRNA MEG3
inhibits cell proliferation and metastasis in chronic myeloid
leukemia via targeting miR-184. Oncol Res. 26:297–305. 2018.
View Article : Google Scholar
|
|
5
|
Zhang JJ, Wang DD, Du CX and Wang Y: Long
noncoding RNA ANRIL promotes cervical cancer development by acting
as a sponge of miR-186. Oncol Res. May 22–2017.Epub ahead of print.
View Article : Google Scholar
|
|
6
|
Yang M, Zhai X, Ge T, Yang C and Lou G:
miR-181a-5p promotes proliferation and invasion, and inhibits
apoptosis of cervical cancer cells via regulating inositol
polyphosphate-5-phosphatase A (INPP5A). Oncol Res. 26:703–712.
2018. View Article : Google Scholar
|
|
7
|
Wang Y, Li J, Xu C and Zhang X:
MicroRNA-139-5p inhibit cell proliferation and invasion by
targeting RHO-associated coiled-coil containing protein kinase 2 in
ovarian cancer. Oncol Res. Jun 14–2017.Epub ahead of print.
View Article : Google Scholar
|
|
8
|
Liu L, Yu D, Shi H, Li J and Meng L:
Reduced lncRNA aim enhances the malignant invasion of
triple-negative breast cancer cells mainly by activating
Wnt/beta-catenin/mTOR/PI3K signaling. Pharmazie. 72:599–603.
2017.
|
|
9
|
Jia L, Tian Y, Chen Y and Zhang G: The
silencing of LncRNA-H19 decreases chemoresistance of human glioma
cells to temozolomide by suppressing epithelial-mesenchymal
transition via the Wnt/beta-catenin pathway. Onco Targets Ther.
11:313–321. 2018. View Article : Google Scholar :
|
|
10
|
Xie H, Liao X, Chen Z, Fang Y, He A, Zhong
Y, Gao Q, Xiao H, Li J, Huang W and Liu Y: LncRNA MALAT1 inhibits
apoptosis and promotes invasion by antagonizing miR-125b in bladder
cancer cells. J Cancer. 8:3803–3811. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu Y, Deng C, Zhang H, Zhang J, Peng B and
Hu C: Long non-coding RNA XIST promotes cell growth and metastasis
through regulating miR-139-5p mediated Wnt/beta-catenin signaling
pathway in bladder cancer. Oncotarget. 8:94554–94568.
2017.PubMed/NCBI
|
|
12
|
Zhai X and Xu W: Long noncoding RNA ATB
promotes proliferation, migration and invasion in bladder cancer by
suppressing microRNA-126. Oncol Res. Jan 10–2018.Epub ahead of
print. View Article : Google Scholar
|
|
13
|
Wang M, Guo C, Wang L, Luo G, Huang C, Li
Y, Liu D, Zeng F, Jiang G and Xiao X: Long noncoding RNA GAS5
promotes bladder cancer cells apoptosis through inhibiting EZH2
transcription. Cell Death Dis. 9:2382018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J, Lu C, Xiao M, Jiang F, Qu L and Ni
R: Long non-coding RNA SNHG20 predicts a poor prognosis for HCC and
promotes cell invasion by regulating the epithelial-to-mesenchymal
transition. Biomed Pharmacother. 89:857–863. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang D, Cao C, Liu L and Wu D:
Up-regulation of LncRNA SNHG20 predicts poor prognosis in
hepatocellular carcinoma. J Cancer. 7:608–617. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Z, Chen X, Chen P, Yu S, Nie F, Lu B,
Zhang T, Zhou Y, Chen Q, Wei C, et al: Long non-coding RNA SNHG20
promotes non-small cell lung cancer cell proliferation and
migration by epigenetically silencing of P21 expression. Cell Death
Dis. 8:e30922017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li C, Zhou L, He J, Fang XQ, Zhu SW and
Xiong MM: Increased long noncoding RNA SNHG20 predicts poor
prognosis in colorectal cancer. BMC Cancer. 16:6552016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He S, Zhao Y, Wang X, Deng Y, Wan Z, Yao S
and Shen H: Up-regulation of long non-coding RNA SNHG20 promotes
ovarian cancer progression via Wnt/β-catenin signaling. Biosci Rep.
38:2018. View Article : Google Scholar
|
|
19
|
Liu J, Liu L, Wan JX and Song Y: Long
noncoding RNA SNHG20 promotes gastric cancer progression by
inhibiting p21 expression and regulating the GSK-3beta/beta-catenin
signaling pathway. Oncotarget. 8:80700–80708. 2017.PubMed/NCBI
|
|
20
|
Guan YX, Zhang ZM, Chen XZ, Zhang Q, Liu
SZ and Zhang YL: Lnc RNA SNHG20 participated in proliferation,
invasion and migration of breast cancer cells via miR-495. J Cell
Biochem. Dec 13–2017.Epub ahead of print. View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Huang T, Liu HW, Chen JQ, Wang SH, Hao LQ,
Liu M and Wang B: The long noncoding RNA PVT1 functions as a
competing endogenous RNA by sponging miR-186 in gastric cancer.
Biomed Pharmacother. 88:302–308. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xue M, Pang H, Li X, Li H, Pan J and Chen
W: Long noncoding RNA UCA1 promotes bladder cancer cell migration
and invasion via hsa-miR-145/ZEB1/2/FSCN1 pathway. Cancer Sci.
107:18–17. 2016. View Article : Google Scholar
|
|
24
|
Liu D, Li Y, Luo G, Xiao X, Tao D, Wu X,
Wang M, Huang C, Wang L, Zeng F and Jiang G: LncRNA SPRY4-IT1
sponges miR-101-3p to promote proliferation and metastasis of
bladder cancer cells through up-regulating EZH2. Cancer Lett.
388:281–291. 2017. View Article : Google Scholar
|
|
25
|
Maurya SK, Tewari M, Sharma B and Shukla
HS: Expression of procaspase 3 and activated caspase 3 and its
relevance in hormone-responsive gallbladder carcinoma chemotherapy.
Korean J Intern Med. 28:573–578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang TQ, Lu XJ, Wu TF, Ding DD, Zhao ZH,
Chen GL, Xie XS, Li B, Wei YX, Guo LC, et al: MicroRNA-16 inhibits
glioma cell growth and invasion through suppression of BCL2 and the
nuclear factor-kappaB1/MMP9 signaling pathway. Cancer Sci.
105:265–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fife CM, McCarroll JA and Kavallaris M:
Movers and shakers: Cell cytoskeleton in cancer metastasis. Br J
Pharmacol. 171:5507–5523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mowers EE, Sharifi MN and Macleod KF:
Functions of autophagy in the tumor microenvironment and cancer
metastasis. FEBS J. 285:1751–1766. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kapoor C, Vaidya S, Wadhwan V, Hitesh,
Kaur G and Pathak A: Seesaw of matrix metalloproteinases (MMPs). J
Cancer Res Ther. 12:28–35. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen CH, Wu JD, Jou YC, Cheng MC, Lin CT,
Chen PC, Tseng YS, Shi CS, Chen SY, Chang DC and Lee YR: The
correlation between TWIST, E-cadherin, and beta-catenin in human
bladder cancer. J BUON. 16:733–737. 2011.
|
|
31
|
Schmitz-Drager BJ, Schulz WA, Jurgens B,
Gerharz CD, van Roeyen CR, Bültel H, Ebert T and Ackermann R: c-myc
in bladder cancer. Clinical findings and analysis of mechanism.
Urol Res. 25(Suppl 1): S45–S49. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mao XW, Xiao JQ, Li ZY, Zheng YC and Zhang
N: Effects of microRNA-135a on the epithelial-mesenchymal
transition, migration and invasion of bladder cancer cells by
targeting GSK3beta through the Wnt/beta-catenin signaling pathway.
Exp Mol Med. 50:e4292018. View Article : Google Scholar
|
|
33
|
Yuan H, Yu S, Cui Y, Men C, Yang D, Gao Z,
Zhu Z and Wu J: Knockdown of mediator subunit Med19 suppresses
bladder cancer cell proliferation and migration by downregulating
Wnt/beta-catenin signalling pathway. J Cell Mol Med. 21:3254–3263.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mao XW, Xiao JQ, Xu G, Li ZY, Wu HF, Li Y,
Zheng YC and Zhang N: CUL4B promotes bladder cancer metastasis and
induces epithelial-to-mesenchymal transition by activating the
Wnt/beta-catenin signaling pathway. Oncotarget. 8:77241–77253.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo Y, Ying L, Tian Y, Yang P, Zhu Y, Wang
Z, Qiu F and Lin J: miR-144 downregulation increases bladder cancer
cell proliferation by targeting EZH2 and regulating Wnt signaling.
FEBS J. 280:4531–4538. 2013. View Article : Google Scholar
|
|
36
|
Costa VL, Henrique R, Ribeiro FR, Carvalho
JR, Oliveira J, Lobo F, Teixeira MR and Jerónimo C: Epigenetic
regulation of Wnt signaling pathway in urological cancer.
Epigenetics. 5:343–351. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hu Y, Yu K, Wang G, Zhang D, Shi C, Ding
Y, Hong D, Zhang D, He H, Sun L, et al: Lanatoside C inhibits cell
proliferation and induces apoptosis through attenuating
Wnt/beta-catenin/c-Myc signaling pathway in human gastric cancer
cell. Biochem Pharmacol. 150:280–292. 2018. View Article : Google Scholar : PubMed/NCBI
|