Introduction
Nasopharyngeal carcinoma (NPC) is the most common
primary malignancy of the nasopharynx, which frequently occurs in
South China and Southeast Asia (1). The main treatment strategy for NPC
is a combination of radiotherapy and chemotherapy; however, due to
distant metastasis and resistance to treatment, the clinical
prognosis of NPC is poor (2). In
addition, numerous therapeutic measures can cause serious side
effects and lead to multidrug resistance (3). Therefore, it is necessary to
identify a novel, safe and effective method to treat NPC. At
present, for the prevention, inhibition or delay of carcinogenesis,
treatment with natural, synthetic or biological chemicals is
regarded as being able to induce relatively effective
chemoprevention (4).
Curcumin,
1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, is
present in turmeric (Curcuma longa), and has been used to
treat various diseases, including allergies, coryza, cough,
anorexia, rhinitis and hepatic conditions, in Asia for centuries
(5). In addition, curcumin is
able to suppress proliferation and promote apoptosis in various
malignant diseases (6-9). However, the functions and effects of
curcumin on NPC cells require further research. Some specific
functions that have been detected in other tumor models include
induction of apoptosis via c-Jun N-terminal kinase and p38
mitogen-activated protein kinase signaling pathway regulation
(10), endoplasmic reticulum (ER)
stress (11) and autophagy
(12).
The ER is an organelle, which can fold and
synthesize transmembrane, intraorganellar and secretory proteins,
and accumulate intracellular calcium. Disturbance of ER homeostasis
leads to ER stress and the subsequent accumulation of unfolded or
misfolded proteins; this is known as the unfolded protein response
(UPR). The UPR induces a series of signaling pathways to maintain
ER homeostasis via the upregulation of molecular chaperones that
can accelerate immunoglobulin folding and protein synthesis
attenuation (13). Three ER
transmembrane receptors inhibit the three signaling pathways that
comprise the UPR; inositol-requiring enzyme 1α (IRE1α), activating
transcription factor 6 and eukaryotic translation initiation factor
2α kinase 3 (14). Initially, UPR
signaling serves a crucial role in restoring ER homeostasis;
however, continuous or prolonged UPR results in cell apoptosis via
caspase-12, caspase-4 and B-cell lymphoma 2 (Bcl-2). The present
study aimed to investigate the effects of the UPR on the toxic
activity of curcumin on NPC cells. The effects of interference in
the UPR pathway on the toxic effects of curcumin on human NPC cells
were also analyzed.
Materials and methods
Cell culture
SUNE1 and SUNE2 NPC cells were purchased from the
American Type Culture Collection (Manassas, VA, USA). These cells
were cultured in Dulbecco's modified Eagle's medium (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) or RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin, and 100 µg/ml streptomycin
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C
in an atmosphere containing 95% air and 5% CO2.
Reagents and antibodies
Curcumin, Z-VAD-FMK (Z-VAD) and MK-2206 were
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Primary antibodies against GRP78 (cat. no. ab21685), GAPDH (cat.
no. ab37168), cleaved poly (ADP-ribose) polymerase (PARP; cat. no.
ab32561), caspase-3 (cat. no. ab90437), caspase-9 (cat. no.
ab25758) and caspase-12 (cat. no. ab62484) were obtained from Abcam
(Cambridge, UK). Antibodies against CCAAT-enhancer-binding protein
homologous protein (CHOP; cat. no. 2895), IRE1 (cat. no. 3294),
phosphorylated (p)-eukaryotic initiation factor 2α (eIF2α; cat. no.
9721), Bcl-2 (cat. no. 2872), protein kinase B (AKT; cat. no. 9272)
and p-AKT (Ser473) (cat. no. 9271) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA).
Measurement of cell viability
Cell viability was analyzed using the Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto,
Japan). Initially, cells (5×103/well) were cultured in
culture medium and were inoculated into 96-well plates. After 24 h,
0, 10, 20, 40 and 80 µM curcumin was added to each well and the
cells were cultured at 37°C for a further 24 h. The original
culture medium was replaced with a mixture of fresh medium and
CCK-8 solution (10 µl). The optical density (OD) index of the cells
was measured at 450 nm after culturing at 37°C for 2 h. The
percentage of inhibition of cell activity was calculated according
to the following formula: (control OD value-experiment OD
value)/control group OD value ×100%.
Cell cycle analysis by flow
cytometry
Human NPC cells were cultured in the aforementioned
culture medium. Subsequently, once the cells reached 50%
confluence, they were treated with 80 µM curcumin at 37°C for 24 h
and were collected in order to analyze the cell cycle. Briefly,
5×104 cells were incubated with DAPI and RNase A (Thermo
Fisher Scientific, Inc.) for 30 min at room temperature, and the
cells were then suspended in 0.5 ml propidium iodide (PI; BD
Biosciences, San Jose, CA, USA) solution and incubated for 30 min
at room temperature in the dark, according to the manufacturer's
protocol. Subsequently, cell cycle distribution was analyzed using
flow cytometry [fluorescence-activated cell sorting (FACS)
analysis; BD FACSDiva software v8.0.1; BD Biosciences].
Apoptotic analysis by flow cytometry
Cells (4×105/well) were seeded in the
aforementioned culture medium in 6-well plates and were incubated
at 37°C for 24 h. Subsequently, cells were treated with fresh
medium containing the required concentrations of curcumin (0, 20,
40 and 80 µM), or the cells were pretreated with 50 µM Z-VAD,
followed by treatment with or without 80 µM curcumin at 37°C for 24
h. Cells were then stained using the Annexin V-fluorescein
isothiocyanate (FITC) apoptosis detection kit (BD Biosciences).
According to the manufacturer's protocol, cells were treated for 15
min with a mixture of Annexin V (5 µl) and PI (5 µl) at room
temperature. Subsequently, the stained cells were observed by flow
cytometry (FACS analysis; BD FACSDiva software v8.0.1, BD
Biosciences).
Western blot analysis
Cells were treated with either curcumin (0-80 µM) or
MK-2206 (5 µM) alone or in combination for 24 h. Alternatively,
cells underwent GRP78 knockdown by transient transfection with
small interfering (si)RNA (20 nM) for 24 h, after which, the
transfected NPC cells were treated with or without curcumin (80 µM)
in complete medium at 37°C for 24 h. The cells were then lysed in
cold radioimmuno-precipitation assay lysis buffer (Sigma-Aldrich;
Merck KGaA) supplemented with 1 nM phenylmethylsufonyl fluoride,
and were centrifuged at 12,000 × g for 10 min at 4°C. The
concentration of proteins extracted from cells was determined using
a bicinchoninic acid assay (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). Total proteins (20-25 µg) were separated by 8-15%
SDS-PAGE and transferred onto a polyvinylidene fluoride membrane
(EMD Millipore, Billerica, MA, USA). The membrane was then blocked
in a mixture of 5% skimmed milk and PBS containing 0.1% Tween-20
for 2 h at room temperature, and was incubated with primary
antibodies at 4°C overnight [1:1,000 dilutions for GRP78, cleaved
PARP, caspase-3, caspase-9, caspase-12, CHOP, IRE1, p-eIF2α, Bcl-2,
AKT, p-AKT (Ser473) and GAPDH]. Membranes were then incubated with
horseradish peroxidase-conjugated secondary antibodies (cat. nos.
7074 and 7076; 1:2,000; Cell Signaling Technology, Inc.) for 1 h at
room temperature, followed by visualization with enhanced
chemiluminescence immunoblotting detection reagents (EMD
Millipore). Protein band intensities were semi-quantified by
densitometric analysis using ImageJ software 1.6.0_20 (National
Institutes of Health, Bethesda, MD, USA).
Immunofluorescence assay
A total of 1×105 human NPC cells were
placed in each well of 6-well chamber slides and were treated with
curcumin (80 µM) at 37°C for 24 h. PBS was used to wash the cells,
and they were then immobilized and permeabilized with 4%
paraformaldehyde and 0.1% Triton X-100 for 15 min at room
temperature. Immunofluorescence staining was then conducted.
Initially, the processed human NPC cells were stained with primary
antibodies (1:1,000 for GRP78; 1:3,000 for CHOP) for 2 h at room
temperature, and were then cultured with secondary antibodies
conjugated to Alexa Fluor 488 (green) (1:200) or Alexa Fluor 546
(red) (1:200) (cat. nos. A28175 and A-11071; Invitrogen; Thermo
Fisher Scientific, Inc.) for detection of CHOP and GRP78 for 2 h at
room temperature, respectively. Subsequently, the cells were
stained with DAPI (Abcam). Fluorescent images of the NPC cells were
captured and analyzed under a light microscope (Olympus
Corporation, Tokyo, Japan).
RNA interference
GRP78-specific (cat. no. sc-29338) and non-silencing
scrambled siRNA (cat. no. sc-37007) were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). According to the
manufacturer's protocol, 4×105 human NPC cells/well were
plated in 6-well plates and transfected with siRNAs using
Lipofectamine® 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) for 24 h. Subsequently, the
transfected NPC cells were exposed to curcumin (80 µM) in complete
medium at 37°C for 24 h.
Statistical analysis
All experiments were repeated in triplicate. Data
are presented as the means ± standard deviation. The differences
among multiple groups were analyzed by one-way analysis of variance
followed by Dunnett's test using SPSS software (version 17.0; SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statically significant difference.
Results
Curcumin reduces the viability of human
NPC cells
In order to investigate the role of curcumin in the
survival of NPC cells in vitro, SUNE1 and SUNE2 cells were
exposed to various concentrations of curcumin (0-80 µM) for 1 day,
and inhibition of cell proliferation was evaluated by CCK-8. As
shown in Fig. 1, inhibition of
NPC cell viability increased with increasing concentrations of
curcumin, from 13.58% inhibition following treatment with 10 µM
curcumin to 40.60% inhibition following treatment with 80 µM
curcumin in SUNE1 cells, and from 15.16% inhibition following
treatment with 10 µM curcumin to 43.27% inhibition following
treatment with 80 µM curcumin in SUNE2 cells.
Curcumin induces NPC cell apoptosis and
cell cycle arrest
In order to determine the role of curcumin in
apoptotic cell death, SUNE1 and SUNE2 cells were exposed to various
concentrations of curcumin (20-80 µM) for 24 h. Apoptotic cell
death was analyzed using the Annexin V/PI analysis. As shown in
Fig. 2A-D, early apoptosis of
SUNE1 cells was markedly increased from 1.49 to 17.45% and late
apoptosis of SUNE1 cells was increased from 0.72 to 21.38%
following exposure to 80 µM curcumin for 24 h. In addition, early
and late apoptosis increased from 1.16 to 25.12% and 2.23 to 15.93%
in 80 µM curcumin-treated SUNE2 cells, respectively. As shown in
Fig. 2E, cell cycle distribution
of curcumin-treated SUNE1 and SUNE2 cells was determined. The
majority of curcumin (80 µM)-treated NPC cells were arrested at
G0/G1 phase; the percentage of cells in
G0/G1 phase was increased to 68.85 and 70.88%
in SUNE1 cells and SUNE2 cells, respectively. These data indicated
that curcumin can induce cell death via apoptosis.
Curcumin induces ER stress in human NPC
cells
To determine the effects of curcumin on ER stress in
human NPC cells, specific ER-associated proteins were detected.
Western blot analysis demonstrated that IRE1, p-eIF2α, CHOP and
GRP78 were increased following curcumin treatment (Fig. 3A-D). To ascertain whether curcumin
can increase the expression of markers that are associated with ER
stress in NPC cells, CHOP and GRP78 were analyzed by
immunofluorescence staining. As shown in Fig. 3E, immunofluorescence staining of
CHOP and GRP78 was markedly increased following curcumin (80 µM)
treatment in SUNE1 and SUNE2 cells. Previous studies have reported
that prolonged ER stress can active caspase-12, and caspase-12 has
an important influence on inducing cell death (15,16). In order to analyze the effects of
curcumin on ER stress and toxicity, and to investigate the
underlying mechanisms, western blotting was used to detect the
expression of the caspase-12 protein in NPC cells. As shown in
Fig. 4A-D, compared with in the
control group, activation of caspase-12 was increased in
curcumin-treated SUNE1 and SUNE2 cells. Furthermore, the expression
levels of the anti-apoptotic protein Bcl-2 were significantly
decreased (Fig. 4A-D). These
results indicated that ER stress may induce apoptosis.
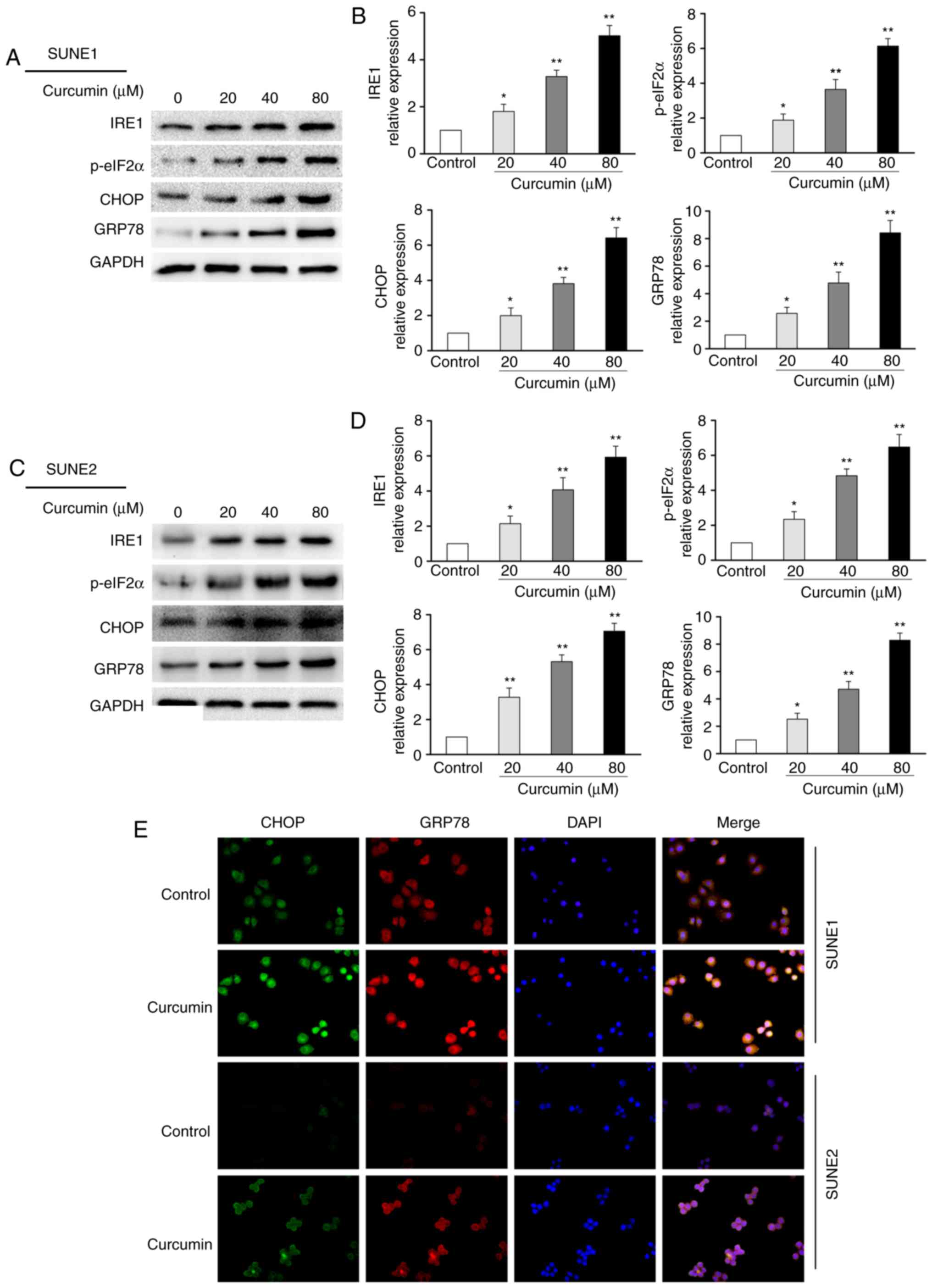 | Figure 3Effects of curcumin on ER
stress-associated signaling molecules in human nasopharyngeal
carcinoma cells. (A and C) Cells were untreated (control) or
treated with curcumin (20-80 µM) for 24 h. To determine the
expression of ER stress-associated molecules, IRE1, p-eIF2α, CHOP
and GRP78, cell lysates were analyzed by western blotting. GAPDH
was used as an internal control. The blots represent the results of
at least three independent tests. (B and D) Band density in each
assay was measured and normalized to that of GAPDH.
*P<0.05 and **P<0.05 vs. the control
group. (E) Immunofluorescence analysis of CHOP (green fluorescence)
and GRP78 protein (red fluorescence) (magnification, ×200). CHOP,
CCAAT-enhancer-binding protein homologous protein; ER, endoplasmic
reticulum; GRP78, glucose-regulated protein 78; IRE1,
inositol-requiring enzyme 1; p-eIF2α, phosphorylated-eukaryotic
initiation factor 2α. |
GRP78 knockdown enhances curcumin-induced
apoptosis of human NPC cells
GRP78, which is the most important marker of the
UPR, has been reported to be associated with chemoresistance
(17,18). siRNA was used to determine the
effects of GRP78 knockdown on curcumin-induced apoptosis of SUNE1
and SUNE2 cells. As shown in Fig.
5A, following GRP78 siRNA transfection, in cells that were
treated with curcumin, cell viability was significantly reduced. In
addition, GRP78 siRNA transfection significantly inhibited the
protein expression levels of GRP78, improved PARP, caspase-3 and
caspase-9 cleavage (Fig. 5B and
C), and increased cell apoptosis (Fig. 6A and B) of curcumin-treated SUNE1
and SUNE2 cells. Subsequently, the pan-caspase inhibitor Z-VAD was
used to investigate the role of curcumin in cell death. As shown in
Fig. 6C and D, a marked reduction
in cell death was detected in SUNE1 and SUNE2 cells pretreated with
Z-VAD. This finding indicated that curcumin-mediated apoptosis may
be associated with caspase activation.
GRP78 knockdown reduces AKT activity in
human NPC cells
The AKT pathway is important in the modification of
cell growth and proliferation (19). Previous studies have reported that
the GRP78 signaling pathway can regulate the AKT signaling pathway
(20,21). Therefore, the present study aimed
to determine whether AKT was involved in GRP78-mediated cell death
during curcumin treatment. As shown in Fig. 7A and B, treatment of SUNE1 and
SUNE2 cells with curcumin (80 µM) significantly suppressed AKT
phosphorylation, whereas total AKT was only slightly altered.
Knockdown of GRP78 via siRNA alongside curcumin treatment led to a
marked reduction in AKT phosphorylation compared with in cells
treated with curcumin only. To further investigate the effects of
GRP78 expression and AKT phosphorylation, MK-2206, a chemical
inhibitor of AKT, was used to treat cells prior to treatment with
curcumin. As shown in Fig. 7C and
D, treating SUNE1 and SUNE2 cells with MK-2206 reduced
activation of AKT. Furthermore, MK-2206 markedly decreased the
expression levels of GRP78 (Fig. 7C
and D). These results indicated that GRP78 knockdown may reduce
AKT activity.
Discussion
NPC is a common cancer of the nasopharynx, with a
five year survival rate of 40-70% (22). Numerous patients with NPC
experience recurrence or metastasis due to resistance to standard
therapy with radiation (23).
Therefore, it is very important to identify novel treatment
modalities. Curcumin, which is a polyphenolic dienone, has been
demonstrated to induce apoptosis in several human malignancies
(24-27); however, the mechanisms underlying
the suppressive effects of curcumin on NPC remain to be completely
explored. The present study demonstrated that curcumin included ER
stress, cell cycle arrest and apoptosis of human NPC cells.
Knockdown of GRP78 by siRNA was able to significantly enhance
curcumin-induced NPC cell apoptosis.
Previous studies have reported that ER stress is not
only conducive to cell survival, but also induces apoptosis in
various cell types (28,29). The aim of the UPR is to reinstate
appropriate ER homeostasis; however, if ER stress perseveres, the
UPR signaling pathways can trigger apoptosis. Therefore, the
balance between the survival signal and the apoptosis signal
determines the survival and apoptosis of cells. GRP78 is an ER
molecular chaperone, which is important in folding and assembling
proteins (30). Previous research
has indicated that constitutive over expression of GRP78 is
associated with chemoresistance in cancer treatment (31), whereas knockdown of GRP78 can
enhance chemosensitivity in several tumor cells (18,32,33). According to the present study,
treatment of human NPC cells with curcumin led to ER stress, and
curcumin-induced ER stress in SUNE1 and SUNE2 cells was associated
with the up regulation of GRP78. In addition, silencing GRP78 using
siRNA significantly enhanced the cytotoxic and apoptotic effects of
curcumin in SUNE1 and SUNE2 cells. These data revealed that GRP78
may be crucial in protecting human NPC cells from curcumin-induced
apoptosis. Therefore, downregulation of GRP78 may markedly improve
the sensitivity of human NPC cells to curcumin.
Activation of the AKT signaling pathway is crucial
in modifying cell proliferation and motility in various types of
cancer; therefore, inhibition of AKT phosphorylation could suppress
tumor cell growth and proliferation (34,35). In addition, it has been
demonstrated that the AKT signaling pathway is associated with
chemoresistance and maintaining GRP78 expression (36,37). The present results revealed that
GRP78 knockdown by siRNA significantly inhibited the
phosphorylation of AKT in curcumin-treated cells. Furthermore,
inhibiting AKT activity using the chemical inhibitor MK-2206
prevented curcumin-mediated GRP78 induction, and suppressed AKT
activity, thus suggesting that GRP78 may function in NPC to
restrict chemotherapeutic-induced cytotoxicity via regulating the
AKT signaling pathway.
In conclusion, this study suggested that curcumin
may inhibit the viability and promote the apoptosis of human NPC
cells. Downregulation of GRP78 may further enhance curcumin-induced
SUNE1 and SUNE2 cell apoptosis. These findings are promising;
however, further research is required to develop novel therapeutic
strategies for human NPC.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81400376) and thee
Shandong Province Natural Science Foundation of China (grant no.
BS2014YY017).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XY performed experiments and analyzed data; XZ
designed the research, interpreted data and edited the manuscript;
LS wrote the manuscript and analyzed the data; LY analyzed the data
and reviewed the manuscript; HW interpreted data and reviewed the
manuscript; YW interpreted data and edited the manuscript. All
authors read and approved the final manuscript
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Dr. Edward C. Mignot
(Shandong University, Jinan, China) for linguistic advice.
References
|
1
|
Lee AW, Fee WE Jr, Ng WT and Chan LK:
Nasopharyngeal carcinoma: Salvage of local recurrence. Oral Oncol.
48:768–774. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu Y, Zhang J, Shi W and Liu Y: Anticancer
effects of 3,3′-diindolylmethane are associated with G1 arrest and
mitochondria-dependent apoptosis in human nasopharyngeal carcinoma
cells. Oncol Lett. 5:655–662. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiao WW, Huang SM, Han F, Wu SX, Lu LX,
Lin CG, Deng XW, Lu TX, Cui NJ and Zhao C: Local control, survival,
and late toxicities of locally advanced nasopharyngeal carcinoma
treated by simultaneous modulated accelerated radiotherapy combined
with cisplatin concurrent chemotherapy: Long-term results of a
phase 2 study. Cancer. 117:1874–1883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang J, Liang ZD, Wu TT, Hoque A, Chen H,
Jiang Y, Zhang H and Xu XC: Tumor-suppressive effect of retinoid
receptor-induced gene-1 (RRIG1) in esophageal cancer. Cancer Res.
67:1589–1593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ammon HP and Wahl MA: Pharmacology of
Curcuma longa. Planta Med. 57:1–7. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He M, Li Y, Zhang L, Li L, Shen Y, Lin L,
Zheng W, Chen L, Bian X, Ng HK and Tang L: Curcumin suppresses cell
proliferation through inhibition of the Wnt/β-catenin signaling
pathway in medulloblastoma. Oncol Rep. 32:173–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qiao Q, Jiang Y and Li G: Curcumin
enhances the response of non-Hodgkin's lymphoma cells to ionizing
radiation through further induction of cell cycle arrest at the
G2/M phase and inhibition of mTOR phosphorylation. Oncol Rep.
29:380–386. 2013. View Article : Google Scholar
|
|
8
|
Du Q, Hu B, An HM, Shen KP, Xu L, Deng S
and Wei MM: Synergistic anticancer effects of curcumin and
resveratrol in Hepa1-6 hepatocellular carcinoma cells. Oncol Rep.
29:1851–1858. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan Y, Xiao J, Liang G, Wang M, Wang D,
Wang S and Yang H: A new curcumin analogue exhibits enhanced
antitumor activity in nasopharyngeal carcinoma. Oncol Rep.
30:239–245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu X, Zhong J, Yan L, Li J, Wang H, Wen Y
and Zhao Y: Curcumin exerts antitumor effects in retinoblastoma
cells by regulating the JNK and p38 MAPK pathways. Int J Mol Med.
38:861–868. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cao A, Li Q, Yin P, Dong Y, Shi H, Wang L,
Ji G, Xie J and Wu D: Curcumin induces apoptosis in human gastric
carcinoma AGS cells and colon carcinoma HT-29 cells through
mitochondrial dysfunction and endoplasmic reticulum stress.
Apoptosis. 18:1391–1402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zanotto-Filho A, Braganhol E, Klafke K,
Figueiró F, Terra SR, Paludo FJ, Morrone M, Bristot IJ, Battastini
AM, Forcelini CM, et al: Autophagy inhibition improves the efficacy
of curcumin/temozolomide combination therapy in glioblastomas.
Cancer Lett. 358:220–231. 2015. View Article : Google Scholar
|
|
13
|
Bortolozzi R, Viola G, Porcù E, Consolaro
F, Marzano C, Pellei M, Gandin V and Bassoj G: A novel copper(I)
complex induces ER-stress-mediated apoptosis and sensitizes B-acute
lymphoblastic leukemia cells to chemotherapeutic agents.
Oncotarget. 5:5978–5991. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu C, Bailly-Maitre B and Reed JC:
Endoplasmic reticulum stress: Cell life and death decisions. J Clin
Invest. 115:2656–2664. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhong J, Dong X, Xiu P, Wang F, Liu J, Wei
H, Xu Z, Liu F, Li T and Li J: Blocking autophagy enhances
meloxicam lethality to hepatocellular carcinoma by promotion of
endoplasmic reticulum stress. Cell Prolif. 48:691–704. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhong J, Xiu P, Dong X, Wang F, Wei H,
Wang X, Xu Z, Liu F, Li T, Wang Y and Li J: Meloxicam combined with
sorafenib synergistically inhibits tumor growth of human
hepatocellular carcinoma cells via ER stress-related apoptosis.
Oncol Rep. 34:2142–2150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li W, Wang W, Dong H, Li Y, Li L, Han L,
Han Z, Wang S, Ma D and Wang H: Cisplatin-induced senescence in
ovarian cancer cells is mediated by GRP78. Oncol Rep. 31:2525–2534.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pi L, Li X, Song Q, Shen Y, Lu X and Di B:
Knockdown of glucose-regulated protein 78 abrogates chemoresistance
of hypo-pharyngeal carcinoma cells to cisplatin induced by unfolded
protein in response to severe hypoxia. Oncol Lett. 7:685–692. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yip PY: Phosphatidylinositol
3-kinase-AKT-mammalian target of rapamycin (PI3K-Akt-mTOR)
signaling pathway in non-small cell lung cancer. Transl Lung Cancer
Res. 4:165–176. 2015.PubMed/NCBI
|
|
20
|
Kelber JA, Panopoulos AD, Shani G, Booker
EC, Belmonte JC, Vale WW and Gray PC: Blockade of Cripto binding to
cell surface GRP78 inhibits oncogenic Cripto signaling via
MAPK/PI3K and Smad2/3 pathways. Oncogene. 28:2324–2336. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wey S, Luo B, Tseng CC, Ni M, Zhou H, Fu
Y, Bhojwani D, Carroll WL and Lee AS: Inducible knockout of
GRP78/BiP in the hematopoietic system suppresses Pten-null
leukemogenesis and AKT oncogenic signaling. Blood. 119:817–825.
2012. View Article : Google Scholar :
|
|
22
|
Zhang W, Yang P, Gao F, Yang J and Yao K:
Effects of epigallocatechin gallate on the proliferation and
apoptosis of the nasopharyngeal carcinoma cell line CNE2. Exp Ther
Med. 8:1783–1788. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma J, Wen ZS, Lin P, Wang X and Xie FY:
The results and prognosis of different treatment modalities for
solitary metastatic lung tumor from nasopharyngeal carcinoma: A
retrospective study of 105 cases. Chin J Cancer. 29:787–795. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yin H, Zhou Y, Wen C, Zhou C, Zhang W, Hu
X, Wang L, You C and Shao J: Curcumin sensitizes glioblastoma to
temozolomide by simultaneously generating ROS and disrupting
AKT/mTOR signaling. Oncol Rep. 32:1610–1616. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peng SF, Lee CY, Hour MJ, Tsai SC, Kuo DH,
Chen FA, Shieh PC and Yang JS: Curcumin-loaded nanoparticles
enhance apoptotic cell death of U2OS human osteosarcoma cells
through the Akt-Bad signaling pathway. Int J Oncol. 44:238–246.
2014. View Article : Google Scholar
|
|
26
|
Wu J, Tang Q, Zhao S, Zheng F, Wu Y, Tang
G and Hahn SS: Extracellular signal-regulated kinase
signaling-mediated induction and interaction of FOXO3a and p53
contribute to the inhibition of nasopharyngeal carcinoma cell
growth by curcumin. Int J Oncol. 45:95–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang SJ, Lee SA, Park MG, Kim JS, Yu SK,
Kim CS, Kim JS, Kim SG, Oh JS, Kim HJ, et al: Induction of
apoptosis by diphenyldifluoroketone in osteogenic sarcoma cells is
associated with activation of caspases. Oncol Rep. 31:2286–2292.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schroder M and Kaufman RJ: The mammalian
unfolded protein response. Annu Rev Biochem. 74:739–789. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang K and Kaufman RJ: The unfolded
protein response: A stress signaling pathway critical for health
and disease. Neurology. 66:S102–S109. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li J and Lee AS: Stress induction of
GRP78/BiP and its role in cancer. Curr Mol Med. 6:45–54. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee AS: GRP78 induction in cancer:
Therapeutic and prognostic implications. Cancer Res. 67:3496–3499.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang KH, Kuo KL, Chen SC, Weng TI, Chuang
YT, Tsai YC, Pu YS, Chiang CK and Liu SH: Down-regulation of
glucose-regulated protein (GRP) 78 potentiates cytotoxic effect of
celecoxib in human urothelial carcinoma cells. PLoS One.
7:e336152012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J, Yin Y, Hua H, Li M, Luo T, Xu L,
Wang R, Liu D, Zhang Y and Jiang Y: Blockade of GRP78 sensitizes
breast cancer cells to microtubules-interfering agents that induce
the unfolded protein response. J Cell Mol Med. 13:3888–3897. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Singh S, Trevino J, Bora-Singhal N,
Coppola D, Haura E, Altiok S and Chellappan SP: EGFR/Src/Akt
signaling modulates Sox2 expression and self-renewal of stem-like
side-population cells in non-small cell lung cancer. Mol Cancer.
11:732012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qin J, Ji J, Deng R, Tang J, Yang F, Feng
GK, Chen WD, Wu XQ, Qian XJ, Ding K and Zhu XF: DC120, a novel AKT
inhibitor, preferentially suppresses nasopharyngeal carcinoma
cancer stem-like cells by downregulating Sox2. Oncotarget.
6:6944–6958. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li B, Li J, Xu WW, Guan XY, Qin YR, Zhang
LY, Law S, Tsao SW and Cheung AL: Suppression of esophageal tumor
growth and chemoresistance by directly targeting the PI3K/AKT
pathway. Oncotarget. 5:11576–11587. 2014.PubMed/NCBI
|
|
37
|
Gray MJ, Mhawech-Fauceglia P, Yoo E, Yang
W, Wu E, Lee AS and Lin YG: AKT inhibition mitigates GRP78
(glucose-regulated protein) expression and contribution to
chemoresistance in endometrial cancers. Int J Cancer. 133:21–30.
2013. View Article : Google Scholar : PubMed/NCBI
|





















